Abstract
Non-neoformans cryptococci were previously considered to be saprophytes and nonpathogenic to humans. Cryptococcus laurentii is frequently used as a biological means to control fruit rot. Interestingly, C laurentii has recently been reported to be a rare cause of infection in humans. The authors report a case of pulmonary cryptococcosis caused by C laurentii in a diabetic AIDS patient who was on antituberculosis and antiretroviral treatments. The sputum smear revealed capsulated yeast cells that were identified as C laurentii. Repeated pleural fluid culture revealed growth of C laurentii. Both respiratory samples were negative for acid-fast bacilli. Moraxella catarrhalis and Klebsiella pneumoniae were also found in the sputum, but not in the pleural fluid. The patient had a good response to oral fluconazole therapy at 600 mg/day for five weeks and was then discharged. The present article is the first to report on the rare pulmonary involvement of C laurentii in the Indian HIV population. These unusual forms of cryptococci create a diagnostic predicament in the rapid diagnosis of pulmonary cryptococcosis. A high degree of suspicion and improvement of techniques for culture and identification will contribute to the early diagnosis and treatment of unusual fungal infections.
Keywords: AIDS, Cryptococcus laurentii, Klebsiella pneumoniae, Moraxella catarrhalis, Non-neoformans cryptococcus
Abstract
Auparavant, on croyait que le Cryptococcus non neoformans était saprophyte et non pathogène chez l’humain. Le Cryptococcus laurentii est sou-vent utilisé pour le contrôle biologique de la pourriture des fruits. Fait intéressant, le C laurentii a récemment été déclaré comme rare cause d’infection chez les humains. Les auteurs font état d’un cas de cryptococcose pulmonaire imputable au C laurentii chez un diabétique sidéen qui suivait un traitement antituberculeux et antirétroviral. Le frottis d’expectoration a révélé des cellules de levure encapsulées identifiées comme un C laurentii. Des cultures répétées du liquide pleural ont révélé la prolifération du C laurentii. Les deux échantillons respiratoires étaient négatifs aux bacilles acidorésistants. On a également trouvé du Moraxella catarrhalis et du Klebsiella pneumoniae dans les expectorations, mais pas dans le liquide pleural. Le patient a bien réagi à la thérapie orale de 600 mg/jour de fluconazole pendant cinq semaines et a obtenu son congé. Le présent article est le premier à rendre compte d’une rare atteinte pulmonaire au C laurentii au sein de la population sidéenne indienne. Ces formes inhabituelles de cryptococcose compliquent le diagnostic rapide de cryptococcose pulmonaire. Un fort degré de présomption et l’amélioration des techniques de culture et de dépistage contribueront au diagnostic et au traitement rapides d’infections fongiques inhabituelles.
Cryptococcosis, usually due to Cryptococcus neoformans, is considered to be one of the most serious fungal infections in immunocompromised patients. In the past, non-neoformans species have been generally regarded as nonpathogenic saprophytes. However, in recent years, opportunistic infections associated with Cryptococcus albidus, Cryptococcus curvatus, Cryptococcus humicolus, Cryptococcus uniguttulatus and Cryptococcus laurentii have been reported (1–6).
C laurentii, a basidiomycetous encapsulated yeast, is present in the droppings and cloacal samples of feral pigeons (7). C laurentii is used as a biopesticide and is efficient in controlling fruit rot in apples (8,9). C laurentii has recently been reported as a cause of pulmonary and cutaneous infections in humans. Interestingly, there are only 16 reported cases of disease caused by C laurentii infection. We report a case of pulmonary cryptococcosis resulting from C laurentii, along with Moraxella catarrhalis and Klebsiella pneumoniae infections, in a diabetic patient with AIDS, in whom complete clinical resolution occurred after oral fluconazole administration.
CASE PRESENTATION
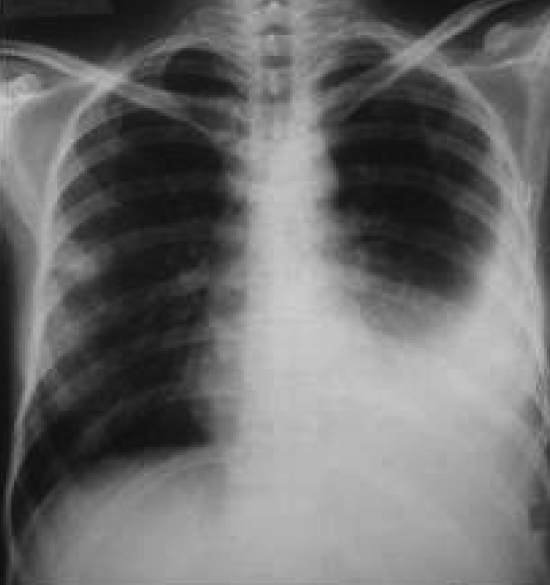
A 35-year-old diabetic woman with clinically proven AIDS was admitted in September 2005 to the inpatient department of the YR Gaitonde Centre for AIDS Research and Education, a specialized AIDS care and research institution in Chennai, India. The patient presented with a febrile illness, breathlessness, dysphagia, odynophagia, vomiting, headache, cough and sputum, night sweats, malaise and left pleuritic chest pain for approximately one week. She was diagnosed with HIV infection in 2001 consequent to bouts of fever, diarrhea, aphthosis, rectal and genital ulcers, and weight loss. The patient complained of producing thick, mucopurulent sputum for the past few months and had been on some form of antiretroviral therapy with zidovudine for the past four years. At the time of admission, she was also on Pneumocystis carinii pneumonia prophylaxis with trimethoprim-sulfamethoxazole (160 mg/day trimethoprim and 800 mg/day sulfamethoxazole) and anti-tuberculosis treatment with two months of daily isoniazid, rifampicin, ethambutol and pyrazinamide, followed by a seven-month continuation phase of daily isoniazid and rifampicin. On examination, the patient had a temperature of 38.5°C, a pulse of 106 beats/min, a blood pressure of 110/70 mmHg and blood oxygen saturation of 96%. She was thin, conscious, oriented and edema-free. A chest examination revealed reduced expansion on the left, quiet breath sounds, dullness to percussion in the left infrascapular region and tan-coloured, thick, mucopurulent sputum. She also presented with rales and coarse crepitations on auscultation. An abdominal examination revealed hepatosplenomegaly. A chest x-ray revealed extensive left pleural effusion (Figure 1). Laboratory examinations revealed that she had a random blood glucose of 9.8 mmol/L (normal values 4.4 mmol/L to 6.6 mmol/L), hemoglobin of 73 g/L (normal values 120 g/L to 150 g/L), total leukocyte count of 6.9×109/L (normal values 4×109/L to 11×109/L), total lymphocyte count of 0.2×109/L (normal values 0.8×109/L to 3.2×109/L), erythrocyte sedimentation rate of greater than 125 mm/h (normal values 0 mm/h to 30 mm/h) and a total platelet count of 161×109/L (normal values 150×109/L to 450×109/L). Her absolute CD4 lymphocyte count (Guava Technologies, USA) was 17 cells/μL (normal values 350 cells/μL to 1600 cells/μL) and her CD4 percentage was less than 14% (normal values 30% to 40%). Her liver function test revealed normal values, namely, alanine aminotransferase of 11 U/L (normal range 4 U/L to 36 U/L), total bilirubin of 6.8 μmol/L (normal values 2 μmol/L to 14 μmol/L) and conjugated bilirubin of 1.7 μmol/L (normal values 0 μmol/L to 4 μmol/L). Her urine creatinine was 8.84 mmol/day (variable). A serum electrolyte investigation revealed a chloride level of 110 mmol/L (normal values 96 mmol/L to 106 mmol/L) and bicarbonate of 15 mmol/L (normal values 22 mmol/L to 29 mmol/L). Serum sodium and potassium levels were normal. Her serum tested positive for HIV-1 antibodies by HIV-1/HIV-2 ELISA and Western blot assays (Immunetics Inc, USA), and was also positive for herpes simplex virus 2 immunoglobulin G by ELISA. The patient was negative for herpes simplex virus 2 immunoglobulin M and rapid plasma reagin antibodies for syphilis.
Figure 1).
Chest x-ray showing extensive left pleural effusion
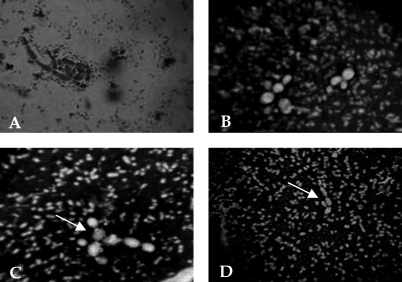
Pleural fluid drained on day 2 (200 mL) did not reveal any bacterial growth. A sputum examination showed Gram-negative, large, round to oval yeast cells (Figure 2A) that were initially misinterpreted as non-albicans Candida. Gram-negative intra-cellular diplococci and capsulated bacilli were also observed in the sputum, and were identified as M catarrhalis and K pneumoniae, respectively. The sputum and pleural fluid were negative for acid-fast bacilli (AFB) by Ziehl-Neelsen staining. The patient felt better after the pleural drainage and underwent blood transfusion on days 3 and 4. On day 5, she developed fever with a severe cough and dyspnea. Sputum smears were negative for AFB. Sputum and pleural fluid cultures on Sabouraud’s dextrose agar with chloramphenicol (without cycloheximide) at 48 h revealed a few 1 mm to 2 mm in diameter, smooth, cream-coloured, poorly grown mucoid colonies and 2 mm to 3 mm in diameter mucoid colonies at 37°C and 25°C, respectively. No yeast cells were observed on pleural smear examination. Nigrosin staining revealed encapsulated, elongated, budding yeast cells with thickened cell walls and capsules; these cells were identified as C laurentii (Figure 2). The yeast was repeatedly encountered in pleural fluid culture. Antifungal therapy with oral fluconazole 600 mg/day for five weeks was started, along with oral ceftrioxone 1 g/day to 2 g/day for clearance of bacteria. Culture of both respiratory samples (BACTEC TB culture system, BD Biosciences, USA) did not reveal AFB. The patient responded well to the treatment and was discharged.
Figure 2).
Cryptococcus laurentii. A Direct examination of sputum revealing Gram-negative, round to oval, yeast-like fungus. B and C Negative staining with 0.5% nigrosin revealed encapsulated, round to oval, budding yeast cells with thick capsules. D Negative staining showing elongated budding yeast cells with capsules. The minute capsulated bacilli seen beside the yeasts were identified as Klebsiella pneumoniae (original magnification ×100, oil immersion). Arrows (C and D) point to the capsulated yeast C laurentii
DISCUSSION
Cryptococci generally occur in soil contaminated with pigeon feces (10) and are transmitted to humans primarily through inhaled fomites. Species other than C neoformans have generally been thought to be nonpathogenic to humans (4,11,12). Although C laurentii has been reported as occurring worldwide, its natural habitat has not yet been thoroughly established. Cryptococcosis, an uncommon disease before the AIDS epidemic, has emerged as an important cause of illness and death in HIV-infected patients. However, there are little data on the isolation of C laurentii from the respiratory tract of AIDS patients; to date, only 16 cases, including the present report, have been published (these reports are summarized in Table 1). Thus, the present report is significant in that it potentially describes the first case of pneumonia resulting from C laurentii in the Indian HIV population.
TABLE 1.
Summary of data from cases of Cryptococcus laurentii infection in humans
| Year (reference) | Age (years) | Sex | Underlying condition(s) | Prior steroid exposure | Prior catheter use | Prior neutropenia | Clinical diagnosis | Clinical presentation | Treatment | Outcome |
|---|---|---|---|---|---|---|---|---|---|---|
| 1977 (2) | 40 | M | – | NR | NR | NR | Cutaneous infection | Cutaneous granuloma, regional lymph node enlargement | D-AmB | Resolved |
| 1980 (12) | 55 | F | Adenocarcinoma, dermatomyositis | Yes | NR | NR | Lung abscess | Asymptomatic right upper lobe cavitary lesion | D-AmB | Resolved |
| 1985 (16) | 37 | M | None known | NR | NR | NR | Pneumonia | NR | Surgery | Resolved |
| 1989 (17) | 13 | F | ESRD, peritoneal dialysis | NR | NR | NR | Peritonitis | Fever, abdominal pain, cloudy dialysate fluid | Catheter removal, D-AmB | Resolved |
| 1989 (18) | 14 | F | ESRD, peritoneal dialysis | NR | NR | NR | Peritonitis | Fever, abdominal pain, cloudy dialysate fluid | Catheter removal, peritoneal lavage with saline | Resolved |
| 1995 (11) | 61 | F | Chronic uveitis | Yes | NR | NR | Endophthalmitis | Deteriorating vision | Fluconazole | Resolved |
| 1997 (19) | 17 | M | Leukemia BMT | NR | Yes | Yes | Fungemia | Fever | Fluconazole | Resolved |
| 1997 (4) | 51 | NR | Diabetes, wore contact lenses | NR | NR | NR | Keratitis | Central corneal ulceration, central descemetocele with trace aqueous leak | Enucleation, D-AmB, miconazole | Resolved |
| 1997 (1) | <1 | M | Premature birth | NR | Yes | NR | Fungemia | Hypotension, tachycardia | Catheter removal, D-AmB | Resolved |
| 1997 (1) | 27 | F | Bacterial endocarditis | NR | Yes | NR | Fungemia | Fever, chills, painful cutaneous nodules | Catheter removal, fluconazole | Resolved |
| 1998 (3) | 34 | M | AIDS | NR | NR | NR | Meningitis | Hypotension, fever, dyspnea, headache, dizziness, diplopia | D-AmB, flucytosine | Resolved |
| 1998 (20) | 26 | M | Solid tumour | NR | Yes | Yes | Fungemia | NR | Catheter removal, fluconazole | Resolved |
| 1998 (20) | 50 | M | Non-Hodgkin lymphoma | Yes | Yes | Yes | Fungemia | NR | Catheter removal, D-AmB | Death |
| 1999 (20) | 57 | M | Acute myelogenous leukemia | Yes | Yes | Yes | Fungemia | NR | Catheter removal, D-AmB | Resolved |
| 2000 (21) | <1 | F | Premature birth | NR | Yes | No | Fungemia | Apnea, bradycardia, hypotension, hypothermia, abdominal distension | Catheter removal, D-AmB | Resolved |
| 2005 (PR) | 35 | F | AIDS, diabetes | NR | NR | NR | Pneumonia | Pleural effusion, fever, chest pain, cough, rales, crepitation, hepatosplenomegaly | Pleural drainage, fluconazole | Resolved |
BMT Bone marrow transplantation; D-AmB Deoxycholate amphotericin B; ESRD End-stage renal disease; F Female; M Male; NR Not reported; PR Present report
Most patients with cryptococcosis suffer from substantial T cell dysfunction, as do patients with AIDS (13). Other immunological defects associated with cryptococcosis are lymphopenia and immune dysfunctions (14). Our patient was severely lymphopenic, as evidenced by laboratory results (total lymphotcyte count of 0.2×109/L). The repeated isolation of C laurentii from pleural fluid and sputum indicated that it was probably the cause of the pneumonic disease in our patient, although the pleural fluid did not reveal the yeast on direct examination. The involvement of AFB in the pleural fluid was also ruled out by negative AFB culture. The pleural fluid was bacteriologically sterile, ruling out other bacterial infections of the pleural space; M catarrhalis and K pneumoniae were isolated only from the sputum, not from the pleural fluid. The patient had initially felt better after pleural drainage, which could be the result of a possible reduction of the yeast population in the fluid. There was no laboratory evidence of P carinii, possibly because the patient was on trimethoprim-sulfamethoxazole prophylaxis.
There is no validated standard treatment for C laurentii infection. Correlations between in vitro antifungal susceptibility test results and treatment outcomes do not exist for C laurentii. However, the patient tolerated oral fluconazole well and, with pleural drainage, the outcome was favourable. Feral pigeons may be carriers of C laurentii (7), but details on the patient’s previous contact with pigeons were unavailable. She did, however, live in close proximity to agricultural areas and pastures. Because isolation of C laurentii from plants and soil has been previously reported (Fell and Statzell-Tallman [15]), a rural habitat and possible exposure to yeast, combined with underlying predisposing conditions, may have made our patient vulnerable to infection. Extensive surveillance of the patient’s living environment could provide more insight into her acquisition of the yeast. A review of the literature showed only two reports pertaining to infection involving the respiratory tract: one involving a lung abscess (12) and the other involving pneumonia (16). Our patient is the second to be diagnosed with an AIDS and C laurentii coinfection; the first was a patient with meningitis (3).
CONCLUSIONS
The present report is the first to describe the rare pulmonary involvement of C laurentii in the Indian HIV population. The pulmonary symptoms may be noncharacteristic, but a high degree of suspicion and improvement of culture and identification techniques will contribute to the early diagnosis, treatment and management of unusual fungal infections in HIV/AIDS patients.
Acknowledgments
The authors acknowledge the financial support (Award No 3/1/[3] 54-2003 MPD-JRF) provided by the Indian Council of Medical Research, New Delhi, India. The authors also acknowledge assistance from Geetha Arumugam, Martin, Usha, Dhanalakshmi, Beula, Rashmi and Selvi at the inpatient department, as well as Mr R Vignesh Ram, Dr Vidya Venkatesh and Dr EM Ponmalar Chandrakumar.
Footnotes
DECLARATION OF ETHICS APPROVAL: This study was carried out after prior approval of the study protocols by the Institutional Review Board of the Voluntary Health Services – YR Gaitonde Centre for AIDS Research and Education Medical Centre, Taramani, Chennai, India. Written informed consent was obtained from the patient and her representatives before clinical specimen collection for the study.
REFERENCES
- 1.Johnson LB, Bradley SF, Kauffmann CA. Fungaemia due to Cryptococcus laurentii and a review of non-neoformans cryptococcaemia. Mycoses. 1998;41:277–80. doi: 10.1111/j.1439-0507.1998.tb00338.x. [DOI] [PubMed] [Google Scholar]
- 2.Kamalam A, Yesudian P, Thambiah AS. Cutaneous infection by Cryptococcus laurentii. Br J Dermatol. 1977;97:221–3. doi: 10.1111/j.1365-2133.1977.tb15070.x. [DOI] [PubMed] [Google Scholar]
- 3.Kordossis T, Avlami A, Velegraki A, et al. First report of Cryptococcus laurentii meningitis and a fatal case of Cryptococcus albidus cryptococcaemia in AIDS patients. Med Mycol. 1998;36:335–9. [PubMed] [Google Scholar]
- 4.Ritterband DC, Seedor JA, Shah MK, Waheed S, Schorr I. A unique case of Cryptococcus laurentii keratitis spread by a rigid gas permeable contact lens in a patient with onychomycosis. Cornea. 1998;17:115–8. doi: 10.1097/00003226-199801000-00017. [DOI] [PubMed] [Google Scholar]
- 5.Velez A, Fernandez-Roldan JC, Linares M, Casal M. Melanonychia due to Candida humicola. Br J Dermatol. 1996;134:375–6. doi: 10.1111/j.1365-2133.1996.tb07639.x. [DOI] [PubMed] [Google Scholar]
- 6.McCurdy LH, Morrow JD. Ventriculitis due to Cryptococcus uniguttulatus. South Med J. 2001;94:65–6. [PubMed] [Google Scholar]
- 7.Mattsson R, Haemig PD, Olsen B. Feral pigeons as carriers of Cryptococcus laurentii, Cryptococcus uniguttulatus and Debaryomyces hansenii. Med Mycol. 1999;37:367–9. doi: 10.1046/j.1365-280x.1999.00241.x. [DOI] [PubMed] [Google Scholar]
- 8.Chand-Goyal T, Spotts RA. Postharvest biological control of blue mold of apple and brown rot of sweet cherry by natural saprophytic yeasts alone or in combination with low doses of fungicides. Biol Control. 1996;6:253–9. [Google Scholar]
- 9.Wilson CL, Wisniewski ME, Droby S, Chalutz E. A selection strategy for microbial antagonists to control postharvest diseases of fruits and vegetables. Sci Hortic. 1993;53:183–9. [Google Scholar]
- 10.Chand-Goyal T, Spotts RA. Enumeration of bacterial and yeast colonists of apple fruits and identification of epiphytic yeasts on pear fruits in the Pacific Northwest United States. Microbiol Res. 1996;151:427–32. doi: 10.1016/S0944-5013(96)80013-9. [DOI] [PubMed] [Google Scholar]
- 11.Custis PH, Haller JA, de Juan E., Jr An unusual case of cryptococcal endophthalmitis. Retina. 1995;15:300–4. doi: 10.1097/00006982-199515040-00006. [DOI] [PubMed] [Google Scholar]
- 12.Lynch JP, III, Schaberg DR, Kissner DG, Kauffman CA. Cryptococcus laurentii lung abscess. Am Rev Respir Dis. 1981;123:135–8. doi: 10.1164/arrd.1981.123.1.135. [DOI] [PubMed] [Google Scholar]
- 13.Rimek D, Haase G, Luck A, Casper J, Podbielski A. First report of a case of meningitis caused by Cryptococcus adeliensis in a patient with acute myeloid leukemia. J Clin Microbiol. 2004;42:481–3. doi: 10.1128/JCM.42.1.481-483.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kontoyiannis DP, Pietsch WK, Reddy BT, et al. Cryptococcosis in patients with cancer. Clin Infect Dis. 2001;32:E145–50. doi: 10.1086/320524. [DOI] [PubMed] [Google Scholar]
- 15.Fell JW, Statzell-Tallman A. Cryptococcus Vuillemin. In: Kurtzman CP, Fell JW, editors. The Yeasts – A Taxonomic Study. 4th edn. Amsterdam: Elsevier; 1998. pp. 742–67. [Google Scholar]
- 16.Winn RE, Rinaldi MG, Galbraith M, Bower JH. Abstract F37. Annual Meeting of the American Society of Microbiology; 1985. p. 370. [Google Scholar]
- 17.Sinnott JT, IV, Rodnite J, Emmanuel PJ, Campos A. Cryptococcus laurentii infection complicating peritoneal dialysis. Pediatr Infect Dis. 1989;8:803–5. doi: 10.1097/00006454-198911000-00017. [DOI] [PubMed] [Google Scholar]
- 18.Morace G, Manzara S, Dettori G. In vitro susceptibility of 119 yeast isolates to fluconazole, 5-flucytosine, amphotericin B and ketoconazole. Chemotherapy. 1991;37:23–31. doi: 10.1159/000238828. [DOI] [PubMed] [Google Scholar]
- 19.Krcmery V, Jr, Kunova A, Mardiak J.Nosocomial Cryptococcus laurentii fungemia in a bone marrow transplant patient after prophylaxis with ketoconazole successfully treated with oral fluconazole Infection 199725130(Lett) [DOI] [PubMed] [Google Scholar]
- 20.Krcmery V, Krupova I, Denning DW. Invasive yeast infections other than Candida spp. in acute leukaemia. J Hosp Infect. 1999;41:181–94. doi: 10.1016/s0195-6701(99)90015-4. [DOI] [PubMed] [Google Scholar]
- 21.Cheng MF, Chiou CC, Liu YC, Wang HZ, Hsieh KS. Cryptococcus laurentii fungemia in a premature neonate. J Clin Microbiol. 2001;39:1608–11. doi: 10.1128/JCM.39.4.1608-1611.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]