Abstract
Recent studies indicate that the transcriptional activity of steroid receptors is governed by proteins called nuclear receptor coactivators. Using immunocytochemistry, we found that on the day of birth (postnatal d 0) males express higher levels of the nuclear receptor coactivator, cAMP response element binding protein-binding protein (CBP), within the ventromedial hypothalamus, medial preoptic area, and arcuate nucleus. Using Western immunoblots, we confirmed that males have higher levels of CBP on postnatal d 0, 1, and 5; however, there was no sex difference on postnatal d 11. To examine the functional role of CBP, we infused oligodeoxynucleotides that were antisense to CBP mRNA or a scrambled sequence as a control into the hypothalamus of female rats on postnatal d 0, 1, and 2. On postnatal d 1, all rats were injected with 100 μg testosterone propionate to both masculinize (increase male) and defeminize (decrease female) sexual behavior. Rats were ovariectomized in adulthood and tested for adult sexual behavior. Neonatal CBP antisense oligodeoxynucleotides treatment interfered with the defeminizing, but not the masculinizing, actions of testosterone. These results indicate that CBP expression in developing rat brain is sexually dimorphic and an important modulator for steroid hormone action.
STEROID HORMONES ACT in the brain to influence a wide variety of behavioral and physiological processes, largely by binding to intracellular steroid receptors (1, 2). It is well established that steroid receptors exert most of their effects in cells by binding to hormone response elements (HREs) on DNA and influencing gene transcription (3). Recent studies indicate that steroid receptors also interact with other regulatory proteins, nuclear receptor coactivators or corepressors, that increase or decrease, respectively, their binding and action at the HRE (4, 5). The nuclear coactivator found to enhance transcriptional activity of progestin receptors when bound to its HRE was termed steroid receptor coactivator-1 (SRC-1) because it also enhances the transcriptional activity of receptors for estrogens, androgens, glucocorticoids, and thyroid hormone (6). Since SRC-1 was first characterized, other coactivators have been identified, such as the SRC-1-related proteins, TIF2 (SRC-2) and SRC-3, and unrelated coactivators such as ARA70, Trip1, and TIF1. The cAMP response element-binding protein (CREB)-binding protein (CBP), which was originally identified as a coactivator for CREB (7, 8), appears to function as a nuclear receptor coactivator by interacting with SRC-1 (9) and synergistically enhance steroid receptor action at the HRE (4, 10).
Nuclear receptor coactivators increase steroid receptor transcription via their intrinsic histone acetyltransferase activity. Acetylation of histones by coactivators removes a positive charge, leading to a reduction in higher-order chromatin structures, and makes DNA more accessible to transcription factors (11-13). Nuclear receptor coactivators also increase transcriptional activity of steroid receptors by interacting with other histone acetyltransferase factors, such as p300/ CBP-associated factor, and general transcription factors, such as TBP and TIFIIB (5). Because the actions of steroid receptors are important for normal reproductive physiology and behavior of both males and females and the actions of steroid receptors at the genome are governed by nuclear receptor coactivators, it is important to determine the expression and function of these nuclear receptor coactivators (i.e. CBP) during brain development.
Although much is known about CBP in vitro, little is known about the functional role of CBP in developing brain. Homozygous mice containing a targeted disruption of the CBP gene die during midgestation (14). Although heterozygous mice survive, they show postnatal disruptions in neurotube development as well as deficits in learning and memory, indicating that CBP is required for normal central nervous system development and function (15). In adult rat brain, CBP is found in many areas that also contain steroid receptors, such as the amygdala, hypothalamus, and hippocampus (16). In addition, CBP appears to be regulated by estradiol because treatment of cultured hippocampal neurons with estradiol increases the level of CBP within 24 h (17). The time course for estradiol-induced CBP correlates with estradiol-induced increases in neuronal dendritic spines, the sites of excitatory synapses, and one of the more pronounced sex differences in the brain is the density of dendritic spines (18, 19). Therefore, we hypothesized that CBP is involved in processes related to sexual differentiation of the brain.
Sexual differentiation of the brain is determined by steroid hormone exposure during a discrete developmental period, between embryonic d 18 and postnatal d 10 (20-22). At birth, male rats are exposed to high levels of testosterone released from the testis, resulting in behavioral masculinization, defined as increased male-typical behaviors, and behavioral defeminization, defined as decreased female-typical behaviors. The absence of a testosterone surge in females results in feminization of the brain. Neonatally castrated male rats grown to adulthood exhibit decreased male and increased female sexual behavior under the appropriate hormonal conditions (23). Likewise, females administered testosterone neonatally do not display female sexual behavior as adults (24, 25) but will display male-typical behaviors if treated with exogenous testosterone. We have recently reported that SRC-1 is critical for steroid-mediated differentiation of sexual behavior. Reducing SRC-1 expression during the first few days of life nearly abolished testosterone's effect on suppressing female sexual behavior in males and androgenized females (26). Interestingly, reducing SRC-1 expression did not interfere with the development of male sexual behavior in males and androgenized females, suggesting the effects were specific to defeminization. Because CBP interacts with SRC-1 in vitro, we have now investigated the expression of CBP and its function in sexual differentiation of neonatal male and female rat brain.
Materials and Methods
Animals
Adult female Sprague Dawley rats (Charles River Laboratories, Inc., Wilmington, MA) were mated in our animal facility and mating confirmed by the presence of sperm in vaginal smears. Pregnant females were allowed to deliver normally. Cages were checked regularly to determine the day of birth. Pups were collected on the afternoon of the day of birth (postnatal d 0, PN0), 24 h later (postnatal d 1), postnatal d 5, or postnatal d 11.
Experiment 1: quantification of CBP using immunocytochemistry
On PN0, brains were collected from newborn male (n=5) and female (n=6) pups and immediately immersed in 5% acrolein for 24 h and then placed into 0.1 M PBS containing 20% sucrose overnight. Brains were sectioned at 50 μm using a cryostat at -20 C and stored in cryoprotectant until processed for CBP immunocytochemistry.
CBP immunocytochemistry
Sections were rinsed three times for 5 min each in 0.1 M PBS (pH 7.6) and placed into 1% H2O2, 20% normal goat serum, and 1% BSA for 30 min to reduce nonspecific staining and endogenous peroxidase activity. Sections were then incubated with an antibody that recognizes the carboxy terminus of CBP (rabbit polyclonal, SC-20; 1:2000 dilution, Santa Cruz Biotechnology, Inc., Santa Cruz, CA) overnight at room temperature in PBS containing 0.3% Triton X-100. After primary incubation, sections were rinsed three times in PBS for 5 min each and then incubated with biotinylated goat antirabbit IgG (7.5 g/ml) diluted in PBS for 90 min at room temperature. Following three additional rinses in PBS, sections were incubated with the DH:biotinylated horseradish peroxidase H complex (1:200 in PBS) for 90 min. Sections were rinsed three more times in PBS for 5 min each and treated with 0.05% diaminobenzidine and 0.05% H2O2 in PBS for 5 min. Developed sections were mounted onto gelatin-coated slides and coverslipped with Permount mounting medium. Omission of CBP antibody and preadsorption with CBP control peptide blocked all immunoreactivity.
Computer-aided image analysis
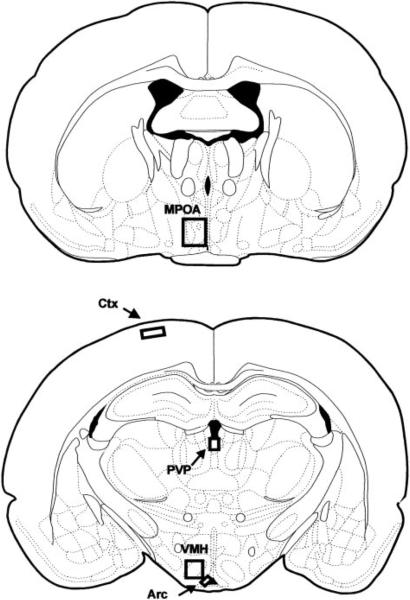
Brain areas examined are illustrated in Fig. 1 and include areas that express high levels of gonadal steroid receptors, such as the medial preoptic area (MPOA), arcuate nucleus (Arc), ventromedial hypothalamus (VMH), and the CA1 and CA3 regions of the hippocampus, and areas that contain little or no gonadal steroid receptor expression, such as the paraventricular thalamic nucleus, posterior part (PVP), and the frontal cortex. One section per area was matched according to the rat brain atlas of Paxinos and Watson (27).
FIG. 1.
Schematic drawing of areas in which CBP immunoreactive cell number was quantified. Figures are adapted from Paxinos and Watson (27). Boxes represent the area used for image analysis within a given region. Regions examined are the MPOA, VMH, Arc, PVP, and a region of the frontal cortex (Ctx).
CBP-immunoreactive cells were counted using a Ziess microscope fitted with an MTI CCD72 camera (Dage-MTI, Michigan City, IN) connected to a Macintosh computer. The software used for analysis was the public domain NIH IMAGE program (http://rsb.info.nih.gov/nih-image). The microscope was set to Kohler illumination at 10 × 10 magnification. Details of this procedure have been published previously (28, 29). Data were analyzed with a two-tailed t test using the SigmaStat Statistical Analysis System 1.01 software (Jandel Scientific, Corta Madera, CA).
Experiment 2: quantification of CBP by Western immunoblotting
Neonatal male and female rat brains (five to six rats per group) were collected on PN0, postnatal d 1, postnatal d 5, and postnatal d 11. The basomedial hypothalamus was dissected out on a chilled surface, snap frozen in isopentane on dry ice, and stored frozen until homogenization with ice-cold lysis buffer consisting of 50 mm Tris-HCl, 1% Na-deoxycholate, 0.25% Nonidet P-40, 150 mm NaCl, 1 mm EDTA, and protease inhibitors (1 μg/ml aprotinin, leupeptin, and pepstatin; 1 mm phenylmethylsulfonyl fluoride). Following tissue homogenization, samples were centrifuged at 2000 × g for 30min at 4C to sediment cellular debris and nuclei. The supernatant fraction was collected and protein concentration determined by a Bradford assay. Twenty micrograms total protein from each rat were gel electrophoresed using a precast SDS-PAGE (8-16% Tris Glycine) and transferred to a polyvinyl difluoride membrane. Membranes were washed briefly in 0.1 M Tris-buffered saline (TBS) and blocked for 1 h in 0.1 M TBS containing 5% nonfat dry milk with constant agitation at room temperature. Membranes were then incubated with CBP antibody (SC-20; 1:3000) in TBS containing 0.05% Tween-20 (TTBS) for 3 h at room temperature with agitation and washed three times for 5 min each in TTBS. Following washes, membranes were incubated in a goat antirabbit horseradish peroxidase-linked secondary antibody for 30 min at room temperature with agitation and then washed three times for 5 min each in TTBS. Immunoreactive bands were detected using an enhanced chemiluminescence kit (New England Biolabs, Inc., Beverly, MA) and membrane exposed to film (Hyperfilm-ECL, Amersham, Arlington Heights, IL). Membranes were then reblotted with a mouse monoclonal antibody against the housekeeping gene, glyceraldehyde-3-phosphate dehydrogenase (GAPDH; 1:100,000, Chemicon International, Inc., Temecula, CA). Films were placed on a light box and analyzed using a gel-plotting macro in the public domain NIH image program. Densitometric areas were then were analyzed with a two-tailed t test.
Experiment 3: functional consequence of reducing CBP protein using antisense oligodeoxynucleotides
Oligodeoxynucleotide infusions
CBP antisense oligodeoxynucleotides (ODNs) (n = 4) or scrambled control ODNs (n = 6) were infused into the hypothalamus of female rats on PN0-2, using a modified stereotaxic apparatus and a 1-μl Hamilton syringe. Rats were cryoanesthetized before each infusion. Bregma is visible through the skin under bright light and was used as a landmark for the hypothalamus. Bilateral injections (1 μg ODNs dissolved in 1 μl 0.9% saline) were aimed at the mediobasal hypothalamus (1.0 mm anterior and 0.8 mm lateral to Bregma; 5.0 mm below the skull). The sequence for the 21-mer antisense oligonucleotide spanning the putative start codon of CBP is 5′-CAG-CAA-GTT-CTC-GGC-CAT-CTT-3′ and the 21-mer scrambled control oligonucleotide is 5′-CCC-TAG-CAG-CTA-CAG-CTT-CGC-3′. Chimeric ODNs were obtained from Oligos etc. (Wilforshire, OR). These second-generation chimeric ODNs contain limited phosphorothioate linkages allowing for greater resistance to nuclease cleavage and increased activation of Rnase H, an enzyme that cleaves the RNA strand of DNA/RNA hybrids. On postnatal d 1, all females were injected with 100 μg testosterone propionate, a dose known to both masculinize and defeminize sexual behavior.
Female sexual behavior
Neonatally treated rats were grown to adulthood and ovariectomized on postnatal d 45 using ketamine/acepromazine anesthesia. Two weeks following surgery, rats were steroid primed with 10 μg estradiol benzoate followed 48 h later by 500 μg progesterone, a paradigm that reliably induces female sexual behavior. Fours hours after progesterone treatment, rats were placed in a testing chamber and allowed to acclimate. Behavioral testing occurred during the dark phase of the light cycle under dim red light. The flanks and perineum were manually stimulated by the experimenter and lordosis quotients and ratings scored. Lordosis quotients indicate number of responses per number of attempts multiplied by 100. Lordosis rating indicates a lordosis intensity range on a scale from 0 to 3, reflecting increasing dorsoflexion of the back. The experimenter scoring the lordosis response was blind to the treatment group.
Male sexual behavior
Following testing for female sexual behavior, rats were implanted with testosterone-filled capsules and 3 wk later tested for male sexual behavior. Rats were then placed in a testing chamber and allowed to acclimate. A sexually receptive female was placed into the testing chamber, and the total number of mounts and latency to first mount were recorded over a 15-min test period. Behavioral testing occurred during the dark phase of the light cycle under dim red light. Behavioral data were analyzed with a two-tailed t test.
Results
Experiment 1: quantification of CBP by immunocytochemistry
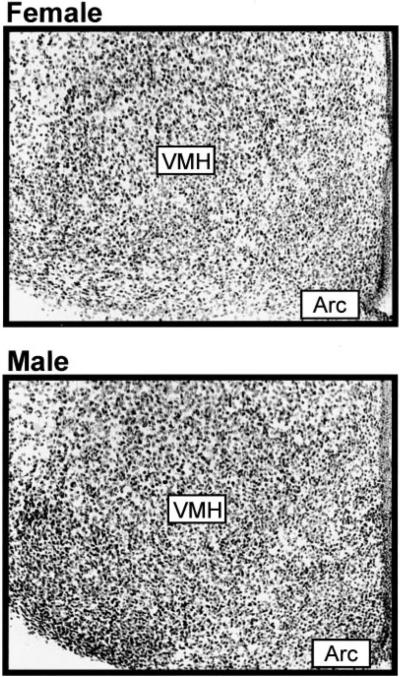
On PN0, CBP immunoreactivity was found to be widely expressed throughout male and female rat brain. A sex difference was found in the number of CBP immunoreactive cells within brain regions that have high levels of gonadal steroid receptors. In the MPOA, males had 53% more CBP immunoreactive cells than females (males 5625 ± sem 447, females 3662 ± 538; P < 0.05). In the VMH, males had 83% more CBP immunoreactive cells (males 3810 ± 205, females 2085 ± 565; P < 0.05; Fig. 2). In the Arc, males had 89% more CBP immunoreactive cells than females (males 567 ± 33, females 299 ± 89; P < 0.05). There were no sex differences found in brain regions that have little to no expression of gonadal steroid receptors, such as the PVP (males 355 ± 42, females 369 ± 38; P > 0.05) or the frontal cortex (males 724 ± 33, females 744 ± 61; P > 0.05; Fig. 3).
FIG. 2.
Photomicrographs of CBP immunoreactive cells within the VMH and the adjacent Arc of female and male rat brain.
FIG. 3.

CBP immunoreactive cell number on the day of birth. Mean number (± SEM) of CBP immunoreactive cells counted bilaterally for each area from males and females on the day of birth. *, P < 0.05.
Experiment 2: quantification of CBP by Western immunoblotting
Confirming immunocytochemical results, males had higher levels of CBP within the basomedial hypothalamus on PN0 (P < 0.05, Figs. 4 and 5A). Males also had higher CBP levels within the basomedial hypothalamus on PN1 and on PN5 (P < 0.05, Fig. 5, B and C). However, this difference was not present on postnatal d 11 (P > 0.05, Fig. 5D), a time point outside the sensitive period for sexual differentiation.
FIG. 4.

Photomicrograph of Western blot showing increased levels of CBP within the hypothalamus of males, contrasted to females on the day of birth. Each lane represents an individual animal. CBP is recognized as a single protein band at approximately 265 kDa.
FIG. 5.
Results of the densitometric analysis of CBP immunoreactive bands shown as a ratio of CBP to the housekeeping gene, GAPDH, within the hypothalamus of males and females on PN0, postnatal d 1, postnatal d 5, and postnatal d 11. *, P < 0.05.
Experiment 3: functional consequence of reducing CBP protein using antisense ODNs
Female sexual behavior
Neonatal infusions of CBP antisense ODNs into the hypothalamus blocked the defeminizing actions of neonatal testosterone treatment on female sexual behavior. Androgenized female rats infused neonatally with CBP antisense ODNs displayed significantly higher levels of lordosis contrasted with scrambled ODNs infused androgenized females (P < 0.05, Fig. 6).
FIG. 6.
Adult female sexual behavior observed in rats that were neonatally treated with testosterone to reduce female sexual behavior. Reducing CBP with bilateral infusions of antisense ODNs to CBP mRNA interferes with the suppressive effects of testosterone on female sexual behavior. Rats infused with CBP antisense ODNs had higher lordosis quotients and ratings, compared with rats infused with scrambled control ODNs. *, P < 0.05.
Male sexual behavior
In contrast to the effects on female sexual behavior, neonatal infusions of CBP antisense ODNs into the hypothalamus did not interfere with the masculinizing actions of neonatal testosterone treatment on male sexual behavior. CBP antisense ODN treatment did not alter the total number of mounts or mount latencies in androgenized female rats (P > 0.05, Fig. 7).
FIG. 7.
Adult male sexual behavior observed in rats that were neonatally treated with testosterone. Reducing CBP with bilateral infusions of antisense ODNs to CBP mRNA did not interfere with the development of male sexual behavior. Rats infused with CBP antisense ODNs had similar number of mounts and latencies to first mount, compared with rats infused with scrambled control ODNs.
Effect of CBP antisense ODNs on CBP level
Using Western immunoblots, we found that neonatal infusions of CBP antisense ODNs into the hypothalamus reduced the expression of CBP in hypothalamic tissue within 24 h by 30%, contrasted with scrambled ODN control (P < 0.05, Fig. 8).
FIG. 8.

CBP antisense ODN infusions decreased the levels of hypothalamic CBP. Histogram represents Western blot analysis of the densitometric area of CBP immunoreactive bands in rats infused bilaterally with CBP antisense ODNs or scrambled control ODNs. The level of CBP is shown as a ratio to GAPDH. *, P < 0.05.
Discussion
We report here a sex difference in the expression of the nuclear receptor coactivator, CBP, during neonatal development of the rat brain. We found that CBP is present at higher levels in steroid receptor-containing brain regions of males, contrasted with females on the day of birth. Males have more CBP immunoreactive cells within the MPOA, VMH, and Arc, regions that express high levels of gonadal steroid receptors; however, there were no sex differences in the number of CBP immunoreactive cells within the PVP or frontal cortex, regions that express little or no gonadal steroid receptors. To confirm and extend the immunocytochemical observations, we used Western immunoblots to determine whether this sex difference persists throughout the sensitive period for sexual differentiation of the brain. We found that CBP levels are higher in the basomedial hypothalamus of males on PN0, postnatal d 1 and postnatal d 5; however, this sex difference is gone by postnatal d 11. These findings indicate that CBP levels are sexually dimorphic within steroid receptor-containing areas during early postnatal development. Because most sex differences in the brain are due to steroid hormone exposure (21, 22), it is likely that steroid hormones contribute to the sex difference found in CBP levels within these sexually dimorphic steroid receptor-containing brain regions.
The sex difference in the levels of CBP within particular brain regions is likely to impact the activity of multiple steroid receptors. In vitro studies (4, 30) indicate that CBP interacts with receptors for glucocorticoids, estrogens, progestins, thyroid hormone, retinoic acid, and retinoid X as well as for androgens (31). An important outcome of ligand-activated steroid receptors is the ability to squelch the activity of other steroid receptors. One possible mechanism for this cross-talk between steroid receptors is by receptors binding to and limiting the availability of coactivators (4, 6). For example, ligand-induced transactivation of progestin receptors in HeLa cells can be squelched by estradiol activation of estrogen receptors, and this phenomenon can be overcome by increasing the levels of SRC-1 (6). Therefore, it appears that a limited amount of coactivators is available to bind to ligand-activated steroid receptors. Our present finding that CBP is regulated in a sex-specific manner during development suggests that the regulation of CBP provides a means by which one steroid receptor can alter the activity of other steroid receptors in developing brain. For example, testosterone, or its metabolites, may increase the response to other steroid hormones by increasing the levels of CBP within particular brain regions.
The immunocytochemical distribution of CBP in neonatal rat brain closely resembles that observed in adults (16). Although CBP immunoreactivity is widely expressed throughout the neonatal rat brain, there appears to be some heterogeneity in the levels of CBP as well as sex differences in these levels. The present immunocytochemical results suggest the sex differences in CBP expression occur only within sexually dimorphic brain regions that contain steroid receptors, such as the MPOA, VMH, and Arc. It is not known whether CBP immunoreactivity is found exclusively within neurons or can be found within glia. The presence of high levels of CBP may be important to promote cellular responses to steroids within cell populations that have low levels of steroid receptors. The distribution of CBP correlates well with the distribution of the coactivator, SRC-1, in rat brain (32). Because SRC-1 interacts with CBP at the genome to enhance steroid receptor-induced transcription, it is not surprising to find a similar distribution of these coactivators within the brain. Another parallel between CBP and SRC-1 is the functional role of these coactivators in modulating steroid-mediated differentiation of sexual behavior.
It is well documented that neonatal testosterone treatment of females leads to the suppression of adult female sexual behavior via its aromatization to estradiol (20, 21, 33). Because CBP is critical for estrogen receptor action in vitro, we examined whether CBP is required for estrogen receptor action in developing brain. If CBP is important for estrogen receptor action, then reducing CBP levels should interfere with the ability of estradiol to suppress female sexual behavior. Therefore, we hypothesized that reducing postnatal CBP levels in testosterone-treated female rats with antisense ODNs would block the suppressive actions of estradiol and lead to increased female sexual behavior. As predicted, testosterone-treated female rats infused with CBP antisense ODNs neonatally exhibited higher levels of lordosis as adults, contrasted with scrambled ODN controls. This indicates that reducing CBP expression interfered with estradiol's ability to defeminize or reduce female sexual behavior. Interestingly, reducing CBP expression with antisense ODN did not interfere with male sexual behavior. There were no differences between rats treated with CBP antisense ODNs or control ODN in the number of mounts directed toward an estrous female, and there were no differences in the latency to mount an estrous female. Taken together, these data suggest that postnatal expression of CBP may be more important for processes leading to defeminization than those associated with masculinization of sexual behavior.
The postnatal disruption of defeminization, but not masculinization, of sexual behavior by reducing CBP expression is consistent with the effects of reducing SRC-1 expression neonatally (26). We previously reported that postnatal reduction of SRC-1 protein interfered with the defeminizing, but not the masculinizing, actions of testosterone. These data are consistent with others (23, 34-37) that report changes in defeminization, but not masculinization, of sexual behavior. As stated above, it is well documented that behavioral defeminization results from estrogen action (23, 38, 39). However, behavioral masculinization by testosterone is more complex, at least in Sprague-Dawley rats (40). Neonatal treatment with an antiandrogen, cyproterone acetate or flut-amide, interferes with behavioral masculinization but has little impact on behavioral defeminization (40, 41). Furthermore, blocking the aromatization of testosterone to estradiol neonatally with 1,4,6-androstatriene-3,17-dione disrupts behavioral defeminization, but not behavioral masculinization, of sexual behavior. That is, neonatally 1,4,6-androstatriene-3,17-dione-treated males exhibit high levels of both male and female sexual behavior (34). Thus, blocking the conversion of testosterone into estradiol produces bisexual males that resemble those treated neonatally with either SRC-1 or CBP antisense ODNs. Although our data do not rule out a possible role for CBP in modulating the development of some components of male sexual behavior, they do suggest that coactivators, such as CBP and SRC-1, may be more important in processes mediating defeminization than those mediating masculinization of sexual behavior.
The lack of an effect on masculinization in androgenized females does not rule out an involvement of CBP in masculinization in males. During development, masculinization of sexual behavior in males appears to be influenced by two surges in the synthesis and release of testosterone, one occurring on embryonic d 18 and the other on the day of birth. Treatment of pregnant rats with an androgen antagonist, cyproterone acetate, to block androgen action on embryonic d 18 interferes with masculinization (42). Furthermore, female rats located near a male within the uterus display increased male sexual behavior in adulthood, and this increase can be blocked by prenatal treatment with an antiandrogen, flutamide (43). These studies suggest that a portion of masculinization is due to prenatal testosterone. Therefore, it is possible that CBP may be involved in mediating some effects of prenatal testosterone on masculinization.
Another point to consider is that the CBP antisense ODN reduced protein expression by 30%, as determined by Western immunoblot. This percent reduction is consistent with what we observed using SRC-1 antisense ODN, which completely blocked the defeminizing, but not the masculinizing, actions of testosterone (26). Typically antisense ODN treatments reduces protein levels by 10-40%, and the behavioral or physiological effects are more profound (44). It has been suggested that newly synthesized proteins may be functionally preferred over older proteins (44). The Western blot technique would detect both old and newly synthesized proteins. Because antisense ODNs interfere preferentially with the synthesis of new protein, it is possible for antisense to have a greater impact on physiology than on protein levels. Although a 30% reduction in CBP attenuated the defeminizing actions of testosterone, a greater reduction of coactivators may be needed to interfere with the masculinization of sexual behavior. Another possibility is that both CBP and SRC-1 may need to be reduced to influence masculinization. Indeed, simultaneous reduction of both CBP and SRC-1 was required to interfere with adult female sexual behavior and estradiol induction of progestin receptors (9).
It is important to note that CBP is not only a coactivator for steroid receptors but is also critically involved in mediating the actions of CREB (7, 8). When CREB is phosphorylated, it binds to a cAMP response element located on DNA and then recruits CBP to the transcriptional complex. Phosphorylation of CREB occurs following estradiol treatment in both adult (45- 47) and immature neurons (17). Because neonatal males have higher levels of estradiol, which is aromatized from testosterone within the brain, it was predicted that males would have higher levels of CREB phosphorylation. Indeed, we have recently reported that CREB phosphorylation is higher in males on the day of birth, contrasted with females in steroid receptor-containing areas that are sexually dimorphic, such as the MPOA, VMH, and the Arc (48). Our present data indicate that males also express higher levels of CBP within these same regions. The increased expression of CBP may act to enhance downstream transcriptional events mediated by estradiol-induced phosphorylated CREB. Taken together, these data suggest that CBP is an important modulator of estradiol action in developing brain.
Acknowledgments
We thank Jesse J. Alt for technical assistance throughout these studies.
This work was supported by NIH Grant MH-02035 and a Women's Health Research Group grant from the University of Maryland, funded by Parke-Davis/Pfizer, Inc. (to A.P.A.); NIH Grant MH-52716 (to M.M.M.); and Grants NSF IBN 0080818 and NIH DK-61935 (to M.J.T.).
Abbreviations
- Arc
Arcuate nucleus
- CBP
CREB-binding protein
- CREB
cAMP response element-binding protein
- GAPDH
glyceraldehyde-3-phosphate dehydrogenase
- HRE
hormone response element
- MPOA
medial preoptic area
- ODN
oligodeoxynucleotide
- PN0
postnatal d 0
- PVP
paraventricular thalamic nucleus, posterior part
- SRC-1
steroid receptor coactivator-1
- TBS
Tris-buffered saline
- TTBS
TBS containing 0.05% Tween-20
- VMH
ventromedial hypothalamus
References
- 1.Pfaff DW, Schwartzgiblin S, McCarthy MM, Kow LM. Cellular and molecular mechanisms of female reproductive behaviors. In: Knobil E, JD Neill, editors. Physiology of reproduction. 2nd ed. vol 1. Raven Press; New York: 1994. pp. 2107–2120. [Google Scholar]
- 2.Blaustein JD, Olster DH. Gonadal steroid hormone receptors and social behaviors. In: Balthazart J, editor. Advances in comparative and environmental physiology. vol 3. Springer-Verlag; Berlin: 1989. pp. 31–104. [Google Scholar]
- 3.Tsai MJ, O'Malley BW. Molecular mechanisms of action of steroid/thyroid receptor superfamily members. Annu Rev Biochem. 1994;63:451–486. doi: 10.1146/annurev.bi.63.070194.002315. [DOI] [PubMed] [Google Scholar]
- 4.Shibata H, Spencer TE, Onate SA, Jenster G, Tsai SY, Tsai MJ, O'Malley BW. Role of co-activators and co-repressors in the mechanism of steroid/ thyroid receptor action. Recent Prog Horm Res. 1997;52:141–164. [PubMed] [Google Scholar]
- 5.McKenna NJ, Lanz RB, O'Malley BW. Nuclear receptor coregulators: cellular and molecular biology. Endocr Rev. 1999;20:321–344. doi: 10.1210/edrv.20.3.0366. [DOI] [PubMed] [Google Scholar]
- 6.Onate SA, Tsai SY, Tsai MJ, O'Malley BW. Sequence and characterization of a coactivator for the steroid hormone receptor superfamily. Science. 1995;270:1354–1357. doi: 10.1126/science.270.5240.1354. [DOI] [PubMed] [Google Scholar]
- 7.Kwok RP, Lundblad JR, Chrivia JC, Richards JP, Bachinger HP, Brennan RG, Roberts SG, Green MR, Goodman RH. Nuclear protein CBP is a coactivator for the transcription factor CREB. Nature. 1994;370:223–226. doi: 10.1038/370223a0. [DOI] [PubMed] [Google Scholar]
- 8.Chrivia JC, Kwok RP, Lamb N, Hagiwara M, Montminy MR, Goodman RH. Phosphorylated CREB binds specifically to the nuclear protein CBP. Nature. 1993;365:855–859. doi: 10.1038/365855a0. [DOI] [PubMed] [Google Scholar]
- 9.Molenda HA, Griffen AL, Auger AP, McCarthy MM, Tetel MJ. Nuclear receptor coactivators modulate hormone-dependent gene expression in brain and female reproductive behavior in rats. Endocrinology. 2002;143:436–444. doi: 10.1210/endo.143.2.8659. [DOI] [PubMed] [Google Scholar]
- 10.Smith CL, Onate SA, Tsai MJ, O'Malley BW. CREB binding protein acts synergistically with steroid receptor coactivator-1 to enhance steroid receptor-dependent transcription. Proc Natl Acad Sci USA. 1996;93:8884–8888. doi: 10.1073/pnas.93.17.8884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Spencer TE, Jenster G, Burcin MM, Allis CD, Zhou J, Mizzen CA, McKenna NJ, Onate SA, Tsai SY, Tsai MJ, O'Malley BW. Steroid receptor coactivator-1 is a histone acetyltransferase. Nature. 1997;389:194–198. doi: 10.1038/38304. [DOI] [PubMed] [Google Scholar]
- 12.Martinez-Balbas MA, Bannister AJ, Martin K, Haus-Seuffert P, Meisterernst M, Kouzarides T. The acetyltransferase activity of CBP stimulates transcription. EMBO J. 1998;17:2886–2893. doi: 10.1093/emboj/17.10.2886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bannister AJ, Kouzarides T. The CBP co-activator is a histone acetyltransferase. Nature. 1996;384:641–643. doi: 10.1038/384641a0. [DOI] [PubMed] [Google Scholar]
- 14.Oike Y, Takakura N, Hata A, Kaname T, Akizuki M, Yamaguchi Y, Yasue H, Araki K, Yamamura K, Suda T. Mice homozygous for a truncated form of CREB-binding protein exhibit defects in hematopoiesis and vasculo-angiogenesis. Blood. 1999;93:2771–2779. [PubMed] [Google Scholar]
- 15.Oike Y, Hata A, Mamiya T, Kaname T, Noda Y, Suzuki M, Yasue H, Nabeshima T, Araki K, Yamamura K. Truncated CBP protein leads to classical Rubinstein-Taybi syndrome phenotypes in mice: implications for a dominant-negative mechanism. Hum Mol Genet. 1999;8:387–396. doi: 10.1093/hmg/8.3.387. [DOI] [PubMed] [Google Scholar]
- 16.Stromberg H, Svensson SP, Hermanson O. Distribution of CREB-binding protein immunoreactivity in the adult rat brain. Brain Res. 1999;818:510–514. doi: 10.1016/s0006-8993(98)01219-0. [DOI] [PubMed] [Google Scholar]
- 17.>Murphy DD, Segal M. Morphological plasticity of dendritic spines in central neurons is mediated by activation of cAMP response element binding protein. Proc Natl Acad Sci USA. 1997;94:1482–1487. doi: 10.1073/pnas.94.4.1482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mong JA, Glaser E, McCarthy MM. Gonadal steroids promote glial differentiation and alter neuronal morphology in the developing hypothalamus in a regionally specific manner. J Neurosci. 1999;19:1464–1472. doi: 10.1523/JNEUROSCI.19-04-01464.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Matsumoto A, Arai Y. Sexual dimorphism in `wiring pattern' in the hypothalamic arcuate nucleus and its modification by neonatal hormonal environment. Brain Res. 1980;190:238–242. doi: 10.1016/0006-8993(80)91173-7. [DOI] [PubMed] [Google Scholar]
- 20.Baum MJ. Differentiation of coital behavior in mammals: a comparative analysis. Neurosci Biobehav Rev. 1979;3:265–284. doi: 10.1016/0149-7634(79)90013-7. [DOI] [PubMed] [Google Scholar]
- 21.Cooke B, Hegstrom CD, Villeneuve LS, Breedlove SM. Sexual differentiation of the vertebrate brain: principles and mechanisms. Front Neuroendocrinol. 1998;19:323–362. doi: 10.1006/frne.1998.0171. [DOI] [PubMed] [Google Scholar]
- 22.McCarthy MM. Molecular aspects of sexual differentiation of the rodent brain. Psychoneuroendocrinology. 1994;19:415–427. doi: 10.1016/0306-4530(94)90029-9. [DOI] [PubMed] [Google Scholar]
- 23.Whalen RE, Edwards DA. Hormonal determinants of the development of masculine and feminine behavior in male and female rats. Anat Rec. 1967;157:173–180. doi: 10.1002/ar.1091570208. [DOI] [PubMed] [Google Scholar]
- 24.Wilson JG, Hamilton JB, Young WC. Influence of age and presence of the ovaries on reproductive function in rats injected with androgens. Yale J Biol Med. 1940;13:189–202. [PMC free article] [PubMed] [Google Scholar]
- 25.Feder HH, Phoenix CH, Young WC. Suppression of feminine behaviour by administration of testosterone propionate to neonatal rats. J Endocrinol. 1966;34:131–132. doi: 10.1677/joe.0.0340131. [DOI] [PubMed] [Google Scholar]
- 26.Auger AP, Tetel MJ, McCarthy MM. Steroid receptor coactivator-1 (SRC-1) mediates the development of sex-specific brain morphology and behavior. Proc Natl Acad Sci USA. 2000;97:7551–7555. doi: 10.1073/pnas.97.13.7551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Paxinos G, Watson C. The rat brain in stereotaxic coordinates. compact ed 3 Academic Press; Sydney, Australia: 1997. [Google Scholar]
- 28.Auger AP, Meredith JM, Snyder GL, Blaustein JD. Oestradiol increases phosphorylation of a dopamine- and cyclic AMP-regulated phosphoprotein (DARPP-32) in female rat brain. J Neuroendocrinol. 2001;13:761–768. doi: 10.1046/j.1365-2826.2001.00700.x. [DOI] [PubMed] [Google Scholar]
- 29.Auger AP, Perrot-Sinal TS, McCarthy MM. Excitatory versus inhibitory GABA as a divergence point in steroid-mediated sexual differentiation of the brain. Proc Natl Acad Sci USA. 2001;98:8059–8064. doi: 10.1073/pnas.131016298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Horwitz KB, Jackson TA, Bain DL, Richer JK, Takimoto GS, Tung L. Nuclear receptor coactivators and corepressors. Mol Endocrinol. 1996;10:1167–1177. doi: 10.1210/mend.10.10.9121485. [DOI] [PubMed] [Google Scholar]
- 31.Fronsdal K, Engedal N, Slagsvold T, Saatcioglu F. CREB binding protein is a coactivator for the androgen receptor and mediates cross-talk with AP-1. J Biol Chem. 1998;273:31853–31859. doi: 10.1074/jbc.273.48.31853. [DOI] [PubMed] [Google Scholar]
- 32.Meijer OC, Steenbergen PJ, De Kloet ER. Differential expression and regional distribution of steroid receptor coactivators SRC-1 and SRC-2 in brain and pituitary. Endocrinology. 2000;141:2192–2199. doi: 10.1210/endo.141.6.7489. [DOI] [PubMed] [Google Scholar]
- 33.MacLusky NJ, Naftolin F. Sexual differentiation of the central nervous system. Science. 1981;211:1294–1302. doi: 10.1126/science.6163211. [DOI] [PubMed] [Google Scholar]
- 34.Vreeburg JT, van der Vaart PD, van der Schoot P. Prevention of central defeminization but not masculinization in male rats by inhibition neonatally of oestrogen biosynthesis. J Endocrinol. 1977;74:375–382. doi: 10.1677/joe.0.0740375. [DOI] [PubMed] [Google Scholar]
- 35.Van der Schoot P. Effects of dihydrotestosterone and oestradiol on sexual differentiation in male rats. J Endocrinol. 1980;84:397–407. doi: 10.1677/joe.0.0840397. [DOI] [PubMed] [Google Scholar]
- 36.Tonjes R, Docke F, Dorner G. Effects of neonatal intracerebral implantation of sex steroids on sexual behaviour, social play behaviour and gonadotrophin secretion. Exp Clin Endocrinol. 1987;90:257–263. doi: 10.1055/s-0029-1210699. [DOI] [PubMed] [Google Scholar]
- 37.Davis PG, Chaptal CV, McEwen BS. Independence of the differentiation of masculine and feminine sexual behavior in rats. Horm Behav. 1979;12:12–19. doi: 10.1016/0018-506x(79)90022-9. [DOI] [PubMed] [Google Scholar]
- 38.Sodersten P. Effects of anti-oestrogen treatment of neonatal male rats on lordosis behaviour and mounting behaviour in the adult. J Endocrinol. 1978;76:241–249. doi: 10.1677/joe.0.0760241. [DOI] [PubMed] [Google Scholar]
- 39.McCarthy MM, Schlenker EH, Pfaff DW. Enduring consequences of neonatal treatment with antisense oligodeoxynucleotides to estrogen receptor messenger ribonucleic acid on sexual differentiation of rat brain. Endocrinology. 1993;133:433–439. doi: 10.1210/endo.133.2.8344188. [DOI] [PubMed] [Google Scholar]
- 40.Brand T, Slob AK. Perinatal flutamide and mounting and lordosis behavior in adult female Wistar and Sprague-Dawley rats. Behav Brain Res. 1991;44:43–51. doi: 10.1016/s0166-4328(05)80238-4. [DOI] [PubMed] [Google Scholar]
- 41.Ward IL, Renz FJ. Consequences of perinatal hormone manipulation on the adult sexual behavior of female rats. J Comp Physiol Psychol. 1972;78:349–355. doi: 10.1037/h0032375. [DOI] [PubMed] [Google Scholar]
- 42.Perakis A, Stylianopoulou F. Effects of a prenatal androgen peak on rat brain sexual differentiation. J Endocrinol. 1986;108:281–285. doi: 10.1677/joe.0.1080281. [DOI] [PubMed] [Google Scholar]
- 43.Clemens LG, Gladue BA, Coniglio LP. Prenatal endogenous androgenic influences on masculine sexual behavior and genital morphology in male and female rats. Horm Behav. 1978;10:40–53. doi: 10.1016/0018-506x(78)90023-5. [DOI] [PubMed] [Google Scholar]
- 44.Morris M, Lucion AB. Antisense oligonucleotides in the study of neuroendocrine systems. J Neuroendocrinol. 1995;7:493–500. doi: 10.1111/j.1365-2826.1995.tb00787.x. [DOI] [PubMed] [Google Scholar]
- 45.Zhou Y, Watters JJ, Dorsa DM. Estrogen rapidly induces the phosphorylation of the cAMP response element binding protein in rat brain. Endocrinology. 1996;137:2163–2166. doi: 10.1210/endo.137.5.8612562. [DOI] [PubMed] [Google Scholar]
- 46.Carlstrom L, Ke Z, Unnerstall JR, Cohen RS, Pandey SC. Estrogen modulation of the cyclic AMP response element-binding protein pathway: effects of long-term and acute treatments. Neuroendocrinology. 2001;74:227–243. doi: 10.1159/000054690. [DOI] [PubMed] [Google Scholar]
- 47.Gu GB, Rojo AA, Zee MC, Yu JH, Simerly RB. Hormonal regulation of CREB phosphorylation in the anteroventral periventricular nucleus. J Neurosci. 1996;16:3035–3044. doi: 10.1523/JNEUROSCI.16-09-03035.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Auger AP, Hexter DP, McCarthy MM. Sex difference in the phosphorylation of cAMP response element binding protein (CREB) in neonatal rat brain. Brain Res. 2001;890:110–117. doi: 10.1016/s0006-8993(00)03151-6. [DOI] [PubMed] [Google Scholar]