Abstract
Introduction
Neurodegeneration occurs after intracerebral hemorrhage (ICH) and tissue-type transglutaminase (tTG) has a role in neurodegenerative disorders. The present study investigated tTG expression after ICH and the effects of a tTG inhibitor, cystamine, on ICH-induced brain edema and neurological deficits.
Methods
This study had two parts. In the first, male Sprague-Dawley rats received an intracaudate injection of 100 µL autologous whole blood or a needle insertion (sham). Rats were killed 3 days later and the brains used for immunohistochemistry, Western blots and real-time quantitative polymerase chain reaction. In the second set, ICH rats were treated intraperitoneally with either a tTG inhibitor, cystamine, or vehicle. Rats underwent behavioral testing and were killed at day-3 for measurement of brain swelling.
Results
tTG positive cells were found in the ipsilateral basal ganglia after ICH and most of those cells were neuron-like. Western blot analysis showed a 3-fold increase in tTG in the ipsilateral basal ganglia (p<0.01 vs. sham) after ICH. tTG mRNA levels were also significantly higher (8.5-fold increase vs. sham). Cystamine treatment attenuated ICH-induced brain swelling (day 3: 14.4±3.2 vs. 21.4±4.0% in vehicle-treated rats, p<0.01), neuronal death and improved functional outcome (forelimb placing score: 47±23 vs. 17±16% in vehicle-treated rats, p<0.05).
Conclusions
ICH induces perihematomal tTG upregulation and cystamine, a tTG inhibitor, reduces ICH-induced brain swelling and neurological deficits.
Keywords: brain swelling, cerebral hemorrhage, cystamine, tissue-type transglutaminase
1. Introduction
Spontaneous intracerebral hemorrhage (ICH) is a common and often fatal stroke subtype. Community based studies have indicated a mortality of more than 40%, and many survivors are left with significant neurological deficits (Mendelow et al., 2005). The appropriate therapy for ICH remains a subject of debate, and an improved understanding of the detailed cellular and biochemical mechanisms leading to neuronal injury after ICH may facilitate the development of improved medical and surgical therapies. Brain edema aggravates brain injury and is associated with poor outcome in patients (Zazulia et al., 1999). There are several phases of edema formation after ICH including coagulation cascade, thrombin production, erythrocyte lysis, and hemoglobin toxicity (Xi et al., 2006). Further, a relationship between ICH and neuronal cell death has been noted (Kingman et al., 1988).
Transglutaminases are a family of cross-linking enzymes catalyzing the formation of γ-glutamyl-ɛ-lysine bonds. Tissue-type transglutaminase (tTG) is the most ubiquitously expressed member of this family. It is abundantly expressed in brain (Kim et al., 1999) and an increase in tTG mRNA levels has been observed during normal aging in humans (Lu et al., 2004). Expression of tTG is implicated in numerous processes including neurodegeneration (Citron et al., 2002; Lesort et al., 2000). An upregulation in tTG has been documented in chronic neuropathological conditions such as Huntington disease, Parkinson’s disease, and Alzheimer’s disease (Citron et al., 2002; Lesort et al., 2000). Because tTG upregulation during cell death processes is well documented in many different models (Piacentini et al., 2005), it has been hypothesized that tTG can facilitate neuronal cell death in neurodegenerative disease as well as in CNS injury. However, the role of tTG in ICH-induced brain injury has not yet been examined.
Cystamine is a competitive inhibitor of TG activity that has been shown to limit the aggregation of proteins with expanded polyglutamine tracts in vitro (Igarashi et al., 1998). Several studies have demonstrated that cystamine treatment is neuroprotective in Huntington disease (Karpuj et al., 2002; Van Raamsdonk et al., 2005; Wang et al., 2005). Furthermore, it has been described that cystamine can also inhibit caspase-3 activity (Lesort et al., 2003), increase intracellular levels of the antioxidants glutathione (Lesort et al., 2003), and increase expression of heat-shock proteins (Karpuj et al., 2002).
In this study, we examined brain protein and mRNA levels of tTG in a rat model of ICH. We also investigated the effects of the tTG inhibitor, cystamine, on brain edema and functional outcomes following ICH.
2. Results
Physiological Variables
All physiological variables were measured immediately before an ICH. Mean arterial blood pressure, blood pH, PaO2, PaCO2, and blood glucose level were controlled within normal ranges (data not shown).
Brain tTG Levels after ICH
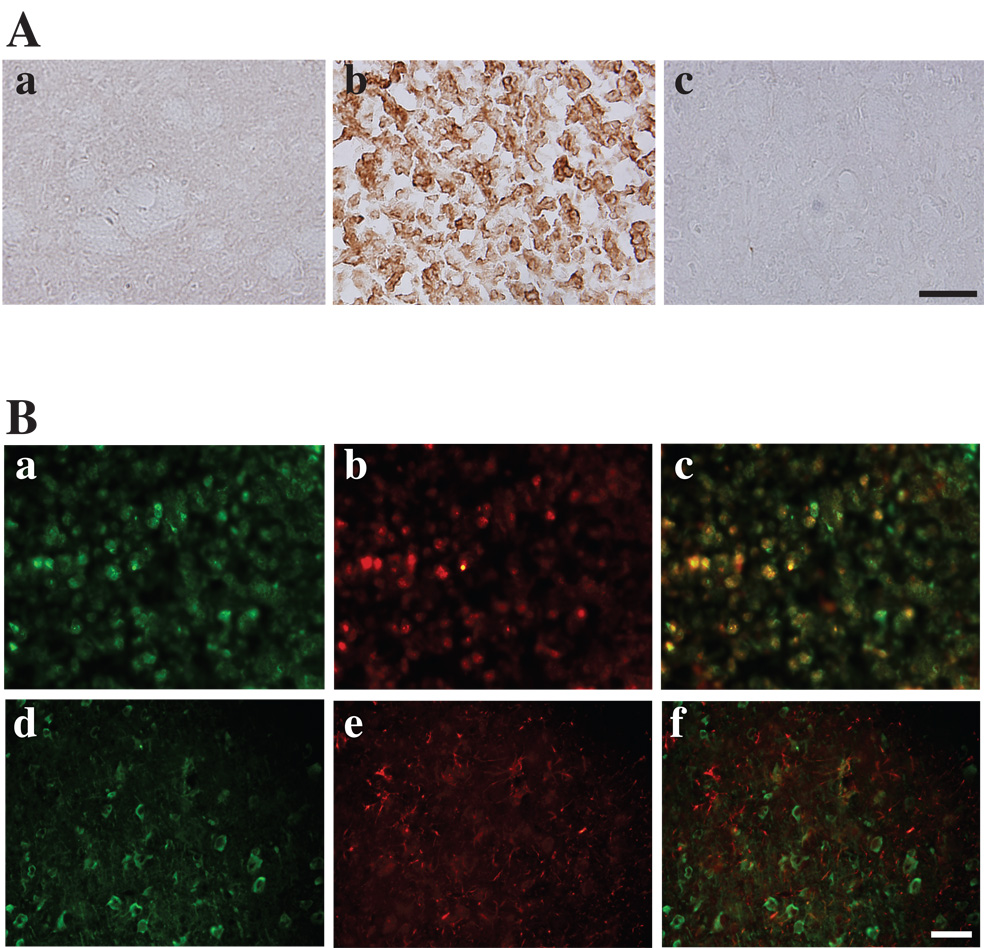
Immunohistochemistry demonstrated that tTG protein was over-expressed in the ipsilateral basal ganglia after ICH (Figure 1Ab) compared with the contralateral basal ganglia (Figure 1Ac) or the ipsilateral basal ganglia after needle insertion (Figure 1Aa). Immuno-fluorescent double labeling showed that some tTG-positive cells were also NSE positive. In contrast tTG-positive cells were not GFAP positive, so tTG appears to be neuronal (Figure 1B).
Figure 1.
(A): Immunoreactivity for tTG in the ipsilateral basal ganglia at 3 days after needle insertion (a), or 100µl blood injection (b), and in the contralateral basal ganglia after blood injection (c), scale bar=50µm. (B): Double immunofluorescent labeling of the perihematomal zone at 3 days after ICH. a) and d) show immunostaining for tTG , b) shows immunostaining for NSE, and e) for GFAP. tTG is colocalized with NSE positive cells (c), but not with GFAP positive cells (f). Scale bar = 50 µm.
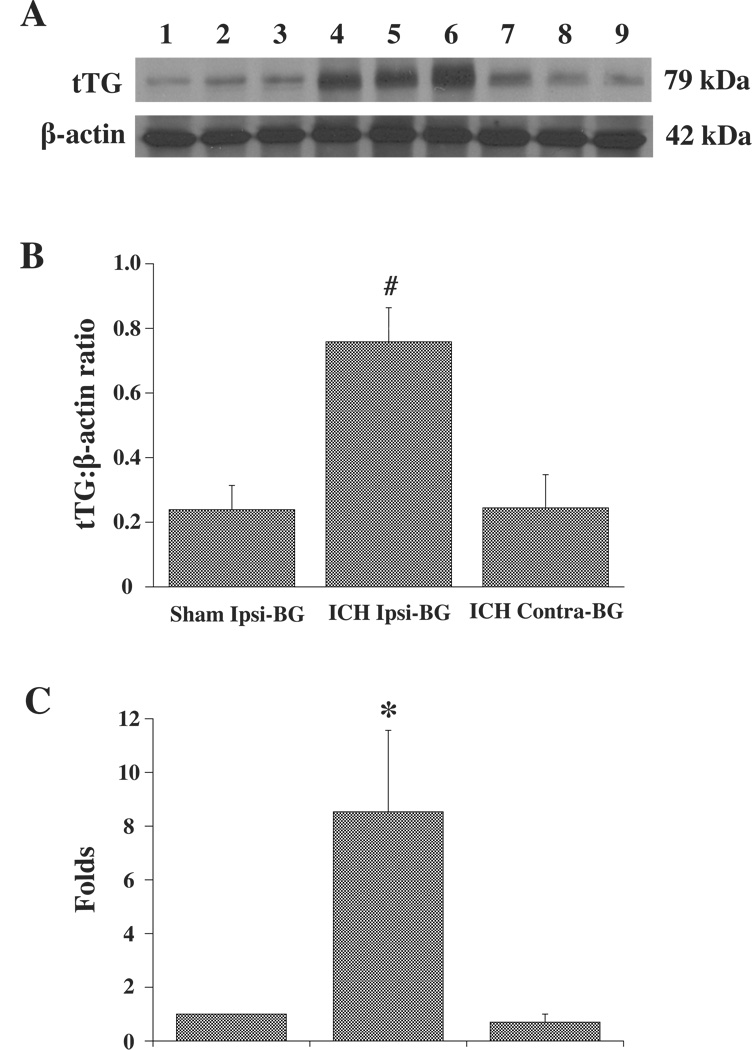
By Western blot analysis, tTG was identified as a ~79 kDa band and β-actin as a ~42 kDa band (Figure 2A). A densitometric analysis showed a marked (3-fold) increase in tTG/β-actin protein ratio in the ipsilateral basal ganglia after ICH (0.76±0.10) compared with the sham control (0.24±0.07, p<0.01), and the contralateral basal ganglia (0.24±0.10, p<0.01; Figure 2B).
Figure 2.
(A): Western blot analysis for tTG in ipsilateral basal ganglia at 3 days after needle insertion (Lane 1–3) or 100µl blood injection (Lane 4–6), and in the contralateral basal ganglia after blood injection (Lane7–9). β-actin is shown as a control for equal loading. (B): Bar graph showing the levels of tTG protein (Western blot; expressed as a ratio to β-actin) in ipsilateral (Ipsi) or contralateral (Contra) basal ganglia (BG) at 3 days after 100µl blood or a needle injected into right basal ganglia. Values are expressed as mean ± SD. # p<0.01 vs. Sham ipsi-BG and contra-BG. (C): tTG mRNA levels (real-time PCR analysis) in the ipsilateral or contralateral basal ganglia at 3 days after 100µl blood injection or in the ipsilateral basal ganglia after needle insertion. Values are expressed as the means ± SD. *p<0.05 vs. Sham ipsi-BG and contra-BG.
RNA was also prepared from the ipsilateral basal ganglia after needle insertion (sham) and the ipsilateral and contralateral basal ganglia after blood injection. The relative amount of tTG mRNA was expressed relative to the sham control. After ICH, tTG mRNA levels were significantly increased in the ipsilateral basal ganglia (8.5±3.0 fold vs. sham control, p<0.05) but not in the contralateral basal ganglia (0.7±0.3 fold change vs. sham; Figure 2C).
Effects of Cystamine Treatment on ICH-Induced Brain Swelling and Neurological Deficits
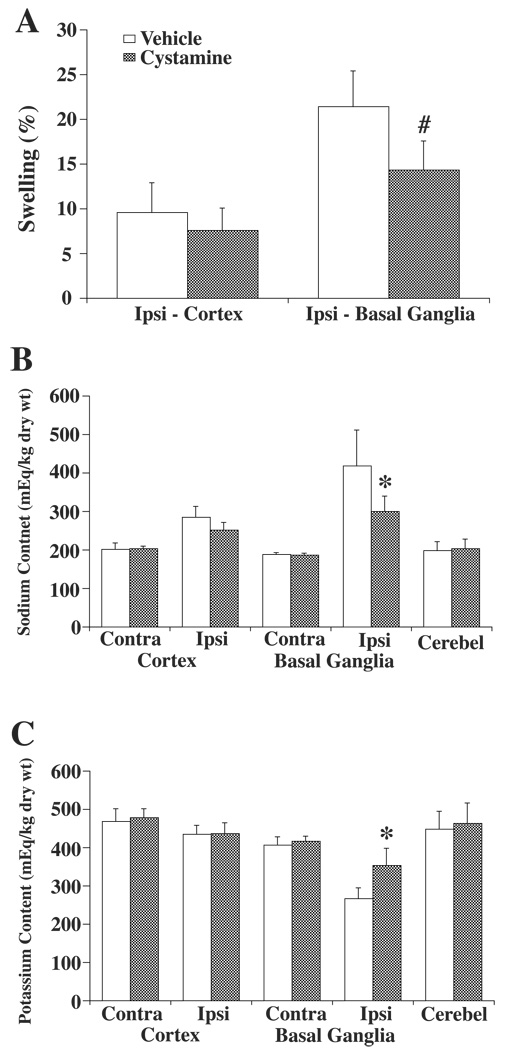
Cystamine treatment reduced brain swelling in the ipsilateral basal ganglia (14.4±3.2%) compared with the vehicle treated group (21.4±4.0%, p<0.01; Figure 3A). This reduced brain swelling was associated with a reduction in sodium accumulation in the ipsilateral basal ganglia (299±40 versus 418±94 mEq/kg dry wt, p<0.05; Figure 3B) and reduction in potassium loss (354±45 versus 267±28 mEq/kg dry wt, p<0.05; Figure 3C).
Figure 3.
Effect of cystamine or vehicle treatment on brain swelling (A), and tissue sodium (B) and potassium (C) contents at 3 days after 100µl blood injection. Brain swelling was measured in the ipsilateral (ipsi) cortex and basal ganglia. Ion contents were measured in the ipsi- and contralateral (contra) cortex and basal ganglia as well the cerebellum (cerebel) which served as a control. Values are given as means ± SD, N=6. # and * indicate differences from the vehicle group at the p<0.01 and p<0.05 levels, respectively.
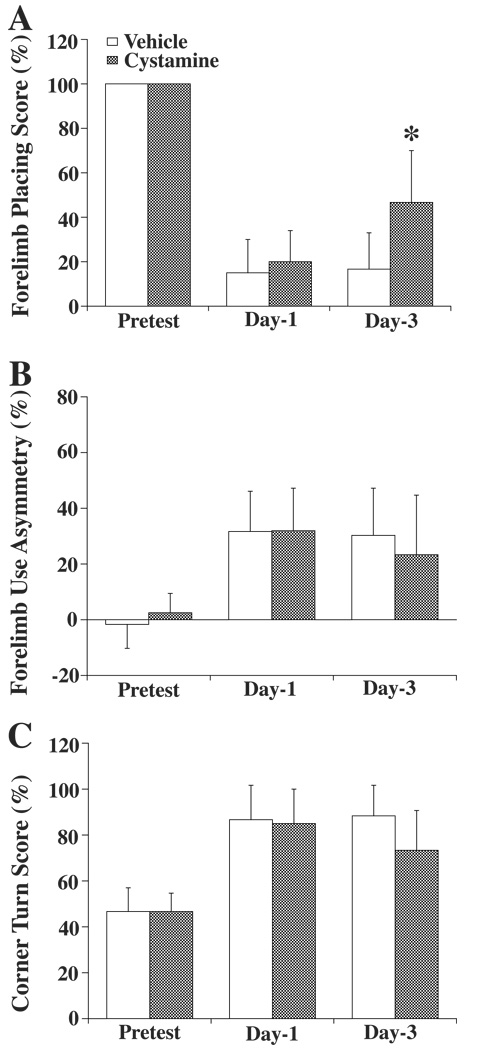
Behavioral tests including using forelimb placing test, forelimb use asymmetry test and corner turn test were performed before, and 1 and 3 days after ICH. Forelimb placing deficits were significantly improved at day-3 in the cystamine treated group (47±23%) compared with that in vehicle treated group (17±16%, p<0.05) (Figure 4A). In the other two tests, there were tendencies for improvement in the cystamine treated rats at day 3, but these did not reach significance (Figure 4B and C).
Figure 4.
Behavioral assessment including using forelimb placing (A), forelimb use asymmetry (B), and corner turn (C) tests before ICH and 1 day or 3 days after ICH. Values are expressed as the means ± SD. *p<0.05 vs. Vehicle group. For the forelimb placing test, a score of 100% indicates no injury and 0% indicates a maximum deficit. For the forelimb use asymmetry test, 0% indicates no injury. For the corner turn test, 50% indicates no injury while a 100% represents a maximum deficit.
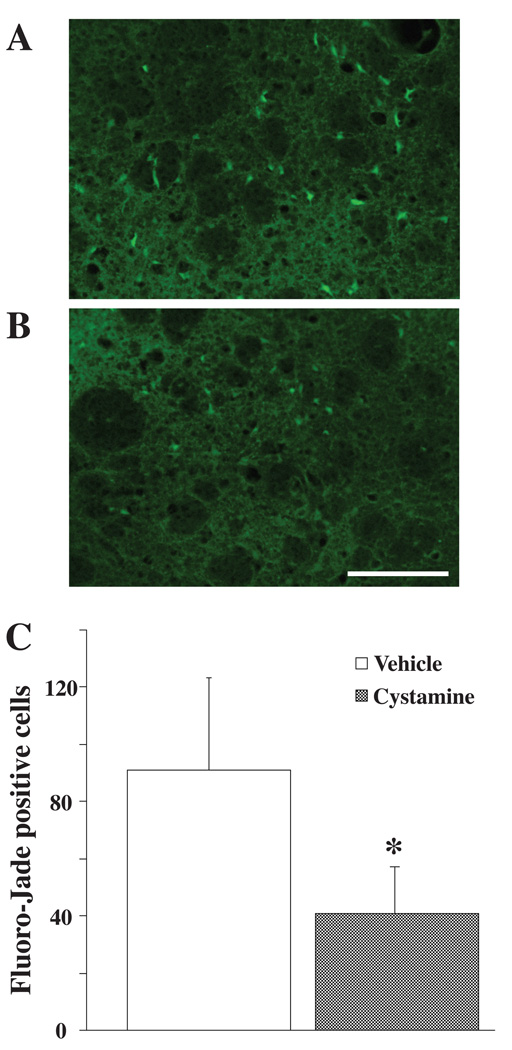
Fluoro-Jade C staining was performed to assess whether cystamine can reduce cell death around the hematoma. The total number of Fluoro-Jade positive cells was significantly decreased in the cystamine treated group (41±17 vs. 91±32 in saline-treated group, p<0.05; Figure 5).
Figure 5.
Fluoro-Jade staining in the ipsilateral basal ganglia at 3 days after ICH in (A) vehicle- treated and (B) cystamine treated rats. Scale bar = 100 µm. C: Quantification of the effect of cystamine and vehicle treatment on Fluoro-Jade staining (number of positive cells). Values are expressed as the means ± SD; n = 4 rats per group. *p<0.05 vs. vehicle group.
3. Discussion
This study demonstrates that brain tTG mRNA and protein levels are increased in the perihematomal area after ICH. Previous studies have shown tTG upregulation in animal models of cerebral ischemia (Ientile et al., 2004; Tolentino et al., 2004), traumatic brain injury (Tolentino et al., 2002), calcium-induced hippocampal damage (Tucholski et al., 2006), and spinal cord injury (Festoff et al., 2002). Evidence is mounting that tTG may have a role in acute brain injury (as explored here for ICH) as well as long-term neurodegeneration (such as in Huntington disease). In rats and humans, tTG is expressed in the central nervous systems and is localized mostly in the cytoplasmic compartment of neurons (Kim et al., 1999; Maggio et al., 2001). Our current study also showed most tTG positive cells after ICH are neuronal.
Several studies have demonstrated that tTG is present in cells and tissues during apoptotic cell degeneration and it is thus considered as a potential marker of apoptosis (Fesus, 1998). In some, but not all, cells tTG activation leads to formation of crossed-linked protein envelopes and subsequent cell death and tTG is, therefore, considered a crucial player in apoptosis (Fesus, 1998). Recently, apoptosis has been detected in the perihematomal zone in experimental ICH models (Gong et al., 2001; Matsushita et al., 2000; Qureshi et al., 2001) and in human ICH (Qureshi et al., 2003). Caspase-3, a cysteine protease, is involved in apoptotic neuronal death (Namura et al., 1998) and a caspase-3 inhibitor, zVADfmk, attenuates the appearance of perihematomal apoptotic cells in ICH (Matsushita et al., 2000). The upregulation of tTG after ICH may play an important role in ICH-induced apoptotic cell death.
The role of tTG induction in the neuronal response to injury is, however, still not well understood. Oxidative stress is a contributing factor to increase of tTG activity, and tTG overexpression sensitizes neuronal cell lines to apoptosis by increasing mitochondrial membrane potential and increasing reactive oxygen species production (Piacentini et al., 2002). Furthermore, increased tTG expression is found both in diseased tissues with inflammation and in cells with inflammatory stress (Kim, 2006). In multiple sclerosis, a CNS disease with a strong inflammatory component, activation of tTG in activated microglia contributes to the development of inflammation (Lee et al., 2004). The effects of tTG on neuroprotection need to be studied further, for example using tTG knockdown technology in neuronal cultures.
Cystamine was used as a competitive inhibitor of TG. Daily intraperitoneal administration of cystamine attenuated brain edema 3 days later. Additionally, cystamine reduced ICH-induced forelimb placing deficits and neuronal death (Fluoro-Jade staining). Brain edema after ICH is known to exacerbate brain injury, and peaks around the third day after ICH in experimental models (Xi et al., 1998; Xi et al., 2001; Yang et al., 1994). The time course of brain edema is correlated closely with the time course of neurological deficits in rats with ICH (Hua et al., 2002). The mechanisms of ICH-induced edema formation have been investigated and several factors are now known to play key roles. Erythrocyte lysis and the release of hemoglobin and hemoglobin breakdown products are major factors in inducing edema and oxidative damage after ICH (Huang et al., 2002; Wu et al., 2002; Xi et al., 1998).
Cystamine is neuroprotective in neurodegenerative diseases such as Huntington disease (Karpuj et al., 2002; Van Raamsdonk et al., 2005; Wang et al., 2005). Cystamine has also been shown to decrease cell death in cultured cells exposed to glutamate (Ientile et al., 2003) or the N-terminal fragment of mutant huntingtin (Zainelli et al., 2005). The therapeutic benefit of cystamine has been attributed to its inhibition of TG activity. The current study demonstrates that cystamine can reduce brain swelling, cell death and neurological deficits after ICH. Although, the mechanism of this effect still remains to be fully elucidated, there is a possibility that cystamine prevented not only apoptotic cell death but also oxidative stress or inflammation involving tTG activity. Interestingly, estrogen can protect neuronal apoptosis in hippocampal tissue following ischemia by down regulating tTG activity (Fujita et al., 2006), and estrogen also reduces ICH-induced brain edema formation through limiting oxidative brain injury (Nakamura et al., 2005). These findings support the possibility that cystamine has protective effects against oxidative brain injury involving tTG activity.
It should be noted, however, that whether cystamine is directly inhibiting TG and whether the therapeutic effects of cystamine are caused by TG inhibition alone remain to be clearly established. Protective effects of cystamine that do not involve inhibition of tTG have been shown (Bailey and Johnson, 2006). For example, cystamine can inhibit caspase-3 activity (Lesort et al., 2003), increase expression of heat-shock protein 40, a suppressor of neuronal cell apoptosis (Karpuj et al., 2002), and increase brain levels of brain-derived neurotrophic factor (Borrell-Pages et al., 2006). In addition, cystamine is able to increase cellular antioxidant activity by increasing the concentration of glutathione and cysteine in vivo (Lesort et al., 2003) and in vitro (Pinto et al., 2005). Further studies will be necessary to clarify the precise mechanism(s) involved in cystamine-induced protection and the relative importance of tTG inhibition in that protection.
In conclusion, ICH increases brain tTG levels and cystamine, a tTG inhibitor, reduces ICH-induced brain edema and neurological deficits. These results suggest a role of tTG in ICH.
4. Experimental Procedure
Animal Preparation and Intracerebral Infusion
Animal use protocols were approved by the University of Michigan Committee on the Use and Care of Animals. A total of thirty male Sprague-Dawley rats (weight, 300 to 350 g; Charles River Laboratories, Portage, MI, U.S.A.) were used in this study. Rats were anesthetized with intraperitoneal pentobarbital (45 mg/kg). The right femoral artery was catheterized for continuous blood pressure monitoring and blood sampling. Blood was obtained from the catheter for analysis of blood pH, PaO2, PaCO2, and blood glucose. Core temperature was maintained at 37°C with use of a feedback-controlled heating pad. Rats were positioned in a stereotactic frame (Kopf Instruments, Tujunga, CA, U.S.A.), and a cranial burr hole (1 mm) was drilled on the right coronal suture 3.5 mm lateral to the midline. A 26-gauge needle was inserted stereotactically into the right basal ganglia (coordinates: 0.2 mm anterior, 5.5 mm ventral, 3.5 mm lateral to the bregma). Autologous whole blood (100 µL) was infused at a rate of 10 µL/minute with the aid of a microinfusion pump (Harvard Apparatus Inc., South Natick, MA, U.S.A.). Sham controls had only a needle insertion. The needle was removed, the burr hole filled with bone wax, and the skin incision closed with sutures.
Experimental Groups
This study was in two parts. In the first part, rats received either a needle insertion (sham, n = 9) or an intracaudate injection of 100µL autologous whole blood (n = 9). The rats were killed 3 days later and the brains sampled for immunostaining, Western blot analysis, and real-time PCR analysis. In the second part, we examined the effect of the transglutaminase inhibitor, cystamine (Sigma Chemical Co., St. Lois, MO, U.S.A.), on ICH-induced brain damage in rats. Cystamine (100 mg/kg in saline; n = 6) or saline (vehicle, n = 6) were injected intraperitoneally 2 hours after the rats received 100 µL blood, and then administered daily for a total of 3 days. Behavioral tests were also performed before ICH and at days 1 and 3 after ICH. At day 3, rats were euthanized for brain swelling and ion content measurement.
Immunohistochemistry
Rats were anesthetized with pentobarbital (60 mg/kg) and underwent transcardiac perfusion with 4% paraformaldehyde in 0.1mol/L (pH 7.4) phosphate-buffered saline. Brains were removed and kept in 4% paraformaldehyde for 6 hours, then immersed in 30% sucrose for 3 to 4 days at 4°C. Brains were then placed in optimal cutting temperature embedding compound (Sakura Finetek, Inc., Torrance, CA, U.S.A.) and sectioned on a cryostat (18µm thick slices). Sections were examined using the avidin-biotin complex technique. The primary antibody was mouse anti-transglutaminase-2 monoclonal antibody (1:500 dilution, Lab Vision, Fremont, CA, U.S.A.) and the secondary antibody was anti-mouse immunoglobulin G antibody (1:500 dilution, Vector Laboratories, Inc., Burlingame, CA, U.S.A.). Normal horse immunoglobulin G (Vector Laboratories, Inc.) was used as a negative control.
Immunofluorescent Double Labeling
For immunofluorescent double labeling, tTG was combined with neuron-specific enolase (NSE) or glial fibrillary acidic protein (GFAP) immunostaining. The primary antibodies were mouse anti-transglutaminase-2 monoclonal antibody (1:500 dilution), rabbit anti-NSE antibody (1:500 dilution, Chemicon International, Inc., Temecula, CA, U.S.A.), and goat anti-GFAP antibody (1:500 dilution, Santa Cruz Biotechnology, Inc., Santa Cruz, CA, U.S.A.). The secondary antibodies were fluorescein isothiocyanate (FITC)-labeled horse anti-mouse antibody (1:500 dilution, Vector Laboratories), rhodamine-conjugated goat anti-rabbit antibody (1:500 dilution, Chemicon International, Inc.), and rhodamine-conjugated rabbit anti-goat antibody (1:500 dilution, Chemicon International, Inc.). The double labeling was analyzed using a fluorescence microscope.
Western Blot Analysis
Animals were anesthetized before undergoing transcardiac perfusion with 0.1 mol/L phosphate-buffered saline. The brains were removed and a 3-mm thick coronal brain slice was cut approximately 4 mm from the frontal pole. The slice was separated into ipsilateral and contralateral basal ganglia. Western blot analysis was performed as previously described (Xi et al., 1999). Briefly, 50-µg proteins for each were separated using sodium dodecyl sulfate polyacrylamide gel electrophoresis and transferred to a Hybond-C pure nitrocellulose membrane (Amersham, Piscataway, NJ, U.S.A.). The membranes were blocked in Carnation nonfat milk and probed with the primary and secondary antibodies. The primary antibodies were mouse anti-transglutaminase-2 monoclonal antibody (1:2000 dilution) and mouse anti-β-actin antibody (1:5000 dilution, Sigma-Aldrich, Inc., St. Louis, MO, USA), and the secondary antibody was peroxidase-conjugated goat anti-mouse antibody (BIO-RAD Laboratories, Hercules, CA, U.S.A.). The antigen/antibody complexes were demonstrated with a chemiluminescence system (Amersham) and exposed to film (X-OMAT; Kodak, Rochester, NY, U.S.A.). The relative densities of the bands were analyzed using NIH image (version 1.62; National Institutes of Health, Bethesda, MD, U.S.A.). Quantification of tTG protein was performed by calculating the ratio between tTG and β-actin protein expression.
Real-time quantitative polymerase chain reaction
Animals were anesthetized and killed by decapitation. The brains were removed and brain tissues were sampled as described previously. Total RNA was extracted with Trizol reagent (Gibco BRL Grand Island, NY, U.S.A.), and 1µg RNA was digested with amplification-grade deoxyribonuclease I (Gibco BRL). Complementary DNA was synthesized by reverse transcription using the digested 1µg RNA (11µL) with 14 µL reaction buffer (Perkin Elmer, Foster City, CA, U.S.A.) containing dNTP (dATP, dCTP, dGTP and dTTP), 25mmol/L magnesium chloride, 10x polymerase chain reaction buffer II, Random Hexamer Primer, ribonuclease inhibitor, and murine leukemia virus reverse transcriptase. The reaction was performed at 42°C for 30 minutes and terminated at 99°C for 5 minutes. Diethylpyrocarbonate water (75µL) was added to dilute the complimentary DNA to 100 µL and stored at −20°C for later use.
Real-time polymerase chain reaction was performed using a thermal cycler system (Mastercycler ep realplex, Eppendorf AG, Hamburg, Germany) according to the manufacturer’s instructions. Reactions were performed in a 20µL volume with 10µM primers, 20x SYBR solution in real master mix (Eppendorf AG), and diethylpyrocarbonate water. Rat TG2 primers used in this study were 5’-AAGGGAAGTCTTCACCAGAGCCAA-3’ (sense) and 5’-CGATGTGGGCAAACACGTCAAAGT-3’ (anti-sense). Rat GAPDH primers (5’-CCGTGCCAAGATGAAATTGGCTGT-3’ (sense), 5’-TGTGCATATGTGCGTGTGTGTGTG-3’ (anti-sense) were used to amplify GAPDH mRNA, a housekeeping gene used as a control. Amplification protocol included a 5 minute 95°C denaturation; one cycle with 95°C denaturation for 5 s, 65°C annealing for 10 s, and 72°C extension for 35 s; one cycle with 95°C denaturation for 5 s, 62.5°C annealing for 10 s, and 72°C extension for 35 s; then 40 cycles of 95°C denaturation for 5 s, and 60°C annealing for 10 s, and 72°C extension for 35 s. Detection of the fluorescent product occurred at the end of the 72°C extension periods. Quantification was performed by online monitoring for identification of the exact time point at which the logarithmic linear phase could be distinguished from the background (crossing point). The crossing point is expressed as a cycle number.
Brain Swelling and Ion Contents
Animals were anesthetized and decapitated to measure brain swelling and ion content. Brains were removed and a coronal tissue slice (3-mm thickness) 4 mm from the frontal pole was cut using a blade. The brain tissue slice was divided into two hemispheres along the midline, and each hemisphere was dissected into cortex and basal ganglia. The cerebellum served as a control. Five tissue samples from each brain were obtained: the ipsilateral and contralateral cortex, the ipsilateral and contralateral basal ganglia, and the cerebellum. Brain samples were immediately weighted on an electric analytical balance (model AE 100; Mettler Instrument, Highstown, NJ, U.S.A.) to obtain the wet weight. Brain samples were dried at 100°C for 24 hours to obtain the dry weight. Brain swelling was expressed as the percentage change in the ratio of wet weight to dry weight between the ipsilateral and contralateral sides.
After weighing, the dehydrated samples were digested in 1 ml of mol/L nitric acid for 1 week. The sodium and potassium contents of this solution were measured using the automatic flame photometer (model IL 943; Instrumentation Laboratory, Lexington, MA, U.S.A.). Ion content was expressed in microequivalents per gram of dehydrated brain tissue (milliequivalent per kilogram dry weight).
Assessment of Neurodegeneration
To assess neuronal degeneration, Fluoro-Jade staining (Schmued et al., 1997) was performed on brain coronal sections at 3 days after ICH. Three high-power images (×40 magnification) were taken around the hematoma using a digital camera. Fluoro-Jade positive cells were counted on these 3 areas from each of 4 cystamine or vehicle treatment rat brain sections.
Behavioral Tests
Intracerebral hemorrhage-induced neurological deficits were assessed using forelimb placing, forelimb use asymmetry and corner turn tests (Hua et al., 2002). In the vibrissae-elicited forelimb placing test, animals were held by their bodies to allow the forelimbs to hang free. Independent testing of each forelimb was conducted by brushing the respective vibrissae on the corner of a table top once per trial for 10 trials. A score of 1 was given each time the rat placed its forelimb onto the edge of the table in response to vibrissae stimulation. The percentage of successful placing responses was determined for the impaired and the unimpaired forelimbs.
For the forelimb use asymmetry test, forelimb use during exploratory activity was analyzed in a standing transparent cylinder. Behavior was quantified by determining the number of times the normal ipsilateral (I) forelimb, the impaired contralateral (C) forelimb, and both (B) forelimbs were used as a percentage of total number of limb usage. A single, overall limb-use asymmetry score was calculated as follows: forelimb use asymmetry score = [I/(I+C+B)]−[C/(I+C+B)]. During the corner turn test, the rat was allowed to proceed into a corner whose angle was 30°. To exit the corner, the animal could turn to either the left or the right, and the direction was recorded. This task was repeated 10 to 15 times, and the percentage of right turns calculated.
Statistical Analysis
All data in this study are presented as means ± SD. Data were analyzed with Student’s t-test or one-way analysis of variance (ANOVA). Differences were considered significant at p<0.05.
Acknowledgements
This study was supported by grants NS-017760, NS-039866, NS-047245 and NS-057539 from the National Institutes of Health (NIH) and 0435354Z and 0840016N from American Heart Association (AHA). The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH and AHA.
Abbreviations
- CNS
central nervous system
- ICH
intracerebral hemorrhage
- tTG
tissue-type transglutaminase
References
- Bailey CD, Johnson GV. The protective effects of cystamine in the R6/2 Huntington's disease mouse involve mechanisms other than the inhibition of tissue transglutaminase. Neurobiol Aging. 2006;27:871–879. doi: 10.1016/j.neurobiolaging.2005.04.001. [DOI] [PubMed] [Google Scholar]
- Borrell-Pages M, Canals JM, Cordelieres FP, Parker JA, Pineda JR, Grange G, Bryson EA, Guillermier M, Hirsch E, Hantraye P, Cheetham ME, Neri C, Alberch J, Brouillet E, Saudou F, Humbert S. Cystamine and cysteamine increase brain levels of BDNF in Huntington disease via HSJ1b and transglutaminase. J Clin Invest. 2006;116:1410–1424. doi: 10.1172/JCI27607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Citron BA, Suo Z, SantaCruz K, Davies PJ, Qin F, Festoff BW. Protein crosslinking, tissue transglutaminase, alternative splicing and neurodegeneration. Neurochem Int. 2002;40:69–78. doi: 10.1016/s0197-0186(01)00062-6. [DOI] [PubMed] [Google Scholar]
- Festoff BW, SantaCruz K, Arnold PM, Sebastian CT, Davies PJ, Citron BA. Injury-induced "switch" from GTP-regulated to novel GTP-independent isoform of tissue transglutaminase in the rat spinal cord. J Neurochem. 2002;81:708–718. doi: 10.1046/j.1471-4159.2002.00850.x. [DOI] [PubMed] [Google Scholar]
- Fesus L. Transglutaminase-catalyzed protein cross-linking in the molecular program of apoptosis and its relationship to neuronal processes. Cell Mol Neurobiol. 1998;18:683–694. doi: 10.1023/A:1020638020024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujita K, Kato T, Shibayama K, Imada H, Yamauchi M, Yoshimoto N, Miyachi E, Nagata Y. Protective effect against 17beta-estradiol on neuronal apoptosis in hippocampus tissue following transient ischemia/recirculation in mongolian gerbils via down-regulation of tissue transglutaminase activity. Neurochem Res. 2006;31:1059–1068. doi: 10.1007/s11064-006-9114-y. [DOI] [PubMed] [Google Scholar]
- Gong C, Boulis N, Qian J, Turner DE, Hoff JT, Keep RF. Intracerebral hemorrhage-induced neuronal death. Neurosurgery. 2001;48:875–883. doi: 10.1097/00006123-200104000-00037. [DOI] [PubMed] [Google Scholar]
- Hua Y, Schallert T, Keep RF, Wu J, Hoff JT, Xi G. Behavioral tests after intracerebral hemorrhage in the rat. Stroke. 2002;33:2478–2484. doi: 10.1161/01.str.0000032302.91894.0f. [DOI] [PubMed] [Google Scholar]
- Huang F, Xi G, Keep RF, Hua Y, Nemoianu A, Hoff JT. Brain edema after experimental intracerebral hemorrhage: role of hemoglobin degradation products. J Neurosurg. 2002;96:287–293. doi: 10.3171/jns.2002.96.2.0287. [DOI] [PubMed] [Google Scholar]
- Ientile R, Campisi A, Raciti G, Caccamo D, Curro M, Cannavo G, Li Volti G, Macaione S, Vanella A. Cystamine inhibits transglutaminase and caspase-3 cleavage in glutamate-exposed astroglial cells. J Neurosci Res. 2003;74:52–59. doi: 10.1002/jnr.10702. [DOI] [PubMed] [Google Scholar]
- Ientile R, Caccamo D, Marciano MC, Curro M, Mannucci C, Campisi A, Calapai G. Transglutaminase activity and transglutaminase mRNA transcripts in gerbil brain ischemia. Neurosci Lett. 2004;363:173–177. doi: 10.1016/j.neulet.2004.04.003. [DOI] [PubMed] [Google Scholar]
- Igarashi S, Koide R, Shimohata T, Yamada M, Hayashi Y, Takano H, Date H, Oyake M, Sato T, Sato A, Egawa S, Ikeuchi T, Tanaka H, Nakano R, Tanaka K, Hozumi I, Inuzuka T, Takahashi H, Tsuji S. Suppression of aggregate formation and apoptosis by transglutaminase inhibitors in cells expressing truncated DRPLA protein with an expanded polyglutamine stretch. Nat Genet. 1998;18:111–117. doi: 10.1038/ng0298-111. [DOI] [PubMed] [Google Scholar]
- Karpuj MV, Becher MW, Springer JE, Chabas D, Youssef S, Pedotti R, Mitchell D, Steinman L. Prolonged survival and decreased abnormal movements in transgenic model of Huntington disease, with administration of the transglutaminase inhibitor cystamine.[erratum appears in Nat Med 2002 Mar;8(3):303] Nat Med. 2002;8:143–149. doi: 10.1038/nm0202-143. [DOI] [PubMed] [Google Scholar]
- Kim SY, Grant P, Lee JH, Pant HC, Steinert PM. Differential expression of multiple transglutaminases in human brain. Increased expression and cross-linking by transglutaminases 1 and 2 in Alzheimer's disease. J Biol Chem. 1999;274:30715–30721. doi: 10.1074/jbc.274.43.30715. [DOI] [PubMed] [Google Scholar]
- Kim SY. Transglutaminase 2 in inflammation. Front Biosci. 2006;11:3026–3035. doi: 10.2741/2030. [DOI] [PubMed] [Google Scholar]
- Kingman TA, Mendelow AD, Graham DI, Teasdale GM. Experimental intracerebral mass: description of model, intracranial pressure changes and neuropathology. J Neuropathol Exp Neurol. 1988;47:128–137. doi: 10.1097/00005072-198803000-00005. [DOI] [PubMed] [Google Scholar]
- Lee J, Kim YS, Choi DH, Bang MS, Han TR, Joh TH, Kim SY. Transglutaminase 2 induces nuclear factor-kappaB activation via a novel pathway in BV-2 microglia. J Biol Chem. 2004;279:53725–53735. doi: 10.1074/jbc.M407627200. [DOI] [PubMed] [Google Scholar]
- Lesort M, Tucholski J, Miller ML, Johnson GV. Tissue transglutaminase: a possible role in neurodegenerative diseases. Prog Neurobiol. 2000;61:439–463. doi: 10.1016/s0301-0082(99)00052-0. [DOI] [PubMed] [Google Scholar]
- Lesort M, Lee M, Tucholski J, Johnson GV. Cystamine inhibits caspase activity. Implications for the treatment of polyglutamine disorders. J Biol Chem. 2003;278:3825–3830. doi: 10.1074/jbc.M205812200. [DOI] [PubMed] [Google Scholar]
- Lu T, Pan Y, Kao SY, Li C, Kohane I, Chan J, Yankner BA. Gene regulation and DNA damage in the ageing human brain. Nature. 2004;429:883–891. doi: 10.1038/nature02661. [DOI] [PubMed] [Google Scholar]
- Maggio N, Sellitti S, Capano CP, Papa M. Tissue-transglutaminase in rat and human brain: light and electron immunocytochemical analysis and in situ hybridization study. Brain Res Bull. 2001;56:173–182. doi: 10.1016/s0361-9230(01)00649-9. [DOI] [PubMed] [Google Scholar]
- Matsushita K, Meng W, Wang X, Asahi M, Asahi K, Moskowitz MA, Lo EH. Evidence for apoptosis after intercerebral hemorrhage in rat striatum. Journal of Cerebral Blood Flow & Metabolism. 2000;20:396–404. doi: 10.1097/00004647-200002000-00022. [DOI] [PubMed] [Google Scholar]
- Mendelow AD, Gregson BA, Fernandes HM, Murray GD, Teasdale GM, Hope DT, Karimi A, Shaw MD, Barer DH, investigators S. Early surgery versus initial conservative treatment in patients with spontaneous supratentorial intracerebral haematomas in the International Surgical Trial in Intracerebral Haemorrhage (STICH): a randomised trial.[see comment] Lancet. 2005;365:387–397. doi: 10.1016/S0140-6736(05)17826-X. [DOI] [PubMed] [Google Scholar]
- Nakamura T, Hua Y, Keep RF, Park JW, Xi G, Hoff JT. Estrogen therapy for experimental intracerebral hemorrhage in rats. J Neurosurg. 2005;103:97–103. doi: 10.3171/jns.2005.103.1.0097. [DOI] [PubMed] [Google Scholar]
- Namura S, Zhu J, Fink K, Endres M, Srinivasan A, Tomaselli KJ, Yuan J, Moskowitz MA. Activation and cleavage of caspase-3 in apoptosis induced by experimental cerebral ischemia. J Neurosci. 1998;18:3659–3668. doi: 10.1523/JNEUROSCI.18-10-03659.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piacentini M, Farrace MG, Piredda L, Matarrese P, Ciccosanti F, Falasca L, Rodolfo C, Giammarioli AM, Verderio E, Griffin M, Malorni W. Transglutaminase overexpression sensitizes neuronal cell lines to apoptosis by increasing mitochondrial membrane potential and cellular oxidative stress. J Neurochem. 2002;81:1061–1072. doi: 10.1046/j.1471-4159.2002.00898.x. [DOI] [PubMed] [Google Scholar]
- Piacentini M, Amendola A, Ciccosanti F, Falasca L, Farrace MG, Mastroberardino PG, Nardacci R, Oliverio S, Piredda L, Rodolfo C, Autuori F. Type 2 transglutaminase and cell death. Prog Exp Tumor Res. 2005;38:58–74. doi: 10.1159/000084233. [DOI] [PubMed] [Google Scholar]
- Pinto JT, Van Raamsdonk JM, Leavitt BR, Hayden MR, Jeitner TM, Thaler HT, Krasnikov BF, Cooper AJ. Treatment of YAC128 mice and their wild-type littermates with cystamine does not lead to its accumulation in plasma or brain: implications for the treatment of Huntington disease. J Neurochem. 2005;94:1087–1101. doi: 10.1111/j.1471-4159.2005.03255.x. [DOI] [PubMed] [Google Scholar]
- Qureshi AI, Ling GS, Khan J, Suri MF, Miskolczi L, Guterman LR, Hopkins LN. Quantitative analysis of injured, necrotic, and apoptotic cells in a new experimental model of intracerebral hemorrhage. Critical Care Medicine. 2001;29:152–157. doi: 10.1097/00003246-200101000-00030. [DOI] [PubMed] [Google Scholar]
- Qureshi AI, Suri MF, Ostrow PT, Kim SH, Ali Z, Shatla AA, Guterman LR, Hopkins LN. Apoptosis as a form of cell death in intracerebral hemorrhage. Neurosurgery. 2003;52:1041–1047. discussion 1047-8. [PubMed] [Google Scholar]
- Tolentino PJ, DeFord SM, Notterpek L, Glenn CC, Pike BR, Wang KK, Hayes RL. Up-regulation of tissue-type transglutaminase after traumatic brain injury. J Neurochem. 2002;80:579–588. doi: 10.1046/j.0022-3042.2001.00726.x. [DOI] [PubMed] [Google Scholar]
- Tolentino PJ, Waghray A, Wang KK, Hayes RL. Increased expression of tissue-type transglutaminase following middle cerebral artery occlusion in rats. J Neurochem. 2004;89:1301–1307. doi: 10.1111/j.1471-4159.2004.02436.x. [DOI] [PubMed] [Google Scholar]
- Tucholski J, Roth KA, Johnson GV. Tissue transglutaminase overexpression in the brain potentiates calcium-induced hippocampal damage. J Neurochem. 2006;97:582–594. doi: 10.1111/j.1471-4159.2006.03780.x. [DOI] [PubMed] [Google Scholar]
- Van Raamsdonk JM, Pearson J, Bailey CD, Rogers DA, Johnson GV, Hayden MR, Leavitt BR. Cystamine treatment is neuroprotective in the YAC128 mouse model of Huntington disease. J Neurochem. 2005;95:210–220. doi: 10.1111/j.1471-4159.2005.03357.x. [DOI] [PubMed] [Google Scholar]
- Wang X, Sarkar A, Cicchetti F, Yu M, Zhu A, Jokivarsi K, Saint-Pierre M, Brownell AL. Cerebral PET imaging and histological evidence of transglutaminase inhibitor cystamine induced neuroprotection in transgenic R6/2 mouse model of Huntington's disease. J Neurol Sci. 2005;231:57–66. doi: 10.1016/j.jns.2004.12.011. [DOI] [PubMed] [Google Scholar]
- Wu J, Hua Y, Keep RF, Schallert T, Hoff J, Xi G. Oxidative brain injury from extravasated erythrocytes after intracerebral hemorrhage. Brain Research. 2002;953:45. doi: 10.1016/s0006-8993(02)03268-7. [DOI] [PubMed] [Google Scholar]
- Xi G, Keep RF, Hoff JT. Erythrocytes and delayed brain edema formation following intracerebral hemorrhage in rats. J Neurosurg. 1998;89:991–996. doi: 10.3171/jns.1998.89.6.0991. [DOI] [PubMed] [Google Scholar]
- Xi G, Keep RF, Hua Y, Xiang JM, Hoff JT. Attenuation of thrombin-induced brain edema by cerebral thrombin preconditioning. Stroke. 1999;30:1247–1255. doi: 10.1161/01.str.30.6.1247. [DOI] [PubMed] [Google Scholar]
- Xi G, Keep RF, Hua Y, Bhasin RR, Duong HK, Hoff JT. Mechanisms of edema formation following intracerebral hemorrhage: Does hemolysate cause ischemia in intracerebral hemorrhage? J Neurosurg. 2001;94:162A. [Google Scholar]
- Xi G, Keep RF, Hoff JT. Mechanisms of brain injury after intracerebral haemorrhage. Lancet Neurol. 2006;5:53–63. doi: 10.1016/S1474-4422(05)70283-0. [DOI] [PubMed] [Google Scholar]
- Yang GY, Betz AL, Chenevert TL, Brunberg JA, Hoff JT. Experimental intracerebral hemorrhage: relationship between brain edema, blood flow, and blood-brain barrier permeability in rats. J Neurosurg. 1994;81:93–102. doi: 10.3171/jns.1994.81.1.0093. [DOI] [PubMed] [Google Scholar]
- Zainelli GM, Dudek NL, Ross CA, Kim SY, Muma NA. Mutant huntingtin protein: a substrate for transglutaminase 1, 2, and 3. J Neuropathol Exp Neurol. 2005;64:58–65. doi: 10.1093/jnen/64.1.58. [DOI] [PubMed] [Google Scholar]
- Zazulia AR, Diringer MN, Derdeyn CP, Powers WJ. Progression of mass effect after intracerebral hemorrhage. Stroke. 1999;30:1167–1173. doi: 10.1161/01.str.30.6.1167. [DOI] [PubMed] [Google Scholar]