Abstract
Matrix metalloproteinases (MMPs) degrade extracellular matrix and regulate many functions including cell signaling. Oxidative stress is implicated in the development of diabetic retinopathy, and MMP-2, the most ubiquitous member of the MMP family, is sensitive to oxidative stress. This study aims to determine the regulation of MMP-2 by oxidative stress in the development of diabetic retinopathy, and the role of MMP-2 in the apoptosis of retinal capillary cells. Effect of mitochondrial superoxide scavenger on glucose-induced alterations in MMP-2, and its proenzyme activator-MT1-MMP and physiological inhibitor-TIMP-2, was determined in retinal endothelial cells, and regulation of their glucose-induced accelerated apoptosis by the inhibitors of MMP-2 was accessed. To confirm in vitro results, effect of antioxidant supplementation on MMP-2, MT1-MMP and TIMP-2 was investigated in the retina of streptozotocin-induced diabetic rats. Glucose-induced activation of retinal capillary cell MMP-2 and MT1-MMP and decrease in TIMP-2 were inhibited by superoxide scavengers, and their accelerated apoptosis was prevented by the inhibitors of MMP-2. Antioxidant therapies, which are shown to inhibit oxidative stress, capillary cell apoptosis and retinopathy in diabetic rats, ameliorated alterations in retinal MMP-2 and its regulators. Thus, MMP-2 has a pro-apoptotic role in the loss of retinal capillary cells in diabetes, and the activation of MMP-2 is under the control of superoxide. This suggests a possible use of MMP-2 targeted therapy to inhibit the development of diabetic retinopathy.
Keywords: Antioxidants, Diabetic Retinopathy, Endothelial cells, Matrix Metalloproteinase, Oxidative stress
Introduction
Oxidative stress plays an important role in diabetic complications (1–7), and reactive oxygen species (ROS) are considered as a causal link between elevated glucose and metabolic abnormalities important in the development of diabetic complications (6). Retina and capillary cells experience increased oxidative damage in diabetic milieu, and antioxidant defense mechanism is impaired (2–4, 7, 8). Administration of antioxidants to diabetic rats prevents retina from oxidative damage, and also the development of retinopathy. Furthermore, retinal mitochondria become dysfunctional and start to leak cytochrome c into the cytosol, and superoxide levels are elevated (4, 8). Overexpression of the enzyme responsible for scavenging mitochondrial superoxide (MnSOD) prevents these diabetes-induced mitochondrial alterations, and histopathology characteristic of diabetic retinopathy (8, 9), thus suggesting a major role of mitochondrial superoxide in the development of diabetic retinopathy.
Matrix metalloproteinases (MMPs), a class of approximately 25 known proteinases, are a family of zinc enzymes that can degrade at least one component of the extracellular matrix. They regulate major biological functions including tissue repair and cell signaling (10). The most ubiquitous of the MMP family is MMP-2, a 72KD gelatinase that cleaves primarily type IV collagen and aids advancing front of the migrating column of endothelial cells to migrate through the basement membrane. MMP-2 is secreted as a latent pro-form that is processed into the active molecule through interaction with membrane type 1–MMP (MT1–MMP) on the cell surface at the location where it is needed (11). MT1-MMP initiates the activation pathway by converting pro-MMP-2 into an activation intermediate that further undergoes autocatalytic conversion to generate the mature enzyme of MMP-2. The interactions of MMP-2 and MT1-MMP are regulated by their physiological tissue inhibitors, TIMPs (12). How MMP-2 and its regulators contribute to the development of diabetic retinopathy remains to be clarified.
MMP-2 and MT1-MMP are sensitive to oxidative stress; low concentrations of ROS activate pro-MMPs by oxidation of the sulfide bond in the pro-domain of the MMP and decrease TIMPs, and peroxynitrite (formed between ROS and nitric oxide) activates pro-MMPs via interacting with cytosolic glutathione (13–16). Increased levels of MMP-2 and MMP-9 are observed in diabetic patients and animal models of diabetic retinopathy, and these increases are suggested to contribute to the disruption of the overall tight junction complex and vascular permeability and maintenance of blood retinal barrier (17–22). In the pathogenesis of diabetic retinopathy, in addition to the impairments in the tight junction and blood retinal barrier, the apoptosis of retinal capillary cells (pericytes and endothelial cells) and other non vascular cells is also accelerated (23–25). Animal models have suggested that the detection of apoptotic capillary cells can serve as a surrogate endpoint to screen efficacy of interventions to inhibit the development of this microvascular complication of diabetes (24).
The purpose of this study is to investigate the potential role of MMP-2 and investigation of the possible mechanism by which it contributes in the development of diabetic retinopathy. Role of mitochondrial superoxide on glucose-induced alterations in MMP-2 and its proenzyme activator, and MMP-2 inhibitors on accelerated apoptosis were determined in retinal capillary cells. The effect of antioxidant therapies, which we have shown to inhibit diabetes-induced increased oxidative stress and the development of retinopathy (26, 27) on the regulation of retinal MMP-2 and its regulators was investigated in the retina of diabetic rats. Since retina is a complex tissue with multiple cell types, to conclusively establish the role of MMP-2 in the pathogenesis of diabetic retinopathy, we have investigated also the effect of diabetes on the activation of MMP-2 in the microvessels isolated from diabetic rat retina.
Methods
Bovine retinal endothelial cells: Retinal endothelial cells were prepared from bovine eyes, and primary cultures of the cells from 4–7th passage were employed for the experiments as routinely used in our laboratory (4, 28). The cells exhibited their typical ‘cobblestone’ morphology and were cultured in Dulbecco’s Modified Eagle Medium (DMEM) containing 15% fetal calf serum (heat inactivated), 5% Nu-serum, heparin (50μg/ml), endothelial growth supplement (25μg/ml) and antibiotic/antimycotic in 95% O2 and 5% CO2. The cells were incubated in the DMEM containing 2% heat inactivated fetal calf serum, 10% Nu-serum, 50μg/ml heparin, 1μg/ml endothelial growth supplement and antibiotic/antimycotic supplemented with 5mM glucose or 20mM glucose for 4–5 days in the presence or absence of inhibitors of MMP-2; 10μM of MMP-2 inhibitor I (cis-octadecenoyl-N-hydroxylamide oleoyl-N-hydroxylamide; EMD Biosciences, Gibbstown, NJ) (29), or 5μM of MMP-2 inhibitor II (4-(4–9Methanesulfonamido) phenyl)methyloxirane) (26), or 200μM of a cell permeable MnSOD mimic- MnTBAP (Biomol, Plymouth Meeting, PA) (4). The medium and the inhibitors were replaced every other day. At the end of incubation medium was collected, and the cells were washed with phosphate buffer saline and collected. Osmotic controls included the cells incubated in identical experimental conditions with 20mM mannitol instead of 20mM glucose.
Human endothelial cells
In order to confirm that human retinal endothelial cells express similar patterns of MMP-2 activation as bovine cells, some of the key experiments were repeated in the endothelial cells obtained from human retina. Endothelial cells were prepared from human eyes by the methods described by Chen and associates (31). The cells were cultured in DMEM containing fetal bovine serum (10%), bovine pituitary endothelial growth factor (15μg/ml) and ITS (insulin/transferrin/selenium), Gluta Max and antibiotic/antimycotic (1% each). Cells from passages 3rd–5th were incubated in the DMEM incubation medium containing 1% fetal bovine serum, 9% Nu-serum, 0.5μg/ml endothelial growth factor, 0.5% ITS, and 1% each Gluta Max and antibiotic/antimycotic, with 5mM glucose or 20mM glucose for 4–5 days in the presence or absence of inhibitors of MMP.
Rats
Rats, male (200 to 220g) were made diabetic by streptozotocin (55mg/kg body weight). To allow slow weight gain while maintaining hyperglycemia (blood glucose levels of 20–25mM), small dose of insulin (1–2 IU, Humulin N, Eli Lilly, IN) was administered 3–5 times/week. After establishment of diabetes (3 days after streptozotocin administration; blood glucose >250mg/dl), a group of diabetic rats received powder diet (Purina 5001) either supplemented with α-lipoic acid (400mg/kg, diabetes+LA) or with micronutrients consisting mainly antioxidants (vitamin C, 50mg; vitamin E, 0.5g; β-carotene, 1.5mg; zinc oxide, 8mg and copper oxide, 0.2mg/kg BW; diabetes+AO). Age-matched normal rats served as controls. Each group had 12 or more rats, and the entire rat colony was housed in metabolic cages. The rats were weighed two times a week and their feeders weighed once every week to calculate the amount of food consumed. Glycated hemoglobin (GHb) was measured at two months of diabetes, and every three months thereafter. The rats were maintained in the experiment for 11–12 months; the retina was isolated by gently separating from choroid, and frozen immediately at −80°C for biochemical measurements, or processed immediately for microvessel isolation (please see below). Treatment of the animals conformed to the Association for Research in Vision and Ophthalmology Resolution on the Use of Animals in Research, and the Institutional guidelines. The severity of hyperglycemia, as reported earlier (26, 27), was similar in diabetes and diabetes+LA or diabetes+AO groups. Portions of the retina from some of these rats have been used by us in our previous studies (26, 27).
Retinal microvessels
Freshly isolated whole retina was incubated in distilled water for one hour at 37°C. This was followed by 5 minutes incubation with DNase (2mg/ml). The retinal vasculature was isolated under a microscope by repetitive inspiration and ejection through Pasteur pipettes. Retinal blood vessels isolated by this method show a normal complement of nuclei and were devoid of nonvascular materials (32).
MMP-gelatinase assay
MMP-2 activity was measured in the culture medium or tissue lysate by zymography. Equal amounts of protein (10–20μg) were electrophoresed under non-reducing conditions into 10% SDS-PAGE gels polymerized with 1mg/ml gelatin. The gels were washed with 2.5% Triton X-100 for 30 minutes with gentle shaking followed by a 30-minute wash with distilled water. The gels were then incubated overnight at 37°C in substrate buffer containing 50mM Tris-HCl, pH 8.0, 5mM CaCl2 and 0.02% NaN3, and stained with Coomassie blue (Simply Blue Safe Stain, Invitrogen, Carlsbad, CA). This was followed by de-staining with distilled water (33). Active MMP-2 (67kD) band was detected in the zymogram and quantified using Un-Scan-It Gel digitizing software.
Gene expressions
Gene expression was determined by semi-quantitative reverse transcription-Polymerase Chain Reaction (PCR) and by real time quantitative RTPCR. RNA extracted from retina or cells using TRizol reagent was converted to cDNA using the High capacity cDNA reverse transcription kit with RNase inhibitor. For semi-quantitative PCR, 0.5μg of cDNA template was added to 10pmol of forward and reverse primer and 1 unit of GoTaq DNA Polymerase (Promega, Madison, WI). The primers for the target and housekeeping genes are provided in Table I. Following the PCR amplification, the DNA was run on a 1.2% agarose gel at 80V. DNA ladder, 100bp, was simultaneously run on each gel. Primers for the target genes were designed using Applied Biosystems software Primer Express 3.0 and synthesized by Integrated DNA Technologies (Coralville, IA). The bands were visualized with the UVP Bio-Doc it Imaging System (UVP LLC., Upland, CA), and band intensities were quantified by UnScan-It software as reported earlier (8, 27).
TABLE 1.
Primers for the target genes and housekeeping gene used for semi-quantitative PCR
| Species | Gene | Primer sequence |
|---|---|---|
| Rat | MMP-2 | fwd 5′-GCA CCG TCG CCC ATC A-3′ rev 5′-GTC TCG ATG GTG TTC TGG TCA A-3′ |
| TIMP-2 | fwd 5″-CAG CCC TAC GAA CCC ACA GA-3′ rev 5′-GCT TGG GAA CCT TGA GAG TGA T-3′ |
|
| MT1-MMP | fwd 5′-ATT TCC CAG GCC CCA ATA TC-3′ rev 5′-GAA GTT CTC GGT GTC CAT CCA-3′ |
|
| Bovine | MMP-2 | fwd 5′-CTT CCC CCG CCA GCC CAA GTG GG-3′ rev 5′-GGT GAA CAG GGC TTC ATG GGG GC-3′ |
| TIMP-2 | fwd 5′-CCT CCT GCT GGG GAC GCT GC-3′ rev 5′-AGT CCT GGT GGC CTG ACG GG-3′ |
|
| MT1-MMP | fwd 5′-AAC ATC AAA GTC TGG GAA GG-3′ rev 5′-GTC TCC TCC TCA GTC CCC TC-3′ |
|
| Human | MMP-2 | fwd 5′-CTG CTG AAG GAC ACA CTA AAG AAG A-3′ rev 5′-TTG CCA TCC TTC TCA AAG TTG TGA G-3′ |
| TIMP-2 | fwd 5-AAA CGA CAT TTA TGG CAA CCC TAT C-3′ rev 5′-ACA GGA GCC GTC ACT TCT CTT GAT G-3′ |
To confirm the effect of diabetes on retinal MMP-2, mRNA levels of MMP-2 and its regulators were also quantified using quantitative real-time PCR by the methods routinely used in our laboratory (27, 33). Each sample was measured in triplicate β-microglobulin (B2M) was used as a housekeeping gene. Genebank accession numbers of Taqman PCR primers for MMP-2, TIMP-2 and MT1-MMP B2M are NM_031054.2, NM_021989.2, NM_031056.1 and NM_012512.1 respectively. The standard PCR conditions included 2 minutes at 50°C and 10 min at 95°C followed by 40 cycles of extension at 95°C for 15 seconds and one minute at 60°C. Threshold lines were automatically adjusted to intersect amplification lines in the linear portion of the amplification curves and cycle to threshold (Ct) were recorded automatically. Data were normalized with B2M mRNA (housekeeping gene) and the fold change in gene expression relative to normal was calculated using the ddCt method.
Protein expression
Expression of MT1-MMP was determined by western blot technique using 25–30μg of retinal proteins separated by SDS-PAGE on a 10% gel. After blotting the proteins onto nitrocellulose membrane and blocking them in 5% nonfat milk, the membranes were incubated with monoclonal antibody against anti MT1-MMP (Calbiochem, Gibbstown, NJ), rinsed, and incubated with anti-mouse horseradish peroxidase-coupled secondary antibody for one hour. The target protein was enhanced by ECL reagent (Santa Cruz Biotechnology, Santa Cruz, CA), and determined by autoradiography. β-actin (Sigma-Aldrich, St Louis, MO) was used as the house-keeping protein (Sigma-Aldrich, St Louis, MO). The band intensity was quantified using Un-Scan-It Gel digitizing software (Silk Scientific Inc, Orem, UT).
Superoxide levels
Mitochondria were isolated from the cells by centrifugation and the levels of superoxide were measured by lucigenin method as reported earlier (4, 8).
Apoptosis
Cell death was determined by Cell Death Detection ELISAPLUS (Roche Diagnostics, Indianapolis, IN) by quantifying cytoplasmic histone-associated DNA fragments formed by internucleosomal degradation of genomic DNA during apoptosis. The cytoplasmic fraction of the cells was transferred onto a streptavidin-coated microtiter plate and incubated for two hours at room temperature with a mixture of peroxidase-conjugated anti-DNA and biotin-labeled anti-histone. The plate was washed, incubated with the ABTS (Roche Diagnostics), and absorbance was measured at 405 nm (4, 28).
Statistical analysis
The results are presented as mean ± SD, and analyzed statistically using the nonparametric Kruskal-Wallis test followed by Mann-Whitney test for multiple group comparisons. Similar conclusions were achieved by using ANOVA with Fisher or Tukey.
Results
High glucose activates MMP-2 and its regulators in bovine retinal endothelial cells
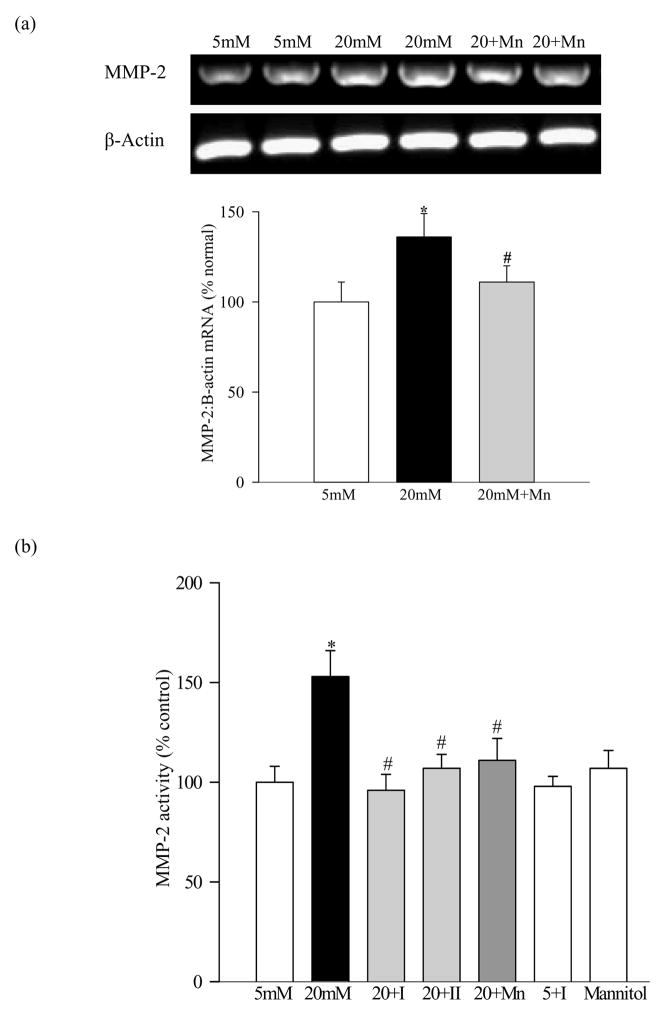
Incubation of endothelial cells in high glucose for five days increased MMP-2 gene expression by 30–35% (Figure 1a) and the gelatinase activity by about 55% (Figure 1b) relative to the cells incubated in 5mM glucose. Furthermore, high glucose altered the major physiologic inhibitor and proenzyme activator of MMP-2; 40% decrease in TIMP-2 (Figure 1c) and 25% elevation in MT1-MMP were observed in the same cell preparations (Figure 1d). Incubation of retinal endothelial cells with 20mm mannitol did not have any effect on the activation of MMP-2 (Figure 1b), suggesting that the increase in MMP-2 or its regulators in high glucose conditions is not due the increased osmolarity, but is the result of elevated glucose itself.
Figure 1.
Effect of high glucose on MMP-2 and its regulators in retinal endothelial cells: Bovine retina endothelial cells from 4–7th passage were incubated in 5mM glucose or 20mM glucose medium for 4–5 days in the presence or absence of 10μM MMP-2 inhibitor I or 5μM MMP-2 inhibitor II, or 200μM MnTBAP. At the end of incubation medium was collected, and the cells were washed with phosphate buffer saline and collected. (a) Gene expression of MMP-2 and that of β-actin in the cells was determined by semi-quantitative PCR using the primers given in table I. (b) The gelatinase activity of MMP-2 was determined in the medium. (c) and (d) The levels of mRNA of TIMP-2 and MT1-MMP were determined by semi-quantitative PCR respectively, and were adjusted to the mRNA levels of β-actin in each sample. Each measurement was made in duplicate in at least three different cell preparations. The values obtained from the cells incubated in 5mM glucose are considered as 100% (control). *P<0.05 compared to the values obtained from the cells incubated in 5mM glucose, and #P<0.05 compared to 20mM glucose. 20+I= 20mM glucose+10μM MMP-2 inhibitor I; 20+II=20mM glucose+5μM MMP-2 inhibitor II; 20+Mn=20mM glucose+200μM MnTBAP; 5+I=5mM glucose+10μM MMP-2 inhibitor I
Glucose-induced alterations in MMP-2 and its regulators are prevented by inhibitors of MMP-2 activation and mitochondrial superoxide
Two distinct inhibitors of MMP-2, MMP-2 inhibitor I and II, attenuated glucose-induced increases in the gelatinolytic activity of MMP-2 (Figure 1b) and mRNA levels of MMP-2 and MT1-MMP (Figures 1a and c), and in retinal endothelial cells. These MMP-2 inhibitors also prevented glucose-induced alterations in TIMP-2 and MT1-MMP gene expressions (Figure 1c and d).
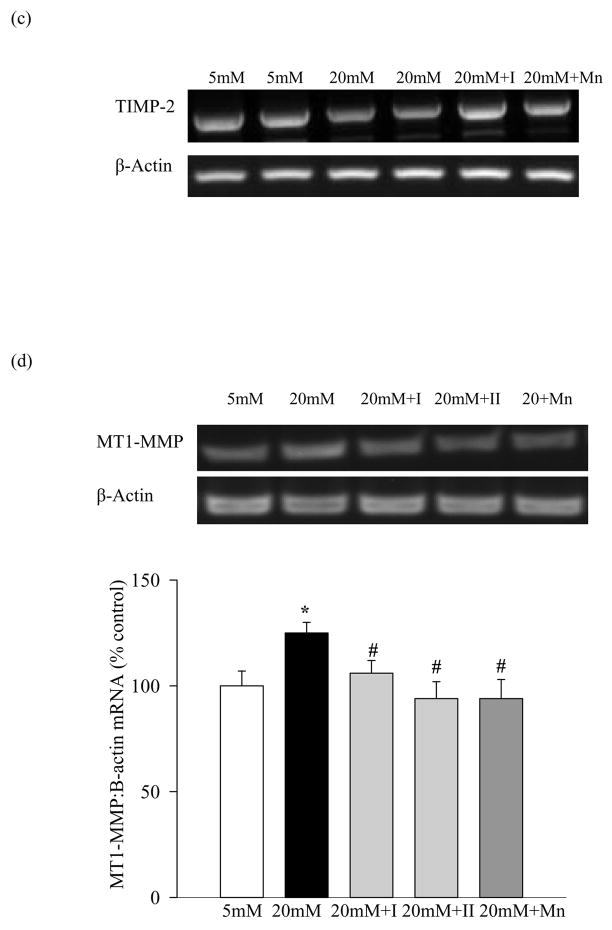
Addition of MnSOD mimic, MnTBAP, which we have shown to prevent glucose-induced mitochondrial dysfunction (4), and increased superoxide levels (Figure 2a) ameliorated elevation in MMP-2 and MT1-MMP experienced by retinal endothelial cells in high glucose conditions, and also prevented reduction in TIMP-2 expression (Figure 1a–1d). However, MMP-2 inhibitor did not produce any inhibitory effect on MMP-2 when the cells were incubated in 5mM glucose (Figure 1b).
Figure 2.
Effect of inhibition of MMP-2 and MnTBAP on the apoptosis of retinal endothelial cells: (a) Apoptosis was measured by performing ELISA for cytoplasmic histone-associated-DNA-fragments using an assay kit from Roche Diagnostics. The graph shows the values obtained from the cells incubated with glucose for five days, and these values were adjusted to the total DNA. (b) Superoxide levels were measured in the mitochondria by lucigenin method. The values obtained from the cells incubated in 5mM glucose are considered as 100%. Each measurement was made in duplicate in at least four different cell preparations. *P<0.05 compared to the values obtained from the cells incubated in 5mM glucose, and #P<0.05 compared to 20mM glucose.
Inhibition of high glucose mediated increase in MMP-2 protects retinal endothelial cells from increased apoptosis
Apoptosis was increased by 75% in the bovine retinal endothelial cells incubated in 20mM glucose for 5 days (Figure 2b), and this is consistent with our previous results. Inclusion of MMP-2 inhibitors (I or II) in high glucose medium prevented the cells from undergoing apoptosis. Percent of apoptotic cells in the presence of either of the two MMP-2 inhibitors was decreased by over 80% compared to the cells that were incubated in high glucose without any inhibitor.
Endothelial cells from human retina present similar effects of high glucose on MMP-2 and TIMP-2 as bovine retina
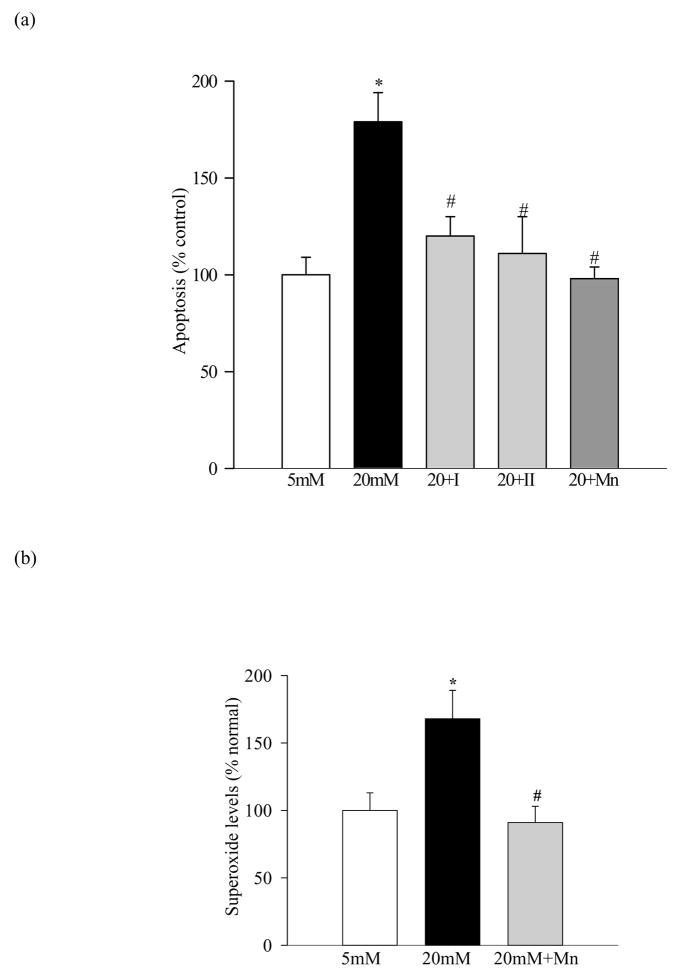
As observed with bovine retinal endothelial cells, endothelial cells prepared from human retina also showed a significant (40%) increase in MMP-2 activity in high glucose conditions, and inhibitor I of MMP-2 prevented such activation (Figure 3a). The gene expression of MMP-2 was elevated by 30% (Figure 3b) and that of TIPM-2 was decreased by 25% (Figure 3c) compared to the cells incubated in 5mM glucose conditions. These results suggest that the effect of high glucose on MMP-2 is not restricted to bovine retina, and human retina capillaries also present increased MMP-2.
Figure 3.
Effect of high glucose on MMP-2 activity in human retinal endothelial cells: Human retinal endothelial cells from 3rd–5th passage were incubated in 5mM glucose or 20mM glucose medium for 5 days in the presence or absence of 10μM MMP-2 inhibitor I. (a) The gelatinase activity was measured in the medium and the gene expression of (b) MMP-2 and (c) TIMP-2 in the RNA isolated from the cells using TRizol reagent by semi-quantitative PCR. The values obtained from the cells incubated in 5mM glucose are considered as 100% (control). The results are presented as mean ±SD of the values obtained from three different cell preparations, with each measurement made in duplicate. *P<0.05 compared to the values obtained from the cells incubated in 5mM glucose, and #P<0.05 compared to 20mM glucose.
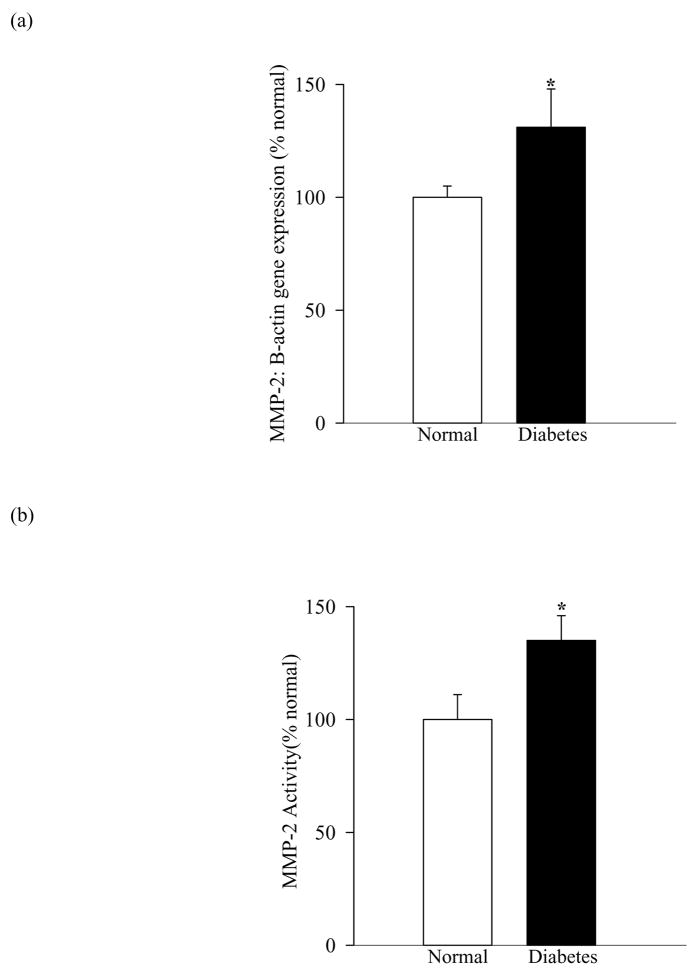
Diabetes activates MMP-2 in the retina and its microvessels and antioxidants inhibit such activation
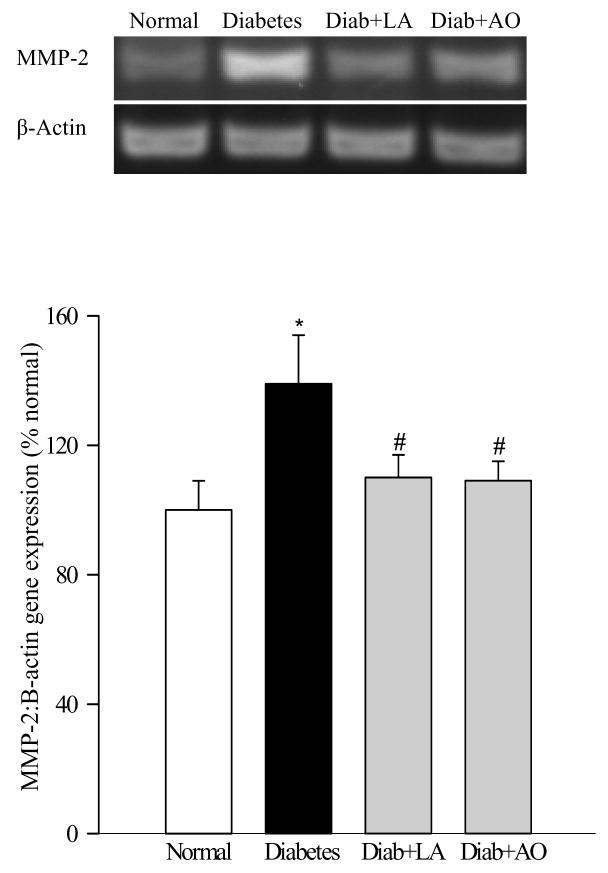
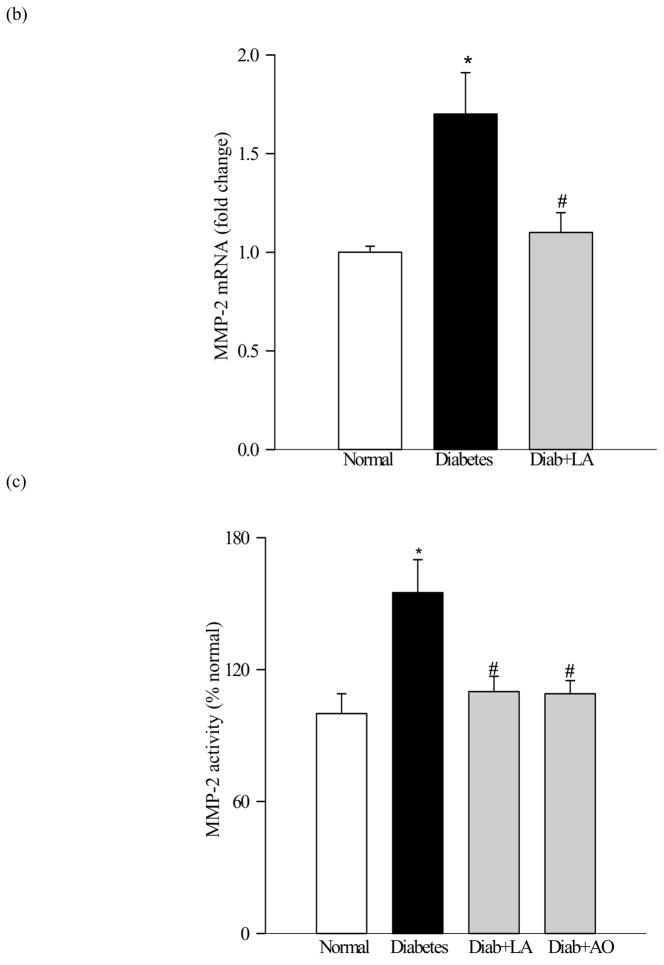
The gene expression of MMP-2 was increased by 35–70% in the retina from diabetic rats as quantified by both semi-quantitative and quantitative PCR (Figures 4a and b). In the same animals the gelatinolytic activity of MMP-2 was increased by about 50% (Figure 4c) compared to the retina from age-matched normal rats. Similar increases in the gene expression and gelatinase activity were observed in the microvessel preparations isolated from diabetic rat retina (Figures 5a and b). Administration of α-lipoic acid or micronutrients immediately after induction of diabetes in rats, which protect the retina from oxidative damage (26, 27), also attenuated diabetes-induced increases in both the gene expression and gelatotinlytic activity of MMP-2 (Figures 4a–c).
Figure 4.
Effect of long-term diabetes on retinal MMP-2: Retina from rats diabetic for 12 months receiving α-lipoic acid (Diab+LA) or antioxidants (Diab+AO), and age-matched normal rats was used to measure (a) gene expression of MMP-2 was determined by RTPCR using the primers given in table I. MMP-2 mRNA levels in each sample were normalized to β-actin levels, and (b) was quantified by real time PCR method using Taqman PCR primers. Fold-change relative to normal age-matched controls was calculated using the ddCt method. (c) Gelatinase activity by zymography. 10–20μg retinal protein was electrophoresed under non reducing conditions, incubated in substrate buffer and stained with Coomassie blue. Active MMP-2 (67kD) band was detected in the zymogram and quantified using Un-Scan-It Gel digitizing software. Retina from five to seven rats in each group was analyzed. *P<0.05 compared to the values obtained from the retina of age-matched normal rats, and #P<0.05 compared to retina of diabetic rats without any supplementation.
Figure 5.
Activation of MMP-2 in retinal microvessels in diabetes: (a) Gene expression of MMP-2 was determined by semi-quantitative PCR using the primers described in table I, and (b) its gelatinase activity by zymography in the microvessels isolated by osmotic shock method from diabetic rats. *P<0.05 compared to the values obtained from the retinal microvessels of age-matched normal rats
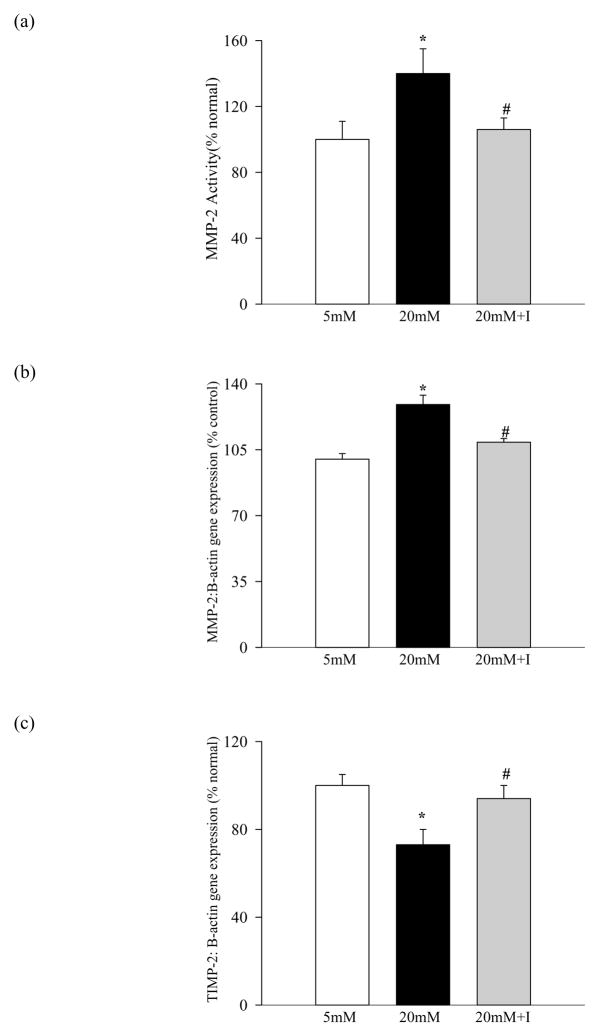
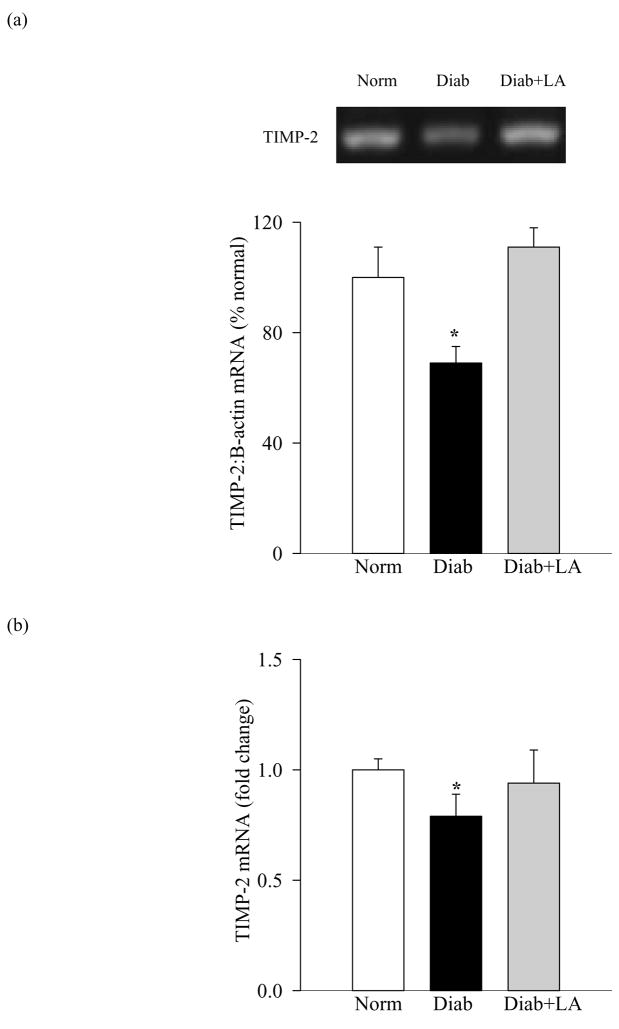
Modulators of MMP-2 are altered in the retina in diabetes, and antioxidant supplementation prevents such alterations
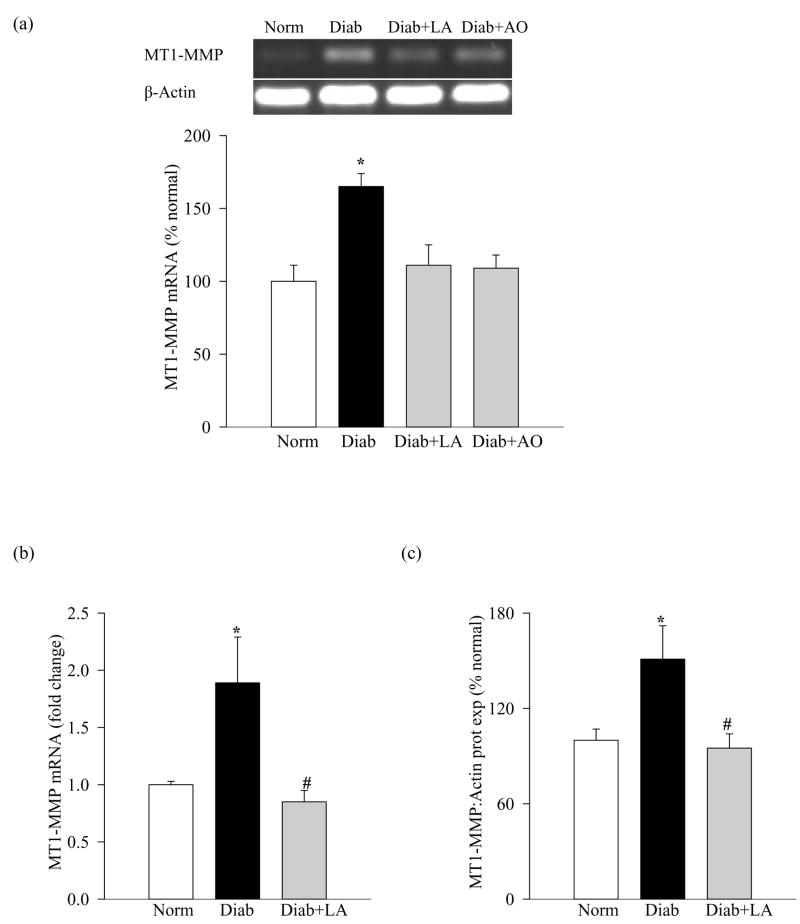
Significant decreases in the gene expression of retinal TIMP-2 (25–30%), as shown in Figure 6a (semi-quantitative PCR) and Figure 6b (quantitative PCR) was observed in diabetic rats compared to the age-matched normal rats. This decrease in TIMP-2 was accompanied by a concomitant increase in the mRNA and protein expression of MT1-MMP compared to the values obtained from normal rat retina (Figures 7a-c), suggesting that activation of retinal MMP-2 is via decrease in its physiological inhibitor and increase in the proenzyme activator.
Figure 6.
Effect of diabetes on retinal TIMP-2: TIMP-2 was measured in the same tissue samples as given in figure 4. Gene expression of TIMP-2 was measured by both semi-quantitative (a) and quantitative (b) PCR methods.
Figure 7.
Activation of MT1-MMP in retina in diabetes: MT1-MMP gene expression was measured in the same retina samples as used in figures 4 and 6 by (a) semi-quantitative PCR, and the histograms represent mRNA levels (mean ±SD) of the MT1-MMP adjusted to that of β-actin in the same sample from six or more rats in each group and quantitative (b) and by quantitative PCR methods (c) Protein expression of MT1-MMP was determined by western blot using mouse monoclonal antibody against MT1-MMP. The histograms represent protein expression of MT1-MMP adjusted to the expression of β-actin in the same sample. *P<0.05 compared to the values obtained from the retina of age-matched normal rats, and #P<0.05 compared to retina of diabetic rats without any supplementation.
Administration of α-lipoic acid or micronutrients prevented diabetes-induced alteration in retinal TIMP-2 and MT1-MMP; the levels of TIMP-2 and MT1-MMP were significantly altered (higher or lower respectively) in diabetic rats receiving antioxidants compared to the diabetic rats without any supplementation (p<0.05; Figures 4, 6 and 7).
Discussion
This is the first report showing a novel role of MMP-2 in the pathogenesis of diabetic retinopathy. The data show that MMP-2 has a pro-apoptotic role in the retinal capillary cells, the cells that show histopathology of diabetic retinopathy, and MMP-2 and its membrane bound proenzyme activator are activated and the physiological activator is down regulated in retinal endothelial cells in high glucose conditions. Regulation of mitochondrial SOD, which we have shown to decrease glucose-induced oxidative stress, mitochondrial dysfunction and apoptosis of capillary cells (4, 9), regulates these alterations in MMP-2, TIMP-2 and MT-1-MMP. Further, we show that inhibition of MMP-2 by its selective inhibitor protects endothelial cells from glucose-induced accelerated apoptosis, a phenomenon that can predict the development of pathology of diabetic retinopathy (20). Results from our in vivo model of diabetic retinopathy show that MMP-2 and MT1-MMP are activated and TIMP-2 is downregulated in the retina at duration of diabetes when retinopathy can be observed in rats, and the therapies that inhibit diabetic retinopathy (26, 27) also inhibit activation of MMP-2 and MT1-MMP. Thus, our in vitro and in vivo data convincingly suggest a critical role of MMP-2 in the pathogenesis of retinopathy in diabetes, and provide a possibility that MMP-2 activation inhibitors could potentially retard the development of diabetic retinopathy.
Increased expression of MMP-2 is reported in retinal endothelial cells incubated in the presence of advanced glycation end products (22), and in retinal pericytes incubated in high glucose or in heavily oxidized and glycated LDL (21, 34). Further, MMPs are activated in the retina, vitreous and fibrovascular tissues in patients with diabetic retinopathy, and activated proMMP-2 is observed also in the neovascular retinal membranes of patients with proliferative diabetic retinopathy (14–20, 34–37). In patients with proliferative diabetic retinopathy activation of proMMP-2 in the fibrovascular tissues is postulated to be via its interaction with MT1-MMP and TIMP-2 (36). Our exciting results show that MMP-2 is activated in the retina and its microvessels at duration of diabetes (10–12 months) when capillary cell apoptosis and histopathology characteristic of diabetic retinopathy can be observed in rats. This implies that MMP-2 is important in the development of diabetic retinopathy, a disease that is largely microvascular (38).
Oxidative stress has been shown to regulate MMP-2 and MT1-MMP; ROS and peroxynitrite activate MT-1-MMP in cultured human coronary smooth muscle cells (39). Sustained exposure of hydrogen peroxide to human endothelial cells increases pro-MMP-2 activation through the induction of MT1-MMP expression (40). A strong relation between oxidative stress and regulation of MMP-2 and MT1-MMP is observed in various other pathological conditions. Low concentrations of ROS are shown to activate pro-MMPs by oxidation of the sulfide bond in the pro-domain of the MMP (13), and also activate MT1-MMP (41). Here, data are provided to show that the therapies that inhibit oxidative stress and peroxynitrite in the retina of diabetic rats (26, 27) also inhibit MMP-2 and MT1-MMP up-regulation and TIMP-2 reduction, suggesting that in diabetes retinal MMP-2 and its regulators are modulated by oxidative stress, and the use of antioxidant mixtures confirms in ‘principle’ the regulation of retinal MMP-2 by oxidative stress. However, how oxidative stress regulates retinal MMP-2 in diabetes is not clear, but similar beneficial effect by lipoic acid, a powerful free radical scavenger that serves as a coenzyme involved in mitochondrial metabolism (42), suggests that MMP-2 regulation in diabetes could include its protection by preventing mitochondrial damage that the retina experiences in diabetes. Furthermore, glucose increases oxidative stress and mitochondrial damage in retinal endothelial cells, and inhibition of mitochondrial superoxide accumulation that inhibits their accelerated apoptosis (4, 43, 44) also inhibits activation of MMP-2 and MT1-MMP. In contrast, a recently published study has suggested that in human retinal endothelial cells oxidative stress is increased by cytokines, but not by glucose (45). The reasons for such discrepancies are not clear, but may include differences in cell preparation and other experimental conditions.
MMPs, the main enzymes that hydrolyze most of the components of the extracellular matrix, are precisely regulated by their main endogenous protein inhibitors, TIMPs (10, 46). TIMP-2 is shown to facilitate the activation of MMP-2 via MT1-MMP and MT1-MMP acts as a receptor for TIMP-2, and forms a tri-molecular complex with MMP-2 and TIMP-2 (47, 48). Dysregulation of MMPs is postulated to contribute to the development of diabetic complications, including diabetic coronary and peripheral arterial disease. Urinary excretion of MMP-2 in diabetic patients is shown to predict intra-renal pathologic processes that are characteristic of nephropathy (49). Reduced level of MMP-2 in the myocardium of diabetic rats is postulated to contribute to collagen degradation and matrix deposition in diabetic myocardiopathy (50). MMP-2 and MMP-9 are also associated with increased angiostatin expression in the human diabetic arterial vasculature, resulting in the pathogenesis of impaired angiogenesis (51). Upregulation of MMPs in the retina is shown to play a role in the proteolytic breakdown of the tight junction protein and vascular permeability (19) and in blood retinal barrier breakdown (22). However, in the pathogenesis of diabetic retinopathy, manifestation of TUNEL-positive retinal capillary cells precedes the appearance of acellular capillaries (23, 24), and our previous studies have demonstrated that inhibition of retinal capillary cell apoptosis predicts the inhibition of the development of the late stages of the retinopathy in diabetic rodents (46). We show that the inhibition of MMP-2 in retinal endothelial cells also attenuates apoptosis of these cells. This suggests that dysregulation of MMP-2 in diabetes is, in part, contributing to the accelerated apoptosis of retinal capillary cells. In concomitant with this in vitro data, our observations from diabetic rats show that administration of α-lipoic acid, which prevents retinal oxidative stress and accelerated apoptosis of capillary cells (27), also diminishes MMP-2 dysregulation. Similar diabetes-induced activation is also observed in the retinal microvasculature, the site of histopathology of diabetic retinopathy, and the data from isolated capillary cells further strengthens the role of MMP-2 in the apoptosis of capillary cells. In support, inhibition of MMP-2 is shown by others to prevent loss of retinal pericytes in diabetic conditions (21, 52). How activation MMP-2 results in the apoptosis of retinal capillary cells is not clear, but could include damage to the mitochondria resulting in leakage of cytochrome c and activation of apoptosis. Others have shown that the activation of MMP-2 cleaves the nuclear poly ADP ribose polymerase (PARP), which induces apoptosis via mitochondrial pathway releasing apoptosis-inducing factor from the mitochondria (53, 54). In the development of diabetic retinopathy, mitochondrial dysfunction and PARP activation are considered important in the accelerated apoptosis of retinal capillary cells (4, 8, 9, 55), thus, further supporting the role of MMP-2 in the apoptosis of capillary cells.
We should acknowledge that although MMP-9, another member of the gelatinase family, is also shown to be upregulated in the retina in diabetes, and MMP-9 is associated with blood retinal barrier breakdown (17–19), the focus of our study, however, was on MMP-2. The role of other MMPs in the development of diabetic retinopathy is beyond the scope of this study, and cannot be ruled out.
In conclusion, the results presented here suggest that MMP-2 is regulated by increased oxidative stress in diabetes, and MMP-2 has a novel pro-apoptotic role in the pathogenesis of diabetic retinopathy. Attenuation of retinal capillary cell apoptosis, a phenomenon that predicts the pathology associated with diabetic retinopathy, by MMP-2 inhibitors, and inhibition of retinal MMP-2 activation in diabetic rats by antioxidant therapies that inhibit capillary cell apoptosis, strongly implicates another novel pro-apoptotic role for MMP-2 in the pathogenesis of diabetic retinopathy. Understanding the role of MMP-2 in the pathogenesis of diabetic retinopathy should help lay ground for MMP-2 targeted therapy to retard the development/progression of retinopathy, the sight threatening complication that is feared by diabetic patients.
Acknowledgments
Authors thank Dr. Bindu Menon for technical help and Dr. Ram Nagaraj (Case Western Reserve University, OH) for providing human retinal endothelial cells. This study was supported in part by grants from the National Institutes of Health, the Juvenile Diabetes Research Foundation, the Thomas Foundation, and Research to Prevent Blindness.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Baynes JW. Role of oxidative stress in development of complications in diabetes. Diabetes. 1991;40:405–412. doi: 10.2337/diab.40.4.405. [DOI] [PubMed] [Google Scholar]
- 2.Kowluru RA, Kern TS, Engerman RL. Abnormalities of retinal metabolism in diabetes or experimental galactosemia. IV. Antioxidant defense system. Free Rad Biol Med. 1991;22:587–592. doi: 10.1016/s0891-5849(96)00347-4. [DOI] [PubMed] [Google Scholar]
- 3.Kowluru RA, Tang J, Kern TS. Abnormalities of retinal metabolism in diabetes and experimental galactosemia. VII. Effect of long-term administration of antioxidants on the development of retinopathy. Diabetes. 2001;50:1938–1942. doi: 10.2337/diabetes.50.8.1938. [DOI] [PubMed] [Google Scholar]
- 4.Kowluru RA, Abbas SN. Diabetes-induced mitochondrial dysfunction in the retina. Inves Ophthal Vis Sci. 2003;44:5327–5334. doi: 10.1167/iovs.03-0353. [DOI] [PubMed] [Google Scholar]
- 5.Feldmen EL. Oxidative stress and diabetic neuropathy: a new understanding of an old problem. J Clin Inves. 2003;111:431–433. doi: 10.1172/JCI17862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brownlee M. The pathobiology of diabetic complications: a unifying mechanism. Diabetes. 2005;54:1615–1625. doi: 10.2337/diabetes.54.6.1615. [DOI] [PubMed] [Google Scholar]
- 7.Caldwell RB, Bartoli M, Behzadian MA, El-Remessy AE, Al-Shabrawey M, Platt DH, Liou GI, Caldwell RW. Vascular endothelial growth factor and diabetic retinopathy: role of oxidative stress. Curr Drug Targets. 2005;6:511–524. doi: 10.2174/1389450054021981. [DOI] [PubMed] [Google Scholar]
- 8.Kanwar M, Chan PS, Kern TS, Kowluru RA. Oxidative damage in the retinal mitochondria of diabetic mice: possible protection by superoxide dismutase. Inves Ophthal Vis Sci. 2007;48:3805–3811. doi: 10.1167/iovs.06-1280. [DOI] [PubMed] [Google Scholar]
- 9.Kowluru RA, Kowluru V, Ho YS, Xiong Y. Overexpression of mitochondrial superoxide dismutase in mice protects the retina from diabetes-induced oxidative stress. Free Rad Biol Med Sci. 2006;41:1191–1196. doi: 10.1016/j.freeradbiomed.2006.01.012. [DOI] [PubMed] [Google Scholar]
- 10.Malemud CJ. Matrix metalloproteinases (MMPs) in health and disease: an overview. Front Biosci. 2006;11:1696–1701. doi: 10.2741/1915. [DOI] [PubMed] [Google Scholar]
- 11.Itoh Y, Seiki M. CMT1-MMP: a potent modifier of pericellular microenvironment. J Cell Physiol. 2006;206:1–8. doi: 10.1002/jcp.20431. [DOI] [PubMed] [Google Scholar]
- 12.Liotta LA. Probing the depths of degradation: matrix metalloproteinase-2 and endometrial menstrual breakdown. J Clin Invest. 1996;97:273–274. doi: 10.1172/JCI118412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pfeilschifter J, Eberhardt W, Huwiler A. Nitric oxide and mechanisms of redox signalling: matrix and matrix-metabolizing enzymes as prime nitric oxide targets. Eur J Pharmacol. 2001;429:279–286. doi: 10.1016/s0014-2999(01)01326-7. [DOI] [PubMed] [Google Scholar]
- 14.Cox MJ, Sood HS, Hunt MJ, Chandler D, Henegar JR, Aru GM, Tyagi SC. Apoptosis in the left ventricle of chronic volume overload causes endocardial endothelial dysfunction in rats. Am J Physiol Heart Circ Physiol. 2002;282:H1197–H205. doi: 10.1152/ajpheart.00483.2001. [DOI] [PubMed] [Google Scholar]
- 15.Ho FM, Liu SH, Lin WW, Liau CS. Opposite effects of high glucose on MMP-2 and TIMP-2 in human endothelial cells. J Cell Biochem. 2007;101:442–450. doi: 10.1002/jcb.21192. [DOI] [PubMed] [Google Scholar]
- 16.Schulz R. Intracellular targets of matrix metalloproteinase-2 in cardiac disease: Rationale and therapeutic approaches. Ann Rev Pharmac Toxico. 2007;47:211–242. doi: 10.1146/annurev.pharmtox.47.120505.105230. [DOI] [PubMed] [Google Scholar]
- 17.Das A, McGuire PG, Eriqat C, Ober R, DeJuan E, Jr, Williams GA, McLamore A, Biswas J, Johnson DW. Human diabetic neovascular membranes contain high levels of urokinase and metalloproteinase enzymes. Invest Ophthal Vis Sci. 1999;40:809–813. [PubMed] [Google Scholar]
- 18.Jin M, Kashiwagi K, Iizuka Y, Tanaka Y, Imai M, Tsukahara S. Matrix metalloproteinases in human diabetic and nondiabetic vitreous. Retina. 2001;21:28–33. doi: 10.1097/00006982-200102000-00005. [DOI] [PubMed] [Google Scholar]
- 19.Giebel SJ, Menicucci G, McGuire PG, Das A. Matrix metalloproteinases in early diabetic retinopathy and their role in alteration of the blood-retinal barrier. Lab Invest. 2005;85:567–607. doi: 10.1038/labinvest.3700251. [DOI] [PubMed] [Google Scholar]
- 20.Shiau MY, Tsai ST, Tsai KJ, Haung ML, Hsu YT, Chang YH. Increased circulatory MMP-2 and MMP-9 levels and activities in patients with type 1 diabetes mellitus. Mt Sinai J Med. 2006;73:1024–1028. [PubMed] [Google Scholar]
- 21.Yang R, Liu H, Williams I, Chaqour B. Matrix metalloproteinase-2 expression and apoptogenic activity in retinal pericytes: implications in diabetic retinopathy. Ann N Y Acad Sci. 2007;1103:196–201. doi: 10.1196/annals.1394.000. [DOI] [PubMed] [Google Scholar]
- 22.Navaratna D, McGuire PG, Menicucci G, Das A. Proteolytic degradation of VE-cadherin alters the blood-retinal barrier in diabetes. Diabetes. 2007;56:2380–2387. doi: 10.2337/db06-1694. [DOI] [PubMed] [Google Scholar]
- 23.Mizutani M, Kern TS, Lorenzi M. Accelerated death of retinal microvascular cells in human and experimental diabetic retinopathy. J Clin Invest. 1996;97:2883–2890. doi: 10.1172/JCI118746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kern TS, Tang J, Mizutani M, Kowluru RA, Nagaraj RH, Romeo G, Podesta F, Lorenzi M. Response of capillary cell death to aminoguanidine predicts the development of retinopathy: comparison of diabetes and galactosemia. Invest Ophtha Vis Sci. 2000;41:3972–3978. [PubMed] [Google Scholar]
- 25.Gardner TW, Antonetti DA, Barber AJ, LaNoue KF, Levison SW. Diabetic retinopathy: more than meets the eye. Surv Ophthalmol. 2002;47:S253–S262. doi: 10.1016/s0039-6257(02)00387-9. [DOI] [PubMed] [Google Scholar]
- 26.Kowluru RA, Odenbach S. Effect of long-term administration of alpha lipoic acid on retinal capillary cell death and the development of retinopathy in diabetic rats. Diabetes. 2004;53:3233–3238. doi: 10.2337/diabetes.53.12.3233. [DOI] [PubMed] [Google Scholar]
- 27.Kowluru RA, Kanwar M, Chan PS, Zhang JP. AREDS-based micronutrients inhibit retinopathy and retinal metabolic abnormalities in diabetic rats. Arch Ophthalmol. 2008;126:1266–1272. doi: 10.1001/archopht.126.9.1266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kowluru RA, Kowluru A, Kanwar M. Small molecular weight G-protein, H-Ras, and retinal endothelial cell apoptosis in diabetes. Mol Cell Biochem. 2007;296:69–76. doi: 10.1007/s11010-006-9299-z. [DOI] [PubMed] [Google Scholar]
- 29.Zheng F, Cornacchia F, Schulman I, Banerjee A, Cheng QL, Potier M, Plati AR, Berho M, Elliot SJ, Li J, Fornoni A, Zang YJ, Zisman A, Striker LJ, Striker GE. Development of albuminuria and glomerular lesions in normoglycemic B6 recipients of db/db mice bone marrow: the role of mesangial cell progenitors. Diabetes. 2004;53:2420–2427. doi: 10.2337/diabetes.53.9.2420. [DOI] [PubMed] [Google Scholar]
- 30.Ikejiri M, Bernardo MM, Bonfil RD, Toth M, Chang M, Fridman R, Mobashery S. Potent mechanism-based inhibitors for matrix metalloproteinases. J Biol Chem. 2005;280:33992–34002. doi: 10.1074/jbc.M504303200. [DOI] [PubMed] [Google Scholar]
- 31.Chen W, Jump DB, Grant MB, Esselman WJ, Busik J. Dyslipidemia, but not hyperglycemia, induces inflammatory adhesion molecules in human retinal vascular endothelial cells. Invest Ophthal Vis Sci. 2003;44:5016–22. doi: 10.1167/iovs.03-0418. [DOI] [PubMed] [Google Scholar]
- 32.Kanwar M, Kowluru RA. Diabetes regulates small molecular weight G-protein, H-Ras in the microvasculature of the retina: implications in the development of retinopathy. Microvasc Res. 2008;76:189–193. doi: 10.1016/j.mvr.2008.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Romanic AM, White RF, Arleth AJ, Ohlstein EH, Barone FC. Matrix metalloproteinase expression increases after cerebral focal ischemia in rats: inhibition of matrix metalloproteinase-9 reduces infarct size. Stroke. 1998;29:1020–1030. doi: 10.1161/01.str.29.5.1020. [DOI] [PubMed] [Google Scholar]
- 34.Barth JL, Yu Y, Song W, Lu K, Dashti A, Huang Y, Argraves WS, Lyons TJ. Oxidised, glycated LDL selectively influences tissue inhibitor of metalloproteinase-3 gene expression and protein production in human retinal capillary pericytes. Diabetologia. 2007;50:2200–2208. doi: 10.1007/s00125-007-0768-z. [DOI] [PubMed] [Google Scholar]
- 35.Grant MB, Caballero S, Tarnuzzer RW, Bass KE, Ljubimov AV, Spoerri PE, Galardy RE. Matrix metalloproteinase expression in human retinal microvascular cells. Diabetes. 1998;47:1311–1317. doi: 10.2337/diab.47.8.1311. [DOI] [PubMed] [Google Scholar]
- 36.Noda K, Ishida S, Inoue M, Obata K, Oguchi Y, Okada Y, Ikeda E. Production and activation of matrix metalloproteinase-2 in proliferative diabetic retinopathy. Invest Ophthal Vis Sci. 2003;44:2163–2170. doi: 10.1167/iovs.02-0662. [DOI] [PubMed] [Google Scholar]
- 37.Noda K, Ishida S, Shinoda H, Koto T, Akoi T, Ouguchi Y, Okada Y, Ikeda E. Hypoxia induces the expression of membrane-type 1 matrix metalloproteinase in retinal glial cells. Invest Ophthal Vis Sci. 2005;46:3817–3824. doi: 10.1167/iovs.04-1528. [DOI] [PubMed] [Google Scholar]
- 38.Kohner EM. Diabetic retinopathy. Br Med Bull. 1989;45:148–173. doi: 10.1093/oxfordjournals.bmb.a072309. [DOI] [PubMed] [Google Scholar]
- 39.Valentin F, Bueb JL, Kieffer P, Tschirhart E, Atkinson J. Oxidative stress activates MMP-2 in cultured human coronary smooth muscle cells. Fundam Clin P harmacol. 2005;19:661–667. doi: 10.1111/j.1472-8206.2005.00371.x. [DOI] [PubMed] [Google Scholar]
- 40.Yoon SO, Park SJ, Yoon SY, Yun CH, Chung AS. Sustained production of H(2)O(2) activates pro-matrix metalloproteinase-2 through receptor tyrosine kinases/phosphatidylinositol 3-kinase/NF-kappa B pathway. J Biol Chem. 2002;277:30271–3082. doi: 10.1074/jbc.M202647200. [DOI] [PubMed] [Google Scholar]
- 41.Valentin F, Bueb JL, Kieffer P, Tschirhart E, Atkinson J. Oxidative stress activates MMP-2 in cultured human coronary smooth muscle cells. Fundam Clin Pharmacol. 2005;19:661–667. doi: 10.1111/j.1472-8206.2005.00371.x. [DOI] [PubMed] [Google Scholar]
- 42.Liu J. The effects and mechanisms of mitochondrial nutrient alpha-lipoic acid on improving age-associated mitochondrial and cognitive dysfunction: an overview. Neurochem Res. 2008;33:194–203. doi: 10.1007/s11064-007-9403-0. [DOI] [PubMed] [Google Scholar]
- 43.Al-Shabrawey M, Bartoli M, El-Remessy AB, Ma G, Matragoon S, Lemtalsi T, et al. Role of NADPH oxidase and Stat3 in statin-mediated protection against diabetic retinopathy. Invest Ophthal Vis Sci. 2008;49:3231–3238. doi: 10.1167/iovs.08-1754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Xie L, Zhu X, Hu Y, Li T, Gao Y, Shi Y, et al. Mitochondrial DNA oxidative damage triggering mitochondrial dysfunction and apoptosis in high glucose-induced HRECs. Invest Ophthal Vis Sci. 2008;49:4203–4209. doi: 10.1167/iovs.07-1364. [DOI] [PubMed] [Google Scholar]
- 45.Busik JV, Mohr S, Grant MB. Hyperglycemia-induced reactive oxygen species toxicity to endothelial cells is dependent on paracrine mediators. Diabetes. 2008;57:1952–1965. doi: 10.2337/db07-1520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Fu X, Parks WC, Heinecke JW. Activation and silencing of matrix metalloproteinases. Semin Cell Dev Biol. 2008;19:2–13. doi: 10.1016/j.semcdb.2007.06.005. [DOI] [PubMed] [Google Scholar]
- 47.Worley JR, Thompkins PB, Lee MH, Hutton M, Soloway P, Edwards DR, Murphy G, Knäuper V. Sequence motifs of tissue inhibitor of metalloproteinases 2 (TIMP-2) determining progelatinase A (proMMP-2) binding and activation by membrane-type metalloproteinase 1 (MT1-MMP) Biochem J. 2003;372:799–809. doi: 10.1042/BJ20021573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Osenkowski P, Toth M, Fridman R. Processing, shedding, and endocytosis of membrane type 1-matrix metalloproteinase (MT1-MMP) J Cell Physiol. 2004;200:2–10. doi: 10.1002/jcp.20064. [DOI] [PubMed] [Google Scholar]
- 49.Thrailkill KM, Bunn RC, Moreau CS, Cockrell GE, Simpson PM, Coleman HN, Frindik JP, Kemp SF, Fowlkes JL. Matrix metalloproteinase-2 dysregulation in type 1 diabetes. Diabetes Care. 2007;30:2321–2326. doi: 10.2337/dc07-0162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Li Q, Sun SJ, Wang Y, Tian YJ, Liu MH. The roles of MMP-2/TIMP-2 in extracellular matrix remodelling in the hearts of STZ-induced diabetic rats. Acta Cardiol. 2007;62:485–91. doi: 10.2143/AC.62.5.2023412. [DOI] [PubMed] [Google Scholar]
- 51.Chung AW, Hsiang YN, Matzke LA, McManus BM, van Breemen C, Okon EB. Reduced expression of vascular endothelial growth factor paralleled with the increased angiostatin expression resulting from the upregulated activities of matrix metalloproteinase-2 and -9 in human type 2 diabetic arterial vasculature. Circ Res. 2006;99:140–148. doi: 10.1161/01.RES.0000232352.90786.fa. [DOI] [PubMed] [Google Scholar]
- 52.Liu H, Yang R, Tinner B, Choudhry A, Schutze N, Chaqour B. Cysteine-rich protein 61 and connective tissue growth factor induce de-adhesion and anoikis of retinal pericytes. Endocrinology. 2008;149:1666–77. doi: 10.1210/en.2007-1415. [DOI] [PubMed] [Google Scholar]
- 53.Kwan JA, Schulze CJ, Wang W, Leon H, Sariahmetoglu M, Sung M, et al. Matrix metalloproteinase-2 (MMP-2) is present in the nucleus of cardiac myocytes and is capable of cleaving poly (ADP-ribose) polymerase (PARP) in vitro. FASEB J. 2004;18:690–692. doi: 10.1096/fj.02-1202fje. [DOI] [PubMed] [Google Scholar]
- 54.Dawson VL, Dawson TM. Deadly conversations: nuclear-mitochondrial cross-talk. J Bioenerg Biomembr. 2004;36:287–294. doi: 10.1023/B:JOBB.0000041755.22613.8d. [DOI] [PubMed] [Google Scholar]
- 55.PZheng L, Szabo C, Kern TS. Poly(ADP-ribose) polymerase is involved in the development of diabetic retinopathy via regulation of nuclear factor-kappaB. Diabetes. 2004;53:2960–67. doi: 10.2337/diabetes.53.11.2960. [DOI] [PubMed] [Google Scholar]