Abstract
Varenicline a partial agonist for α4β2 nicotinic acetylcholine receptors (nAChRs) and full agonist for α7 nAChRs, has been approved for the treatment of smoking cessation. While recent clinical trials support the efficacy of varenicline for managing global nicotine withdrawal symptoms and for smoking cessation, varenicline effects on specific withdrawal-associated behaviors in animal models have not been tested. In mice and humans, withdrawal from chronic nicotine disrupts cognitive processing; in mice, this can be measured by changes in contextual fear conditioning. To elucidate potential mechanisms underlying the clinical efficacy of varenicline, the present study evaluated the effects of varenicline on contextual fear conditioning when administered alone and when administered 24 hours after withdrawal from chronic nicotine administration (6.3 mg/kg/day). Varenicline (0.01, 0.1, 1.0 mg/kg) had no effect on contextual fear conditioning when administered alone. However, varenicline dose-dependently prevented nicotine withdrawal-associated deficits in contextual fear conditioning. These data demonstrate that varenicline reverses nicotine withdrawal-induced deficits in an animal model and suggest that varenicline may be effective at treating nicotine withdrawal-associated deficits in learning and memory.
Keywords: Nicotine, Withdrawal, Hippocampus, Fear Conditioning, Addiction, Acetylcholine, Cognition
Introduction
As the leading preventable cause of death in the United States (CDC, 2005), cigarette smoking has dire consequences to both individuals and society. In the United States, over 435,000 deaths are attributed to smoking annually (Mokdad et al., 2004) and the combined direct and indirect economic costs of cigarette smoking are estimated to be 138 billion dollars per year (Rice, 1999). Until recently, the only available FDA-approved pharmacotherapies for smoking cessation included nicotine replacement therapies (NRTs) and bupropion (Frishman et al., 2006; Wu et al., 2006). Varenicline, a partial agonist for α4β2 nicotinic acetylcholine receptors (nAChRs) and full agonist for α7 nAChRs (Mihalak et al., 2006) is the newest FDA-approved medication. Clinical trial data document its superiority to bupropion and NRTs, with quit rates of 50% at the end of treatment and 35% at 6-month follow-up (Gonzales et al., 2006; Jorenby et al., 2006). However, even with pharmacotherapy and counseling, the vast majority of smokers who make a quit attempt will relapse (Schnoll and Lerman, 2006). An increased understanding of the mechanisms through which varenicline facilitates smoking cessation could provide information valuable to medication development for nicotine dependence.
Withdrawal from nicotine, the chemical responsible for tobacco dependence (Benowitz, 1988; DHHS, 1987) produces multiple negative symptoms, including sleep disturbances, negative affect, increased appetite, and cravings (Hughes et al., 1994; Jarvis, 2004), and varenicline can reduce the severity of these symptoms (Gonzales et al., 2006; Jorenby et al., 2006; Nakamura et al., 2007). Additional studies have established that alterations of cognition, including difficulty in concentration (Hughes et al., 1991; Hughes et al., 1994), impairments in attention (Rukstalis et al, 2005; Jacobsen et al., 2005), and deficits in learning and memory (Jacobsen et al., 2005; Mendrek et al., 2006; Jacobsen et al., 2007) also occur in humans during nicotine withdrawal. Importantly, these symptoms can increase the risk of relapse in smokers who attempt to quit (Rukstalis et al, 2005). However, no studies to date have investigated the effects of varenicline on nicotine withdrawal-related deficits in cognition.
Although current research in humans has not addressed the effects of varenicline on nicotine withdrawal-associated deficits in cognition, animal models have been used effectively to evaluate the effects of other smoking cessation drugs (Lerman et al, 2007). One such animal model is Pavlovian fear conditioning, in which a conditioned stimulus (CS) is paired with an aversive unconditioned stimulus (US). Subjects trained in this procedure acquire two associations: an association between the training context and the US (contextual conditioning), and an association between the CS and US (cued conditioning). Research has demonstrated that acute nicotine enhances contextual conditioning, but not cued conditioning (Gould and Wehner, 1999; Gould and Higgins, 2003; Wehner et al., 2004). Conversely, withdrawal from chronic nicotine administration, at a dose comparable to that reported in human smokers (Benowitz et al., 1989; Henningfield and Keenan, 1993; Davis et al., 2005), produces deficits in contextual conditioning but does not affect cued conditioning (Davis et al., 2005; Davis and Gould, 2007; Davis et al., 2007; Portugal and Gould, 2007; Portugal et al., 2008). Nicotine withdrawal-related deficits in contextual conditioning can be ameliorated by nicotine replacement (Davis et al., 2005), by the norepinephrine and dopamine reuptake inhibitor bupropion (Portugal and Gould, 2007), and by the norepinephrine reuptake inhibitor atomoxetine (Davis and Gould, 2007).
As no studies have reported the effects of varenicline on withdrawal-associated cognitive deficits or the effects of varenicline in an animal model of nicotine withdrawal, the goal of the present study was to examine the effects of varenicline on nicotine withdrawal-induced deficits in contextual conditioning in C57BL/6 mice. Additional experiments evaluated whether varenicline administered only on training day or testing day would ameliorate nicotine withdrawal-associated deficits in contextual conditioning.
Methods
Subjects
Male C57BL/6 mice were trained at 8-12 weeks of age (n =7-12). Mice were provided with ad libitum access to food and water, maintained on a 12 hour light-dark cycle (lights on at 7:00am), and housed in groups of two or four. Behavioral procedures occurred during the light phase of the cycle. All behavioral and surgical procedures were approved by the Temple University Institutional Animal Care and Use Committee.
Apparatus
The training and testing of contextual fear conditioning was conducted in four identical conditioning chambers (Med-Associates, St. Albans, VT). The chamber floors were composed of 18 stainless steel bars, connected to a shock generator and scrambler (Med-Associates), through which a 2 second 0.57 mA footshock (US) was administered. Speakers attached to the right wall of each chamber provided an 85 dB white noise (CS). Ventilation fans, which provided background noise (69 dB), were mounted on the right wall of each sound-attenuating box. All stimulus administrations were controlled by a computer running Med-PC software (Med-Associates).
Behavioral Procedures: Contextual Fear Conditioning
During training and testing mice were observed for freezing at 10-second intervals (Gould and Wehner, 1999). Freezing was defined as the absence of all movement except for respiration (Blancard and Blanchard, 1969). Training began with the activation of a house light. Baseline activity was scored for 120 s. Next, two co-terminating CS-US pairings, separated by a 120 s inter-trial interval, were presented. CS presentation lasted for 30 s and during the final two seconds the US was presented. Immediate freezing was scored during the 120 s inter-trial interval. Thirty seconds following the second CS-US pairing the house light was extinguished and mice were returned to their home cages. To test for contextual fear conditioning mice were placed in the training apparatus 24 hours following training and observed for freezing for 5 minutes. At the beginning of the testing session the house light was activated. During testing no CS was presented. Cued fear conditioning was not assessed because multiple studies have shown that withdrawal from chronic nicotine does not alter cued fear conditioning (Davis et al., 2005; Davis and Gould, 2007; Davis et al., 2007; Portugal and Gould, 2007; Portugal et al., 2008).
Drug Administration
Varenicline, generously provided by Pfizer (New York, NY), was dissolved in saline and administered subcutaneously one hour before training and/or testing for all experiments. During experiments in which varenicline was administered at both training and testing, mice were treated with 0, 0.01, 0.1, or 1 mg/kg varenicline; only the 0.1 mg/kg dose or saline was used in experiments where varenicline was administered only at training or at testing. Doses and timing of drug administration were based on communications with Dr. Hans Rolema, of Pfizer.
For experiments involving nicotine withdrawal, nicotine hydrogen tartrate salt (Sigma, St. Louis, MO) was dissolved in saline and administered via mini-osmotic pumps (model 1002; Alzet, Cupertino, CA) at a dose of 6.3 mg/kg/day (dose reported in freebase). The selection of this dose of chronic nicotine was based on previous studies that reported nicotine withdrawal deficits in contextual fear conditioning (Davis et al., 2005; Davis and Gould, 2007; Portugal and Gould, 2007; Portugal et al., 2008) and research demonstrating that this dose of chronic nicotine produces plasma nicotine levels within the range observed in smokers (Benowitz et al., 1989; Henningfield and Keenan, 1993; Davis et al., 2005). On day 1, mini-osmotic pumps were implanted subcutaneously into anesthetized mice. Chronic nicotine or saline was administered for 12 days following pump implantation. All pumps were removed on day 12. Training and testing took place on days 13 and 14, respectively.
Experimental Design
The first experiment examined if varenicline at training and testing in control and nicotine withdrawn mice altered fear conditioning. Because the middle dose of varenicline ameliorated nicotine withdrawal deficits in learning, a second series of experiments were conducted to test whether varenicline alters processes involved in acquisition or recall in nicotine-withdrawn mice. Thus, for one condition, control mice and nicotine-withdrawn mice received varenicline or saline at training and for the other condition control and withdrawn mice received varenicline or saline at testing.
Statistical Analyses
Data were analyzed with one-way ANOVAs. Homogeneity of variance was tested with the Levene statistic (Cohen, 2001). Tukey post hoc tests were conducted on data sets meeting the homogeneity of variance assumption (Cohen, 2001). Games-Howell post hoc tests were conducted on data sets not meeting the homogeneity of variance assumption (Maxwell & Delaney, 2003). Differences between groups exceeding (p < 0.05) are reported as significantly different.
Results
The effects of varenicline on contextual conditioning
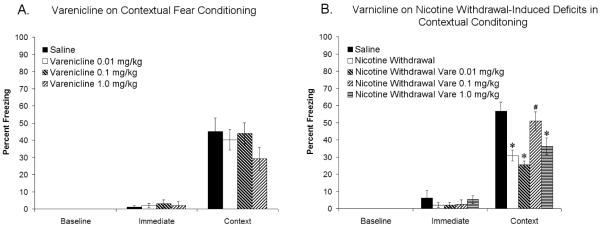
To determine if varenicline altered contextual conditioning, varenicline (0, 0.01, 0.1, or 1 mg/kg) was administered to drug-naïve mice prior to training and testing of contextual fear conditioning. Varenicline had no significant effect on measures of baseline, immediate, or contextual freezing (Figure 1A), suggesting that varenicline has no effect on contextual conditioning at these doses.
Figure 1.
Varenicline at doses of 0.01, 0.1, and 1.0 mg/kg does not affect contextual fear conditioning (Figure 1A). Deficits in contextual fear conditioning produced by withdrawal of chronic nicotine administration are ameliorated by varenicline (0.1 mg/kg) (Figure 1B). Significant difference (p < 0.05) from saline treated groups denoted with (*), significant difference (p < 0.05) from nicotine withdrawal groups denoted by (#).
The effects of varenicline on nicotine withdrawal-induced deficits in contextual conditioning
To determine the effect of varenicline on nicotine withdrawal-induced deficits in contextual conditioning, varenicline (0, 0.01, 0.1 & 1 mg/kg) was administered to nicotine-withdrawn mice prior to the training and testing of contextual fear conditioning. An ANOVA revealed significant differences in contextual freezing between drug treatment groups [F(4, 34) = 10.72, p < 0.05], but showed no effect of drug condition on either baseline or immediate freezing. Tukey’s post-hoc tests demonstrated that nicotine-withdrawn mice receiving 0, 0.01, or 1 mg/kg varenicline froze significantly less than saline-withdrawn mice, and that nicotine-withdrawn mice receiving 0.1 mg/kg varenicline were significantly different from nicotine-withdrawn mice receiving 0, 0.01, or 1 mg/kg varenicline and not significantly different from mice withdrawn from saline (Figure 1B). These results suggest that varenicline dose-dependently ameliorates nicotine withdrawal-induced deficits in contextual conditioning.
The effects of varenicline on the acquisition or recall of contextual fear conditioning
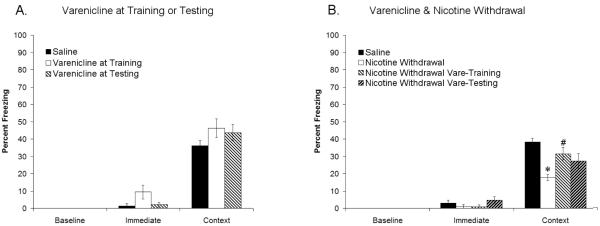
As we were interested in determining whether the effects of varenicline on nicotine withdrawal-induced deficits in contextual conditioning occur during training or testing, we first administered varenicline (0.1 mg/kg) to drug-naïve mice prior to either training or testing of contextual conditioning to determine if varenicline had any effect if administered at those time points. Varenicline, administered at either training or testing, did not significantly alter baseline, immediate, or contextual freezing (Figure 2A), suggesting that varenicline (0.1 mg/kg) has no effect on contextual conditioning if administered only at training or testing.
Figure 2.
Varenicline (0.1 mg/kg) has no effect on contextual fear conditioning if administered only at training or testing (Figure 2A). Deficits in contextual fear conditioning produced by withdrawal of chronic nicotine administration are ameliorated by varenicline at training (Figure 2B). Significant difference (p < 0.05) from saline treated groups denoted with (*), significant difference (p < 0.05) from nicotine withdrawal groups denoted by (#).
The effect of varenicline on acquisition or recall during withdrawal from chronic nicotine
To determine if varenicline ameliorates nicotine withdrawal-induced deficits in contextual conditioning by affecting processes that occur during training or testing, we administered varenicline (0.1 mg/kg) to nicotine-withdrawn mice prior to either training or testing of contextual conditioning. An ANOVA revealed significant differences in contextual freezing between drug treatment groups [F(3,33) = 6.08, p < 0.05], but no differences in baseline or immediate freezing. Games-Howell post hoc tests showed that nicotine-withdrawn mice froze significantly less than saline-withdrawn controls, that varenicline administered at training to nicotine-withdrawn mice resulted in freezing that was no different than saline-withdrawn controls, and that varenicline treatment at testing of nicotine-withdrawn mice resulted in an intermediate level of freezing which was not significantly different from saline- or nicotine-withdrawn mice (Figure 2B). These results suggest that varenicline ameliorates nicotine withdrawal-induced deficits in processes related to the training of contextual conditioning.
Discussion
This study is the first to demonstrate that varenicline ameliorates nicotine withdrawal-induced deficits in cognitive processing. Specifically, varenicline reversed withdrawal-induced deficits in contextual conditioning in C57BL/6 mice. These findings suggest that varenicline may be effective in ameliorating the nicotine-withdrawal associated cognitive deficits that are reported in human smokers (Bell et al, 1999; Blake & Smith, 1997; Hughes et al, 1989; Jacobsen et al, 2005; Mendrek et al, 2006; Rukstalis et al, 2005). In addition, varenicline administered only at training is sufficient to ameliorate nicotine withdrawal-induced deficits in contextual conditioning, suggesting that varenicline may ameliorate nicotine withdrawal-induced deficits in processes related to the acquisition or consolidation of contextual learning. Although varenicline administration at testing did not reverse nicotine withdrawal-induced deficits in contextual conditioning, an intermediate effect was observed (Figure 2B). This suggests that varenicline may also act to ameliorate nicotine withdrawal-induced deficits in retrieval of contextual learning.
The mechanisms underlying varenicline’s amelioration of nicotine withdrawal-induced deficits in contextual conditioning have yet to be determined. Varenicline is a partial agonist for α4β2 nAChRs (i.e. high-affinity nAChRs) (Mihalak et al., 2006) and the high-affinity nAChR antagonist DHβE precipitates deficits in chronic nicotine treated mice. Further, nicotine withdrawal-induced deficits in contextual conditioning do not occur in β2 knockout mice (Portugal et al, 2008). Thus, it seems likely that varenicline ameliorates nicotine withdrawal-induced deficits in contextual conditioning through its action on the α4β2 nAChR. However even though α7 nAChRs are not critically involved in contextual fear conditioning (Davis & Gould; 2007; Wehner et al, 2004), it is possible that varenicline also acts on the α7 nAChR to ameliorate nicotine withdrawal-induced deficits in this task. Further research is necessary to determine which of these nicotinic receptors is involved in the effects of varenicline on nicotine withdrawal-induced deficits in contextual conditioning.
This study adds to previous research suggesting that contextual fear conditioning could be a useful screening tool for potential smoking cessation agents. First, deficits in contextual fear conditioning can be induced by withdrawal of a dose of chronic nicotine that produces plasma nicotine levels within the range reported in human smokers (Benowitz et al, 1982; Benowitz, 1988; Davis et al, 2005). Additionally, withdrawal-induced deficits in contextual fear conditioning can be ameliorated by smoking cessation therapies including; acute nicotine (NRT), bupropion, and varenicline (Davis et al, 2005; Portugal & Gould, 2007). Another strength of this animal model is that the effects of nicotine withdrawal on learning observed in our model are consistent with the disruption of cognition that is reported during human smoking cessation (Hughes et al, 1991; Jacobsen et al, 2005; Mendrek et al, 2006, Hughes, 2007; Jacobsen et al, 2007). In contrast, other models have focused on somatic withdrawal symptoms (Malin et al, 1992; Malin, 2001), which may not reflect the typical symptoms reported during nicotine withdrawal in humans (West and Gossop, 1004; Hughes, 2007). Therefore, an animal model of cognitive withdrawal symptoms may identify different neural substrates of nicotine withdrawal and potential therapeutic targets than those indicated by a model of somatic withdrawal signs.
Acknowledgements
The authors would like to acknowledge grant support from the National Institute on Drug Abuse (DA017949 TG), the National Cancer Institute and National Institute on Drug Abuse (P5084718 PI: Caryn Lerman Ph.D), additionally, Jonathan D. Raybuck was supported by a NIH/NIDA training grant (T32DA07237), and varenicline was generously provided by Pfizer.
Footnotes
Disclosure/Conflict of Interest
Dr. Lerman has served as a paid consultant for pharmaceutical companies, including Pfizer, GlaxoSmith Kline, and Astra Zeneca, on topics unrelated to the present study. The other authors report no conflicts of interest.
References
- Arnold HM, Nelson CL, Sarter M, Bruno JP. Sensitization of cortical acetylcholine release by repeated administration of nicotine in rats. Psychopharmacology (Berl) 2003;165:346–358. doi: 10.1007/s00213-002-1260-6. [DOI] [PubMed] [Google Scholar]
- Balfour DJ, Benwell ME, Birrell CE, Kelly RJ, Al-Aloul M. Sensitization of the mesoaccumbens dopamine response to nicotine. Pharmacol Biochem Behav. 1998;59:1021–1030. doi: 10.1016/s0091-3057(97)00537-6. [DOI] [PubMed] [Google Scholar]
- Bell SL, Taylor RC, Singleton EG, Henningfield JE, Heishman SJ. Smoking after nicotine deprivation enhances cognitive performance and decreases tobacco craving in drug abusers. Nicotine Tob Res. 1999;1(1):45–52. doi: 10.1080/14622299050011141. [DOI] [PubMed] [Google Scholar]
- Benowitz NL, Kuyt F, Jacob P., 3rd Circadian blood nicotine concentrations during cigarette smoking. Clin Pharmacol Ther. 1982;32(6):758–64. doi: 10.1038/clpt.1982.233. [DOI] [PubMed] [Google Scholar]
- Benowitz NL. Pharmacologic aspects of cigarette smoking and nicotine addiction. The new England Journal of Medicine. 1988;319(20):1319. doi: 10.1056/NEJM198811173192005. [DOI] [PubMed] [Google Scholar]
- Benowitz NL, Porchet H, Sheiner L, Jacob P., 3rd Nicotine absorption and cardiovascular effects with smokeless tobacco use: comparison with cigarettes and nicotine gum. Clin Pharmacol Ther. 1988;44(1):23–8. doi: 10.1038/clpt.1988.107. [DOI] [PubMed] [Google Scholar]
- Benowitz NL, Porchet H, Jacob P., 3rd Nicotine dependence and tolerance in man: pharmacokinetic and pharmacodynamic investigations. Prog Brain Res. 1989;79:279–287. doi: 10.1016/s0079-6123(08)62487-5. [DOI] [PubMed] [Google Scholar]
- Blake J, Smith A. Effects of smoking deprivation on the articulatory loop of working memory. Human Psychopharmacology. 1997;12:259–264. [Google Scholar]
- Blanchard RJ, Blanchard DC. Crouching as an index of fear. J Comp Physiol Psychol. 1969;67:370–375. doi: 10.1037/h0026779. [DOI] [PubMed] [Google Scholar]
- CDC Annual smoking-attributable mortality, years of potential life lost, and productivity losses--United States, 1997-2001. MMWR Morb Mortal Wkly Rep. 2005;54:625–628. [PubMed] [Google Scholar]
- Cohen BH. Explaining psychological statistics. 2nd ed. Wiley; New York: 2001. [Google Scholar]
- Collins AC, Romm E, Wehner JM. Dissociation of the apparent relationship between nicotine tolerance and up-regulation of nicotinic receptors. Brain Res Bull. 1990;25:373–379. doi: 10.1016/0361-9230(90)90222-l. [DOI] [PubMed] [Google Scholar]
- Davis JA, Gould TJ. Atomoxetine reverses nicotine withdrawal-associated deficits in contextual fear conditioning. Neuropsychopharmacology. 2007;32:2011–2019. doi: 10.1038/sj.npp.1301315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis JA, Kenney JW, Gould TJ. Hippocampal alpha4beta2 nicotinic acetylcholine receptor involvement in the enhancing effect of acute nicotine on contextual fear conditioning. J Neurosci. 2007;27:10870–10877. doi: 10.1523/JNEUROSCI.3242-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis JA, James JR, Siegel SJ, Gould TJ. Withdrawal from chronic nicotine administration impairs contextual fear conditioning in C57BL/6 mice. J Neurosci. 2005;25:8708–8713. doi: 10.1523/JNEUROSCI.2853-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Department of Health and Human Services. Public Health Service . The health consequences of smoking: nicotine addiction: A report of the surgeon general. Government printing office; Washington, DC: 1988. DHHS publication no. (CDC) 88-8406. [Google Scholar]
- Flores CM, Rogers SW, Pabreza LA, Wolfe BB, Kellar KJ. A subtype of nicotinic cholinergic receptor in rat brain is composed of alpha 4 and beta 2 subunits and is up-regulated by chronic nicotine treatment. Mol Pharmacol. 1992;41:31–37. [PubMed] [Google Scholar]
- Frishman WH, Mitta W, Kupersmith A, Ky T. Nicotine and non-nicotine smoking cessation pharmacotherapies. Cardiol Rev. 2006;14:57–73. doi: 10.1097/01.crd.0000172309.06270.25. [DOI] [PubMed] [Google Scholar]
- Gentry CL, Wilkins LH, Jr., Lukas RJ. Effects of prolonged nicotinic ligand exposure on function of heterologously expressed, human alpha4beta2- and alpha4beta4-nicotinic acetylcholine receptors. J Pharmacol Exp Ther. 2003;304:206–216. doi: 10.1124/jpet.102.041756. [DOI] [PubMed] [Google Scholar]
- Gonzales D, Rennard SI, Nides M, Oncken C, Azoulay S, Billing CB, Watsky EJ, Gong J, Williams KE, Reeves KR. Varenicline, an alpha4beta2 nicotinic acetylcholine receptor partial agonist, vs sustained-release bupropion and placebo for smoking cessation: a randomized controlled trial. Jama. 2006;296:47–55. doi: 10.1001/jama.296.1.47. [DOI] [PubMed] [Google Scholar]
- Gould TJ. Nicotine and hippocampus-dependent learning: implications for addiction. Mol Neurobiol. 2006;34:93–107. doi: 10.1385/MN:34:2:93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gould TJ, Wehner JM. Nicotine enhancement of contextual fear conditioning. Behav Brain Res. 1999;102:31–39. doi: 10.1016/s0166-4328(98)00157-0. [DOI] [PubMed] [Google Scholar]
- Gould TJ, Higgins SJ. Nicotine enhances contextual fear conditioning in C57BL/6J mice at 1 and 7 days post-training. Neurobiol Learn Mem. 2003;80:147–157. doi: 10.1016/s1074-7427(03)00057-1. [DOI] [PubMed] [Google Scholar]
- Henningfield JE, Keenan RM. Nicotine delivery kinetics and abuse liability. J Consult Clin Psychol. 1993;61:743–750. doi: 10.1037//0022-006x.61.5.743. [DOI] [PubMed] [Google Scholar]
- Henningfield JE, Fant RV, Buchhalter AR, Stitzer ML. Pharmacotherapy for nicotine dependence. CA Cancer J Clin. 2005;55:281–299. doi: 10.3322/canjclin.55.5.281. quiz 322-283, 325. [DOI] [PubMed] [Google Scholar]
- Hughes JR, Keenan RM, Yellin A. Effect of tobacco withdrawal on sustained attention. Addict Behav. 1989;14(5):577–80. doi: 10.1016/0306-4603(89)90079-8. [DOI] [PubMed] [Google Scholar]
- Hughes JR, Gust SW, Skoog K, Keenan RM, Fenwick JW. Symptoms of tobacco withdrawal. A replication and extension. Arch Gen Psychiatry. 1991;48:52–59. doi: 10.1001/archpsyc.1991.01810250054007. [DOI] [PubMed] [Google Scholar]
- Hughes JR, Higgins ST, Bickel WK. Nicotine withdrawal versus other drug withdrawal syndromes: similarities and dissimilarities. Addiction. 1994;89:1461–1470. doi: 10.1111/j.1360-0443.1994.tb03744.x. [DOI] [PubMed] [Google Scholar]
- Jacobsen LK, Krystal JH, Mencl WE, Westerveld M, Frost SJ, Pugh KR. Effects of smoking and smoking abstinence on cognition in adolescent tobacco smokers. Biol Psychiatry. 2005;57:56–66. doi: 10.1016/j.biopsych.2004.10.022. [DOI] [PubMed] [Google Scholar]
- Jacobsen LK, Mencl WE, Constable RT, Westerveld M, Pugh KR. Impact of smoking abstinence on working memory neurocircuitry in adolescent daily tobacco smokers. Psychopharmacology (Berl) 2007;193:557–566. doi: 10.1007/s00213-007-0797-9. [DOI] [PubMed] [Google Scholar]
- Jarvis MJ. Why people smoke. Bmj. 2004;328:277–279. doi: 10.1136/bmj.328.7434.277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jorenby DE, Hays JT, Rigotti NA, Azoulay S, Watsky EJ, Williams KE, Billing CB, Gong J, Reeves KR. Efficacy of varenicline, an alpha4beta2 nicotinic acetylcholine receptor partial agonist, vs placebo or sustained-release bupropion for smoking cessation: a randomized controlled trial. Jama. 2006;296:56–63. doi: 10.1001/jama.296.1.56. [DOI] [PubMed] [Google Scholar]
- Lerman C, LeSage MG, Perkins KA, O’Malley SS, Siegel SJ, Benowitz NL, Corrigall WA. Translational research in medication development for nicotine dependence. Nat Rev Drug Discov. 2007;6(9):746–62. doi: 10.1038/nrd2361. [DOI] [PubMed] [Google Scholar]
- Lopez-Hernandez GY, Sanchez-Padilla J, Ortiz-Acevedo A, Lizardi-Ortiz J, Salas-Vincenty J, Rojas LV, Lasalde-Dominicci JA. Nicotine-induced up-regulation and desensitization of alpha4beta2 neuronal nicotinic receptors depend on subunit ratio. J Biol Chem. 2004;279:38007–38015. doi: 10.1074/jbc.M403537200. [DOI] [PubMed] [Google Scholar]
- Malin DH, Lake JR, Newlin-Maultsby P, Roberts LK, Lanier JG, Carter VA, Cunningham JS, Wilson OB. Rodent model of nicotine abstinence syndrome. Pharmacol Biochem Behav. 1992;43(3):779–84. doi: 10.1016/0091-3057(92)90408-8. [DOI] [PubMed] [Google Scholar]
- Malin DH. Nicotine dependence: studies with a laboratory model. Pharmacol Biochem Behav. 2001;70(4):551–9. doi: 10.1016/s0091-3057(01)00699-2. [DOI] [PubMed] [Google Scholar]
- Mamede M, Ishizu K, Ueda M, Mukai T, Iida Y, Kawashima H, Fukuyama H, Togashi K, Saji H. Temporal Change in Human Nicotinic Acetylcholine Receptor After Smoking Cessation: 5IA SPECT Study. J Nucl Med. 2007;48:1829–1835. doi: 10.2967/jnumed.107.043471. [DOI] [PubMed] [Google Scholar]
- Marks MJ, Stitzel JA, Collins AC. Time course study of the effects of chronic nicotine infusion on drug response and brain receptors. J Pharmacol Exp Ther. 1985;235:619–628. [PubMed] [Google Scholar]
- Marks MJ, Pauly JR, Gross SD, Deneris ES, Hermans-Borgmeyer I, Heinemann SF, Collins AC. Nicotine binding and nicotinic receptor subunit RNA after chronic nicotine treatment. J Neurosci. 1992;12:2765–2784. doi: 10.1523/JNEUROSCI.12-07-02765.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maxwell SE, Delaney HD. Designing experiments and analyzing data: A model comparison perspective. 2nd ed. Erlbaum; Mahwah,NJ: 2003. [Google Scholar]
- Mendrek A, Monterosso J, Simon SL, Jarvik M, Brody A, Olmstead R, Domier CP, Cohen MS, Ernst M, London ED. Working memory in cigarette smokers: comparison to non-smokers and effects of abstinence. Addict Behav. 2006;31:833–844. doi: 10.1016/j.addbeh.2005.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mihalak KB, Carroll FI, Luetje CW. Varenicline is a partial agonist atalpha4beta2 and a full agonist at alpha7 neuronal nicotinic receptors. Mol Pharmacol. 2006;70:801–805. doi: 10.1124/mol.106.025130. [DOI] [PubMed] [Google Scholar]
- Mokdad AH, Marks JS, Stroup DF, Gerberding JL. Actual causes of death in the United States, 2000. Jama. 2004;291:1238–1245. doi: 10.1001/jama.291.10.1238. [DOI] [PubMed] [Google Scholar]
- Nakamura M, Oshima A, Fujimoto Y, Maruyama N, Ishibashi T, Reeves KR. Efficacy and tolerability of varenicline, an alpha4beta2 nicotinic acetylcholine receptor partial agonist, in a 12-week, randomized, placebo-controlled, dose-response study with 40-week follow-up for smoking cessation in Japanese smokers. Clin Ther. 2007;29:1040–1056. doi: 10.1016/j.clinthera.2007.06.012. [DOI] [PubMed] [Google Scholar]
- Nguyen HN, Rasmussen BA, Perry DC. Subtype-selective up-regulation by chronic nicotine of high-affinity nicotinic receptors in rat brain demonstrated by receptor autoradiography. J Pharmacol Exp Ther. 2003;307:1090–1097. doi: 10.1124/jpet.103.056408. [DOI] [PubMed] [Google Scholar]
- Nides M, Oncken C, Gonzales D, Rennard S, Watsky EJ, Anziano R, Reeves KR. Smoking cessation with varenicline, a selective alpha4beta2 nicotinic receptor partial agonist: results from a 7-week, randomized, placebo- and bupropion-controlled trial with 1-year follow-up. Arch Intern Med. 2006;166:1561–1568. doi: 10.1001/archinte.166.15.1561. [DOI] [PubMed] [Google Scholar]
- Olale F, Gerzanich V, Kuryatov A, Wang F, Lindstrom J. Chronic nicotine exposure differentially affects the function of human alpha3, alpha4, and alpha7 neuronal nicotinic receptor subtypes. J Pharmacol Exp Ther. 1997;283:675–683. [PubMed] [Google Scholar]
- Peng X, Gerzanich V, Anand R, Whiting PJ, Lindstrom J. Nicotine-induced increase in neuronal nicotinic receptors results from a decrease in the rate of receptor turnover. Mol Pharmacol. 1994a;46:523–530. [PubMed] [Google Scholar]
- Peng X, Katz M, Gerzanich V, Anand R, Lindstrom J. Human alpha 7 acetylcholine receptor: cloning of the alpha 7 subunit from the SH-SY5Y cell line and determination of pharmacological properties of native receptors and functional alpha 7 homomers expressed in Xenopus oocytes. Mol Pharmacol. 1994b;45:546–554. [PubMed] [Google Scholar]
- Peng X, Gerzanich V, Anand R, Wang F, Lindstrom J. Chronic nicotine treatment up-regulates alpha3 and alpha7 acetylcholine receptor subtypes expressed by the human neuroblastoma cell line SH-SY5Y. Mol Pharmacol. 1997;51:776–784. doi: 10.1124/mol.51.5.776. [DOI] [PubMed] [Google Scholar]
- Perry DC, Davila-Garcia MI, Stockmeier CA, Kellar KJ. Increased nicotinic receptors in brains from smokers: membrane binding and autoradiography studies. J Pharmacol Exp Ther. 1999;289:1545–1552. [PubMed] [Google Scholar]
- Pietila K, Lahde T, Attila M, Ahtee L, Nordberg A. Regulation of nicotinic receptors in the brain of mice withdrawn from chronic oral nicotine treatment. Naunyn Schmiedebergs Arch Pharmacol. 1998;357:176–182. doi: 10.1007/pl00005152. [DOI] [PubMed] [Google Scholar]
- Portugal GS, Gould TJ. Bupropion dose-dependently reverses nicotine withdrawal deficits in contextual fear conditioning. Pharmacol Biochem Behav. 2007;88:179–187. doi: 10.1016/j.pbb.2007.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Portugal GS, Kenney JW, Gould TJ. beta2 subunit containing acetylcholine receptors mediate nicotine withdrawal deficits in the acquisition of contextual fear conditioning. Neurobiol Learn Mem. 2008;89(2):106–113. doi: 10.1016/j.nlm.2007.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rice DP. Economic costs of substance abuse, 1995. Proc Assoc Am Physicians. 1999;111:119–125. doi: 10.1046/j.1525-1381.1999.09254.x. [DOI] [PubMed] [Google Scholar]
- Rukstalis M, Jepson C, Patterson F, Lerman C. Increases in hyperactive-impulsive symptoms predict relapse among smokers in nicotine replacement therapy. J Subst Abuse Treat. 2005;28(4):297–304. doi: 10.1016/j.jsat.2005.02.002. [DOI] [PubMed] [Google Scholar]
- Schnoll RA, Lerman C. Current and emerging pharmacotherapies for treating tobacco dependence. Expert Opin Emerg Drugs. 2006;11(3):429–44. doi: 10.1517/14728214.11.3.429. [DOI] [PubMed] [Google Scholar]
- Tonstad S, Tonnesen P, Hajek P, Williams KE, Billing CB, Reeves KR. Effect of maintenance therapy with varenicline on smoking cessation: a randomized controlled trial. Jama. 2006;296:64–71. doi: 10.1001/jama.296.1.64. [DOI] [PubMed] [Google Scholar]
- Wehner JM, Keller JJ, Keller AB, Picciotto MR, Paylor R, Booker TK, Beaudet A, Heinemann SF, Balogh SA. Role of neuronal nicotinic receptors in the effects of nicotine and ethanol on contextual fear conditioning. Neuroscience. 2004;129:11–24. doi: 10.1016/j.neuroscience.2004.07.016. [DOI] [PubMed] [Google Scholar]
- West R, Gossop M. Overview: a comparison of withdrawal symptoms from different drug classes. Addiction. 1994;89(11):1483–9. doi: 10.1111/j.1360-0443.1994.tb03747.x. [DOI] [PubMed] [Google Scholar]
- Whiteaker P, Sharples CG, Wonnacott S. Agonist-induced up-regulation of alpha4beta2 nicotinic acetylcholine receptors in M10 cells: pharmacological and spatial definition. Mol Pharmacol. 1998;53:950–962. [PubMed] [Google Scholar]
- Wooltorton JR, Pidoplichko VI, Broide RS, Dani JA. Differential desensitization and distribution of nicotinic acetylcholine receptor subtypes in midbrain dopamine areas. J Neurosci. 2003;23:3176–3185. doi: 10.1523/JNEUROSCI.23-08-03176.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu P, Wilson K, Dimoulas P, Mills EJ. Effectiveness of smoking cessation therapies: a systematic review and meta-analysis. BMC Public Health. 2006;6:300. doi: 10.1186/1471-2458-6-300. [DOI] [PMC free article] [PubMed] [Google Scholar]