Abstract
This review summarises features of networks of commissural interneurones co-ordinating muscle activity on both sides of the body as an example of feline elementary spinal interneuronal networks. The main feature of these elementary networks is that they are interconnected and incorporated into more complex networks as their building blocks. Links between networks of commissural interneurones and other networks are quite direct, with mono- and disynaptic input from the reticulospinal and vestibulospinal neurones, disynaptic from the contralateral and ipsilateral corticospinal neurones and fastigial neurones, di- or oligosynaptic from the mesencephalic locomotor region and mono-, di- or oligosynaptic from muscle afferents. The most direct links between commissural interneurones and motoneurones are likewise simple: monosynaptic and disynaptic via premotor interneurones with input from muscle afferents. By such connections a particular elementary interneuronal network may subserve a wide range of movements, from simple reflex and postural adjustments to complex centrally initiated phasic and rhythmic movements, including voluntary movements and locomotion. Other common features of the commissural and other interneuronal networks investigated so far is that input from several sources is distributed to their constituent neurones in a semi-random fashion and that there are several possibilities of interactions between neurones both within and between various populations. Neurones of a particular elementary network are located at well defined sites but intermixed with neurones of other networks and distributed over considerable lengths of the spinal cord, which precludes the topography to be used as their distinguishing feature.
Keywords: Spinal cord, interneuron, commissural neuron, networks
1. Introduction
Elementary spinal interneuronal networks are very simple. In the simplest cases there are just one or two interneurones in series between input neurones and motoneurones. However, even in the simplest networks there is a number of interneurones of each kind in parallel and these neurones integrate somewhat different combinations of information, from not only their main sources of input, e.g. muscle and skin afferents, but also from other neuronal networks. They forward it also to somewhat different combinations of their target neurones including interneurones of other neuronal networks. Because of their links with other networks, all elementary networks may thus be considered to be components of more complex networks.
This arrangement may be illustrated with any of the previously investigated networks of spinal interneurones, from Renshaw cells and interneurones mediating Ia reciprocal inhibition which were among the first interneurones to be analysed (for references see Jankowska, 1992), through cervical propriospianal neurones (Lundberg, 1979) and interneurones mediating reflex actions of group II muscle spindle afferents (Jankowska et al., 2002), to mention only those known in most detail. In this review it will be illustrated with the recently investigated networks of commissural interneurones. These networks have become of particular interest as being attributed a critical role in locomotor networks (for references see Buchanan, 1999; Grillner, 2003; Kiehn, 2006; Soffe et al., 1984) because they are needed to adjust rhythmic activity of neurones on both sides of the spinal cord and because they are one of the major targets of reticulospinal neurones that are involved in initiation of locomotion. There is also a growing body of evidence that commissural interneurones may be of as critical importance for other centrally or reflexly initiated phasic movements, including voluntary movements and postural adjustments, and that individual commissural interneurones may contribute to several of these movements.
2. Networks of commissural interneurones as examples of spinal elementary networks
2.1. Functional differentiation of the population of commissural interneurones
As other spinal interneuronal populations, the population of commissural interneurones is not homogenous. It includes subpopulations of both excitatory (glutamatergic) and inhibitory (glycinergic) neurones (Bannatyne et al., 2003, 2006; Butt and Kiehn, 2003; Nissen et al., 2005; Roberts et al., 1988; Sugiuchi et al., 1995), at different locations (Bannatyne et al., 2003, 2006; Harrison et al., 1986; Huang et al., 2000; Kiehn and Butt, 2003; Lu et al., 2001; Ohta et al., 1991; Stokke et al., 2002), with different target cells (Bannatyne et al., 2003, 2006; Birinyi et al., 2003; Butt et al., 2002; Butt and Kiehn, 2003; Matsuyama et al., 2006; Matsuyama et al., 2004; Stokke et al., 2002) and with different types of input (Harrison et al., 1986; Jankowska et al., 2005; Jankowska and Noga, 1990). For instance, commissural interneurones of the L3–L6 segments that target contralateral motoneurones in caudal lumbar segments fall into two main subpopulations, those with monosynaptic input from reticulospinal (RS) neurones, vestibulospinal (VS) neurones and group I afferents, and those with monosynaptic input from group II muscle afferents (Jankowska et al., 2005).
In the adult cat, rat and mouse the majority of commissural interneurones are located in lamina VIII on one side of the grey matter (Harrison et al., 1986; Hoover and Durkovic, 1992; Puskar and Antal, 1997; Stokke et al., 2002) and target neurones on the other side (Bannatyne et al., 2003; Matsuyama et al., 2006; Matsuyama et al., 2004; Nissen et al., 2005), as illustrated in Fig. 1A,B. This is true for both excitatory and inhibitory lamina VIII commissural interneurones but occasional bilateral projections have recently been reported (in one out of 34 lamina VIII neurones analysed by Matsuyama (2006). Bilateral projections have also been found in the case of two groups of interneurones with input from group II afferents: inhibitory (but not excitatory) dorsal horn interneurones (Bannatyne et al., 2006; Fig. 1C and D) and excitatory (but not inhibitory) lamina VII interneurones (B.A. Bannatyne, D.J. Maxwell K. Stecina, I. Hammar and E. Jankowska unpublished). Contralateral projections have also been demonstrated for unidentified, primarily inhibitory dorsal horn neurones in the adult rat (Petko and Antal, 2000; Petko et al., 2004) but without specifying whether the same neurones projected ipsilaterally.
Figure 1. Axonal projections of commissural interneurones.
A, an example of exclusively contralateral projections of lamina VIII neurobiotin labelled interneurones with input from reticulospinal neurones in adult cat, with the trajectory of the main axonal branches, as indicated, and terminal projection areas shaded (Modified from Fig. 9 in Bannatyne et al., 2003). B. summary of axonal branching of intraaxonally labelled adult laminae VIII interneurones with input from reticulospinal neurones; all collaterals given of contralaterally (to the left of the dashed lines indicating the midline (Modified from Fig. 6 in Matsuyama et al., 2004). C and D, bilateral projections of inhibitory dorsal horn interneurones with input from group II afferents but only ipsilateral of excitatory ones (Modified from Fig. 7 & 8 in Bannatyne et al., 2006). Shaded areas in C and D indicate the total terminal projected areas of the reconstructed axonal branches. E and F. bilateral projections of lamina VIII commissural interneurones in a newborn kitten and mouse (Golgi staining; modified from Fig. 5 and 8 in Scheibel and Scheibel, 1966).
In contrast to projections in adult animals, projections of lamina VIII interneurones in neonatal animals appear to be more often bilateral, at least as judged by anatomical studies using Golgi technique (Cajal, 1953; Scheibel and Scheibel, 1966), with examples in Fig. 1E and F. This may indicate that ipsilateral axon collaterals of these interneurones withdraw at some stage during the development. Bilateral projections may also be more frequent in genetically modified Epha4 knock out mice exhibiting synchronous (rabbit- or kangaroo-like) rather than alternating gait (Dottori et al., 1998; Kullander et al., 2003).
2.2. Network connections indicated by patterns of input to lamina VIII commissural interneurones targeted by reticulospinal neurones
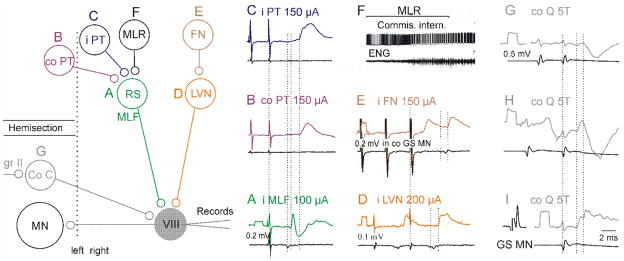
Network connections of commissural interneurones have been found to be generally very extensive but differ for various subpopulations of these neurones. Fig. 2 summarizes some of the connections for interneurones with monosynaptic input from RS and VS neurones. The figure shows that commissural interneurones monosynaptically excited by RS neurones (A) (Jankowska et al., 2005; Jankowska et al., 2003) may be disynaptically excited by both ipsilateral (B) and contralateral (C) pyramidal tract (PT) neurones (Cabaj et al., 2006; Edgley et al., 2004; Jankowska et al., 2006) and thus incorporated in networks of neurones initiating voluntary movements. Rhythmic activation of many of these neurones by stimuli applied in the mesencephalic locomotor region (MLR)(F) (Matsuyama et al., 2004) shows that they are also incorporated in networks of locomotion.
Figure 2. Incorporation of commissural interneurones with monosynaptic input from reticulospinal neurones into other spinal networks.
The grey circle represents commissural interneurones targeting contralateral motoneurones. Other circles represent commissural interneurones (Co C) on the opposite side of the spinal grey matter, reticulospinal (RS) neurones with axons in the ipsilateral medial longitudinal fascicle (MLF), neurones in the lateral Vestibular nucleus (LVN), ipsilateral and contralateral pyramidal tract (PT) neurones, and neurones in the ipsilateral mesencephalic locomotor region (MLR) and cerebellar fastigial nucleus (FN). Records A–D and G–I, are from commissural interneurones which were antidromically activated from the contralateral GS motor nuclei, while those in E are from a GS motoneurone and in F from an unspecified commissural neurone. They show PSPs (or action potentials in F) evoked by stimulation of the indicated structures. Dotted lines in A–E indicate: stimulus artefacts, descending volleys following the MLF or VS stimuli, onset of monosynaptic EPSPs and onset of di- or trisynaptically evoked EPSPs. Dotted lines in G–I indicate afferent volleys from the Q nerve and onset of disynaptic EPSPs and IPSPs evoked by them. The records are: A–C from Fig. 6 in Jankowska et al.(2006); D from Fig. 1 in Krutki et al. (2003); E from Fig. 5 in Matsuyama and Jankowska (2004); F from Fig. 8 in Matsuyama et al.(2004); G–I from Figs. 5 & 7 in Jankowska et al. (2005).
Commissural interneurones monosynaptically excited by VS neurones (E) (Krutki et al., 2003) will contribute to postural adjustments evoked by signals from the vestibular receptors and neck afferents and, as they are disynaptically excited by fastigial neurones (FN) (F) (Matsuyama and Jankowska, 2004), they are also incorporated in the networks of motor control and learning that depend on the cerebellum.
Fig. 2 shows only the most direct connections, monosynaptic and disynaptic, but there are also possibilities of an additional indirect coupling at any of the linking sites and of incorporation of the network of these neurones into even more complex neuronal systems, e.g. of locomotion steered by cortical neurones (Matsuyama et al., 2004) or scratching initiated by contralateral afferents (Stein et al., 1995).
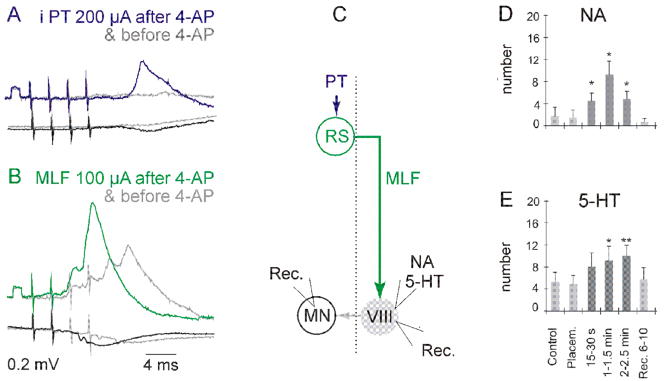
Synaptic actions evoked by only one source of input to commissural interneurones were generally weak. However, in several cases the neurones responded not only with small intracellularly recorded EPSPs but also with extracellularly recorded action potentials, showing that these weak synaptic actions may nonetheless be suprathreshold. Furthermore, activation of commissural interneurones was considerably facilitated when 2 sources of input were jointly activated (e.g. MLF and VS, or both ipsilateral and contralateral PT neurones; Edgley et al., 2004; Jankowska et al., 2005; Krutki et al., 2003). Synaptic transmission in their networks could also be enhanced, e.g. by the K+ channel blocker 4-AP (Jankowska et al., 2005) and by monoamines (Hammar et al., 2004), as illustrated in Figure 3.
Figure 3. Enhancement of synaptic actions in the double crossed pathways between ipsilateral PT neurones and motoneurones.
A and B, enhancement of EPSPs evoked by pyramidal tract (PT) and medial longitudinal fascicle (MLF) stimuli in a GS motoneurone after systemic application of the K+ channel blocker 4-aminopyridine (4-AP) Modified from Fig. 2 in Jankowska et al.(2005). C. diagram of connections in these pathways, via reticulospinal (RS) neurones and lamina VIII commissural interneurones and sites of recording from a motoneurone (MN) in A and B and of recording from interneurones and of ionophoresis in D and E. D and E. Histograms of mean numbers of spike potentials evoked in commissural interneurones by 20 stimuli applied within the MLF before (light grey; control & placement), during (dark grey) and after (light grey; recovery) ionophoresis of noradrenaline (NA) and serotonin (5-HT) Modified from Fig. 4 in Hammar et al. (2004)
Strengthening and prolonging activation of commissural interneurones by delayed actions of some sources of input to them might be particularly important for triggering voltage dependent persistent inward current and plateau potentials in motoneurones (Hounsgaard et al., 1988; Hultborn, 1999; Hultborn et al., 2003; Schwindt and Crill, 1980) which in turn give rise to long-lasting discharges. This is because the critical time of depolarization needed for the persistent inward current in motoneurones apparently exceeds 10 ms for even the strongest sources of the depolarization and over 100 ms for the weaker ones (Hultborn et al., 2003; Lee and Heckman, 1996; Schwindt and Crill, 1980). Doubling or tripling of the period of activation of commissural interneurones, and hence of their actions on motoneurones, by utilising several parallel pathways with different overall conduction time might thus be beneficial for this purpose.
2.3. Network connections indicated by output from lamina VIII commissural interneurones activated by reticulospinal neurones
Reconstruction of terminal branching of intracellularly labelled lamina VIII interneurones projecting to contralateral hindlimb motor nuclei revealed that their projection areas are not only in lamina IX but extend to laminae VI–VIII (Bannatyne et al., 2003, 2006; Matsuyama et al., 2006; Matsuyama et al., 2004: see also Birinyi et al., 2003)), and overlap with the areas of location of several populations of premotor interneurones (for references see Jankowska, 1992).
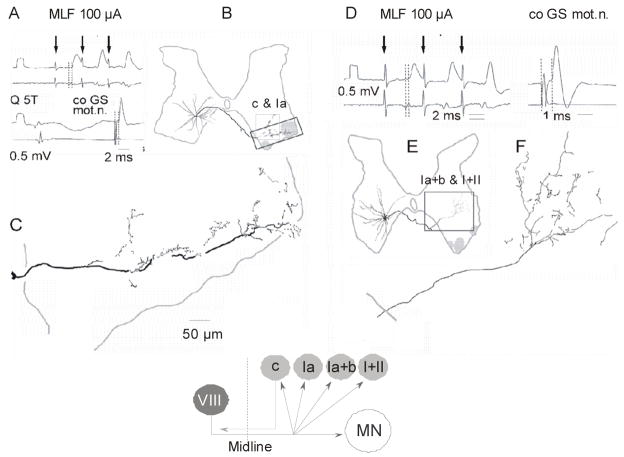
At locations indicated in Fig. 4E commissural interneurones might target interneurones mediating nonreciprocal inhibition from group I muscle afferents and interneurones in pathways from group II afferents. Intracellular records from interneurones in this area have shown that stimuli applied in the MLF did indeed evoked disynaptic EPSPs (Fig. 5A and D) or IPSPs (Fig. 5B and E) in interneurones with input from group Ib or II afferents (Cabaj et al., 2006). These EPSPs and IPSPs were evoked at the same latencies as in motoneurones (Fig. 5G and H), indicating that both excitatory and inhibitory lamina VIII commissural neurones act in parallel on motoneurones and on these interneurones. Interneurones excited by commissural interneurones might thus add to their actions on motoneurones with an additional synaptic delay, as indicated in Fig. 5I. Excitatory premotor interneurones would amplify excitatory actions of commissural interneurones, while inhibitory interneurones might either amplify inhibitory actions or counteract the excitatory ones. The illustrated records are from interneurones with characteristics of premotor interneurones but which have not been identified as exciting or inhibiting motoneurones. However, evidence that both excitatory and inhibitory premotor interneurones mediate actions of commissural interneurones has been provided by the demonstration that disynaptic EPSPs and/or IPSPs evoked by group Ia, Ib or II afferents in motoneurones are enhanced by a preceding stimulation of neuronal systems that activate commissural interneurones (Cabaj et al., 2006). This is illustrated in Fig. 5J–L with enhancement of disynaptic IPSPs evoked from Ib afferents.
Figure 4. Terminal projection areas of lamina VIII commissural interneurones with input from RS neurones outside motor nuclei.
A and D, records from two excitatory interneurones with monosynaptic EPSPs from the MLF (<1 ms latency from the descending volleys indicated by the first dotted lines). Both were antidromically activated from the gastrocnemius-soleus GS) motor nuclei, but only the first was found to project to motor nuclei in the L5 segment (B) and their projections outside motor nuclei differed. In the L5 segment (B) they were in the ventral horn dorsal and medial to motor nuclei (upper box) and in the L4 segment (E) in the more dorsal part of lamina VII. In both areas a considerable number of terminal were found (C and F). Modified from Fig. 7 and 9 in Bannatyne et al. (2003). Diagram shows hypothetical target cells (light grey) of lamina VIII commissural interneurones (dark grey) outside motor nuclei; dotted line indicates midline.
Figure 5. Examples of disynaptic PSPs evoked from the MLF via commissural interneurones - at the same latency in motoneurones and in interneurones in pathways from group Ib and II afferents and thus indicating collateral actions of commissural interneurones on other premotor interneurones.
A–F, Intracellular records (upper traces) from four interneurones with input from group Ib afferents (illustrated in C) or group II afferents (illustrated in F) in which disynaptic EPSPs (left panels) or IPSPs (middle panels) were evoked from the MLF. As judged by antidromic activation from motor nuclei such interneurones were premotor interneurones in pathways from group Ib or II afferents. G–H, Records from two GS motoneurones aligned with respect to MLF volleys evoked by the 3rd stimulus; the volleys are indicated by the first dotted lines and the onset of the PSPs by the second. I, Diagram showing collateral connections between MLF and the two kinds of interneurones illustrated in A–F. The circles represent subpopulations of interneurones of the various populations but the indicated connections apply to individual interneurones of these subpopulations. J–L, Evidence that disynaptic Ib IPSPs evoked in motoneurones (K) are mediated by interneurones co-excited from the MLF because they are facilitated (L) when preceded by MLF stimuli. Note much greater amplitude of IPSPs evoked when stimulation of Ib afferents was preceded by stimulation of the MLF (black traces in L) than when it was not (K and grey traces in L). Such records substantiate connections indicated in I and postulated on the basis of the data illustrated in Fig. 4. Modified from Figs 3, 4 and 8 in Cabaj et al. (2006).
As a consequence of their actions on premotor interneurones, commissural interneurones might modify the operation of the whole networks of these premotor interneurones; they might not only enhance PSPs evoked by them in motoneurones but also the degree of mutual inhibitory interactions between Ib interneurones (Brink et al., 1983) or between Ib interneurones and group II interneurones (Edgley and Jankowska, 1987).
At locations indicated in Fig. 4B commissural interneurones might target contralateral commissural interneurones and interneurones mediating Ia reciprocal inhibition. Their actions on such interneurones were demonstrated by disynaptic or trisynaptic actions of contralateral afferents on commissural interneurones (Harrison et al., 1986) and by enhancement of disysynaptic IPSPs evoked in motoneurones by Ia afferents and of disynaptic or trisynaptic EPSPs and IPSPs evoked from contralateral group II afferents (via Ia inhibitory interneurones and commissural interneurones respectively (Jankowska et al., 2005; Jankowska et al., 2005). The Ia interneurones were in addition shown to be the last order interneurones of IPSPs evoked in motoneurones via contralateral commissural interneurones activated from the MLF and VS as well as from both PTs. The evidence was depression of these IPSPs by a preceding activation of Renshaw cells (Fig. 6B–D), which counteract actions of Ia inhibitory interneurones (Hultborn et al., 1971) but do not depress direct actions of commissural interneurones (Fig. 6A).
Figure 6. Depression of IPSPs evoked in motoneurones from the MLF, LVN and PT by Renshaw cells activated by stimulation of motor axons as the evidence that the IPSPs are mediate by Ia inhibitory interneurones.
Top row, test IPSPs alone. Middle row IPSPs evoked by the same stimuli but after conditioning stimulation of a muscle nerve followed by activation of Renshaw cells. Bottom row, superposition of the test (black) and conditioned (grey, smaller) IPSPs. All the records are averages of 20 single records. Left diagram, pathways via which IPSPs illustrated in A–D were evoked. These from reticulospinal (RS) neurones with axons in the medial longitudinal fascicle (MLF) excited by pyramidal tract (PT) neurones or from vestibulospinal (VS) neurones via lamina VIII commissural interneuronesand Ia inhibitory interneurones, the latter two represented by the grey circles labelled VIII and Ia, to alpha motoneurones. Right diagram, network of Ia inhibitory interneurones (Ia) and Renshaw cells (R) with which the networks to the left were linked. A and B, modified from Fig. 2 in Jankowska et al. (2005)
By exciting Ia inhibitory interneurones commissural interneurones would also influence operation of their whole networks; enhancing actions of Ia interneurones on interneurones that mediate inhibition of agonists and counteracting the depression of their actions by Renshaw cells (Fig. 13, right diagram; Hultborn et al., 1976). As Ia inhibitory interneurones are last order interneurones of several contralateral and ipsilateral neuronal networks (see Lindstrom, 1973), the network of commissural interneurones would work in concert with them, e.g. during postural adjustments evoked by signals from the vestibular receptors (by ipsilateral VS neurones; Hultborn and Udo, 1972), and voluntary movements (by PT neurones; Hultborn et al., 1976; Hultborn and Udo, 1972) or fictive locomotion (Degtyarenko et al., 1998; Feldman and Orlovsky, 1975) or scratching (Deliagina and Orlovsky, 1980).
At locations indicated in Fig. 4B commissural interneurones might also target contralateral Renshaw cells, as postulated by Nishimaru et al.(2006). However, as stated by the authors, more studies would be needed before relations between commissural interneurones and Renshaw cells are established.
2.4. Network connections indicated by input to and output from commissural interneurones activated by group II muscle afferents
Only a small proportion of commissural interneurones with monosynaptic input from ipsilateral group II muscle afferents were found to be excited by reticulospinal neurones and if so only di-or polysynaptically. The network of these neurones appears therefore to be only to a small extent, and only indirectly, incorporated in the networks steered by descending tract neurones illustrated in Fig. 2. This is also indicated by differences in modulatory actions of monoamines (Hammar et al., 2004) and of presynaptically acting GABAergic neurones (Edgley et al., 2003; Jankowska et al., 2002) on commissural interneurones with monosynaptic input from group II afferents and from the MLF.
Activity of subpopulations of commissural interneurones with monosynaptic input from ipsilateral group II muscle afferents on both sides of the spinal cord might nevertheless be linked. This is indicated by disynaptic EPSPs and IPSPs from contralateral group II afferents in commissural interneurones with monosynaptic input from the ipsilateral MLF (illustrated in Fig. 2G) or from the contralateral MLF in commissural interneurones with monosynaptic input from group II afferents (Jankowska et al., 2005), which implicate monosynaptic actions of contralaterally located commissural interneurones which mediate them. These observations extend the original evidence for mutual interactions between networks of commissural interneurones on both sides of the body based on demonstration of disynaptic EPSPs and IPSPs from contralateral group I afferents (Harrison et al., 1986) and are substantiated by morphological demonstration of synaptic contacts between commissural interneurones on both sides of the spinal cord (Birinyi et al., 2003; Matsuyama et al., 2006), even if the interconnected neurones have not been specified.
The subpopulations of commissural interneurones with input from the MLF and from group II muscle afferents likewise appear to target the same populations of premotor interneurones, those mediating actions of ipsilateral group Ia, Ib and II muscle afferents on motoneurones. This is indicated by as effective facilitation of IPSPs evoked by Ia afferents (Bruggencate et al., 1969) and of EPSPs or IPSPs evoked by group Ib and II afferents (Cabaj et al., 2006) by conditioning stimulation of contralateral group II afferents as of the MLF. Disynaptic EPSPs recorded in premotor interneurones with group II input from contralateral group II afferents fully supported this conclusion (Arya et al., 1991; Bajwa et al., 1992)..
3. Comparison of internal organization of elementary interneuronal networks
One of the common features of the so far analysed elementary interneuronal networks is that input to each interneuronal population is drawn from a number of sources, and that input from any of these sources is distributed to several populations, although in different combinations. E.g. both Ia and Ib afferents provide input to interneurones mediating non-reciprocal inhibition of motoneurones (Jankowska et al., 1981), but Ia afferents are the main source of peripheral input to interneurones mediating reciprocal inhibition (Hultborn, 1972) while Ib afferents excite also interneurones in pathways from group II afferents. In a given population input from different sources appears to be distributed, with practically at random connections between afferents, or neurones that provide it, and individual interneurones of this population (Edgley, 2001; Harrison and Jankowska, 1985; Harrison et al., 1986; Hultborn, 1972; Jankowska et al., 2005).
Another common feature is that of internal control of operation of neurones within the network. Mutual inhibitory interactions have been found between Renshaw cells (Ryall, 1970), Ia inhibitory interneurones (Hultborn et al., 1976), Ib interneurones (Brink et al., 1983), group II interneurones (Edgley and Jankowska, 1987) and commissural interneurones (Harrison et al., 1986; Jankowska et al., 2005). In several cases they were found between interneurones with the same input. e.g. from group Ib afferents, (Harrison and Jankowska, 1985) or group II afferents (Edgley and Jankowska, 1987) of the same nerve and might represent a kind of negative feedback. In other cases they might reflect interactions between interneurones subserving opposite actions (e.g. Ia inhibition of either flexors or extensors, (Hultborn et al., 1976) or commissural interneurones on the left and right sides (Edgley and Jankowska, 1987; Harrison et al., 1986). Mutual excitatory interactions have also been found but these might be more likely between interneurones of distinct neuronal populations (Bannatyne et al., 2006; Jankowska et al., 2002).
4. Which neurones do and which do not belong to a neuronal network
Boundaries between different neuronal networks may be considered as not being sharp, especially when individual neurones form part of different networks under different circumstances and when neuronal networks change their configuration and elements depending on which movements they sub-serve. It may thus be a matter of personal preferences whether different kinds of neurones are classified as belonging to the same, or to different networks. However, independently of how spinal interneuronal networks are defined, there is no doubt that their constituent neurones cannot be distinguished by mere topographical factors. Interneurones that appear to belong to one interneuronal population are as a rule intermixed with other types of neurones, are distributed over considerable lengths of the spinal cord and do not form nuclear complexes in any parts of the spinal grey matter, even if they are preferentially located in more rostral or more caudal segments, and in more dorsal or more ventral Rexed′s laminae.
Although neither functional not morphological features allow an unequivocal delimitation of the interneuronal networks discussed above, the general conclusion that elementary interneuronal networks are building blocks of larger networks would hold true. It might therefore be more heuristic to look for these elementary networks while analyzing more complex networks rather than to try to identify constituent neurones of the complex networks “from scratch”.
Acknowledgments
The studies carried out in the author′s laboratory were supported by grants from the NINDS/NIH (R01 NS040863) and the Swedish Research Council (15393-01A).
Abbreviations
- 4-AP
4 aminopyridine
- 5-HT
5 hydroxytryptamine (serotonin)
- c
commissural
- co
contralateral
- EPSP
excitatory postsynaptic potential
- FN
fastigial nucleus
- GABA
gamma aminobutyric acid
- GS
gastrocnemius-soleus
- i
ipsilateral
- IPSP
inhibitory postsynaptic potential
- L
lumbar
- LVN
lateral vestibular nucleus
- MLF
medial longitudinal fascicle
- MLR
mesencephalic locomotor region
- MN
motoneuron
- NA
noradrenaline
- Q
quadriceps nerve
- PT
pyramidal tract
- RS
reticulospinal
- T
threshold
- VS
vestibulospinal
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Arya T, Bajwa S, Edgley SA. Crossed reflex actions from group II muscle afferents in the lumbar spinal cord of the anaesthetized cat. J Physiol (Lond) 1991;444:117–131. doi: 10.1113/jphysiol.1991.sp018869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bajwa S, Edgley SA, Harrison PJ. Crossed actions on group II-activated interneurones in the midlumbar segments of the cat spinal cord. J Physiol (Lond) 1992;455:205–217. doi: 10.1113/jphysiol.1992.sp019297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bannatyne BA, Edgley SA, Hammar I, Jankowska E, Maxwell DJ. Networks of inhibitory and excitatory commissural interneurons mediating crossed reticulospinal actions. Eur J Neurosci. 2003;18:2273–2284. doi: 10.1046/j.l460-9568.2003.02973.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bannatyne BA, Edgley SA, Hammar I, Jankowska E, Maxwell DJ. Differential projections of excitatory and inhibitory dorsal horn interneurons relaying information from group II muscle afferents in the cat spinal cord. J Neurosci. 2006;26:2871–2880. doi: 10.1523/JNEUROSCI.5172-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birinyi A, Viszokay K, Weber I, Kiehn O, Antal M. Synaptic targets of commissural interneurons in the lumbar spinal cord of neonatal rats. J Comp Neurol. 2003;461:429–440. doi: 10.1002/cne.10696. [DOI] [PubMed] [Google Scholar]
- Brink E, Jankowska E, McCrea DA, Skoog B. Inhibitory interactions between interneurones in reflex pathways from group Ia and group Ib afferents in the cat. J Physiol (Lond) 1983;343:361–373. doi: 10.1113/jphysiol.1983.sp014897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruggencate Gt, Burke R, Lundberg A, Udo M. Interaction between the vestibulospinal tract, contralateral flexor reflex afferents and la afferents. Brain Res. 1969;14:529–532. doi: 10.1016/0006-8993(69)90131-0. [DOI] [PubMed] [Google Scholar]
- Buchanan JT. Commissural interneurons in rhythm generation and intersegmental coupling in the lamprey spinal cord. J Neurophysiol. 1999;81:2037–2045. doi: 10.1152/jn.1999.81.5.2037. [DOI] [PubMed] [Google Scholar]
- Butt SJ, Harris-Warrick RM, Kiehn O. Firing properties of identified interneuron populations in the mammalian hindlimb central pattern generator. J Neurosci. 2002;22:9961–9971. doi: 10.1523/JNEUROSCI.22-22-09961.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butt SJ, Kiehn O. Functional identification of interneurons responsible for left-right coordination of hindlimbs in mammals. Neuron. 2003;38:953–963. doi: 10.1016/s0896-6273(03)00353-2. [DOI] [PubMed] [Google Scholar]
- Cabaj A, Stecina K, Jankowska E. Same spinal interneurons mediate reflex actions of group Ib & II afferents and crossed reticulospinal actions. J Neurophysiol. 2006;95:3911–3922. doi: 10.1152/jn.01262.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cajal SR. Histologie du systeme nerveux de l’homme & des vertebres. Instituto Ramon y Cajal; Madrid: 1953. pp. 956–993. [Google Scholar]
- Degtyarenko AM, Simon ES, Norden Krichmar T, Burke RE. Modulation of oligosynaptic cutaneous and muscle afferent reflex pathways during fictive locomotion and scratching in the cat. J Neurophysiol. 1998;79:447–463. doi: 10.1152/jn.1998.79.1.447. [DOI] [PubMed] [Google Scholar]
- Deliagina TG, Orlovsky GN. Activity of Ia inhibitory interneurons during fictitious scratch reflex in the cat. Brain Res. 1980;193:439–447. doi: 10.1016/0006-8993(80)90176-6. [DOI] [PubMed] [Google Scholar]
- Dottori M, Hartley L, Galea M, Paxinos G, Polizzotto M, Kilpatrick T, Bartlett PF, Murphy M, Kontgen F, Boyd AW. EphA4 (Sek1) receptor tyrosine kinase is required for the development of the corticospinal tract. Proc Natl Acad Sci USA. 1998;95:13248–13253. doi: 10.1073/pnas.95.22.13248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edgley SA. Organisation of inputs to spinal interneurone populations. J Physiol (Lond) 2001;533:51–56. doi: 10.1111/j.1469-7793.2001.0051b.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edgley SA, Jankowska E. An interneuronal relay for group I and II muscle afferents in the midlumbar segments of the cat spinal cord. J Physiol (Lond) 1987;389:647–674. doi: 10.1113/jphysiol.1987.sp016676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edgley SA, Jankowska E, Hammar I. Ipsilateral actions of feline corticospinal tract neurons on limb motoneurons. J Neurosci. 2004;24:7804–7813. doi: 10.1523/JNEUROSCI.1941-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edgley SA, Jankowska E, Krutki P, Hammar I. Both dorsal horn and lamina VIII interneurones contribute to crossed reflexes from group II muscle afferents. J Physiol (Lond) 2003;552:961–974. doi: 10.1113/jphysiol.2003.048009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feldman AG, Orlovsky GN. Activity of interneurons mediating reciprocal 1a inhibition during locomotion. Brain Res. 1975;84:181–194. doi: 10.1016/0006-8993(75)90974-9. [DOI] [PubMed] [Google Scholar]
- Grillner S. The motor infrastructure: from ion channels to neuronal networks. Nat Rev Neurosci. 2003;4:573–586. doi: 10.1038/nrn1137. [DOI] [PubMed] [Google Scholar]
- Hammar I, Bannatyne BA, Maxwell DJ, Edgley SA, Jankowska E. The actions of monoamines and distribution of noradrenergic and serotoninergic contacts on different subpopulations of commissural interneurons in the cat spinal cord. Eur J Neurosci. 2004;19:1305–1316. doi: 10.1111/j.l460-9568.2004.03239.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison PJ, Jankowska E. Organization of input to the interneurones mediating group I non-reciprocal inhibition of motoneurones in the cat. J Physiol (Lond) 1985;361:403–418. doi: 10.1113/jphysiol.1985.sp015652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison PJ, Jankowska E, Zytnicki D. Lamina VIII interneurones interposed in crossed reflex pathways in the cat. J Physiol (Lond) 1986;371:147–166. doi: 10.1113/jphysiol.1986.sp015965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoover JE, Durkovic RG. Retrograde labeling of lumbosacral interneurons following injections of red and green fluorescent microspheres into hindlimb motor nuclei of the cat. Somatosens Mot Res. 1992;9:211–226. doi: 10.3109/08990229209144772. [DOI] [PubMed] [Google Scholar]
- Hounsgaard J, Hultborn H, Jespersen B, Kiehn O. Bistability of alpha-motoneurones in the decerebrate cat and in the acute spinal cat after intravenous 5-hydroxytryptophan. J Physiol (Lond) 1988;405:345–367. doi: 10.1113/jphysiol.1988.sp017336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang A, Noga BR, Carr PA, Fedirchuk B, Jordan LM. Spinal cholinergic neurons activated during locomotion: localization and electrophysiological characterization. J Neurophysiol. 2000;83:3537–3547. doi: 10.1152/jn.2000.83.6.3537. [DOI] [PubMed] [Google Scholar]
- Hultborn H. Convergence on interneurones in the reciprocal Ia inhibitory pathway to motoneurones. Acta Physiol ScandSuppl. 1972;375:1–42. doi: 10.1111/j.1748-1716.1972.tb05298.x. [DOI] [PubMed] [Google Scholar]
- Hultborn H. Plateau potentials and their role in regulating motoneuronal firing. Prog Brain Res. 1999;123:39–48. doi: 10.1016/s0079-6123(08)62842-3. [DOI] [PubMed] [Google Scholar]
- Hultborn H, Denton ME, Wienecke J, Nielsen JB. Variable amplification of synaptic input to cat spinal motoneurones by dendritic persistent inward current. J Physiol (Lond) 2003;552:945–952. doi: 10.1113/jphysiol.2003.050971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hultborn H, Illert M, Santini M. Convergence on interneurones mediating the reciprocal Ia inhibition of motoneurones. I. Disynaptic Ia inhibition of Ia inhibitory interneurones. Acta Physiol Scand. 1976;96:193–201. doi: 10.1111/j.1748-1716.1976.tb10188.x. [DOI] [PubMed] [Google Scholar]
- Hultborn H, Illert M, Santini M. Convergence on interneurones mediating the reciprocal Ia inhibition of motoneurones. III. Effects from supraspinal pathways. Acta Physiol Scand. 1976;96:368–391. doi: 10.1111/j.1748-1716.1976.tb10206.x. [DOI] [PubMed] [Google Scholar]
- Hultborn H, Jankowska E, Lindstrom S. Recurrent inhibition from motor axon collaterals of transmission in the Ia inhibitory pathway to motoneurones. J Physiol (Lond) 1971;215:591–612. doi: 10.1113/jphysiol.1971.sp009487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hultborn H, Udo M. Convergence in the reciprocal Ia inhibitory pathway of excitation from descending pathways and inhibition from motor axon collaterals. Acta Physiol Scand. 1972;84:95–108. doi: 10.1111/j.1748-1716.1972.tb05159.x. [DOI] [PubMed] [Google Scholar]
- Jankowska E. Interneuronal relay in spinal pathways from proprioceptors. Prog Neurobiol. 1992;38:335–378. doi: 10.1016/0301-0082(92)90024-9. [DOI] [PubMed] [Google Scholar]
- Jankowska E, Cabaj A, Pettersson LG. How to enhance ipsilateral actions of pyramidal tract neurons. J Neurosci. 2005;25:7401–7405. doi: 10.1523/JNEUROSCI.1838-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jankowska E, Edgley SA, Krutki P, Hammar I. Functional differentiation and organization of feline midlumbar commissural interneurones. J Physiol (Lond) 2005;565:645–658. doi: 10.1113/jphysiol.2005.083014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jankowska E, Hammar I, Slawinska U, Maleszak K, Edgley SA. Neuronal basis of crossed actions from the reticular formation upon feline hindlimb motoneurons. J Neurosci. 2003;23:1867–1878. doi: 10.1523/JNEUROSCI.23-05-01867.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jankowska E, Johannisson T, Lipski J. Common interneurones in reflex pathways from group 1a and 1b afferents of ankle extensors in the cat. J Physiol. 1981;310:381–402. doi: 10.1113/jphysiol.1981.sp013556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jankowska E, Krutki P, Matsuyama K. Relative contribution of Ia inhibitory interneurones to inhibition of feline contralateral motoneurones evoked via commissural interneurones. J Physiol (Lond) 2005;568:617–628. doi: 10.1113/jphysiol.2005.088351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jankowska E, Noga BR. Contralaterally projecting lamina VIII interneurones in middle lumbar segments in the cat. Brain Res. 1990;535:327–330. doi: 10.1016/0006-8993(90)91618-q. [DOI] [PubMed] [Google Scholar]
- Jankowska E, Slawinska U, Hammar I. Differential presynaptic inhibition of actions of group II afferents in di- and polysynaptic pathways to feline motoneurones. J Physiol (Lond) 2002;542:287–299. doi: 10.1113/jphysiol.2001.014068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jankowska E, Slawinska U, Hammar I. On organization of a neuronal network in pathways from group II muscle afferents in feline lumbar spinal segments. J Physiol (Lond) 2002;542:301–314. doi: 10.1113/jphysiol.2001.014076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jankowska E, Stecina K, Cabaj A, Pettersson LG, Edgley SA. Neuronal relays in double-crossed pathways between feline motor cortex and ipsilateral hindlimb motoneurons. J Physiol (Lond) 2006;575:527–541. doi: 10.1113/jphysiol.2006.112425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiehn O. Locomotor circuits in the Mammalian spinal cord. Annu Rev Neurosci. 2006;29:279–306. doi: 10.1146/annurev.neuro.29.051605.112910. [DOI] [PubMed] [Google Scholar]
- Kiehn O, Butt SJ. Physiological, anatomical and genetic identification of CPG neurons in the developing mammalian spinal cord. Prog Neurobiol. 2003;70:347–361. doi: 10.1016/s0301-0082(03)00091-1. [DOI] [PubMed] [Google Scholar]
- Krutki P, Jankowska E, Edgley SA. Are crossed actions of reticulospinal and vestibulospinal neurons on feline motoneurons mediated by the same or separate commissural neurons? J Neurosci. 2003;23:8041–8050. doi: 10.1523/JNEUROSCI.23-22-08041.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kullander K, Butt SJ, Lebret JM, Lundfald L, Restrepo CE, Rydstrom A, Klein R, Kiehn O. Role of EphA4 and EphrinB3 in local neuronal circuits that control walking. Science. 2003;299:1889–1892. doi: 10.1126/science.1079641. [DOI] [PubMed] [Google Scholar]
- Lee RH, Heckman CJ. Influence of voltage-sensitive dendritic conductances on bistable firing and effective synaptic current in cat spinal motoneurons in vivo. J Neurophysiol. 1996;76:2107–2110. doi: 10.1152/jn.1996.76.3.2107. [DOI] [PubMed] [Google Scholar]
- Lindstrom S. Recurrent control from motor axon collaterals of Ia inhibitory pathways in the spinal cord of the cat. Acta Physiol Scandin. 1973;(Suppl392):1–43. [PubMed] [Google Scholar]
- Lu Y, Inokuchi H, McLachlan EM, Li JS, Higashi H. Correlation between electrophysiology and morphology of three groups of neuron in the dorsal commissural nucleus of lumbosacral spinal cord of mature rats studied in vitro. J Comp Neurol. 2001;437:156–169. doi: 10.1002/cne.1276. [DOI] [PubMed] [Google Scholar]
- Lundberg A. Integration in propiospinal motor centre controlling the forelimb in the cat. In: Asanuma H, Wilson VS, editors. Integration in the Nervous System. Igaru-Shoin; Tokyo, New York: 1979. pp. 47–65. [Google Scholar]
- Matsuyama K, Jankowska E. Coupling between feline cerebellum (fastigial neurons) and motoneurons innervating hindlimb muscles. J Neurophysiol. 2004;91:1183–1192. doi: 10.1152/jn.00896.2003. [DOI] [PubMed] [Google Scholar]
- Matsuyama K, Kobayashi S, Aoki M. Projection patterns of lamina VIII commissural neurons in the lumbar spinal cord of the adult cat: an anterograde neural tracing study. Neuroscience. 2006;140:203–218. doi: 10.1016/j.neuroscience.2006.02.005. [DOI] [PubMed] [Google Scholar]
- Matsuyama K, Mori F, Nakajima K, Drew T, Aoki M, Mori S. Locomotor role of the corticoreticular-reticulospinal-spinal interneuronal system. Prog Brain Res. 2004;143:239–249. doi: 10.1016/S0079-6123(03)43024-0. [DOI] [PubMed] [Google Scholar]
- Matsuyama K, Nakajima K, Mori F, Aoki M, Mori S. Lumbar commissural interneurons with reticulospinal inputs in the cat: Morphology and discharge patterns during fictive locomotion. J Comp Neurol. 2004;474:546–561. doi: 10.1002/cne.20131. [DOI] [PubMed] [Google Scholar]
- Nishimaru H, Restrepo CE, Kiehn O. Activity of Renshaw cells during locomotor-like rhythmic activity in the isolated spinal cord of neonatal mice. J Neurosci. 2006;26:5320–5328. doi: 10.1523/JNEUROSCI.5127-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nissen UV, Mochida H, Glover JC. Development of projection-specific interneurons and projection neurons in the embryonic mouse and rat spinal cord. J Comp Neurol. 2005;483:30–47. doi: 10.1002/cne.20435. [DOI] [PubMed] [Google Scholar]
- Ohta Y, Dubuc R, Grillner S. A new population of neurons with crossed axons in the lamprey spinal cord. Brain Res. 1991;564:143–148. doi: 10.1016/0006-8993(91)91364-7. [DOI] [PubMed] [Google Scholar]
- Petko M, Antal M. Propriospinal afferent and efferent connections of the lateral and medial areas of the dorsal horn (laminae I–IV) in the rat lumbar spinal cord. J Comp Neurol. 2000;422:312–325. doi: 10.1002/(sici)1096-9861(20000626)422:2<312::aid-cne11>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- Petko M, Veress G, Vereb G, Storm-Mathisen J, Antal M. Commissural propriospinal connections between the lateral aspects of laminae III–IV in the lumbar spinal cord of rats. J Comp Neurol. 2004;480:364–377. doi: 10.1002/cne.20356. [DOI] [PubMed] [Google Scholar]
- Puskar Z, Antal M. Localization of last-order premotor interneurons in the lumbar spinal cord of rats. J Comp Neurol. 1997;389:377–389. doi: 10.1002/(sici)1096-9861(19971222)389:3<377::aid-cne2>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- Roberts A, Dale N, Ottersen OP, Storm-Mathisen J. Development and characterization of commissural interneurones in the spinal cord of Xenopus laevis embryos revealed by antibodies to glycine. Development. 1988;103:447–461. doi: 10.1242/dev.103.3.447. [DOI] [PubMed] [Google Scholar]
- Ryall RW. Renshaw cell mediated inhibition of Renshaw cells: patterns of excitation and inhibition from impulses in motor axon collaterals. J Neurophysiol. 1970;33:257–270. doi: 10.1152/jn.1970.33.2.257. [DOI] [PubMed] [Google Scholar]
- Scheibel ME, Scheibel AB. Spinal motorneurons, interneurons and Renshaw cells. A Golgi study. Arch Ital Biol. 1966;104:328–353. [Google Scholar]
- Schwindt PC, Crill WE. Properties of a persistent inward current in normal and TEA-injected motoneurons. J Neurophysiol. 1980;43:1700–1724. doi: 10.1152/jn.1980.43.6.1700. [DOI] [PubMed] [Google Scholar]
- Soffe SR, Clarke JD, Roberts A. Activity of commissural interneurons in spinal cord of Xenopus embryos. J Neurophysiol. 1984;51:1257–1267. doi: 10.1152/jn.1984.51.6.1257. [DOI] [PubMed] [Google Scholar]
- Stein PS, Victor JC, Field EC, Currie SN. Bilateral control of hindlimb scratching in the spinal turtle: contralateral spinal circuitry contributes to the normal ipsilateral motor pattern of fictive rostral scratching. J Neurosci. 1995;15:4343–4355. doi: 10.1523/JNEUROSCI.15-06-04343.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stokke MF, Nissen UV, Glover JC, Kiehn O. Projection patterns of commissural interneurons in the lumbar spinal cord of the neonatal rat. J Comp Neurol. 2002;446:349–359. doi: 10.1002/cne.10211. [DOI] [PubMed] [Google Scholar]
- Sugiuchi Y, Izawa Y, Shinoda Y. Trisynaptic inhibition from the contralateral vertical semicircular canal nerves to neck motoneurons mediated by spinal commissural neurons. J Neurophysiol. 1995;73:1973–1987. doi: 10.1152/jn.1995.73.5.1973. [DOI] [PubMed] [Google Scholar]