Abstract
Theoretical and empirical evidence indicates that competing species can coexist if dispersal, migration, and competitive interactions occur over relatively small spatial scales. In particular, spatial structure appears to be critical to certain communities with nontransitive competition. A typical nontransitive system involves three competing species that satisfy a relationship similar to the children’s game of rock–paper–scissors. Although the ecological dynamics of nontransitive systems in spatially structured communities have received some attention, fewer studies have incorporated evolutionary change. Here we investigate evolution within toxic bacterial biofilms using an agent-based simulation that represents a nontransitive community containing three populations of Escherichia coli. In structured, nontransitive communities, strains evolve that do not maximize their competitive ability: They do not reduce their probability of death to a minimum or increase their toxicity to a maximum. That is, types evolve that exercise restraint. We show that nontransitivity and spatial structure (in the form of localized interactions) are both necessary for the evolution of restraint in these biofilms.
Keywords: Allelopathy, bacteriocin, biofilms, colicin, competitive restraint, nontransitivity, rock-paper-scissors, survival of the weakest
Ecological dynamics depend both on the nature of ecological interactions and on how often different interactions occur. The spatial scale of ecological processes, such as dispersal and migration, profoundly influences the frequency of different interactions. Ecologists have investigated the effect of spatial structure on community dynamics for a suite of different interactions, including competition, predation, parasitism, and mutualism (Tilman and Kareiva 1997; Dieckmann et al. 2000). Both theoretical and empirical research has demonstrated that space can be a critical factor in the invasion of rare types, the coexistence of interacting species and the stability of communities (Durrett and Levin 1997; Pagie and Hogeweg 1999; Czárán et al. 2002; Johnson and Seinen 2002; Kerr et al. 2002).
Although the ecological dynamics of spatially structured communities has received much attention (Durrett and Levin 1997; Kerr et al. 2002; Lenski and Riley 2002; Laird and Schamp 2006; Reichenbach et al. 2006), fewer studies have incorporated evolutionary change. Specifically, the assumption that ecological interactions are static has been a starting point in much of this research. However, mutations that lead to new phenotypes within a species may alter the nature of the ecological interactions themselves. Interestingly, the precise evolutionary path of a given species may depend on the degree of spatial structure within its community (Kerr et al. 2006; Hansen et al. 2007).
Assemblages of allelopathic bacteria are ideal systems to explore the role of spatial structure on the ecological and evolutionary dynamics within communities. These microbial communities are often found in structured environments (e.g., biofilms). As an example, populations of enteric bacteria (many of which produce narrow-spectrum toxins) are spatially structured, both between hosts and along the intestinal tract within a host (Sweeney et al. 1996; Gordon and Riley 1999; Kirkup and Riley 2004). Theoretical and empirical research on these systems has shown that spatial structure is a key factor in the maintenance of strain diversity, the invasion of rare types, and population dynamics (Chao and Levin 1981; Durrett and Levin 1997; Pagie and Hogeweg 1999; Czárán et al. 2002; Kerr et al. 2002; Kerr 2007).
In these communities, allelopathy is achieved through the production of toxins (or bacteriocins) by specific strains of bacteria. The best-studied class of bacteriocins are the colicins of Escherichia coli. In colicinogenic strains of E. coli, genes encoding the colicin and a constituitively expressed “immunity” protein are found on a plasmid along with a lysis gene (usually expressed under conditions of stress, causing lysis of the cell and subsequent release of the colicin to the external milieu; James et al. 1996). Colicinogenic strains potentially incur several costs including the maintenance of the plasmid, constant immunity expression, and the suicidal act of toxin release. These colicins kill sensitive cells, that is, those lacking the plasmid (and thus lacking immunity). However, these sensitive cells can mutate to generate a strain resistant to the colicin. Resistance is achieved through the loss or alteration of a membrane protein that binds or translocates the toxin. Such resistance can also carry a cost, for example, compromising nutrient acquisition by altering membrane components (James et al. 1996; Feldgarden and Riley 1998, 1999).
These three strains of bacteria (toxin-producer, sensitive, and resistant) can form a nontransitive competitive system. This occurs when the cost of resistance is less than the cost of toxin production. In such a case, the resistant strain will have a growth rate between the sensitive and producing strains (Riley and Gordon 1999; Kerr et al. 2002; Kirkup and Riley 2004; Kerr 2007). The sensitive strain will outgrow the resistant strain, the resistant strain will outgrow the producer, and the producer can displace a sensitive strain through killing. This nontransitive relationship resembles the children’s game of rock–paper–scissors (where rock crushes scissors, scissors cut paper, and paper covers rock) and has been found to hold in E. coli, both in vitro (Kerr et al. 2002) and in vivo (Kirkup and Riley 2004). Spatial structure was predicted to be essential for coexistence within this rock–paper–scissors system (Durrett and Levin 1997), a result later confirmed empirically (Kerr et al. 2002). Thus, the ecological dynamics of this nontransitive community play out differently in structured and unstructured environments.
The evolutionary dynamics of this nonhierarchical triplet have remained unexplored. This is in spite of the fact that there is solid evidence for variation in the cost of resistance in resistant strains and in the level of toxicity in producer strains (Tan and Riley 1997a, b; Feldgarden and Riley 1998; Gordon and O’Brien 2006; Cascales et al. 2007). In nontransitive communities, if the growth rate of one of the three competitors is increased, the density of this same strain can actually decrease (Tainaka 1993, 1995; Frean and Abraham 2001). Some insight into this occurrence is offered by the adage “the enemy of my enemy is my friend.” Specifically, by improving its growth, a strain more effectively displaces its victim, which in turn liberates its own enemy (the victim of its victim).
In this article we explore how factors such as the cost of resistance and the level of toxicity (factors known to vary among bacterial isolates) evolve in a spatially structured habitat. Further, we will explore cases in which there are functional dependencies between various strain properties. Specifically, we will consider cases in which the level of toxicity is a function of the cost of colicinogeny. Of particular interest will be cases in which it is impossible for a producer (due to functional constraints) to simultaneously achieve restraint in growth and toxicity. By investigating evolutionary trends in this well-studied system, we hope to shed light on how evolutionary changes in the nature of ecological interactions influence community behavior.
Agent-Based Simulation
To investigate evolution within toxic biofilms of bacteria, we use agent-based simulation (Durrett and Levin 1997; Johnson and Seinen 2002; Kerr et al. 2002; Kerr 2007). Virtual bacteria are embedded in a square lattice composed of L × L nodes or points. Initially, every node in the lattice is independently assigned one of the following states with preset probabilities: {S, P, R, E}, where S represents a point occupied by a sensitive cell, P is a point occupied by a producer, R is a point occupied by a resistant cell, and E is an empty lattice point. Subsequently, the lattice is updated asynchronously, that is, a random point in the lattice is selected and its state is changed probabilistically. There are only two types of transitions for any selected point: “birth” or “death” events. Births occur when an empty point becomes filled. Deaths occur when a filled point becomes empty. The probabilities of state change at a focal point depend not only on its current state, but also potentially on the states of the points in its neighborhood. Thus, the neighborhood (the set of points around a focal point that influences its probability of change) is a key variable in our simulations. By manipulating the size of a neighborhood we can control the scale of ecological interactions (e.g., natal dispersal, allelopathy). Making the neighborhood small forces natal dispersal, competition for space, and toxic interactions to be spatially restricted. Making the neighborhood large allows these same interactions to take place over greater distances. In this article we explore the extremes of spatial scale: In one set of simulations we use the eight nearest lattice points surrounding a focal point (a “local” neighborhood, referred to as a “structured community”). In another set of simulations we use the entire lattice (excluding the focal point) as the neighborhood (a “global” neighborhood, referred to as an “unstructured community”); here the community behaves like a well-mixed system.
Once the neighborhood is defined, we can specify state transitions (see Table 1). Here we define fi to be the fraction of points in a focal’s neighborhood occupied by strain i. The probability that an empty point becomes filled with strain i (i ∈ {S, P, R}) is fi (a “birth” event). A point occupied by strain i “dies” with probability Δi (ΔP and ΔR do not depend on neighborhood composition, but ΔS does). A sensitive cell surrounded by several colicin-producing cells has a higher probability of death than one surrounded by empty nodes. We assume that the death rate of a sensitive cell increases linearly with the fraction of producers in its neighborhood:
| (1) |
where ΔS,0 is the intrinsic probability of death of a sensitive cell, and τ scales with the toxicity of producers in the neighborhood. In ecological models, the above parameters (and thus the nature of ecological interactions) are kept constant. We could, however, allow ΔP, ΔR, ΔS,0, and τ to evolve. If τ varies, equation (1) becomes ΔS = ΔS,0 + τ̄fP, where τ̄ is the average toxicity in the focal sensitive cell’s neighborhood. These genetic parameters would represent the genotypes of individuals: ΔP and τ for P cells, ΔR for R cells, and ΔS,0 for S cells. Our goal is to track the evolution of these quantities while maintaining the nontransitivity of the system (see Table 1 legend).
Table 1.
Transition probability from current state (rows) to future state (columns).
| S | P | R | E | |
|---|---|---|---|---|
| S | 1−( ΔS,0+τ fP ) | 0 | 0 | ΔS,0+τ fP |
| P | 0 | 1−ΔP | 0 | ΔP |
| R | 0 | 0 | 1−ΔR | ΔR |
| E | fS | fP | fR | 1− ( fS+ fP+ fR) |
which constrains the community to a nontransitive dynamic. The conditions above imply that the sensitive strain has the highest net growth, followed by the resistant strain, with the producer at the bottom. However, the producer possesses a toxicity above a critical level (τ > (ΔP − ΔS,0)/(1 − ΔP)), which guarantees that a sufficiently dense population of producers displaces a sensitive population in an unstructured environment. In a well-mixed environment, there is a case of bistability if the producer’s toxicity is above the critical value τc = (ΔP − ΔS,0)/(1 − ΔP). That is, if producers are sufficiently dense, they fix; otherwise, sensitive cells fix. However, in a spatial system, this bistability can disappear and producers can fix from very small starting frequencies (see Kerr 2007).
To incorporate evolution in our simulations, we allow every cell to carry its own genotype g and when an offspring is “born,” a mutation can occur to change the genotype. For instance, the genotype for an R cell would be its death rate (g = ΔR). If gpar is the genotype of the parent, we assume that the genotype of the offspring goff is:
| (2) |
where μg is the probability of mutation and Zg is a random variable (Zg ~ Unif(−φg, φg)), where φg relates to the amount g can change due to a single mutation). We require g to remain between gmin and gmax, where these values are chosen to maintain the nontransitivity of the bacterial community (see Table 2 and the legend to Table 1). Table 2 summarizes the parameters used in our simulations with their typical ranges.
Table 2.
Parameters or variables used and their values or ranges.
| Symbol | Definition | Value or Range |
|---|---|---|
| ΔS,0 | Intrinsic death rate of a sensitive cell | 0.25 |
| τ | Toxicity of a producer | 0.35–0.75 |
| ΔP | Death rate of a producer | 0.3333–0.3433 |
| ΔR | Death rate of a resistant cell | 0.275–0.329 |
| μΔR | Probability of mutation of death rate in resistant cells | 0.001–0.1 |
| μτ | Probability of mutation of toxicity in producers | 0.001–0.1 |
| φΔR | Amount ΔR can change due to a single mutation | 0.0001–0.1 |
| φτ | Amount τ can change due to a single mutation | 0.0001–0.1 |
| L | Number of nodes in one dimension of the square lattice | 300 |
| m | Slope of linear function relating death rate to toxicity of producers | −0.025 or 0.025 |
| fi | Fraction of cells of type i in the neighborhood of a focal point |
We define an epoch as L × L asynchronous updates to the lattice. In other words, the average waiting time for a focal point to be updated is an epoch. Most of our simulations were terminated at 100,000 epochs, but some were run for longer periods to verify convergence of the evolving parameters.
Evolution of Average Death Rate in Resistant Cells
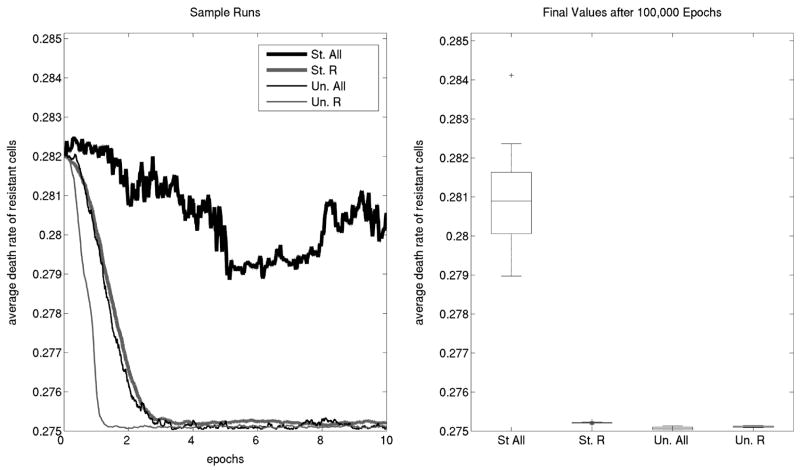
Empirical evidence points to variability in the cost of colicin resistance (Feldgarden and Riley 1998; Cascales et al. 2007). Here we represent this variability in cost by allowing the death rate of R (ΔR) to evolve, that is, ΔR is the genotype of an R cell (for simplicity, we keep all other parameters constant in this set of simulations). We expect an evolvable death rate to approach its minimum value over time. Indeed when R is alone in an unstructured habitat, that is precisely what happens (Fig. 1). However, in a structured world where R, S, and P are present, Δ̄R never reaches its minimum value. Given that birth rate is constant for all strains, when death rate does not evolve to its minimum, then net growth rate (birth minus death) is not maximized. Thus, we consider a population with an elevated death rate to be “competitively restrained.” In a complete structured community, the resistant population has evolved significant competitive restraint.
Figure 1.
The evolution of competitive restraint in death rate. Shown are the results of lattice-based simulations allowing the resistant strain to evolve its death rate. The parameters used are ΔP = 1/3, ΔS,0 = 1/4, τ = 0.65, μΔR = 0.001, φΔR = 0.001 and L = 300. Left: In an unstructured world composed of R cells only (abbreviated “Un. R”), the average death rate (Δ̄R ) evolves immediately to its minimum (thin gray line). On the other hand, in a structured community with all three strains (thick black line, “St. All”), ΔR does not evolve to its minimum. Structure alone does not cause restraint (thick gray line, “St. R”), and neither does the presence of all three strains in an unstructured community (thin black line, “Un. All”). Both structure and a complete community are necessary for restraint. Right: Final average death rate of resistant cells from 10 simulations for each community type. Horizontal lines in the boxes represent the upper quartile (top of the box), median, and lower quartile values (bottom of the box), while vertical lines extending from each box cover all datapoints within 1.5 units of the interquartile range beyond the box. Outliers appear as crosses beyond the vertical lines.
One may ask what causes this: are both structure and the presence of all three strains needed? In a structured world where only R is present, the average death rate Δ̄R is significantly lower than in a complete structured community, so structure alone is not sufficient to produce restraint of the degree seen in Figure 1. Is it merely the presence of all three strains that keeps Δ̄R from evolving to its minimum? To address this question, we ran simulations in which all three strains were present in an unstructured community. However, we found that one or two strains were quickly lost (see Kerr et al. 2002). To keep other types in the system (a complete community), we employed a reseeding strategy. Specifically, we repopulated the entire lattice at defined intervals (60 epochs) to bring back the average frequencies achieved during structured simulations with the three strains (see Appendix I for details). We found that Δ̄R quickly evolved to its minimum value.
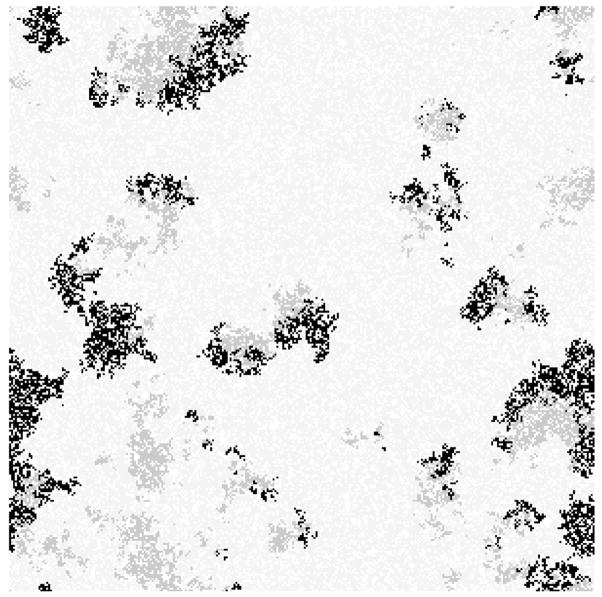
Thus, it is not the mere presence of three strains that produces the evolution of restraint in resistant cells. Rather there is restraint in the average death rate Δ̄R only in structured communities with all three strains present. To understand this, it helps to consider the distribution of individuals in a structured complete community. The three strains form clusters that chase one another at the boundaries: S clusters chase R clusters, R clusters chase P clusters, and P clusters chase S clusters (Kerr 2007 and Fig. 2). Imagine two distinct resistant patches with death rates Δ̄R1 < Δ̄R2. Because patch 1 has a higher average net growth than patch 2, its members will displace more quickly their neighboring P cells. By doing so, R cells in patch 1 come face to face more rapidly with sensitive S cell neighbors (which outgrow them) than cells in patch 2, and thus experience extinction more readily. Members of patch 2, by maintaining a higher death rate, are not able to displace their P neighbors as effectively and thus avoid being surrounded by competitively superior S cells. Thus, the presence of producer and sensitive strains in a spatially structured community can select for competitive restraint in the resistant strain.
Figure 2.
Snapshot of the lattice in a simulation with a complete (all three strains) and structured community at epoch 33,000. S is light gray, P is black, and R is dark gray; empty lattice points are white. Strains form clusters that chase one another around the lattice.
On the other hand, in a well-mixed habitat with all three strains present, same-strain clusters do not readily form. Consequently, unrestrained resistant cells do not disproportionately experience the costs of their lack of restraint. Resistant cells in an unstructured environment, regardless of death rate, are competing for the same vacant space. Therefore, there is universal selection for faster growth and unrestrained variants flourish.
To verify that our simulations were consistent and genotypes converged to their asymptotic values, we ran 10 simulations for each of four different community types: (1) structured and complete (i.e., with all three strains), (2) structured with only resistant cells, (3) unstructured and complete, and (4) unstructured with only resistant cells. Figure 1 shows the final values of the cost of resistance after 100,000 epochs for all 40 simulations. We find that significantly restrained growth only evolves in the complete structured community (P < 0.002 for all tests between the complete structured community and each of the other three community types; here and in all other tests, we use a two-sided Wilcoxon rank sum test with the appropriate Bonferroni correction. Outliers were included in these nonparametric tests).
Evolution of Toxicity When Independent of Death Rate
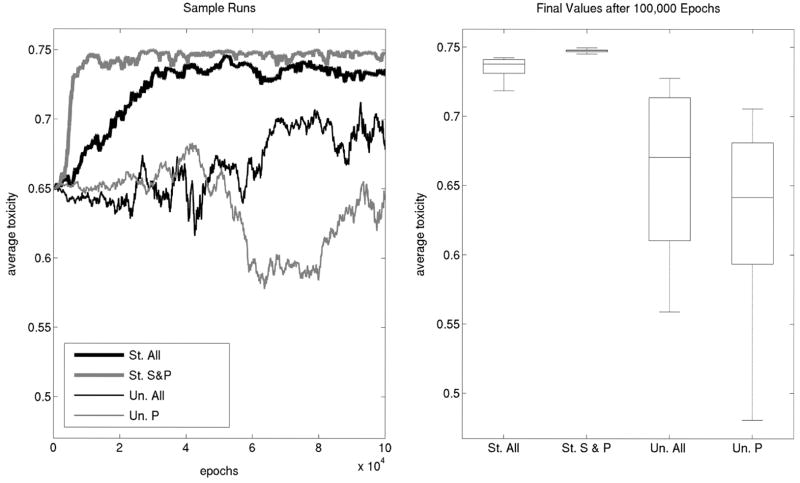
There is evidence for variability in toxicity within colicin-producing strains (Tan and Riley 1997a, 1997b; Gordon and O’Brien 2006; Cascales et al. 2007). We capture this diversity by allowing toxicity (τ) to evolve. Here τ is the genotype of a P cell. To keep things simple, we allow τ to evolve (as shown in eq. 2) while maintaining ΔR constant (Fig. 3). In these first simulations we make the assumption that toxicity is independent of the death rate of producers (i.e., ΔP is constant). As a result, in an unstructured world where only P cells are present there is no selective advantage or disadvantage for increased toxicity, so we expect τ to drift. Indeed that is what we observe and there is large variance in the final toxicity after 100,000 epochs. In a structured environment, toxicity has the effect of “clearing” sensitive cells and allowing producers prime access to the cleared real estate. Thus, we consider what happens in a structured habitat composed of S and P cells only. Obviously, P cells will quickly extinguish S cells from the community. To maintain both strains for an indefinite period of time, we reseed the entire lattice at defined intervals (100 epochs; see Appendix I). In this case, we find that average toxicity of the producer cells evolves to its maximum possible value (τ̄ ≈ 0.75). What happens if we simply add R cells to the community? Interestingly, τ evolves to a high level, but does not reach its maximum value; there is significant restraint (P < 0.002 for a test between the complete structured community and the structured community with S and P cells only).
Figure 3.
The evolution of restraint in toxicity when independent of producer death rate. Shown are the results of lattice-based simulations allowing the colicin-producing strain to evolutionarily change toxicity. The parameters used are ΔP = 1/3, ΔS,0 = 1/4, ΔR = 0.312, μτ= 0.001, φτ = 0.03 and L = 300. Left: In a structured community composed of S and P, average toxicity (τ̄) evolves to its maximum (thick gray line, “St. S & P”). Adding resistant cells to the above community causes τ̄ to evolve restraint (thick black line, “St. All”). In the absence of structure, τ̄ simply drifts whether the community has all three strains present (thin black line, “Un. All”), or P is evolving alone (thin gray line, “Un. P”). Right: Final average toxicity from 10 simulations for each community type.
Again, we can ask whether both structure and the presence of all three strains are necessary for this restraint. When simulating a complete community in an unstructured world, average toxicity also drifts. Thus, in an unstructured world there is no selection on τ and in a structured world with no resistant cells, producers evolve to be significantly more toxic than in the presence of resistant cells. To understand this, imagine two separate patches with τ̄1 < τ̄2. Cells in patch 2 are more successful in clearing out space around them, making it available to related producers, and as a result this patch expands more rapidly in the short run. However, by killing S cells more efficiently, cells in patch 2 are more readily surrounded by R cells, which outgrow them. Colicin-producing cells in patch 1 are less efficient in eliminating their S neighbors and by doing so keep some sensitive cells around which buffer them from their enemy R cells. In this way, cells in patch 1 survive for longer periods of time. In the long run, patches of producers with restrained toxicity remain in the community.
In an unstructured habitat with all three strains present, highly toxic producers disproportionately experience neither the benefits of locally cleared space nor the costs of coming face-to-face with their enemy (no clusters form). Consequently, toxicity drifts. In a structured environment with only sensitive and producer cells present, highly toxic producers do disproportionately receive the local benefits of clearing real estate (clusters form here), but avoid the costs (as no resistant cells are present). Therefore, toxicity evolves to a maximum. Only when resistant cells are added to the structured habitat are the costs realized and some degree of restraint evolves.
Evolution of Toxicity When Dependent on Death Rate
In the above set of simulations, we have assumed that toxicity (τ) is independent of death rate (ΔP). This may not be the case as colicin production could potentially affect the death rate of producer cells. To simulate this dependency, we use the following relationship:
| (3) |
where m is the slope of a linear function (i.e., if m > 0, higher toxicity causes higher death rates; if m < 0, higher toxicity causes a drop in death rates) and is the value of ΔP at τ = τmin (Fig. 4).
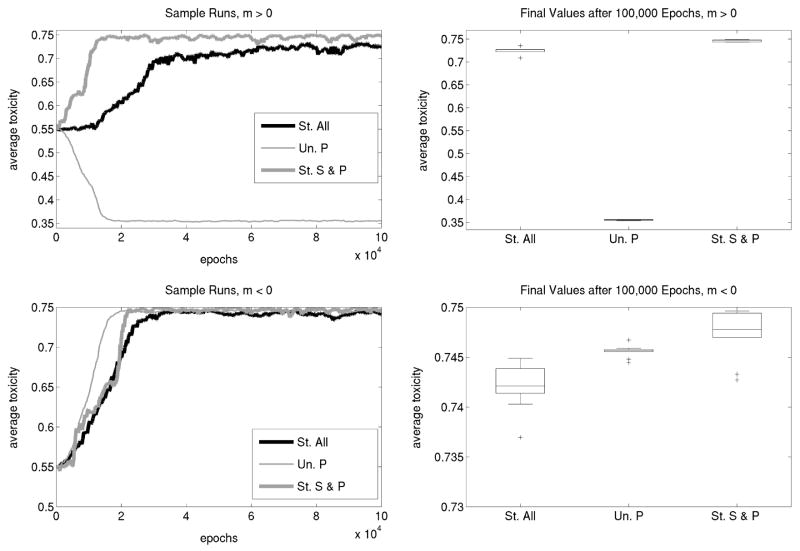
Figure 4.
The evolution of restraint in toxicity when death rate of the producer is a function of toxicity. Shown are the results of lattice-based simulations allowing the colicin-producing strain to evolve toxicity. The parameters used are as in Figure 3 except for ΔP. Top graphs use the equation ΔP = 0.025(τ − 0.35) + 0.3333, while bottom graphs use the equation ΔP = −0.025(τ − 0.35) + 0.3433. Top left: In a structured community composed of S and P cells, average toxicity (τ̄) evolves to its maximum (thick gray line, “St. S & P”). Adding resistant cells to the above community causes restraint in toxicity (thick black line, “St. All”). For comparison, the producing strain quickly evolves to minimize both death rate (Δ̄P ) and average toxicity (τ̄) when alone in an unstructured habitat (thin gray line, “Un. P”). Top right: Final average toxicity from 10 simulations for each community (when m > 0). Bottom left: With benefits associated with toxic production, toxicity evolves to near the maximum in all three communities. Nevertheless, there is still restraint in the complete structured community. Bottom right: Final average toxicity from 10 simulations for each community (when m < 0). Note the different scales on the vertical axes of the two bottom graphs.
We start with the case in which it is impossible for a producer to simultaneously achieve restraint in growth and toxicity (m > 0 in eq. 3). In the absence of other strains, we expect unstructured communities consisting only of P cells to minimize the cost of colicin production. This is indeed what we observe, and they achieve it by reducing both average death rate (Δ̄P ) and average toxicity (τ̄), the latter variable evolving close to its minimum value (τ̄ ≈ 0.35). On the other hand, in a structured community with S and P cells (maintained by the same reseeding strategy mentioned above), toxicity evolves to its maximum possible level as long as we are in the regime of nontransitivity. Colicin-producing cells evolve to maintain high levels of toxicity despite the concomitant high death rates. In this case, producers incur a higher death rate in exchange for higher toxicity (which clears more space for their offspring). Note that it is toxicity and not competition (i.e., a lower death rate ΔP) that gives P cells an advantage over S cells. As long as nontransitivity holds, even if P cells were to evolve ΔP to its minimum value, S cells still have a growth advantage. However, the presence of R cells (in addition to S cells) in a structured community prevents the producing cells from reaching the maximum toxicity allowed. In other words, we observe significant restraint again (P < 0.006 for a test between the complete structured community and the structured community with S and P cells only).
In a different scenario, we allow a producer to simultaneously evolve low death rates and high toxicity (m < 0 in eq. 3). As above, we expect unstructured communities consisting only of P cells to minimize their average death rate. Indeed, this is what we observe and because toxicity is negatively correlated with production cost, P cells increase toxicity to a near-maximum. Adding S cells and structure to the community causes average toxicity to evolve close to its maximum value again. Here, there are two advantages to higher toxicity: (1) a lower death rate, and (2) more cleared real estate. However, in a structured community with all three strains, average toxicity does not evolve to the same high levels as in the previous two habitats. Again, toxicity is significantly restrained in spite of the fact that such restraint causes higher mortality in P cells (P < 0.006 for a test between the complete structured community and the structured community with S and P cells only; P < 0.001 for a test between the complete structured community and the P-only community; there was no significant difference in the final value of toxicity between the P-only community and the community with S and P cells only).
Discussion
In structured communities with three strains exhibiting a non-transitive dynamic, we find that strains do not evolve the “most competitive” strategy. At the level of a patch of cells, the most competitive strategy is to minimize death rate or maximize toxicity. These strategies make sense locally. However, patches within the lattice that contain these “local optimizers” become extinct whereas restrained patches survive. These findings are the result of two factors: (1) the negative feedback generated by the presence of all three strains and (2) the spatial structure of the community that constrains the scale of potential ecological interactions among organisms. In a nontransitive community, there is always selection to disregard the adage “the enemy of my enemy is my friend.” That is, short-term benefits accrue to mutants with improved competitive ability. However when the community is spatially structured, those clusters that are the first to “ignore” the adage (i.e., the clusters in which mutation generates the most competitive types) are more prone to disappear as they come face-to-face with their victim’s victim (i.e., their enemy). Thus, localized dispersal and interactions (which gives rise to a population that is separated into many clusters) can lead to the persistence of restrained types in longer-lived clusters. Even if locally “ignored,” the adage is “heeded” at a global level in structured communities (see Johnson and Seinen 2002 for more discussion of this point).
We argue that selection favors survival of the “weaker” in terms of the local competitive optimum. Cells exercising some restraint remain in the community the longest. The weakest members of the community (with high death rates) go extinct early, whereas the strongest members “improve themselves to death.” Similarly, colicin-producing cells that exercise restraint by limiting their toxicity also survive the longest. Less-toxic P cells go extinct early because they are incapable of clearing sufficient space. The most toxic colicinogenic cells clear substantially more space for their own lineages, but as mentioned above, come more readily into contact with their enemy R cells and thus go extinct earlier. We therefore see survival of the “weaker” in complete and structured communities. By running simulations with other parameter values, we find that survival of the “weaker” is a relatively, but not completely, robust result (see Appendix II for exploration of altered mutational parameters).
In all of our simulations, we forced our community to maintain a nontransitive relationship. However, it is possible that evolutionary change could lead to a violation of the nontransitivity. For instance, a mutation might make the death rate of a resistant cell equal to (or even lower than) the death rate of sensitive cells. How important are these forced constraints to the evolution of restraint? Interestingly, we find that when we simulate a community where it is possible to break the nontransitivity, there are cases in which restraint can still evolve. Specifically, in one set of simulations (data not shown), we set ΔR, min < ΔS,0 such that it was possible for mutations to generate resistant cells that grew faster than sensitive cells. However, if resistant cells started with a death rate above sensitive cells (and if mutation parameters are small), then restraint still evolves. In this case, restraint appears to be an evolutionarily stable strategy. Also, all three strains persist despite the fact that a mutant, which is superior to all strains, is allowed. On the other hand, if mutation parameters are larger (specifically if it is possible for a resistant mutant to have a death rate very different from its parent), then restraint will not evolve and the community will collapse into a monoculture.
As mentioned above, empirical evidence points to variability in the cost of colicin resistance (Feldgarden and Riley 1998; Cascales et al. 2007). Given this variability, it is possible to perform an experiment to explore the evolution of restraint in resistant cells. This can be achieved by selecting three strains of E. coli (e.g., a colicin-producing strain (P), a sensitive strain (S), and a resistant strain (R)), which satisfy a rock–paper–scissors competitive relationship (Kerr et al. 2002). Biofilm growth on petri dishes mimics an environment in which natal dispersal and interactions are primarily local. A complete community could consist of all three strains present in the plate, whereas in a separate plate another community would be created with resistant cells only. After several generations, one could compare growth rates of resistant cells from the two treatments. If restraint had evolved, the cells from the complete community should exhibit lower growth rates.
The presence of nontransitivity in a competitive context has been found in many systems including side-blotched lizards (Sinervo and Lively 1996), sessile marine invertebrates (Buss and Jackson 1979), epiphytes of intertidal alga (Stebbing 1973), and yeast (Paquin and Adams 1983). Sinervo and Calsbeek (2006) argue that rock–paper–scissors dynamics are common in nature. Nontransitive dynamics are not only important in competitive interactions among morphs or strains of the same species, but also may play a role in mate preferences (Kirkpatrick et al. 2006), direct and indirect reciprocity (Brandt and Sigmund 2006), and volunteering in public goods games (Hauert et al. 2002; Semmann et al. 2003). Nontransitivity may also be critical to the dynamics of altruism, mutualism, antagonism, Batesian mimicry, and competition between two species for the same resource (Sinervo and Calsbeek 2006). Many communities for which nontransitivity has been discovered are also spatially structured (e.g., microbes in biofilms, territorial animals, and sessile marine invertebrates). In fact, Johnson and Seinen (2002) hypothesize that competitive restraint of the type we discuss may evolve in subtidal hard-bottom marine communities. These communities frequently possess competitive nontransitivity and are spatially structured (Johnson and Seinen 2002); both of these features are critical to Johnson and Seinen’s hypothesis of evolutionary restraint.
As another example of nontransitivity outside a competitive context, consider a victim–exploiter relationship (e.g., host–pathogen, prey–predator, etc.) in which exploiters cause local extinction in a subpopulation of victims (see Sella and Lachmann 2000). In a metapopulation, there will be three basic classes of subpopulations: (1) uncolonized subpopulations (which we label “U”), (2) subpopulations with only victims (which we label “V”), and (3) subpopulations with exploiters (which we label “E”). We note that E subpopulations may contain some victims; however, over time any victims in the E subpopulations go extinct due to overexploitation (by assumption). Without victims (and if no other victims immigrate into the E subpopulation), then, the exploiter subpopulation is also doomed to eventual extinction. Thus, E sub-populations transition into U subpopulations over time. However, U subpopulations can become V subpopulations when victims immigrate from a V subpopulation. Finally, V subpopulations can become E subpopulations when exploiters immigrate from an E subpopulation. Thus, we have a type of rock–paper–scissors relationship at the subpopulation scale through the processes of immigration and decay: U is beaten by V, V is beaten by E, and E is “beaten” by U. Kerr et al. (2006) experimentally studied a host–pathogen metapopulation with precisely these dynamics. They manipulated the scale of migration between subpopulations of bacteria and virus (that infected the bacteria). They found that under spatially restricted migration, the virus (i.e., exploiter) evolved significant competitive restraint (in pairwise competition for hosts with a marked virus) compared to virus evolved under spatially unrestricted migration. Thus, the results reported in our model may have applications to evolution within systems with fundamentally different ecological interactions.
More generally, restraint can be viewed as a form of cooperation. Types that are prudent with the use of common resources suffer selective disadvantages relative to unrestrained types competing for the same resources. However, collections with many restrained types can be more productive than collections with many unrestrained types. The explanation of group-beneficial, self-detrimental traits has been a central focus of multi-level selection theory (Wilson 1975; Sober and Wilson 1998; Okasha 2006). Many models using a multilevel framework have shown that population structure can favor the evolution of cooperation (Maynard Smith 1964; Eshel 1972; Wilson 1975; Sella and Lachmann 2000; Kerr and Godfrey-Smith 2002). Most of these models assume that discrete groups form (i.e., boundaries exist). Our work suggests that some of the insights from these models carry over to the case of contiguous spatial structure. Specifically, restraint in our resistant cells is always locally disadvantageous; however, in a spatially structured habitat with three strains present, restraint evolves because prudent clusters persist longer.
The case of restraint given costly toxin production (m > 0) is particularly interesting with respect to the topic of cooperation. A high level of toxicity is a form of cooperation (a producer incurs a fitness cost for a higher τ when m > 0, but it benefits other producers by clearing space). When only sensitive and producer cells are present in a spatially structured habitat, a high level of toxicity can evolve (as the cells disproportionately benefiting from cleared real estate also tend to be highly toxic). However, when resistant cells are added to the structured environment, the short-term benefits of cooperative clearing of sensitive cells become balanced against the long-term costs of coming face-to-face with resistant cells. In these circumstances restraint in a trait (toxicity) with immediate prosocial consequences (cleared space) leads to longer-term prosocial consequences (maintenance of a buffering layer of sensitive cells between the producer cluster and its resistant enemy).
Finally, theoretical work has shown that nontransitive interactions can promote biodiversity (Huisman and Weissing 1999; Huisman et al. 2001; Zhang et al. 2006). In allelopathic E. coli, costly toxin production and costly resistance favor the formation of nontransitive communities. However, nontransitivity alone is not sufficient to maintain diversity in bacteriocin communities. The other necessary ingredient is spatial structure. We show here that this second ingredient also influences the evolutionary trajectories of members in a nontransitive community. In general, space can play an important role in the evolution of ecological interactions (Kerr et al. 2006; Hansen et. al 2007). In our system, both nontransitivity and spatial structure are necessary for the evolution of restraint.
Acknowledgments
We thank J. Bergelson, C. Eshelman, J. HilleRisLambers, H. Kokko, K. Laegreid, B. Miner, J. Nahum, R. Vouk, J. West and two anonymous reviewers for many useful comments on previous versions of this manuscript.
APPENDIX I
RESEEDING STRATEGIES
Reseeding strategy to keep all three strains in an unstructured lattice
To maintain all three strains for an indefinite period of time in an unstructured habitat, we repopulate the entire grid every 60 epochs. Reseeding more frequently slows the speed at which genotypes converge to their asymptotic values whereas reseeding less frequently permits the collapse of the community. During reseeding, the entire lattice is scanned and either all the resistant cells or all the producers (depending on which parameter is evolving) are counted and copied into a “storage” array. Next, the entire lattice is scanned again, and frequencies for the three types of cells are reset as follows: 0.6 for S cells, 0.05 for P cells, and 0.13 for R cells when ΔR is evolving, or 0.2 for S cells, 0.25 for P cells, and 0.22 for R cells when τ is evolving. These values are close to the long-term average frequencies found in complete and structured communities. If there is an excess of the evolving strain at reseeding time, they are probabilistically removed. If there is a deficit, they are probabilistically added and the death rates of the new R cells or the toxicities of the new P cells are randomly picked from the storage array.
Reseeding strategy to keep producers and sensitive cells in a structured lattice
To maintain sensitive and producing strains for an indefinite period of time in a structured community, we repopulate the entire grid every 100 epochs. As above, reseeding more frequently slows the speed at which genotypes converge to their asymptotic values whereas reseeding less frequently permits the collapse of the community because of the extinction of sensitive cells. During reseeding, the entire lattice is scanned and all the producing cells are counted and copied into a storage array. Next, the entire lattice is scanned again, and frequencies for both types of cells are reset as follows: 0.89 for S cells, 0.01 for P cells. If there is an excess of P cells at reseeding time, they are probabilistically removed and replaced by S cells or empty spaces. In the rare event that there is a deficit of P cells, they are probabilistically added and the toxicities of the new P cells are randomly picked from the storage array. This strategy generally reduces the size of producer clusters, leaving behind a few P cells that subsequently recolonize the space held by the old cluster, increasing their numbers until the next reseeding. In a sense, therefore, a semblance of the original community structure is maintained.
APPENDIX II
EXPLORING MUTATION PARAMETERS
To verify that restraint in the average death rate of resistant cells evolved under different mutational regimes, we ran additional simulations varying the probability of mutation μΔR, the amount of change caused by a single mutation (i.e., mutation distance) φΔR, or both. We consistently observed restraint in the average death rate (Δ̄R ) when the probability of mutation and mutation distance were high. We also observed restraint when μΔR was high and φΔR was low. In these simulations, all three types remained in the lattice for 1,000,000 epochs. However, with a low probability of mutation and a high mutation distance, the community eventually collapsed to a monoculture in 7 out of 10 simulations. Interestingly, in all 10 cases the resistant cells did not evolve restraint (Δ̄R reached its minimum value). These eventual collapses to a monoculture appear to be stochastic.
LITERATURE CITED
- Brandt H, Sigmund K. The good, the bad and the discriminator—Errors in direct and indirect reciprocity. J Theor Biol. 2006;239:183–194. doi: 10.1016/j.jtbi.2005.08.045. [DOI] [PubMed] [Google Scholar]
- Buss LW, Jackson JBC. Competitive networks: non-transitive competitive relationships in cryptic coral-reef environments. Am Nat. 1979;113:223–234. [Google Scholar]
- Cascales E, Buchanan SK, Duche D, Kleanthous C, Lloubès R, Postle K, Riley M, Slatin S, Cavard D. Colicin biology. Microb Mol Biol Rev. 2007;71:158–229. doi: 10.1128/MMBR.00036-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chao L, Levin BR. Structured habitats and the evolution of anticompetitor toxins in bacteria. Proc Natl Acad Sci USA. 1981;78:6324–6328. doi: 10.1073/pnas.78.10.6324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Czárán TL, Hoekstra RF, Pagie L. Chemical warfare between microbes promotes biodiversity. Proc Natl Acad Sci USA. 2002;99:786–790. doi: 10.1073/pnas.012399899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dieckmann U, Law R, Metz JAJ. The Geometry of Ecological Interactions: Simplifying Spatial Complexity. Cambridge Univ. Press; Cambridge, U.K: 2000. [Google Scholar]
- Durrett R, Levin S. Allelopathy in spatially distributed populations. J Theor Biol. 1997;185:165–171. doi: 10.1006/jtbi.1996.0292. [DOI] [PubMed] [Google Scholar]
- Eshel I. On the neighborhood effect and the evolution of altruistic traits. Theor Pop Biol. 1972;3:258–277. doi: 10.1016/0040-5809(72)90003-2. [DOI] [PubMed] [Google Scholar]
- Feldgarden M, Riley MA. High levels of colicin resistance in Escherichia coli. Evolution. 1998;52:1270–1276. doi: 10.1111/j.1558-5646.1998.tb02008.x. [DOI] [PubMed] [Google Scholar]
- Feldgarden M, Riley MA. The phenotypic and fitness effects of colicin resistance in Escherichia coli. K-12 Evolution. 1999;53:1019–1027. doi: 10.1111/j.1558-5646.1999.tb04517.x. [DOI] [PubMed] [Google Scholar]
- Frean M, Abraham ER. Rock–scissors–paper and the survival of the weakest. Proc R Soc Lond Series B. 2001;268:1323–1327. doi: 10.1098/rspb.2001.1670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon DM, Riley MA. A theoretical and empirical investigation of the invasion dynamics of colicinogeny. Microbiology-Sgm. 1999;145:655–661. doi: 10.1099/13500872-145-3-655. [DOI] [PubMed] [Google Scholar]
- Gordon DM, O’Brien CL. Bacteriocin diversity and the frequency of multiple bacteriocin production in Escherichia coli. Microbiology-Sgm. 2006;152:3239–3244. doi: 10.1099/mic.0.28690-0. [DOI] [PubMed] [Google Scholar]
- Hansen SK, Rainey PB, Haagensen JAJ, Molin S. Evolution of species interactions in a biofilm community. Nature. 2007;445:533–536. doi: 10.1038/nature05514. [DOI] [PubMed] [Google Scholar]
- Hauert C, De Monte S, Hofbauer J, Sigmund K. Volunteering as red queen mechanism for cooperation in public good games. Science. 2002;296:1129–1132. doi: 10.1126/science.1070582. [DOI] [PubMed] [Google Scholar]
- Huisman J, Weissing FJ. Biodiversity of plankton by species oscillations and chaos. Nature. 1999;402:407–410. [Google Scholar]
- Huisman J, Johansson AM, Folmer EO, Weissing FJ. Towards a solution of the plankton paradox: the importance of physiology and life history. Ecol Lett. 2001;40:408–411. [Google Scholar]
- James R, Kleanthous C, Moore GR. The biology of E. colicins: paradigms and paradoxes. Microbiology-UK. 1996;142:1569–1580. doi: 10.1099/13500872-142-7-1569. [DOI] [PubMed] [Google Scholar]
- Johnson CR, Seinen I. Proc R Soc Lond Series B. Vol. 269. 2002. Selection for restraint in competitive ability in spatial competition systems; pp. 655–663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerr B. The ecological and evolutionary dynamics of model bacteriocin communities. In: Riley MA, Chavan MA, editors. Bacteriocins: ecology and evolution. Springer; Berlin: 2007. pp. 111–134. [Google Scholar]
- Kerr B, Godfrey-Smith P. On individualist and multi-level perspectives on selection in structured populations. Biol Philos. 2002;17:477–517. [Google Scholar]
- Kerr B, Riley MA, Feldman MW, Bohannan BJM. Local dispersal promotes biodiversity in a real-life game of rock–paper–scissors. Nature. 2002;418:171–174. doi: 10.1038/nature00823. [DOI] [PubMed] [Google Scholar]
- Kerr B, Neuhauser C, Bohannan BJM, Dean AM. Local migration promotes competitive restraint in a host-pathogen ‘tragedy of the commons. Nature. 2006;442:75–78. doi: 10.1038/nature04864. [DOI] [PubMed] [Google Scholar]
- Kirkpatrick M, Stanley Rand A, Ryan MJ. Mate choice rules in animals. Anim Behav. 2006;71:1215–1225. [Google Scholar]
- Kirkup BC, Riley MA. Antibiotic-mediated antagonism leads to a bacterial game of rock–paper–scissors in vivo. Nature. 2004;428:412–414. doi: 10.1038/nature02429. [DOI] [PubMed] [Google Scholar]
- Lenski RE, Riley MA. Chemical warfare from an ecological perspective. Proc Natl Acad Sci USA. 2002;99:556–558. doi: 10.1073/pnas.022641999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laird RA, Schamp BS. Competitive intransitivity promotes species coexistence. Am Nat. 2006;168:182–193. doi: 10.1086/506259. [DOI] [PubMed] [Google Scholar]
- Maynard Smith J. Group selection and kin selection. Nature. 1964;201:1145–1147. [Google Scholar]
- Okasha S. Evolution and the levels of selection. Oxford Univ. Press; Oxford, U.K: 2006. [Google Scholar]
- Pagie L, Hogeweg P. Colicin diversity: a result of eco-evolutionary dynamics. J Theor Biol. 1999;196:251–261. doi: 10.1006/jtbi.1998.0838. [DOI] [PubMed] [Google Scholar]
- Paquin CE, Adams J. Relative fitness can decrease in evolving asexual populations of S. cerevisiae. Nature. 1983;306:368–371. doi: 10.1038/306368a0. [DOI] [PubMed] [Google Scholar]
- Reichenbach T, Mobilia M, Frey E. Coexistence versus extinction in the stochastic cyclic Lotka-Volterra model. Phys Rev E. 2006;74:051907–1–051907–11. doi: 10.1103/PhysRevE.74.051907. [DOI] [PubMed] [Google Scholar]
- Riley MA, Gordon DM. The ecological role of bacteriocins in bacterial competition. Trends Microbiol. 1999;7:129–133. doi: 10.1016/s0966-842x(99)01459-6. [DOI] [PubMed] [Google Scholar]
- Sella G, Lachmann M. On the dynamic persistence of cooperation: how lower individual fitness induces higher survivability. J Theor Biol. 2000;206:465–485. doi: 10.1006/jtbi.2000.2130. [DOI] [PubMed] [Google Scholar]
- Semmann D, Krambeck H, Milinski M. Volunteering leads to rock–paper–scissors dynamics in a public goods game. Nature. 2003;425:390–393. doi: 10.1038/nature01986. [DOI] [PubMed] [Google Scholar]
- Sinervo B, Calsbeek R. The developmental, physiological, neural, and genetic causes and consequences of frequency-dependent selection in the wild. Annu Rev Ecol Evol Syst. 2006;37:581–610. [Google Scholar]
- Sinervo B, Lively CM. The rock–paper–scissors game and the evolution of alternative male strategies. Nature. 1996;380:240–243. [Google Scholar]
- Sober E, Wilson DS. Unto others: the evolution and psychology of unselfish behavior. Harvard Univ. Press; Cambridge, MA: 1998. [Google Scholar]
- Stebbing ARD. Competition for space between epiphytes of Fucus serratus. L J Mar Biol Assoc UK. 1973;53:247–261. [Google Scholar]
- Sweeney NJ, Klemm P, McCormick BA, MollerNielsen E, Utley M, Schembri MA, Laux DC, Cohen PS. The Escherichia coli K-12 gntP gene allows E-coli F-18 to occupy a distinct nutritional niche in the streptomycin-treated mouse large intestine. Inf Imm. 1996;64:3497–3503. doi: 10.1128/iai.64.9.3497-3503.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tainaka K. Indirect effect in cyclic voter models. Phys Lett A. 1993;176:303–306. [Google Scholar]
- Tainaka K. Paradoxical effect in a 3-candidate voter model. Phys Lett A. 1995;207:53–57. [Google Scholar]
- Tan Y, Riley MA. Nucleotide polymorphism in colicin E2 gene clusters: evidence for nonneutral evolution. Mol Biol Evol. 1997a;14:666–673. doi: 10.1093/oxfordjournals.molbev.a025806. [DOI] [PubMed] [Google Scholar]
- Tan Y, Riley MA. Positive selection and recombination: major molecular mechanisms in colicin diversification. Trends Ecol Evol. 1997b;12:348–351. doi: 10.1016/s0169-5347(97)01127-0. [DOI] [PubMed] [Google Scholar]
- Tilman D, Kareiva P. Spatial ecology: the role of space in population dynamics and interspecific interactions. Princeton Univ. Press; Princeton, NJ: 1997. [Google Scholar]
- Wilson DS. A theory of group selection. Proc Natl Acad Sci USA. 1975;72:143–146. doi: 10.1073/pnas.72.1.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang F, Li ZZ, Hui C. Spatiotemporal dynamics and distribution patterns of cyclic competition in metapopulation. Eco Model. 2006;193:721–735. [Google Scholar]