Abstract
Although theory suggests a link between social anxiety and social dominance, direct empirical evidence for this link is limited. The present experiment tested the hypothesis that socially anxious individuals, particularly men, would respond to a social-dominance threat by exhibiting decrements in their testosterone levels, an endocrinological change that typically reflects pronounced social submission in humans and other animals. Participants were randomly assigned to either win or lose a rigged face-to-face competition with a confederate. Although no zero-order relationship between social anxiety and level of testosterone was observed, testosterone levels showed a pronounced drop among socially anxious men who lost the competition. No significant changes were observed in nonanxious men or in women. This research provides novel insight into the nature and consequences of social anxiety, and also illustrates the utility of integrating social psychological theory with endocrinological approaches to psychological science.
Social anxiety plays a major role in regulating—and sometimes dysregulating—many forms of human social interaction. People high in social anxiety perceive a variety of everyday social situations to be threatening and often respond to social situations with exaggerated worry, distress, physiological hyperarousal, and avoidant behavior (Heimberg, Liebowitz, Hope, & Schneier, 1995; Hofmann, 2007; Liebowitz, Gorman, Fyer, & Klein, 1985).
What kinds of social threat cause worry in people with social anxiety? Most theories suggest that socially anxious individuals are concerned primarily with being evaluated negatively and, ultimately, with being socially excluded or rejected (Barlow, 2002; Baumeister & Tice, 1990; Leary, 1990). Indeed, highly anxious people are very vigilant to the possibility of negative evaluation, especially when social acceptance is perceived to be at stake (e.g., Maner, DeWall, Baumeister, & Schaller, 2007).
A much smaller body of theory, however, suggests that in addition to concerns about general social evaluation or social acceptance, social anxiety may involve concerns pertaining specifically to social dominance (Barkow, 1975; Öhman, 1986; cf. Leary, Cottrell, & Phillips, 2001). An evolutionary perspective, for example, suggests that social anxiety may reflect concerns about one's place in the social hierarchy and, moreover, that social anxiety may lead people to respond in maladaptive ways when their dominance is threatened. Yet few studies have tested this hypothesis empirically.
In this article, we provide evidence that social anxiety shapes responses to social-dominance threat. We hypothesized that people high in social anxiety would respond to dominance threat by displaying signs of exaggerated social submission. We tested this hypothesis by manipulating social-dominance threat and examining changes in levels of testosterone, a hormone known to mediate social dominance.
Dominance and Social Anxiety
The social structures of many species are organized hierarchically (de Waal, 1982), and navigating one's place in the dominance hierarchy is an important adaptive challenge faced by people in many social groups. Because there are myriad benefits to being dominant, individuals often compete over dominance, and these competitions can involve significant interpersonal conflict. Whether competition is explicit (e.g., a sporting event) or implicit (e.g., being compared with another person; e.g., Antony, Rowa, Liss, Swallow, & Swinson, 2005), defeat can pose salient psychological and interpersonal threats, in part because defeat can reduce one's level of dominance.
How might people respond to dominance threats when they occur? Imagine losing an important tennis match to a rival. One might imagine that some people would respond with renewed interest in reasserting their dominance, perhaps by attempting to compete again (e.g., Mehta & Josephs, 2006). One might also imagine, however, that some people instead would respond by adopting a submissive stance, presumably as a way of avoiding further social harm. Indeed, in responding to social defeat, there is an important trade-off. Although striving to reassert one's dominance could help one regain status, it could also increase one's vulnerability to harm and could further damage one's stature. Thus, when likelihood of success seems low, people may become submissive in order to avoid potential harm, especially harm from more dominant individuals (Öhman, 1986).
We suggest that the way individuals navigate this trade-off is influenced by their level of social anxiety. We hypothesize that socially anxious individuals will respond to dominance threat with signs of pronounced social submission, rather than with interest in reasserting their dominance. Socially anxious individuals typically respond to threat with pessimism and a pronounced lack of self-efficacy (e.g., Maddux, Norton, & Leary, 1988). Social anxiety, therefore, may lead people to become submissive after a defeat, because their expectations for future success are likely to be low. This would not be unlike a lion who, upon losing a dominance competition, rolls onto his back, communicating to the victor that he has no interest in further competition and would prefer to live to see another day.
The Role of Testosterone
Assessment of endocrine responses provides a particularly valuable means of evaluating the submissiveness that we hypothesize occurs among anxious individuals. It is well known that in many species, testosterone levels mediate the expression of dominance, at one end of the spectrum (high testosterone), and submission, at the other end (low testosterone; Mazur & Booth, 1998; Schultheiss et al., 2005).
Numerous rodent-based studies have demonstrated that deficits in testosterone are associated with anxious responses to threat, as indicated by behavioral freezing, fear-induced analgesia, increased startle response, and inhibited exploratory behavior (Edinger & Frye, 2005; Toufexis, Myers, & Davis, 2006). Although the link between testosterone and anxiety has been documented in the rodent literature, only a few studies have examined this link in humans. One study found that low basal testosterone was associated with anxiety in male adolescents (Granger et al., 2003), and another suggested that low prenatal testosterone in utero may be associated with anxiety in adulthood (de Bruin, Verheij, Wiegman, & Ferdinand, 2006). Although there is some suggestive evidence, however, the link between social anxiety and testosterone in humans has not been well established.
How might socially anxious individuals modulate their level of testosterone when faced with dominance threat? Previous studies indicate that when people's dominance is threatened by social defeat, some individuals respond with increases in testosterone levels, but others respond with decreases (e.g., Mehta & Josephs, 2006). Few studies have identified stable personality characteristics that determine whose testosterone increases and whose testosterone decreases following defeat. We propose that endocrinological responses to defeat may be shaped by social anxiety and, in particular, that highly socially anxious individuals may display decreases in testosterone following defeat.
Gender Differences
There have been few investigations of gender differences in social anxiety, although it has been shown that women experience social anxiety more frequently and more severely than men (Turk et al., 1998; see also Moscovitch, Hofmann, & Litz, 2005). Few studies have investigated the possibility that social anxiety is linked with different responses to social threat in men versus women.
There are clear reasons to suspect that the links among social anxiety, social-dominance threat, and decrements in testosterone are stronger in males than in females. Although levels of testosterone are associated with dominance in both men and women (e.g., Cashdan, 1995), men tend to be more concerned than women about their level of social dominance (Mazur & Booth, 1998). An evolutionary perspective implies that this gender difference is rooted ultimately in differences between men's and women's reproductive strategies (Wilson & Daly, 1992).
Because men are more concerned with their dominance than women are, we expected to observe greater reactivity to dominance threat among men than among women. Specifically, we expected dominance threat to promote decreases in testosterone among socially anxious men, but did not predict similar reactivity among women. Thus, our primary hypotheses were that (a) social-dominance threat would be associated with decreased levels of testosterone among individuals high in social anxiety (but not among those low in social anxiety), and that (b) this response would be specific to males.
Method
In the experiment reported here, participants competed with a confederate and were randomly assigned to either win (control condition) or lose (dominance-threat condition) by a wide margin. Changes in salivary testosterone were assessed.
Participants
Sixty-four undergraduate students participated in exchange for course credit. Because of computer malfunction, 5 participants' social-anxiety scores were not recorded. One participant had an extremely high social-anxiety score (4 standard deviations above the mean) and was excluded. Fifty-eight participants remained (35 women, 23 men; average age = 18.9 years). To prepare for the experiment, participants refrained from activities known to affect hormone levels: They did not eat food or drink caffeinated beverages or alcohol for 2 hr prior to testing, exercise for 12 hr prior to testing, or smoke for 6 hr prior to testing.
Materials and Procedure
To reduce diurnal variability in testosterone, we scheduled participants to arrive between noon and 4:30 p.m. Participants were told that the study was an investigation of leadership styles, personality, and hormones, and they were shown how to provide saliva samples by spitting into collection vials (approximately 4 ml per sample). After receiving these instructions, participants completed a well-validated measure of social anxiety—the Social Phobia Scale (SPS; Mattick & Clarke, 1998). The SPS includes 20 items assessing anxiety in social settings (e.g., “I get tense when I speak in front of other people”). Responses were recorded on 5-point scales (1 = not at all, 5 = extremely). Social-anxiety scores were generated by summing responses across items (α = .88).
After completing the SPS, participants provided a baseline saliva sample, after which they underwent the experimental manipulation, which involved a face-to-face competition with a same-gender confederate. The competition consisted of a rigged number-tracking task used in previous studies (e.g., Josephs, Sellers, Newman, & Mehta, 2006). In this task, participants traced through a set of numbers in sequential order until a designated target number was reached. They were told that fast completion of this task reflected overall competence and leadership ability, and that winners would serve as group leaders in a subsequent team task, whereas losers would serve a subordinate role. In reality, the competition was rigged, and participants were randomly assigned to either win or lose.
Following this manipulation, participants completed filler questionnaires, and then provided a saliva sample, approximately 15 min after the manipulation. The time delay was included because typically it takes approximately 15 min before changes in testosterone concentration are detectable in saliva (e.g., Schultheiss et al., 2005).
Testosterone Measurement
We used a conventional approach for assaying salivary hormones. Saliva samples were frozen at −20 °C. To precipitate mucins, we thawed the samples and centrifuged them at 3,000 rpm for 10 min. The supernatant was stored in 250-μl aliquots at −20 °C until assayed. Commercially available solid-phase radioimmunoassay kits were used to measure concentrations of testosterone (in nanograms per deciliter). These kits have minimal cross-reactivity to other steroid hormones. All samples were processed in duplicate using a high-throughput, automated gamma counter. The lower limit of sensitivity of the radioimmunoassay kits was 0.2 ng/dL.
Results
Levels of social anxiety were typical for a normative university sample (M = 31.67, SD = 8.53), as were basal testosterone levels (M = 8.31 ng/dL, SD = 3.45 for men; M = 1.58 ng/dL, SD = 1.04 for women). No significant zero-order correlation was observed between social anxiety and basal testosterone in men (r = .12, p = .60) or women (r = −.23, p = .19).
We examined change in testosterone from baseline to posttest by conducting an analysis of variance with factors of measurement occasion, experimental condition, gender, and level of social anxiety (a continuous variable). The four-way interaction was significant, F(1, 50) = 10.29, p = .002. The three-way interaction among measurement occasion, experimental condition, and social anxiety was significant among males, F(1, 19) = 8.98, p = .007, but not among females, F < 1.
To interpret this pattern, we used multiple regression to evaluate the simple effect of manipulated dominance threat among men and women who were high versus low in social anxiety (1 standard deviation above and below the mean). The difference between testosterone at baseline and posttest served as the dependent variable. These analyses revealed a clear pattern: Among anxious males, dominance threat evoked a substantial drop in testosterone, b = −3.48, t(50) = −4.43, p < .001, pr = −.53. No other significant effects were observed. The manipulation had no observable effect on men low in social anxiety, b = 0.68, t < 1, p = .33; women high in social anxiety, b = 0.40, t < 1, p = .56; or women low in social anxiety, b = −0.05, t < 1, p = .90.
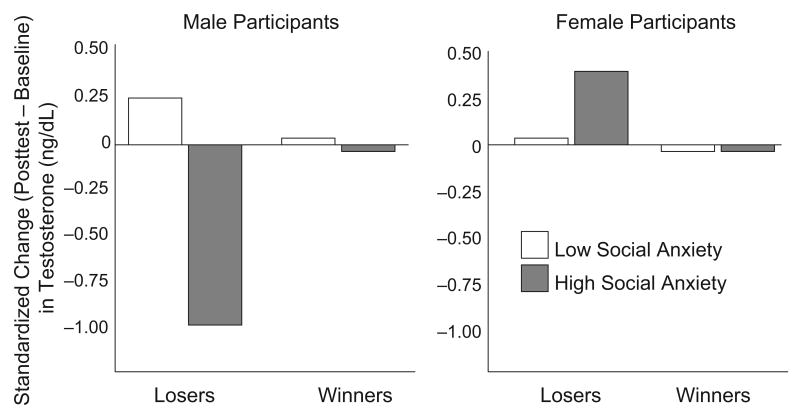
Raw-score variability in testosterone was substantially lower in women than in men, so to ensure that even small but systematic changes in testosterone in women would be detectable, we conducted additional analyses using testosterone levels standardized within gender. These analyses revealed the same pattern: Dominance threat evoked a significant drop in testosterone among anxious males, b = −1.00, t(50) = −2.20, p < .05, pr = −.30. In no other case did the effect of dominance threat approach significance (all ps > .33; see Fig. 1).
Fig. 1.
Change in testosterone level as a function of experimental condition (losers vs. winners) and social anxiety (1 SD above the mean vs. 1 SD below the mean). Results are presented separately for men and women. Change scores were standardized within each gender.
Discussion
Although many previous studies have documented the manner in which socially anxious individuals respond to negative social evaluation, the current study is the first to provide direct evidence that social anxiety shapes responses to social-dominance threat. Socially anxious men responded to dominance threat with substantial decreases in testosterone. Many studies have shown that such decrements reflect submissiveness and a desire to avoid further competition (e.g., Mehta & Josephs, 2006), so this response of socially anxious men seems to reflect a pronounced orientation toward social submission.
On the one hand, submitting to defeat is a good strategy if one's intention is to avoid potential social conflict or further loss of dominance. On the other hand, submission could prove maladaptive, because exaggerated submissiveness could place a strain on interpersonal relationships and could jeopardize one's status within the group. Further research is needed to investigate these possibilities directly. It will be important for studies to evaluate the extent to which decrements in testosterone among socially anxious individuals are associated with subjective distress or social withdrawal (cf. van Honk, Peper, & Schutter, 2005; Wirth & Schultheiss, 2007).
Notably, men low in social anxiety showed no signs of decreased testosterone following dominance threat, and, in fact, the trend was in the opposite direction. This result is consistent with evidence that defeat does not always evoke submission and that some individuals respond to defeat with increased testosterone and a desire for further competition (Mehta & Josephs, 2006). Although the increase in testosterone among men low in social anxiety was not statistically significant, the current findings do suggest that increases in testosterone following defeat are more likely to occur among individuals low in social anxiety than among individuals high in social anxiety.
Although social anxiety moderated hormone responses to dominance threat, we observed no relationship between social anxiety and basal testosterone. This is interesting, because it suggests that social anxiety is linked not so much to a person's baseline level of dominance as to the manner in which a person responds to dominance threats when they occur.
Decrements in testosterone following defeat were observed in men, but not women. This fits with evolutionary perspectives suggesting that, reproductively speaking, men have more to gain from achieving dominance than women do, and that men therefore tend to be more concerned with their level of dominance. Although additional research is needed, the current findings have implications for understanding gender differences in social anxiety and suggest that social anxiety may be linked more strongly with concerns about social dominance in men than in women (cf. Kivlighan, Granger, & Booth, 2005).
Future research would benefit from examining how fluctuations in testosterone might interact with changes in other hormones, such as cortisol and oxytocin, both of which have been shown to affect important social processes (e.g., Mehta & Josephs, 2006; Taylor et al., 2000). Such hormones likely work in concert to shape behavioral and psychological responses to threat.
Although the current work focused on social dominance, future studies should examine hormone responses to other forms of threat. Endocrinological changes are likely to mediate reactions to a range of social and nonsocial challenges, and we suspect the moderator variables that shape such reactions may be domain-specific. Whereas endocrinological responses to social threat appear to be moderated by social anxiety, responses to other types of threat (e.g., physical threat) may be moderated by other theoretically meaningful individual differences (e.g., fear of physical harm).
References
- Antony MM, Rowa K, Liss A, Swallow SR, Swinson RP. Social comparison processes in social phobia. Behaviour Therapy. 2005;36:65–75. [Google Scholar]
- Barkow JH. Prestige and culture: A biosocial interpretation. Current Anthropology. 1975;16:553–562. [Google Scholar]
- Barlow DH. Anxiety and its disorders: The nature and treatment of anxiety and panic. 2nd. New York: Guilford Press; 2002. [Google Scholar]
- Baumeister RF, Tice DM. Anxiety and social exclusion. Journal of Social and Clinical Psychology. 1990;9:165–195. [Google Scholar]
- Cashdan E. Hormones, sex, and status in women. Hormones and Behavior. 1995;29:354–366. doi: 10.1006/hbeh.1995.1025. [DOI] [PubMed] [Google Scholar]
- de Bruin EI, Verheij F, Wiegman T, Ferdinand RF. Differences in finger length ratio between males with autism, pervasive developmental disorder-not otherwise specified, ADHD, and anxiety disorders. Developmental Medicine & Child Neurology. 2006;48:962–965. doi: 10.1017/S0012162206002118. [DOI] [PubMed] [Google Scholar]
- de Waal F. Chimpanzee politics: Power and sex among apes. New York: Harper and Row; 1982. [Google Scholar]
- Edinger KL, Frye CA. Testosterone's anti-anxiety and analgesic effects may be due in part to actions of its 5α-reduced metabolites in the hippocampus. Psychoneuroendocrinology. 2005;30:418–430. doi: 10.1016/j.psyneuen.2004.11.001. [DOI] [PubMed] [Google Scholar]
- Granger DA, Shirtcliff EA, Zahn-Waxler C, Usher B, Klimes-Dougan B, Hastings P. Salivary testosterone diurnal variation and psychopathology in adolescent males and females: Individual differences and developmental effects. Development and Psychopathology. 2003;13:431–449. [PubMed] [Google Scholar]
- Heimberg RG, Liebowitz MR, Hope DA, Schneier FR. Social phobia: Diagnosis, assessment, and treatment. New York: Guilford Press; 1995. [Google Scholar]
- Hofmann SG. Enhancing exposure-therapy from a translational research perspective. Behaviour Research and Therapy. 2007;45:1987–2001. doi: 10.1016/j.brat.2007.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Josephs RA, Sellers JG, Newman ML, Mehta PH. The mismatch effect: When testosterone and status are at odds. Journal of Personality and Social Psychology. 2006;90:999–1013. doi: 10.1037/0022-3514.90.6.999. [DOI] [PubMed] [Google Scholar]
- Kivlighan KT, Granger DA, Booth A. Gender differences in testosterone and cortisol response to competition. Psychoneuroendocrinology. 2005;30:58–71. doi: 10.1016/j.psyneuen.2004.05.009. [DOI] [PubMed] [Google Scholar]
- Leary MR. Responses to social exclusion: Social anxiety, jealousy, loneliness, depression, and low self-esteem. Journal of Social and Clinical Psychology. 1990;9:221–229. [Google Scholar]
- Leary MR, Cottrell CA, Phillips M. Deconfounding the effects of dominance and social acceptance on self-esteem. Journal of Personality and Social Psychology. 2001;81:898–909. doi: 10.1037//0022-3514.81.5.898. [DOI] [PubMed] [Google Scholar]
- Liebowitz MR, Gorman JM, Fyer AJ, Klein DF. Social phobia: Review of a neglected anxiety disorder. Archives of General Psychiatry. 1985;42:729–736. doi: 10.1001/archpsyc.1985.01790300097013. [DOI] [PubMed] [Google Scholar]
- Maddux JE, Norton LW, Leary MR. Cognitive components of social anxiety: An investigation of the integration of self-presentation theory and self-efficacy theory. Journal of Social and Clinical Psychology. 1988;6:180–190. [Google Scholar]
- Maner JK, DeWall CN, Baumeister RF, Schaller M. Does social exclusion motivate interpersonal reconnection? Resolving the “porcupine problem”. Journal of Personality and Social Psychology. 2007;92:42–55. doi: 10.1037/0022-3514.92.1.42. [DOI] [PubMed] [Google Scholar]
- Mattick RP, Clarke JC. Development and validation of measures of social phobia scrutiny fear and social interaction anxiety. Behaviour Research and Therapy. 1998;36:455–470. doi: 10.1016/s0005-7967(97)10031-6. [DOI] [PubMed] [Google Scholar]
- Mazur A, Booth A. Testosterone and social dominance in men. Behavioral and Brain Sciences. 1998;21:353–397. [PubMed] [Google Scholar]
- Mehta P, Josephs R. Testosterone change after losing predicts the decision to compete again. Hormones and Behavior. 2006;50:684–692. doi: 10.1016/j.yhbeh.2006.07.001. [DOI] [PubMed] [Google Scholar]
- Moscovitch DA, Hofmann SG, Litz BT. The impact of self-construals on social anxiety: A gender-specific interaction. Personality and Individual Differences. 2005;38:659–672. [Google Scholar]
- Öhman A. Face the beast and fear the face: Animal and social fears as prototypes for evolutionary analyses of emotion. Psychophysiology. 1986;23:123–145. doi: 10.1111/j.1469-8986.1986.tb00608.x. [DOI] [PubMed] [Google Scholar]
- Schultheiss OC, Wirth MM, Torges CM, Pang JS, Villacorta MA, Welsh KM. Effects of implicit power motivation on men's and women's implicit learning and testosterone changes after social victory or defeat. Journal of Personality and Social Psychology. 2005;88:174–188. doi: 10.1037/0022-3514.88.1.174. [DOI] [PubMed] [Google Scholar]
- Taylor SE, Klein LC, Lewis BP, Gruenewald TL, Gurung RAR, Updegraff JA. Biobehavioral responses to stress in females: Tend-and-befriend, not fight-or-flight. Psychological Review. 2000;107:411–429. doi: 10.1037/0033-295x.107.3.411. [DOI] [PubMed] [Google Scholar]
- Toufexis DJ, Myers KM, Davis M. The effect of gonadal hormones and gender on anxiety and emotional learning. Hormones and Behavior. 2006;50:539–549. doi: 10.1016/j.yhbeh.2006.06.020. [DOI] [PubMed] [Google Scholar]
- Turk C, Heimberg R, Orsillo S, Holt C, Gitow A, Street L, et al. An investigation of gender differences in social phobia. Journal of Anxiety Disorders. 1998;12:209–223. doi: 10.1016/s0887-6185(98)00010-3. [DOI] [PubMed] [Google Scholar]
- van Honk J, Peper JS, Schutter DJLG. Testosterone reduces unconscious fear but not consciously experienced anxiety: Implications for the disorders of fear and anxiety. Biological Psychiatry. 2005;58:218–225. doi: 10.1016/j.biopsych.2005.04.003. [DOI] [PubMed] [Google Scholar]
- Wilson M, Daly M. Competitiveness, risk-taking, and violence: The young male syndrome. Ethology and Sociobiology. 1992;6:59–73. [Google Scholar]
- Wirth MM, Schultheiss OC. Basal testosterone moderates responses to anger faces in humans. Physiology & Behavior. 2007;90:496–505. doi: 10.1016/j.physbeh.2006.10.016. [DOI] [PubMed] [Google Scholar]