Abstract
The present report describes a case of severe airway obstruction caused by endobronchial tuberculosis in an 11-year-old girl who was successfully treated by bronchoscopic balloon dilation. This case illustrates the insidious presentation and the increasingly important role of bronchoscopic intervention in the management of endobronchial tuberculosis. In addition, a brief literature review of the condition in the pediatric age group is included.
Keywords: Airway obstruction, Bronchial stenosis, Bronchoscopic dilation, Bronchoscopic stenting, Endobronchial tuberculosis, Tuberculosis
Abstract
Le présent rapport décrit un cas de grave encombrement des voies aériennes causé par une tuberculose endobronchique chez une fillette de 11 ans qui a été traitée avec succès par dilatation bronchoscopique au ballonnet. Ce cas illustre la présentation insidieuse et le rôle de plus en plus important de l’intervention bronchoscopique dans la prise en charge de la tuberculose endobronchique. De plus, le rapport comporte une brève analyse bibliographique de la maladie au sein du groupe d’âge pédiatrique.
CASE PRESENTATION
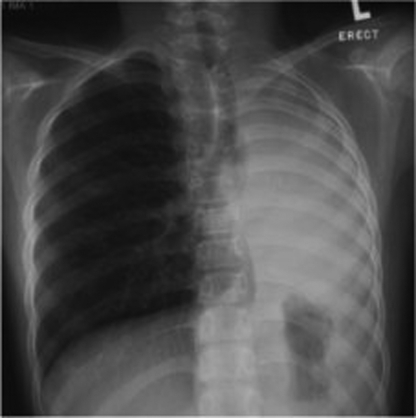
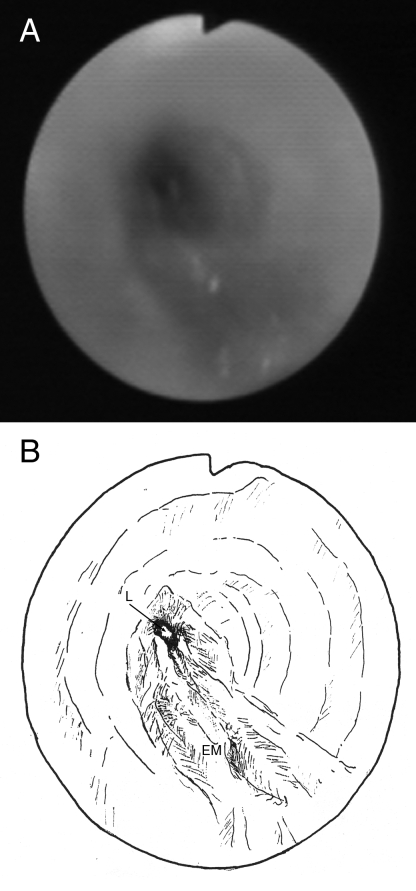
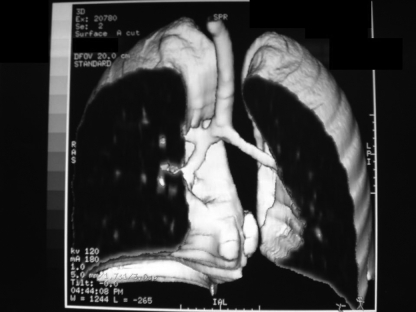
A previously healthy 11-year-old girl presenting with persistent cough, low-grade fever for three months and mild weight loss was referred for further management. On admission, a chest x-ray showed diffuse, left lower zone haziness. Subsequently, sputum culture grew Mycobacterium tuberculosis (TB) and she was started on isoniazid, rifampicin, pyrazinamide and ethambutol for the initial two months, followed by four months of isoniazid and rifampicin according to the culture sensitivities. Her symptoms improved considerably, and serial chest x-ray showed a reduction of left lung haziness. The patient remained well after five months of treatment, until incidentally, the chest x-ray showed complete whiteout of the left lung with left tracheal deviation (Figure 1). The patient had good drug compliance monitored daily by the TB chest clinic at the Prince of Wales Hospital (New Territories, Hong Kong). Interestingly, no significant decrease in exercise tolerance was reported and she was not dyspneic. Computed tomography (CT) of the thorax showed abrupt tapering of the left main bronchus with complete collapse of the left lung. There was no enlarged mediastinal or hilar lymph node to suggest extrinsic compression. Flexible bronchoscopy under local anesthesia confirmed the complete luminal obstruction of the left main bronchus 1 cm from the carina due to edematous and hyperemic mucosa (Figure 2). The stenosis was passed with a guidewire and dilated with a MaxForce (Boston Scientific, USA) 6 mm balloon dilation catheter, inflating up to 12 atm. The left lung re-expanded quickly following bronchoscopic dilation. Bronchial aspirate taken during the procedure showed no growth following prolonged TB culture. The patient remained asymptomatic and completed the six-month anti-TB antibiotics treatment. Bronchoscopy at eight months after the initial dilation showed no residual stenosis of the left main bronchus. At two years follow-up, the patient was well and the CT scan showed only a minor irregularity of the left main bronchus (Figure 3).
Figure 1).
Chest x-ray showing complete whiteout of the left lung with left tracheal deviation at five months after starting antituberculosis medication
Figure 2).
A Bronchoscopic view of the obstructed left main bronchus with surrounding hyperemic edematous mucosa. B Illustrative drawing of the bronchoscopic view. EM Edematous mucosa; L Lumen
Figure 3).
Computed tomography with three-dimensional reconstruction two years following bronchoscopic intervention showing only a minor irregularity of the left main bronchus
DISCUSSION
Endobronchial tuberculosis (EBTB) is a common complication of pediatric pulmonary TB. In children with active pulmonary TB, the incidence of EBTB was reported to be between 41.7% and 43% (1,2). Airway obstruction may occur and children are particularly at risk because their airways are small and more compressible. In the younger age group, bronchial obstruction is commonly caused by extrinsic compression of the bronchus by hilar lymphadenopathy (3). Nevertheless, EBTB- and TB-related tracheobronchomalacia are also important causes of airway obstruction.
The pathogenesis of EBTB is not fully understood, however, sources of EBTB may include the spread of tubercle bacilli from an adjacent mediastinal lymph node, bronchial lymph node or pulmonary parenchymal lesion (4). Clinical symptoms and chest radiographs are not sensitive in detecting EBTB, and bronchoscopy remains the diagnostic tool of choice (1). Endoscopically, EBTB may manifest as edematous mucosa, ulcerative mucosa, inflamed mucosa with granuloma, caseating mucosa or fibrostenotic airway (4), which can result in the main complication of bronchial obstruction. Bronchoscopy and CT are useful modalities in differentiating between the causes of obstruction. Furthermore, bronchoscopy offers the advantage of endobronchial intervention, while CT may provide additional anatomical information on the mediastinum and its lymph nodes, as well as on the condition of airways beyond the obstruction. The management of EBTB differs according to the various etiologies mentioned below.
Obstruction by caseum, mucus and granuloma
Caseum and mucus plugging can cause airway obstruction, especially in children with small airways and poor coughing effort. In this instance, bronchoscopic toileting can give immediate relief of the obstruction (1,2). Conversely, endobronchial granuloma usually does not cause significant airway obstruction unless the granuloma becomes large. Different reports have suggested that airway obstruction can be relieved by endoscopic resection of the granuloma (4,5).
Obstruction by edematous mucosa
In edematous hyperemic EBTB, the bronchial lumen is constantly narrowed due to severe mucosal swelling with surrounding hyperemia, occasionally causing complete obstruction of the airway with lung collapse (4). Endoscopic treatment for this kind of obstruction is not well-documented in the literature. In the present case, we tried a course of balloon dilation with immediate relief of the left main bronchus obstruction, which led to satisfactory airway patency upon follow-up. Therefore, bronchoscopic dilation seems to be an effective strategy in the initial management for this condition. Furthermore, bronchoscopic dilation in children may be facilitated by the elasticity of their airways.
Of note, the role of bronchoscopic stenting in this condition remains to be defined. Endobronchial stents have been used successfully in the past to reduce the risk of restenosis in adult patients (6). However, complications associated with bronchoscopic stenting, such as mucolization, stent migration, crusting and granulation, can cause significant morbidity necessitating repeated bronchoscopic interventions (7,8), which are particularly undesirable in children. Furthermore, in pediatric patients, the stent may require replacement with progressively larger prostheses as the patient grows.
Obstruction due to bronchial fibrostenosis
Bronchial fibrostenosis is the end sequelae of EBTB. It causes permanent bronchial stenosis that can lead to lung parenchymal destruction when prolonged. Currently, the use of steroids in the prevention and treatment of bronchial fibrostenosis is still controversial (9,10). However, EBTB patients responsive to steroid therapy are usually those treated early on in the disease process. In adults, bronchoscopic dilation has been shown to be effective in the initial management of EBTB, but the recurrence rate is relatively high (11). Endobronchial stenting can be used to reduce the risk of restenosis; nevertheless, stent-related complications, such as those previously described, may cause significant morbidity to the patient (6). In children, successful cases of bronchial stenting for benign conditions like tracheomalacia, vascular compression and anastomotic strictures have been reported (5,12), but the role of bronchoscopic treatment for EBTB fibrostenosis remains unclear. Surgery remains the definitive treatment for significant fibrostenosis, which involves excision and primary bronchial anastomosis for simple strictures. In more complex cases, bronchoplasty and resection of the destroyed lung parenchyma may be required (3).
SUMMARY
Airway obstruction is a major complication of pediatric EBTB. The presentation of this disease may be insidious and clinicians should closely monitor their patients for early signs of development. In the past, the role of bronchoscopy in EBTB was mainly for diagnostic purposes. With recent advances in technology and refinement of endoscopic techniques, it now plays a therapeutic role in pediatric patients with EBTB. Indications for choosing from among the numerous available procedures remain to be defined.
REFERENCES
- 1.Chan S, Abadco DL, Steiner P. Role of flexible fiberoptic bronchoscopy in the diagnosis of childhood endobronchial tuberculosis. Pediatr Infect Dis J. 1994;13:506–9. doi: 10.1097/00006454-199406000-00008. [DOI] [PubMed] [Google Scholar]
- 2.Tagarro Garcia A, Barrio Gomez de Aguero MI, Martinez Carrasco C, et al. Fiberoptic bronchoscopy in childhood endobronchial tuberculosis. An Pediatr (Barc) 2004;61:314–9. doi: 10.1016/s1695-4033(04)78394-0. [DOI] [PubMed] [Google Scholar]
- 3.Papagiannopoulos KA, Linegar AG, Harris DG, Rossouw GJ. Surgical management of airway obstruction in primary tuberculosis in children. Ann Thorac Surg. 1999;68:1182–6. doi: 10.1016/s0003-4975(99)00684-0. [DOI] [PubMed] [Google Scholar]
- 4.Chung HS, Lee JH. Bronchoscopic assessment of the evolution of endobronchial tuberculosis. Chest. 2000;117:385–92. doi: 10.1378/chest.117.2.385. [DOI] [PubMed] [Google Scholar]
- 5.Freixinet J, Varela A, Lopez Rivero L, Caminero JA, Rodriguez de Castro F, Serrano A. Surgical treatment of childhood mediastinal tuberculous lymphadenitis. Ann Thorac Surg. 1995;59:644–6. doi: 10.1016/0003-4975(94)00993-7. [DOI] [PubMed] [Google Scholar]
- 6.Wan IY, Lee TW, Lam HC, Abdullah V, Yim AP. Tracheobronchial stenting for tuberculous airway stenosis. Chest. 2002;122:370–4. doi: 10.1378/chest.122.1.370. [DOI] [PubMed] [Google Scholar]
- 7.Jacobs JP, Quintessenza JA, Botero LM, et al. The role of airway stents in the management of pediatric tracheal, carinal, and bronchial disease. Eur J Cardiothorac Surg. 2000;18:505–12. doi: 10.1016/s1010-7940(00)00534-0. [DOI] [PubMed] [Google Scholar]
- 8.Fayon M, Donato L, de Blic J, et al. French experience of silicone tracheobronchial stenting in children. Pediatr Pulmonol. 2005;39:21–7. doi: 10.1002/ppul.20136. [DOI] [PubMed] [Google Scholar]
- 9.Rikimaru T. Therapeutic management of endobronchial tuberculosis. Expert Opin Pharmacother. 2004;5:1463–70. doi: 10.1517/14656566.5.7.1463. [DOI] [PubMed] [Google Scholar]
- 10.Park IW, Choi BW, Hue SH. Prospective study of corticosteroid as an adjunct in the treatment of endobronchial tuberculosis in adults. Respirology. 1997;2:275–81. doi: 10.1111/j.1440-1843.1997.tb00089.x. [DOI] [PubMed] [Google Scholar]
- 11.Lee KH, Ko GY, Song HY, Shim TS, Kim WS. Benign tracheobronchial stenoses: Long-term clinical experience with balloon dilation. J Vasc Interv Radiol. 2002;13:909–14. doi: 10.1016/s1051-0443(07)61774-6. [DOI] [PubMed] [Google Scholar]
- 12.Kumar P, Bush AP, Ladas GP, Goldstraw P. Tracheobronchial obstruction in children: Experience with endoscopic airway stenting. Ann Thorac Surg. 2003;75:1579–86. doi: 10.1016/s0003-4975(02)04891-9. [DOI] [PubMed] [Google Scholar]