Abstract
Illuminating the neural mechanisms subserving lexico-semantic processing is requisite to further understanding the neurophysiological basis of the dyslexias. Yet, despite numerous functional neuroimaging experiments, the location and temporal behavior of brain regions mediating word-level language processing remain an area of debate. Such investigations typically utilize the word/pseudoword contrast within hemodynamic measurements, and report several left hemisphere regions that respond more strongly to pseudowords, but fail to replicate neural areas unique to real word processing. The present experiment addressed this problem from a different perspective. Mainly, we hypothesized that the time course, but not the neuroanatomy would show within-subject across-condition disparities. For that purpose, we applied dipole-modeling techniques to high-density magnetoencephalographic recordings of healthy subjects, and utilized excellent spatiotemporal accuracy to demonstrate significant across-condition differences in the time domain, along with indistinguishable neural correlates within-subject. In all participants, both words and pseudowords elicited activity in left perisylvian language areas, with words consistently activating these regions ~100 msec earlier than pseudowords. Considerable functional heterogeneity was also observed, and this may underlie the inconsistencies among previous studies. We conclude that the neural distinction in word/pseudoword processing is not in spatial localization, but is better conceptualized as a dynamic difference in processing time.
Keywords: dyslexia, language, magnetoencephalography (MEG), perisylvian, word
Introduction
Many functional neuroimaging experiments have contrasted words (e.g., table) and pronounceable pseudowords (e.g., baper) to elucidate the neural mechanisms subserving lexicality, semantics, phonology, and/or other subcomponents of the language processing faculties. The most consistent observation amongst this data is that relative to words, pseudowords elicit more robust activation in the left inferior temporal gyrus (ITG) (Price et al., 1996; Brunswick et al., 1999; Paulesu et al., 2000; Xu et al., 2001) and the left inferior frontal gyrus (IFG) (Herbster et al., 1997; Rumsey et al., 1997; Brunswick et al., 1999; Fiez et al., 1999; Hagoort et al., 1999; Paulesu et al., 2000; Xu et al., 2001; Binder et al., 2003). Although other brain regions (e.g., right cerebellum, left supramarginal gyrus) have shown significant activation to pseudowords on a study-specific basis, this contrast has mostly yielded widely replicable results (i.e., IFG and ITG activation). To the contrary, brain areas exhibiting enhanced activity to words relative to pseudowords have been entirely study-specific. For example, one group observed greater activity in vast regions of the temporal lobe bilaterally (Hagoort et al., 1999), another study reported widespread left parahippocampal activation (Rumsey et al., 1997), whereas other groups found no brain regions exhibiting heightened activity to common words (Fiebach et al., 2002; Mechelli et al., 2003). In sum, the data on this comparison (word > pseudoword) does not qualify any specific neural area as uniquely burdened during word processing.
While investigations focusing on this comparison have added much to our understanding, they have also functioned as a venue for highlighting inconsistent findings within the cognitive neuroscience of language processing (Binder et al., 2003; Mechelli et al., 2003). On the surface, this contrast seems straightforward with minimal possibility of confounds. However, as recently reviewed, a multitude of factors could underlie the divergent results (Mechelli et al., 2003). For example, nonlexical aspects of the stimuli can introduce confounds (e.g., bigram frequency differences, number of syllables, etc.), utilizing low statistical thresholds by not correcting for multiple comparisons can allow false-positives to obscure the results (e.g., all studies cited above report uncorrected p values, except Mechelli et al., 2003), and finally variability in the duration or presentation rate of stimuli can lead to distinct outcomes in different experiments.
Another possibility is that true effects are simply inconsistent across subject (i.e., different reading strategies, and/or heterogeneity in the neural basis of the construct of interest). Early lesion work investigating the acquired dyslexias initially suggested this explanation (Coslett, 2000). Neuropsychological investigators almost globally recognize the behavioral manifestations of both phonological and surface dyslexia: in phonological dyslexia, patients are able to read both regular (e.g., table) and irregular words (e.g., yacht), but are unable to pronounce even simple pseudowords (Funnell, 1983); in contrast, surface dyslexics can read pseudowords and regular words, but not irregular words (Patterson et al., 1985). Different theoretical models of reading attempt to explain how these lesion-induced disorders may arise in dissimilar ways, but the behavioral aspects of the two disorders are well characterized. Contrary to the agreement in behavioral symptomatology, the necessary lesion site for the occurrence of each disorder is virtually unknown. Patients with the same disorder often have largely distinct lesions, and patients exhibiting different versions of acquired dyslexia often have lesions in more overlapping locations (Coslett, 2000). By virtue of this disparity, much of the available neuropsychological data directly suggests substantial heterogeneity in the neural basis of word-level language processing. In addition, further evidence supporting this position is now available in the neuroimaging literature. By analyzing their functional magnetic resonance imaging (fMRI) data in a random-effect fashion, and investigating single-subject data when group analyses reflected null results, Mechelli et al. (2003) demonstrated highly significant effects (e.g., Z scores > 8) for words relative to pseudowords that were subject-specific in over one-third of their 20-subject sample. Such responses in individual subjects could skew group results in other experiments, especially those employing < 10 subjects, and easily cause divergent observations across hemodynamic studies.
However, true effects in the spatial domain may be inconsistent for a completely different reason when the word/pseudoword contrast is employed within neuroimaging paradigms. Data from positron-emission tomography (PET) and fMRI experiments identify neural regions sufficient for a given cognitive process, but cannot discriminate which areas are absolutely necessary for that cognitive process. Conversely, neuropsychological lesion data can suggest necessary brain regions, yet often such conclusions are arguably confounded. Now, it is possible that identical neural areas activate obligatorily during both word and pseudoword processing, but that only a subset of these regions are necessary for the processing of each word class. From this perspective, the enhanced activation consistently reported for pseudowords (left ITG and IFG) indicates neural populations common to both word and pseudoword processing that are more extensively recruited in processing the unfamiliar stimuli, whereas the existence of both phonological and surface dyslexia indicates that the necessary neural regions for word and pseudoword processing can be selectively damaged. If common neural circuitry is activated obligatorily by both words and pseudowords, then true effects in the spatial domain should be difficult to obtain in hemodynamic measurements.
More established differences are available from behavioral investigations that made use of this same contrast. These studies used reaction time (RT) or voice-onset latency as the dependent measure, and consistently observed slower processing for pseudowords relative to real words (Coltheart et al., 2001). To date, the majority of imaging research has relied on hemodynamic approaches (i.e., PET and fMRI), which provide superb spatial resolution, but insufficient temporal precision for direct comparison with behavioral studies. This lack of temporal resolution prevents these studies from demonstrating processing differences in brain space as a function of time. In contrast, studies using event-related potentials (ERP) or event-related fields (ERF), evoked by words of differing frequency and/or pseudowords, have reported processing differences as a function of time (Assadollahi and Pulvermüller, 2001; Embick et al., 2001; Pylkkänen et al., 2002). However, these differences are typically ascertained through inspection of across condition peak-latency differences in certain components of the ERP/ERF (e.g., the M350), and without more detailed anatomical information, it is tenuous to conclude that comparable neural regions contribute to the time course differences.
More recently, neurolinguistic researchers have incorporated dipole-modeling techniques into their analyses, moving beyond the limited anatomical information provided through sensor/electrode waveforms. Most of these investigations relied on the multi-dipole model, but a few utilized the single-moving-dipole approach. However, the vast majority of this work used whole sentences as stimuli, or compared the processing of words and unpronounceable constant strings (Helenius et al., 1998, 1999a, 1999b; Tarkiainen et al., 1999, 2002; Cornelissen et al., 2003), limiting the utility of comparison with the present study. Nonetheless, one group has compared Finnish words and pronounceable pseudowords using the multi-dipole framework, and another has made the same comparison with English stimuli and the single-moving-dipole model. The multi-dipole study found no reliable neuroanatomical distinctions between the two word classes, but did observe left superior temporal lobe activation of greater duration and amplitude during the processing of pseudowords (Wydell et al., 2003). In contrast, results obtained through the single-moving-dipole approach showed clear spatial differences, but no significant temporal disparity in the processing of English words and pronounceable pseudowords (Simos et al., 2002). In this study, both word classes activated a large network of left hemisphere brain regions, but only words activated the posterior portion of the left middle temporal gyrus. These two studies applied distinct dipole models and were conducted in different languages (Finnish versus English), which could account for some of the discrepancies. Multi-dipole models allow a group of fixed sources to be quantified across conditions and time, provided the sources are reasonably distant from one another, and/or possess distinct orientations. This class of models outputs a series of source waveforms, which show the amplitude of each source as a function of time, thus preserving the temporal resolution of the original recording. However, the multi-dipole model is prone to the assumption that the spatial location of each source is constant across conditions and time, which is an assumption unlikely to be totally accurate (for a discussion, see Halgren et al., 2002). In contrast, the single-moving-dipole approach does not assume the locale of individual sources to be constant across time and conditions. This model allows consecutive data points to be modeled individually, and can illuminate distinct sources that are too close to be discerned through the application of a multi-dipole model. Unfortunately, this advantage is incurred at the expense of the time course of the original recording. The model typically outputs spatially contiguous ‘independent’ sources, which makes accurate source waveform calculation impossible because sources too close produce interference or cancellation in the final computation. However, since each dipole represents neural activity culminating at a known period in time, at least some aspects of the time course are preserved using the single dipole approach. Of course, each dipole-modeling technique has advantages and disadvantages, but without a priori data showing the spatial distribution of individual sources across time and conditions, the single-moving-dipole model may be a safer and more reliable technique.
In this study, we measured the brain basis of word/pseudoword processing in both time and space by applying the single-moving-dipole model to 248-channel neuromagnetometer data. Magnetoencephalography (MEG) is a technique with excellent temporal resolution (~2 msec in the current study) and good spatial accuracy. Under optimal conditions, the spatial precision of MEG is 2–3 mm (Wagner et al., 1997; Leahy et al., 1998). MEG non-invasively measures magnetic fields that emerge from postsynaptic currents generated through the activity of parallel-oriented pyramidal cells of the neocortex. In a recent assessment of MEG’s sensitivity, Hillebrand and Barnes (2002) observed only thin strips (~2 mm wide) at the very crest of gyri that were poorly resolvable with MEG. These areas constituted less than 5% of the entire cortical mantle (Hillebrand and Barnes, 2002). The observed magnetic field distributions, arising from these cortical activations, are often dipolar in nature and can be modeled with equivalent-current dipoles (ECDs). ECD models are both a physiologically and physically reasonable approximation of current flowing within a limited cortical structure being recorded from a distance of several centimeters (Hämäläinen et al., 1993). Each ECD indicates the three-dimensional location, orientation, and amplitude of the center of a cortical current.
The aim of this MEG investigation was two-fold. First, we wanted to characterize the neural mechanisms subserving word/pseudoword processing through a design that allowed both within-subject and across-subject comparisons in each of our conditions. To this end, we utilized the single-moving ECD technique in each subject for each condition. ECD models provide the capacity to compare the anatomical locale of sources within-subject across-condition, while also allowing source locations to be compared across subjects within-condition. Through this method, we can investigate the spatial relationship of neural regions activated by words and/or pseudowords, compute the time course of these regions (see below), and in addition possibly provide some insight into whether the inconsistent hemodynamic results are a product of unreliable true effects (i.e., functional heterogeneity), or whether the existence of false-positives due to low statistical thresholds are a more plausible explanation. This method can also gauge the spatial distribution of individual sources across conditions and time, which will provide valuable data to those utilizing multi-dipole modeling techniques in this paradigm. Our second goal was to establish the time course of neural regions involved in word and pseudoword processing. It is quite possible, given the plethora of behavioral data, that the most reliable across condition differences will emerge in the time course in which common neural regions are recruited. MEG data normally indicates primary visual responses occurring at ~100 msec, and behavioral data typically reveals that the lexical decision task (word/non-word judgment) can be solved in ~600 msec by normal subjects (faster for words). Thus, we looked within this time-window (i.e., 100–600 msec) for within-subject, across-condition differences in the timing of source areas common to the two conditions. In sum, our data analysis approach provided the capacity to recognize commonalities and differences in word/pseudoword elicited spatiotemporal patterns of brain activity within-subject, and subsequently to compare these patterns across-subject to elucidate consistent trends in our sample. In doing so, we observed that both word classes elicited ECDs in left perisylvian cortex, and that within-subject these ECDs were localized to indistinguishable neural regions. In contrast to the similarity in spatial localization, the time course differentiated word and pseudoword processing in all participants, with words activating nodes of the dynamic network significantly earlier than pseudowords.
Materials and Methods
Participants
Eleven native English speakers age 18–41 years (mean age = 28 years) were paid to participate in the experiment (8 males and 3 females). One male subject’s data was discarded due to poor signal-noise ratios. All subjects were strongly right-handed as assessed by the Edinburgh Handedness Inventory (range: 75–100; Oldfield, 1971). All subjects had normal or corrected to normal vision, and denied any history of neurological or psychiatric disease (including drug/alcohol abuse). Each subject provided informed consent to a protocol approved by the Institutional Review Boards of the University of Minnesota and the Veterans Affairs Medical Center, Minneapolis, Minnesota.
Stimuli
The stimulus set consisted of 100 concrete nouns (4–6 letters), 100 pseudowords (4–6 letters; all were pronounceable), and 100 consonant strings (4–6 letters; hereafter, non-words). The non-word condition provided a means to discern responses common to all orthographic stimuli from those specific to pronounceable linguistic stimuli. The two-syllable concrete nouns were all of high-frequency (range: 1.20 – 1.77 log; mean: 1.48 log; Kucera and Francis, 1967). We created pseudowords by shuffling the phonemes of the concrete nouns; thus, the phonemic units present in the corpus of concrete nouns were preserved in the pseudowords. Additionally, particular care was taken to ensure that the pseudowords resembled real English words in all respects, with the exception of lexical and semantic status (i.e., the stimulus set was screened for pseudohomophones and other ‘special’ pseudowords). For each condition (i.e., words, pseudowords, and non-words), 20 stimuli were randomly chosen as oddballs and the remaining 80 were chosen as targets. The font size of oddballs was twice that of targets (i.e., 72-point). All target stimuli (80 per condition) were of the same absolute length and height, and all were displayed in 36-point Courier font.
Procedure
During MEG data acquisition, participants were supine in a dimly lit, magnetically shielded room. The experiment consisted of 6 blocks, each of which lasted approximately 60 seconds with a 15-second inter-block interval. Thus, the overall recording time was approximately 8 minutes. In each block, 40 targets and 10 oddballs were randomly presented. Participants saw, on average, the same number of targets and oddballs from each stimulus condition within each block. No stimulus was repeated. Participants were instructed to view each stimulus and respond with a button press each time an oddball was observed (i.e., go/no-go task). This task ensures that vigilance is maintained, limits the total amount of required motor responses (to avoid motor contamination in the neural data), and does not emphasize any particular aspect of linguistic processing (e.g., phonology). Stimulus presentation alternated with a white fixation cross, and all stimuli were presented in the middle of the screen in lowercase white letters on a black background (stimulus duration = 600 msec; stimulus-onset asynchrony = 1200 msec). Before MEG acquisition began, subjects were verbally asked to refrain from blinking during stimulus presentation to reduce artifacts associated with eye-blinks. However, during the 15-second inter-block intervals, participants were told via visual display that they were free to blink during this period. An LCD projector outside the magnetically shielded room projected all stimuli onto a screen ~60 cm above each participant.
MEG Recording Parameters
Neuromagnetic signals were recorded continuously using a whole-head neuromagnetometer employing 248 axial-gradiometers (Magnes 3600 WH, 4-D Neuroimaging., San Diego, CA). Each axial-gradiometer is coupled to a SQUID sensor (superconductive quantum interference device), which acts as a low-noise magnetic flux-to-voltage converter. Neuromagnetic responses were sampled at 508 Hz with an acquisition passband of 0.1–200 Hz. Along with magnetic signals, vertical and horizontal electrooculogram (EOG) was also recorded. All data were stored for off-line analysis.
MEG Data Analysis
All MEG data were subjected to a global noise filter subtracting the external, non-biological noise obtained through the MEG reference channels. MEG data were low-pass filtered at 60 Hz and split into conditional epochs. All epochs were of 1000 msec duration, including a 100 msec pre-stimulus baseline period. Zero was defined as the time-point of stimulus onset as recorded by a photodetector draped in the LCD projector’s output path. Individual epochs with an EOG level > 100 uV or MEG level > 2.0 pT on one or more MEG channels were discarded from analysis. Further, epochs in which the participant responded (oddballs) were also excluded. In the end, 3 distinct average bins per subject were created (1 for each experimental condition), each of which contained a minimum of 60 trials (out of 80 possible trials).
For signal analysis, the brain was approximated as a spherically symmetric conductor. In MEG, spherical head models and realistic head models typically yield equivalent results (for examples and discussion, see Hämäläinen and Sarvas, 1989; Tarkiainen et al., 1999). The center of the sphere was defined as the midpoint of the anterior commissure – posterior commissure line on each individual subject’s MRI.
The procedure for source localization started with visual inspection of the contour maps from each condition. We identified time periods with clear dipolar field patterns and minimal interference from nearby simultaneously active brain areas. Once time windows for all dipolar field distributions were identified, each was modeled as an ECD using the Downhill-Simplex optimization algorithm with five Simplex turns. The same dipolar distribution was never modeled repeatedly. It is not uncommon to observe steady, prolonged (i.e., > 25 msec) dipolar field patterns, which raises the question of how one distinguishes a single sustained source from multiple distinct sources peaking at different times in virtually the same anatomical vicinity. In this study, a dipolar field had to exhibit significant dissipation and subsequent reorganization to be acknowledged as a distinct source and entered into a separate ECD model. Sustained dipolar field distributions (i.e., those not showing dissipation/reorganization) were modeled only once, using the temporal window surrounding the moment of peak flux.
The precise temporal window entered into the ECD model was determined by the time-point when the magnetic flux was at its maximum (i.e., the time-point of greatest field strength for the maxima/minima). Once this time-point was identified, the two following and preceding time-points were also selected, and all five time-points were entered into the ECD model (so, five neuromagnetic measurements, or ~10 msec of brain activity went into each model). For each temporal window, we applied the ECD algorithm to the magnetic flux measurements of the subset of sensors (18–70 axial-gradiometers) covering both magnetic flux extrema. Figure 1 depicts four representative dipolar distributions, which were successfully fitted with ECDs. Each of the four contour maps correspondents to a different point in the time course, and was taken from the same subject in the word condition. The subset of sensors selected for each dipole fit is enclosed within the white circle (Figure 1); note that perisylvian sources were modeled with a much narrower range of sensors (range: 18–42 sensors; mean: 34 sensors) relative to other sources (e.g., primary visual responses were typically modeled with 70 sensors). ECD solutions were considered satisfactory upon meeting the 0.90 goodness-of-fit (GOF) criterion. In other words, to be accepted, the ECD had to account for at least 90% of the variance observed over the ~10 msec window in the subset of sensors entered into the model. We used the Brain Electrical Source Analysis (BESA 2000, Version 4.2) software (MEGIS Software GmbH, Gräfelfing, Germany) for all MEG data pre-processing and source modeling, and the Standard Version of SPSS for Windows (Release 11.0.1, Chicago, IL) for all statistical analyses.
Figure 1.
Four representative contour maps (A-D) indicate how subgroups of sensors were chosen for individual dipole fits. These four left hemisphere maps are derived from four distinct time points in the averaged epoch for the word condition in the same subject. On each map, the subgroup of sensors used for fitting the ECD is enclosed within a white circle. Blue indicates magnetic flux directed into the brain (negative flux), while red shows flux directed out of the brain (positive flux). These ‘snapshots’ depict dipolar distributions peaking at four different time points in the left hemisphere. Again, all maps are from the same subject during the word condition.
Alignment of MEG and Anatomical Data
Anatomic images of the brain were acquired on a Signa Horizons LX 1.5T MR scanner (General Electric, Milwaukee, WI) using a neuro-vascular head coil. Sagittal scout images (T1 weighted 2D spin echo sequence, TE = minfull, TR = 400 ms, FOV = 240 × 240 mm, matrix = 256×256, slice thickness/gap = 5.0/2.5, NEX = 1) were taken to determine the number and placement of subsequent axial slices. The volume covered extended from the top of the head to the bottom of the cerebellum, including the external auditory meati bilaterally (thus, all MEG fiducial points were within coverage). T1 weighted axial images were then acquired using a 3-dimensional SPGR sequence with the following parameters: TE = minfull, TR = 20 ms, Flip angle = 30 deg., FOV = 240 × 240 mm, matrix = 256×256, slice thickness/gap = 1.5/0, NEX = 1. The resulting voxel resolution was 0.94 × 0.94 × 1.5 mm.
Prior to MEG measurement, five small coils were attached to the participant’s head and the locations of these coils, together with the three fiducial points (i.e., the nasion and left/right periauricles) and the scalp surface, were determined with a 3-D digitizer (Fastrak 3SF0002, Polhemus Navigator Sciences, Colchester, VT, USA). Once the participant was positioned inside the helmet of the neuromagnetometer, a small electric current was fed to the coils, which induced a measurable magnetic field. This allowed the coils to be located in reference to the sensors. Since the coil locations were also known in head coordinates, all MEG measurements could be transformed into a common coordinate system. Moreover, since the head coordinate system (including the digitized scalp surface points) could be mapped onto the participant’s MRI, individual MEG responses could also be mapped onto the structural MRI. Coregistration of the functional MEG data and the structural MRI data was performed with the BrainVoyager 2000 (Version 4.9) software (Brain Innovation B.V., Maastricht ,The Netherlands).
Results
Behavioral Data
Due to the nature of our task, the RT data are not directly comparable to most other studies that utilized similar stimuli. Typically, such studies require participants to make a lexical decision (word/non-word judgment), or read aloud the presented word. In contrast, the current task only required subjects to monitor the font size of stimuli. We chose this task mainly because it does not probe any particular aspect of linguistic processing (e.g., rhyming tasks emphasize phonological processing), but given the stimuli’s simplicity (i.e., 4–6 letter high-frequency words, pseudowords, and non-words) it is reasonable to assume that participants recognized the stimuli as words or non-words.
Error rates for the oddball detection task were too low (0.9%) to allow further analyses. Participants noticed large font stimuli most slowly when they were non-words (mean RT: words = 522 msec; pseudowords = 527 msec; non-words = 576 msec). The repeated-measures ANOVA was significant F(2,18) = 26.532 (p < 0.001), and pairwise comparisons showed non-words were noticed significantly slower than both pseudowords and words (p < 0.001), and that large font words and pseudowords did not significantly differ (p > 0.5). Again, it is problematic to compare these results to related studies due to the nature of our task, and the limited number of trials per subject per condition (i.e., 20 trials). Nevertheless, the relative mean RT for each condition was consistent with similar studies using more conventional tasks (e.g., lexical decision), and consonant with both the word- and pseudoword superiority effect (Maris, 2002).
MEG Data
Across the three conditions, individual brain contour maps were equivalent until ~200 msec post-stimulus onset. This entailed bilateral activation of posterior occipital cortices within the first 150 msec, followed by activity in basal occipito-temporal cortices. Around 200 msec, brain contour maps began to differentiate the non-words from the other two word classes. After 200 msec, the non-word stimuli induced predominantly right-lateralized activation, whereas both words and pseudowords elicited strongly left-lateralized activity in parietal regions, lateral occipital regions, temporal regions, and inferior frontal regions (see Fig. 2). Given the present focus, we will concentrate on the spatial and quantitative data from the pronounceable conditions and provide only a brief summary of findings in the non-word condition toward the end of the results section.
Figure 2.
Sources active 200–600 msec post-stimulus onsets, in all subjects, have been projected to the surface of a standardized 3-D rendering of a participant’s MRI for easier visualization. The different colors represent different participants (i.e., all sources detected in the same participant are the same color). (A) Sources in the non-word condition were relatively scarce, typically right-lateralized, and displayed little consistency across subjects. (B) Sources with white centers denote pseudoword-induced responses, whereas ECDs depicted in solid colors represent sources detected in the word condition. Again, each participant’s data is portrayed in a different color. ECDs detected in both the word and pseudoword conditions were mostly left-lateralized, localizing to parietal regions, lateral occipital regions, temporal regions, and inferior frontal regions.
Spatial data
On inspection of single-subject source configurations, we observed the most striking pattern of responses in left temporal regions. Both pronounceable word classes elicited activity in virtually identical regions of the superior temporal gyrus (STG), superior temporal sulcus (STS), and/or the pars opercularis (contingent on subject, see Fig. 2B). Given the spatial accuracy of MEG, we used a dipole plot size of 2.5 mm (radius of sphere), which provided a criterion for judging the spatial relationship of ECDs detected across the two conditions, within-subject. The dipole plot size is the relative size in which the source is depicted in the MRI. Thus, if two ECDs coincide in an individual’s MRI (as discerned through visual inspection), one can reasonably say their centers of mass represent the same activated neural area; or, conversely, that the sources arose from distinct tissue too close to be resolved with MEG. While source configurations across the two conditions were notably spatially contiguous within-subject, considerable variability existed in the locale of these responses across-subject (see Fig. 3). In most subjects, several ECDs were localized to the posterior portions of the left STG (STGp) and/or the left STS (STSp) in each condition. In other subjects, the two conditions induced significant and highly overlapping neural activity in the left pars opercularis accompanied by either left STGp activation, left STSp activation, or both.
Figure 3.
Three representative cases. In each, the subject’s functional data is displayed on the individual’s MRI. ECDs detected in the word condition are depicted in red, while those to pseudowords are blue in all figures. The two-dimensional MRI plots are shown in radiological convention. The cylindrical bar on each ECD in the three-dimensional renditions represents the orientation of the dipolar source. (A) Both words and pseudowords elicited responses in the mid-to-posterior STS in this subject. As shown, source areas across condition overlapped almost entirely. (B) In this subject, both conditions evoked responses on the superior bank of the posterior STG (i.e., sylvian fissure). Again, substantial overlap in source areas across-condition is apparent. (C) Both conditions induced responses in several neural regions in this subject. Word and pseudoword ECDs were detected in the pars opercularis, the inferior bank of the posterior STS, and several other regions. With the exception of the pars triangularis, overlap in neural tissue is extensive across the two conditions.
Quantitative analyses
Remarkably, while the activated neuroanatomy did not distinguish the two word classes, the within-subject time course clearly separated the two pronounceable conditions in all subjects. Specifically, activity in left perisylvian cortex (i.e., the STG, STS, IFG, and supramarginal gyrus) began much earlier in the word condition relative to the pseudoword condition. Collapsed across subjects, the average dipole latency for the initial left perisylvian ECD was 236 msec in the word condition (range: 203–269 msec), and 342 msec in the pseudoword condition (range: 251–395 msec). Thus, on average, words activated left perisylvian cortex 106 msec earlier than pseudowords (range: 48–172 msec; median = 108 msec).
For statistical evaluation, all word/pseudoword elicited left perisylvian ECDs were grouped into one of three latency bins (i.e., dipoles peaking between 200–300 msec, 301–400 msec, or 401–600 msec). The onset and duration of these bins reflect the facts that none of our subjects activated these neural regions before 200 msec (regardless of condition), and that activation significantly diminished between 450–550 msec in all subjects (with substantial variation between subjects). Thus, the latest time bin has a longer duration to account for the between-subject variability in the precise time in which activity declined; note however that activity during the extended time bin was still weaker relative to the other latency bins of shorter duration. After binning the data, we performed repeated-measures ANOVA with condition (2 factors) and latency bin (3 factors) as within-subject variables, and number of reliable sources (i.e., 0.90 GOF) as the dependent measure. The validity of our dependent variable as a metric of regional activation has been repeatedly demonstrated (Simos et al., 1998, 1999; Zouridakis et al., 1998; Breier et al., 1999, 2001; Papanicolaou et al., 1999). Since number of sources is a ‘count’ variable, it is appropriate to re-express the data using at a minimum the square-root transformation (Tukey, 1977). This transformation stabilizes the variance and decreases skewness associated with the nature of ‘count’ variables. Thus, we applied the square-root transformation before we calculated the ANOVA reported below; but, for clarity, we also computed the ANOVA using the raw values and the significant results were identical.
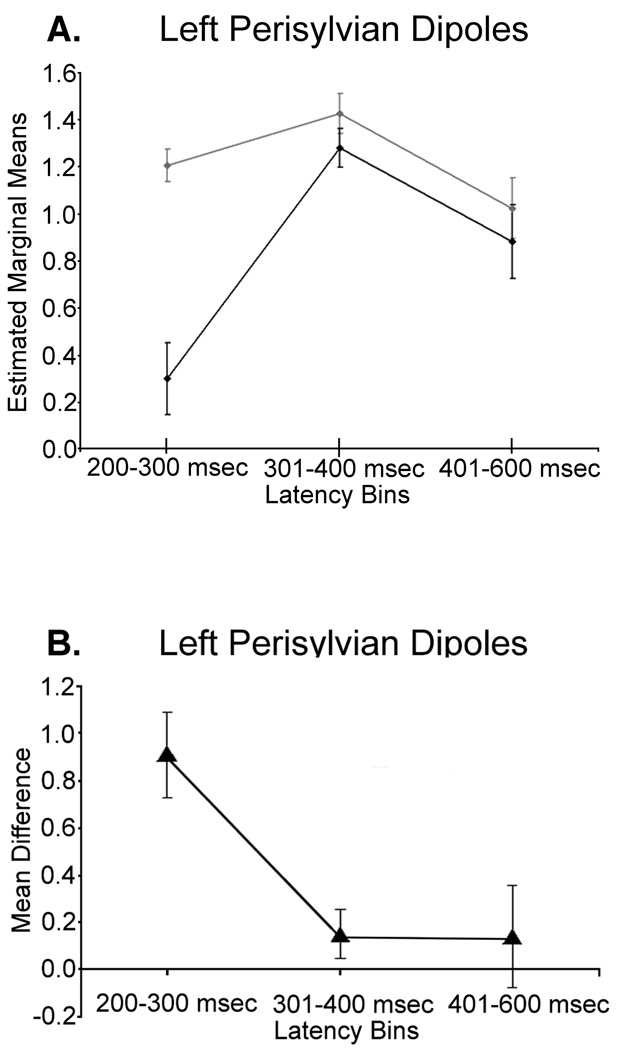
Mauchly’s test of sphericity indicated the assumption of sphericity held in our data set, thus all reported values assume sphericity. The main effect of condition was significant F(1,9) = 12.71 (p < 0.01), indicating significantly more sources in the word condition. The effect of latency bin was also significant F(2,18) = 11.98 (p < 0.001), and pairwise comparisons revealed significantly more sources in the 301–400 msec latency bin, relative to the 200–300 msec (p < 0.01) and 401–600 msec bins (p < 0.02). The condition-by-latency bin interaction effect was significant F(2,18) = 7.51 (p < 0.01), and within-subject contrasts indicated significant linear F(1,9) = 8.80 (p < 0.02) and quadratic F(1,9) = 5.18 (p < 0.05) components; thus, mean differences started high (200–300 msec bin), decreased substantially (301–400 msec bin), and then stabilized in 401–600 msec latency bin (see Fig. 4A–B).
Figure 4.
Profile plots for left perisylvian sources detected in each of the three latency bins for both words and pseudowords. Error bars represent one standard error of the mean. Note that the duration of each latency bin was equivalent across conditions, but that the 401–600 msec bin has a longer duration than the earlier bins (in both conditions). Thus, although weaker, activity in the latest latency bin has actually bin collapsed across 200 msec, which is twice the duration of the earlier bins. (A) Profile plot showing the condition-by-latency bin interaction effect. The gray line represents the word condition and the black line shows the pseudoword condition. The ordinate displays estimated marginal means of the dependent measure (i.e., number of sources detected per latency bin, after the square-root transformation). Both the linear (p < 0.02) and quadratic (p < 0.05) terms of the interaction effect reached significance. (B) Each triangle corresponds to the mean difference between pseudowords and words for the respective latency bin (dependent measure same as in (A)).
To explore the interaction effect, we contrasted the two pronounceable conditions in each latency bin using paired-samples t-tests. As shown in Figure 5, this set of analyses indicated significantly more word-elicited sources in the 200–300 msec latency bin t(9) = 5.02 (p < 0.01), and that the number of sources per condition did not reliably differ in the other latency bins (all p > 0.15). We also contrasted the three latency bins within each of the two conditions. For words, significantly more sources occurred in the 301–400 msec range relative to the 401–600 msec bin, t(9) = 2.25 (p < 0.05). None of the other comparisons reached significance. For pseudowords, significantly more sources culminated in both the 301–400 msec latency range t(9) = −5.63 (p < 0.001) and the 401–600 msec window t(9) = −3.32 (p < 0.01), relative to the 200–300 msec latency bin.
Figure 5.
Number of detected sources in left perisylvian cortex collapsed across the entire sample. The latency bins are the same as described in Figure 4. The only significant condition-by-latency bin effect occurred in the early temporal window (i.e., 200–300 msec), with more ECDs in the word condition. All other significant differences occurred within-condition across latency bin (see text for greater detail). Note that the t-test reported in the text used transformed data, but that the figure displays the actual values. The actual values are easier to interpret and the significance of the results was not affected by the transformation.
* = (p < 0.01)
Numerous studies involving control subjects and patients demonstrate the validity of using the number of reliable sources localizing to a given neural region as a metric of that region’s activation. Thus, we chose a priori to use this measure as our primary index of regional activation for each condition. However, given prior observations of highly significant and subject-specific responses to words (Mechelli et al., 2003), we expanded our investigation to include the predicted amplitude of cortical currents represented by each dipole. In doing so, we discerned a trend analogous to that observed previously (Mechelli et al., 2003), in that words, but not pseudowords, induced left perisylvian responses of very high amplitude in several subjects. These sources culminated in both the early and late latency bins (i.e., 200–300 msec and 401–600 msec), with strengths more than four times greater than the mean ECD amplitude collapsed across the two pronounceable conditions (left perisylvian ECDs only). Given this, we computed an amplitude by condition t-test using only the left perisylvian ECDs peaking in the 301–400 msec range (the significantly most active time-bin in both conditions), and detected significantly greater amplitude for pseudoword-induced dipoles, t(9) = −2.54 (p < 0.05).
Non-word condition
The focus of the current study was the word/pseudoword comparison. However, we included a non-word condition to discern responses common to all orthographic visual stimuli from those specific to pronounceable linguistic stimuli. Given this feature of our design, the results from the non-word condition will only be briefly summarized. Activation unique to non-words emerged at ~200 msec post-stimulus onset and was predominantly right-lateralized, paired-samples t(9) = −2.61 (p < 0.05). Sources tended to cluster in right superior temporal regions, but there was substantial variability across subjects (see Fig. 2A). In addition, the number of reliable sources (i.e., 0.90 GOF) was highly variable across our sample (range: 3–11).
Discussion
We contrasted dynamic spatiotemporal activation patterns elicited by words and pseudowords within-subject, then compared the condition-specific activation patterns across-subject to illuminate consistent trends in the sample. The spatial data indicated that brain regions subserving word and pseudoword processing were essentially indistinguishable within-subject, but also revealed a considerable degree of across-subject variability in the precise site of these neural correlates. The time course was more straightforward; both within-subject and across-subject, words always activated left perisylvian cortex earlier than pseudowords. On average, word processing recruited left perisylvian cortices at 236 msec, whereas pseudoword processing delayed this region’s involvement some 106 msec. Words also elicited significantly more reliable sources in left perisylvian cortex, but the average amplitude of these sources was significantly greater for pseudowords, which prevented a unanimous interpretation of the activation intensity data.
The different time course for word/pseudoword processing was not surprising. An extensive behavioral literature has established that pseudowords are rejected more slowly in lexical decision tasks (word/non-word judgment), and pronounced more slowly in word reading tasks (Coltheart et al., 2001). Given these behavioral observations, one would expect the time course of the neural correlates to parallel the behavioral response (i.e., earlier activation for words), and this is precisely what we observed. Within-subject, ECDs localizing to the same neural regions always occurred earlier for words relative to pseudowords. Interestingly, the different time course of left perisylvian activation followed a quadratic trend: large across-condition differences early in the epoch (200–300 msec), a linear decrease toward the middle of the epoch (301–400 msec), and finally an era of stabilization at the end of the analysis period (401–600 msec). Such a trend may indicate that the early left perisylvian processing, unique to real words, is mediated by more direct connections from extrastriate areas specialized for encoding linguistic items encountered frequently in normal reading (i.e., high-frequency words).
The spatial domain of our data is more complicated, but can be summarized by three core observations. First, in individual subjects, the neural substrates underlying word/pseudoword processing were either identical, or too spatially contiguous to be segregated with the 248-channel neuromagnetometer. Second, across our sample, the brain regions subserving word/pseudoword processing were very similar on a gross level (e.g., STG or STS), but quite heterogeneous at a finer level (e.g., medial/lateral or superior/inferior STG). Lastly, the involvement of the pars opercularis (i.e., posterior Broca’s area) in word/pseudoword processing was subject-specific. Only a subset of the sample showed activation in this region, and when present it typically occurred for both pronounceable conditions; moreover, activity in the pars opercularis region was always accompanied by activation of either left STGp, left STSp, or both.
To compare present findings with those from subtraction-based hemodynamic studies and other dipole-based electromagnetic research, we utilized two distinct aspects of the activation intensity data. First, we tallied the number of dipoles per region for each condition, which is an established index of regional activation (Simos et al., 1998, 1999; Zouridakis et al., 1998; Breier et al., 1999, 2001; Papanicolaou et al., 1999). Subsequently, we incorporated the predicted amplitude of the cortical current represented by each dipole, which is a less understood and more contentious metric of activation intensity (see below). The most reliable hemodynamic observations have been pseudowords eliciting greater activation relative to words in several regions, and neural areas showing enhanced activation to words being non-replicable across studies. Meanwhile, the two most relevant dipole studies have reached conflicting results, with one showing a neural area recruited by words but not pseudowords (Simos et al., 2002), and another indicating time course and dipole amplitude differences (Wydell et al., 2003). In the current study, words elicited significantly more left perisylvian sources, but the average ECD amplitude in this region was significantly greater during pseudoword processing.
Attempting to relate neuromagnetic data with that from hemodynamic studies raises two highly interrelated questions. First, in neuromagnetic research, what is greater activation? Should the absolute number of dipoles reliably localizing to a given neural region be considered the unitary measure of regional activation, or should the average amplitude of these dipoles be considered an additional or adjacent metric of activation? Single-moving-dipole studies have access to both metrics, yet the latter is temporally discontinuous; whereas multi-dipole investigations lack the former index, but have a continuous and temporally precise measure of dipole amplitude. Second, how would each of these metrics be reflected in metabolically based measurements? For the first measure, transient high-amplitude responses are typically dipolar and readily localized, but could occasionally go undetected in imaging techniques yoked to slowly responding parameters (e.g., blood-flow). In contrast, the neural mechanism underlying the second measure, dipole amplitude, is more likely transparent to hemodynamic techniques in a definitive fashion. The level of synchronization in a given neural network could fluctuate without significant changes in mean neuronal firing rates, and concomitantly without changes in metabolic parameters (Hari and Salmelin, 1997). Meanwhile, simulation studies indicate that dipole amplitude is strongly affected by the degree of synchrony in neural networks mediating the response (Hari et al., 1997). If dipole amplitude is a measure dominated by synchronicity, it may not have a meaningful connection with the ‘activation’ described in hemodynamic studies (for a similar view, see Hari et al., 2000). Thus, for the present purpose, the tally of reliably localized dipoles per region is likely the more appropriate metric of regional activation. As for the dipole amplitude data, it should be interpreted as indicating enhanced synchronization within regions of left perisylvian cortex during the processing of pseudowords; which is an interesting observation that corroborates other evidence (Wydell et al., 2003), but is beyond the scope of the current paper.
If one accepts the synchronization argument, and thereby assumes hemodynamic techniques to be insensitive to dipole amplitude, than the remaining data indicates that words elicited significantly more activity in left perisylvian cortex. This observation stands in stark contrast to the most consistent finding of relevant PET/fMRI studies. A possible explanation for this disparity involves our selection of the dipole technique for source localization. In this study, we relied on the dipole model because of its capacity to provide spatial accuracy on the 2–3 mm scale (Wagner et al., 1997; Leahy et al., 1998). Although, a limitation inherent in this approach is that all sources are represented as focal, or not at all, on the output side of the model. In short, regardless of the actual nature of the neural activity (i.e., very focal, limited to several adjacent columns or widespread, spanning an entire gyrus or more) the dipole model will yield a focal solution in reasonable cases, and no reliable solution when activation is extremely diffuse. Thus, if pseudowords induce extremely diffuse activation in left perisylvian cortices, than it is reasonable to suspect that some sources may not be fully captured by the metric of regional activation used in the current study. However, it seems unlikely that this shortcoming could account for our significant difference in regional activation, as validation studies have not suggested an effect of this magnitude (Simos et al., 1998, 1999; Zouridakis et al., 1998; Breier et al., 1999, 2001; Papanicolaou et al., 1999). Alternatively, it may be that the center of activation (i.e., what the dipole reflects) is identical for words and pseudowords in individual subjects, but that pseudowords actually activate a larger span of tissue. Larger areas of activation would lead to more overlap in activated regions across the sample, and would enhance the probability of adjacent voxels surviving the across-subject averaging common in hemodynamic studies. Presumably, pseudowords induce a search process in which the linguistic system attempts to match the input stimulus to a possible lexical candidate. Such processing could potentially be indicated by more distributed activation within neural regions mediating lexicality. Perhaps neural areas showing heightened activation to words are rarely reported because the sources are more focal, and the slight functional heterogeneity that exists in all samples causes the activity to wash out in the across-subject averages (i.e., lack of overlap in activated regions). If the neural correlates of pseudoword processing are more distributed, than this would partially reconcile the present observations with those from hemodynamic studies.
The spatial domain of our data is also enlightening in the context of the neuropsychological language literature. There is little agreement as to the lesion site/extent necessary to induce either phonological or surface dyslexia (Coslett, 2000). Furthermore, roughly the same statement can be made in regard to the different aphasias (Alexander, 2000). In the present study, both words and pseudowords activated virtually identical brain regions when comparisons were made within-subject. However, given the dissociable pattern of behavioral symptoms associated with each of the acquired dyslexias, certain neural areas seem necessary for the processing of pseudowords but not words. As a corollary, the indistinguishable activations we observed across the two conditions most probably indicate that neural areas not necessary for processing words are activated obligatorily by word stimuli. Of course, most imaging techniques currently used in human studies cannot discriminate necessary brain regions from those simply sufficient for a given cognitive process. Nevertheless, the neural correlates of word/pseudoword processing were considerably variable across subjects in the current study, which suggests the exact locale of any necessary region varies considerably from person to person. Such an observation could indicate that the related neuropsychological data, concerning lesion site/extent necessary for a given disorder, may be contradictory for a valid reason. Lastly, data from two of our subjects was especially intriguing when considered in the present discussion. For both words and pseudowords, activation in left perisylvian cortex was restricted to a small area of the STSp in one subject, and to a very circumscribed region of the STGp in another subject (see Fig. 3B). Presumably, these limited regions of left perisylvian cortex subserve most of the more specialized aspects of word-level language processing in these subjects. Although speculative, these two exemplars may be informative as to the mechanisms by which relatively small lesions are sometimes able to induce profound aphasias.
Taken together, the current results provide new insight into the neural mechanisms subserving lexico-semantic processing. Using the most validated MEG techniques and a task that did not emphasize any single aspect of linguistic processing (e.g., phonological processing), we were able to characterize a common dynamic network underlying both word and pseudoword processing. The spatial extent of this dynamic network did not distinguish the two word classes, but the time course by which this network operated clearly differentiated words from pseudowords. High-density MEG provides investigators with excellent spatiotemporal resolution, and thus allows the actual dynamics of human information processing to be simultaneously characterized on both the millimeter and millisecond scale. With such techniques, the questions addressed throughout cognitive neuroscience, and in the language processing domain in particular, will certainly broaden. For instance, there has been much interest in attributing specific linguistic functions (e.g., phonology) to certain neural areas, and the capacity to discern the time course will greatly facilitate the field’s ability to achieve such goals. In our data set, a natural question concerned the sequential order in which left perisylvian areas were recruited. The presence of sequential order would argue against strict parallel processing accounts of linguistic function, and also provide insight into the specific aspect of language processing mediated by each neural area (assuming that certain functions precede other functions; e.g., phonology, lexicality, then semantics). In regard to this latter point, we observed activation in posterior Broca’s area (i.e., the pars opercularis) preceding activation in other left perisylvian areas, which supports the view that this region subserves phonological processing (Devlin et al., 2003). However, this data should be considered preliminary and interpreted with caution because only a subset of our sample exhibited activation in this region. Furthermore, questions of this sort are probably better addressed through employing task manipulations, and focusing on how the dynamic time course changes as a function of task modifications. For example, experiments using the lexical decision task and different rhyming tasks (which put a premium on lexical and phonological processing, respectively) are currently underway in our laboratory. Investigating how the time course changes as specific linguistic functions are selectively burdened will provide novel insight, and hopefully facilitate the integration of time into the quest of matching brain structure with brain function. We conclude that the neural distinction in word/pseudoword processing is best conceptualized as a difference in the time course in which cell populations are recruited, and not in the locale of these neural correlates.
Acknowledgements
TWW was supported by a pre-doctoral traineeship through the Center for Cognitive Sciences (NIH training grant T32 HD007151). Funding for ACL and PJP was provided by the Mental Illness and Neuroscience Discovery (MIND) Institute. This work was also supported by the U.S. Department of Veterans Affairs, the American Legion Auxiliary, and the American Legion Brain Sciences Chair.
References
- Alexander MP. Aphasia I: Clinical and anatomic issues. In: Farah MJ, Feinberg TE, editors. Patient-based approaches to cognitive neuroscience. Cambridge: MIT Press; 2000. pp. 165–181. [Google Scholar]
- Assadollahi R, Pulvermüller F. Neuromagnetic evidence for early access to cognitive representations. Neuroreport. 2001;12:207–213. doi: 10.1097/00001756-200102120-00007. [DOI] [PubMed] [Google Scholar]
- Binder JR, McKiernan KA, Parsons ME, Westbury CF, Possing ET, Kaufman JN, et al. Neural correlates of lexical access during visual word recognition. J Cogn Neurosci. 2003;15:372–393. doi: 10.1162/089892903321593108. [DOI] [PubMed] [Google Scholar]
- Breier JI, Simos PG, Papanicolaou AC, Zouridakis G, Wilmore LJ, Wheless JW, et al. Cerebral laterality assessment using magnetoencephalography: A comparison with the intracarotid amobarbital procedure. Neurology. 1999;22:938–945. [Google Scholar]
- Breier JI, Simos PG, Wheless JW, Constantinou JEC, Papanicolaou AC. Hemispheric language dominance in children determined by magnetic source imaging. J Child Neurol. 2001;16:124–130. doi: 10.1177/088307380101600211. [DOI] [PubMed] [Google Scholar]
- Brunswick N, McCrory E, Price CJ, Frith CD, Frith U. Explicit and implicit processing of words and pseudowords by adult developmental dyslexics: A search for Wernicke’s wortschatz. Brain. 1999;122:1901–1917. doi: 10.1093/brain/122.10.1901. [DOI] [PubMed] [Google Scholar]
- Coltheart M, Rastle K, Perry C, Langdon R, Ziegler J. DRC: A dual route cascaded model of visual word recognition and reading aloud. Psychol Rev. 2001;108:204–256. doi: 10.1037/0033-295x.108.1.204. [DOI] [PubMed] [Google Scholar]
- Cornelissen PL, Tarkiainen A, Helenius P, Salmelin R. Cortical effects of shifting letter position in letter strings of varying length. J Cogn Neurosci. 2003;15:731–746. doi: 10.1162/089892903322307447. [DOI] [PubMed] [Google Scholar]
- Coslett HB. Acquired dyslexia. In: Patient-based approaches to cognitive neuroscience. In: Farah MJ, Feinberg TE, editors. Cambridge: MIT Press; 2000. pp. 235–246. [Google Scholar]
- Devlin JT, Matthews PM, Rushworth MFS. Semantic processing in the left inferior prefrontal cortex: A combined functional magnetic resonance imaging and transcranial magnetic stimulation study. J Cogn Neurosci. 2003;15:71–84. doi: 10.1162/089892903321107837. [DOI] [PubMed] [Google Scholar]
- Embick D, Hackl M, Schaeffer J, Kelepir M, Marantz A. A MEG component whose latency reflects lexical frequency. Cogn Brain Res. 2001;10:345–348. doi: 10.1016/s0926-6410(00)00053-7. [DOI] [PubMed] [Google Scholar]
- Fiebach CJ, Friederici AD, Müller K, von Cramon DY. fMRI evidence for dual routes to the mental lexicon in visual word recognition. J Cogn Neurosci. 2002;14:11–23. doi: 10.1162/089892902317205285. [DOI] [PubMed] [Google Scholar]
- Fiez JA, Balota DA, Raichle ME, Peterson SE. Effects of lexicality, frequency, and spelling-to-sound consistency on the functional anatomy of reading. Neuron. 1999;24:205–218. doi: 10.1016/s0896-6273(00)80833-8. [DOI] [PubMed] [Google Scholar]
- Funnell E. Phonological processes in reading: New evidence from acquired dyslexia. Br J Psychol. 1983;74:159–180. doi: 10.1111/j.2044-8295.1983.tb01851.x. [DOI] [PubMed] [Google Scholar]
- Hagoort P, Indefrey P, Brown C, Herzog H, Steinmetz H, Seitz RJ. The neural circuitry involved in the reading of German words and pseudowords: A PET study. J Cogn Neurosci. 1999;11:383–398. doi: 10.1162/089892999563490. [DOI] [PubMed] [Google Scholar]
- Halgren E, Dhond RP, Christensen N, Van Petten C, Marinkovic K, Lewine JD, et al. N400-like magnetoencephalography responses modulated by semantic context, word frequency, and lexical class in sentences. Neuroimage. 2002;17:1101–1116. doi: 10.1006/nimg.2002.1268. [DOI] [PubMed] [Google Scholar]
- Hämäläinen MS, Sarvas J. Realistic conductivity geometry model of the human head for interpretations of neuromagnetic data. IEEE Trans Biomed Eng. 1989;36:165–171. doi: 10.1109/10.16463. [DOI] [PubMed] [Google Scholar]
- Hämäläinen M, Hari R, Ilmoniemi RJ, Knuutila J, Lounasmaa OV. Magnetoencephalography: Theory, instrumentation, and applications to noninvasive studies of the working human brain. Rev Mod Phys. 1993;65:413–497. [Google Scholar]
- Hari R, Salmelin R. Human cortical oscillations: A neuromagnetic view through the skull. Trends Neurosci. 1997;20:44–49. doi: 10.1016/S0166-2236(96)10065-5. [DOI] [PubMed] [Google Scholar]
- Hari R, Salmelin R, Mäkelä JP, Salenius S, Helle M. Magnetoencephalographic cortical rhythms. Int J Psychophysiol. 1997;26:51–62. doi: 10.1016/s0167-8760(97)00755-1. [DOI] [PubMed] [Google Scholar]
- Hari R, Levänen S, Raij T. Timing of human cortical functions during cognition: Role of MEG. Trends Cogn Sci. 2000;4:455–462. doi: 10.1016/s1364-6613(00)01549-7. [DOI] [PubMed] [Google Scholar]
- Helenius P, Salmelin R, Service E, Connolly JF. Distinct time courses of word and context comprehension in the left temporal cortex. Brain. 1998;121:1133–1142. doi: 10.1093/brain/121.6.1133. [DOI] [PubMed] [Google Scholar]
- Helenius P, Salmelin R, Service E, Connolly JF. Semantic cortical activation in dyslexic readers. J Cogn Neurosci. 1999a;11:535–550. doi: 10.1162/089892999563599. [DOI] [PubMed] [Google Scholar]
- Helenius P, Tarkiainen A, Cornelissen P, Hansen PC, Salmelin R. Dissociation of normal feature analysis and deficient processing of letter-strings in dyslexic adults. Cereb Cortex. 1999b;9:476–483. doi: 10.1093/cercor/9.5.476. [DOI] [PubMed] [Google Scholar]
- Herbster AN, Mintun MA, Nebes RD, Becker JT. Regional cerebral blood flow during word and nonword reading. Hum Brain Mapp. 1997;5:84–92. doi: 10.1002/(sici)1097-0193(1997)5:2<84::aid-hbm2>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- Hillebrand A, Barnes GR. A quantitative assessment of the sensitivity of whole-head MEG to activity in the adult human cortex. Neuroimage. 2002;16:638–650. doi: 10.1006/nimg.2002.1102. [DOI] [PubMed] [Google Scholar]
- Kucera H, Francis WN. Computational analysis of present-day american english. Providence: Brown University Press; 1967. [Google Scholar]
- Leahy RM, Mosher JC, Spencer ME, Huang MX, Lewine JD. A study of dipole localization accuracy for MEG and EEG using a human skull phantom. Electroencephalogr Clin Neurophysiol. 1998;107:159–173. doi: 10.1016/s0013-4694(98)00057-1. [DOI] [PubMed] [Google Scholar]
- Maris E. The role of orthographic and phonological codes in the word and pseudoword superiority effect: An analysis by means of multinomial processing tree models. J Exp Psychol Hum Percept Perform. 2002;28:1409–1431. doi: 10.1037//0096-1523.28.6.1409. [DOI] [PubMed] [Google Scholar]
- Mechelli A, Gorno-Tempini ML, Price CJ. Neuroimaging studies of word and pseudoword reading: Consistencies, inconsistencies, and limitations. J Cogn Neurosci. 2003;15:260–271. doi: 10.1162/089892903321208196. [DOI] [PubMed] [Google Scholar]
- Oldfield RC. The assessment and analysis of handedness: The Edinburgh inventory. Neuropsychologia. 1971;9:97–113. doi: 10.1016/0028-3932(71)90067-4. [DOI] [PubMed] [Google Scholar]
- Papanicolaou AC, Simos PG, Breier JI, Zouridakis G, Willmore LJ, Wheless JW. Magnetoencephalographic mapping of the language-specific cortex. J Neurosurg. 1999;90:85–93. doi: 10.3171/jns.1999.90.1.0085. [DOI] [PubMed] [Google Scholar]
- Patterson KE, Marshall JC, Coltheart M. Surface dyslexia: Cognitive and neuropsychological studies of phonological reading. Hove: Erlbaum Press; 1985. [Google Scholar]
- Paulesu E, McCrory E, Fazio F, Menoncello L, Brunswick N, Cappa SF, et al. A cultural effect on brain function. Nat Neurosci. 2000;3:91–96. doi: 10.1038/71163. [DOI] [PubMed] [Google Scholar]
- Price CJ, Wise RJS, Frackowiak RSJ. Demonstrating the implicit processing of visually presented words and pseudowords. Cereb Cortex. 1996;6:62–70. doi: 10.1093/cercor/6.1.62. [DOI] [PubMed] [Google Scholar]
- Pylkkänen L, Stringfellow A, Marantz A. Neuromagnetic evidence for the timing of lexical activation: An MEG component sensitive to phonotactic probability but not neighborhood density. Brain Lang. 2002;81:666–678. doi: 10.1006/brln.2001.2555. [DOI] [PubMed] [Google Scholar]
- Rumsey JM, Horwitz B, Donohue C, Nace K, Maisog JM, Andreason P. Phonological and orthographic components of word recognition: A PET–rCBF study. Brain. 1997;120:739–759. doi: 10.1093/brain/120.5.739. [DOI] [PubMed] [Google Scholar]
- Simos PG, Breier JI, Zouridakis G, Papanicolaou AC. Assessment of cerebral dominance for language using magnetoencephalography. J Clin Neurophysiol. 1998;15:364–372. doi: 10.1097/00004691-199807000-00009. [DOI] [PubMed] [Google Scholar]
- Simos PG, Papanicolaou AC, Breier JI, Wilmore LJ, Wheless JW, Constantinou JC, et al. Localization of language-specific cortex using MEG and intraoperative stimulation mapping. J Neurosurg. 1999;91:787–796. doi: 10.3171/jns.1999.91.5.0787. [DOI] [PubMed] [Google Scholar]
- Simos PG, Breier JI, Fletcher JM, Foorman BR, Castillo EM, Papanicolaou AC. Brain mechanisms for reading words and pseudowords: An integrated approach. Cereb Cortex. 2002;12:297–305. doi: 10.1093/cercor/12.3.297. [DOI] [PubMed] [Google Scholar]
- Tarkiainen A, Helenius P, Hansen PC, Cornelissen PL, Salmelin R. Dynamics of letter string perception in the human occipitotemporal cortex. Brain. 1999;122:2119–2132. doi: 10.1093/brain/122.11.2119. [DOI] [PubMed] [Google Scholar]
- Tarkiainen A, Cornelissen PL, Salmelin R. Dynamics of visual feature analysis and object-level processing in face versus letter-string perception. Brain. 2002;125:1125–1136. doi: 10.1093/brain/awf112. [DOI] [PubMed] [Google Scholar]
- Tukey JW. Exploratory data analysis. Reading: Addison-Wesley Publishing; 1977. [Google Scholar]
- Wagner H, Eiselt M, Zwienger U. Exactness of source analysis of biomagnetic signals of epileptiform spikes by the method of spatial filtering: A computer simulation. Med Biol Eng Comput. 1997;35:708–714. doi: 10.1007/BF02510982. [DOI] [PubMed] [Google Scholar]
- Wydell TN, Vuorinen T, Helenius P, Salmelin R. Neural correlates of letter string length and lexicality during reading in a regular orthography. J Cogn Neurosci. 2003;15:1052–1062. doi: 10.1162/089892903770007434. [DOI] [PubMed] [Google Scholar]
- Xu B, Grafman J, Gaillard WD, Ishii K, Vega-Bermudez F, Pietrini P, et al. Conjoint and extended neural networks for the computation of speech codes: The neural basis of selective impairment in reading words and pseudowords. Cereb Cortex. 2001;11:267–277. doi: 10.1093/cercor/11.3.267. [DOI] [PubMed] [Google Scholar]
- Zouridakis G, Simos PG, Breier JI, Papanicolaou AC. Cerebral dominance assessment using magnetoencephalography. Brain Topogr. 1998;11:57–65. doi: 10.1023/a:1022270620396. [DOI] [PubMed] [Google Scholar]