Abstract
Objectives
The purpose of this study was to determine the effects of a long-term exercise intervention on two prominent biomarkers of inflammation (C-reactive protein and interleukin-6) in elderly men and women.
Design
Single-blind, randomized, controlled trial: The Lifestyle Interventions and Independence for Elders trial (LIFE).
Setting
The Cooper Institute, Dallas, Texas; Stanford University, Stanford, California; University of Pittsburgh, Pittsburgh, Pennsylvania; and Wake Forest University, Winston-Salem, North Carolina
Participants
Elderly (70–89 yrs), non-disabled, community-dwelling men and women at risk for physical disability (n=424).
Intervention
A 12-month moderate-intensity physical activity (PA) intervention compared to a successful aging (SA) health education intervention.
Measurements
CRP and IL-6
Results
After adjustment for baseline IL-6, gender, clinic site, diabetes, treatment group, visit, and group by visit interaction, the PA intervention resulted in a lower (P=0.02) IL-6 concentration compared to the SA intervention. The adjusted mean IL-6 at month 12 was 8.5% (0.21 pg/ml) higher in the SA compared to the PA group. There were no significant differences in CRP between the groups at 12 months (P=0.09). Marginally significant interaction effects of the PA intervention by baseline functional status (P=0.051) and IL-6 (above versus below the median; P=0.06) were observed. There was a greater effect of the PA intervention in participants with a lower functional status and those with a higher baseline IL-6.
Conclusion
Increased physical activity results in reduced systemic concentrations of IL-6 in elderly individuals and this benefit is most pronounced in those individuals at the greatest risk for disability and subsequent loss of independence.
Keywords: exercise training, inflammation, aging, interleukin-6, C-reactive protein
INTRODUCTION
Chronic, low-grade inflammation is an independent predictor of several aging-related diseases, including coronary heart disease and stroke,1, 2 diabetes,3 Alzheimer’s,4 and osteoarthritis.5 In addition, inflammation predicts loss of function and onset of disability,6–10 as well as mortality.8 While there are several systemic biomarkers that are indicative of an up-regulated inflammatory state, C-reactive protein (CRP) and interleukin-6 (IL-6) show the most consistent association with both disease and disability in older individuals.1, 4, 11 Moreover, circulating concentrations of both CRP and IL-6 are higher in older persons,12–16 and there is especially strong evidence that IL-6 (which has been coined a “cytokine for gerontologists”17) is elevated with advancing age as there is a dramatic increase in the number of individuals with elevated IL-6 in those over the age of 70 years.15 Thus, given the widespread health risks of elevated CRP and IL-6, identification of successful therapies that reduce inflammation may be especially important in this age group.
While use of certain pharmacological agents may reduce inflammation, side effects of these medications may limit their clinical application for the on-going treatment of chronic inflammation.18 On the other hand, there is promising evidence that participation in regular physical activity lowers both CRP and IL-6. Observational data in both young and elderly persons show that a higher volume of physical activity is associated with lower CRP and IL-6 levels.19–25 In addition, several small or uncontrolled studies show an effect of aerobic exercise training on reducing CRP and IL-6 in middle-aged or older persons.26–34 However, to date, there is no randomized, controlled trial evidence for an effect of exercise training on CRP or IL-6 levels in older persons. Therefore, the purpose of this study was to determine whether a 12-month physical activity intervention decreases systemic concentrations of CRP and IL-6 compared to a no-exercise health education intervention in elderly men and women.
METHODS
Study Design
The presented findings are from an ancillary study to The Lifestyle Interventions and Independence for Elders pilot (LIFE-P) study, a four-site, single-blind, randomized, controlled clinical trial comparing a 12-month physical activity (PA) intervention to a successful aging (SA) intervention in 424 elderly, non-disabled, community-dwelling men and women at risk for physical disability. The study design and main findings on physical function of the LIFE-P study have been published.35, 36 The study was approved by the local Institutional Review Boards at the clinic sites (Wake Forest University, Cooper Institute, University of Pittsburgh, and Stanford University) and all study participants gave written informed consent to participate.
Study Participants
Detailed inclusion/exclusion criteria and a flow diagram of specific numbers of individuals screened and reasons for exclusion have been published.36 Briefly, the major inclusion criteria were: 1) 70 to <90 yrs; 2) lower functional performance based on a short physical performance battery (SPPB) score <10 (on a scale of 0 (worst) to 12 (best)); 3) sedentary lifestyle; 4) ability to complete a 400m walk test within 15 minutes without sitting and without using an assistive device; 5) completion of a behavioral run-in which required tracking/logging of healthy behaviors; and 6) willingness to be randomized to either treatment group. The major exclusion criteria were: 1) living in a nursing home; 2) self-reported inability to walk a mile; 3) significant cognitive impairment; 4) severe hearing or visual impairment; 5) severe cardiac, pulmonary, neurologic, orthopedic, renal, or psychiatric disease.
A total of 3141 persons were screened via telephone and 566 completed two screening clinic visits with a total of 424 (106% of study goal) participants randomized (PA=213; SA=211). All of the 424 randomized participants consented to the baseline blood draw and a sufficient blood sample was successfully collected from 369 participants (87%). Blood samples were available from 345 participants at the 6-month follow-up (6-month retention rate of 93%) and from 335 participants at the 12-month follow-up (12-month retention rate of 91%). Of the 79 participants with missing 6-month data, 2 were deceased, 22 withdrew consent or dropped from the study, and 55 did not have a blood sample drawn due to technical difficulties. Of the 89 participants with missing 12-month data, 4 were deceased, 25 withdrew consent or dropped from the study, and 60 did not have a blood sample drawn due to technical difficulties.
Interventions
The Physical Activity (PA) intervention consisted of a combination of aerobic, strength, balance, and flexibility exercises and was divided into 3 phases. For the first 2 months (adoption phase), 3 center-based exercise sessions (40–60 min) per week were conducted in a supervised setting. During the next 4 months (transition phase), the number of center-based sessions were reduced (2/week) and home-based exercises (≥3/week) were started. The subsequent maintenance phase (week 25-trial end) consisted of the home-based intervention, optional 1–2/week center-based sessions, and monthly telephone contacts.
The PA intervention included group-based behavioral counseling sessions (1x/week for the first 10 weeks) that focused on PA participation. The intervention focused on walking as the primary mode of exercise and the goal was to engage in walking for at least 150 minutes/week. Each session was preceded by a brief warm-up and followed by a brief cool down period. Participants also completed lower extremity strengthening exercises, followed by lower extremity stretching exercises. The intensity of training was gradually increased over the first 2–3 weeks. Perceived exertion was used to regulate the intensity of exercise. Participants were asked to walk at a target intensity of 12–13 (somewhat hard), and they were discouraged from exercising at levels ≥15 (hard) or ≤11 (fairly light). Strengthening exercises were performed at a perceived exertion of 15–16.
A “Successful Aging” (SA) health education intervention was used as the active control. Participants met in groups weekly for the first 26 weeks and met monthly for the remaining weeks. Sessions included health topics relevant to older adults such as nutrition, medications, foot care, and recommended preventive health care. At the end of each session, a short instructor-led intervention (5–10 min) of gentle upper extremity stretching was delivered. Phone calls were made after each missed session to encourage regular participation and participants received a monthly newsletter.
Measurements
CRP and IL-6
Blood samples were collected from LIFE study participants in the early morning (between 7–9 AM) after a 12-hour fast at the baseline, 6-month and 12-month assessment visits. The 6- and 12-month blood samples were collected at least 24 hours after the last acute bout of exercise training and blood sampling was postponed (1–2 weeks after recovery of symptoms) in the event of an acute respiratory, urinary tract, or other infection. All blood was collected, processed, divided into aliquots, and stored locally at −80°C until shipment to the Biological Specimen Repository at Wake Forest University where samples were placed for long-term storage at −80°C until analysis.
Plasma CRP was determined using an automated immunoanalyzer (IMMULITE®, Diagnostics Products Corporation, Los Angeles). This assay has a sensitivity of 0.1 mg/L with a calibration range up to 250 mg/L. In our laboratory, the inter-assay and intra-assay coefficients of variation (CV) for the CRP assay are 6.7% and 3.5%, respectively. Five samples (2 baseline, 1 6-month and 2 12-month timepoints) were below the lower limit of detection for CRP and were not included in analyses. Plasma IL-6 was determined using the Quantikine® high-sensitivity enzyme-linked immunosorbent assay kit from R&D Systems (Minneapolis, MN). This assay has a sensitivity of 0.10 pg/ml with a detection range of 0–10.0 pg/ml. In our laboratory, the inter-assay and intra-assay CVs for IL-6 are 9.8% and 3.0%, respectively. IL-6 was not detectable for one sample (6-month timepoint). All samples were measured in duplicate and the average of the two values was used for data analyses. Duplicate samples that did not provide a coefficient of variation of <15% were re-analyzed and all values were averaged for data analyses.
Short Physical Performance Battery (SPPB)
The short physical performance battery is based on a timed short-distance walk, repeated chair stands and balance test.37 Each of the performance measures is assigned a score ranging from 0 to 4, with 4 indicating the highest level of performance and 0 the inability to complete the test. The categories computed for walking speed and chair stands are derived from cut-points based on quartiles of the time to perform each task assessed in the Established Populations for Epidemiologic Studies of the Elderly (EPESE) studies.37 A summary score ranging from 0 (worst) to 12 (best) is calculated by summing all scores.
Body Composition
Total body fat mass and lean mass were measured using dual x-ray absorptiometry (DXA) at baseline and at the 12-month follow-up in a subset (n=222) of the LIFE participants (3 sites—Wake Forest University, Cooper Institute, and University of Pittsburgh).
Statistical Analyses
Statistical analyses were performed by using SAS software, version 9.1 (Cary, NC). The sample means and standard deviations were computed for the continuous descriptive characteristics, and the count and proportions were calculated for the discrete descriptive characteristics, by intervention groups. To minimize the heterogeneity of variance and best approximate the conditional normality assumption, CRP and IL-6 were log-transformed for the primary statistical analyses. Comparisons of 12-month changes in log-transformed CRP and log-transformed IL-6 between intervention groups were performed using the two-sample t-test. Raw values and log-transformed values for each intervention group at each time point were reported as means ± standard deviations. Differences in mean CRP and IL-6 between intervention groups were estimated using repeated measures analysis of covariance with the baseline measure, gender (stratifying variable for randomization), clinic site, diabetes, intervention assignment, visit, and an intervention by visit interaction included in the model. Hypothesis tests for intervention effects at the 6- and 12-month assessment visits were performed using contrasts of the 6- and 12-month intervention means. Overall comparisons between groups for CRP and IL-6 across follow-up visits were obtained using a contrast to compare average effects across both follow-up visits. In addition, we also tested for an interaction between baseline SPPB and intervention group by additional adjustment for the main effect of SPPB and interaction effect of SPPB and intervention group in the model, and then further stratified the analysis by SPPB score (≤ 7 vs > 7). We repeated the same analysis to test for an interaction between baseline IL-6 group (<median vs ≥median) and intervention group. The analysis stratified by baseline IL-6 was also performed. No gender interaction was found, so no subgroup analysis by gender was performed.
RESULTS
Baseline Characteristics and Relationships (Table 1 and Table 2)
Table 1.
Baseline Descriptive Characteristics by Treatment Group.
| Physical Activity (N=183) |
Successful Aging (N=186) |
|
|---|---|---|
| Age (yrs) | 76.4 ± 4.1 | 77.0 ± 4.4 |
| Gender, Female | 126 (68.9) | 125 (67.2) |
| Race, White | 141 (77.1) | 141 (75.8) |
| Body mass index (kg/m2) | 30.7 ± 6.0 | 29.8 ± 5.5 |
| Total fat mass (kg)+ | 30.3 ± 9.0 | 29.5 ± 9.5 |
| Total lean mass (kg)+ | 47.9 ± 10.9 | 46.6 ± 10.7 |
| Smoking | ||
| Never | 148 (80.9) | 157 (84.4) |
| Former | 28 (15.3) | 25 (13.4) |
| Current | 7 (3.8) | 4 (2.15) |
| MMSE score | 27.0 ± 2.3 | 27.5 ± 2.1 |
| Prevalent co-morbidities | ||
| Hypertension | 127 (69.8) | 129 (69.4) |
| Diabetes | 52 (28.4) | 32 (17.2)* |
| Cancer | 28 (15.3) | 32 (17.3) |
| Myocardial infarction | 22 (12.1) | 12 (6.5) |
| Stroke | 8 (4.4) | 12 (6.5) |
| CHF | 11 (6.1) | 12 (6.5) |
| COPD | 26 (14.4) | 27 (14.6) |
| SPPB score | 7.61 ± 1.45 | 7.46 ± 1.41 |
Data are N(%) or Mean±St. Dev;
P=0.01 between groups
N=114 for Physical Activity and N=108 for Successful Aging
MMSE=Mini-Mental State Examination; CHF=congestive heart failure; COPD=chronic obstructive pulmonary disease; SPPB=Short Physical Performance Battery
Table 2.
Unadjusted Actual Values and Log-transformed Values of Plasma C-reactive Protein (CRP) and Interleukin-6 (IL-6) Concentrations by Treatment Group at Baseline, 6 Months and 12 Months.
| Physical Activity | Successful Aging | |||||
|---|---|---|---|---|---|---|
| Actual value | Median (IQR)† | N | Actual value | Median (IQR)† | N | |
| CRP (mg/L) | ||||||
| Baseline | 5.78 ± 11.53 | 1.97 (4.47) | 182 | 4.38 ± 5.29 | 2.66 (4.13) | 185 |
| 6 Months | 3.57 ± 4.15 | 2.01 (3.77) | 173 | 4.85 ± 8.96 | 2.23 (4.68) | 171 |
| 12 Months | 4.21 ± 5.53 | 2.25 (3.48) | 172 | 4.08 ± 4.89 | 2.43 (3.94) | 161 |
| Δ CRP* (Month 12–baseline) |
−1.87 ± 12.25 | 153 | −0.37 ± 5.82 | 153 | ||
| IL-6 (pg/ml) | ||||||
| Baseline | 3.39 ± 4.03 | 2.46 (1.92) | 183 | 3.36 ± 4.01 | 2.45 (2.27) | 186 |
| 6 Months | 3.26 ± 3.59 | 2.50 (2.06) | 173 | 3.75 ± 5.15 | 2.67 (2.15) | 171 |
| 12 Months | 2.97 ± 1.91 | 2.51 (1.92) | 173 | 3.59 ± 4.65 | 2.49 (1.96) | 162 |
| Δ IL-6* (Month 12–baseline) |
−0.53 ± 4.19 | 155 | 0.42 ± 3.27 | 155 | ||
IQR = inter-quartile range
Data are Mean ± St. Dev;
Change between groups P=0.173 for CRP and P=0.027 for IL-6.
The two treatment groups had similar baseline characteristics, except for a slightly higher prevalence of diabetes in the PA group (Table 1). In addition, there were no differences in baseline CRP or IL-6 concentrations between treatment groups (Table 2). In all participants, there was a significant positive pair-wise correlation between CRP and IL-6 (r=0.39, P<0.001). In addition, IL-6 was positively related to age (r=0.13, P=0.015) and BMI (r=0.18, P<0.001), while CRP was related to BMI (r=0.10, P=0.05), but not age (r=−0.01, P=0.81). IL-6 was higher in men than women (3.60±3.93 pg/ml vs. 3.27±4.06 pg/ml; P=0.046), and there were no racial/ethnic differences in CRP or IL-6. In addition, there was no relationship between baseline functional status (SPPB score) and either CRP (r= −0.02, P=0.63) or IL-6 (r= −0.09, P=0.09).
Effects of Physical Activity Intervention on CRP and IL-6 (Table 2 and Figure 1)
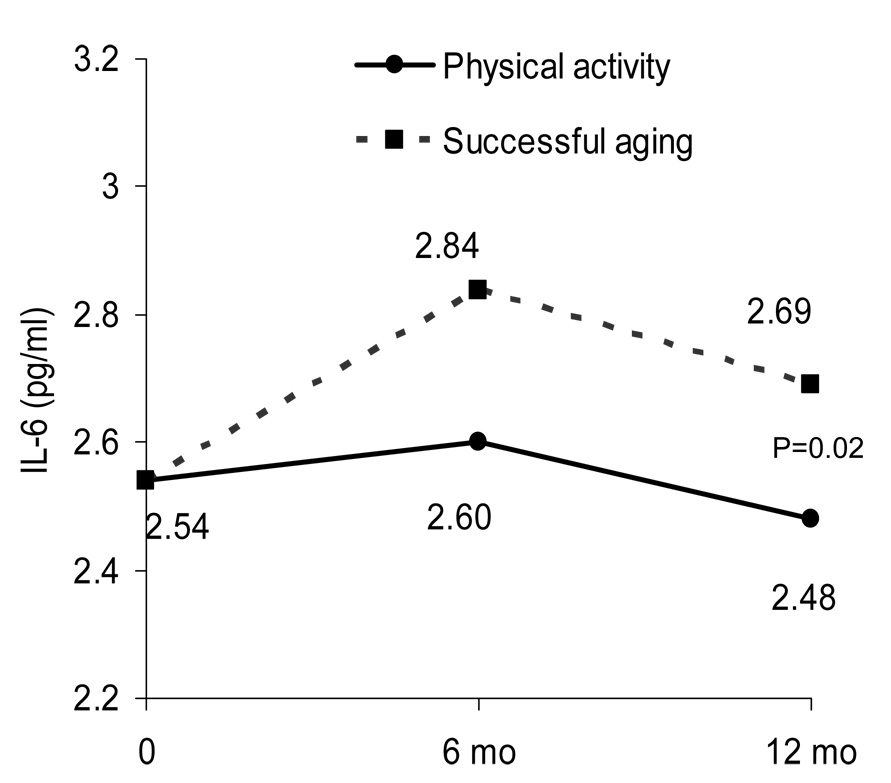
Figure 1.
Adjusted IL-6 means for each treatment group estimated from repeated measures ANCOVA adjusted for baseline IL-6, gender, clinic site, diabetes, treatment group, visit, and treatment by visit interaction. On average, the PA group had lower IL-6 levels over time compared to the SA group.
Compliance to the PA and SA interventions was previously reported.36 In the PA group, attendance during the adoption and transition phases averaged 71% and 61%, and during the maintenance phase, participants engaged in an average of 3.7 walking sessions/week and walked an average of 138±149 minutes/week (median =119 mins/wk, inter-quartile range=123 mins/wk). Attendance to the SA group sessions averaged 70% for weeks 1 to 26 and 73% for weeks 27 to 52. The estimated calories expended engaging in moderate PA was similar in the two groups at baseline and significantly higher in the PA group during follow-up.36 There were no changes in body weight as result of either intervention (PA: Baseline= 82.6±18.1 kg, 6 months= 83.7±17.7 kg, 12 months= 83.6±18.3 kg; SA: Baseline= 81.8±17.9 kg, 6 months= 82.0±18.1 kg, 12 months= 81.1±16.9 kg). Likewise, there were no changes in body composition and no effect of PA on total body fat mass (PA: Baseline= 30.3±9.0 kg, 12 months= 29.9±9.1 kg; SA: Baseline= 29.5±9.5 kg, 12 months= 29.0±9.5 kg; P=0.20) or lean mass (PA: Baseline= 47.9±10.9 kg, 12 months= 47.6±10.8 kg; SA: Baseline= 46.6±10.7 kg, 12 months= 46.4±10.7 kg; P=0.68) in the subset of participants (n=222) with measures of body composition.
Table 2 shows the unadjusted means (actual and log values) for CRP and IL-6 in each treatment group at baseline and at each follow-up assessment. On average, the PA intervention resulted in a 32% reduction in CRP and a 16% reduction in IL-6 by the end of the 12 months, while the SA intervention resulted in an 8% reduction in CRP, and a 13% increase in IL-6. Unadjusted overall changes (12-month minus baseline) in CRP were not significantly different between treatment groups, but changes in IL-6 were significantly different between groups (P=0.027; Table 2).
After adjustment for baseline IL-6, gender, clinic site, diabetes, treatment group, visit, and group by visit interaction, the PA intervention resulted in a lower (P=0.02) IL-6 concentration compared to the SA intervention (Figure 1). The adjusted mean IL-6 at month 12 was 8.5% (0.21 pg/ml) higher in the SA compared to the PA group. However, there were no significant differences in CRP concentrations between the groups at 12 months (P=0.09).
Sub-Group Analyses (Figure 1 & Figure 2)
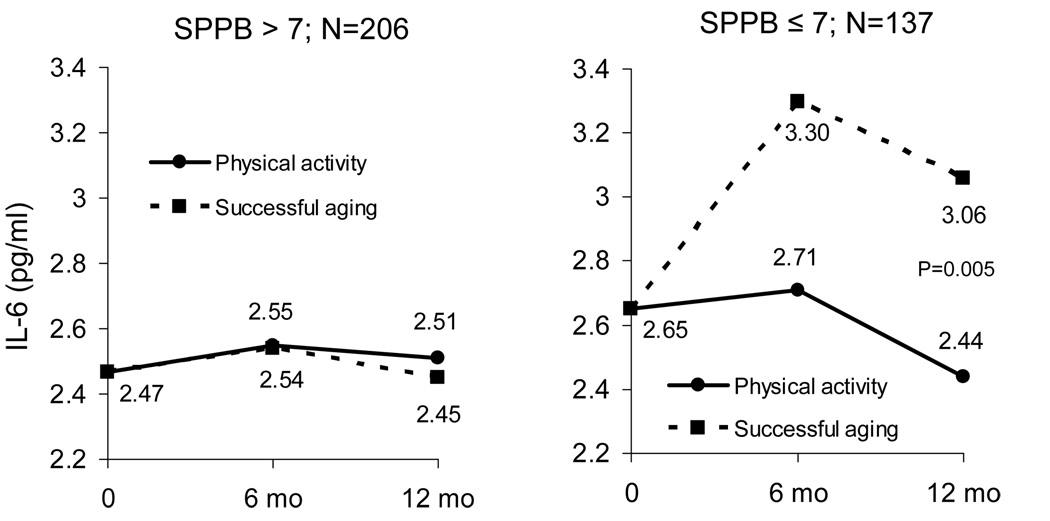
Figure 2.
Adjusted IL-6 means for each treatment group stratified by baseline functional status (SPPB score). Means are estimated from repeated measures ANCOVA adjusted for baseline IL-6, gender, clinic site, diabetes, treatment group, visit, treatment by visit interaction. P for interaction=0.051.
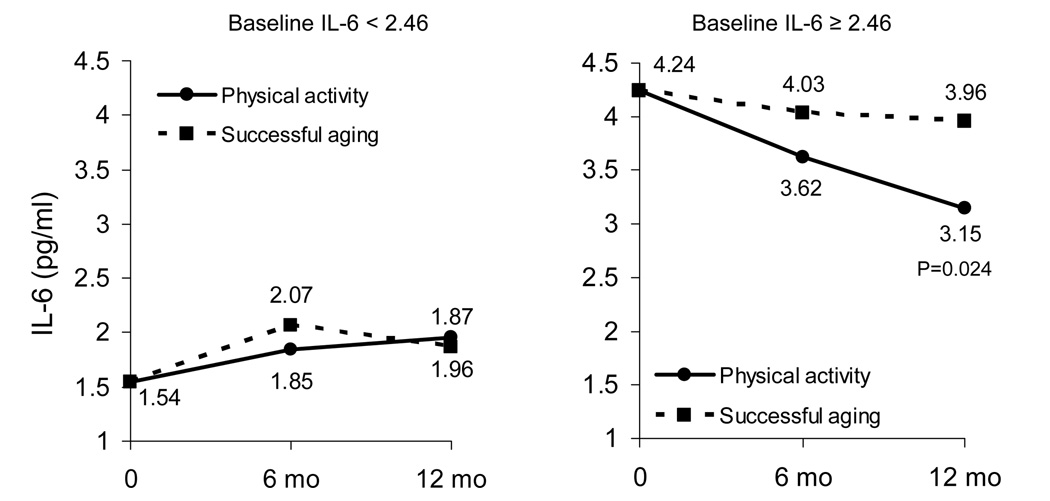
We performed further analyses (using the same covariates as above in our main model) to determine if the effects of the PA intervention on IL-6 concentrations were similar between men and women, between those with higher (SPPB > 7) and lower (SPPB ≤ 7) overall physical function at baseline, and between those with higher and lower baseline IL-6 (by median IL-6 value: 2.46 pg/ml). There was no significant interaction of gender (P=0.648) for the effects of PA on IL-6, but there was marginal statistical evidence for an interaction with both baseline functional status (P=0.051) and IL-6 (above versus below the median; P=0.06). There was a much greater effect of the PA intervention in participants with a lower functional status (SPPB ≤ 7; Figure 2), and in those with a higher baseline IL-6 (IL-6 ≥ 2.46 pg/ml; Figure 3). There was no observed effect of the PA intervention in persons with a higher SPPB or a lower IL-6. Because SPPB was marginally (P=0.086) related to IL-6 at baseline (e.g., persons with lower function tended to have higher IL-6 levels), we included both interaction terms (treatment by SPPB score and treatment by median IL-6) in the same model to ascertain whether these interactions were independent of one another. The analyses showed that both interaction terms remained marginally significant (P=0.056 and P=0.062, respectively). In addition, mean baseline IL-6 values were similar between the SPPB groups (3.35±2.67 pg/ml and 3.39±4.76 pg/ml), indicating that the effects of baseline functional status on the differential IL-6 response to the PA intervention were likely not due to differences in baseline IL-6.
Figure 3.
Adjusted IL-6 means for each treatment group stratified by baseline IL-6 (median IL-6). Means are estimated from repeated measures ANCOVA adjusted for baseline IL-6, gender, clinic site, diabetes, treatment group, visit, treatment by visit interaction. P for interaction=0.063.
DISCUSSION
These findings provide randomized controlled trial evidence that a one-year physical activity intervention results in reduced systemic concentrations of IL-6 in elderly individuals at risk for disability. However, the effect of the exercise intervention on IL-6 was driven by the subgroup of participants with a lower functional status (SPPB score ≤ 7), and those with a higher baseline IL-6, as there was no observed effect in persons with a higher SPPB or a lower baseline IL-6. There was no interaction of gender for the effects of physical activity on IL-6, so similar responses were observed between men and women. Thus, regular physical activity—even in the absence of weight loss—is an effective therapy for reducing systemic concentrations of IL-6, an important biological predictor of increased risk for disability and mortality.6–10 Further, this benefit of regular physical activity is most beneficial in those older individuals at the greatest risk for disability and subsequent loss of independence.
It is well-known that a single bout of strenuous exercise acutely increases systemic IL-6 levels, as well as concentrations of other cytokines and acute phase reactants.38 However, as our observation of a reduction in systemic IL-6 with long-term exercise training is consistent with some previously published data from smaller and uncontrolled studies of a shorter duration,28, 31, 34 it appears that chronic muscle contractions performed on a regular basis decrease systemic IL-6 concentrations. Additionally, our findings extend those of previous studies in several ways. First, our data provide evidence from a randomized, controlled design which causally links the observed decrease in IL-6 to the increased physical activity. In addition, the intervention was of sufficient length (12 months), but did not change body weight, indicating that exercise training does not need to result in weight loss to have an effect on IL-6. Finally, we found that the effect of the increased physical activity on IL-6 was much more pronounced in the subset of persons with higher baseline IL-6, and in those with worse physical function. Of note, there was a mean increase in IL-6 levels in the Successful Aging group, especially in those with lower function at baseline, suggesting that physical activity may result in lower IL-6 by preventing an age-related increase. As seen in other published studies,6, 14–16 concentrations of IL-6 were positively related to age at baseline in the individuals enrolled in this study.
The mechanism by which chronic exercise alter IL-6 concentrations in the circulation must be through either an inhibitory effect on IL-6 production or through a stimulatory effect on IL-6 clearance. Adipose tissue is a significant source of circulating IL-6 and individuals with more fat have higher levels of IL-6.39 In the present study, IL-6 was directly related to BMI at baseline, but the intervention did not cause weight loss—nor changes in body composition (measured in a subset of LIFE participants)—making it unlikely that the observed reduction in IL-6 could be solely attributed to a loss of body fat. In addition, although data are limited, it does not appear that exercise in the absence of fat loss influences production or release of IL-6 by adipose tissue per se.40, 41 On the other hand, data are beginning to show that chronic exercise may decrease pro-inflammatory cytokine production from peripheral mononuclear cells.42, 43
Although there was a trend for the PA intervention to lower CRP levels, we did not find a statistically significant treatment effect for CRP, either in the entire sample or in subgroups of individuals with higher CRP or with lower physical function at baseline. On average, the physical activity intervention reduced CRP by nearly 2.0 mg/L; however, this reduction was not different from the control group. Using this effect size, post-hoc power analyses indicate that statistical power with the present sample size was 27% and that, in order to reach 80% power with the same effect size, we would need to study 643 participants in each intervention group. Despite a large body of cross-sectional evidence showing that a higher volume of physical activity is associated with a lower CRP concentration,19–25 not all, prior intervention studies show an effect of increasing physical activity for reducing CRP.27, 30, 32, 34, 44, 45 However, these studies were conducted with smaller sample sizes (n=16–140) than the current study. Previously published intervention studies showing that exercise training reduced CRP either did not compare individuals randomized to exercise or a non-exercise control group,26, 28, 29, 46–48 or the intervention also resulted in slight to moderate decreases in body weight/fat.26, 33, 47–49 Thus, exercise training interventions that result in even a slight amount of weight reduction are beneficial for reducing CRP levels, whereas it appears that increasing physical activity alone has a small, often undetectable, effect on CRP.
While our findings indicate that increasing physical activity can be advocated as an effective therapy for reducing systemic IL-6, we did not test whether this reduction resulted in an improvement in risk factors for adverse health conditions associated with inflammation. An elevated blood concentration of IL-6 can be indicative of several aging-related diseases, but is an especially strong risk factor for subsequent cardiovascular disease1, 2 and disability6–10 in older persons. While there is currently no established IL-6 cut-point used to identify an increase in disease or disability risk, one study suggested that the elevated risk of mobility-disability associated with IL-6 was nonlinear, with the risk rising rapidly beyond a concentration of 2.5 pg/ml—individuals above this level were approximately 62% more likely to develop disability over the next four years.7 The average baseline IL-6 in the individuals enrolled in the LIFE trial was 3.38 pg/ml, suggesting that, overall, they are at high risk for development of disability. Furthermore, the mean decrease in IL-6 in all individuals in the physical activity intervention group was 0.53 pg/ml (~16%), down to an average of 2.97 pg/ml. Unfortunately, there are no available data regarding whether this magnitude of decline in IL-6 is associated with a lowered risk of subsequent disability or other aging-related adverse health condition. Thus, longitudinal studies are needed to determine whether there is a delay in the onset of physical disability or a reduction in disease incidence associated with this reduction in IL-6 seen with regular exercise.
These findings should be interpreted in light of certain aspects of the study and potential limitations. First, the entire sample was older (>70 yrs) and the effects of the intervention may not directly translate to another age group, and they may not be observed in all older persons. Moreover, because the observed IL-6 response to physical activity was driven by the subset of individuals with lower physical function, this effect may have been underestimated as missing blood samples may have disproportionally come from these impaired individuals. In addition, the physical activity intervention in the LIFE study utilized a combination of aerobic (mainly walking) and light lower extremity resistance exercise. This intervention does not allow us to distinguish whether one type of training has greater anti-inflammatory effects over another, nor does it provide information regarding possible dose-response effects of increasing the exercise intensity or the overall caloric expenditure of physical activity. Despite these caveats, this study points to the benefit of regular physical activity—even in the absence of weight loss—as an effective therapy for reducing systemic concentrations of IL-6 in the elderly, especially in those with the greatest risk for disability.
ACKNOWLEDGMENTS
The Lifestyle Interventions and Independence for Elders Pilot (LIFE-P) Study was funded by a grant from the National Institutes of Health/National Institute on Aging (U01 AG22376) and supported in part by the Intramural Research Program, National Institute on Aging, NIH. The ancillary study was supported, in part, by the WFU Claude D. Pepper Older Americans Independence Center (P30 AG21332) and by NIH grant 1R01 AG027529 to Dr. Nicklas.
Funding: NIH grant U01 AG22376; WFU Claude D. Pepper Older Americans Independence Center (P30 AG21332); and NIH grant 1R01 AG027529
Footnotes
Author Contributions:
BN: Acquisition of data, study concept and design, analysis and interpretation of data, preparation of manuscript
FCH: study concept and design, analysis and interpretation of data, preparation of manuscript
TB: Acquisition of data, analysis and interpretation of data, preparation of manuscript
TC: Acquisition of data, study concept and design, analysis and interpretation of data, preparation of manuscript
BG: Acquisition of data, study concept and design, preparation of manuscript
SK: Acquisition of data, study concept and design, preparation of manuscript
MP: Acquisition of data, study concept and design, preparation of manuscript
Presented in part at: The Gerontological Society of America annual meeting; San Francisco, CA, November 19, 2007
Conflict of Interest: The editor in chief has reviewed the conflict of interest checklist provided by the authors and has determined that the authors have no financial or any other kind of personal conflicts with this paper.
References
- 1.Kritchevsky SB, Cesari M, Pahor M. Inflammatory markers and cardiovascular health in older adults. Cardiovasc Res. 2005;66:265–275. doi: 10.1016/j.cardiores.2004.12.026. [DOI] [PubMed] [Google Scholar]
- 2.Cesari M, Penninx BW, Newman AB, et al. Inflammatory markers and onset of cardiovascular events. Results from the Health ABC study. Circulation. 2003;108:2317–2322. doi: 10.1161/01.CIR.0000097109.90783.FC. [DOI] [PubMed] [Google Scholar]
- 3.Schmidt MI, Duncan BB, Sharrett AR, et al. Markers of inflammation and prediction of diabetes mellitus in adults (Atherosclerosis Risk in Communities study). A cohort study. Lancet. 1999;353:1649–1652. doi: 10.1016/s0140-6736(99)01046-6. [DOI] [PubMed] [Google Scholar]
- 4.Dziedzic T. Systemic inflammatory markers and risk of dementia. Am J Alzheimers Dis Other Demen. 2006;21:258–262. doi: 10.1177/1533317506289260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sharif M, Shepstone L, Elson CJ, et al. Increased serum C reactive protein may reflect events that precede radiographic progression in osteoarthritis of the knee. Ann Rheum Dis. 2000;59:71–74. doi: 10.1136/ard.59.1.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cohen HJ, Pieper CF, Harris T, et al. The association of plasma IL-6 levels with functional disability in community-dwelling elderly. J Gerontol A Biol Sci Med Sci. 1997;52:M201–M208. doi: 10.1093/gerona/52a.4.m201. [DOI] [PubMed] [Google Scholar]
- 7.Ferrucci L, Harris TB, Guralnik JM, et al. Serum IL-6 level and the development of disability in older persons. J Am Geriatr Soc. 1999;47:639–646. doi: 10.1111/j.1532-5415.1999.tb01583.x. [DOI] [PubMed] [Google Scholar]
- 8.Harris TB, Ferrucci L, Tracy RP, et al. Associations of elevated Interleukin-6 and C-reactive protein levels with mortality in the elderly. Am J Med. 1999;106:506–512. doi: 10.1016/s0002-9343(99)00066-2. [DOI] [PubMed] [Google Scholar]
- 9.Taaffe DR, Harris TB, Ferrucci L, et al. Cross-sectional and prospective relationships of Interleukin-6 and C-reactive protein with physical performance in elderly persons: MacArthur studies of successful aging. J Gerontol. 2000;55A(12):M709–M715. doi: 10.1093/gerona/55.12.m709. [DOI] [PubMed] [Google Scholar]
- 10.Penninx BW, Kritchevsky SB, Newman AB, et al. Inflammatory markers and incident mobility limitation in the elderly. J Am Geriatr Soc. 2004;52:1105–1113. doi: 10.1111/j.1532-5415.2004.52308.x. [DOI] [PubMed] [Google Scholar]
- 11.Maggio M, Guralnik JM, Longo DL, et al. Interleukin-6 in aging and chronic disease: A magnificent pathway. J Gerontol A Biol Sci Med Sci. 2006;61:575–584. doi: 10.1093/gerona/61.6.575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Grimble RF. Inflammatory response in the elderly. Curr Opin Clin Nutr Metab Care. 2003;6:21–29. doi: 10.1097/00075197-200301000-00005. [DOI] [PubMed] [Google Scholar]
- 13.Wener MH, Daum PR, McQuillan GM. The influence of age, sex, and race on the upper reference limit of serum C-reactive protein concentration. J Rheumatol. 2000;27:2351–2359. [PubMed] [Google Scholar]
- 14.Ferrucci L, Corsi A, Lauretani F, et al. The Origins of Age-Related Pro-Inflammatory State. Blood. 2005;105:2294–2299. doi: 10.1182/blood-2004-07-2599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Giuliani N, Sansoni P, Girasole G, et al. Serum interleukin-6, soluble interleukin-6 receptor and soluble gp130 exhibit different patterns of age- and menopause-related changes. Exp Gerontol. 2001;36:547–557. doi: 10.1016/s0531-5565(00)00220-5. [DOI] [PubMed] [Google Scholar]
- 16.Forsey RJ, Thompson JM, Ernerudh J, et al. Plasma cytokine profiles in elderly humans. Mech Ageing Dev. 2003;124:487–493. doi: 10.1016/s0047-6374(03)00025-3. [DOI] [PubMed] [Google Scholar]
- 17.Ershler WB. Interleukin-6: A cytokine for gerontologists. J Am Geriatr Soc. 1993;41:176–181. doi: 10.1111/j.1532-5415.1993.tb02054.x. [DOI] [PubMed] [Google Scholar]
- 18.Canvin JM, el Gabalawy HS. Anti-inflammatory therapy. Phys Med Rehabil Clin N Am. 1999;10:301–317. [PubMed] [Google Scholar]
- 19.Geffken D, Cushman M, Burke G, et al. Association between physical activity and markers of inflammation in a healthy elderly population. Am J Epidemiol. 2001;153:242–250. doi: 10.1093/aje/153.3.242. [DOI] [PubMed] [Google Scholar]
- 20.Wannamethee SG, Lowe GD, Whincup PH, et al. Physical activity and hemostatic and inflammatory variables in elderly men. Circulation. 2002;105:1785–1790. doi: 10.1161/hc1502.107117. [DOI] [PubMed] [Google Scholar]
- 21.Abramson JL, Vaccarino V. Relationship between physical activity and inflammation among apparently healthy middle-aged and older US adults. Arch Intern Med. 2002;162:1286–1292. doi: 10.1001/archinte.162.11.1286. [DOI] [PubMed] [Google Scholar]
- 22.Church TS, Barlow CE, Earnest CP, et al. Associations between cardiorespiratory fitness and C-reactive protein in men. Arterioscler Thromb Vasc Biol. 2002;22:1869–1876. doi: 10.1161/01.atv.0000036611.77940.f8. [DOI] [PubMed] [Google Scholar]
- 23.King DE, Carek P, Mainous AG, III, et al. Inflammatory markers and exercise: differences related to exercise type. Med Sci Sports Exerc. 2003;35:575–581. doi: 10.1249/01.MSS.0000058440.28108.CC. [DOI] [PubMed] [Google Scholar]
- 24.Reuben DB, Judd-Hamilton L, Harris TB, et al. The associations between physical activity and inflammatory markers in high-functioning older persons: MacArthur Studies of Successful Aging. J Am Geriatr Soc. 2003;51:1125–1130. doi: 10.1046/j.1532-5415.2003.51380.x. [DOI] [PubMed] [Google Scholar]
- 25.Kasapis C, Thompson PD. The effects of physical activity on serum C-reactive protein and inflammatory markers: A systematic review. J Am Coll Cardiol. 2005;45:1563–1569. doi: 10.1016/j.jacc.2004.12.077. [DOI] [PubMed] [Google Scholar]
- 26.Milani RV, Lavie CJ, Mehra MR. Reduction in C-reactive protein through cardiac rehabilitation and exercise training. J Am Coll Cardiol. 2004;43:1056–1061. doi: 10.1016/j.jacc.2003.10.041. [DOI] [PubMed] [Google Scholar]
- 27.Hammett CJ, Oxenham HC, Baldi JC, et al. Effect of six months' exercise training on C-reactive protein levels in healthy elderly subjects. J Am Coll Cardiol. 2004;44:2411–2413. doi: 10.1016/j.jacc.2004.09.030. [DOI] [PubMed] [Google Scholar]
- 28.Goldhammer E, Tanchilevitch A, Maor I, et al. Exercise training modulates cytokines activity in coronary heart disease patients. Int J Cardiol. 2005;100:93–99. doi: 10.1016/j.ijcard.2004.08.073. [DOI] [PubMed] [Google Scholar]
- 29.Lakka TA, Lakka HM, Rankinen T, et al. Effect of exercise training on plasma levels of C-reactive protein in healthy adults: The HERITAGE Family Study. Eur Heart J. 2005;26:2018–2025. doi: 10.1093/eurheartj/ehi394. [DOI] [PubMed] [Google Scholar]
- 30.Fairey AS, Courneya KS, Field CJ, et al. Effect of exercise training on C-reactive protein in postmenopausal breast cancer survivors: A randomized controlled trial. Brain Behav Immun. 2005;19:381–388. doi: 10.1016/j.bbi.2005.04.001. [DOI] [PubMed] [Google Scholar]
- 31.Kohut ML, McCann DA, Russell DW, et al. Aerobic exercise, but not flexibility/resistance exercise, reduces serum IL-18, CRP, and IL-6 independent of beta-blockers, BMI, and psychosocial factors in older adults. Brain Behav Immun. 2006;20:201–209. doi: 10.1016/j.bbi.2005.12.002. [DOI] [PubMed] [Google Scholar]
- 32.Zoppini G, Targher G, Zamboni C, et al. Effects of moderate-intensity exercise training on plasma biomarkers of inflammation and endothelial dysfunction in older patients with type 2 diabetes. Nutr Metab Cardiovasc Dis. 2006;16:543–549. doi: 10.1016/j.numecd.2005.09.004. [DOI] [PubMed] [Google Scholar]
- 33.Oberbach A, Tonjes A, Kloting N, et al. Effect of a 4 week physical training program on plasma concentrations of inflammatory markers in patients with abnormal glucose tolerance. Eur J Endocrinol. 2006;154:577–585. doi: 10.1530/eje.1.02127. [DOI] [PubMed] [Google Scholar]
- 34.Dekker MJ, Lee S, Hudson R, et al. An exercise intervention without weight loss decreases circulating interleukin-6 in lean and obese men with and without type 2 diabetes mellitus. Metabolism. 2007;56:332–338. doi: 10.1016/j.metabol.2006.10.015. [DOI] [PubMed] [Google Scholar]
- 35.Rejeski WJ, Fielding RA, Blair SN, et al. The lifestyle interventions and independence for elders (LIFE) pilot study: Design and methods. Contemp Clin Trials. 2005;26:141–154. doi: 10.1016/j.cct.2004.12.005. [DOI] [PubMed] [Google Scholar]
- 36.Pahor M, Blair SN, Espeland M, et al. Effects of a physical activity intervention on measures of physical performance: Results of the lifestyle interventions and independence for Elders Pilot (LIFE-P) study. J Gerontol A Biol Sci Med Sci. 2006;61:1157–1165. doi: 10.1093/gerona/61.11.1157. [DOI] [PubMed] [Google Scholar]
- 37.Guralnik JM, Ferrucci L, Pieper CF, et al. Lower extremity function and subsequent disability: consistency across studies, predictive models, and value of gait speed alone compared with the short physical performance battery. J Gerontol A Biol Sci Med Sci. 2000;55:M221–M231. doi: 10.1093/gerona/55.4.m221. [DOI] [PubMed] [Google Scholar]
- 38.Pedersen BK, Steensberg A, Schjerling P. Exercise and interleukin-6. Curr Opin Hematol. 2001;8:137–141. doi: 10.1097/00062752-200105000-00002. [DOI] [PubMed] [Google Scholar]
- 39.Mohamed-Ali V, Goodrick S, Rawesh A, et al. Subcutaneous adipose tissue releases interleukin-6, but not tumor necrosis factor-alpha, in vivo. J Clin Endocrinol Metab. 1997;82:4196–4200. doi: 10.1210/jcem.82.12.4450. [DOI] [PubMed] [Google Scholar]
- 40.Polak J, Klimcakova E, Moro C, et al. Effect of aerobic training on plasma levels and subcutaneous abdominal adipose tissue gene expression of adiponectin, leptin, interleukin 6, and tumor necrosis factor alpha in obese women. Metabolism. 2006;55:1375–1381. doi: 10.1016/j.metabol.2006.06.008. [DOI] [PubMed] [Google Scholar]
- 41.Klimcakova E, Polak J, Moro C, et al. Dynamic strength training improves insulin sensitivity without altering plasma levels and gene expression of adipokines in subcutaneous adipose tissue in obese men. J Clin Endocrinol Metab. 2006;91:5107–5112. doi: 10.1210/jc.2006-0382. [DOI] [PubMed] [Google Scholar]
- 42.Smith JK, Dykes R, Douglas JE, et al. Long-term exercise and atherogenic activity of blood mononuclear cells in persons at risk of developing ischemic heart disease. JAMA. 1999;281:1722–1727. doi: 10.1001/jama.281.18.1722. [DOI] [PubMed] [Google Scholar]
- 43.Sloan RP, Shapiro PA, Demeersman RE, et al. Aerobic exercise attenuates inducible TNF production in humans. J Appl Physiol. 2007;103:1007–1011. doi: 10.1152/japplphysiol.00147.2007. [DOI] [PubMed] [Google Scholar]
- 44.Marcell TJ, McAuley KA, Traustadottir T, et al. Exercise training is not associated with improved levels of C-reactive protein or adiponectin. Metabolism. 2005;54:533–541. doi: 10.1016/j.metabol.2004.11.008. [DOI] [PubMed] [Google Scholar]
- 45.Rauramaa R, Halonen P, Vaisanen SB, et al. Effects of aerobic physical exercise on inflammation and atherosclerosis in men: The DNASCO Study: A six-year randomized, controlled trial. Ann Intern Med. 2004;140:1007–1014. doi: 10.7326/0003-4819-140-12-200406150-00010. [DOI] [PubMed] [Google Scholar]
- 46.Mattusch F, Dufaux B, Heine O, et al. Reduction of the plasma concentration of C-reactive protein following nine months of endurance training. Int J Sports Med. 2000;21:21–24. doi: 10.1055/s-2000-8852. [DOI] [PubMed] [Google Scholar]
- 47.Okita K, Nishijima H, Murakami T, et al. Can exercise training with weight loss lower serum C-reactive protein levels? Arterioscler Thromb Vasc Biol. 2004;24:1868–1873. doi: 10.1161/01.ATV.0000140199.14930.32. [DOI] [PubMed] [Google Scholar]
- 48.Obisesan TO, Leeuwenburgh C, Phillips T, et al. C-reactive protein genotypes affect baseline, but not exercise training-induced changes, in C-reactive protein levels. Arterioscler Thromb Vasc Biol. 2004;24:1874–1879. doi: 10.1161/01.ATV.0000140060.13203.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Giannopoulou I, Fernhall B, Carhart R, et al. Effects of diet and/or exercise on the adipocytokine and inflammatory cytokine levels of postmenopausal women with type 2 diabetes. Metabolism. 2005;54:866–875. doi: 10.1016/j.metabol.2005.01.033. [DOI] [PubMed] [Google Scholar]