Abstract
Stressful social experiences early in life, such as maternal separation and social isolation, have enduring effects on the development of the brain and behavior. In the present study in socially monogamous male prairie voles (Microtus ochrogaster), we found that following six weeks of social isolation after weaning males spent more time in the closed arms and less time in the open arms during an elevated plus maze (EPM) test, moved more frequently from central to peripheral squares in an open field test, and diminished their preferences for the empty chamber during a two-chamber affiliation test, compared to control males that were housed with siblings. This increased behavioral anxiety in socially isolated males was also associated with enhanced mRNA expression for vasopressin (AVP), oxytocin (OT), corticotrophin releasing factor (CRF), and tyrosine hydroxylase (TH) in the paraventricular nucleus of the hypothalamus (PVN). Together, these data illustrate the importance of the post-weaning social environment on anxiety-related behavior and suggest a potential role of neurochemical systems in the PVN in the regulation of this behavior.
Keywords: elevated plus maze, vasopressin, oxytocin, corticotropin releasing factor, tyrosine hydroxylase, PVN
Stressful early life experiences, such as social isolation, have long lasting effects on the development of the brain and behavior in a variety of mammalian species [19, 23, 40]. In rodents, for example, social isolation has been found to induce disturbances in subsequent behavioral and neuroendocrine functions [12, 23], and such effects are influenced by social structure; social animals usually have stronger responses to social isolation than do less social ones [44]. As social isolation is stressful [12, 23], such experience is often associated with altered activity of the neurochemical systems involved in anxiety and stress responses. For example, social isolation or stress early in life has been found to alter the activity of corticotrophin releasing factor (CRF) in the hypothalamus [24, 38]. Central vasopressin (AVP) and oxytocin (OT) activity also changes with social experience and during behavioral responses to social stimuli and stress [5, 28]. Furthermore, central dopamine may also play a role in the response to stress as social isolation has been shown to up-regulate the mRNA and protein expression of tyrosine hydroxylase (TH), the rate-limiting enzyme for dopamine synthesis, in the brain [2, 42]. It is important to note that social isolation may affect several neurochemical systems, which interact to mediate the activity of the hypothalamic-pituitary-adrenal (HPA) axis in the response to stress [34, 48].
Prairie voles (Microtus ochrogaster) are a socially monogamous rodent species [16]. In the field, male-female pairs share a nest after mating, stay together throughout breeding seasons, and participate in biparental care of offspring [14]. Additionally, the majority of prairie vole juveniles remain in the parental nest throughout adulthood, forming extended family units [14, 17, 33]. In the lab, members of prairie vole families are found to have high levels of social interactions through display of biparental, alloparental, play, and affiliative behaviors [7].
Although previous studies in the prairie vole have demonstrated that a social environment is important for normal behavior, HPA activity, and neurogenesis in adulthood [13, 20, 27, 39, 45], little is known about the effects of social isolation during early development. As the majority of prairie vole offspring remain in the parental nest throughout adulthood [14, 18, 46], the social environment encountered in the post-weaning period may be particularly important. Indeed, social variables such as the presence or absence of siblings, the population density, and the reproductive status of others in a communal group have all been shown to alter juvenile behavior in this species [14, 15, 33]. Therefore, the present study was designed to examine the effects of post-weaning social isolation on anxiety-related behaviors in the male prairie vole. Additionally, we examined the mRNA expression for CRF, AVP, OT and TH in the brain to reveal their potential roles in mediating environment-behavior interactions.
Male prairie voles that were offspring from our laboratory breeding colony were weaned at 21 days of age and then randomly assigned into either the control (housed with a same sex sibling, n=8) or social isolation (singly housed, n=9) group for 6 weeks. All animals were housed in plastic cages (29×18×13 cm) with cedar chip bedding and maintained under a 14L:10D photoperiod (lights on at 0700) at about 21 °C. Food and water were provided ad libitum. Following 6 weeks of social manipulation, subjects were tested for their affiliative and anxiety-related behaviors over three consecutive days, with one test per day.
The apparatus for the affiliation test consisted of two chambers (13×18×29 cm) connected with a hollow tube (7.5×16 cm). A novel male prairie vole was tethered in one chamber while another chamber remained empty. Each subject was put into the empty chamber and allowed to move freely through the apparatus for 60 min. A customized computer program using a series of light beams across the connecting tube was used to monitor subject’s movement between chambers. The duration that subjects spent in each chamber and frequency of chamber entries were recorded.
The open field test was conducted to examine locomotor activity and anxiety-related behavior [36]. The open-field was made of plastic (56×56×20 (H) cm) and its floor was divided by lines into 16 squares (14×14 cm). Each subject was placed individually in the center of the arena. The time that subjects spent in the center squares, number of squares crossed, and entries from central to peripheral squares were recorded during a 10-min test.
The Elevated Plus Maze (EPM) test was conducted to examine anxiety-related behavior [45]. The EPM apparatus was comprised of two open arms (35×6.5 cm) and two closed arms (35×6.5×15 (H) cm) that crossed in the middle, and was elevated 45 cm off the ground. The subject was placed on the central platform facing a closed arm. The number of subject’s entries into open and closed arms and time spent in each arm or on the center platform were recorded during a 10-min test. All behavioral testing apparatuses were cleaned thoroughly between subjects.
Following the EPM test, subjects were put back into the home cage for 30 min without disturbance. Thereafter, they were rapidly decapitated. Their brains were harvested, frozen on dry ice, and subsequently cut on a cryostat into coronal sections (14um in thickness) which were thaw-mounted onto slides. Four sets of brain sections at 98 μm intervals were processed for in situ hybridization labeling of AVP, OT, CRF, and TH mRNAs using previously established methods [9, 31]. The AVP and OT oligoprobes were 3’-end-labeled with 35S-dATP and the CRF and TH riboprobes were labeled with 35S-CTP. Detailed procedures for the probe labeling and purification, and for the in situ hybridization method used were described previously [9, 31]. Brain sections were fixed in 4% paraformaldehyde and hybridized overnight at 55°C. After washing, rinsing, and dehydrating, slides were air-dried and exposed to BioMax MR film (Kodak) for 1-7 days to generate autoradiograms.
AVP, OT, CRF and TH mRNA labeling was visualized from the autoradiograms and subsequently quantified using a computerized image program (NIH IMAGE 1.64) from selected brain areas, including the paraventricular (PVN) and supraoptic (SON) nuclei of the hypothalamus, central nucleus of the amygdala (CeA), and ventral tegmental area (VTA). The optical density of mRNA labeling in each brain area was quantified from 3-7 matched brain sections per subject [31]. Group differences in the density of the mRNA labeling as well as in all behavioral measurements were analyzed by t tests.
Social isolation affected anxiety-related behavior. During the 2-chamber affiliation test, control males spent more time in the empty chamber than in the chamber with a novel male (t=2.2, p<0.05), whereas such a preference disappeared in males that were socially isolated post-weaning (Fig 1a). The two groups did not differ in the frequency of chamber crossing (Fig 1b). They also did not differ in the time spent in the central squares and in the frequency of square crossing during the open field test (Table 1). However, socially isolated males made more entries from central to peripheral squares than did control males (t=2.4, p<0.05). Socially isolated males also spent more time in the closed arms (t=2.6, p<0.05) and less time in the open arms (t=2.2, p<0.05) than did control males during the EPM test (Fig 1c). The frequency of arm entries did not differ between the two groups (Fig 1d).
Figure 1.
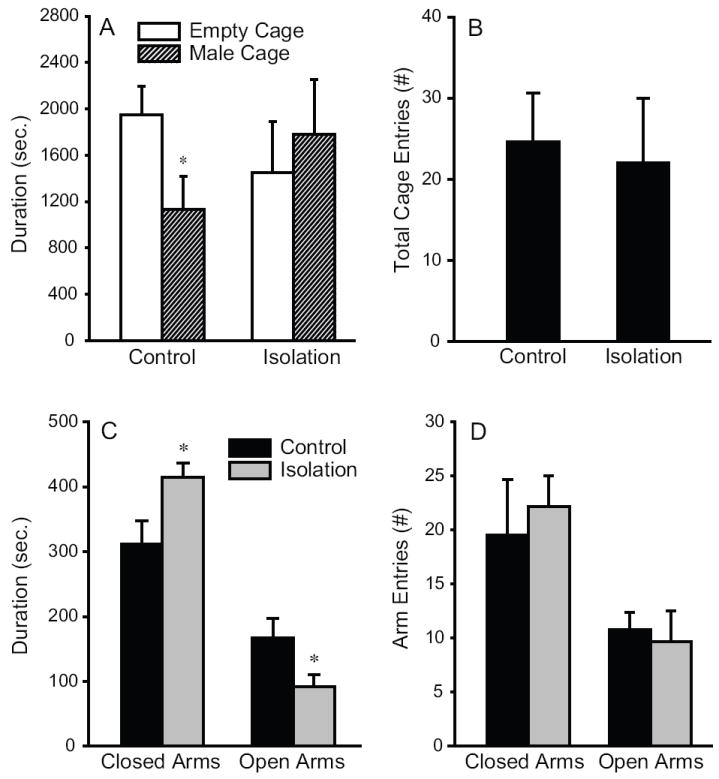
Post-weaning social isolation affects the behavior of male prairie voles. (A) During a two-chamber affiliation test, control males that were housed with a same sex sibling cage mate spent significantly more time in the empty chamber than in the chamber containing a novel male. Six weeks of post-weaning social isolation diminished such preferences. (B) The two groups did not differ in their locomotor activities, as indicated by total cage entries during the affiliation test. (C) During an elevated plus maze (EPM) test, socially isolated males spent significantly more time in closed arms and less time in open arms than did control males. (D) No group differences were found in the frequency of arm entries during the EPM test. *: p<0.05.
Table 1.
Behavior in the open field test
| Control | Isolation | p | ||
|---|---|---|---|---|
| Central Square | duration | 58.5 ± 18.1* | 64.4 ± 13.6 | ns |
| Square Crossing | frequency | 167.1 ± 26.1 | 274.1 ± 49.2 | ns |
| Central to Peripheral | frequency | 8.9 ± 1.6 | 21.9 ± 4.8 | <0.05 |
: mean±SEM in seconds.
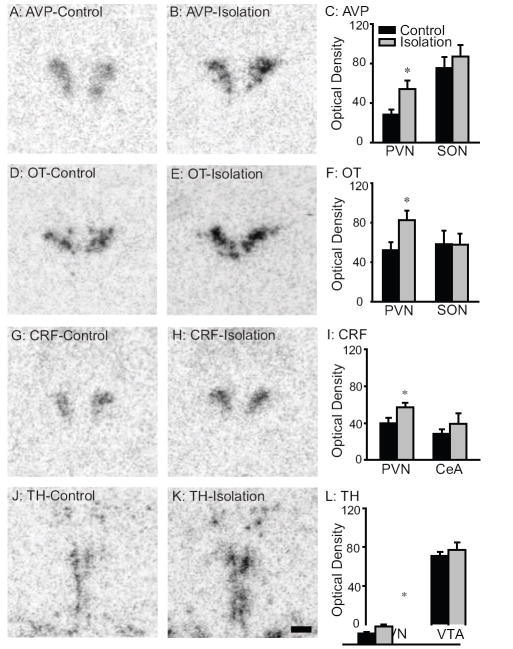
Dense clusters of AVP, OT, CRF, and TH mRNA labeling were found in the vole brains, as described in our previous study [31]. Socially isolated males expressed higher levels of AVP mRNA (t=2.5, p<0.05) and OT mRNA (t=2.5, p<0.05) labeling in the PVN, but not the SON, than did control males (Fig 2a-f). In addition, socially isolated males showed higher levels of CRF mRNA (t=2.2, p<0.05) and TH mRNA (t=3.0, p<0.01) labeling in the PVN, compared to the control males (Fig 2g-l). No group differences were found for CRF mRNA labeling in the CeA or TH mRNA labeling in the VTA (Fig 2i & 2l).
Figure 2.
Representative photomicrographs illustrating vasopressin (AVP; A & B), oxytocin (OT; D & E), corticotrophin releasing factor (CRF; G & H) and tyrosine hydroxylase (TH; J & K) mRNA labeling in the paraventricular nucleus of the hypothalamus (PVN) in the brain of male prairie voles that were housed with a same sex sibling cage mate (A, D, G, & J) or were socially isolated for 6 weeks post-weaning (B, E, H & K). Socially isolated males expressed higher levels of AVP mRNA (C), OT mRNA (F), CRF mRNA (I), and TH mRNA (L) labeling in the PVN. No group differences were found in AVP and OT mRNA labeling in the supraoptic nucleus of the hypothalamus (SON), CRF mRNA labeling in the central nucleus of the amygdala (CeA), and TH mRNA labeling in the ventral tegmental area (VTA). *: p<0.05. Scale bar=400μm.
Our data indicate that 6 weeks of post-weaning social isolation increased anxiety-related behavior and enhanced gene expression for AVP, OT, CRF, and TH in the PVN of the male prairie vole. Therefore, post-weaning social isolation is stressful, and this stress may alter neuroendocrine systems that modulate behavior in male prairie voles, as in other rodent species [3, 21, 47].
EPM and open field tests exploit rodents’ conflicting propensities to explore their surroundings and avoid open areas, and as such have been successfully used as ethologically relevant tests of anxiety in a variety of rodent species [6], including prairie voles [36, 45]. In the present study, increased anxiety in socially isolated male prairie voles was indicated by their spending more time in the closed arms and less time in the open arms during an EPM test and making more central to peripheral entries during an open field test, compared to control males. These data are consistent with that from adult prairie voles showing isolation enhanced anxiety- and depression-related behaviors [20, 27, 45]. Therefore, social isolation, either during the post-weaning period or in adulthood, represents a stressful experience that induces anxiety in prairie voles.
Our data from the affiliation test are intriguing. The preference for the empty chamber displayed by the control males may represent their tendency to avoid novel males, a mechanism that may underlie group cohesion in naturally occurring communal groups [8]. It may also indicate that they were less fearful of exploring a novel environment, i.e. the empty chamber. Interestingly, socially isolated males showed impaired preferences for the empty chamber. This can be explained by increased anxiety resulting from social isolation, which is consistent with the data from the EPM and open field tests. Additionally, it may also be due to their increased tendency to interact with novel conspecifics [8] as, in the field, socially excluded males may frequently engage in agonistic encounters with unfamiliar males [14].
Post-weaning social isolation increased the mRNA expression for CRF, AVP, OT and TH, particularly in the PVN of male prairie vole brains. Although our experimental design did not allow us to determine whether these alterations in mRNA expression were a result of social isolation alone, or the combination of isolation plus behavioral testing, our findings are in accordance with those noted in other rodent species [1, 4, 25, 41, 49].
Increased CRF mRNA expression in the PVN likely represents enhanced HPA activity, which is supported by the increased level of circulating corticosterone following social isolation and anxiety testing in adult male prairie voles [45]. Therefore, hyperactivity of the HPA axis may be a characteristic response to anxiety following social isolation during youth and adulthood in prairie voles. As the majority of juvenile male prairie voles tend to remain in the parental nest, surrounded by extended family members throughout adulthood [14, 33], it is not surprising that post-weaning social isolation would be stressful for members of this species.
Elevated AVP expression in the PVN of socially isolated male prairie voles may also be closely tied to their increase in anxiety-related behavior. PVN AVP has been implicated in anxiety-related behavior [29, 48] by having direct actions [11, 30] or through synergistic interactions with CRF [1]. Similarly, enhanced OT mRNA expression in the PVN may be involved in mediating anxiety-related behaviors, as PVN OT has been implicated in the response to acute stress [35] and EPM testing seems to be an acute and mild stressor for prairie voles [45]. Finally, as TH neurons in the PVN can be activated by different stimuli including mild stressors [37], increased TH mRNA expression may indicate enhanced dopamine release which, in turn, may play a role in activation of CRF neurons in the PVN, mediating stress responses [10].
It is important to note that, in the present study, group differences in neurochemical mRNA labeling were only found in the PVN. These data suggest that social isolation and anxiety induce neurochemical gene activation in a region-specific manner, further illustrating the importance of the PVN in mediating the HPA activity and stress responses following social isolation in prairie voles. Although CRF in the CeA [22, 32], AVP and OT in the SON [26], and dopamine in the VTA [43] have been implicated in a variety of stress responses in other rodent species, it is possible that social isolation in prairie voles may represent a mild stressor not potent enough to increase neurochemical gene expression in those brain areas. It is also possible that social isolation only induced a transient increase in the neurochemical gene expression in those brain areas, which could not be detected by our 6-week paradigm. These questions need to be addressed in future studies.
Acknowledgments
This work was supported by National Institutes of Health grants MHR01-58616, DAR01-19627, and DAK02-23048 to ZXW.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Aguilera G, Rabadan-Diehl C. Vasopressinergic regulation of the hypothalamic-pituitary-adrenal axis: implications for stress adaptation. Regul Pept. 2000;96:23–9. doi: 10.1016/s0167-0115(00)00196-8. [DOI] [PubMed] [Google Scholar]
- 2.Angulo JA, Printz D, Ledoux M, McEwen BS. Isolation stress increases tyrosine hydroxylase mRNA in the locus coeruleus and midbrain and decreases proenkephalin mRNA in the striatum and nucleus accumbens. Brain Res Mol Brain Res. 1991;11:301–8. doi: 10.1016/0169-328x(91)90039-z. [DOI] [PubMed] [Google Scholar]
- 3.Anisman H, Zaharia MD, Meaney MJ, Merali Z. Do early-life events permanently alter behavioral and hormonal responses to stressors? Int J Dev Neurosci. 1998;16:149–64. doi: 10.1016/s0736-5748(98)00025-2. [DOI] [PubMed] [Google Scholar]
- 4.Bartanusz V, Jezova D, Bertini LT, Tilders FJ, Aubry JM, Kiss JZ. Stress-induced increase in vasopressin and corticotropin-releasing factor expression in hypophysiotrophic paraventricular neurons. Endocrinology. 1993;132:895–902. doi: 10.1210/endo.132.2.8425502. [DOI] [PubMed] [Google Scholar]
- 5.Bester-Meredith JK, Marler CA. Vasopressin and the transmission of paternal behavior across generations in mated, cross-fostered Peromyscus mice. Behav Neurosci. 2003;117:455–63. doi: 10.1037/0735-7044.117.3.455. [DOI] [PubMed] [Google Scholar]
- 6.Carobrez AP, Bertoglio LJ. Ethological and temporal analyses of anxiety-like behavior: the elevated plus-maze model 20 years on. Neurosci Biobehav Rev. 2005;29:1193–205. doi: 10.1016/j.neubiorev.2005.04.017. [DOI] [PubMed] [Google Scholar]
- 7.Carter CS, Getz LL. Monogamy and the prairie vole. Sci Am. 1993;268:100–106. doi: 10.1038/scientificamerican0693-100. [DOI] [PubMed] [Google Scholar]
- 8.DeVries AC, Johnson CL, Carter CS. Familiarity and gender influence social preferences in prairie voles (Microtus ochrogaster) Can J Zool. 1997;75:295–301. [Google Scholar]
- 9.Dietz DM, Tapocik J, Gaval-Cruz M, Kabbaj M. Dopamine transporter, but not tyrosine hydroxylase, may be implicated in determining individual differences in behavioral sensitization to amphetamine. Physiol Behav. 2005;86:347–55. doi: 10.1016/j.physbeh.2005.08.005. [DOI] [PubMed] [Google Scholar]
- 10.Eaton MJ, Cheung S, Moore KE, Lookingland KJ. Dopamine receptor-mediated regulation of corticotropin-releasing hormone neurons in the hypothalamic paraventricular nucleus. Brain Res. 1996;738:60–6. doi: 10.1016/0006-8993(96)00765-2. [DOI] [PubMed] [Google Scholar]
- 11.Engelmann M, Landgraf R, Wotjak CT. The hypothalamic-neurohypophysial system regulates the hypothalamic-pituitary-adrenal axis under stress: an old concept revisited. Front Neuroendocrinol. 2004;25:132–49. doi: 10.1016/j.yfrne.2004.09.001. [DOI] [PubMed] [Google Scholar]
- 12.Fone KC, Porkess MV. Behavioural and neurochemical effects of post-weaning social isolation in rodents-relevance to developmental neuropsychiatric disorders. Neurosci Biobehav Rev. 2008;32:1087–102. doi: 10.1016/j.neubiorev.2008.03.003. [DOI] [PubMed] [Google Scholar]
- 13.Fowler CD, Liu Y, Ouimet C, Wang Z. The effects of social environment on adult neurogenesis in the female prairie vole. J Neurobiol. 2002;51:115–28. doi: 10.1002/neu.10042. [DOI] [PubMed] [Google Scholar]
- 14.Getz LL, Carter CS. Prairie-vole partnerships. American Scientist. 1996;84:56–62. [Google Scholar]
- 15.Getz LL, Carter CS. Social organization in Microtus ocrogaster populations. The Biologist. 1980;62:56–69. [Google Scholar]
- 16.Getz LL, Carter SC, Gavish L. The mating system of the prairie vole, Microtus ochrogaster: field and laboratory evidence for pair-bonding. Behav Ecol Sociobiol. 1981;8:189–194. [Google Scholar]
- 17.Getz LL, Hofmann JE. Social organizations in free-living prairie voles, Microtus ochrogaster. Behav Ecol Sociobiol. 1986;18:275–282. [Google Scholar]
- 18.Getz LL, Mc Guire B, Pizzuto T, Hofmann JE, Frase B. Social organization of the prairie vole (Microtus ochrogaster) J Mamm. 1993;74:44–58. [Google Scholar]
- 19.Gilmer WS, McKinney WT. Early experience and depressive disorders: human and non-human primate studies. J Affect Disord. 2003;75:97–113. doi: 10.1016/s0165-0327(03)00046-6. [DOI] [PubMed] [Google Scholar]
- 20.Grippo AJ, Gerena D, Huang J, Kumar N, Shah M, Ughreja R, Carter CS. Social isolation induces behavioral and neuroendocrine disturbances relevant to depression in female and male prairie voles. Psychoneuroendocrinology. 2007;32:966–80. doi: 10.1016/j.psyneuen.2007.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hall FS. Social deprivation of neonatal, adolescent, and adult rats has distinct neurochemical and behavioral consequences. Crit Rev Neurobiol. 1998;12:129–62. doi: 10.1615/critrevneurobiol.v12.i1-2.50. [DOI] [PubMed] [Google Scholar]
- 22.Hand GA, Hewitt CB, Fulk LJ, Stock HS, Carson JA, Davis JM, Wilson MA. Differential release of corticotropin-releasing hormone (CRH) in the amygdala during different types of stressors. Brain Res. 2002;949:122–30. doi: 10.1016/s0006-8993(02)02972-4. [DOI] [PubMed] [Google Scholar]
- 23.Heidbreder CA, Weiss IC, Domeney AM, Pryce C, Homberg J, Hedou G, Feldon J, Moran MC, Nelson P. Behavioral, neurochemical and endocrinological characterization of the early social isolation syndrome. Neuroscience. 2000;100:749–68. doi: 10.1016/s0306-4522(00)00336-5. [DOI] [PubMed] [Google Scholar]
- 24.Heim C, Nemeroff CB. The impact of early adverse experiences on brain systems involved in the pathophysiology of anxiety and affective disorders. Biol Psychiatry. 1999;46:1509–22. doi: 10.1016/s0006-3223(99)00224-3. [DOI] [PubMed] [Google Scholar]
- 25.Hesketh S, Jessop DS, Hogg S, Harbuz MS. Differential actions of acute and chronic citalopram on the rodent hypothalamic-pituitary-adrenal axis response to acute restraint stress. J Endocrinol. 2005;185:373–82. doi: 10.1677/joe.1.06074. [DOI] [PubMed] [Google Scholar]
- 26.Jezova D, Skultetyova I, Tokarev DI, Bakos P, Vigas M. Vasopressin and oxytocin in stress. Ann N Y Acad Sci. 1995;771:192–203. doi: 10.1111/j.1749-6632.1995.tb44681.x. [DOI] [PubMed] [Google Scholar]
- 27.Kim JW, Kirkpatrick B. Social isolation in animal models of relevance to neuropsychiatric disorders. Biol Psychiatry. 1996;40:918–22. doi: 10.1016/0006-3223(95)00546-3. [DOI] [PubMed] [Google Scholar]
- 28.Kramer KM, Yamamoto Y, Hoffman GE, Cushing BS. Estrogen receptor alpha and vasopressin in the paraventricular nucleus of the hypothalamus in Peromyscus. Brain Res. 2005;1032:154–61. doi: 10.1016/j.brainres.2004.10.070. [DOI] [PubMed] [Google Scholar]
- 29.Landgraf R. The involvement of the vasopressin system in stress-related disorders. CNS Neurol Disord Drug Targets. 2006;5:167–79. doi: 10.2174/187152706776359664. [DOI] [PubMed] [Google Scholar]
- 30.Landgraf R, Neumann ID. Vasopressin and oxytocin release within the brain: a dynamic concept of multiple and variable modes of neuropeptide communication. Front Neuroendocrinol. 2004;25:150–76. doi: 10.1016/j.yfrne.2004.05.001. [DOI] [PubMed] [Google Scholar]
- 31.Liu Y, Curtis JT, Fowler CD, Spencer C, Houpt T, Wang ZX. Differential expression of vasopressin, oxytocin and corticotrophin-releasing hormone messenger RNA in the paraventricular nucleus of the prairie vole brain following stress. J Neuroendocrinol. 2001;13:1059–65. doi: 10.1046/j.1365-2826.2001.00729.x. [DOI] [PubMed] [Google Scholar]
- 32.Makino S, Shibasaki T, Yamauchi N, Nishioka T, Mimoto T, Wakabayashi I, Gold PW, Hashimoto K. Psychological stress increased corticotropin-releasing hormone mRNA and content in the central nucleus of the amygdala but not in the hypothalamic paraventricular nucleus in the rat. Brain Res. 1999;850:136–43. doi: 10.1016/s0006-8993(99)02114-9. [DOI] [PubMed] [Google Scholar]
- 33.McGuire B, Getz LL, Hofmann J, Pizzuto T, Frase B. Natal dispersal and philopatry in prairie voles (Microtus ochrogaster) in relation to population density, season, and natal social environment. Behav Ecol Sociobiol. 1993;32:293–302. [Google Scholar]
- 34.Neumann ID. Involvement of the brain oxytocin system in stress coping: interactions with the hypothalamo-pituitary-adrenal axis. Prog Brain Res. 2002;139:147–62. doi: 10.1016/s0079-6123(02)39014-9. [DOI] [PubMed] [Google Scholar]
- 35.Neumann ID, Torner L, Toschi N, Veenema AH. Oxytocin actions within the supraoptic and paraventricular nuclei: differential effects on peripheral and intranuclear vasopressin release. Am J Physiol Regul Integr Comp Physiol. 2006;291:R29–36. doi: 10.1152/ajpregu.00763.2005. [DOI] [PubMed] [Google Scholar]
- 36.Olazabal DE, Young LJ. Variability in “spontaneous” maternal behavior is associated with anxiety-like behavior and affiliation in naive juvenile and adult female prairie voles (Microtus ochrogaster) Dev Psychobiol. 2005;47:166–78. doi: 10.1002/dev.20077. [DOI] [PubMed] [Google Scholar]
- 37.Pirnik Z, Kiss A. Fos expression variances in mouse hypothalamus upon physical and osmotic stimuli: co-staining with vasopressin, oxytocin, and tyrosine hydroxylase. Brain Res Bull. 2005;65:423–31. doi: 10.1016/j.brainresbull.2005.02.023. [DOI] [PubMed] [Google Scholar]
- 38.Plotsky PM, Thrivikraman KV, Nemeroff CB, Caldji C, Sharma S, Meaney MJ. Long-term consequences of neonatal rearing on central corticotropin-releasing factor systems in adult male rat offspring. Neuropsychopharmacology. 2005;30:2192–204. doi: 10.1038/sj.npp.1300769. [DOI] [PubMed] [Google Scholar]
- 39.Ruscio MG, Sweeny T, Hazelton J, Suppatkul P, Sue Carter C. Social environment regulates corticotropin releasing factor, corticosterone and vasopressin in juvenile prairie voles. Horm Behav. 2007;51:54–61. doi: 10.1016/j.yhbeh.2006.08.004. [DOI] [PubMed] [Google Scholar]
- 40.Sanchez MM, Ladd CO, Plotsky PM. Early adverse experience as a developmental risk factor for later psychopathology: evidence from rodent and primate models. Dev Psychopathol. 2001;13:419–49. doi: 10.1017/s0954579401003029. [DOI] [PubMed] [Google Scholar]
- 41.Sawchenko PE, Brown ER, Chan RK, Ericsson A, Li HY, Roland BL, Kovacs KJ. The paraventricular nucleus of the hypothalamus and the functional neuroanatomy of visceromotor responses to stress. Prog Brain Res. 1996;107:201–22. doi: 10.1016/s0079-6123(08)61866-x. [DOI] [PubMed] [Google Scholar]
- 42.Segal DS, Knapp S, Kuczenski RT, Mandell AJ. The effects of environmental isolation on behavior and regional rat brain tyrosine hydroxylase and tryptophan hydroxylase activities. Behav Biol. 1973;8:47–53. doi: 10.1016/s0091-6773(73)80005-7. [DOI] [PubMed] [Google Scholar]
- 43.Serova LI, Nankova BB, Feng Z, Hong JS, Hutt M, Sabban EL. Heightened transcription for enzymes involved in norepinephrine biosynthesis in the rat locus coeruleus by immobilization stress. Biol Psychiatry. 1999;45:853–62. doi: 10.1016/s0006-3223(98)90360-2. [DOI] [PubMed] [Google Scholar]
- 44.Shimozuru M, Kikusui T, Takeuchi Y, Mori Y. Effects of isolation-rearing on the development of social behaviors in male Mongolian gerbils (Meriones unguiculatus) Physiol Behav. 2008;94:491–500. doi: 10.1016/j.physbeh.2008.03.003. [DOI] [PubMed] [Google Scholar]
- 45.Stowe JR, Liu Y, Curtis JT, Freeman ME, Wang Z. Species differences in anxiety-related responses in male prairie and meadow voles: the effects of social isolation. Physiol Behav. 2005;86:369–78. doi: 10.1016/j.physbeh.2005.08.007. [DOI] [PubMed] [Google Scholar]
- 46.Wang ZX, Novak MA. Alloparental care and the influence of father presence on juvenile prairie voles, Microtus ochrogaster. Anim Behav. 1994;47:281–288. [Google Scholar]
- 47.Weiss IC, Pryce CR, Jongen-Relo AL, Nanz-Bahr NI, Feldon J. Effect of social isolation on stress-related behavioural and neuroendocrine state in the rat. Behav Brain Res. 2004;152:279–95. doi: 10.1016/j.bbr.2003.10.015. [DOI] [PubMed] [Google Scholar]
- 48.Wigger A, Sanchez MM, Mathys KC, Ebner K, Frank E, Liu D, Kresse A, Neumann ID, Holsboer F, Plotsky PM, Landgraf R. Alterations in central neuropeptide expression, release, and receptor binding in rats bred for high anxiety: critical role of vasopressin. Neuropsychopharmacology. 2004;29:1–14. doi: 10.1038/sj.npp.1300290. [DOI] [PubMed] [Google Scholar]
- 49.Wotjak CT, Naruo T, Muraoka S, Simchen R, Landgraf R, Engelmann M. Forced swimming stimulates the expression of vasopressin and oxytocin in magnocellular neurons of the rat hypothalamic paraventricular nucleus. Eur J Neurosci. 2001;13:2273–81. doi: 10.1046/j.0953-816x.2001.01613.x. [DOI] [PubMed] [Google Scholar]