Abstract
Retinoic acid, a key morphogen in early vertebrate development and tissue regeneration, mediates its effects through the binding of receptors that act as ligand-induced transcription factors. These binding events function to recruit an array of transcription co-regulatory proteins to specific gene promoters. One such co-regulatory protein, neuronal proliferation and differentiation control-1 (NPDC-1), is broadly expressed during mammalian development and functions as an in vitro repressor of retinoic acid receptor (RAR)-mediated transcription. To obtain comparative and developmental insights about NPDC-1 function, we cloned the axolotl (Ambystoma mexicanum) orthologue and measured transcript abundances among tissues sampled during the embryonic and juvenile phases of development, and also during spinal cord regeneration. Structurally, the axolotl orthologue of NPDC-1 retained sequence identity to mammalian sequences in all functional domains. Functionally, we observed that axolotl NPDC-1 mRNA expression peaked late in embryogenesis, with highest levels of expression occurring during the time of limb development, a process regulated by retinoic acid signaling. Also similar to what has been observed in mammals, axolotl NPDC-1 directly interacts with axolotl RAR, modulates axolotl RAR DNA binding, and represses cell proliferation and axolotl RAR-mediated gene transcription. These data justify axolotl as a model to further investigate NPDC-1 and its role in regulating retinoic acid signaling.
Keywords: axolotl, Ambystoma mexicanum, co-regulator, embryogenesis, NPDC-1, retinoic acid, retinoic acid receptor, transcription factor
INTRODUCTION
Retinoids function in a variety of cellular and developmental processes that include differentiation, proliferation, and patterning (Sporn MM 1984; DeLuca 1988; Sporn MM 1994; Sun et al. 2002). Retinoids exert their effects by binding to members of the steroid/nuclear receptor family of ligand-induced transcription factors. These receptors are divided into two subgroups, the retinoic acid receptors (RARs) and retinoid “X” receptors (RXRs). Retinoid receptors, like most nuclear receptor family members, function as ligand induced transcription factors. RAR-RXR heterodimers localize primarily to the nucleus and evidence suggests, in the absence of ligand, that these heterodimers are bound to DNA (Ribeiro et al. 1995). Ligand binding to RAR-RXR heterodimers is thought to alter their conformational state and permit recruitment of specific co-regulatory complexes (Minucci et al. 1998). These complexes consist of co-activators or co-repressors, depending on the promoter being regulated, and they function by integrating information from external signaling events, by facilitating communication between mediator and basal transcription complexes, and by remodeling chromatin (Dilworth et al. 2001).
Previously, we showed that (mammalian) NPDC-1 functions as a retinoid receptor co-repressor protein in vitro. NPDC-1 directly binds to retinoid receptor family members, affects retinoid receptor DNA binding, and represses retinoid receptor-mediated transcription (Henry et al. 2003). Prior to our work, NPDC-1 was shown to regulate neural cell proliferation and differentiation. In particular, NPDC-1 is capable of inhibiting proliferation of neural cells in vitro and NPDC-1 transcript levels change significantly at the time of neuronal cell differentiation (Galiana et al. 1995). Although NPDC-1 has been associated primarily with neural-specific functions (Evrard et al. 2005), NPDC-1 transcripts are expressed among multiple tissues and patterns of expression differ spatially and temporarily among mammalian models (Qu et al. 2001; Henry et al. 2003). These data suggest that NPDC-1 functions extend beyond neuronal cell differentiation and are possibly associated with retinoid receptor signaling.
To date, all studies of NPDC-1 have been performed with mammalian cells and tissues. To gain broader comparative perspective on NPDC-1 function, we cloned and analyzed the Mexican axolotl (Ambystoma mexicanum) orthologue. The axolotl is a classic salamander model for early vertebrate development and tissue regeneration (Armstrong et al. 1989). Studies of the axolotl have established the importance of retinoic acid in multiple developmental and cellular processes, including regeneration (Niazi et al. 1978; Maden 1982; Thoms et al. 1984; Crawford et al. 1998). Here we show that axolotl NPDC-1 is differentially expressed during development and among multiple tissues, it binds axolotl retinoic acid receptor gamma (RARγ) and affects axolotl RARγ DNA binding, and it represses axolotl RARγ-mediated transcription and cell proliferation. These data support the hypothesis that NPDC-1 plays a role in regulating retinoic acid signaling during axolotl development.
MATERIALS AND METHODS
Tissues and RNA isolation
Axolotl RNAs were isolated from tissues that were collected under University of Kentucky IACUC protocols 00907L2005 and 01040L2006. Total RNA was obtained using TRIzol reagent (Sigma) and RNeasy columns (Qiagen).
Chemicals, Enzymes and Antibodies
Standard chemicals used throughout these studies, unless otherwise stated, were purchased from Sigma-Aldrich (St Louis, MO, USA) or Fisher Scientific. Restriction enzymes, polymerases, ligases and kinases used, unless otherwise stated, were purchased from New England Biolabs (Beverly, MA, USA). Benzonase nuclease was purchased from Novagen (San Diego, CA). Anti-thioredoxin antibody was purchased from Santa Cruz (Santa Cruz, CA, USA). Secondary antibodies were purchased from Cell Signaling (Danvers, MA, USA).
NPDC-1 Antibody
The peptide sequence NH2-EENEDGDFTVYECPGLAPT-COOH located in the PEST region of NPDC-1 and observed to be conserved from axolotl to human, was used as an immunogen to generate a rabbit polyclonal affinity purified antibody directed against NPDC-1. Peptide synthesis, antibody development, and affinity purification were performed by Washington Biotechnology (Baltimore, MD, USA).
Cloning the axolotl NPDC-1 and RARγ cDNAs
Partial cDNA sequence information for the 3′ end of the axolotl NPDC-1 gene was obtained from BLAST searches of the Ambystoma EST Database (http://salamander.uky.edu/ESTdb). From this information, PCR primers were designed and used in 5′RACE (Invitrogen) with 500 ng total RNA from juvenile (40 mm) axolotl brain to synthesize a full-length axolotl NPDC-1 cDNA. The 5′RACE protocol used was essentially as described in the manufacturer’s instruction manual. Three gene-specific primers were utilized in the first round of 5′RACE: GSP1: 5′-GTG AAA TCA CCA TCC TCA TT-3′; GSP2, 5′-CTC CTC GTC AGA GGT CGT GT-3′; GSP3, 5′-GAG TCC GGC AGC TTT GGT-3′. The second 5′RACE procedure included: GSP1.2, 5′-TGC CGT CAT TAT TCT CCA CA-3′; GSP2.2, 5′-CTT TAG GAG GGT GCA GTC CA-3′; GSP2.3, 5′-AGC ATT AAC ATC CCG CAG AG-3′. PCR products and the full-length axolotl NPDC-1 gene were cloned into the pGEM-T vector system (Promega; Madison, WI, USA).
Axolotl RARγ2 was cloned from juvenile (40 mm) axolotl lung RNA using axolotl RARγ-specific RT-PCR and the following primers, forward: 5′-AAG CGG CCG CGT ACG ACT GCA TGG AGG CC-3′ and reverse: 5′-AAG GAT CCC TAG AGC TCT TTG GAA CT-3′. RT-PCR reactions were performed using the SuperScript™ One-Step RT-PCR kit (Invitrogen) according to manufacturer’s instructions. The amplified fragment was digested with NotI and BamHI and ligated into the NotI/BamHI sites of the p3XFLAG-CMV-10 vector (Sigma) to generate a pFLAG-aRARγ mammalian expression construct. The integrity of cloning and all subcloned constructs was confirmed by DNA sequencing.
Plasmids and Constructs
To generate the pcDNA3.1-aRARγ and pcDNA3.1-HA-aRARγ mammalian expression constructs for axolotl RAR gamma, and the pGEX-aRARγ and pET32-aRARγ bacterial expression constructs for axolotl RAR gamma, we used the pFLAG-aRARγ construct as template in a PCR reaction with the following primers: forward 5′-AAG GAT CCA TGT ACG ACT GCA TGG AGG CC-3′ and reverse 5′-AAC TCG AGC TAG AGC TCT TTG GAA CT-3′. The PCR fragment was digested with BamHI/XhoI and subloned into the BamHI/XhoI sites of the mammalian expression vectors pcDNA3.1/Zeo (Invitrogen) and pcDNA3.1/Zeo/3HA (a gift from Dr. Douglas Andres at the University of Kentucky), and the bacterial expression vectors pGEX-KG (Amersham) and pET32a (Novagen).
To create a full-length pET-NPDC-1 bacterial expression construct, the pGEM-NPDC-1 construct was digested with AvrII, filled-in with Klenow and then digested with NotI. This fragment was subcloned into the Eco RV/Not I sites of the bacterial protein expression vector pET32c (Novagen). An axolotl NPDC-1 bacterial expression helix-loop-helix deletion construct (pET-NPDC-1ΔHLH: deletion of amino acids 1–97) was constructed by isolating a SmaI/NotI fragment from the full-length pET-NPDC-1 construct and inserting the fragment into the EcoRV/NotI sites of the bacterial protein expression vector pET32a. To generate the pET-NPDC-1ΔPEST PEST deletion construct (deletion of amino acids 209–288), PCR primers (forward 5′-AAA GGA TCC GAG CCT CCC AGG GCG ACC-3′ and reverse 5′-AAG GAC TCG AGT TAT TAT TAT TGT GCC AAC TTC TTG TC-3′) were used to amplify the first 209 amino acids of axolotl NPDC-1. This PCR fragment was digested with BamHI/XhoI and cloned into the BamHI/XhoI sites of the bacterial protein expression vector pET32a. To generate pcDNA3.1-NPDC-1 mammalian expression construct, full length axolotl NPDC-1 was digested from the pET-NPDC-1 construct with SacII, filled in with Klenow, digested with NotI and inserted into the EcoRV/NotI sites of the mammalian expression vector pcDNA3.1/Zeo. To generate pcDNA-NPDCΔPEST, pET-NPDC-1ΔPEST was digested with BglII/EcoRI. The resulting fragment was inserted into the BamHI/EcoRI sites of pcDNA3.1/Zeo.
RT-PCR assays
We used semi-quantitative PCR analysis to estimate NPDC-1 transcripts derived from 8 axolotl embryonic stages (15, 22, 23, 27, 29, 38, 41, 43) and 2 post-embryonic stages (hatching and 2 months post-hatching). cDNA was synthesized using 1μg of total RNA and the iScript cDNA synthesis kit (Biorad) exactly as described in the manufacturer’s manual. This cDNA was used as template (1/20 dilution of original synthesis) in PCR using the following primers: NPDC-1 forward 5′-AGC GCC CTA GGC TTT GAG, NPDC-1 reverse (5′-TCT AAG CCA CAG TAG CGC C-3′, aRARγ forward 5′-CCC AGA CAA ATA TAC AGA AAC TGC-3′ and aRARγ reverse 5′-CCA TGC ACC ACA GGC CAT TTG AGA-3′. As a control, the axolotl elongation factor 1α (EF-1α) was used as previously described (Carlson et al. 2001). PCR products were resolved on a 1.2% agarose gel and detected using the Gel logic gel imaging system (Kodak). Primers for axolotl NPDC, axolotl RARγ, and axolotl elongation factor-1 alpha (EF-1α) yielded PCR products of 896, 539 and 297 bp, respectively.
Quantitative real-time RT-PCR was performed to estimate changes in axolotl NPDC-1 transcript abundance during spinal cord regeneration as we have previously described (Monaghan et al. 2006). Reactions included cDNA that was synthesized from 10ng total RNA, 300nM primers, and iQ SYBR-Green real-time PCR mix (BioRad). All PCR reactions were run in duplicate on a BioRad I Cycler real-time RT-PCR system. Normalization was performed against glyceraldehyde-3-phosphate dehydrogenase and MC01187, and fold changes were calculated using the ΔΔCt method. Statistical significance was assessed by Student’s unpaired t-test using two normalized biological replicates for 24hrs, day 7, and day 0 samples.
Recombinant protein expression
GST-tagged and/or thioredoxin-tagged recombinant proteins were generated as we have previously detailed (Spencer et al. 2004). Briefly, bacterial expression constructs in pGEX or pET vector systems were used to transform BL21-RP E. coli cells (Novagen). Cells were grown to a 0.6 OD600, in 500 mL of Luria broth, brought to 0.01mM IPTG (isopropyl-beta-D-thiogalactopyranoside) and incubated for 6h. Cells were harvested, lysed for 30 min at room temperature in 20mL of Triple Detergent Lysis buffer [50mM Tris-HCl pH 8.0, 150mM NaCl, 0.02% sodium azide (v/v), 1% NP-40 (v/v), 0.5% sodium deoxycholate (v/v), 0.001mM PMSF, 1X protease inhibitor cocktail (Roche Applied Science) and 500 units Benzonase Nuclease (Novagen)]. Lysates were clarified by ultracentrifugation for 1h at 100,000 xg in a Ti70 rotor and proteins were purified by Fast Performance Liquid Chromatography (FPLC) on either GSTrap Fast Flow columns (GE Life Sciences) for GST fusion proteins or HiTrap chelating HP columns (Amersham Biosciences) for pET vector fusion proteins. Purified proteins were aliquoted and stored at −70°C.
In vitro binding assays
Recombinant GST-tagged axolotl RARγ and GST-tagged human RXRα proteins were prebound to glutathione sepharose beads (50% slurry in APB buffer) [APB: 50mM Tris-HCl pH 7.5, 150mM NaCl, 10% glycerol (v/v), 1% NP-40 (v/v)] by rotating for 1h at 4°C. To the GST prebound proteins, equimolar amounts of thioredoxin or thioredoxin-tagged axolotl NPDC-1 proteins were added as described in the results and figure legends. The proteins were allowed to interact by rotating for at least 1h at 4°C. The beads were recovered by centrifugation in a microcentrifuge and washed 5 times with 1mL APB buffer. Protein concentrations were determined using a commercial Bradford Assay technology (Advance, Cytoskeleton Inc) exactly as described in the manufacturer’s protocol. The bound proteins were analyzed by western blot analyses probing with antisera for thio-probe (Santa Cruz).
Western blot analysis and Bradford Assay
Western blot analyses were performed as we have previously detailed (Spencer et al. 2004). Briefly, proteins were separated by SDS-PAGE and electroblotted onto nitrocellulose membranes (Stratagene Inc). Blocking was performed in PC buffer (1% casein in 1X PBS) [PBS: 2.5mM NaH2PO4, 8.1mM Na2HPO4, 154mM NaCl, pH 7.2–7.4] overnight. Primary antibody was diluted in PCT buffer (1% casein in 1X PBS, 0.01% Tween-20) and incubated for 1h. Blots were subsequently washed with TBST buffer (20mM Tris pH7.5, 137mM NaCl, 0.1% Tween-20), and incubated for 1hr in PCT buffer containing an appropriate secondary antibody coupled to horseradish peroxidase. Blots were washed 3 times in TBST buffer and reacted with ECL reagents (Pierce) per manufacturer’s instructions. chemiluminescence was detected on X-ray film by autoradiography.
Electromobility Shift Assays (EMSA)
EMSAs were performed as we have detailed previously (Henry et al. 2003). Briefly, a βRE (a double-stranded oligonucleotide encompassing a RAR DR5 (direct repeat separated by 5 base pairs) response element found in the promoter region of the RAR beta gene (5′-AGCTTCAGGTCAGAAGGTCAGAGAGCT-3′) containing 5′ overhangs, was radioactively labeled with [α-32P]-dCTP using a standard Klenow fill-in reaction (Yan et al. 1995). The radioactive response element was purified by centrifugation through a microspin G-25 column (Amersham) according to manufacturer’s instructions. Labeled response element was incubated in a 20μL reaction mixture containing 5mM Tris pH 7.9, 15mM HEPES-KOH pH 7.9, 5mM EDTA, 3.5mM MgCl2, 5mM DTT, 0.1% Tween-20, 10% glycerol, 50mM KCl, 0.001mM all-trans retinoic acid and 10μg recombinant axolotl RARγ, human RXRα, axolotl NPDC-1, axolotl NPDCΔPEST or axolotl NPDCΔHLH NPDC as indicated. The protein/DNA complexes were separated from free radiolabeled DNA by electrophoresis in 5% non-denaturing polyacrylamide gels. The gels were dried and the results visualized by autoradiography on X-ray film. For supershift analyses, DNA binding reactions were supplemented with 1μg of an affinity purified anti-NPDC-1 antibody.
Mammalian Transcription Assays
Cos7 cells were transfected using SuperFect reagent (Qiagen) and a modification of the manufacturer’s protocol. Briefly, 5μg total DNA was combined with 150μL OptiMEM (Gibco) and vortexed briefly. To the OptiMEM-DNA mixture 20μL of SuperFect reagent was added and incubated at room temperature for 20 min. During this time, Cos7 cells were washed with 1XPBS once, and then 3mL of OptiMEM was added. To the OptiMEM-DNA mixture, 2mL of OptiMEM was added and after mixing by pipetting up and down, the mixture was added dropwise to Cos7 cells (60–80% confluent in 10 cm tissue culture plates). After 4h incubation at 37°C, the OptiMEM-DNA mixture was removed and cells were washed with 1XPBS 3 times. Cells were removed from the plate by trypsinization, resuspended in 10mL DMEM containing 10% charcoal absorbed fetal calf serum, and 20μL cells/well were plated into a 96 well plate with and without 0.001mM all-trans retinoic acid (atRA). The cells were incubated for 24h in the presence of retinoic acid, and then luciferase and normalizing β-galactosidase activities were determined as we have previously detailed (Noonan et al. 2004). Activity was expressed as the average of luciferase response normalized to the average β-galactosidase response/minute.
Proliferation Assay
HEK293 cells were transfected using a standard calcium phosphate precipitation protocol as we have extensively detailed previously (Noonan et al. 2004). Briefly, HEK293 cells grown on 10 cm tissue culture plates at 70% confluence were transfected with a HEPES buffered calcium phosphate solution (18.75mM HEPES pH 6.95, 137mM NaCl, 0.7mM NaPO4, 120mM CaCl2) containing 2μg of either pcDNA vector, pcDNA-aRARγ or pcDNA-aNPDC plasmids and 18μg of pUC 18 plasmid. The calcium phosphate/DNA mix was allowed to incubate with the cells for 6h at 37°C, the cells were washed three times with 1X PBS and fresh cell culture media were added. Transfections were incubated overnight and cells were replated into 24 well tissue culture plates at a density of 50,000 cells per well. Cells were then incubated in the presence of 2μCi of [3H] thymidine and with or without 1μM atRA. At 6h, 24h and 48h following treatment the cells were harvested and lysed. DNA was precipitated using 10% TCA and radioactivity was trapped by filtration through a vacuum filter apparatus (Hoefer Scientific Instruments) on glass fiber filter circles (GF/C: Fisherbrand). Total filter-bound radioactivity was measured using a scintillation counter.
Statistics
Data from the in vitro transcription and DNA proliferation assays were subjected to one-way analysis of variance (ANOVA) to test for significance, followed by the Tukey-Kramer test. Differences were accepted as significant at p < 0.05. All data are expressed as the mean ± SEM.
RESULTS
Cloning of axolotl NPDC-1 and RARγ
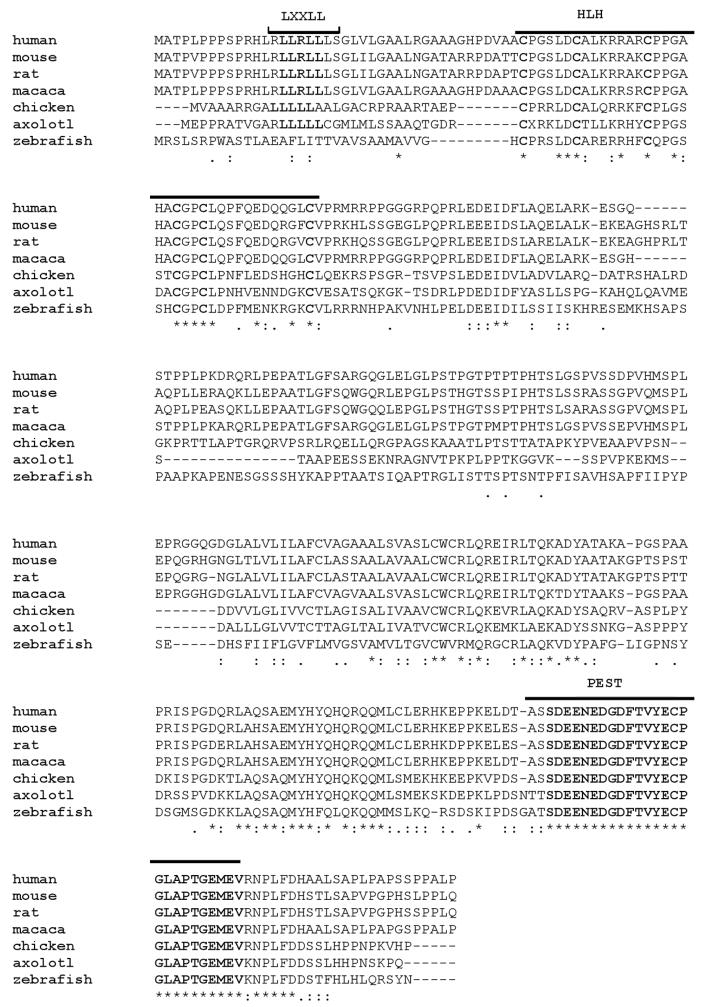
We cloned presumptive full-length axolotl NPDC-1 using partial EST sequence information (Smith et al. 2005) for primer design and 5′ RACE. The reading frame specifies a coding sequence that is shorter than human NPDC-1 (288 vs 326 aa) and the two sequences are 36.3% identical considering all aligned sites (Fig. 1). This percent identity is much lower than the average identity of human-salamander full-length orthologues (~80%, unpublished data). Given this weak evidence for orthology, genetic linkage mapping was used to determine the location of NPDC-1 within the Ambystoma mexicanum genome (data not shown). We mapped NPDC-1 to a region of Ambystoma Linkage Group 7 (Smith et al. 2005) that shows conserved synteny with the region of HSA 9 that human NPDC-1 maps in the human genome (Fig. 2). These linkage data strongly suggest that we identified the Ambystoma orthologue of NPDC-1.
Fig. 1. Protein alignment for human, mouse, rat and axolotl NPDC-1.
Identical ( * ), conserved ( : ) and semiconserved ( . ) amino acids are denoted. The conserved LXXLL, β-helix-loop-helix (HLH), and PEST domains within these proteins are as indicated by lines and bolded letters.
Fig. 2. Conserved synteny of NPDC-1 between axolotl and human.
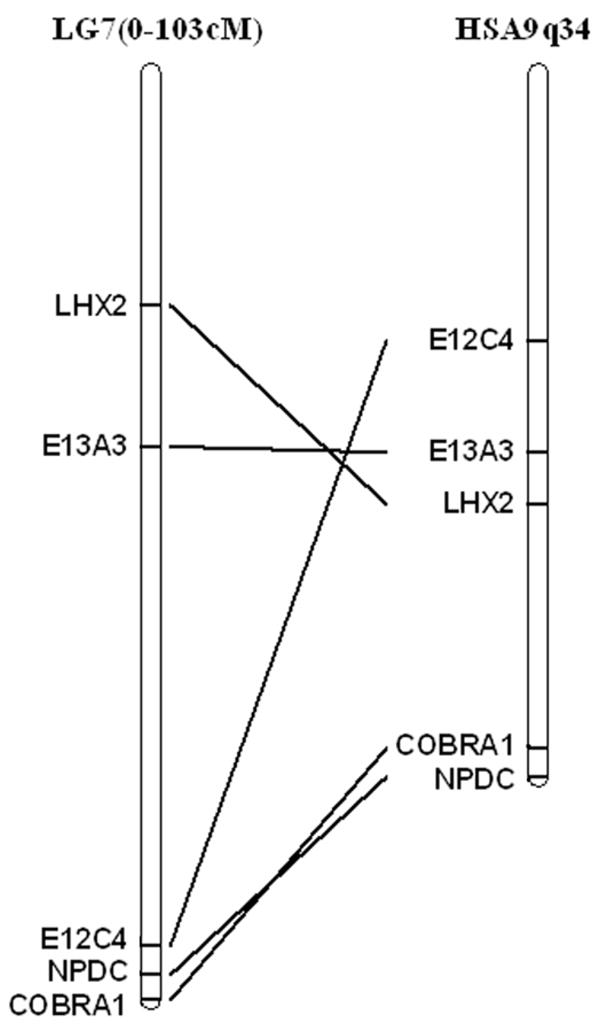
Linkage mapping positions axolotl NPDC-1 on Ambystoma mexicanum linkage group 7 (LG7). The human NPDC-1 orthologue and other syntenic loci from LG7 map to the tip of human chromosome 9 (Hsa9q34).
All of the previously characterized functional domains of mammalian NPDC-1 were identified in axolotl NPDC-1. These included: a) a LXXLL motif that may mediate interactions between nuclear receptors and co-regulators (Zhang et al. 2000), b) an HLH motif that functions as both a protein interaction site and a binding site for DNA, c) a MAPK target sequence, that is presumably involved in regulation of cell cycle signaling, and d) a PEST domain that targets proteins for degradation by the ubiquitin-proteosome system (Spencer et al. 2004). Thus, axolotl NPDC-1 has a domain structure that is identical to mammalian NPDC-1. Moreover, the putative LXXLL, the essential cysteines and spacing of the HLH domain, as well as the putative PEST domain, all displayed 100% amino acid conservation with mammalian NPDC-1.
Tissue Distribution of Axolotl NPDC-1 and RARγ
The distribution of axolotl NPDC-1 and axolotl RARγ transcripts among juvenile axolotl tissues was investigated using semi-quantitative RT-PCR (Fig. 3A, B). Axolotl NPDC-1 expression was higher in heart, brain, eye, skin, and gill than it was in spinal cord, lung, muscle and liver. No expression was observed for spleen. axolotl RARγ expression was detected in all tissues examined, with the highest expression observed in heart (Fig. 3C, D). These data show tissue-specific differences in the abundances of NPDC-1 and axolotl RARγ transcripts, and co-expression among many of the same juvenile axolotl tissues.
Fig. 3. Tissue expression of axolotl NPDC-1 and axolotl RARγ in axolotl.
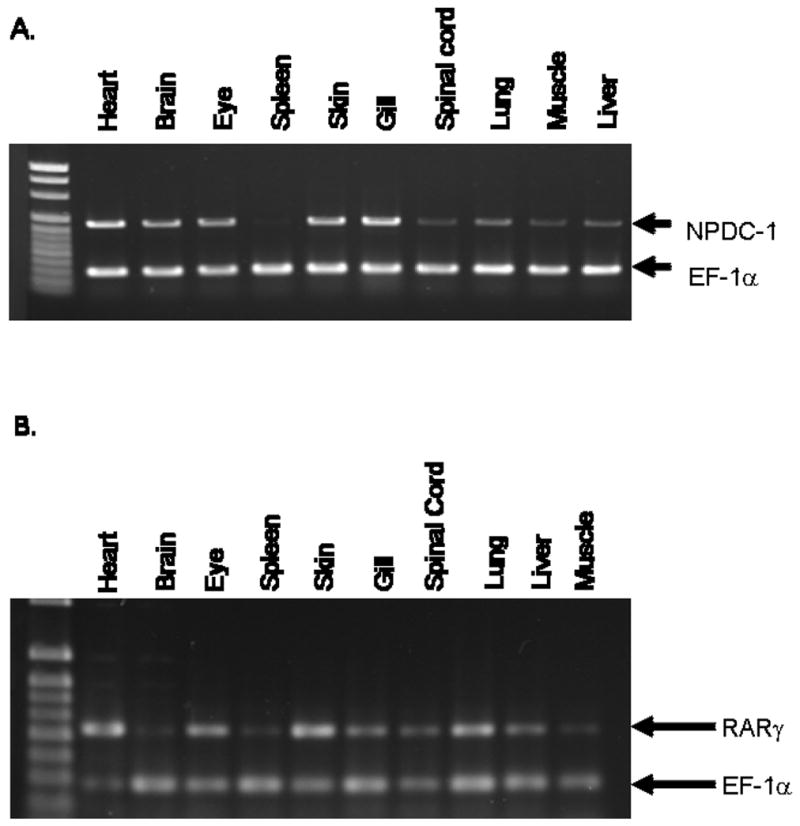
Semi-quantitative RT-PCR analysis of NPDC-1 (A) or RARγ (B) mRNA expression in axolotl tissues. The relative intensities of axolotl NPDC-1- and axolotl RARγ-specific PCR bands were normalized to control axolotl EF-1α bands.
Developmental expression of NPDC-1 during axolotl embryogenesis and regeneration
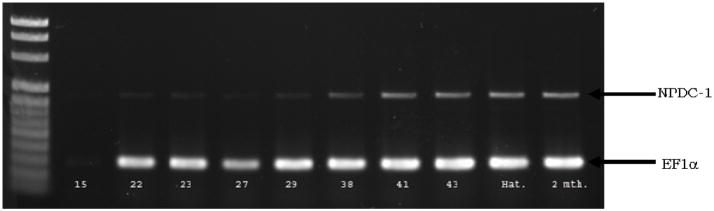
Previous studies using mammalian tissues showed that NPDC-1 mRNA expression varies with development and higher levels are detected in adulthood (Dupont et al. 1998). We observed a similar pattern of expression during axolotl embryogenesis (Fig. 4A, B). In axolotl NPDC-1 expression during development is low during early development (neurulation), but increases during tailbud stages (stages 29–32). Around the time of hatching (stages 41–43), expression increases dramatically and transcript levels remain high at least through the first two months of the larval period. Thus, levels of NPDC-1 correlate with developmental changes that are initiated relatively late in embryogenesis and that continue into early larval development.
Fig. 4. Developmental Expression of axolotl NPDC-1.
Semi-quantitative RT-PCR analysis of NPDC-1 during embryogenesis and early larval development of axolotl. The relative intensities of NPDC-1 specific PCR bands were normalized to the EF-1α bands for the various embryo stages.
In mammals, increased NPDC-1 mRNA expression is thought to be associated with neuronal proliferation and differentiation (Galiana et al. 1995). Quantitative RT-PCR was used to estimate transcript abundances for NPDC-1 during early axolotl spinal cord regeneration, when there is considerable cell proliferation and neurogenesis. We found that NPDC-1 transcript abundances at day 0 were not significantly different from levels measured at 24 hours or 7 days after tail amputation (24 hours, mean fold change = 0.83, p = 0.16; 7 days, mean fold change = 0.71, p = 0.42). These results validate microarray estimates of NPDC-1 during early spinal cord regeneration (One-way ANOVA, n = 5, p = 0.77; D1 FC = 1.02; D3 FC = 0.98; D5 FC = 1.05; D7 FC = 0.88; (Monaghan et al. 2006). Thus, there appears to be no significant increase in NPDC-1 mRNA expression during the neuronal proliferation and differentiation events occurring in early axolotl spinal cord regeneration.
Analysis NPDC-1 and axolotl RARγ Interactions
Previous studies showed that human NPDC-1 complexes with human RARβ and human RXRα (Henry et al. 2003). To examine whether this interaction occurs between axolotl NPDC-1 and axolotl RARγ proteins, in vitro pull-down assays were performed in which GST-aRARγ bound to glutathione sepharose beads was mixed with equimolar amounts of thioredoxin or thioredoxin-tagged NPDC-1 proteins (Fig. 5). Full-length NPDC-1 (NPDC-CDS) and NPDC-1 minus the PEST domain (NPDCΔPEST), were able to complex with axolotl RARγ. However, NPDC-1 minus the helix-loop-helix domain (NPDCΔHLH) failed to bind axolotl RARγ. These data suggest that the primary domain of interaction between axolotl RARγ and NPDC-1 resides within the amino terminus (amino acids 1–97) of NPDC-1.
Fig. 5. Direct in vitro interaction between axolotl RARγ and axolotl NPDC-1.
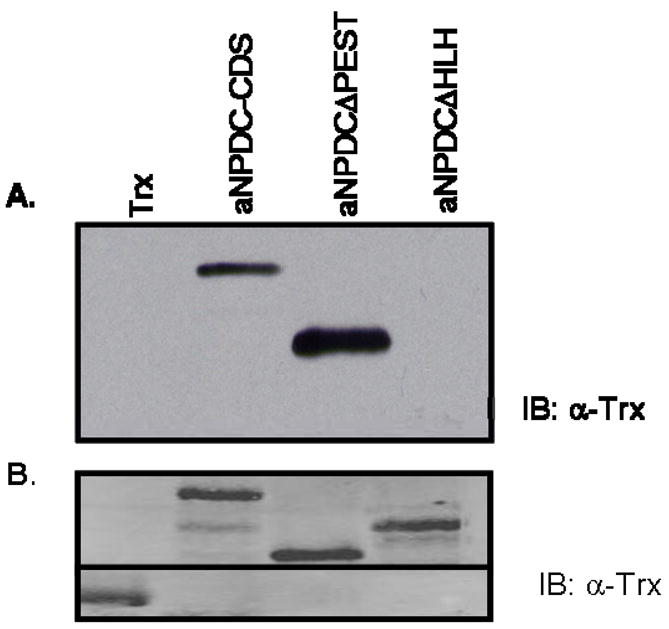
(A). Glutathione bead-bound GST-RARγ was used in pull-down assays with equimolar amounts of thioredoxin (Trx), or thioredoxin-tagged axolotl NPDC-1, thioredoxin-tagged axolotl NPDCΔPEST and thioredoxin-tagged axolotl NPDCΔHLH recombinant proteins. Bound proteins were resolved on a SDS-PAGE gel, Western blotted and blots were probed with an anti-thioredoxin antibody. (B) In an independent experiment, equivalent amounts of thioredoxin and thioredoxin-tagged proteins used in the pull-down assay seen in (A), were Western blotted and probed for thioredoxin as described in (A). These results are representative of experiments repeated at least three times.
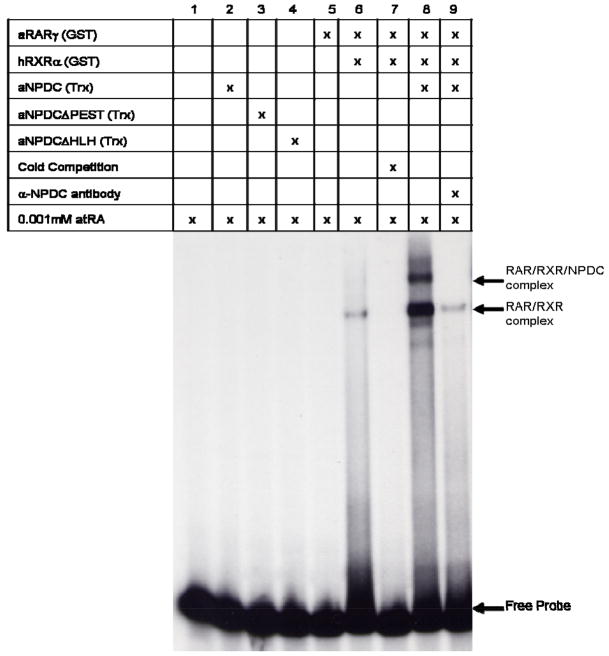
Analysis of the impact NPDC-1 has on axolotl RARγ DNA binding
Previously, we showed that human NPDC-1 facilitates RAR/RXR heterodimer DNA binding, but does not shift the DNA binding complex (Henry et al. 2003). We investigated whether a similar result is observed between axolotl RARγ and axolotl NPDC-1 (Fig. 6). None of the tested proteins (axolotl RARγ, axolotl NPDC-1, and axolotl NPDC-1 with domain deletions) were able to independently complex with the βRE DNA response element (lanes 2–5). The combination of human RXRα and axolotl RARγ recombinant proteins was observed to form a shift complex (lane 6). Specificity of the complex was shown by cold competition with unlabelled oligonucleotide (lane 7). Addition of axolotl NPDC-1 made the axolotl RARγ/human RXRα complex stronger in intensity and generated a second higher mobility complex (lane 8). The possibility that this higher molecular weight complex contains axolotlNPDC-1 is supported by the observation that anti-NPDC-1 antibody (raised against the conserved PEST region) eliminates the upper complex (lane 9) and diminishes the lower complex. These data support the hypothesis that axolotl NPDC-1, like rodent and human NPDC-1, modulates retinoid receptor DNA binding. Additionally, formation of a second NPDC-1-specific higher mobility shift complex is a novel result that may be unique to axolotl. Finally, the observation that anti-NPDC-1 antibody also reduces the intensity of the lower band in these complexes is interesting because it suggests that NPDC-1 can facilitate axolotl RARγ/RXRα DNA binding and form novel RARγ/RXRα/NPDC-1 complexes.
Fig. 6. Electromobility shift assay analyzing the impact axolotl NPDC-1 has on axolotl RARγ DNA binding.
A 32P-labeled oligonucleotide containing an established retinoid receptor DNA binding motif (βRE) was used in an EMSA assay with recombinant GST-tagged human RXRα, GST-tagged axolotl RARγ, thioredoxin-tagged axolotl NPDC-1 (Trx-NPDC-1), thioredoxin-tagged axolotl NPDCΔPEST (Trx-NPDCΔPEST) and thioredoxin-tagged axolotl NPDCΔHLH (Trx-NPDCΔHLH) proteins as described in the Methods section. DNA:protein complexes were resolved on a non-denaturing polyacrylamide gel and bands were visualized by autoradiography on Kodak Xar-5 film. Also, where indicated, binding reactions were supplemented with unlabelled oligonucleotide (cold βRE) or antisera specific for NPDC-1 (α-NPDC). In all reactions 0.001mM atRA was included. These results are representative of experiments repeated at least three times.
Analysis of the impact NPDC-1 has on transcription events mediated by axolotl RARγ
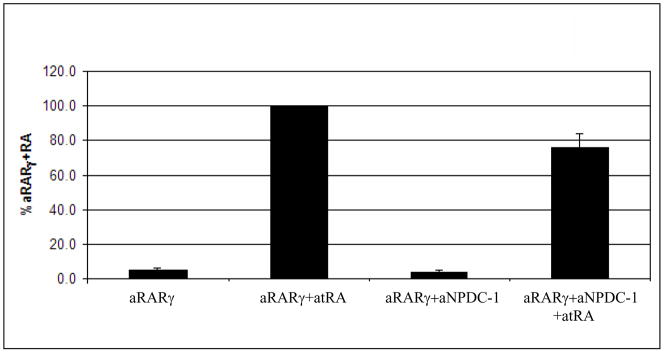
The ability of rat and human NPDC-1 to repress retinoid receptor mediated transcription has been previously reported (Henry et al. 2003). To investigate whether NPDC-1 can similarly affect axolotl RARγ-mediated transcription, in vitro transcription analyses were performed. Cos7 cells that were transfected with mammalian expression constructs for axolotl RARγ and/or axolotl NPDC-1 were not capable of activating transcription of the reporter gene in the absence of atRA (Fig. 7). After stimulating transfected cells with 1μM atRA, axolotl RARγ was capable of activating reporter gene transcription. Relative to this level, transcription was lowered by 30% when axolotl RARγ and axolotl NPDC-1 were co-transfected in the presence of 1μM atRA. These data support a role for NPDC-1 in repression of axolotl RARγ mediated transcription.
Fig. 7. Analysis of the impact NPDC-1 has on axolotl RARγ-mediated transcription.
Cos 7 cells were co-transfected with a βRE-tk-luc reporter plasmid and with mammalian expression plasmids for axolotl NPDC-1, axolotl RARγ, and β-galactosidase. Transfected cells were incubated in the presence of 0.001mM atRA and assayed for luciferase and β-galactosidase activity as described in the Methods section. Luciferase data for quadruplicate samples was normalized to β-galactosidase activity for these respective samples, and data were reported as a % of the atRA-induced activation of the axolotl RARγ plasmid. The error bars represent ± standard errors of the mean for the quadruplicate samples. These results are representative of experiments repeated at least three times.
Proliferation Assay
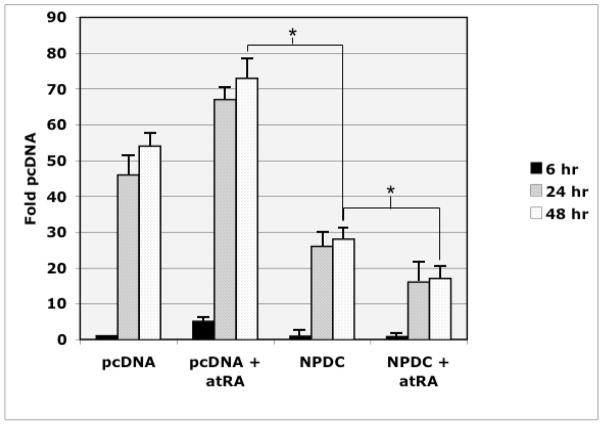
A hallmark of rodent NPDC-1 is functional inhibition of cell proliferation (Galiana et al. 1995). To evaluate this with respect to axolotl NPDC-1, HEK293 cells were transfected with a mammalian expression plasmids for axolotl NPDC-1. Transfected cells were incubated for varying times in the presence [3H]-thymidine, and in the presence and absence of atRA (Fig. 8). Over-expression of NPDC-1 significantly reduced [3H]-thymidine incorporation at 24h and 48h. The presence of retinoic acid also appeared to significantly facilitate NPDC-1 suppression of proliferation at the 24h and 48h time points. These data support a role for NPDC-1 in suppression of cell proliferation.
Fig. 8. Analysis of the impact axolotl NPDC-1 has on the proliferation rate of HEK293 cells.
HEK 293 cells transfected with pcDNA or pcDNAaNPDC mammalian expression plasmids were distributed (4 wells per transfection) into 24 well tissue culture plates and incubated with [3H]-thymidine for 6h, 24h, 48h with or without 0.001mM atRA. Cells were lysed and assayed by TCA precipitation as described in the Methods section. Data are expressed as a percent of incorporation of [3H]-thymidine for the 6h vector (pcDNA) transfection in the absence of atRA. Statistics were performed on the results from three independent experiments and the error bars represent ± standard errors of the mean, * = p < 0.05.
DISCUSSION
Previous studies have established that mammalian NPDC-1 is associated with cell proliferation and neuronal differentiation, and it can regulate retinoic acid signaling. The general importance of retinoic acid signaling during vertebrate development led us to examine NPDC-1 function in a model amphibian system (Ambystoma). We isolated a full-length NPDC-1 gene from A. mexicanum and verified its correspondence to mammalian orthologues by sequence comparison and genome mapping. Although axolotl NPDC-1 shows low sequence similarity to mammal orthologues, key domains of the protein are conserved, including the LXXLL motif, MAPK domain, PEST sequence and the helix-loop-helix motif. These structural data suggest that axolotl and mammalian NPDC-1 orthologues perform similar functions and indeed, we observed similar patterns of gene expression and protein function in in vitro assays. Below, we discuss these similarities and also point out a few important differences that justify additional studies of NPDC-1 function and its relation to retinoic acid signaling, in axolotls.
Although mammalian NPDC-1 was isolated initially as a neuronal protein, independent groups (Qu et al. 2001; Henry et al. 2003) have documented a more diverse tissue distribution, with expression observed in brain, lung, heart muscle and kidney. In addition, NPDC-1 expression in mouse brain is known to increase during development with peak levels occurring at adulthood (Dupont et al. 1998). We also observed a diverse tissue distribution among axolotl tissues, with the highest levels observed in heart, brain, skin, gill, lung and eye. We also observed NPDC-1 transcripts to increase during early axolotl development, but not during spinal cord regeneration. It is possible that NPDC-1 is not associated with neuronal proliferation and differentiation in axolotl, or it is regulated post-translationally during these events, or perhaps it is only associated with early neural fate decisions during embryogenesis. In axolotl, NPDC-1 transcript abundances were low throughout the embryonic period until stage 36, at which time they dramatically increased. High levels of NPDC-1 were sustained across developmental stages at which hatching occurs in Ambystoma and remained high through the first 2 months. Interestingly, forelimb development is initiated in Ambystoma around the time of hatching and hindlimb development follows about 1 month later. It is well known that salamander retinoic acid receptors are up regulated during salamander limb development and that retinoids affect patterning and cellular differentiation (Giguere et al. 1989; Hill et al. 1993; Laudet et al. 2002). Temporal co-expression of NPDC-1 and retinoic acid receptors is expected if the products of these genes function to mediate retinoic acid signaling during development. Additional studies are needed to locate NPDC-1 and retinoic acid receptor gene expression to specific cell populations of tissues. However, observation of NPDC-1 and axolotl RAR co-expression among many of the same juvenile axolotl tissues suggests a functional link between NPDC-1 and retinoid signaling.
In mammals, NPDC-1 has been shown to suppress cellular proliferation (Galiana et al. 1995). In a preliminary analysis of this parameter for axolotl NPDC-1, overexpression of axolotl NPDC-1 in HEK293 cells was observed to substantially decrease [3H]-thymidine incorporation, an established measure of cellular proliferation rate. Furthermore, atRA appeared to facilitate NPDC-1 suppression of proliferation, suggesting NPDC-1’s impact on proliferation might be linked in some way to retinoid signaling. The potential mechanism for NPDC-1’s actions in inhibiting cellular proliferation is not fully understood, but NPDC-1 has been reported to associate either directly or indirectly with cell cycle regulatory proteins such as E2F-1, cyclin A, cyclin D1 and cdk2 (Dupont et al. 1998; Sansal et al. 2000).
Previous studies of mammalian NPDC-1 demonstrated it could affect retinoic acid signaling through direct binding to RXR and RAR-RXR heterodimers (Henry et al. 2003). Here we demonstrate that axolotl NPDC-1 can also bind axolotl RAR, and that this binding event can be localized to the amino terminus within amino acids 1–97. Two highly conserved domains occur within this amino terminus region. The first is an LXXLL motif. This motif may be an important nuclear receptor binding site for a variety of transcription co-regulatory proteins (Laudet et al. 2002), although Henry et al (Henry et al. 2003) demonstrated that, at least with respect to human NPDC-1 and RAR signaling, this motif appears not to be critical for transcriptional regulatory events modulated by NPDC-1. The second motif is a helix-loop-helix (HLH) domain that occurs downstream of the LXXLL motif. This domain has been shown to function as a binding site for DNA and proteins belonging to the HLH family. These proteins are key regulators of development, particularly cell type determination, terminal differentiation and sex determination (Qu et al. 2001). Further studies are needed to establish what, if any role the HLH domain might be playing in axolotl NPDC-1- axolotl RARγ binding events.
Most co-regulatory proteins mediate their regulation of transcription through modulation of protein complexes that associate with specific DNA regulatory sequences (Zilliacus et al. 1995). Here we demonstrate that the mechanism for NPDC-1 regulation of axolotl RARγ-mediated transcription appears complex and may involve both direct associations with axolotl RARγ, as well as facilitating complex formation on RAR response elements. The presence of NPDC-1 increased the amount of response element the RAR-RXR heterodimer complex bound, suggesting that NPDC-1 is capable of altering the DNA binding capacity or affinity of the RAR-RXR heterodimers. These results parallel what have been published previously for mammals (Henry et al. 2003) and suggest that NPDC-1 is able to alter the DNA binding capacity of the RAR-RXR complex without altering the mobility of the complex. However, unique to this study, a novel shift in the axolotl RAR-RXR complex was observed with the addition of axolotl NPDC-1. Elimination of this novel complex by a NPDC-1-specific antibody suggests that the increase in complex size is due to the binding of NPDC-1. Thus, NPDC-1 orthologues similarly affect RAR-RXR DNA binding, but axolotl NPDC-1 appears to complex with axolotl RAR-RXR heterodimers in a novel manner. One caveat to these studies is our use of a human RXR isoform. The lack of a cloned axolotl RXR isoform relegated the use of this human homolog, but previously published data strongly support the existence of RXR homologs in axolotls as well as their homology with human isoforms (Alfaro et al. 2002). In this study the authors demonstrated that antibodies developed to the human RXR alpha, beta and gamma isoforms uniquely identified proteins of comparable size and molecular weight in Axolotl protein extracts. Furthermore, the high conservation of RXR across species (especially at the DNA and ligand binding domains examined in this assay), as well as the ligand-dependent in vitro transcription response we obtain in human cell lines with this axolotl RAR clone, strongly suggests the human RXR functions as a suitable substitute for axolotl RXR.
Our study shows that a functional consequence of axolotl NPDC-1 binding is repression of axolotl RARγ-mediated transcription. We demonstrated that DR5-driven reporter gene transcription is moderately repressed by over-expression of NPDC-1 in an in vitro transcription analysis. This is similar to what has been reported for human NPDC-1 (Henry et al. 2003), although the repression observed for NPDC-1 is not as great as the repression observed for human NPDC-1 (Henry et al. 2003). This could be due to a number of factors, not the least of which is the expression of axolotl proteins in mammalian cells. Further studies will be required to fully establish the biochemical mechanism for repression of RAR-mediated transcription events by NPDC-1.
In summary, this study presents the first evidence for interaction between NPDC-1 and RARγ in axolotl. Retinoic acid signaling is an established regulator of chordate development, and the studies presented here support a conserved role for NPDC-1 in this process extending from humans to axolotl. Overall, these studies represent an important first step in understanding the role of NPDC-1 on RAR mediated signaling in a developmental context.
Acknowledgments
These studies were supported by grants from the National Institutes of Health (NIH) (HL67321), the LAM Foundation (LAM052), and the Kentucky Lung Cancer Research Program to D.J.N. Aspects of this project were also supported by grants to S.R.V.: IBN-0242833 from the National Science Foundation (NSF) CAREER Award program and Grant Number 5-R24-RR016344-06 from the National Center for Research Resources (NCRR), a component of the NIH. The contents of this study are solely the responsibility of the authors and do not necessarily represent the official views of NCRR or NIH or NSF. We also acknowledge the support of the NSF funded Ambystoma Genetic Stock Center at University of Kentucky.
Abbreviations
- NPDC-1
neuronal proliferation and differentiation control-1
- RAR
retinoic acid receptor
- RXR
retinoid X receptor
- 5′RACE
Rapid amplification of 5′ complementary DNA ends
- IPTG
isopropyl-beta-D-thiogalactopyranoside
- PMSF
phenymethanesulfonyl fluoride
- EMSA
electromobility shift assay
- MAPK
mitogen activated protein kinase
- PEST
rich in proline, glutamine, serine and threonine
- HLH
helix-loop-helix
- LXXLL
leucine-X-X-leucine-leucine containing sequence
- atRA
all-trans retinoic acid
- DR5
direct repeat separated by 5 base pairs
- RARE
RAR-specific DNA response element
- Trx
thioredoxin
- EF1α
elongation factor 1 alpha
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Armstrong JB, Malacinski GM. Developmental biology of the axolotl. Oxford University Press; New York: 1989. [Google Scholar]
- Carlson MR, Komine Y, Bryant SV, Gardiner DM. Expression of Hoxb13 and Hoxc10 in developing and regenerating Axolotl limbs and tails. Dev Biol. 2001;229:396–406. doi: 10.1006/dbio.2000.0104. [DOI] [PubMed] [Google Scholar]
- Crawford K, Vincenti DM. Retinoic acid and thyroid hormone may function through similar and competitive pathways in regenerating axolotls. J Exp Zool. 1998;282:724–738. doi: 10.1002/(sici)1097-010x(19981215)282:6<724::aid-jez8>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- DeLuca HF. The vitamin D story: a collaborative effort of basic science and clinical medicine. FASEB J. 1988;2:224–236. [PubMed] [Google Scholar]
- Dilworth FJ, Chambon P. Nuclear receptors coordinate the activities of chromatin remodeling complexes and coactivators to facilitate initiation of transcription. Oncogene. 2001;20:3047–3054. doi: 10.1038/sj.onc.1204329. [DOI] [PubMed] [Google Scholar]
- Dupont E, Sansal I, Evrard C, Rouget P. Developmental pattern of expression of NPDC-1 and its interaction with E2F-1 suggest a role in the control of proliferation and differentiation of neural cells. J Neurosci Res. 1998;51:257–267. doi: 10.1002/(SICI)1097-4547(19980115)51:2<257::AID-JNR14>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- Evrard C, Rouget P. Subcellular localization of neural-specific NPDC-1 protein. J Neurosci Res. 2005;79:747–755. doi: 10.1002/jnr.20405. [DOI] [PubMed] [Google Scholar]
- Galiana E, Vernier P, Dupont E, Evrard C, Rouget P. Identification of a neural-specific cDNA, NPDC-1, able to down-regulate cell proliferation and to suppress transformation. Proc Natl Acad Sci USA. 1995;92:1560–1564. doi: 10.1073/pnas.92.5.1560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giguere V, Ong ES, Evans RM, Tabin CJ. Spatial and temporal expression of the retinoic acid receptor in the regenerating amphibian limb. Nature. 1989;337:566–569. doi: 10.1038/337566a0. [DOI] [PubMed] [Google Scholar]
- Henry IK, Spencer ML, Theodosiou M, Lou D, Noonan DJ. A neuronal-specific differentiation protein that directly modulates retinoid receptor transcriptional activation. Nucl Recept. 2003;1:7. doi: 10.1186/1478-1336-1-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill DS, Ragsdale CW, Jr, Brockes JP. Isoform-specific immunological detection of newt retinoic acid receptor delta 1 in normal and regenerating limbs. Development. 1993;117:937–945. doi: 10.1242/dev.117.3.937. [DOI] [PubMed] [Google Scholar]
- Laudet V, Gronemeyer H. The nuclear receptor factsbook. Academic Press; San Diego: 2002. [Google Scholar]
- Maden M. Vitamin A and pattern formation in the regenerating limb. Nature. 1982;295:672–675. doi: 10.1038/295672a0. [DOI] [PubMed] [Google Scholar]
- Minucci S, Wong J, Blanco JC, Shi YB, Wolffe AP, Ozato K. Retinoid receptor-induced alteration of the chromatin assembled on a ligand-responsive promoter in Xenopus oocytes. Mol Endocrinol. 1998;12:315–324. doi: 10.1210/mend.12.3.0074. [DOI] [PubMed] [Google Scholar]
- Monaghan JR, Walker JA, Page RB, Putta S, Beachy CK, Voss SR. Early gene expression during natural spinal cord regeneration in the salamander Ambystoma mexicanum. 2006 doi: 10.1111/j.1471-4159.2006.04344.x. (in review) [DOI] [PubMed] [Google Scholar]
- Niazi IA, Saxena S. Abnormal hind limb regeneration in tadpoles of the toad, Bufo andersoni, exposed to excess vitamin A. Folia Biol (Krakow) 1978;26:3–8. [PubMed] [Google Scholar]
- Noonan DJ, Henry K, Twaroski ML. A high-throughput mammalian cell-based transient transfection assay. Methods Mol Biol. 2004;284:51–65. doi: 10.1385/1-59259-816-1:051. [DOI] [PubMed] [Google Scholar]
- Qu X, Zhang C, Zhai Y, Xing G, Wei H, Yu Y, Wu S, He F. Characterization and tissue expression of a novel human gene npdc1. Gene. 2001;264:37–44. doi: 10.1016/s0378-1119(01)00324-9. [DOI] [PubMed] [Google Scholar]
- Ribeiro RC, Kushner PJ, Baxter JD. The nuclear hormone receptor gene superfamily. Annu Rev Med. 1995;46:443–453. doi: 10.1146/annurev.med.46.1.443. [DOI] [PubMed] [Google Scholar]
- Sansal I, Dupont E, Toru D, Evrard C, Rouget P. NPDC-1, a regulator of neural cell proliferation and differentiation, interacts with E2F-1, reduces its binding to DNA and modulates its transcriptional activity. Oncogene. 2000;19:5000–5009. doi: 10.1038/sj.onc.1203843. [DOI] [PubMed] [Google Scholar]
- Spencer ML, Theodosiou M, Noonan DJ. NPDC-1, a novel regulator of neuronal proliferation, is degraded by the ubiquitin/proteasome system through a PEST degradation motif. J Biol Chem. 2004;279:37069–37078. doi: 10.1074/jbc.M402507200. [DOI] [PubMed] [Google Scholar]
- Sporn MM, Robert AB, Goodman DS. The Retinoids: Biology, Chemistry, and Medicine. Raven Press, Ltd; New York: 1984. [Google Scholar]
- Sun SY, Lotan R. Retinoids and their receptors in cancer development and chemoprevention. Crit Rev Oncol Hematol. 2002;41:41–55. doi: 10.1016/s1040-8428(01)00144-5. [DOI] [PubMed] [Google Scholar]
- Thoms SD, Stocum DL. Retinoic acid-induced pattern duplication in regenerating urodele limbs. Dev Biol. 1984;103:319–328. doi: 10.1016/0012-1606(84)90320-8. [DOI] [PubMed] [Google Scholar]
- Yan R, Qureshi S, Zhong Z, Wen Z, Darnell JE., Jr The genomic structure of the STAT genes: multiple exons in coincident sites in Stat1 and Stat2. Nucleic Acids Res. 1995;23:459–463. doi: 10.1093/nar/23.3.459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J, Lazar MA. The mechanism of action of thyroid hormones. Annu Rev Physiol. 2000;62:439–466. doi: 10.1146/annurev.physiol.62.1.439. [DOI] [PubMed] [Google Scholar]
- Zilliacus J, Wright AP, Carlstedt-Duke J, Gustafsson JA. Structural determinants of DNA-binding specificity by steroid receptors. Mol Endocrinol. 1995;9:389–400. doi: 10.1210/mend.9.4.7659083. [DOI] [PubMed] [Google Scholar]