Abstract
The present study demonstrates that oral gavage of 6 μmol D,L-Sulforaphane (SFN), a synthetic analogue of cruciferous vegetable-derived L-isomer, thrice per week beginning at six weeks of age, significantly inhibits prostate carcinogenesis and pulmonary metastasis in TRAMP mice without causing any side effects. The incidence of the prostatic intraepithelial neoplasia (PIN) and well-differentiated (WD) carcinoma were ~23-28% lower (P<0.05 compared with control by Mann Whitney test) in the dorsolateral prostate (DLP) of SFN-treated mice compared with controls, which was not due to the suppression of T-antigen expression. The area occupied by the WD carcinoma was also ~44% lower in the DLP of SFN-treated mice relative to that of control mice (P=0.0011 by Mann Whitney test). Strikingly, the SFN-treated mice exhibited an approximate 50% and 63% decrease, respectively, in pulmonary metastasis incidence and multiplicity compared with control mice (P<0.05 by t-test). The DLP from SFN-treated mice showed decreased cellular proliferation and increased apoptosis when compared with that from control mice. Additionally, SFN administration enhanced cytotoxicity of co-cultures of natural killer cells and dendritic cells (DC) against TRAMP-C1 target cells, which correlated with infiltration of T-cells in the neoplastic lesions and increased levels of interleukin-12 production by the DC. In conclusion, the results of the present study indicate that SFN administration inhibits prostate cancer progression and pulmonary metastasis in TRAMP mice by reducing cell proliferation and augmenting NK cell lytic activity.
Keywords: Sulforaphane, Prostate Cancer, Chemoprevention
Introduction
Epidemiological studies continue to support the premise that dietary intake of cruciferous vegetables may be protective against different types of malignancies including prostate cancer (1-3). The anticarcinogenic effect of cruciferous vegetables is attributed to organic isothiocyanates (ITCs), which are generated via myrosinase-mediated hydrolysis of corresponding glucosinolates (4,5). The ITC compound (-)-1-isothiocyanato-(4R)-(methylsulfinyl)-butane (L-SFN) and its synthetic analogue D,L-sulforaphane (SFN) have generated a great deal of research interest because of their remarkable anticancer effects (4-7). For example, L-SFN and SFN are equipotent inducers of quinone reductase in Hepa1c1c7 murine hepatoma cells (6). The L-SFN or SFN administration has been shown to offer protection against 9,10-dimethyl-1,2-benzanthracene-induced mammary cancer in rats, azoxymethane-induced colonic aberrant crypt foci in rats, and benzo[a]pyrene-induced forestomach cancer in mice (8-10). Dietary feeding of SFN and its N-acetylcysteine conjugate inhibited malignant progression of lung adenomas induced by tobacco carcinogens in A/J mice (11).
Recent studies including those from our laboratory have documented novel cellular responses to SFN treatment in cultured human cancer cells, including growth arrest, induction of apoptosis and autophagy, inhibition of histone deacetylase, binding to proteins, and sensitization of cells to TRAIL-induced apoptosis (12-21). The mechanism by which SFN causes growth arrest and apoptosis induction has been extensively studied in human prostate cancer cells (13,14,16-18,22). For example, we have shown previously that exposure of human prostate cancer cells to SFN results in G2/M phase cell cycle arrest via checkpoint kinase 2-mediated phosphorylation of cell division cycle 25C (13). We also demonstrated that the SFN-induced apoptosis was selective towards prostate cancer cells and linked to generation of reactive oxygen species (15,16). Moreover, oral gavage of SFN in male nude mice significantly retarded the growth of PC-3 human prostate cancer xenografts in vivo (22).
Demonstration of in vivo efficacy of potential cancer chemopreventive agents in suitable animal models is a prerequisite for their further clinical development. The present study was designed to test whether SFN administration offers protection against prostate carcinogenesis in a transgenic mouse model of prostate cancer (Transgenic Adenocarcinoma of Mouse Prostate; commonly abbreviated as TRAMP). We now demonstrate that oral gavage of SFN significantly prevents prostate cancer progression and pulmonary metastasis in TRAMP mice without causing any side effects or affecting the T-antigen expression.
Materials and Methods
Synthesis of SFN
SFN was synthesized as described by Conaway et al. (11). The structure of SFN was confirmed by proton NMR and MS. The purity of the SFN (>99% pure) preparation was determined by HPLC as described by Conaway et al (11). SFN was stored at -20°C and found to be stable for at least six months.
Cell viability and apoptosis assays
Monolayer cultures of TRAMP-C1 cells, a generous gift from Dr. Barbara Foster (Roswell Park Cancer Institute, Buffalo, NY), were maintained in DMEM supplemented with 10% heat-inactivated fetal bovine serum, 10−8 mol/L 5α-androstan-17ß-ol-3-one, and antibiotics in a humidified atmosphere of 95% air and 5% CO2. Stock solution of SFN was prepared in dimethyl sulfoxide (DMSO) and an equal volume of DMSO (final concentration <0.1%) was added to controls. Effect of SFN treatment on TRAMP-C1 cell viability was determined by trypan blue dye exclusion assay as described by us previously (23). The proapoptotic effect of SFN was assessed by analysis of cytoplasmic histone-associated DNA fragmentation and sub-diploid cells as described by us previously (23,24).
Immunoblotting
The control and SFN-treated cells were lysed as described by us previously (25). Prostate/tumor tissues were thawed on ice and samples weighing approximately 100 mg were minced and homogenized in 5 volumes of ice-cold phosphate buffered saline (PBS) containing protease and phosphatase inhibitors. Tissue homogenates were centrifuged at 5,000×g for 10 min at 4°C to remove tissue debris followed by centrifugation at 14,000×g for 30 min to obtain clear supernatants. Immunoblotting was performed as described by us previously (23-25).
SFN treatment
We selected TRAMP mice for the studies described herein because: (a) a well-defined course of disease progression with similarities to human prostate carcinogenesis from histological prostatic intraepithelial neoplasia (PIN) to well-differentiated (WD) and poorly-differentiated (PD) carcinoma and distant site metastasis renders TRAMP mice suitable for chemoprevention studies (26-28), (b) the chemoprevention studies in TRAMP mice can be completed in a reasonable time frame (27,28), and (c) the TRAMP model has been used successfully to test chemopreventive efficacy of several synthetic and natural agents (29-32). Male TRAMP[C57BL/6xFVB]F1 hybrid mice were generated by breeding female TRAMP on a C57BL/6 background with non-transgenic FVB male mice. Male TRAMP mice were identified by PCR using DNA obtained from tail clippings (27). After transgene verification, male TRAMP mice were maintained in a climate-controlled environment with a 12 h light /12 h dark cycle and fed food and water ad libitum. At 6-7 weeks of age, mice were randomized into two groups. Mice in the control group (n= 14) received 0.1 mL PBS by oral gavage three times per week (Monday, Wednesday, and Friday) while the experimental mice (n= 13) received 6 μmol SFN in 0.1 mL PBS by oral gavage three times per week for 17-19 weeks. Body weights of the control and SFN-treated mice were recorded once each week beginning at the onset of the study. The mice were sacrificed 24 h after the last administration of the vehicle or SFN by CO2 inhalation. A portion of the prostate/tumor tissue was placed in 10% neutral buffered formalin and paraffin-embedded.
Pathological evaluation and scoring of tumor stage
Ten randomly selected fields of H&E stained sections of the dorsolateral prostate (DLP) from individual mice of both control and SFN-treated groups were independently scored by two researchers for incidence and percentage of area corresponding to each pathological stage. Pathological grading was performed according to the recommendations of Greenberg and colleagues (27,28) as described by us previously (32). Lung and pelvic lymph nodes were evaluated for the presence of metastasis by two independent researchers.
Pharmacokinetic study
Groups of male non-transgenic mice (n= 3; 6-8 weeks old) were orally gavaged with 6 μmol SFN in 0.1 mL PBS. Mice were sacrificed at specified time intervals and plasma and lung tissue were collected and kept frozen at -80°C. Plasma (0.1 mL) or a 1:3 (w/v) homogenate of lung tissue in PBS (0.2 mL) were mixed with 10 μL of 10 μg/mL alyssin (internal standard; LKT Laboratories). Lung homogenates were mixed with 1 mL of acetonitrile and centrifuged at 16,000×g for 5 min at room temperature. The resulting supernatants were evaporated to dryness under a gentle stream of nitrogen at 37 °C and reconstituted in 200 μL distilled water:formic acid (200:10, v/v). Plasma samples were mixed with 5 μL formic acid. Each reconstituted lung or plasma sample was applied to a Bond Elute (C2, 500 mg, 3 mL) solid phase cartridge (Varian, Palo Alto, CA) preconditioned with 2 mL of methanol and 2 mL of 1% formic acid. The cartridges were washed with 2 mL of distilled water:methanol (95:5, v/v). The SFN and the internal standard were eluted with 2 mL of acetonitrile, and the eluates were evaporated to dryness under a gentle stream of nitrogen at 37°C. Each dried residue was reconstituted in 100 μL of acetonitrile:distilled water (15:85, v/v), transferred to an autosampler vial, and 10 μL were injected into the HPLC system. The HPLC system consisted of an Agilent model 1100 autosampler and binary pump (Agilent Technologies, Palo Alto, CA) and a Phenomenex Hydro-Synergi RP (4 μm, 100×2 mm) reverse-phase analytical column. A gradient mobile phase consisting of solvent A (acetonitrile:formic acid; 99.9%/0.1%) and solvent B (water:formic acid; 99.9%/0.1%) was employed for separation. The initial condition was 15% solvent A (2 min), which increased linearly in 8 min to 40% solvent A. This condition was held for 5 min and then solvent A was linearly returned to 15% at 16 min. This condition was held for 8 min. The flow rate was 0.2 mL/min. The SFN and the internal standard were detected using a ThermoFinnigan MSQ Mass Spectrometer (Thermo Electron, San Jose, CA) operating in positive electrospray, single-ion mode monitoring m/z 177.7 for SFN and m/z 191.7 for alyssin. Concentrations of SFN were determined by calculating the ratio of SFN peak area to that of the internal standard and back-calculating the concentration of SFN in the unknown samples with a concomitantly analyzed standard curve in the respective matrix. Standard curves were linear over the range of 10–10,000 ng/mL, and displayed acceptable performance (accuracy within 15% of nominal concentrations and precision <15% at all concentrations). Pharmacokinetic data were analyzed non-compartmentally with PK Solutions 2.0 (Summit Research Services, Montrose, CO; www.summitPK.com).
Immunohistochemical analyses of T-antigen, proliferating cell nuclear antigen (PCNA), CD31, and E-cadherin expression and quantitation of apoptotic bodies
Tissues were sectioned at 4-5 μm thickness, deparaffinized, and rehydrated. Immunohistochemical analysis of T-antigen, PCNA, CD31 and E-cadherin expression was performed as described by us previously (32). Apoptotic bodies were visualized by TUNEL staining as described by us previously (32). The images were analyzed using Image ProPlus 5.0 software (Media Cybernetics, Silver Spring, MD) for quantitation of T-antigen, PCNA, and E-cadherin expression as well as for analysis of microvessel number (CD31 staining).
Cytotoxicity of natural killer (NK) cells and dendritic cells (DC)
After transgene verification, male TRAMP mice (6-7 weeks old) were randomized into two groups with six mice per group. The mice were gavaged thrice weekly (Monday, Wednesday, and Friday) with either 6 μmol SFN in 0.1 mL PBS or vehicle (control) for thirteen weeks. We reasoned that SFN-mediated inhibition of prostate carcinogenesis in TRAMP mice may be preceded by augmentation of NK/DC function, which prompted us to terminate this experiment at an earlier time point. The mice were then sacrificed and the spleen and bone marrow were harvested from individual mice of each group for isolation of NK cells and DC, respectively, as described by us previously (32). Cytotoxicity of NK and/or DC towards TRAMP-C1 target cells was determined using the Cytotox 96 non-radioactive assay as described previously by us (32). Quantitation of T-cell and NK cell infiltration in the DLP was performed by immunohistochemical analysis of CD3+ and CD57+ cells, respectively, using the Double Stain kit from Biocare Medical (Concord, CA) according to the manufacturer’s protocol.
Quantitation of interleukin-12 (IL-12) and interferon γ (IFNγ) release
Spleen-derived NK cells and bone marrow-derived DC from control and SFN-treated mice were cultured for 5 days and the culture media were then collected and analyzed for IFNγ and IL-12 release, respectively, using ELISA kits from Biosource (Carlsbad, California).
Results
SFN treatment decreased viability of TRAMP-C1 cells by causing apoptosis
As can be seen in Fig. 1A, the viability of TRAMP-C1 cells was decreased significantly after a 24 h exposure to SFN in a dose-dependent manner. The SFN-mediated suppression of TRAMP-C1 cell viability correlated with apoptosis induction as revealed by a dose-dependent increase in cytoplasmic histone-associated DNA fragmentation (Fig. 1B) and enrichment of sub-diploid apoptotic fraction (Fig. 1C). Moreover, consistent with the results in human prostate cancer cells (16,22), SFN treatment caused induction of multidomain proapoptotic protein Bax and down-regulation of anti-apoptotic Bcl-xL protein (Fig. 1D).
Figure 1.
A, Effect of SFN (24 h treatment) on viability of TRAMP-C1 cells. B, cytoplasmic histone-associated DNA fragmentation in TRAMP-C1 cells resulting from a 24 h exposure to DMSO or SFN. C, percentage of sub-diploid apoptotic cells in TRAMP-C1 treated with DMSO or 20 μmol/L SFN for the indicated time periods. D, immunoblotting for Bax and Bcl-xL using lysates from TRAMP-C1 cells treated with 20 μmol/L SFN for the indicated time periods. Densitometric scanning data after correction for actin loading control are shown on top of the bands. Columns, mean (n= 3 for panel A-C and n= 2 for panel D); bars, SE. *Significantly different (P<0.05) compared with control by one-way ANOVA followed by Dunnett’s test.
Oral administration of SFN inhibited prostate cancer progression in TRAMP mice
Next, we proceeded to determine the effect of SFN administration on prostate carcinogenesis using TRAMP mice. We have shown previously that thrice weekly oral gavage of 6 μmol SFN is highly effective in suppressing growth of PC-3 xenografts in male nude mice (22). We employed a similar dosing regimen in the present study to determine efficacy of SFN against prostate carcinogenesis in TRAMP mice. Representative H&E staining in sections of DLP from control and SFN-treated mice are depicted in Fig. 2A. The DLP of the vehicle-treated control TRAMP mice exhibited low-and high-grade PIN, and WD and PD carcinomas along with areas consistent with normal prostate. The incidence of the PIN and WD carcinoma in the DLP of mice treated with SFN was lower by 28% (P= 0.006 by Mann Whitney test) and 23% (P= 0.0104), respectively, in comparison with control mice (Fig. 2B). The area occupied by the WD in the DLP of SFN-treated mice was lower by about 44% (P= 0.0011 by Mann Whitney test) compared with control mice (Fig. 2C). Even though the area occupied by the PIN in the DLP of SFN-treated mice was lower by about 24% compared with control, the difference did not reach statistical significance. The body weights of the control and SFN-treated mice did not differ significantly throughout the experimental protocol (results not shown). The mice of both groups were monitored on alternate days for signs of stress throughout the experimental protocol. Except for some mice that were moribund due to large tumor burden prior to the termination of the experiment, control as well as SFN-treated mice did not exhibit signs of stress including food and water withdrawal, impaired posture or movement, ruffled fur, areas of redness or swelling, and indigestion. Moreover, the vital organs (liver, kidney, lung, and heart) from control and SFN treated mice were histologically normal except for microscopic evidence of pulmonary metastasis. However, the SFN-mediated inhibition of prostate carcinogenesis was not due to suppression of T-antigen expression (Fig. 2D).
Figure 2.
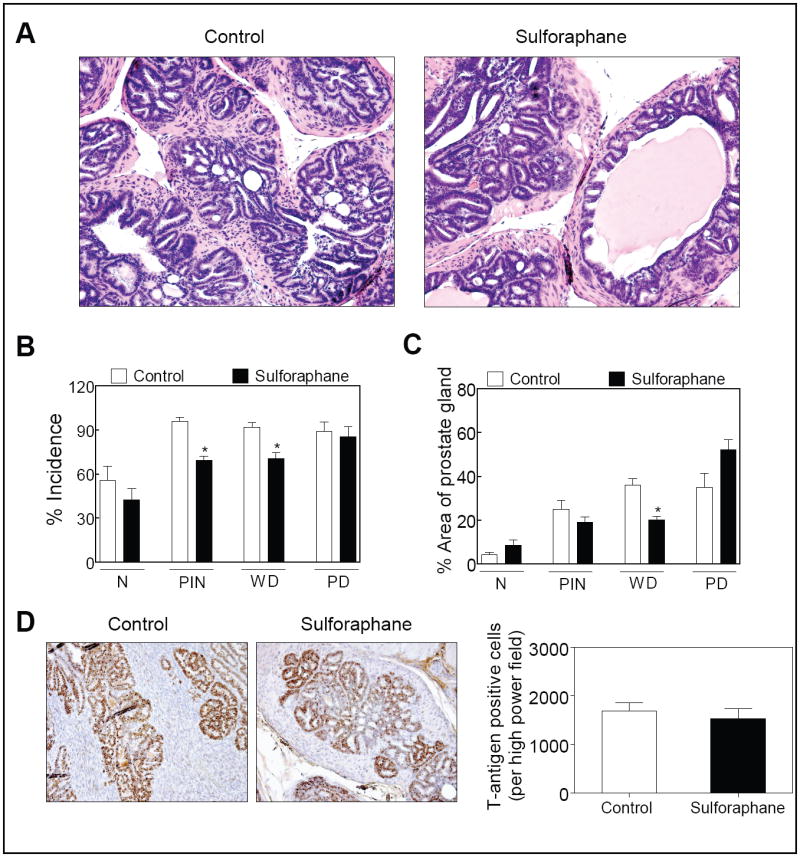
A, H&E staining in the dorsolateral prostate from representative mouse of both vehicle-treated control and SFN treatment groups (200× magnification). B, incidence of histological grades classified as normal prostate (N), PIN, WD carcinoma, and PD carcinoma in the dorsolateral prostate from vehicle-treated control mice and SFN-treated mice. Ten representative fields of each section were scored for incidence of each histological grade. The error bars represent variability of results in histological grading between the two researchers. C, percentage of the area corresponding to the normal prostate, PIN, WD carcinoma, and PD carcinoma in the dorsolateral prostate of control and SFN-treated mice. The scores of the two researchers were averaged and expressed as mean ± SE. In panels B and C *significantly different compared with control by Mann Whitney test. D, T-antigen expression in representative dorsolateral prostate of a control and a SFN-treated TRAMP mouse (200× magnification). The bar graph represents quantitative analysis of T-antigen expression. Columns, mean (n= 4), bars, SE.
SFN administration inhibited pulmonary metastasis
Fig. 3A depicts H&E staining in lung section of a representative mouse of both the control and SFN treatment group. The incidence of pulmonary metastasis was ~80% in vehicle-treated control mice, which was reduced to about 40% in the SFN treatment group (Fig. 3B). The incidence of metastasis to pelvic lymph nodes was comparable in control and SFN-treated mice (Fig. 3B). Multiplicity of pulmonary metastatic lesions in mice treated with SFN was lower by about 63% (P= 0.042 by t-test) compared with control mice (Fig. 3C).
Figure 3.

A, representative H&E stain showing metastatic lesions (marked by arrows) in the lungs of control and SFN-treated mice. Incidence of metastasis (B) and pulmonary metastasis multiplicity (C) in control and SFN-treated mice. Columns, mean (n=12-14); bars, SE. *Significantly different (P<0.05) compared with the vehicle-treated control group by t-test. D, plasma and lung concentrations of SFN versus time after oral gavage of male non-transgenic mice with 6 μmol SFN. Points, mean (n= 3); bars, SE.
Pharmacokinetic measurements
We found that SFN was absorbed rapidly and reached a peak plasma concentration (Cmax) of 3410 ng/mL at a Tmax of approximately 42 min (Fig. 3D). The terminal elimination half-life of SFN in plasma was approximately 255 min. The Cmax of SFN in the lung was approximately 3686 ng/g with a Tmax of about 15 min. The area under the concentration versus time curve between 0 and 8 h (AUC0-8) of SFN in lung (5.80 μg/g•h) was 47% higher than the AUC0-8 of SFN in plasma (3.94 μg/mL•h).
SFN administration decreased cell proliferation
We determined expression of PCNA, a well-accepted marker of cell proliferation (33), by immunohistochemistry to test whether SFN-mediated suppression of prostate carcinogenesis was due to inhibition of cellular proliferation. As can be seen in Fig. 4A, PCNA expression was about 40% lower in the DLP of mice treated with SFN compared with control mice (P= 0.028 by t-test). The fraction of TUNEL positive apoptotic bodies was about 1.9-fold higher in the DLP of SFN-treated mice compared with the controls but the difference was statistically insignificant due to large data scatter especially in the SFN treatment group (Fig. 4B).
Figure 4.
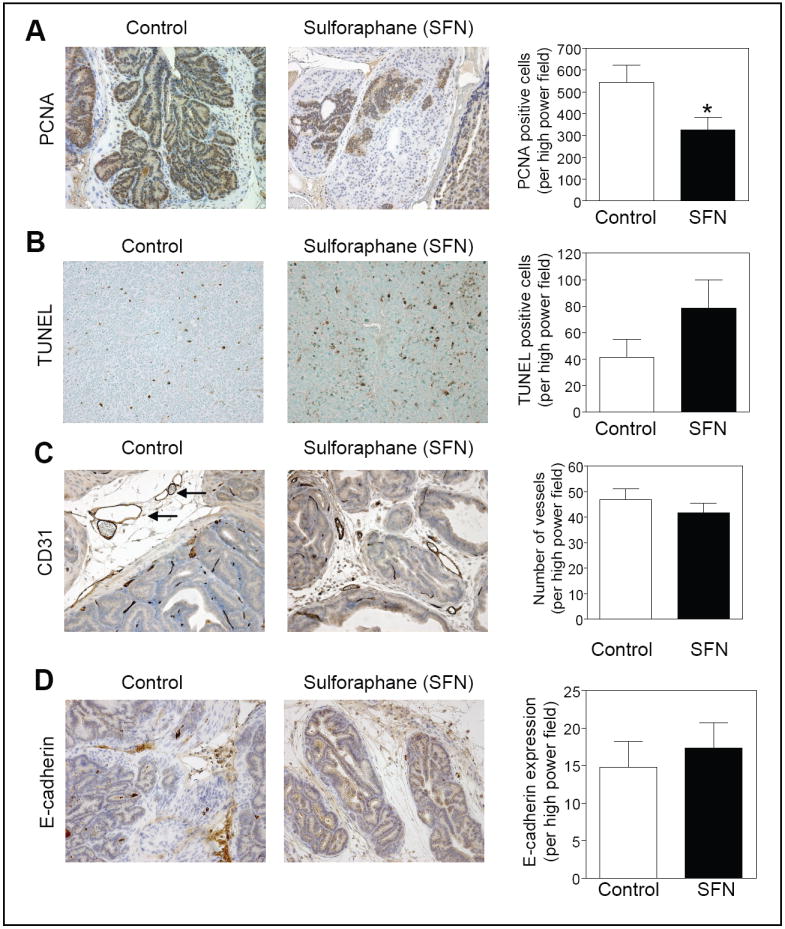
Analyses for (A) expression of PCNA, (B) TUNEL-positive apoptotic bodies, (C) CD31 to visualize blood vessels (identified by arrows), and (D) E-cadherin expression in the dorsolateral prostate of a representative mouse of both the control and SFN treatment groups (200× magnification). The panels on the right show quantitative results for each parameter. Columns, mean (n= 6-7), bars, SE. *Significantly different (P<0.05) compared with control by t-test.
SFN exhibits anti-angiogenic activity in vitro and in vivo (34). To test whether SFN administration caused suppression of neovascularization in vivo, the DLP from vehicle-treated control mice and SFN-treated mice were stained for angiogenic marker CD31. Immunohistochemical staining for CD31 in the DLP of representative mouse from both the control and SFN groups are shown in Fig. 4C (vessels are identified by arrows). The average number of vessels in the DLP did not differ significantly between control and SFN-treated mice (Fig. 4C).
E-cadherin is considered a suppressor of invasion and growth of many epithelial cancers (35). We therefore compared expression of E-cadherin in the DLP of control and SFN-treated mice. Expression of E-cadherin was slightly higher in the DLP of SFN-treated mice compared with that of control mice but the difference was statistically insignificant. These results indicated that the SFN-mediated inhibition of prostate carcinogenesis correlated with decreased cellular proliferation as well as a modest increase in the number of apoptotic bodies in the DLP.
Effect of SFN administration on levels of Bcl-2 family proteins
We compared expression of Bcl-2 family proteins using prostate/tumor supernatants to determine the mechanism of SFN-mediated apoptosis. As can be seen in Fig. 5, the levels of proapoptotic Bcl-2 family proteins Bad, Bak, Bax, and Bid were higher by about 1.33-, 1.16-, 1.27-and 1.9-fold, respectively, in the prostate of SFN treated mice compared with controls. Additionally, SFN administration resulted in a marked decrease (about 63% decrease compared with control) in the level of anti-apoptotic protein Mcl-1 compared with control (Fig. 5). The level of cleaved PARP was about 2.7-fold higher in the prostate of SFN-treated mice compared with the control (Fig. 5).
Figure 5.
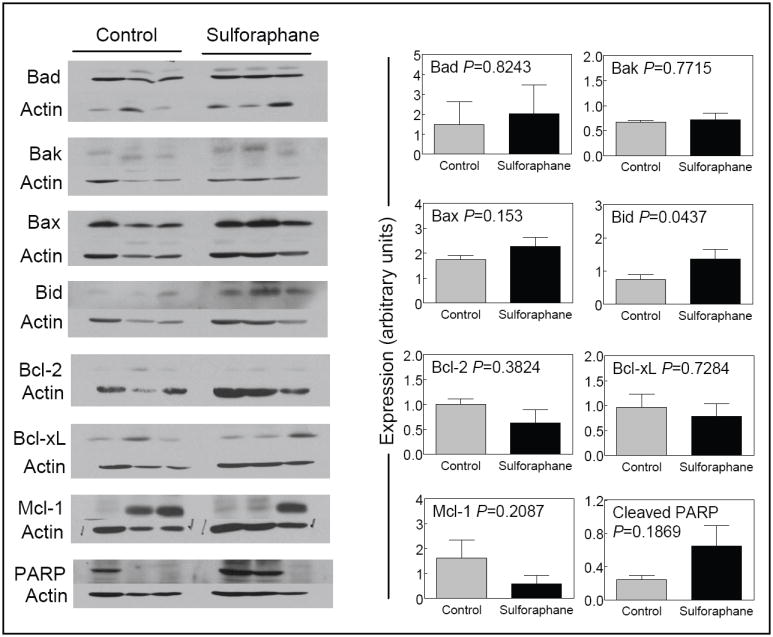
Immunoblotting for Bcl-2 family proteins and cleaved PARP using prostate/tumor supernatants from 3 different mice of both control and SFN-treated groups. The blots were stripped and re-probed with anti-actin antibody to normalize for differences in protein loading. The panels on the right show quantitative results for each protein. Columns, mean (n= 3), bars, SE.
SFN augmented cytotoxic activity of NK:DC co-cultures against TRAMP-C1 cells
The NK cells play an important role in immune surveillance during tumorigenesis (36,37). The NK cells mediate cytotoxicity against tumor cells by multiple mechanisms, including granule-mediated exocytosis and induction of apoptosis (36). To assess if SFN administration affected NK cell/DC function, we determined the cytotoxicity of NK cells and/or DC isolated from control and SFN-treated mice towards TRAMP-C1 as a target cell line. The results in Fig. 6A show that the NK cells isolated from the spleen of SFN-treated TRAMP mice were more cytotoxic against TRAMP-C1 cells compared with the NK cells isolated from control mice (23% versus 50% cytotoxicity for NK cells isolated from control and SFN-treated mice, respectively; P<0.05 by t-test). It is well established that interactions of NK cells with DC result in co-activation of both cell types (38). We therefore determined the lytic functions of NK cell and DC co-cultures. The cytotoxic activity of NK cells against TRAMP-C1 cells was increased even more with co-culture of NK cells with DC at 10:8 ratio compared with NK cells alone. This effect correlated with a significant increase in IL-12 production by the DC isolated from the bone marrow of SFN-treated mice compared with control mice (Fig. 6B, left panel). On the other hand, the IFNγ production by the NK cells did not differ significantly between control and SFN-treated mice (Fig. 6B, right panel). Altogether, these results indicate that SFN administration stimulates immune response by augmenting NK cell lytic function through DC-mediated IL-12 production.
Figure 6.
A, cytotoxicity of NK cell alone and NK cell:DC co-cultures towards TRAMP-C1 target cells. The NK:DC were incubated together at the desired ratio for 24 h prior to the addition of TRAMP-C1 targets cells. B, IL-12 (left panel) and IFNγ (right panel) levels in DC and NK culture medium after 5 days, respectively. C, immunohistochemical analysis for CD3 positive T-cell infiltration and CD57 positive NK cell infiltration in representative prostate of a control mouse and a SFN-treated mouse. The CD3 positive and CD57 positive cells are identified by yellow and black arrowheads, respectively (400× magnification). D, quantification of CD3+ T-cell and CD57+ NK cell infiltration in prostate tumor of control and SFN-treated animal. Ten representative fields of each section were evaluated and averaged for CD3+ and CD57+ staining. Columns, mean (n= 6 for panels A-C and n=3 for data in panel D); bars, SE; *, P<0.05, significantly different compared with control by t-test.
SFN administration caused T-cell infiltration in the prostate tumor
Next, we explored the possibility of whether the prostate tumor from SFN-treated mice was infiltrated by carriers of an adaptive immune response such as T-cells. We stained prostate tumors from control and SFN treated mice using anti-CD3 (a marker of T-cells) and anti-CD57 (a marker of NK cells) antibodies. The results can be visualized in Fig. 6C where NK cells are stained in brown and the T-cells are stained in pink. The prostate tumors from SFN-treated mice exhibited a modest yet statistically significant increase in T-cell, but not NK cell, infiltration compared with control (Fig. 6D). Taken together our results indicate that SFN administration increases immune surveillance by boosting both the NK cell lytic function and T-cell infiltration.
Discussion
The present study demonstrates that oral gavage of SFN not only suppresses the growth of a TRAMP tumor-derived cell line in culture but also prevents incidence and/or burden of PIN and well-differentiated carcinoma and incidence and multiplicity of lung metastasis in male TRAMP mice without causing weight loss or affecting the T-antigen expression. The possibility that higher concentration of SFN and/or a more intensive dosing regimen (e.g., daily administration) results in better chemopreventive response cannot be ruled out. Likewise, it is possible that earlier onset of SFN administration (e.g., starting at 4-5 weeks of age) leads to even greater protection against prostate carcinogenesis in TRAMP mice. Additional work is needed to systematically explore these possibilities.
The bioavailability of SFN in TRAMP mice is yet to be evaluated, but this parameter has been determined in rats. The SFN was detectable in the plasma after 1 h of oral gavage of rats with 50 μmol SFN (39). The maximal plasma concentration of SFN was found to be approximately 20 μmol/L after 4 h of oral gavage, and declined with a half-life of 2.2 h (39). Hanlon et al. (40) have shown that SFN is rapidly absorbed with an absolute bioavailability of approximately 82% in rats (40). The maximal plasma concentration of SFN and its cysteine conjugate varied between 0.65 and 0.82 μmol/L in healthy human volunteers who consumed a test meal of broccoli soup (150 mL) containing 100 g of broccoli (41). The SFN concentration effective against prostate carcinogenesis and pulmonary metastasis in TRAMP mice is well within the range that can not only be generated through dietary intake of cruciferous vegetables but can also be safely administered to humans without any harmful side effects (5,42).
We have shown previously that SFN treatment suppresses growth of cultured human prostate cancer cells by causing G2/M phase cell cycle arrest and apoptosis induction (13,16,21,22). The present study reveals that a cell line derived from the prostate tumor of a TRAMP mouse is also highly sensitive to growth inhibition and apoptosis induction by SFN treatment. We also found that oral administration of SFN causes a statistically significant decrease in cell proliferation in the DLP of TRAMP mice as evidenced by reduced expression of PCNA. Moreover, the average number of TUNEL-positive apoptotic bodies was markedly higher in the DLP of SFN-treated mice in comparison with controls. Thus, it is reasonable to conclude that SFN-mediated inhibition of prostate carcinogenesis in TRAMP mice in vivo is mediated by reduced cellular proliferation and increased apoptosis.
Distant site metastasis is a major cause of death in prostate cancer patients (43). The pathogenesis of metastasis is complex and involves a series of molecular events including synthesis and secretion of angiogenic factors to promote neovascularization, motility and local invasion of the host stroma, entry into the circulation, detachment, and extravasation (44). Steps leading to metastasis are complex and regulated by multiple molecules including growth factors, matrix metalloproteinases, and cell adhesion molecules (44). For example, loss of expression of cell adhesion molecules, especially E-cadherin, is believed to be important for development of metastatic lesions (35). Inhibition of prostate carcinogenesis and metastasis by the plant flavonoid apigenin in TRAMP mice was shown to correlate with retained expression of E-cadherin (29). The present study reveals that the SFN administration inhibits pulmonary metastasis incidence and multiplicity in TRAMP mice. However, the inhibitory effect of SFN administration against pulmonary metastasis seems independent of changes in E-cadherin expression or inhibition of angiogenesis. Delineation of the precise mechanisms by which SFN administration inhibits pulmonary metastasis awaits further work.
The present study demonstrates, for the first time, that treatment of TRAMP mice with SFN leads to increased production of IL-12 by DC. This effect results in augmentation of NK cell cytotoxicity towards a prostate cancer cell line and increased efficiency of DC activation of NK cell cytotoxic function following their co-culture. Previous studies have documented the capacity for DC to internalize, process and present tumor cell-derived antigens, and thereby to stimulate the anti-tumor function of both cytotoxic T-lymphocytes and IFNγ-producing NK cells (45,46). In addition to the stimulatory effects of SFN administration on in vitro functions of NK cells and DC, we observed an increase in T-cell infiltration in the prostate tumors of SFN-treated TRAMP mice in comparison with control mice. Taken together, our results suggest that SFN can enhance immune function towards prostate tumors by stimulating IL-12 release by DC, leading to an increased level of cytotoxicity of NK cells and ultimately to a greater infiltration of prostate tumors by T cells, which results in a lower tumor burden and less metastases.
In conclusion, the results of the present study indicate that oral administration of SFN significantly inhibits prostate carcinogenesis and pulmonary metastasis in TRAMP mice without causing any harmful side effects or reducing T-antigen expression.
Acknowledgments
Grant support: This investigation was supported in part by the US PHS grants CA115498 and CA101753 awarded by the National Cancer Institute.
References
- 1.Verhoeven DT, Goldbohm RA, van Poppel G, Verhagen H, van den Brandt PA. Epidemiological studies on Brassica vegetables and cancer risk. Cancer Epidemiol Biomarkers Prev. 1996;5:733–48. [PubMed] [Google Scholar]
- 2.Kolonel LN, Hankin JH, Whittemore AS, et al. Vegetables, fruits, legumes and prostate cancer: a multiethnic case-control study. Cancer Epidemiol Biomarkers Prev. 2000;9:795–804. [PubMed] [Google Scholar]
- 3.Ambrosone CB, McCann SE, Freudenheim JL, Marshall JR, Zhang Y, Shields PG. Breast cancer risk in premenopausal women is inversely associated with consumption of broccoli, a source of isothiocyanates, but is not modified by GST genotype. J Nutr. 2004;134:1134–8. doi: 10.1093/jn/134.5.1134. [DOI] [PubMed] [Google Scholar]
- 4.Hecht SS. Inhibition of carcinogenesis by isothiocyanates. Drug Metab Rev. 2000;32:395–411. doi: 10.1081/dmr-100102342. [DOI] [PubMed] [Google Scholar]
- 5.Conaway CC, Yang YM, Chung FL. Isothiocyanates as cancer chemopreventive agents: their biological activities and metabolism in rodents and humans. Curr Drug Metab. 2002;3:233–55. doi: 10.2174/1389200023337496. [DOI] [PubMed] [Google Scholar]
- 6.Zhang Y, Talalay P, Cho CG, Posner GH. A major inducer of anticarcinogenic protective enzymes from broccoli: isolation and elucidation of structure. Proc Natl Acad Sci USA. 1992;89:2399–403. doi: 10.1073/pnas.89.6.2399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brooks JD, Paton VG, Vidanes G. Potent induction of phase 2 enzymes in human prostate cells by sulforaphane. Cancer Epidemiol Biomarkers Prev. 2001;10:949–54. [PubMed] [Google Scholar]
- 8.Zhang Y, Kensler TW, Cho CG, Posner GH, Talalay P. Anticarcinogenic activities of sulforaphane and structurally related synthetic norbornyl isothiocyanates. Proc Natl Acad Sci USA. 1994;91:3147–50. doi: 10.1073/pnas.91.8.3147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chung FL, Conaway CC, Rao CV, Reddy BS. Chemoprevention of colonic aberrant crypt foci in Fischer rats by sulforaphane and phenethyl isothiocyanate. Carcinogenesis. 2000;21:2287–91. doi: 10.1093/carcin/21.12.2287. [DOI] [PubMed] [Google Scholar]
- 10.Fahey JW, Haristoy X, Dolan PM, et al. Sulforaphane inhibits extracellular, intracellular, and antibiotic-resistant strains of Helicobacter pylori and prevents benzo[a]pyrene-induced stomach tumors. Proc Natl Acad Sci USA. 2002;99:7610–5. doi: 10.1073/pnas.112203099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Conaway CC, Wang CX, Pittman B, et al. Phenethyl isothiocyanate and sulforaphane and their N-acetylcysteine conjugates inhibit malignant progression of lung adenomas induced by tobacco carcinogens in A/J mice. Cancer Res. 2005;65:8548–57. doi: 10.1158/0008-5472.CAN-05-0237. [DOI] [PubMed] [Google Scholar]
- 12.Gamet-Payrastre L, Li P, Lumeau S, et al. Sulforaphane, a naturally occurring isothiocyanate, induces cell cycle arrest and apoptosis in HT29 human colon cancer cells. Cancer Res. 2000;60:1426–33. [PubMed] [Google Scholar]
- 13.Singh SV, Herman-Antosiewicz A, Singh AV, et al. Sulforaphane-induced G2/M phase cell cycle arrest involves checkpoint kinase 2 mediated phosphorylation of Cdc25C. J Biol Chem. 2004;279:25813–22. doi: 10.1074/jbc.M313538200. [DOI] [PubMed] [Google Scholar]
- 14.Myzak MC, Hardin K, Wang R, Dashwood RH, Ho E. Sulforaphane inhibits histone deacetylase activity in BPH-1, LNCaP and PC-3 prostate epithelial cells. Carcinogenesis. 2006;27:811–9. doi: 10.1093/carcin/bgi265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Choi S, Singh SV. Bax and Bak are required for apoptosis induction by sulforaphane, a cruciferous vegetable derived cancer chemopreventive agent. Cancer Res. 2005;65:2035–43. doi: 10.1158/0008-5472.CAN-04-3616. [DOI] [PubMed] [Google Scholar]
- 16.Singh SV, Srivastava SK, Choi S, et al. Sulforaphane-induced cell death in human prostate cancer cells is initiated by reactive oxygen species. J Biol Chem. 2005;280:19911–24. doi: 10.1074/jbc.M412443200. [DOI] [PubMed] [Google Scholar]
- 17.Xu C, Shen G, Chen C, Gelinas C, Kong AN. Suppression of NF-kappaB and NF-kappaB-regulated gene expression by sulforaphane and PEITC through IkappaBalpha, IKK pathway in human prostate cancer PC-3 cells. Oncogene. 2005;24:4486–95. doi: 10.1038/sj.onc.1208656. [DOI] [PubMed] [Google Scholar]
- 18.Herman-Antosiewicz A, Johnson DE, Singh SV. Sulforaphane causes autophagy to inhibit release of cytochrome c and apoptosis in human prostate cancer cells. Cancer Res. 2006;66:5828–35. doi: 10.1158/0008-5472.CAN-06-0139. [DOI] [PubMed] [Google Scholar]
- 19.Kim H, Kim EH, Eom YW, et al. Sulforaphane sensitizes tumor necrosis factor-related apoptosis-inducing ligand (TRAIL)-resistant hepatoma cells to TRAIL-induced apoptosis through reactive oxygen species-mediated up-regulation of DR5. Cancer Res. 2006;66:1740–50. doi: 10.1158/0008-5472.CAN-05-1568. [DOI] [PubMed] [Google Scholar]
- 20.Mi L, Wang X, Govind S, et al. The role of protein binding in induction of apoptosis by phenethyl isothiocyanate and sulforaphane in human non-small lung cancer cells. Cancer Res. 2007;67:6409–16. doi: 10.1158/0008-5472.CAN-07-0340. [DOI] [PubMed] [Google Scholar]
- 21.Choi S, Lew KL, Xiao H, et al. D,L-Sulforaphane-induced cell death in human prostate cancer cells is regulated by inhibitor of apoptosis family proteins and Apaf-1. Carcinogenesis. 2007;28:151–62. doi: 10.1093/carcin/bgl144. [DOI] [PubMed] [Google Scholar]
- 22.Singh AV, Xiao D, Lew KL, Dhir R, Singh SV. Sulforaphane induces caspase-mediated apoptosis in cultured PC-3 human prostate cancer cells and retards growth of PC-3 xenografts in vivo. Carcinogenesis. 2004;25:83–90. doi: 10.1093/carcin/bgg178. [DOI] [PubMed] [Google Scholar]
- 23.Xiao D, Choi S, Johnson DE, et al. Diallyl trisulfide-induced apoptosis in human prostate cancer cells involves c-Jun N-terminal kinase and extracellular-signal regulated kinase-mediated phosphorylation of Bcl-2. Oncogene. 2004;23:5594–606. doi: 10.1038/sj.onc.1207747. [DOI] [PubMed] [Google Scholar]
- 24.Herman-Antosiewicz A, Singh SV. Checkpoint kinase 1 regulates diallyl trisulfide-induced mitotic arrest in human prostate cancer cells. J Biol Chem. 2005;280:28519–28. doi: 10.1074/jbc.M501443200. [DOI] [PubMed] [Google Scholar]
- 25.Xiao D, Srivastava SK, Lew KL, et al. Allyl isothiocyanate, a constituent of cruciferous vegetables, inhibits proliferation of human prostate cancer cells by causing G2/M arrest and inducing apoptosis. Carcinogenesis. 2003;24:891–7. doi: 10.1093/carcin/bgg023. [DOI] [PubMed] [Google Scholar]
- 26.Klein RD. The use of genetically engineered mouse models of prostate cancer for nutrition and cancer chemoprevention research. Mutat Res. 2005;576:111–9. doi: 10.1016/j.mrfmmm.2005.02.012. [DOI] [PubMed] [Google Scholar]
- 27.Greenberg NM, DeMayo F, Finegold MJ, et al. Prostate cancer in a transgenic mouse. Proc Natl Acad Sci USA. 1995;92:3439–43. doi: 10.1073/pnas.92.8.3439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kaplan-Lefko PJ, Chen TM, Ittmann MM, et al. Pathobiology of autochthonous prostate cancer in a pre-clinical transgenic mouse model. Prostate. 2003;55:219–37. doi: 10.1002/pros.10215. [DOI] [PubMed] [Google Scholar]
- 29.Shukla S, MacLennan GT, Flask CA, et al. Blockade of beta-catenin signaling by plant flavonoid apigenin suppresses prostate carcinogenesis in TRAMP mice. Cancer Res. 2007;67:6925–35. doi: 10.1158/0008-5472.CAN-07-0717. [DOI] [PubMed] [Google Scholar]
- 30.Raina K, Singh RP, Agarwal R, Agarwal C. Oral grape seed extract inhibits prostate tumor growth and progression in TRAMP mice. Cancer Res. 2007;67:5976–82. doi: 10.1158/0008-5472.CAN-07-0295. [DOI] [PubMed] [Google Scholar]
- 31.Kumar AP, Bhaskaran S, Ganapathy M, et al. Akt/cAMP-responsive element binding protein/cyclin D1 network: a novel target for prostate cancer inhibition in transgenic adenocarcinoma of mouse prostate model mediated by Nexrutine, a Phellodendron amurense bark extract. Clin Cancer Res. 2007;13:2784–94. doi: 10.1158/1078-0432.CCR-06-2974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Singh SV, Powolny AA, Stan SD, et al. Garlic constituent diallyl trisulfide prevents development of poorly-differentiated prostate cancer and pulmonary metastasis multiplicity in TRAMP mice. Cancer Res. 2008;68:9503–11. doi: 10.1158/0008-5472.CAN-08-1677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chuang LC, Yew PR. Proliferating cell nuclear antigen recruits cyclin-dependent kinase inhibitor Xic1 to DNA and couples its proteolysis to DNA polymerase switching. J Biol Chem. 2005;280:35299–309. doi: 10.1074/jbc.M506429200. [DOI] [PubMed] [Google Scholar]
- 34.Jackson SJT, Singletary KW, Venema RC. Sulforaphane suppresses angiogenesis and disrupts endothelial mitotic progression and microtubule polymerization. Vas Pharmacol. 2006;46:77–84. doi: 10.1016/j.vph.2006.06.015. [DOI] [PubMed] [Google Scholar]
- 35.Wheelock MJ, Johnson KR. Cadherins as modulators of cellular phenotype. Annu Rev Cell Dev Biol. 2003;19:207–35. doi: 10.1146/annurev.cellbio.19.011102.111135. [DOI] [PubMed] [Google Scholar]
- 36.Terabe M, Berzofsky JA. NKT cells in immunoregulation of tumor immunity: a new immunoregulatory axis. Trends Immunol. 2007;28:491–6. doi: 10.1016/j.it.2007.05.008. [DOI] [PubMed] [Google Scholar]
- 37.Guerra N, Tan YX, Joncker NT, et al. NKG2D-Deficient mice are defective in tumor surveillance in models of spontaneous malignancy. Immunity. 2008;28:571–80. doi: 10.1016/j.immuni.2008.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Fernandez NC, Lozier A, Flament C, et al. Dendritic cells directly trigger NK cell functions: cross-talk relevant in innate anti-tumor immune responses in vivo. Nat Med. 1999;5:405–11. doi: 10.1038/7403. [DOI] [PubMed] [Google Scholar]
- 39.Hu R, Hebbar V, Kim BR, et al. In vivo pharmacokinetics and regulation of gene expression profiles by isothiocyanate sulforaphane in the rat. J Pharmacol Expt Ther. 2004;310:263–71. doi: 10.1124/jpet.103.064261. [DOI] [PubMed] [Google Scholar]
- 40.Hanlon N, Coldham N, Gielbert A, et al. Absolute bioavailability and dose-dependent pharmacokinetic behavior of dietary doses of the chemopreventive isothiocyanate sulforaphane in rat. Br J Nutr. 2008;99:559–64. doi: 10.1017/S0007114507824093. [DOI] [PubMed] [Google Scholar]
- 41.Al Janobi AA, Mithen RF, Gasper AV, et al. Quantitative measurement of sulforaphane, iberin and their mercapturic acid pathway metabolites in human plasma and urine using liquid chromatography-tandem electrospray ionization mass spectrometry. J Chromatogr B. 2006;844:223–34. doi: 10.1016/j.jchromb.2006.07.007. [DOI] [PubMed] [Google Scholar]
- 42.Cornblatt BS, Ye L, Dinkova-Kostova AT, et al. Preclinical and clinical evaluation of sulforaphane for chemoprevention in the breast. Carcinogenesis. 2007;28:1485–90. doi: 10.1093/carcin/bgm049. [DOI] [PubMed] [Google Scholar]
- 43.Nelson WG, DeMarzo A, Isaacs WB. Prostate cancer. N Engl J Med. 2003;349:366–81. doi: 10.1056/NEJMra021562. [DOI] [PubMed] [Google Scholar]
- 44.Fidler IJ, Kim SJ, Langley RR. The role of the organ microenvironment in the biology and therapy of cancer metastasis. J Cell Biochem. 2007;101:927–36. doi: 10.1002/jcb.21148. [DOI] [PubMed] [Google Scholar]
- 45.Nouri-Shirazi M, Banchereau J, Bell D, et al. Dendritic cells capture killed tumor cells and present their antigens to elicit tumor-specific immune responses. J Immunol. 2000;165:3797–803. doi: 10.4049/jimmunol.165.7.3797. [DOI] [PubMed] [Google Scholar]
- 46.Karimi K, Boudreau JE, Fraser K, et al. Enhanced antitumor immunity elicited by dendritic cell vaccines is a result of their ability to engage both CTL and IFN gamma-producing NK cells. Mol Ther. 2008;16:411–8. doi: 10.1038/sj.mt.6300347. [DOI] [PubMed] [Google Scholar]




