Abstract
Forkhead box transcription factor FOXO3A is an important regulator of cellular function, is thought to act as a tumor suppressor. We studied whether alterations in FOXO3A activity occur in prostate tumorigenesis. Our studies demonstrate that FOXO3A activity is negatively regulated by Akt/PKB through posttranslational modifications. In prostate cancer cells, Akt activation causes increased accumulation of FOXO3A and its binding chaperone protein 14-3-3 in the cytosol. Higher levels of FOXO3A in the cytosol correlated with phosphorylation at Ser253, which accounted for its nuclear exclusion. Dominant negative Akt approach in PC-3 cells increased FOXO3A accumulation in the nucleus, causing upregulation of the downstream target, MnSOD. Conversely, stable DU145-Akt over-expressing cells exhibited decreased FOXO3A levels in the nucleus. Similar findings were noted in prostate tumor specimens, in which marked cytoplasmic accumulation of FOXO3A and 14-3-3 in prostate tumors was observed with increasing Gleason grade, in contrast to exclusively nuclear accumulation in benign prostate cells. These findings correlate with decreased FOXO3A DNA binding activity along with down modulation of FOXO3A transcriptional activity with increasing tumor grade. Our findings demonstrate that tumor associated alterations and redistribution of FOXO3A are frequent events in the etiology of prostate cancer.
Keywords: Forkhead transcription factor, prostate cancer, tumor suppressor, Akt/PKB, 14-3-3
Introduction
FOXO (Forkhead box, class ‘O’) comprise a subgroup of the winged helix or forkhead transcription factors that regulate a wide range of biological functions, including development, growth, stress resistance, apoptosis, cell cycle, immunity, metabolism, and aging (1, 2). In humans, four members have been identified: FOXO1 (FKHR), FOXO3A (FKHRL1), FOXO4 (AFX) and FOXO6. These proteins bind via their forkhead domains to a cognate DNA binding sequence termed the FOXO-recognized element to initiate gene transcription (3) The first three members were found at the site of chromosomal translocations in various tumors, suggesting that they may play a role in oncogenesis (4). Simultaneous deletion of FOXO1, FOXO3A and FOXO4 is associated with a predisposition to certain neoplasms such as thymic lymphomas and hemangiomas, suggesting that FOXOs are tumor suppressors (5). In contrast, activation of FOXO proteins leads to G1 cell cycle arrest, apoptosis, and gluconeogenesis through upregulation of cyclin dependent kinase inhibition p27/kip1 and apoptosis-related protein viz. FAS ligand, tumor necrosis factor-related apoptosis inducing ligand (TRAIL), BIM, and insulin-like growth factor binding protein-1 (6). FOX transcription factors are regulated by various external stimuli such as insulin, insulin-like growth factor (IGF-I), other growth factors, nutrients, cytokines and oxidative stress (7).
In response to survival growth factors such as IGF-I, Akt/PKB and the related kinase phosphorylate FOXO protein at the first and second phosphorylation sites (Thr32 and Ser253 in FOXO3A) which creates a binding site for the chaperone protein 14-3-3 (8). Furthermore, 14-3-3 binds to FOXO factors in the nucleus, resulting in its nuclear exclusion and consequent inability to bind DNA. Besides phosphorylation, FOXO proteins can undergo further posttranslational modifications such as acetylation and deacetylation (9). These steps are critical for the specificity of FOXO protein activation of downstream target genes such as p27/kip1, MnSOD, catalase, FasL, BIM and GADD45α (10). Posttranslational inactivation and nuclear exclusion of FOXO proteins has been observed in cancers of the hematopoietic system and bone, as well as carcinomas of the breast, stomach, thyroid, lung and prostate (11-17).
Prostate cancers are heterogeneous, with considerable variability in histological characteristics and malignant behavior (18). In prostate tumor cells Akt/PKB is often constitutively activated either through deletion or mutation in phosphatase and tensin homologue on chromosome 10 (PTEN) or through the activity of autocrine growth factors (19). Constitutive Akt activation in prostate cancer cells promotes cellular differentiation and survival through regulation of a number of transcription factors, including FOXO (20). FOXO1A inhibits androgen- and androgen receptor- mediated gene regulation and has been shown to be inactivated by Akt-mediated phosphorylation (21). Prostate cancer progression from androgen-dependence to androgen-independence is associated with decreased FOXO3A expression and reduced p27/kip1 promoter transactivation (22). In contrast, over-expression of FOXO3A and FOXO1 in prostate cancer cells causes apoptosis and induction of genes that affect cellular proliferation (23). Expression of FOXO1 (FKHR) and its phosphorylated form p-FKHR has been demonstrated in clinical prostate cancer specimens (17). Although FOXO3A protein has been shown to be highly expressed in prostate cancer, there are no reports on FOXO3A and its deregulation during disease progression. In this study, we investigated the regulation of FOXO3A in human prostate cancer cells and investigated FOXO3A expression and function in benign and malignant prostate tissue.
Materials and Methods
Cell culture
Human prostate cancer cells, LNCaP, 22Rv1, DU145 and PC-3 were purchased from the American Type Culture Collection (Manassas, VA) and cultured in RPMI 1640 medium with 10% heat-inactivated fetal bovine serum,100μg/ml penicillin–streptomycin (Invitrogen, Carlsbad, CA). Additionally, normal human prostate epithelial cells (NHPE) were purchased from Lonza Inc., Allendale, NJ and cultured in prostate epithelial cell growth medium containing growth medium and supplements (Cat# CC-3166), maintained in an incubator with a humidified atmosphere of 95% air and 5% CO2 at 37°C. The cells were cultured on 100mm dishes and grown to approximately 60% confluence and lysates were prepared for further analysis.
Transient transfection
Androgen-refractory human prostate cancer DU145 cells, which have lower basal levels of Akt; and PC-3 cells which possess higher constitutive expression of Akt were used in the study. Briefly, DU145 cells were plated in 100mm plates and allowed to attach overnight. Approximately 60% confluent cells were transiently transfected with 8μg of either pLNCX vector containing Akt-expression plasmid (kindly provided by Dr William Sellers, Harvard Medical School, MA) or empty vector, whereas, PC-3 cells were transfected with Dominant negative Akt in pUSEamp (K179M mutant) (DN-Akt) and the empty vector pUSEamp (Upstate Cell Signaling, Lake Placid, NY) using Lipofectamine™ 2000 transfection reagent (Invitrogen, Carlsbad, CA). After 5 h, the medium was replaced with fresh complete culture medium, and the cells were incubated overnight at 37°C in a humidified incubator. Later, the cells were processed for immunoblot analysis.
Human prostate tissue specimens
Samples of discarded human prostate tissue were received from the Tissue Procurement Facility of University Hospitals Case Medical Center and the Midwestern Division of the Cooperative Human Tissue Network. Patients from whom these tissues were procured had undergone surgical procedures and had not received any form of adjuvant therapy. The Gleason grade and score of adenocarcinoma in tissue specimens were assigned by a surgical pathologist experienced in genitourinary pathology. Immediately after procurement, samples were snap frozen in liquid nitrogen and stored at -150°C till further use. For IHC studies a human prostate tissue microarray was procured from Zymed Laboratories, San Francisco, CA, including cores of normal prostate, benign hyperplastic prostate tissue, low-grade cancers and high-grade prostate cancers. In these specimens, immunohistochemical analysis was performed according to the manufacturer's protocol (Biocare Medicals, Concord, CA).
Immunoblot analysis
Tumor lysates as well as cells lysates were prepared and subjected to immunoblot analysis. 40μg of protein from total cell lysates or human prostate tumor lysates was resolved over 4-20% SDS–PAGE and transferred to nitrocellulose membrane. After blocking with blocking buffer (PBS containing 0.1% Tween 20 and 10% FBS) for 2 h, the membrane was incubated with primary antibody for 2 h at room temperature. The antibodies used were FOXO1(Cat# Q12778; Millipore, Danvers, MA); MnSOD (Cat# SOD110; Stressgen, Ann Arbor, MI); 14-3-3β (Cat# SC-629; Santa Cruz Biotechnology, Santa Cruz, CA); p-FOXO3A-Ser253 (Cat# 06-953; Upstate Biotechnology, Danvers, MA); FOXO3A (Cat# 9467) FOXO4 (Cat# 9472) and p-GSK3β (Cat# 9331) from Cell Signaling Technology, Danvers, MA). The membrane was then incubated with HRP-conjugated secondary antibody for another 2 h at room temperature. The protein was detected by ECL substrate reagents (Amersham Biosciences, Arlington Heights, IL, USA).
Immunoprecipitation assay
Human prostate cancer cells were grown up to 60% confluence and cytosolic fractions were prepared as previously described (). Equal amounts of cytosolic proteins (100μg) from all the cells fractions were incubated with 5μl anti-14-3-3β antibody at 4°C for 2 h. 20μl of Protein A/G–agarose (Cat # SC-2003; Santa Cruz Biotechnology, Santa Cruz, CA) beads were then added to each sample, incubated overnight at 4°C, and centrifuged the next day at 3,000 rpm for 10 min. Supernatant was aspirated from each sample and the immunoprecipitates were washed four times with cytosolic buffer and subjected to SDS–PAGE followed by immunoblotting with anti-FOXO3A antibody.
FOXO3A DNA binding activity
Nuclear fractions were isolated from normal human prostate epithelial cells, prostate cancer cells and from the human prostate tissue specimens to evaluate FOXO3A DNA binding by using FoxO3a EZ-TFA Transcription Factor Assay Chemiluminescent Kit (Cat # 70-653; Upstate Biotechnology, Danvers, MA) according to the manufacturers protocol.
Reverse transcription-polymerase chain reaction
Semi-quantitative reverse transcription polymerase chain reaction (RT-PCR) was performed to determine the transcript levels of FOXO1, FOXO3A, and FOXO4 in human prostate tissue specimens of different Gleason grades and the amplification of GAPDH transcripts were used as the control to normalize the transcript levels of these FOXOs. Tissues were cut into small pieces and placed in Melt, a total nucleic acid isolation system (Ambion, Austin, TX), according to the manufacturers protocol. RT was performed by using the oligo-dT primer (Invitrogen, Carlsbad, CA), and 0.5μg total RNA in a 25μL reaction mixture, containing 50mM Tris-HCl (pH 8.3), 75mM KCl, 10mM DTT, 5mM MgCl2, 2.5mL dNTP (10mM), 10U RNAsin, and 200U MMLV reverse transcriptase (Invitrogen). The RT reaction was carried out at 39°C for 1 h to synthesize cDNAs. Then, PCR was performed to amplify cDNAs in a 25μL reaction mixture containing 50nmol of each gene-specific primer, 3mL RT product, 2.5mM dNTP, 1X PCR buffer (5mM Tris-HCl pH 8.3, 42.5mM KCl, 0.1% Triton X-100), 0.5mL Taq polymerase (Promega, Madison, WI) 2mM MgCl2 along with primers used in the PCR reaction. The sequences of gene-specific primers for the FOXO1 Forward: 5′-GCAGATCTACGAGTGGATGGTC-3′ and Reverse: 5′-AAACTGTGATCCAGGGCTGTC-3′; FOXO3a Forward: 5′-CTTCAAGGATAAGGGCGACAG-3 and Reverse: 5′-CGTCCTGGACTTCATCCAAC-3′; FOXO4 Forward: 5′-AGCGACTGACACTTGCCCAGAT-3′ and Reverse: 5′-AGGGTTCAGCATCCACCAAGAG-3′; GAPDH Forward: 5′-ATGACCCCTTCATTGACCTCA-3′and Reverse: 5′-GAGATGATGACCCTTTTGGCT-3′. FOXO3A and FOXO4 transcripts were all amplified for 19 cycles (1 min at 94°C, 1 min at 59°C, and 1 min at 72°C), and the cDNAs of GAPDH transcripts were amplified for 17 cycles (1 min at 94°C, 1 min at 55°C, and 1 min at 72°C). The PCR cycling numbers had been optimized to avoid amplification saturation. Ten micro-liters of the RT-PCR product was separated on 2% agarose gels, which were subsequently stained with ethidium bromide. Gels were visualized and band intensities were measured under the Kodak 2000R image station.
Statistical analysis
All measures were summarized as means ± standard deviations (or mean + range). Graphical summaries of the distributions of measure were made using boxplots and bar plots. Differences in levels of nuclear FOXO3A cells between benign prostate, low-grade cancer and high-grade cancers were examined by T-test. The FOXO3A DNA binding activity between two groups (i.e. NHPE, benign tumor tissues and human prostate cancer cell lines) was compared using T-test as well. The test is two-sided and a p-value ≤ 0.05 was considered statistically significant.
Results
Basal levels of FOXO proteins in human prostate cancer cells
FOXO1A, FOXO3A and FOXO4 protein levels were measured in the total lysates obtained from normal human prostate epithelial (NHPE) cells and compared with various human prostate cancer cell lines, specifically 22Rv1, LNCaP, DU145 and PC-3, by Western blot analysis. As shown in figure 1A, the total protein levels of FOXO1A and FOXO3A were considerably higher in prostate cancer cells compared to NHPE cells. FOXO1A and FOXO3A were detected both as a lower band (endogenous form) and an upper, perhaps phoshorylated form. The endogenous levels of FOXO3A protein (corresponding to the lower band in the FOXO3A blot) were predominantly expressed in all four prostate cancer cell lines. The endogenous levels of FOXO1A were expressed only in the androgen-refractory prostate cancer DU145 and PC-3 cell lines. The high levels of FOXO1A and FOXO3A correlated with high levels of p-Akt (Ser473) expression in LNCaP and PC-3 cells, suggesting that the levels of these proteins might be under the transcriptional control of the PI3K-Akt signaling pathway. No significant alterations in FOXO4 protein levels was observed in androgen-refractory prostate cancer DU145 and PC-3, whereas the levels of this protein were higher in androgen-responsive 22Rv1 and LNCaP cells compared to NHPE cells. Higher levels of the chaperone protein 14-3-3 were also observed in cancer cells compared to NHPE cells. These findings suggest that deregulation of FOXO transcription factors and their high levels in the total cell lysates might be due to high endogenous production and/or nuclear exclusion.
Figure 1.
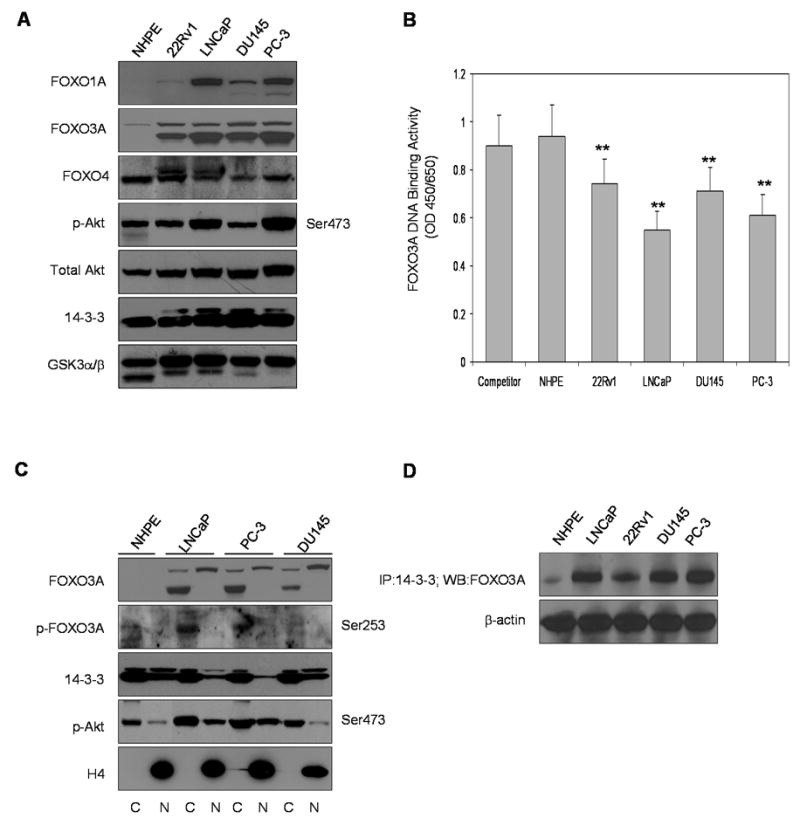
Expression, sub-cellular distribution and DNA binding of Forkhead transcription factor ‘O’ in normal human prostate epithelial NHPE cells and in various human prostate cancer cell lines. (A) FOXO1A, FOXO3A and FOXO4, total Akt, p-Akt (Ser473) and 14-3-3 expression in NHPE cells and in human prostate cancer 22Rv1, LNCaP, DU145 and PC-3 cells as analyzed by Western blotting. Assay with glycogen synthase kinase (GSK)-3α/β was performed to check the viability of these cells. (B) Nuclear FOXO3A DNA binding in NHPE and in various human prostate cancer cell lines. The results of DNA binding are mean ± SD of 3-4 determinations **P<0.001, compared to competitor (C) Sub-cellular distribution of FOXO3A, p-FOXO3A (Ser253), 14-3-3 and p-Akt in NHPE, LNCaP, PC-3 and DU145 cells. Anti-H4 antibody was used as loading control. (D) Binding of FOXO3A with 14-3-3. The details are described in “Materials and Methods” section. To ensure equal protein loading, the membrane was stripped and reprobed with anti-β-actin antibody.
Next we measured FOXO3A binding in various prostate cancer cell lines and in NHPE cells. FOXO3A DNA binding was significantly reduced in all prostate cancer cell lines with the highest reduction in LNCaP cells (∼40%) followed by PC-3 cells (∼33%) compared to 21-23% in 22Rv1 and DU145 cells, indicating that FOXO3A may be inactivated by Akt-mediated phosphorylation, resulting in its nuclear exclusion (Figure 1B).
Since it has been demonstrated that Akt/PKB and related kinase phosphorylate FOXO proteins at Ser253 in FOXO3A (24), we measured the levels of FOXO3A protein in the nucleus and cytosol along with its phosphorylated form. Compared to NHPE, cytosolic fractions of endogenous FOXO3A, which is mostly phosphorylated at Ser253, were significantly higher in LNCaP and PC-3 cells. Similarly the cytosolic fractions of 14-3-3 levels were also higher than the nuclear fractions in these cell lines. An interesting observation was the presence of phosphorylated Akt Ser473 in the nuclear fraction of prostate cancer cells (Figure 1C). It is probable that the nuclear presence of phosphorylated Ser473 Akt induces FOXO3A phosphorylation inside the nucleus and its exclusion after binding with 14-3-3 chaperone protein. To further assess whether there is increased binding of FOXO3A with the chaperone protein 14-3-3 after its phosphorylation, we immunoprecipitated 100μg of the cytosolic fractions of NHPE and the 4 cancer cell lines with anti-14-3-3 antibody and probed for FOXO3A protein expression. We found that prostate cancer LNCaP and PC-3 cells, which over-express Akt Ser473, have higher FOXO3A binding with the 14-3-3 chaperone protein (Figure1D).
Deregulation of FOXO3A by hyperphosphorylated Akt in human prostate cancer cells
To determine whether the hyperphosphorylated form of Akt causes deregulation of FOXO3A, we undertook pharmacological and genetic approaches using PC-3 cells. Treatment of cells with 20μM LY294002 for 24 h and expression of DN-Akt reduced Akt phosphorylation and its nuclear presence. Inhibition of Akt Ser473 resulted in upregulation of FOXO3A in the nucleus along with reductions in the endogenous levels of FOXO3A, which predominantly resides in the cytosol. The nuclear level of MnSOD was also increased after DN-Akt transfection of PC-3 cells (Figure 2A).
Figure 2.
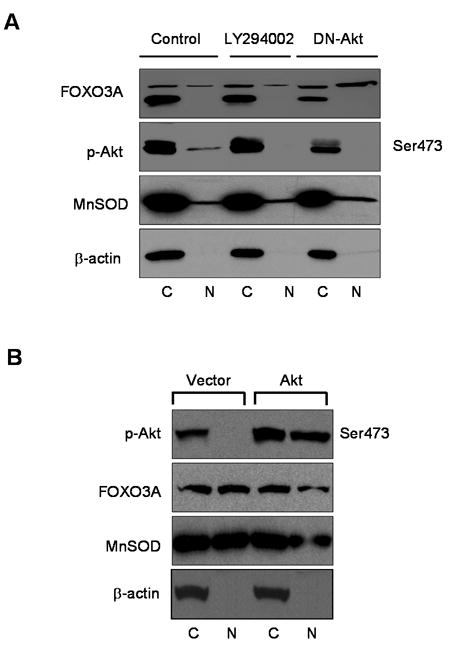
AKT-dependent deregulation of FOXO3A in human prostate cancer PC-3 and DU145 cells. (A) PC-3 cells were treated with 20μM LY294002 or transfected with DN-Akt pUSEamp and control pUSEamp for 24 h. Cytosolic and nuclear fractions were isolated and subjected to Western blotting. For protein loading the blots were stripped and reprobed with β-actin antibody. (B) DU145 cells transfected with either pLNCX vector containing Akt expression plasmid or empty vector. Cytosolic and nuclear fractions were isolated and subjected to Western blotting. The details are described in “Materials and Methods” section.
Using another approach, we induced transient over-expression of Akt, using Akt expression plasmid, in DU145 cells which normally exhibit low levels of phosphorylated Akt Ser473. Over-expression of Akt resulted in significant downmodulation of FOXO3A in DU145-Akt cells, which correlated with decreased MnSOD, essentially confirming that FOXO3A is under the transcriptional regulation of Akt (Figure 2B).
Expression of FOXO3A protein in benign and tumor specimens
Next we measured the levels of FOXO3A and its phosphorylated form in the clinical specimens removed surgically. We performed Western blot analyses for FOXO3A and p-FOXO3A (Ser253), activated form of Akt (Ser473) and chaperone protein 14-3-3 in prostate cancer specimens. As shown in figure 3A, levels of FOXO3A were significantly higher in the cytosolic fraction than in the nucleus. In particular, the endogenous levels of FOXO3A, corresponding to the lower band, was high in the cytosol and correlated with its phosphorylated form. We observed very low levels of FOXO3A in the nucleus of high-grade tumor as compared to their levels in low-grade tumor specimens. Furthermore, 14-3-3 chaperone protein levels were higher in the cytosolic fraction, whereas the majority of prostate tumor specimens had high levels of the phosphorylated form of Akt in the nuclear fractions. The relative density scan obtained for FOXO3A demonstrated higher levels of FOXO3A in the cytosol fractions than in the nuclear fractions, with complete loss of FOXO3A in the nuclei of high-grade tumors (Figure 3B).
Figure 3.
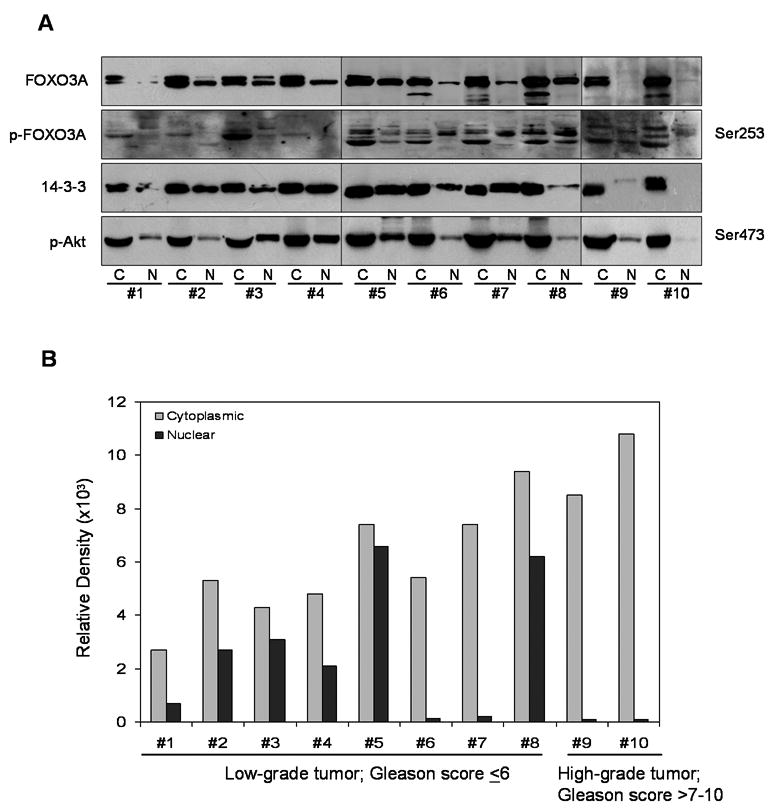
Protein expression of (A) FOXO3A and its phosphorylation, 14-3-3 and p-Akt (Ser473) in benign prostate tissue and prostate cancer specimens. Cytosolic and nuclear fractions were isolated from these specimens and subjected to Western blotting. Cancer tissue is sub-divided into low-grade cancer (Gleason score <7; #1-8) and high-grade cancer (Gleason score 7-10; #9, 10). (B) Relative density of FOXO3A in these specimens. The details are described in “Materials and Methods” section.
Next we analyzed FOXO3A expression by immunohistochemical staining of the paraffin-embedded tissue sections. FOXO3A expression was observed in either the nucleus or the cytoplasm or both, but was predominantly observed in the cytoplasm of cancer cells (Figure 4A). Compared to benign tissue, a progressive increase in FOXO3A expression was observed in the cytoplasm with increasing tumor grade. Overall, FOXO3A expression was higher in carcinoma cells than in benign prostatic epithelium, a finding that we had previously observed in cell culture studies.
Figure 4.
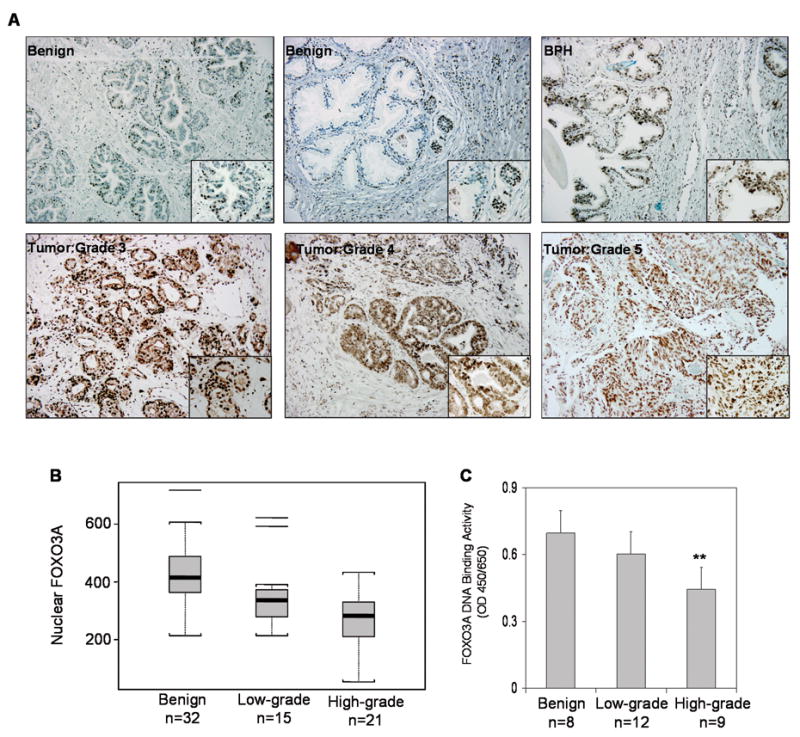
Expression of FOXO3A and its DNA binding in human prostate cancer specimens. (A) Paraffin embedded (4.0 micron) sections from benign, BPH and prostate cancer of various Gleason grades were used for FOXO3A expression by immunohistochemistry. A strong nuclear and cytoplasmic staining was observed in Gleason grade 3 whereas diffuse and weak FOXO3A staining was observed in high-grade cancer (Gleason grade 4 and 5, respectively). Magnified at ×20 and ×40 (B) Statistical Box plot analysis of FOXO3A nuclear presence was performed by counting FOXO3A positive nuclear stained cells from various locations from benign, low-grade and high-grade specimens. I Min-Max, □ 25%-75% and ⚊ Median value (C) FOXO3A nuclear DNA binding in benign, low-grade and high-grade tumors. The results of DNA binding are mean ± SD of various tissue in duplicate **P<0.001, compared to benign tissue Details are described in ‘Materials and Methods’.
Next we evaluated the nuclear levels of FOXO3A in the various histologic components of the tissue samples by counting the number of nuclei showing positive FOXO3A expression at 40X magnification, and analyzed the counts statistically by mean and standard deviation as well as box plot (Figure 4B). Differences in the numbers of cells showing nuclear FOXO3A expression in benign prostatic epithelium, low grade cancers, and high grade cancers were examined by T-test. The test was two sided and p-value <0.05 was considered significant. Benign specimens (n=32) exhibited a mean of 425.03 stained nuclei (range 213-718); low-grade cancers (n=15) showed a mean of 350.0 stained nuclei (range 213-622); and high-grade cancers (n=21) showed a mean of 273.38 stained nuclei (range 53-433). The nuclear FOXO3A expression in benign tissues was significantly higher than in low-grade cancers and in high-grade cancers, with p=0.039 and p<0.0001, respectively.
Next we performed FOXO3A DNA binding assay on benign and tumor specimens. As shown in figure 4C, a progressive decrease in DNA binding was observed in the tumor specimens compared to benign tissue. The decrease was much more pronounced in high-grade tumor compared to low-grade and benign tissue.
Transcriptional downregulation of FOXO3A in benign and tumor specimens
Next we determined whether FOXO3A is deregulated at the protein level or is being modulated at the transcriptional levels as well. We preformed RT-PCR for mRNA expression for FOXO1A, FOXO3A and FOXO4. As shown in figure 5, the mRNA transcript for FOXO3A was significantly down-regulated in high-grade cancers compared to low-grade cancers and benign tissue. No significant difference in mRNA levels of FOXO3A was observed between low-grade cancers and benign tissue. Similar trends in mRNA expression of FOXO1A as compared to FOXO3A expression were noted. However, no difference in the FOXO4 mRNA levels was observed between benign and malignant tissues. The relative density analysis demonstrated a marked decrease in mRNA transcript levels of FOXO3A and FOXO1A in high-grade cancers, compared to low-grade cancers and benign tissue (Figure 5B).
Figure 5.
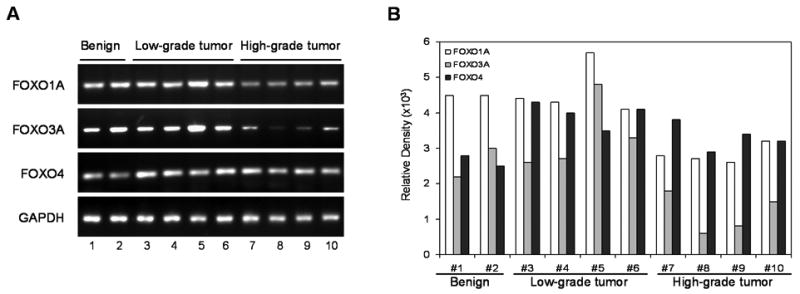
mRNA expression of (A) FOXO1A, FOXO3A and FOXO4 in benign, low-grade and high-grade prostate specimens subjected to the RT-PCR analysis and the product obtained was visualized in gels. (B) Relative density of bands showing mRNA expression in benign and cancer specimens. The details are described in “Materials and Methods” section.
Discussion
Our study is the first to investigate FOXO3A expression in clinical prostate cancer specimens. The results of our study suggest that FOXO3A is over-expressed during the early stages of prostate cancer and sub-cellular re-distribution occurs as evidenced by the presence of high phosphorylated levels in the cytosol and reduced DNA binding in the nucleus. A loss of FOXO3A at the transcriptional level was observed in high grade prostate cancers, which are more likely to be associated with higher clinical stages.
The Forkhead box subgroup ‘O’ of forkhead transcription factors plays a central role in cell-cycle control, differentiation, metabolism control, stress response, and apoptosis (1-7). The function of these important molecules is tightly controlled by a wide range of protein–protein interactions and posttranslational modifications including phosphorylation, acetylation and ubiquitination [9]. Regulation of the sub-cellular localization and transcriptional activity of FOXO protein is achieved primarily by posttranslational modifications, such as phosphorylation and acetylation (4, 9). Our studies demonstrate posttranslational modification in FOXO3A associated with higher levels of phosphorylation at Ser253 in clinical prostate cancer specimens and human prostate cancer cells. Deregulation of FOXO3A may be due to defects in signal transduction.
We have previously demonstrated constitutive Akt activation during prostate cancer progression which could be the result of signaling defects related to the activities of autocrine growth factors, possibly through deletion or mutation in phosphatase and tensin homologue (PTEN) on chromosome 10 (25, 26). In prostate cancer, LNCaP and PC-3 cells exhibit high levels of p-Akt due to loss of PTEN activity; this may lead to the deregulation of FOXO factors. Previous studies have demonstrated FOXO1A as a direct substrate of Akt and an association between these two proteins has been observed in some human cancers including glioblastoma, soft tissue sarcomas and breast cancer, but no such association has been observed in prostate cancer (27, 28). The present study demonstrates that in prostate cancer, FOXO3A is constitutively phosphorylated and is inactivated as a result of Akt hyperactivation. Furthermore, inhibition of Akt/PKB by dominant negative approach in prostate cancer cells caused translocation of FOXO3A into the nucleus and its binding to DNA induced the expression of MnSOD, a FOXO-responsive gene. In contrast, Akt over-expression in DU145 cells, which normally exhibit low levels of Akt, caused a reduction in FOXO3A protein expression. Our studies demonstrate that Akt plays an important role in the regulation of FOXO3A activity, and can phosphorylate and deactivate FOXO3A in prostate cancer. Akt is not the only kinase that regulates FOXO factors: several other kinases, including IKK, MAPK, and serum- and glucocorticoid- inducible kinase (SKG) can also phoshorylate FOXO factors (29-31). Whether these kinases regulate FOXO factors in prostate cancer is not currently known, but may be worthy of investigation.
Upon activation, Akt/PKB phosphorylates a number of key regulatory proteins, leading to 14-3-3 binding and ultimately altering cellular function (32, 33). For example, phosphorylation of Bad by Akt/PKB at Ser136 and Ser155 induces 14-3-3 binding, neutralizing the pro-apoptotic function of Bad (34). In our studies, we observed increased FOXO3A binding with 14-3-3, which may be a consequence of phosphorylation of FOXO3A by Akt/PKB at Ser253. Following 14-3-3 protein binding, the FOXO-14-3-3 complex is transported out of the nucleus and retained within the cytoplasm. It has also been suggested that the 14-3-3 protein may prevent nuclear re-import of FOXO proteins by masking their nuclear localization signal. Decreased FOXO3A nuclear DNA binding observed in prostate cancer cells, compared to levels noted in benign prostatic epithelium, suggests that low transcriptional activity of FOXO3A is associated with biologically more aggressive prostate cancer. Clearly further detailed studies of FOXO3A phosphorylation by Akt/PKB and its regulation by 14-3-3 warrants further investigation.
Studies have shown that FOXO3A function is compromised during the transition of prostate cancer cells from androgen dependence to androgen independence (22). Studies have demonstrated androgen receptor dependent repression of FOXO factors, associated with hyperphosphorylation of FOXO3A protein and marked reduction in their levels, as well as significant down-regulation in the activity of the FOXO-responsive p27/kip1 promoter during prostate cancer progression (22). It has also been shown that knockdown of the astrocyte-elevated gene-1 inhibits prostate cancer progression through upregulation of FOXO3A activity (35). These studies outline mechanisms whereby FOXO factors regulate cellular function by controlling various downstream targets. FOXO3A transcription factor deregulation during prostate carcinogenesis may influence additional transcriptional genes that regulate prostate function.
The results obtained from this study underscore the importance of FOXO3A activity in prostate cancer cell biology and indicate that further investigations of the signaling mechanisms involved in FOXO3A regulation in order to understand the molecular basis for its tumor suppressive role are warranted. Understanding these mechanisms may contribute to the development of preventive and therapeutic strategies in the management of prostate cancer.
Acknowledgments
The authors are thankful to Dr Ata Abbas for his technical assistance during the work.
Financial Support: This work was supported by grants from United States Public Health Service CA108512, Sullivan Foundation for the Study of Prostatitis and The James & Eilleen Dicke Research Endowment funds.
Abbreviations
- FOX
forkhead box
- PKB
protein kinase B
- MnSOD
manganese superoxide dismutase
- PTEN
phosphatase and tensin homologue on chromosome 10
- NHPE
normal human prostate epithelial cells
- SKG
serum- and glucocorticoid- inducible kinase
- TRAIL
tumor necrosis factor-related apoptosis inducing ligand
References
- 1.Calnan DR, Brunet A. The FoxO code. Oncogene. 2008;27:2276–2288. doi: 10.1038/onc.2008.21. [DOI] [PubMed] [Google Scholar]
- 2.Partridge L, Brüning JC. Forkhead transcription factors and ageing. Oncogene. 2008;27:2351–2363. doi: 10.1038/onc.2008.28. [DOI] [PubMed] [Google Scholar]
- 3.Birkenkamp KU, Coffer PJ. Regulation of cell survival and proliferation by the FOXO (Forkhead box, class O) subfamily of Forkhead transcription factors. Biochem Soc Trans. 2003;31:292–297. doi: 10.1042/bst0310292. [DOI] [PubMed] [Google Scholar]
- 4.Myatt SS, Lam EW. The emerging roles of forkhead box (Fox) proteins in cancer. Nat Rev Cancer. 2007;7:847–859. doi: 10.1038/nrc2223. [DOI] [PubMed] [Google Scholar]
- 5.Paik JH, Kollipara R, Chu G, Ji H, Xiao Y, Ding Z, Miao L, Tothova Z, Horner JW, Carrasco DR, Jiang S, Gilliland DG, Chin L, Wong WH, Castrillon DH, DePinho RA. FoxOs are lineage-restricted redundant tumor suppressors and regulate endothelial cell homeostasis. Cell. 2007;128:309–323. doi: 10.1016/j.cell.2006.12.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fu Z, Tindall DJ. FOXOs, cancer and regulation of apoptosis. Oncogene. 2008;27:2312–2319. doi: 10.1038/onc.2008.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gross DN, van den Heuvel AP, Birnbaum MJ. The role of FoxO in the regulation of metabolism. Oncogene. 2008;27:2320–2336. doi: 10.1038/onc.2008.25. [DOI] [PubMed] [Google Scholar]
- 8.Gu TL, Tothova Z, Scheijen B, Griffin JD, Gilliland DG, Sternberg DW. NPM-ALK fusion kinase of anaplastic large-cell lymphoma regulates survival and proliferative signaling through modulation of FOXO3a. Blood. 2004;103:4622–4629. doi: 10.1182/blood-2003-03-0820. [DOI] [PubMed] [Google Scholar]
- 9.Vogt PK, Jiang H, Aoki M. Triple layer control: phosphorylation, acetylation and ubiquitination of FOXO proteins. Cell Cycle. 2005;4:908–913. doi: 10.4161/cc.4.7.1796. [DOI] [PubMed] [Google Scholar]
- 10.Murphy CT. The search for DAF-16/FOXO transcriptional targets: approaches and discoveries. Exp Gerontol. 2006;41:910–921. doi: 10.1016/j.exger.2006.06.040. [DOI] [PubMed] [Google Scholar]
- 11.Bakker WJ, Blázquez-Domingo M, Kolbus A, Besooyen J, Steinlein P, Beug H, Coffer PJ, Löwenberg B, von Lindern M, van Dijk TB. FoxO3a regulates erythroid differentiation and induces BTG1, an activator of protein arginine methyl transferase 1. J Cell Biol. 2004;164:175–184. doi: 10.1083/jcb.200307056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Birkenkamp KU, Essafi A, van der Vos KE, da Costa M, Hui RC, Holstege F, Koenderman L, Lam EW, Coffer PJ. FOXO3a induces differentiation of Bcr-Abl-transformed cells through transcriptional down-regulation of Id1. J Biol Chem. 2007;282:2211–2220. doi: 10.1074/jbc.M606669200. [DOI] [PubMed] [Google Scholar]
- 13.Zou Y, Tsai WB, Cheng CJ, Hsu C, Chung YM, Li PC, Lin SH, Hu MC. Forkhead box transcription factor FOXO3a suppresses estrogen-dependent breast cancer cell proliferation and tumorigenesis. Breast Cancer Res. 2008;10:21–23. doi: 10.1186/bcr1872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kim JH, Kim MK, Lee HE, Cho SJ, Cho YJ, Lee BL, Lee HS, Nam SY, Lee JS, Kim WH. Constitutive phosphorylation of the FOXO1A transcription factor as a prognostic variable in gastric cancer. Mod Pathol. 2007;20:835–842. doi: 10.1038/modpathol.3800789. [DOI] [PubMed] [Google Scholar]
- 15.Karger S, Weidinger C, Krause K, Sheu SY, Aigner T, Gimm O, Schmid K, Dralle H, Fuehrer D. FOXO3a: a novel player in thyroid carcinogenesis? Endocr Relat Cancer. 2008 Oct 9; doi: 10.1677/ERC-07-0283. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 16.van der Horst A, de Vries-Smits AM, Brenkman AB, van Triest MH, van den Broek N, Colland F, Maurice MM, Burgering BM. FOXO4 transcriptional activity is regulated by monoubiquitination and USP7/HAUSP. Nat Cell Biol. 2006;8:1064–1073. doi: 10.1038/ncb1469. [DOI] [PubMed] [Google Scholar]
- 17.Li R, Erdamar S, Dai H, Wheeler TM, Frolov A, Scardino PT, Thompson TC, Ayala GE. Forkhead protein FKHR and its phosphorylated form p-FKHR in human prostate cancer. Hum Pathol. 2007;38:1501–1507. doi: 10.1016/j.humpath.2007.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Liu AY, Roudier MP, True LD. Heterogeneity in primary and metastatic prostate cancer as defined by cell surface CD profile. Am J Pathol. 2004;165:1543–1556. doi: 10.1016/S0002-9440(10)63412-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.McMenamin ME, Soung P, Perera S, Kaplan I, Loda M, Sellers WR. Loss of PTEN expression in paraffin-embedded primary prostate cancer correlates with high Gleason score and advanced stage. Cancer Res. 1999;59:4291–4296. [PubMed] [Google Scholar]
- 20.Trotman LC, Alimonti A, Scaglioni PP, Koutcher JA, Cordon-Cardo C, Pandolfi PP. Identification of a tumour suppressor network opposing nuclear Akt function. Nature. 2006;441:523–527. doi: 10.1038/nature04809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dong XY, Chen C, Sun X, Guo P, Vessella RL, Wang RX, Chung LW, Zhou W, Dong JT. FOXO1A is a candidate for the 13q14 tumor suppressor gene inhibiting androgen receptor signaling in prostate cancer. Cancer Res. 2006;66:6998–7006. doi: 10.1158/0008-5472.CAN-06-0411. [DOI] [PubMed] [Google Scholar]
- 22.Lynch RL, Konicek BW, McNulty AM, Hanna KR, Lewis JE, Neubauer BL, Graff JR. The progression of LNCaP human prostate cancer cells to androgen independence involves decreased FOXO3a expression and reduced p27KIP1 promoter transactivation. Mol Cancer Res. 2005;3:163–169. doi: 10.1158/1541-7786.MCR-04-0163. [DOI] [PubMed] [Google Scholar]
- 23.Yang L, Xie S, Jamaluddin MS, Altuwaijri S, Ni J, Kim E, Chen YT, Hu YC, Wang L, Chuang KH, Wu CT, Chang C. Induction of androgen receptor expression by phosphatidylinositol 3-kinase/Akt downstream substrate, FOXO3a, and their roles in apoptosis of LNCaP prostate cancer cells. J Biol Chem. 2005;280:33558–33565. doi: 10.1074/jbc.M504461200. [DOI] [PubMed] [Google Scholar]
- 24.Van Der Heide LP, Hoekman MF, Smidt MP. The ins and outs of FoxO shuttling: mechanisms of FoxO translocation and transcriptional regulation. Biochem J. 2004;380:297–309. doi: 10.1042/BJ20040167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shukla S, Maclennan GT, Marengo SR, Resnick MI, Gupta S. Constitutive activation of P I3 K-Akt and NF-kappaB during prostate cancer progression in autochthonous transgenic mouse model. Prostate. 2005;64:224–239. doi: 10.1002/pros.20217. [DOI] [PubMed] [Google Scholar]
- 26.Shukla S, Maclennan GT, Hartman DJ, Fu P, Resnick MI, Gupta S. Activation of PI3K-Akt signaling pathway promotes prostate cancer cell invasion. Int J Cancer. 2007;121:1424–1432. doi: 10.1002/ijc.22862. [DOI] [PubMed] [Google Scholar]
- 27.Wayne CM, Fan HY, Cheng X, Richards JS. Follicle-stimulating hormone induces multiple signaling cascades: evidence that activation of Rous sarcoma oncogene, RAS, and the epidermal growth factor receptor are critical for granulosa cell differentiation. Mol Endocrinol. 2007;21:1940–1957. doi: 10.1210/me.2007-0020. [DOI] [PubMed] [Google Scholar]
- 28.Reagan-Shaw S, Ahmad N. RNA interference-mediated depletion of phosphoinositide 3-kinase activates forkhead box class O transcription factors and induces cell cycle arrest and apoptosis in breast carcinoma cells. Cancer Res. 2006;66:1062–1069. doi: 10.1158/0008-5472.CAN-05-1018. [DOI] [PubMed] [Google Scholar]
- 29.Hu MC, Lee DF, Xia W, Golfman LS, Ou-Yang F, Yang JY, Zou Y, Bao S, Hanada N, Saso H, Kobayashi R, Hung MC. IkappaB kinase promotes tumorigenesis through inhibition of forkhead FOXO3a. Cell. 2004;117:225–237. doi: 10.1016/s0092-8674(04)00302-2. [DOI] [PubMed] [Google Scholar]
- 30.Yang JY, Zong CS, Xia W, Yamaguchi H, Ding Q, Xie X, Lang JY, Lai CC, Chang CJ, Huang WC, Huang H, Kuo HP, Lee DF, Li LY, Lien HC, Cheng X, Chang KJ, Hsiao CD, Tsai FJ, Tsai CH, Sahin AA, Muller WJ, Mills GB, Yu D, Hortobagyi GN, Hung MC. ERK promotes tumorigenesis by inhibiting FOXO3a via MDM2-mediated degradation. Nat Cell Biol. 2008;10:138–148. doi: 10.1038/ncb1676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mounier C, Dumas V, Posner BI. Regulation of hepatic insulin-like growth factor-binding protein-1 gene expression by insulin: central role for mammalian target of rapamycin independent of forkhead box O proteins. Endocrinology. 2006;147:2383–2391. doi: 10.1210/en.2005-0902. [DOI] [PubMed] [Google Scholar]
- 32.Woods YL, Rena G. Effect of multiple phosphorylation events on the transcription factors FKHR, FKHRL1 and AFX. Biochem Soc Trans. 2002;30:391–397. doi: 10.1042/bst0300391. [DOI] [PubMed] [Google Scholar]
- 33.Luhn P, Wang H, Marcus AI, Fu H. Identification of FAKTS as a novel 14-3-3-associated nuclear protein. Proteins. 2007;67:479–489. doi: 10.1002/prot.21288. [DOI] [PubMed] [Google Scholar]
- 34.Zhou XM, Liu Y, Payne G, Lutz RJ, Chittenden T. Growth factors inactivate the cell death promoter BAD by phosphorylation of its BH3 domain on Ser155. J Biol Chem. 2000;275:25046–25051. doi: 10.1074/jbc.M002526200. [DOI] [PubMed] [Google Scholar]
- 35.Kikuno N, Shiina H, Urakami S, Kawamoto K, Hirata H, Tanaka Y, Place RF, Pookot D, Majid S, Igawa M, Dahiya R. Knockdown of astrocyte-elevated gene-1 inhibits prostate cancer progression through upregulation of FOXO3a activity. Oncogene. 2007;26:7647–7655. doi: 10.1038/sj.onc.1210572. [DOI] [PubMed] [Google Scholar]


