Abstract
Lens epithelial cells withdraw from the cell cycle to differentiate into secondary fibre cells in response to vitreal factors. Fibroblast growth factor (FGF) in the vitreous has been shown to induce lens fibre differentiation in vivo and in vitro through the activation of defined intracellular signalling, namely via MAPK/ERK1/2 and PI3-K/Akt pathways. To better understand the role of these growth factor-activated signalling pathways in lens fibre differentiation, FGF- and vitreous-induced lens fibre differentiation was examined in primary rat lens epithelial cell explants. The induction of cell elongation and fibre specific β- and γ-crystallin expression in lens explants was accompanied by distinct phosphorylation profiles for ERK1/2 and Akt. Using selective inhibitors (U0126 and LY294002) in blocking studies, these pathways were shown to be required for different aspects of lens fibre differentiation. Furthermore, a short ‘pulse’ treatment of explants with FGF showed that the activation of ERK1/2 over 24 hours was not sufficient for the progression of lens fibre differentiation and that cyclic ERK1/2 phosphorylation was required throughout the extended differentiation process. In conclusion, these results support a key role for both ERK1/2 and PI3-kinase/Akt signalling pathways in FGF- and vitreous-induced lens fibre differentiation.
Keywords: lens fibre differentiation, vitreous humour, fibroblast growth factor, ERK1/2, PI3-kinase/Akt
Introduction
The distinct polarity of the lens is characterised by a monolayer of cuboidal epithelial cells that overlies a mass of elongated fibre cells, all surrounded by a thick lens capsule. The anteriorly situated epithelium is bathed by the aqueous, while the posterior fibre cells are primarily bathed by the vitreous. The peripheral epithelial cells in the germinative zone proliferate and subsequently exit the cell cycle as they enter the transitional zone. Here they start to elongate and express β- and γ-crystallins as they differentiate into secondary fibres (Lovicu and McAvoy, 2005).
Early lens inversion experiments by Coulombre and Coulombre (1963) demonstrated that the distinctive polarity of the lens is determined by the ocular environment, with the vitreous important for inducing the differentiation of the epithelial cells into fibre cells. The vitreous is a virtually acellular, highly hydrated extracellular matrix, containing a number of different growth factors, including fibroblast growth factor (FGF; Mascarelli et al., 1987), insulin-like growth factor (IGF; Beebe et al., 1987), platelet-derived growth factor (PDGF; Cassidy et al., 1998), epidermal growth factor (EGF; Majima K 1997), bone morphogenetic protein (BMP; Belecky-Adams et al., 2002) and transforming growth factor β (TGFβ; Lutty et al., 1983). To date, FGF is the only growth factor capable of initiating lens fibre differentiation in the mammalian lens, exerting its effects in a dose-dependent manner (Chamberlain and McAvoy, 1989). For example, a low dose of FGF can induce cell proliferation, whereas a higher dose of FGF is required to induce fibre cell differentiation (Chamberlain and McAvoy, 1989). Moreover, FGF purified from the vitreous can also induce fibre cell differentiation in rat lens epithelial explants (Schulz et al., 1993; Lovicu et al., 1995). Further support for this comes from transgenic mouse studies overexpressing FGF in the eye, which result in disruption of the normal development of the lens (Robinson et al., 1995a; Lovicu and Overbeek, 1998). In these mouse models, increased levels of FGF induce the epithelial cells to inappropriately exit from the cell cycle and differentiate into fibre cells. Other studies using a similar transgenic approach have overexpressed either truncated FGF receptors (FGFRs) or secreted forms of the FGFR (Chow et al., 1995; Robinson et al., 1995b; Stolen and Griep, 2000; Govindarajan and Overbeek, 2001), effectively blocking FGF-mediated signalling and subsequent lens differentiation. More recent studies support an essential role for FGF in lens development, with conditional deletion of FGFR-1, -2 and -3 in the early stages of lens morphogenesis (Zhao et al., 2003).
Growth factors such as FGFs activate a cascade of intracellular regulatory molecules by binding to specific high affinity receptor tyrosine kinases (RTKs). Growth factor binding results in receptor dimerisation leading to intracellular tyrosine autophosphorylation, subsequently activating a number of different signalling pathways including the phospholipase Cγ (PLCγ) pathway, the mitogen-activated protein kinase/extracellular regulated kinase (MAPK/ERK) pathway and the phosphatidylinositol 3-kinase (PI3-K) signalling pathway (Haugh, 2002). MAPK/ERK1/2 signalling is a central pathway that is mediated by the small, membrane-anchored guanosine triphosphatase (GTPase) Ras, which acts as a molecular switch, cycling between active (GTP bound) and inactive forms (GDP bound) (Foschi et al., 1997). Ras activates Raf which subsequently phosphorylates MAPK kinases1 and 2 (MEK1/2). MEK1/2 in turn phosphorylates ERK1/2 which then enters the nucleus to activate transcription factors, initiating specific gene expression (Yoon and Seger, 2006). FGF-induced MAPK/ERK1/2 signalling has been shown to be required for lens epithelial cell proliferation and fibre differentiation in both chickens and rats (Le and Musil, 2001; Lovicu and McAvoy, 2001; Iyengar et al., 2006). Further studies showed that different concentrations of FGF, leading to different cellular outcomes, were associated with differences in the duration of ERK1/2 activation (Iyengar et al., 2007). A high fibre-differentiating concentration of FGF activated a sustained phosphorylation of ERK1/2, whereas a lower proliferating dose of FGF resulted in a shorter period of ERK1/2 phosphorylation.
Another RTK-activated signalling pathway, the PI3-K pathway, has been shown to regulate mitogenesis, differentiation, vesicular trafficking, cell migration, tubule formation, and prevention of apoptosis (Franke et al., 1997). The PI3-K pathway can be activated by PDGF, EGF, FGF and IGF/insulin (Hanada et al., 2004). PI3-K is a heterodimeric protein comprising of a 110 kD catalytic subunit (p110) and an 85 kD regulatory subunit (p85). PI3-K promotes the conversion of membrane lipid phosphatidylinositol 4, 5-biphosphate (PIP2) into the second messenger phosphatidylinositol 3, 4, 5-triphosphate (PIP3). Increasing levels of PIP3 result in translocation of the serine/threonine kinase Akt to the cell membrane, through interactions between PIP3 and the pleckstrin homology domains of these proteins (Neufeld, 2003). At the plasma membrane, the activation of Akt requires phosphorylation of two conserved residues, Thr308 and Ser473 (Brunet et al., 2001). Once Akt is activated, it relocates to several subcellular sites to phosphorylate its substrates, including Forkhead transcription factors (FOXO), GSK-3, BAD and MDM2 (Toker et al., 2006). The PI3-kinase signalling pathway has been shown to be crucial for lens epithelial cell proliferation and fibre differentiation (Chandrasekher and Sailaja, 2004; Iyengar et al., 2006; Weber and Menko, 2006). PI3-K signalling is required for lens epithelial proliferation induced by many different growth factors such as FGF, IGF, PDGF or EGF (Iyengar et al., 2006). The initiation of lens cell differentiation is regulated by the PI3-K pathway through reorganisation of the actin cytoskeleton from stress fibres to cortical fibres (Weber and Menko, 2006).
Previous studies have demonstrated the importance of both ERK1/2 and PI3-K signalling in lens fibre differentiation, but little is known of the vitreous-induced signalling pathways involved in this process. To better understand the function of ERK1/2 and PI3-K/Akt signalling pathways in lens fibre differentiation, in this present study we examined these two pathways using the lens epithelial explant system. Here we show that both ERK1/2 and Akt signalling pathways are differentially activated in lens cells in response to vitreous or a fibre differentiating dose of FGF. Both vitreous and FGF induce a sustained ERK1/2 activation profile. Using selective pharmacological inhibitors for these signalling pathways, we show that both ERK1/2 and Akt are required for lens fibre differentiation. Using a tyrosine phosphatase inhibitor, we also demonstrate that prolonging the activation of ERK1/2, even in a ligand-independent fashion, is sufficient to induce lens fibre differentiation. Contrary to this, although exposing lens cells to a short pulse treatment with FGF was shown to activate a sustained ERK1/2 phosphorylation profile within 24 hours, this condition was not sufficient to induce a lens fibre differentiation response over 5 days of culture. We demonstrate that a continual cycle of ERK1/2 phosphorylation is required for the fibre differentiation process to progress. By comparing the ERK1/2 and Akt phosphorylation profiles induced by FGF and vitreous, we also show that FGF is involved in vitreous-induced lens fibre differentiation; however, FGF can not completely replace the effects of vitreous. Other growth factors in the vitreous most likely contribute to this process. Overall our studies support that tight regulation of both ERK1/2 and PI3-K/Akt signalling pathways are required for lens fibre differentiation.
Materials and Methods
All experimental procedures conformed to the National Health and Medical Research Council (NHMRC, Australia) guidelines, and the Association for Research in Vision and Ophthalmology (ARVO, USA) handbook, for the use of animals in biomedical research. All protocols were approved by the Animal Ethical Review Committee of the University of Sydney, Australia.
Reagents were obtained from the following sources: recombinant human FGF-2 (R&D system, Inc, USA); sheep anti-rabbit IgG-HRP conjugated antibody, mouse anti-phospho-ERK1/2 (pERK1/2; Thr202/Tyr204) monoclonal antibody, rabbit anti-phospho-Akt (pAkt; Ser473) polyclonal antibody and rabbit anti-ERK1/2 polyclonal antibody (p44/p42 MAP kinase;Cell Signalling Technology, MA, USA), bisbenzimide (Hoechst dye, Calbiochem, CA, USA); goat anti-mouse IgG-HRP conjugated antibody (Zymed, CA, USA); Goat anti-rabbit IgG conjugated with fluorescein-isothiocyanate (FITC, Sigma, Australia); Micro BCA protein assay kit (Pierce, IL, USA).
Preparation of lens epithelial explants
All tissue culture was performed using medium 199 with Earle’s salts (M199; Trace Scientific, NSW, Australia), supplemented with 0.1% bovine serum albumin (BSA; Sigma), 0.1 μg/ml L-glutamine, 50 IU/ml penicillin, 50 μg/ml streptomycin and 2.5 μg/ml Amphostat B (all from Trace Scientific). Methodology has been described previously by Iyengar et al. (2006). In brief, ten-day old Wistar rats were sacrificed. Eyes were removed and placed in pre-warmed culture medium. Lenses were isolated and the posterior lens capsule was torn to remove the fibre cell mass. The remaining lens capsule (containing the adherent epithelial cell monolayer) was pinned flat to the base of the culture dish with fine forceps. Culture medium was replaced with 1 ml of fresh, equilibrated (37°C, 5% CO2) medium.
Collection of vitreous humour
Bovine eyes (Wilberforce Meats, NSW, Australia) were kept on ice immediately after harvesting until use. The method for collection of vitreous humour has been described previously (Schulz et al., 1993). In summary, following the removal of the aqueous humour with a syringe, scissors were used to dissect the anterior portion of the eye with the lens attached. To avoid any contamination with retinal tissue, approximately half of the vitreous humour (vitreous normally situated closest to the lens) was placed in a sterile dish and pooled with vitreous from at least another 20 eyes. The collected vitreous was then homogenized by passing it through a 19-guage needle, before storing it at −20°C until required.
Protein extraction
Explants to be used for SDS-PAGE and western blotting were cultured for different times (between 5 min to 24 h). At the end of the culture period, explants were rinsed in cold PBS and cell proteins extracted in lysis buffer [1 mM EDTA, 10 mM Tris-HCl (pH 7.5), 0.15 M NaCl, 1% IGEPAL (Sigma), 1 mM Na3VO4 (Sigma) and protease inhibitor cocktail (Roche, Basel, Switzerland)] for 60 min at 4°C. Protein concentration were determined using the Micro-BSA protein assay according to the manufacturer’s instructions (Pierce).
SDS-PAGE and western blotting
The phosphorylation status (activation) of ERK1/2 in explant extracts was assayed using SDS-PAGE and western blotting with commercially available antibodies specific for phosphorylated and non-phosphorylated forms of ERK1/2. In brief, for each lane, up to 3 μg of protein was electrophoresed through a 10% SDS-PAGE gel before being transferred to a PVDF membrane (Millipore Australia, NSW) for western blot analysis. The membrane was blocked with 2.5% BSA in TBST (0.1% Tween 20 in Tris-buffered saline, pH7.6) for 1 h before an overnight incubation at 4°C with a monoclonal antibody specific to phospho-ERK1/2 (diluted 1:1000) or a polyclonal antibody specific for total ERK1/2 (diluted 1:1000). The membrane was then incubated for 2 h at room temperature with a secondary antibody; for chemiluminescence we used horseradish peroxidase (HRP)-conjugated goat anti-mouse IgG (Zymed; diluted 1:10,000) or sheep anti-rabbit IgG (Cell Signalling Technology; diluted 1:5,000). Signals were detected using the ECL system (Amersham Bioscience, UK).
For re-labelling of blots, primary blots were stripped with stripping buffer (2% SDS, 60 mM Tris (pH 6.7), and 100 mM β-mercaptoethanol) for 20 min at 60°C. Following three rinses with TBST, the blot was incubated in blocking buffer and further immunolabelled as required.
In all cases, the experiments were carried out at least three times and representative blots were presented and analysed. The intensity of the protein labelled was quantified using AlphaEaseFc Software (Alpha Innotech, CA). Each band was measured in triplicate. The relative density was obtained using the formula: (DpAkt/DERK1/2)sample/(DpAkt/DERK1/2)control., where D represents the density of each band. DpAkt/DERK1/2 adjusts the density of each band against reference bands (e.g. for total ERK1/2 which we used as our standard).
Immunofluorescence
Explants to be used for immunofluorescent labelling were cultured for 5 days. At the end of the culture period, explants were fixed in 10% neutral buffered formalin (NBF) for 20 min and rinsed in phosphate buffered saline (PBS) supplemented with BSA (PBS/BSA), followed by two rinses in PBS/BSA supplemented with 0.1% Tween 20 (PBS-T) and another in PBS/BSA. Explants were incubated with 3% normal goat serum (NGS) for 30 min to reduce non-specific labelling. Excess NGS was removed and explants were incubated overnight at 4°C with a polyclonal antibody specific to β- or γ-crystallin (diluted 1:40 with PBS/BSA). After a brief rinse in PBS/BSA, explants were incubated for 2 h at room temperature with an anti-rabbit IgG antibody conjugated to FITC (Sigma), rinsed again in PBS/BSA and counter-stained with 1μg/ml Hoechst dye to label cell nuclei. Explants were examined using fluorescence microscopy.
To better demonstrate the changes in morphology of cells in our explants, representative treated lens epithelial explants were re-used following several rinses in PBS/BSA. Explants were labelled for 10 minutes for lectin binding using a TRITC-conjugated Triticum vulgaris lectin (30ug/ml; Sigma). Images of labelled cells were obtained using a Zeiss LSM5 Pascal laser confocal microscope (Carl Zeiss, NSW, Australia).
Inhibitor treatment
U0126 (1,4-diamino-2,3-dicyano-1,4-bis[2-aminophenylthio] butadiene; Promega, NSW, Australia) is a highly selective inhibitor of both MEK1/2 and ERK1/2 (Favata et al., 1998; Duncia et al., 1998). LY294002 (2-(4-Morpholinyl)-8-phenyl-4 H-1-benzopyran-4-one; Calbiochem, La Jolla, USA), is a potent and selective cell-permeable inhibitor of PI3-kinase, competitively inhibiting ATP binding to the catalytic subunit of PI3-kinase, p110. LY294002 has been shown not to inhibit PI4-kinase, MAPK, S6 kinase, EGFR or c-src tyrosine kinases (Vlahos et al., 1994, 1995; Fruman et al., 1998; Rameh and Cantley, 1999). For inhibitor studies, 2 h before addition of growth factors, 50 μM U0126 or 25 μM LY294002 was added. The inhibitor and growth factors were only added at the start of the culture period and once added were present for the duration of the culture period. Control dishes, with no inhibitor added, were supplemented with an equivalent volume of dimethylsulfoxide (DMSO; the recommended solvent for the inhibitors). A phosphatase inhibitor, Na3VO4 (12.5 μM; Sigma), was used to block the de-phosphorylation of proteins, and SU5402 (20 μM; Calbiochem) was used to block FGF receptor signalling (Iyengar et al., 2007).
Results
In earlier studies in vitro, we have shown that the duration of ERK1/2 phosphorylation in lens cells determines their fate (Iyengar et al., 2007). In the present study, to better understand the contribution of other signalling pathways in the lens fibre differentiation process, we compared FGF- and vitreous-induced ERK1/2 and PI3-K/Akt signalling during lens fibre differentiation in lens epithelial explants.
FGF- and vitreous-induced lens fibre differentiation
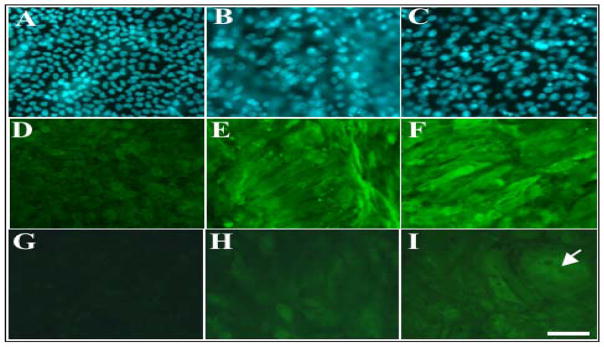
Lens epithelial explants were incubated with a high fibre differentiating dose (100ng/ml) of FGF or 50% vitreous for up to 10 days and labelled for β- and γ-crystallins; key molecular markers for lens fibre differentiation (McAvoy, 1980). In the presence of FGF or vitreous, as previously reported, cells elongated (see Figure 1) and accumulated β-crystallin after 5 days (Figure 2E and 2F) and γ-crystallin after 10 days (Figure 2H and 2I), displaying a distinct lens fibre cell morphology. It was also noticed that many vitreous-treated cells were larger in size than FGF-treated cells after the 10 days of culture (Figure 2I, arrow), implicating the different abilities of FGF and vitreous to induce lens fibre differentiation. In control explants, cultured without growth factors, cells retained their cuboidal shape (see Figure 1) and did not express β- and γ-crystallins (Figure 2D and 2G).
Figure 1.
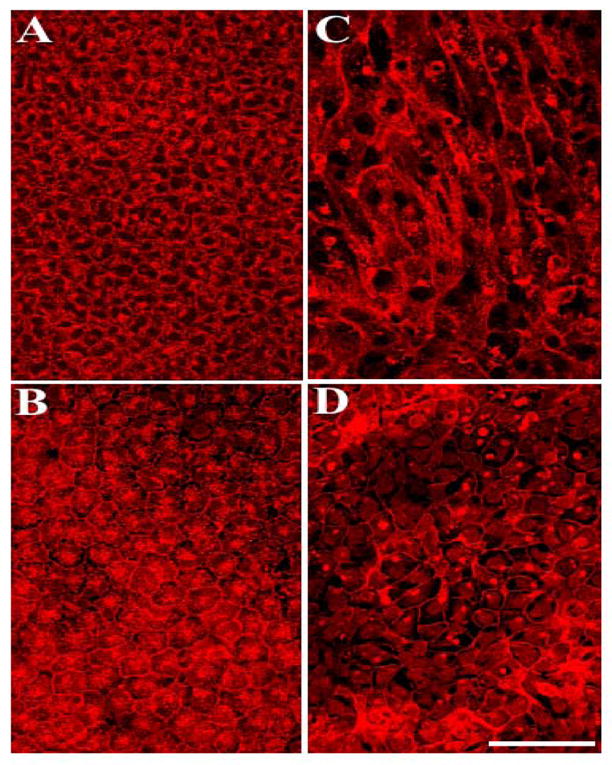
U0126 blocks FGF-induced lens cell elongation. Representative micrographs of cells in lens explants not exposed to growth factors (A,B) or exposed to 100 ng/ml of FGF (C,D), in the presence of U0126 (B,D), labelled with TRITC-conjugated lectin. After 5 days culture, FGF-induced cell elongation (C) was effectively blocked in the presence of U0126 (D), with cells retaining an epithelial cobblestone morphology. Scale bar, 50μm.
Figure 2.
FGF- and vitreous-induced phosphorylation of Akt and ERK1/2 during lens fibre differentiation. Immunofluorescent labelling of β-crystallin (D, E, F) or γ-crystallin (G, H, I) in lens explants cultured without growth factors (A, D, G), with 100ng/ml FGF (B, E, H) or with 50% vitreous (C, F, I). Cell nuclei were counterstained with Hoechst dye (A, B, C). Cells in control explants remain cuboidal in shape and do not express β– and γ-crystallin (D, G). FGF- and vitreous-treated cells elongate and accumulate β– (E, F) and γ-crystallin (H, I). Vitreous-induced fibre differentiation results in the presence of larger cells (I, arrow). Scale bar, 50 μm.
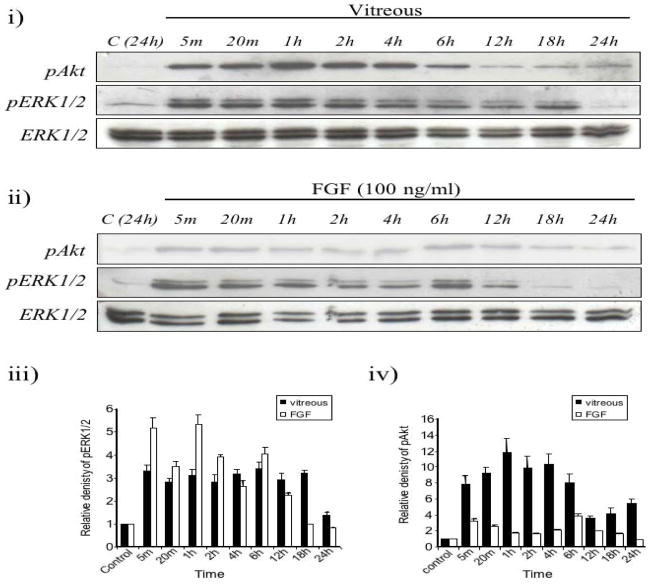
Vitreous-induced Akt and ERK1/2 phosphorylation profiles were examined over a 24 h culture period using western blotting (Figure 3i). In all cases presented, the levels of total ERK1/2 did not change in response to any treatment. As with previous studies, total Akt levels also remained constant under these same experimental conditions (data not shown). Control explants displayed a low level of Akt and ERK1/2 phosphorylation. In response to vitreous, Akt was strongly phosphorylated in lens epithelial cells within 5 min and maintained for up to 6 h before dropping to lower levels (Figure 3i, upper panel and 3iii). Although ERK1/2 phosphorylation was also sustained after addition of vitreous, it was activated within 5 min, but was maintained for up to 18 h. By 24 h, ERK1/2 phosphorylation was reduced to basal levels, comparable to levels observed in control explants (Figure 3i, middle panel).
Figure 3.
(i) Representative western blots of explants cultured with no growth factors (control) or 50% vitreous, from 5 min up to 24 h, assayed for phosphorylated Akt (pAkt, upper panel), phosphorylated ERK1/2 (pERK1/2, middle panel) or total ERK1/2 (lower panel). Vitreous-induced Akt phosphorylation was strongly activated by 5 min and was maintained for up to 6 h, decreasing to a lower level by 12 h. Phosphorylated ERK1/2 was detected after 5 min of vitreous treatment and was maintained for up to 18 h. Total ERK1/2 did not change over this time course and served as a useful loading control. (ii) Representative western blots of explants cultured with no growth factors (control) or 100 ng/ml FGF, from 5 min up to 24 h, were assayed for pAkt (upper panel), pERK1/2 (middle panel) or total ERK1/2 (lower panel). Akt phosphorylation was detected after 5 min, peaking at 6 h. ERK1/2 phosphorylation was also detected at 5 min and was maintained for up to 12 h. Total ERK1/2 did not change much over this time course and served as a useful loading control. (iii) Comparison of ERK1/2 phosphorylation induced by vitreous and FGF. The quantification of the western blots showed that 50% vitreous (black bars) induced a more sustained level of ERK1/2 phosphorylation compared to 100ng/ml FGF-2 (white bars). The graph represents the relative density (means ± S.D.) of ERK1/2 protein taken from three separate western blots. (iv) Comparison of Akt phosphorylation induced by vitreous and FGF. The quantification of the western blots showed that vitreous (black bars) induced a stronger level of Akt phosphorylation compared to FGF (white bars). The graph represents the relative density (means ± S.D.) of Akt protein taken from three separate western blots.
FGF induced sustained ERK1/2 phosphorylation that was activated within 5 min and maintained for up to 12 h (Figure 3ii, middle panel). Although sustained, the duration of ERK1/2 phosphorylation induced by 100 ng/ml of FGF was somewhat shorter than the ERK1/2 phosphorylation profile induced by 50% vitreous. Compared to vitreous, FGF-induced Akt phosphorylation was relatively much weaker over 24 hours, peaking at 6 hours (Figure 3ii, upper panel and 3iii). Interestingly, compared with the other times assayed, the levels of phospho-Akt appeared slightly higher at 5 min and 6 h; however, the significance of this subtle biphasic response, if any, is unclear at present.
U0126 can block FGF- and vitreous-induced fibre differentiation
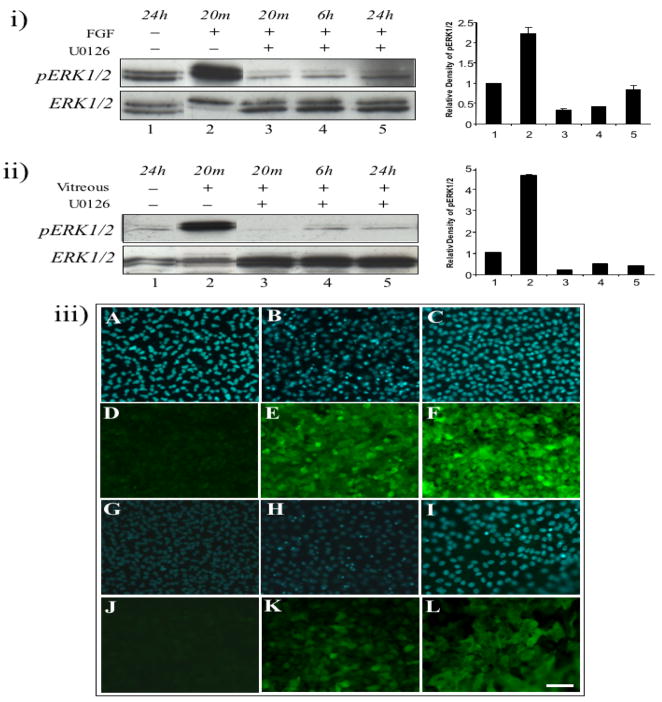
To obtain a better understanding of the role of the different signalling pathways involved in the lens fibre differentiation process, we used a selective ERK1/2 signalling inhibitor (U0126). In the presence of U0126, both FGF- and vitreous-activated ERK1/2 phosphorylation remained at basal levels over a 24 h period, similar to that observed in control explants (Figure 4i and ii, upper panels, lanes 3–5).
Figure 4.
U0126 blocks FGF- and vitreous- induced lens fibre differentiation. (i) Representative western blots of explants exposed to no FGF (lane 1) or 100 ng/ml of FGF for 20 min (lanes 2 and 3), 6 h (lane 4) and 24 h (lane 5), in the absence (lanes 1 and 2) or presence of U0126 (lanes 3, 4 and 5), assayed for phosphorylated ERK1/2 (upper panel) or total ERK1/2 (lower panel). (ii) Representative western blots of explants exposed to no vitreous (lane 1) or 50% vitreous for 20 min (lanes 2 and 3), 6 h (lane 4) and 24 h (lane 5), in the absence (lanes 1 and 2) or presence of U0126 (lanes 3, 4 and 5), assayed for phosphorylated ERK1/2 (upper panel) or total ERK1/2 (lower panel). (iii) U0126 blocks FGF- and vitreous-induced cell elongation, but not β- and γ-crystallin expression. Representative micrographs of cells in lens explants not exposed to growth factors (A, D, G, J) or exposed to 100 ng/ml of FGF (B, E, H, K) or 50% vitreous (C, F, I, L) in the presence of U0126, immunofluorescently labelled for β-crystallin (D–F), γ-crystallin (J–L) or counterstained with Hoechst dye (A–C, G–I). After 5 (A–F) or 10 (G–L) days culture, cell elongation was blocked in the presence of U0126, however, expression of β- and γ-crystallins was not blocked (E, F, K, L). All representative data was taken from experiments undertaken at least three times. Scale bar, 50μm.
Lens explants cultured for 5 days were labelled for β-crystallin. Compared to control explants comprised of a monolayer of cuboidal-shaped epithelial cells (see Figure 1) that did not express β-crystallin (Figure 2A and 2D), cells in explants treated with FGF or vitreous elongated (see Figure 1) and accumulated β-crystallin (see Figure 2E and 2F). Consistent with earlier studies (Lovicu and McAvoy, 2001; Iyengar et al., 2007), U0126 appeared to have no effect on cells in control explants (Figure 1, Figure 4iii, A and D); however, FGF- and vitreous-induced cell elongation was inhibited in the presence of U0126 (Figure 1, Figure 4iii, B, C, E and F). Although cells in these explants tended to retain their cuboidal shape, similar to control explants (Figure 1), β-crystallin still accumulated in these cells (Figure 4iii, E and F). To determine whether this phenomenon was still evident at later stages of fibre differentiation, we cultured the lens explants for up to 10 days and labelled for γ-crystallin. In the absence of any inhibitors, vitreous- and FGF-treated lens cells continued to elongate (see Figure 1), and expressed the later fibre cell marker, γ-crystallin (see Figure 2H and 2I). In the presence of U0126, FGF- and vitreous-induced cell elongation and multilayering was still blocked over 10 days (data not shown); however, cells continued to express γ-crystallin (Figure 4iii, H, I, K and L).
As β- and γ-crystallin accumulation was not blocked by U0126, this suggested that ERK1/2-independent signalling pathways were required for the regulation of FGF- or vitreous-induced β– and γ-crystallin expression. To address this, we more closely examined the PI3-kinase pathway in the lens fibre differentiation process.
LY294002 can block FGF- and vitreous-induced fibre differentiation
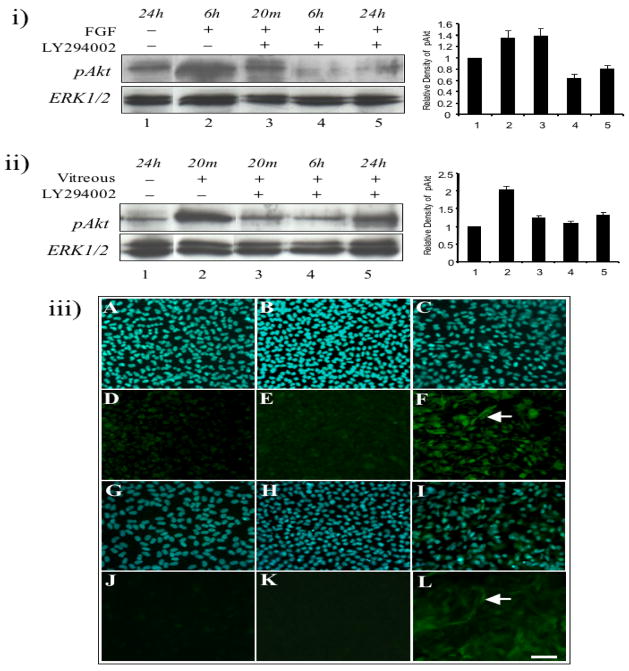
FGF can activate Akt phosphorylation over a 6 h period (Figure 5i, upper panel, lane 2). In the presence of LY294002, added two hours prior to FGF or vitreous treatment, FGF-induced Akt phosphorylation in lens cells was similar to basal levels seen in control explants at 20 min (Figure 5i, upper panel, lane 3), with a drop in levels at 6 h through to 24 h (Figure 5i, upper panel, lanes 4 and 5). Vitreous strongly activated Akt phosphorylation within 20 min (Figure 5ii, upper panel, lane 2); however, in the presence of LY294002, Akt phosphorylation was comparable to basal levels of control explants at 20 min, and these low levels persisted with a slightly stronger label at 24 h (Figure 5ii, upper panel, lanes 3 to 5).
Figure 5.
LY294002 blocks FGF- and vitreous-induced lens fibre differentiation. (i) Representative western blots of explants exposed to no FGF for 24 h (lane 1) or 100 ng/ml of FGF for 20 min (lane 3), 6 h (lanes 2 and 4) and 24 h (lane 5), in the absence (lanes 1 and 2) or presence of LY294002 (lanes 3, 4 and 5), assayed for phosphorylated Akt (upper panel) or total ERK1/2 (lower panel). (ii) Representative western blots of explants exposed to no vitreous for 24 h (lane 1) or 50% vitreous for 20 min (lanes 2 and 3), 6 h (lane 4) and 24 h (lane 5), in the absence (lanes 1 and 2) or presence of LY294002 (lanes 3, 4 and 5), assayed for phosphorylated ERK1/2 (upper panel) or total ERK1/2 (lower panel). Note than in some instances, individual lanes from the same blots were reorganised digitally to more effectively present the data. (iii) Representative micrographs of cells in lens explants not exposed to growth factor (A, D, G, J) or exposed to 100 ng/ml of FGF (B, E, H, K) or 50% vitreous (C, F, I, L) in the presence of LY294002, immunofluorescently labelled for β-(D–F) and γ-crystallin (J–L), or counterstained with Hoechst dye (A–C, G–I). After 5 (A–F) or 10 (G–L) days culture, cell elongation and crystallin expression induced by FGF was blocked in the presence of LY294002 (E and K). Vitreous-induced cell elongation and β- and γ-crystallin expression was only reduced in the presence of LY294002 (F and L). All representative data was taken from experiments undertaken at least three times. Scale bar, 50μm.
Epithelial cells in control explants treated with DMSO (inhibitor diluent) or LY294002 retained their cuboidal shape and remained as a monolayer (see Figure 1, Figure 5iii, A, D, G and J). In the presence of LY294002, FGF-induced lens fibre cell differentiation was completely blocked (Figure 5iii, B and E), including a block in cell elongation (data not shown) and the expression of β-crystallin (compare with Figure 2E showing cells cultured with FGF in the absence of LY294002). For vitreous-induced cells treated with LY294002, many cells remained cuboidal in shape and did not express β-crystallin; however, some cells did elongate and continued to express β-crystallin (Figure 5iii, F, arrow). These cells tended to be smaller and thinner than those normally seen in explants cultured with only vitreous (compare with Figure 2F showing cells cultured with vitreous in the absence of LY294002). After 10 days culture, we also examined for the expression of γ-crystallin in lens explants. LY294002 blocked FGF-induced γ-crystallin expression (Figure 5iii, K; compare with Figure 2H showing cells cultured with FGF in the absence of LY294002), and reduced vitreous-induced γ-crystallin expression and cell enlargement (Figure 5iii, L, arrow). The elongated cells in vitreous-treated explants containing LY294002 were again thinner and smaller than those cultured without LY294002 (see Figure 2I showing cells cultured with vitreous in the absence of LY294002).
Sustained ERK1/2 phosphorylation is required for fibre differentiation
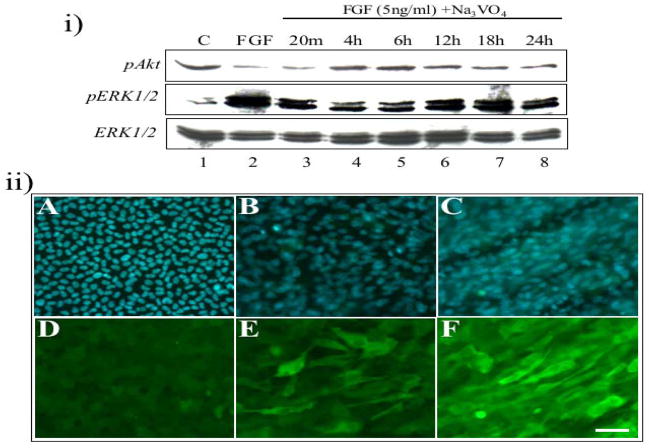
Both FGF (a high ‘fibre cell differentiating’ dose; 100 ng/ml) and vitreous can induce sustained ERK1/2 phosphorylation that leads to lens fibre differentiation. To determine whether this extended activation of ERK1/2 was required for lens fibre differentiation, we used a phosphatase inhibitor, Na3VO4 (sodium orthovanadate), to block the dephosphorylation of ERK1/2, and hence prolong the duration of ERK1/2 activation in a ligand-independent fashion. Lens explants were treated with no growth factors or a lower ‘proliferating’ concentration of FGF (5ng/ml) in the presence or absence of Na3VO4. When we examined the profiles of ERK1/2 and Akt phosphorylation under these conditions, ERK1/2 phosphorylation induced by a low dose of FGF was prolonged, being activated within 20 min and maintained for up to 24 h (Figure 6i, middle panel). This represents a much longer period than the 6h ERK1/2 phosphorylation profile normally induced by this lower dose of FGF on its own (see Iyengar et al., 2006). Similarly, increased Akt phosphorylation was activated after 4 h and maintained for up to 24 h (Figure 6i, upper panel); again this being an extended period of Akt phosphorylation compared with a low dose of FGF in the absence of Na3VO4 (see Iyengar et al., 2006). When we examined cells in the presence of Na3VO4, with no growth factor treatment, they too demonstrated elevated levels of Akt and ERK1/2 phosphorylation (data not shown). Consistent with this, when we examined these cells after 5 days culture in the presence of Na3VO4, with no growth factor treatment (Figure 6ii, E) or with a low dose of FGF (Figure 6ii, F), cells had elongated and expressed β-crystallin (Figure 6ii, E and F).
Figure 6.
Low dose of FGF-induced lens fibre differentiation, in the presence of Na3VO4. (i) Representative western blots of explants exposed to no growth factors (lane 1), 5 ng/ml of FGF for 20 min (lane 2), or FGF in the presence of 12.5μM Na3VO4 (from 20 min to 24 h; lanes 3 to 8), assayed for phosphorylated Akt (upper panel), phosphorylated ERK1/2 (middle panel) and total ERK1/2 (lower panel). (ii) Representative micrographs of cells in lens explants not exposed to growth factor (A, B, D, E) or exposed to 5 ng/ml of FGF (C, F), in the absence (A, D) or presence (B, C, E, F) of 12.5 μM Na3VO4, immunofluorescently labelled for β-crystallin (D–F) or counterstained with Hoechst dye (A–C). All representative data was taken from experiments undertaken at least three times. Scale bar, 50μm.
Sustained ERK1/2 phosphorylation is not sufficient for lens fibre differentiation
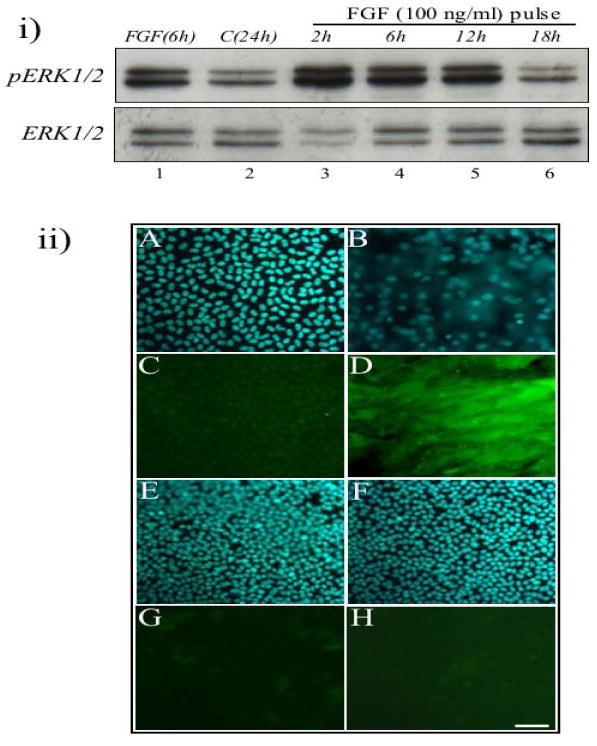
To address whether a sustained ERK1/2 phosphorylation was sufficient for lens fibre differentiation, we examined the ERK1/2 profile induced with only a ‘pulse treatment’ of FGF. ‘Pulse treatment’ of explants involved a short exposure of cells (30 min) to a fibre differentiating dose of FGF (100 ng/ml), followed by washing out the FGF and replacing it with fresh culture medium, with no growth factors, for the remainder of the culture period. Using western blotting, after a ‘pulse treatment’ with FGF, ERK1/2 phosphorylation was activated and maintained for up to 12 h (Figure 7i, lanes 3–5), followed by a drop to basal levels by 18 h (Figure 7i, lane 6), similar to the ERK1/2 profile of explants treated with FGF for the whole 24h.
Figure 7.
Pulse treatment of lens explants with FGF. (i) Representative western blots of lens explants cultured continuously with 100 ng/ml FGF (lane 1), without growth factor (lane 2) and with a pulse treatment of 100 ng/ml FGF for 30 min, collected after 2 h to 18 h (lanes 3–6), assayed for phosphorylated ERK1/2 (upper panel) and total ERK1/2 (lower panel). Pulse treatment of FGF for 30 min is sufficient to activate sustained ERK1/2 phosphorylation for 12 h. (ii) Representative micrographs of cells in lens explants cultured with no growth factor (A, C), with 100 ng/ml FGF for 5 days (B, D), with a pulse-treatment of 100 ng/ml FGF for 30 min (E, G) or for 24 h (F, H), assayed for β-crystallin (C, D, G, H) or counterstained with Hoechst dye (A, B, E, F) after 5 days culture. For pulse treatment of FGF, the cells were cultured with FGF for 30 min or 24 h, then washed with M199 and further cultured in M199 without growth factor for 5 days. These cells did not elongate and express β-crystallin (G and H), similar to control explants (C). All representative data was taken from experiments undertaken at least three times. Scale bar, 50μm.
Immunofluorescent labelling of explants showed that cells treated with a short (either 30 min or 24 h) pulse of FGF did not elongate or express β-crystallin (Figure 7ii, G and H) over the 5 day culture period, unlike the cells treated with FGF for the entire culture period, that did elongate and accumulate β-crystallin (Figure 7ii, B and D) after 5 days. Although a sustained ERK1/2 phosphorylation profile was induced with a pulse of FGF, this was not sufficient to induce lens cell differentiation over the 5 day culture period.
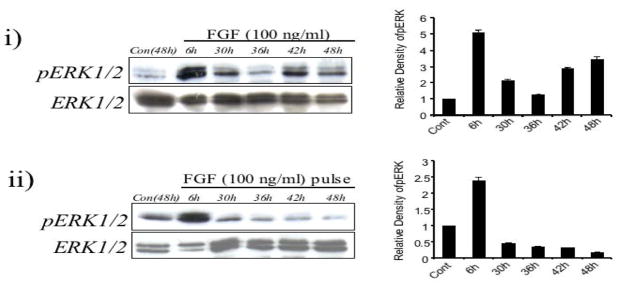
To determine the basis for this ongoing requirement for FGF to induce lens fibre differentiation, we compared the phosphorylation profile of ERK1/2 in cells treated either with FGF over the entire period of tissue culture or with a short pulse of FGF. When FGF was present throughout the culture period, although levels of ERK1/2 phosphorylation dropped to control levels after 12 h (refer to Figure 3ii), these levels were once again noticeably increased relative to basal levels after 30 h and remained higher throughout the culture period (Figure 8i). In contrast, the 30-minute pulse treatment with FGF, for example, failed to further activate ERK1/2 phosphorylation from 30 h after its initial drop to basal levels (Figure 8ii).
Figure 8.
Comparison of ERK1/2 phosphorylation profiles induced by a high dose of FGF (100 ng/ml) present throughout the culture period (i) or for a defined period of time (ii, FGF pulse treatment). (i) Representative western blot of lens explants cultured with no growth factors or with FGF for 6h, 30h, 36h, 42h and 48h. Elevated ERK1/2 phosphorylation was detected well after 6h, with high levels still detected at 42h. (ii) Representative western blot of lens explants cultured with no growth factors or with a 30min pulse treatment of FGF and assayed after 6h, 30h, 36h, 42h and 48h. Elevated ERK1/2 phosphorylation was detected at 6h, and dropped to basal levels throughout the remainder of the culture period.
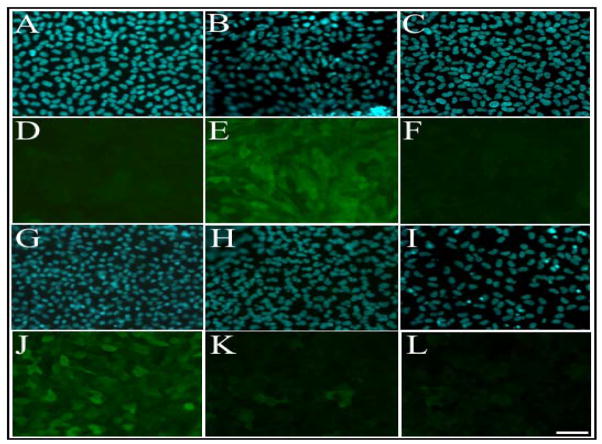
In an additional experiment, to determine whether the ERK1/2 or PI3K signalling pathways were required to maintain the lens fibre differentiation process, we used selective inhibitors to block these pathways at later stages of lens fibre differentiation. Explants were treated with a fibre differentiating dose of FGF to induce ERK1/2 phosphorylation. After 16 h of culture, the period when ERK1/2 activation returns to basal levels, U0126 or LY294002 was added to block any further ERK1/2 and PI3-K signalling, respectively. These inhibitors were also substituted with SU5402 to block FGFR signalling at this time. Although FGF was still present in the medium, in the presence of SU5402, cells did not undergo fibre differentiation (Figure 9C and 9F), indicating that FGF-induced signalling, consistent with our pulse experiments, is required throughout the fibre differentiation process. When U0126 was added after 16h of FGF treatment, cells continued to accumulate β-crystallin, but cell elongation was blocked over the remainder of the culture period (Figure 9G and 9J). When LY294002 was added after 16 h of FGF treatment, cell differentiation was mostly blocked (Figure 9H and 9K). Similarly, if cells were treated with a combination of both U0126 and LY294002, no differentiation was observed in explants (Figure 9I and 9L), comparable to explants treated with SU5402 (Figure 9C and 9F). These results indicate that both ERK1/2 and PI3-K signalling are required to maintain the process of FGF-induced lens fibre cell differentiation.
Figure 9.
Maintenance of the fibre differentiation process is dependent on ERK1/2 and Akt activation. Representative micrographs of lens explants cultured with no growth factor (A, D) or 100 ng/ml of FGF (B, E) for 16 h, followed with treatment of inhibitor SU5402 (C, F), U0126 (G, J), LY294002 (H, K) or both U0126 and LY294002 (I, L), for up to 5 days. After 16 h of FGF treatment, SU5402 completely blocked lens fibre differentiation in explants (C, F). U0126 blocked cell elongation but not expression of β-crystallin (G, J). LY294002 blocked fibre cell elongation and crystallin expression (H, K), similar to the FGFR inhibitor SU5402, as did a combination of U0126 and LY294002 (I, L). Scale bar, 50μm.
Discussion
The ocular environment is important for the establishment and maintenance of lens growth patterns and polarity. Posteriorly, the vitreous influences lens fibre cell differentiation. To date, FGF has been identified as the only growth factor to induce lens fibre differentiation in mammals (Chamberlain and McAvoy, 1989; Lovicu and McAvoy, 1993; Schulz et al., 1993). FGF can activate both ERK1/2 and PI3-K pathways that have been shown to be required for lens fibre differentiation (Lovicu and McAvoy, 2001; Le and Musil, 2001; Weber and Menko, 2006).
In this study, we examined the time-course profile of ERK1/2 and Akt phosphorylation during the process of fibre differentiation in vitro. Both vitreous and a high dose of FGF can induce lens fibre differentiation that is associated with PI3-kinase/Akt activation and the sustained phosphorylation of ERK1/2. When we compared the morphology of differentiating lens cells, we found that vitreous had a stronger ability to induce cell fibre differentiation. The ERK1/2 phosphorylation profile induced by FGF or vitreous is similar in that both induced a sustained phosphorylation of ERK1/2. This phosphorylation is maintained for up to 12 h with FGF and this extends to 18 h when cells are cultured with vitreous. Earlier studies have shown this difference in duration of ERK1/2 phosphorylation to be related to the concentration of FGF; even higher concentrations of FGF than that used in the present study (e.g. 150 ng/ml) can promote a sustained ERK1/2 phosphorylation profile identical to that of vitreous (see Iyengar et al., 2007). Unlike the ERK1/2 profiles, FGF and vitreous show different profiles of Akt phosphorylation; vitreous induced a strong and sustained Akt phosphorylation that was maintained for up to 6 h, whereas, FGF induced a relatively much weaker Akt phosphorylation over this same period. These differences in levels of Akt phosphorylation were also observed using immunofluorescent labelling (data not shown). As vitreous contains multiple growth factors, it is most likely that a number of other growth factors may contribute to the increased phosphorylation of Akt induced by vitreous.
Our blocking experiments showed that ERK1/2 phosphorylation is required for FGF- and vitreous-induced lens fibre differentiation. Consistent with our earlier studies, fibre cell elongation is dependent on ERK1/2 signalling, but β-crystallin expression is not (Lovicu and McAvoy, 2001). Moreover, we have now shown that a later marker of the fibre differentiation process, γ-crystallin accumulation, is also not dependent on ERK1/2 activation. Both FGF- and vitreous-induced lens fibre differentiation also require the PI3-K pathway. LY294002 can completely block FGF-induced cell elongation and β- and γ-crystallin expression, although the same concentration of LY294002 will only reduce the effects of vitreous on cell elongation and crystallin accumulation. This may be accounted for by the fact that vitreous can induce a higher and sustained Akt phosphorylation, compared to FGF which induces a lower Akt phosphorylation, and may be more readily blocked.
Vanadate has been shown in many studies to selectively inhibit phosphatases acting on phosphotyrosines. Although higher concentrations of vanadate may act less specifically (e.g. concentrations of 1mM can block Na-K-ATPase and PMCA activity; personal communication, Dr. Shigeo Tamiya) and are toxic to cells, this compound is widely adopted as an effective inhibitor of protein phosphotyrosine phosphatases (Gordon, 1991). Low doses of FGF have been shown to induce lens epithelial cell proliferation and not fibre differentiation in vitro, and this is associated with a reduced ERK1/2 phosphorylation profile that only extends up to 4–6 h (Iyengar et al., 2007). In the presence of Na3VO4, a low proliferating dose of FGF displays an extended duration of ERK1/2 phosphorylation to 24 h and this is now sufficient to induce fibre cell differentiation over 5 days. The ability of Na3VO4 to block the de-phosphorylation of ERK1/2 results in prolonged activation of ERK1/2 leading to lens fibre differentiation. This phenomenon is not dependent on the presence of exogenous FGF as control cells in explants treated with Na3VO4 and no growth factors, were also induced to elongate and accumulate β-crystallin, in a ligand-independent fashion. In all cases, we noticed that Akt phosphorylation was also prolonged when Na3VO4 was present in the medium, suggesting an important function of PI3-K signalling during lens fibre differentiation.
Our results show that both FGF and vitreous can induce lens fibre differentiation through ERK1/2 and PI3-K/Akt signalling pathways; however, FGF can not completely substitute for vitreous. Differences in Akt phosphorylation between FGF and vitreous suggest that other growth factors in the vitreous are responsible for activating PI3-kinase signalling during fibre differentiation. We know that other growth factors in the vitreous, including IGF, PDGF or EGF, can also activate the ERK1/2 pathway (Iyengar et al., 2006). IGF and PDGF can also potentiate FGF-induced lens fibre differentiation (Richardson et al., 1993; Liu et al., 1996; Kok et al., 2002). Future studies will be focussed on identifying which of these other growth factors, or combination of factors, is able to better reproduce the signalling (in particular Akt phosphorylation) induced by vitreous leading to fibre cell differentiation.
Our pulse experiments in the present study have shown that the lens fibre differentiation process may require successive stages of signalling activity, with an initiation stage followed by a maintenance stage. Earlier studies showed also that a short exposure or ‘pulse treatment’ with FGF could not induce cell differentiation; however, if this short FGF treatment was followed by IGF/insulin, for example, epithelial cells elongated and expressed β-crystallin (Leenders et al., 1997; Klok et al., 1998). This led to the suggestion that FGF initiates lens fibre differentiation but that continued exposure to FGF or IGF/insulin is required to maintain this process (Leenders et al., 1997; Klok et al., 1998; Civil et al., 2000). Our pulse experiments showed that sustained ERK1/2 phosphorylation within 24 h was not sufficient to induce cell differentiation and that further ERK1/2 signalling was required to allow this process to progress. Whether this is the same for PI3-K/Akt signalling is yet to be determined. Irrespective of this, both ERK1/2 and PI3-K/Akt pathways appear to be required to initiate and maintain cell elongation and allow for the expression of β- and γ-crystallins.
The initial and maintenance stages of lens fibre differentiation involve differential expression of specific genes. For the initial stage, cells need to withdraw from the cell cycle and express such proteins as pRb and Kip2/p57 (Pan and Griep, 1994; Lovicu and McAvoy, 1999). For the maintenance stage, epithelial cells elongate and express other specialised proteins such as MIP and β-crystallin. ERK1/2 signalling appears to control the cell elongation process but not crystallin expression (Lovicu and McAvoy, 2001), which may be dependent on PI3-K signalling. PI3-K signalling in lens fibre differentiation may work together with Wnt signalling, as Wnt-conditioned media has also been shown to induce the expression of β-crystallin in lens epithelial cells (Lyu and Joo, 2004). A likely molecule linking the PI3-K pathway to the Wnt signalling pathway may be GSK-3 β, that normally targets β-catenin for proteasome-mediated degradation. When GSK-3 β is inactivated following its phosphorylation, β-catenin will enter the nucleus to bind to the LEF/TCF family of transcription factors to activate specific genes (Dailey et al., 2005). GSK-3 β can be phosphorylated by an upstream effector, Dishevelled, which is involved in Wnt signalling, or it can be phosphorylated by Akt associated with PI3-K signalling. It is observed in neural cells and human endothelial cells, that FGF can increase the nuclear level of β-catenin by reducing GSK-3 β activity (Hashimoto et al., 2002; Holnthoner et al., 2002). It will be interesting to further characterise the cross-talk between the PI3-K and Wnt signalling pathways during lens fibre differentiation.
The PI3-K/Akt pathway has been shown to regulate the TSC1/2 pathway that is required for protein synthesis (Potter et al., 2003). Blocking the PI3-K pathway may affect the activation of the TSC1/2 pathway, leading to the inhibition of protein synthesis, hence reduction in crystallin accumulation. It is plausible that PI3-K signalling is important for the accumulation of β-crystallin and other proteins synthesised during lens fibre differentiation. This is consistent with the fact that the higher levels of Akt phosphorylation induced by vitreous may result in increased protein synthesis contributing to the larger size of cells observed in explants.
In conclusion, we showed that both the ERK1/2 and PI3-K pathways are required for lens fibre differentiation. Although a high dose of FGF can induce lens fibre differentiation, it cannot completely reproduce the effects of vitreous. There are many factors that may contribute to this difference in ability, including differences in presentation of FGF to lens cells in situ, compared to its addition to cells in vitro; however, the most obvious variable is the presence of other growth factors in the vitreous that may be activating other intracellular signalling pathways, effectively potentiating its lens fibre differentiating ability. Future studies will be focussed on characterising the function of some of these other growths factors, such as IGF, PDGF or EGF, and their specific contribution(s) to the differentiating effects of the vitreous.
Acknowledgments
The authors would like to acknowledge the support of the National Health and Medical Research Council (NHMRC, Australia), the Sydney Foundation for Medical Research (Australia) and NIH, USA (R01 EY0-3177) and the Ophthalmic Research Institute of Australia. This research was undertaken as part of the Vision Cooperative Research Centre, New South Wales, Sydney, Australia, supported by the Australian Federal Government through the Cooperative Research Centres Programme.
We would also like to take this opportunity to acknowledge the significant contribution Prof. George Duncan has made to the field of intracellular signalling, amongst others, in lens biology. His research accomplishments and presentations at meetings, together with the many discussions with his peers and junior researchers alike, have encouraged and inspired many to explore this developing area of research. We have lost one of the true identities and characters in the field and he will be greatly missed.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Beebe DC, Silver MH, Belcher KS, Van Wyk JJ, Svoboda ME, Zelenka PS. Lentropin, a protein that controls lens fiber formation, is related functionally and immunologically to the insulin-like growth factors. Proc Natl Acad Sci U S A. 1987;84:2327–2330. doi: 10.1073/pnas.84.8.2327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belecky-Adams TL, Adler R, Beebe DC. Bone morphogenetic protein signaling and the initiation of lens fiber cell differentiation. Development. 2002;129:3795–3802. doi: 10.1242/dev.129.16.3795. [DOI] [PubMed] [Google Scholar]
- Brunet A, Datta SR, Greenberg ME. Transcription-dependent and independent control of neuronal survival by the PI3K-Akt signaling pathway. Curr Opin Neurobiol. 2001;11:297–305. doi: 10.1016/s0959-4388(00)00211-7. [DOI] [PubMed] [Google Scholar]
- Cassidy L, Barry P, Shaw C, Duffy J, Kennedy S. Platelet derived growth factor and fibroblast growth factor basic levels in the vitreous of patients with vitreoretinal disorders. Br J Ophthalmol. 1998;82:181–185. doi: 10.1136/bjo.82.2.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chamberlain CG, McAvoy JW. Induction of lens fibre differentiation by acidic and basic fibroblast growth factor (FGF) Growth Factors. 1989;1:125–134. doi: 10.3109/08977198909029122. [DOI] [PubMed] [Google Scholar]
- Chandrasekher G, Sailaja D. Phosphatidylinositol 3-kinase (PI-3K)/Akt but not PI-3K/p70 S6 kinase signaling mediates IGF-1-promoted lens epithelial cell survival. Invest Ophthalmol Vis Sci. 2004;45:3577–3588. doi: 10.1167/iovs.04-0279. [DOI] [PubMed] [Google Scholar]
- Chow RL, Roux GD, Roghani M, Palmer MA, Rifkin DB, Moscatelli DA, Lang RA. FGF suppresses apoptosis and induces differentiation of fibre cells in the mouse lens. Development. 1995;121:4383–4393. doi: 10.1242/dev.121.12.4383. [DOI] [PubMed] [Google Scholar]
- Civil A, van Genesen ST, Klok EJ, Lubsen NH. Insulin and IGF-I affect the protein composition of the lens fibre cell with possible consequences for cataract. Exp Eye Res. 2000;70:785–794. doi: 10.1006/exer.2000.0846. [DOI] [PubMed] [Google Scholar]
- Coulombre JL, Coulombre AJ. Lens development: fiber elongation and lens orientation. Science. 1963;142:1489–1490. doi: 10.1126/science.142.3598.1489. [DOI] [PubMed] [Google Scholar]
- Dailey L, Ambrosetti D, Mansukhani A, Basilico C. Mechanisms underlying differential responses to FGF signaling. Cytokine Growth Factor Rev. 2005;16:233–247. doi: 10.1016/j.cytogfr.2005.01.007. [DOI] [PubMed] [Google Scholar]
- Duncia JV, Santella JB, 3rd, Higley CA, Pitts WJ, Wityak J, Frietze WE, Rankin FW, Sun JH, Earl RA, Tabaka AC, Teleha CA, Blom KF, Favata MF, Manos EJ, Daulerio AJ, Stradley DA, Horiuchi K, Copeland RA, Scherle PA, Trzaskos JM, Magolda RL, Trainor GL, Wexler RR, Hobbs FW, Olson RE. MEK inhibitors: the chemistry and biological activity of U0126, its analogs, and cyclization products. Bioorg Med Chem Lett. 1998;8:2839–2844. doi: 10.1016/s0960-894x(98)00522-8. [DOI] [PubMed] [Google Scholar]
- Favata MF, Horiuchi KY, Manos EJ, Daulerio AJ, Stradley DA, Feeser WS, Van Dyk DE, Pitts WJ, Earl RA, Hobbs F, Copeland RA, Magolda RL, Scherle PA, Trzaskos JM. Identification of a novel inhibitor of mitogen-activated protein kinase kinase. J Biol Chem. 1998;273:18623–18632. doi: 10.1074/jbc.273.29.18623. [DOI] [PubMed] [Google Scholar]
- Foschi M, Chari S, Dunn MJ, Sorokin A. Biphasic activation of p21ras by endothelin-1 sequentially activates the ERK cascade and phosphatidylinositol 3-kinase. EMBO J. 1997;16:6439–6451. doi: 10.1093/emboj/16.21.6439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franke TF, Kaplan DR, Cantley LC. PI3K: downstream AKTion blocks apoptosis. Cell. 1997;88:435–437. doi: 10.1016/s0092-8674(00)81883-8. [DOI] [PubMed] [Google Scholar]
- Fruman DA, Meyers RE, Cantley LC. Phosphoinositide kinases. Annu Rev Biochem. 1998;67:481–507. doi: 10.1146/annurev.biochem.67.1.481. [DOI] [PubMed] [Google Scholar]
- Gordon JA. Use of vanadate as protein-phosphotyrosine phosphatase inhibitor. Methods Enzymol. 1991;201:477–482. doi: 10.1016/0076-6879(91)01043-2. [DOI] [PubMed] [Google Scholar]
- Govindarajan V, Overbeek PA. Secreted FGFR3, but not FGFR1, inhibits lens fiber differentiation. Development. 2001;128:1617–1627. doi: 10.1242/dev.128.9.1617. [DOI] [PubMed] [Google Scholar]
- Hanada M, Feng J, Hemmings BA. Structure, regulation and function of PKB/AKT--a major therapeutic target. Biochim Biophys Acta. 2004;1697:3–16. doi: 10.1016/j.bbapap.2003.11.009. [DOI] [PubMed] [Google Scholar]
- Hashimoto M, Sagara Y, Langford D, Everall IP, Mallory M, Everson A, Digicaylioglu M, Masliah E. Fibroblast growth factor 1 regulates signaling via the glycogen synthase kinase-3beta pathway. Implications for neuroprotection. J Biol Chem. 2002;277:32985–32991. doi: 10.1074/jbc.M202803200. [DOI] [PubMed] [Google Scholar]
- Haugh JM. Localization of receptor-mediated signal transduction pathways: the inside story. Mol Intervent. 2002;2:292–307. doi: 10.1124/mi.2.5.292. [DOI] [PubMed] [Google Scholar]
- Holnthoner W, Pillinger M, Groger M, Wolff K, Ashton AW, Albanese C, Neumeister P, Pestell RG, Petzelbauer P. Fibroblast growth factor-2 induces Lef/Tcf-dependent transcription in human endothelial cells. J Biol Chem. 2002;277:45847–45853. doi: 10.1074/jbc.M209354200. [DOI] [PubMed] [Google Scholar]
- Iyengar L, Patkunanathan B, Lynch OT, McAvoy JW, Rasko JE, Lovicu FJ. Aqueous humour- and growth factor-induced lens cell proliferation is dependent on MAPK/ERK1/2 and Akt/PI3-K signalling. Exp Eye Res. 2006;83:667–678. doi: 10.1016/j.exer.2006.03.008. [DOI] [PubMed] [Google Scholar]
- Iyengar L, Wang Q, Rasko JE, McAvoy JW, Lovicu FJ. Duration of ERK1/2 phosphorylation induced by FGF or ocular media determines lens cell fate. Differentiation. 2007;75:662–668. doi: 10.1111/j.1432-0436.2007.00167.x. [DOI] [PubMed] [Google Scholar]
- Klok E, Lubsen NH, Chamberlain CG, McAvoy JW. Induction and maintenance of differentiation of rat lens epithelium by FGF-2, insulin and IGF-1. Exp Eye Res. 1998;67:425–431. doi: 10.1006/exer.1998.0534. [DOI] [PubMed] [Google Scholar]
- Kok A, Lovicu FJ, Chamberlain CG, McAvoy JW. Influence of platelet-derived growth factor on lens epithelial cell proliferation and differentiation. Growth Factors. 2002;20:27–34. doi: 10.1080/08977190290022202. [DOI] [PubMed] [Google Scholar]
- Le AC, Musil LS. FGF signaling in chick lens development. Dev Biol. 2001;233:394–411. doi: 10.1006/dbio.2001.0194. [DOI] [PubMed] [Google Scholar]
- Leenders WP, van Genesen ST, Schoenmakers JG, van Zoelen EJ, Lubsen NH. Synergism between temporally distinct growth factors: bFGF, insulin and lens cell differentiation. Mech Dev. 1997;67:193–201. doi: 10.1016/s0925-4773(97)00121-4. [DOI] [PubMed] [Google Scholar]
- Liu J, Chamberlain CG, McAvoy JW. IGF enhancement of FGF-induced fibre differentiation and DNA synthesis in lens explants. Exp Eye Res. 1996;63:621–629. doi: 10.1006/exer.1996.0156. [DOI] [PubMed] [Google Scholar]
- Lovicu FJ, McAvoy JW. Localization of acidic fibroblast growth factor, basic fibroblast growth factor, and heparan sulphate proteoglycan in rat lens: implications for lens polarity and growth patterns. Invest Ophthalmol Vis Sci. 1993;34:3355–3365. [PubMed] [Google Scholar]
- Lovicu FJ, Chamberlain CG, McAvoy JW. Differential effects of aqueous and vitreous on fiber differentiation and extracellular matrix accumulation in lens epithelial explants. Invest Ophthalmol Vis Sci. 1995;36:1459–1469. [PubMed] [Google Scholar]
- Lovicu FJ, Overbeek PA. Overlapping effects of different members of the FGF family on lens fiber differentiation in transgenic mice. Development. 1998;125:3365–3377. doi: 10.1242/dev.125.17.3365. [DOI] [PubMed] [Google Scholar]
- Lovicu FJ, McAvoy JW. Spatial and temporal expression of p57(KIP2) during murine lens development. Mech Dev. 1999;86:165–169. doi: 10.1016/s0925-4773(99)00106-9. [DOI] [PubMed] [Google Scholar]
- Lovicu FJ, McAvoy JW. FGF-induced lens cell proliferation and differentiation is dependent on MAPK (ERK1/2) signalling. Development. 2001;128:5075–5084. doi: 10.1242/dev.128.24.5075. [DOI] [PubMed] [Google Scholar]
- Lovicu FJ, McAvoy JW. Growth factor regulation of lens development. Developmental Biology. 2005;280:1–14. doi: 10.1016/j.ydbio.2005.01.020. [DOI] [PubMed] [Google Scholar]
- Lutty GA, Merges C, Threlkeld AB, Crone S, McLeod DS. Heterogeneity in localization of isoforms of TGF-beta in human retina, vitreous, and choroid. Invest Ophthalmol Vis Sci. 1993;34:477–487. [PubMed] [Google Scholar]
- Lyu J, Joo CK. Wnt signaling enhances FGF2-triggered lens fiber cell differentiation. Development. 2004;131:1813–1824. doi: 10.1242/dev.01060. [DOI] [PubMed] [Google Scholar]
- Majima K. Presence of growth factor in human vitreous. Ophthalmologica. 1997;211:226–228. doi: 10.1159/000310795. [DOI] [PubMed] [Google Scholar]
- Mascarelli F, Rauais D, Counis MF, Courtois Y. Characterization of acidic and basic fibroblast growth factors in brain, retina and vitreous chick embryo. Biochem Biophys Res Commun. 1987;146:478–486. doi: 10.1016/0006-291x(87)90554-7. [DOI] [PubMed] [Google Scholar]
- McAvoy JW. Beta- and gamma-crystallin synthesis in rat lens epithelium explanted with neural retinal. Differentiation. 1980;17:85–91. doi: 10.1111/j.1432-0436.1980.tb01084.x. [DOI] [PubMed] [Google Scholar]
- Neufeld TP. Body building: regulation of shape and size by PI3K/TOR signaling during development. Mech Dev. 2003;120:1283–1296. doi: 10.1016/j.mod.2003.07.003. [DOI] [PubMed] [Google Scholar]
- Pan H, Griep AE. Altered cell cycle regulation in the lens of HPV-16 E6 or E7 transgenic mice: implications for tumor suppressor gene function in development. Genes Dev. 1994;8:1285–1299. doi: 10.1101/gad.8.11.1285. [DOI] [PubMed] [Google Scholar]
- Potter CJ, Pedraza LG, Huang H, Xu T. The tuberous sclerosis complex (TSC) pathway and mechanism of size control. Biochem Soc Trans. 2003;31:584–586. doi: 10.1042/bst0310584. [DOI] [PubMed] [Google Scholar]
- Rameh LE, Cantley LC. The role of phosphoinositide 3-kinase lipid products in cell function. J Biol Chem. 1999;274:8347–8350. doi: 10.1074/jbc.274.13.8347. [DOI] [PubMed] [Google Scholar]
- Richardson NA, Chamberlain CG, McAvoy JW. IGF-1 enhancement of FGF-induced lens fiber differentiation in rats of different ages. Invest Ophthalmol Vis Sci. 1993;34:3303–3312. [PubMed] [Google Scholar]
- Robinson ML, Overbeek PA, Verran DJ, Grizzle WE, Stockard CR, Friesel R, Maciag T, Thompson JA. Extracellular FGF-1 acts as a lens differentiation factor in transgenic mice. Development. 1995a;121:505–514. doi: 10.1242/dev.121.2.505. [DOI] [PubMed] [Google Scholar]
- Robinson ML, MacMillan-Crow LA, Thompson JA, Overbeek PA. Expression of a truncated FGF receptor results in defective lens development in transgenic mice. Development. 1995b;121:3959–3967. doi: 10.1242/dev.121.12.3959. [DOI] [PubMed] [Google Scholar]
- Schulz MW, Chamberlain CG, de Iongh RU, McAvoy JW. Acidic and basic FGF in ocular media and lens: implications for lens polarity and growth patterns. Development. 1993;118:117–126. doi: 10.1242/dev.118.1.117. [DOI] [PubMed] [Google Scholar]
- Stolen CM, Griep AE. Disruption of lens fiber cell differentiation and survival at multiple stages by region-specific expression of truncated FGF receptors. Developmental Biology. 2000;217:205–220. doi: 10.1006/dbio.1999.9557. [DOI] [PubMed] [Google Scholar]
- Toker A, Yoeli-Lerner M. Akt signaling and cancer: surviving but not moving on. Cancer Res. 2006;66:3963–3966. doi: 10.1158/0008-5472.CAN-06-0743. [DOI] [PubMed] [Google Scholar]
- Vlahos CJ, Matter WF, Hui KY, Brown RF. A specific inhibitor of phosphatidylinositol 3-kinase, 2-(4-morpholinyl)-8-phenyl-4H-1-benzopyran-4-one (LY294002) J Biol Chem. 1994;269:5241–5248. [PubMed] [Google Scholar]
- Vlahos CJ, Matter WF, Brown RF, Traynor-Kaplan AE, Heyworth PG, Prossnitz ER, Ye RD, Marder P, Schelm JA, Rothfuss KJ, Serlin BS, Simpson PJ. Investigation of neutrophil signal transduction using a specific inhibitor of phosphatidylinositol 3-kinase. J Immunol. 1995;154:2413–2422. [PubMed] [Google Scholar]
- Weber GF, Menko AS. Phosphatidylinositol 3-kinase is necessary for lens fiber cell differentiation and survival. Invest Ophthalmol Vis Sci. 2006;47:4490–4499. doi: 10.1167/iovs.06-0401. [DOI] [PubMed] [Google Scholar]
- Yoon S, Seger R. The extracellular signal-regulated kinase: multiple substrates regulate diverse cellular functions. Growth Factors. 2006;24:21–44. doi: 10.1080/02699050500284218. [DOI] [PubMed] [Google Scholar]
- Zhao H, Rossant J, Ornitz DM, Beebe DC, Robinson ML. Different FGFR genes play an essential but redundant role in postinduction lens development. Invest Ophthalmol Visual Sci, Suppl. 2003;44:954. [Google Scholar]