Abstract
The constitutive androstane receptor [(CAR) NR1I3] is a hepatic transcription factor that controls the expression of numerous drug-metabolizing enzymes and transporters in response to xenobiotic exposures. In primary hepatocytes and intact liver, CAR resides in the cytoplasm under basal condition and translocates to the nucleus upon exposure to inducers. However, CAR spontaneously accumulates in the nucleus of immortalized cell lines and exhibits constitutive activation in the absence of activators, which makes the identification of CAR activators extremely challenging. Here, we have established an efficient screening method for determining the nuclear translocation of human (h) CAR in human primary hepatocytes (HPHs). Our results demonstrated that adenoviral-enhanced yellow fluorescent protein-tagged hCAR (Ad/EYFP-hCAR) infects HPHs with high efficiency, and the majority of Ad/EYFP-hCAR (>80%) is expressed in the cytoplasm of noninduced HPHs and is translocated to the nucleus in response to activators and antagonists of hCAR. Furthermore, 22 compounds including known hCAR activators, nonactivators, CYP2B inducers, and deactivators were evaluated in this system. Our results indicated that chemical-mediated Ad/EYFP-hCAR translocation in HPHs significantly correlated with hCAR activation and target gene induction. Compared with cell-based reporter assays in cell lines and in vitro ligand-binding assays, the established Ad/EYFP-hCAR translocation assay in HPHs exhibits apparent advantages such as sensitivity to chemical activators and responses to both direct and indirect hCAR activators. Thus, nuclear translocation of Ad/EYFP-hCAR in HPHs represents an efficient means for in vitro prediction of chemical-mediated hCAR nuclear accumulation.
Predominantly expressed in the liver, the constitutive androstane receptor [(CAR) NR1I3] is defined as an important xenobiotic sensor that transfers endogenous and exogenous stimuli into cellular responses by regulating the expression of numerous hepatic genes. Upon xenobiotic stimulation, CAR and its closest relative pregnane X receptor [(PXR) NR1I2] coordinate the cellular defensive response by enhancing the expression of a broad and overlapping set of drug-metabolizing enzymes (DMEs) and transporters (Honkakoski et al., 2003; Stanley et al., 2006). In addition to xenobiotic detoxification, activation of CAR is also involved in other hepatic functions, such as gluconeogenesis, fatty acid oxidation, biotransformation, and clearance of steroid hormones and bilirubin (Sugatani et al., 2001; Ueda et al., 2002; Huang et al., 2003; Kodama et al., 2004; Tien and Negishi, 2006), as well as chemical-mediated tumor promotion (Yamamoto et al., 2004; Huang et al., 2005). Therefore, it is of great interest to develop an efficient screening method for identifying drug candidates as human (h) CAR activators at an early stage of drug development.
In contrast to PXR, CAR expresses a high level of constitutive transcriptional activity in immortalized cell lines and can be accumulated spontaneously into nuclei of these cells without the presence of xenobiotic activators (Baes et al., 1994; Wang and Negishi, 2003). Moreover, CAR could be activated through either direct ligand binding, such as the selective hCAR agonist 6-(4-chlorophenyl) imidazo[2,1-b][1,3]thiazole-5-carbaldehyde-O-(3,4-dichlorobenzy-l)oxime (CITCO) and the antimalarial artemisinin (ART), or ligand-independent (indirect) mechanisms, such as phenobarbital (PB)-type activators (Yamamoto et al., 2003; Simonsson et al., 2006; Merrell et al., 2008). These characteristics of CAR significantly decreased the value of the cell-based reporter assay and the in vitro ligand binding assays for assessing xenobiotic-mediated CAR activation. Nonetheless, CAR is sequestered primarily in the cytoplasm of noninduced hepatocytes in vivo and in primary culture conditions and undergoes a two-step activation process after exposure to activators. The initial step in response to chemical activators is the translocation of CAR from the cytoplasm to the nucleus (Kawamoto et al., 1999).
Significant efforts have been centered on elucidating the molecular mechanisms underlying the chemical-mediated nuclear translocation of CAR. It has recently been proposed that the CAR cytoplasmic complex is composed of heat shock protein 90, cytoplasmic CAR retention protein, membrane-associated subunit of protein phosphatase 1, and other unknown proteins (Kobayashi et al., 2003; Sueyoshi et al., 2008). Apparently, direct ligand binding seems not to be essential for a drug to stimulate CAR nuclear translocation. For example, PB does not bind to either mouse (m) or human CAR but stimulates translocation of both receptors to the nucleus (Kawamoto et al., 1999; Moore et al., 2000; Tzameli et al., 2000). Although the exact mechanisms of nuclear translocation are still elusive, cytoplasmic retention of CAR in the primary hepatocyte cultures and accumulation in the nucleus after CAR activation provide a valuable in vitro system for investigating the signaling pathway involved in the activation of CAR.
To date, mounting evidence indicates that identification of hCAR activators has been extremely complicated because of the significant species differences in CAR activation. For instance, 1,4-bis [2-(3,5-dichlorpyridyloxy)] benzene (TCPOBOP) activates mouse but not human CAR, whereas CITCO activates human but not mouse CAR. Several compounds including androstanol, progesterone, and testosterone showed potent repression of the constitutive activity of mCAR in cell lines. However, such chemical tools pertaining to hCAR are limited. We recently showed that PK11195, a known ligand for the peripheral benzodiazepam receptor, exhibits potent and selective repression of hCAR activity in HepG2 cells, but the repressed hCAR activity was only reestablished in the presence of direct activators such as CITCO and ART not by indirect activators such as PB and phenytoin (PHN) (Li et al., 2008). Given that a large number of hCAR activators function through ligand-independent mechanisms, antagonistic repressors of hCAR only provide limited value in screening hCAR activation.
In this report, we generated a functional adenoviral-enhanced yellow fluorescent protein-hCAR (Ad/EYFP-hCAR) construct. Taking advantage of the human primary hepatocytes (HPHs) in which CAR resides in the cytoplasm before activation, we have established a method for assessing the activation of hCAR in adenoviral-transduced HPHs. Our results showed that Ad/EYFP-hCAR is primarily expressed in the cytoplasm of HPHs, and the chemical-mediated Ad/EYFP-hCAR nuclear accumulation correlated well with hCAR activation and target gene induction in HPHs. This method exhibits clear advantages over cell-based reporter assays and in vitro ligand-binding assays in determining hCAR activation.
Materials and Methods
Chemicals and Biological Reagents. PB, PK11195, TCPOBOP, PHN, rifampicin (RIF), ART, carbamazepine (CMZ), Wy-14,643, chenodeoxycholic acid (CDCA), 22(R)-hydroxycholesterol (HOC), 3-methylcholanthrene (3MC), butylated hydroxyanisole (BHA), clotrimazole (CLZ), diazepam (DZP), meclizine, and chlorpromazine (CPZ) were purchased from Sigma-Aldrich (St. Louis, MO). Okadaic acid (OA) was purchased from Calbiochem (Gibbstown, NJ). CITCO was obtained from BIOMOL Research Laboratories (Plymouth Meeting, PA). Efavirenz (EFV) was purchased from Toronto Research Chemicals (Toronto, ON, Canada), and nevirapine (NVP) was purchased from U.S. Pharmacopeia (Rockville, MD). Fluconazole (FLU) and myclobutanil (MCB) were purchased from LKT Laboratories (St. Paul, MN). Oligonucleotide primers were synthesized by Integrated DNA Technologies, Inc. (Coralville, IA). The Dual-Luciferase Reporter Assay System was purchased through Promega (Madison, WI). FuGENE 6 transfection reagent was obtained from Roche Diagnostics (Basel, Switzerland). Matrigel, insulin, and insulin transferrin selenium were obtained from BD Biosciences (Bedford, MA). Other cell culture reagents were purchased from Invitrogen (Carlsbad, CA) or Sigma-Aldrich.
Plasmids and Generation of Adenovirus-EYFP-Tagged hCAR. The pCR3-hCAR and the EYFP-hCAR expression plasmids were kindly provided by Dr. Masahiko Negishi (National Institute of Environmental and Health Sciences, National Institutes of Health, Research Triangle Park, NC). As reported previously, the CYP2B6-2.2 kb firefly luciferase construct is composed of the phenobarbital-responsive enhancer module containing 1.8 kb of the native promoter, and the 400-bp distal xenobiotic-responsive enhancer module region (Wang et al., 2003). The pRL-TK Renilla luciferase plasmids used to normalize firefly luciferase activities were from Promega. The EYFP-tagged hCAR was subcloned into pShuttle-CMV expression vector at SaII and XbaI sites. The linearized shuttle vector and AdEasy vector were then cotransformed into BJ5183 cells. Positive recombinant Ad/EYFP-hCAR plasmids were selected and transduced into HEK293 cell cultures for virus packaging and amplification. Viruses were purified by using a ViraBind Adenovirus Purification Kit (Cell Biolabs, Inc., San Diego, CA).
Human Primary Hepatocyte Culture and Treatments. Human liver tissues were obtained after surgical resection by qualified pathology staff after diagnostic criteria were met and with prior approval from the institutional review board at the University of Maryland at Baltimore. Hepatocytes were isolated from human liver specimens by a modification of the two-step collagenase digestion method as described previously (LeCluyse et al., 2005). Hepatocytes were seeded at 3.75 × 105 cells/well in 24-well BioCoat plates in Dulbecco's modified Eagle's medium supplemented with 5% fetal bovine serum, 100 U/ml penicillin, 100 μg/ml streptomycin, 4 μg/ml insulin, and 1 μM dexamethasone and cultured as described previously (Wang et al., 2003). Hepatocyte cultures were infected with 2 μl of Ad/EYFP-hCAR for 12 h before treatment with the vehicle control (0.1% DMSO) or test compounds. After 24 h of treatment, cells were washed twice with phosphate-buffered saline and fixed for 30 min in 4% buffered paraformaldehyde. The cells were then stained with 4,6-diamidine-2-phenylindole dihydrochloride for 30 min. Twenty-two various compounds as classified in Table 1 were tested in Ad/EYFP-hCAR-infected HPHs. In separate experiments, HPHs seeded in six-well BioCoat plates were treated with BHA (100 μM), DZP (50 μM), FLU (50 μM), MCB (50 μM), RIF (10 μM), or 0.1% DMSO as vehicle control for real-time PCR and Western blotting analysis.
TABLE 1.
Classification of compounds used in the screening test
| Classification | Compounds | References |
|---|---|---|
| Known hCAR activators | CITCO; artemisinin; phenobarbital; phenytoin; chlorpromazine; carbamazepine; efavirenz; nevirapine | Wang et al., 2003; Yamamoto et al., 2003; Maglich et al., 2003; Simonsson et al., 2006; Faucette et al., 2007 |
| Selective activators of other nuclear receptors | Rifampicin; 3-methylcholanthrene; Wy-14,643; chenodeoxycholic acid; 22(R)-hydroxycholesterol | Tirona and Kim, 2005 |
| Selective mouse CAR activators and known hCAR deactivators | TCPOBOP and meclizine (mCAR activator); clotrimazole; PK11195; okadaic acid | Kawamoto et al., 1999; Honkakoski et al., 2003; Huang et al., 2004 |
| Rodent CYP2B inducers without available CAR information | Fluconazole; diazepam; myclobutanil; butylated hydroxyanisole | Sun and Fukuhara, 1997; Parkinson et al., 2006; Sun et al., 2006, 2007 |
Confocal Laser Scanning Microscopy Imaging. Confocal laser scanning microscopy was performed with a Nikon C1-LU3 instrument based on an inverted Nikon Eclipse TE2000 microscope. The confocal system was equipped with three fluorescence detection channels (photomultipliers) and a nonconfocal transmitted light detector. One of the photomultipliers was used to collect fluorescence signals from the green and yellow region of the fluorescence emission, and the nonconfocal transmitted light detector was used to collect brightfield images.
Real-Time PCR Analysis. Total RNA was isolated from treated hepatocytes using the RNeasy Mini Kit (QIAGEN, Valencia, CA) and reverse-transcribed using a High Capacity cDNA Archive Kit (Applied Biosystems, Foster City, CA) following the manufacturer's instructions. CYP2B6 and CYP3A4 mRNA expression was normalized against that of glyceraldehyde-3-phosphate dehydrogenase (GAPDH). Real-time PCR assays were performed in 96-well optical plates on an ABI Prism 7000 Sequence Detection System with SYBR Green PCR Master Mix (Applied Biosystems). Primers for CYP2B6, CYP3A4, and GAPDH mRNA detection are as follows: CYP2B6 1215 to 1319 bp, 5′-AGACGCCTTCAATCCTGACC-3′ (forward) and 5′-CCTTCACCAAGACAAATCCGC-3′ (reverse); CYP3A4 213 to 462 bp, 5′-GTGGGGCTTTTATGATGGTCA-3′ (forward) and 5′-GCCTCAGATTTCTCACCAACACA-3′ (reverse); and GAPDH 217 to 501 bp, 5′-CCCATCACCATCTTCCAGGAG-3′ (forward) and 5′-GTTGTCATGGATGACCTTGGC-3′ (reverse). -Fold induction values were calculated according to the equation 2ΔΔCt, where ΔCt represents the differences in cycle threshold numbers between the target gene and GAPDH and ΔΔCt represents the relative change in these differences between control and treatment groups.
Western Blot Analyses. Homogenate proteins (20 μg) from treated HPHs were resolved on SDS-polyacrylamide gels and electrophoretically transferred onto Immobilon-P polyvinylidene difluoride membranes. Subsequently, membranes were incubated with specific antibodies against CYP2B6 or CYP3A4 (Millipore Bioscience Research Reagents, Temecula, CA) diluted 1:4000 and 1:5000, respectively. β-Actin (Sigma-Aldrich) was used as an internal control. Blots were washed and incubated with horseradish peroxidase goat anti-rabbit IgG antibody diluted 1:4000. Blots were developed using ECL Western blotting detection reagent (GE Healthcare, Chalfont St. Giles, UK).
Transfection Assays in Cell Lines. HepG2 cells in 24-well plates were transfected with hCAR expression vector, and CYP2B6-2.2-kb reporter construct using a FuGene 6 Transfection Kit following the manufacturer's instruction. Twenty-four hours after transfection, cells were treated with solvent (0.1% DMSO) or test compounds at indicated concentrations (see Fig. 6D) in the absence or presence of PK11195 (5 μM). Twenty-four hours later, cell lysates were assayed for firefly activities normalized against the activities of cotransfected Renilla luciferase using a Dual-Luciferase kit (Promega). Data are represented as the mean ± S.D. of three individual transfections.
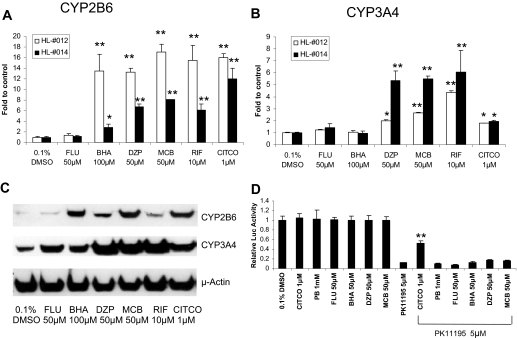
Fig. 6.
Effects of FLU, BHA, DZP, and MCB on CYP2B6/CYP3A4 expression in HPHs, and hCAR reactivation in HepG2 cells. HPHs (HL-#012 and HL-#014) were treated with FLU (50 μM), BHA (100 μM), DZP (50 μM), MCB (50 μM), RIF (10 μM), CITCO (1 μM), or vehicle control (0.1% DMSO). Total RNA extracted after 24-h treatments was subjected to real-time PCR analysis (A and B). Homogenates (20 μg) harvested after 72-h treatments were prepared for CYP2B6 and CYP3A4 immunoblot analysis (C). In a separate experiment, HepG2 cells were transfected with CAR expression plasmid along with CYP2B6-2.2-kb reporter plasmid as described under Materials and Methods. Cells were treated with vehicle (0.1% DMSO) and test compounds alone or in combination with PK11195 (5 μM). Luciferase activities were determined and expressed relevant to control (D). Data represent the mean ± S.D. of three independent transfections. (*, p < 0.05; **, p < 0.01).
Statistical Analysis. Experimental data are presented as a mean of triplicate determinations ± S.D. unless otherwise noted. Statistical comparisons were made using one-way analysis of variance. The statistical significance was set at p values <0.05 or <0.01.
Results
Localization of Exogenous hCAR in HepG2 Cells and HPHs. Spontaneous nuclear accumulation and activation of CAR has been one of the major obstacles in studying CAR activation in immortalized cell lines. As demonstrated in Fig. 1, our recombinant Ad/EYFP-hCAR infected both HepG2 cells and HPHs with high efficiency (Fig. 1A). Consistent with previous observations (Kawamoto et al., 1999; Kanno et al., 2005), the expression of Ad/EYFP-hCAR was primarily observed in the nuclei of HepG2 cells (nucleus = 98%; mixed = 2%). In contrast, the Ad/EYFP-hCAR has been visualized predominantly in the cytoplasm of human primary hepatocytes before activation (nucleus = 4%; cytoplasm = 87%; mixed = 9%). Taken together, the unique characteristics of hCAR distribution and the direct visualization of EYFP-hCAR plus the high efficiency of Ad/EYFP-hCAR infection of HPHs offer an attractive avenue for in vitro identification of hCAR activators.
Fig. 1.
Localization of Ad/EYFP-hCAR in HepG2 cells and HPHs. HepG2 cells and HPHs (HL-#009) were infected with Ad/EYFP-hCAR as described under Materials and Methods. A, confocal images depict the localization of Ah/EYFP-hCAR in HepG2 and HPHs. Left panels, Ad/EYFP-hCAR expression (green); center panels, nuclear staining (red); right panels, merged images. B, 100 hCAR-expressing cells from each group were counted and classified by cytosolic, nuclear, or mixed (cytosolic + nuclear) hCAR cellular localizations.
Ad/EYFP-hCAR Response to Known hCAR Activators. A series of 22 compounds have been chosen to evaluate the correlation between chemical-mediated hCAR nuclear accumulation and target gene induction in current studies. These compounds include known hCAR activators, hCAR deactivators, selective mouse CAR activators, selective activators of other nuclear receptors, and known or suspected CYP2B inducers for which information regarding hCAR activation is not available (Table 1). With no activation, the profusely expressed Ad/EYFP-CAR was observed primarily in the cytoplasm of cultured hepatocytes (84.5%), whereas approximately 15.5% infected cells displayed nucleus or mixed (nucleus + cytoplasm) allocation (Fig. 2, A and B). On the contrary, all of the eight known hCAR activators resulted in remarkable nuclear accumulation after 24 h of treatment, for which nucleus and mixed hCAR distribution accounts for approximately 90% of the infected hepatocytes (Fig. 2C). Representative images of hCAR localization were demonstrated in Fig. 2, A and B, after treatment with the vehicle control (0.1% DMSO), the typical hCAR activator PB (1 mM), or CITCO (1 μM). It is noteworthy that the selective hCAR agonist, CITCO, revealed a unique pattern of hCAR translocation in which up to 64.5% of infected cells exhibit mixed hCAR distribution (Fig. 2B).
Fig. 2.
Known hCAR activators promote nuclear translocation of Ad/EYPF-hCAR in HPHs. HPHs (HL-#009 and HL-#014) were infected with Ad/EYFP-hCAR as described under Materials and Methods and treated with vehicle (0.1% DMSO) or eight known hCAR activators at the indicated concentrations. After 24 h of treatment, hepatocytes were stained with 4,6-diamidine-2-phenylindole dihydrochloride and subjected to confocal microscopy. A, representative Ad/EYFP-hCAR localizations from vehicle control, PB (1 mM)-, or CITCO (1 μM)-treated HPHs. Three panels are shown for each treatment: left, Ad/EYFP-hCAR (green); center, nuclear staining (red); and right, merged image. B and C, for each treatment, 100 hCAR-expressing cells were counted and classified based on cytosolic, nuclear, or mixed (cytosolic + nuclear) hCAR cellular localizations.
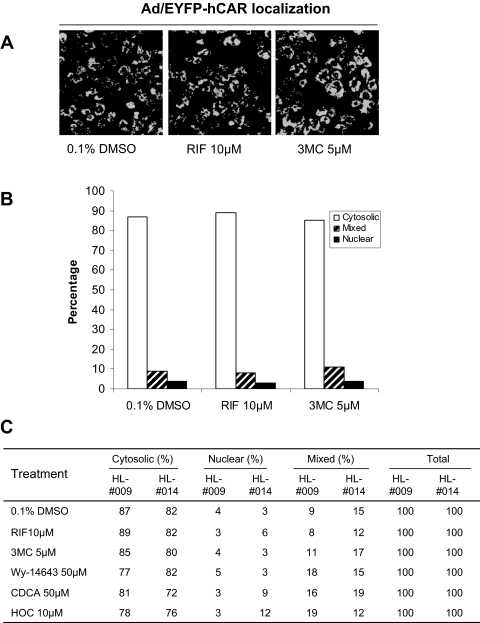
Ad/EYFP-hCAR Response to Selective Activators of Other Nuclear Receptors. Liver is enriched with various transcription factors that govern the regulation of both constitutive and inducible DME expression (Tirona and Kim, 2005). To determine the specificity of chemical-mediated hCAR translocation, several typical activators of other nuclear receptors including RIF (10 μM) for PXR, CDCA (50 μM) for farnesoid X receptor, HOC (10 μM) for liver X receptor, 3MC (5 μM) for aryl hydrocarbon receptor (AhR), and Wy-14,643 (50 μM) for peroxisome proliferator-activated receptor α (PPARα) were characterized in HPHs infected with Ad/EYFP-hCAR (Fig. 3). Representative images of hCAR localization are demonstrated in Fig. 3, A and B, after treatment with the vehicle control (0.1% DMSO), the typical hPXR activator RIF (10 μM), or the AhR activator 3MC (5 μM). As demonstrated in Fig. 3C, none of the selective activators of these other nuclear receptors caused a significant shift of Ad/EYFP-hCAR from the cytoplasm to the nucleus compared with the vehicle control, indicating the chemical selectivity of Ad/EYFP-hCAR nuclear accumulation in HPHs.
Fig. 3.
Ad/EYFP-hCAR was not translocated by typical activators of other nuclear receptors. HPHs (HL-#009 and HL-#014) were infected with Ad/EYFP-hCAR as described under Materials and Methods and treated with vehicle (0.1% DMSO) or five typical activators of other nuclear receptors at the indicated concentrations. After 24 h of treatment, hepatocytes were subjected to confocal microscopy. A, representative Ad/EYFP-hCAR localizations from vehicle control, RIF (10 μM)-, and 3MC (5 μM)-treated HPHs. B and C, For each treatment, 100 hCAR-expressing cells were counted and classified based on cytosolic, nuclear, or mixed (cytosolic + nuclear) hCAR localizations.
Translocation of Ad/EYFP-hCAR by mCAR Agonist and hCAR Antagonists. TCPOBOP is a known selective and potent mCAR agonist and robustly induces Cyp2b10 expression in mouse liver and cultured hepatocytes (Honkakoski et al., 2003). To assess the species specificity of Ad/EYFP-hCAR translocation, infected HPHs were treated with TCPOBOP (250 nM) for 24 h. As shown in Fig. 4, treatment of TCPOBOP was not associated with nuclear accumulation of Ad/EYFP-hCAR in human hepatocytes. Intriguingly, a recent study showed that the typical PPARα ligand, Wy-14,643, remarkably translocated mCAR from the cytoplasm to the nucleus in mouse liver (Guo et al., 2007), whereas our results revealed that Wy-14,643 neither enhanced the nuclear accumulation of hCAR in HPHs (Fig. 3) nor induced the expression of CYP2B6 in HPHs (data not shown). Together, these data underscore the species selectivity of Ad/EYFP-hCAR translocation in HPHs. Conversely, two recently reported hCAR antagonists, CLZ and PK11195, resulted in a significant nuclear accumulation of Ad/EYFP-hCAR in cultured human hepatocytes (Fig. 4C), signifying the complexity of the mechanisms underlying CAR activation (Moore et al., 2000; Li et al., 2008). In a parallel experiment, the indirect CAR deactivator, OA (10 nM) alone exhibited no effects on the translocation of Ad/EYFP-hCAR (Fig. 4A). Intriguingly, after a 1-h pretreatment with OA (10 nM), the cotreatment of OA plus PB (0.5 or 1 mM) failed to inhibit PB-mediated hCAR nuclear translocation in HPHs (Supplemental Fig. 1).
Fig. 4.
Translocation of Ad/EYFP-hCAR in HPHs after treatment with mCAR activator and hCAR deactivators. HPHs (HL-#009 and HL-#014) were infected with Ad/EYFP-hCAR as described under Materials and Methods and treated with vehicle (0.1% DMSO) or tested compounds at the indicated concentrations. After 24 h of treatment, hepatocytes were subjected to confocal microscopy analysis. A, representative Ad/EYFP-hCAR localizations from vehicle control, PK1195 (10 μM)-, OA (0.01 μM)-, or TCPOBOP (250 nM)-treated HPHs. B and C, for each treatment, 100 hCAR-expressing cells were counted and classified based on cytosolic, nuclear, or mixed (cytosolic + nuclear) hCAR localizations.
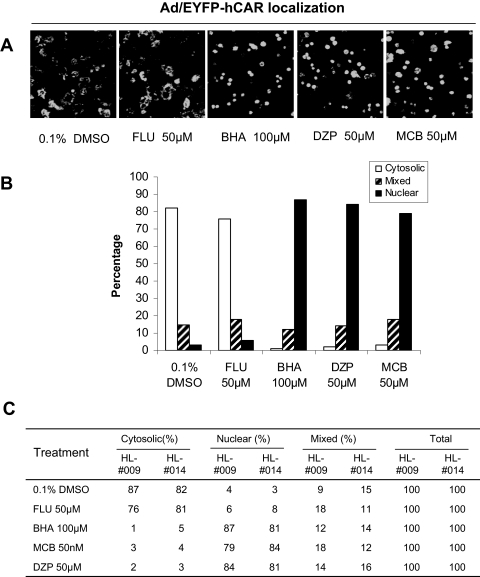
Correlation between hCAR Translocation and CYP2B6 Induction. The CYP2B genes are typical CAR targets in different species. Although other nuclear receptors such as PXR also mediate CYP2B induction, activation of CAR is closely associated with the induction of CYP2B in a species-specific manner. In the current study, a number of known or suspected CYP2B inducers without available hCAR activation data have been evaluated by the Ad/EYFP-hCAR translocation assays. These compounds include four reported rodent CYP2B inducers, MCB, BHA, DZP, and FLU (Sun and Fukuhara, 1997; Parkinson et al., 2006; Sun et al., 2006, 2007). As shown in Fig. 5, three of four compounds (BHA, DZP, and MCB) exhibited a remarkable capacity for translocating hCAR to the nucleus of HPHs. However, one of the four rodent CYP2B inducers, FLU, did not shift Ad/EYFP-hCAR toward the nucleus of treated HPHs.
Fig. 5.
Ad/EYFP-hCAR localization in HPHs after treatment with CYP2B inducers. HPHs (HL-#009 and HL-#014) were infected with Ad/EYFP-hCAR as described under Materials and Methods and treated with vehicle (0.1% DMSO) or tested compounds at the indicated concentrations. After 24 h of treatment, hepatocytes were subjected to confocal microscopy analysis. A, representative images illustrate Ad/EYFP-hCAR localizations in HPHs treated with vehicle control, FLU (50 μM), BHA (100 μM), DZP (50 μM), or MCB (50 μM). B and C, for each treatment, 100 hCAR-expressing cells were counted and classified based on cytosolic, nuclear, or mixed (cytosolic + nuclear) hCAR localizations.
To determine whether hCAR nuclear accumulation correlates with its target induction, HPHs were treated with the four rodent CYP2B inducers as described under Materials and Methods. Total RNA and proteins were prepared from the treated cells for determining the induction of CYP2B6 and CYP3A4 in human liver. The results demonstrated that DZP and MCB significantly enhanced the expression of CYP2B6 and CYP3A4 at both mRNA and protein levels, whereas BHA exhibited selective induction of CYP2B6 over CYP3A4 (Fig. 6, A–C). In contrast, FLU (50 μM) had little, if any induction effects on either CYP2B6 or CYP3A4. Taken together, these data indicate that nuclear translocation of Ad/EYFP-hCAR correlates well with its target gene inducible expression.
Activation of hCAR in Cell-Based Reporter Assays. HepG2 cells transfected with hCAR expression and CYP2B6 reporter constructs exhibited high basal reporter activity and were not sensitive to chemical-mediated activation, as expected (Kawamoto et al., 1999). On the other hand, we previously observed that the potent hCAR deactivator, PK11195, significantly inhibited the constitutive activity of hCAR in HepG2 cells, and the inhibited hCAR activity could only be reactivated by direct activator CITCO but not by indirect activators such as PB (Li et al., 2008). In the current reporter experiment, little or no reactivation was observed after the cotreatment with PK11195 and BHA, DZP, or MCB at 50 μM each (Fig. 6D). Combined with the observed hCAR translocation data in HPHs, these results indicate that BHA, DZP, and MCB most likely activate hCAR through PB-type indirect mechanisms.
Discussion
Although there is an emerging need for efficient screening of hCAR activators at the early stage of drug development, CAR is constitutively activated in all of the immortalized cell lines independent of xenobiotic stimulation. In addition, CAR displays unique activation mechanisms compared with those of other nuclear receptors, where CAR could be activated through either direct ligand binding or indirect phosphorylation/dephosphorylation-related signaling pathways (Kawamoto et al., 1999; Qatanani and Moore, 2005). These CAR features significantly lowered the value of cell-based reporter and in vitro ligand binding assays, making the investigation of CAR activation much more challenging. In contrast to the observations in immortalized cells, CAR is primarily compartmentalized in the cytoplasm of primary cultured hepatocytes and intact liver in vivo and only accumulates in the nucleus upon chemical-mediated activation. Nuclear translocation in hepatocytes seems to be the essential first step of xenobiotic-induced CAR activation and may offer a novel avenue for predicting CAR activation. However, in vitro detection of hCAR translocation has been difficult and time-consuming, partly because of the quiescent nature of human primary hepatocyte cultures. In this report, we have generated an Ad/EYFP-hCAR construct that infects HPHs with high efficiency and maintains hCAR distribution characteristics in a physiologically relevant manner.
Several lines of evidence indicate that activation of CAR is a multistep process, with nuclear accumulation as the essential first stride (Kawamoto et al., 1999; Sueyoshi et al., 2002). To determine the value of Ad/EYFP-hCAR-transduced HPHs as a tool for screening hCAR activators in vitro, in the current study we evaluated 22 compounds including known hCAR activators, deactivators, typical activators of other nuclear receptors, mCAR activators, and known or suspected CYP2B inducers without available CAR activation data for the correlation between hCAR nuclear translocation and target gene induction. Thirteen compounds resulted in significant nuclear accumulation of Ad/EYFP-hCAR in HPHs. Among them, eight compounds (PB, CITCO, ART, PHN, EFV, NVP, CPZ, and carbamazepine) are known hCAR activators and CYP2B6 inducers (Sueyoshi et al., 1999; Maglich et al., 2003; Wang et al., 2004; Faucette et al., 2007), and three compounds (BHA, DZP, and MCB) are newly established human CYP2B6 inducers by the current studies, which display a total of 85% (11 of 13) correlation of Ad/EYFP-hCAR nuclear translocation with hCAR target gene induction (Sun and Fukuhara, 1997; Parkinson et al., 2006; Sun et al., 2007). In contrast, we also observed that two reported hCAR deactivators (CLZ and PK11195) remarkably translocated Ad-EYFP-hCAR to the nucleus of HPHs, which is consistent with our previous reports (Li et al., 2008; Wang and Tompkins, 2008). Likewise, Guo et al. (2007) reported that the typical PPARα agonist Wy-14,643 acts as an antagonist of mCAR and stimulates mCAR nuclear translocation without accompanying transcriptional enhancement of the CAR target gene in mouse liver. Conversely, the known indirect CAR deactivator, OA, alone has no effects on Ad/EYFP-hCAR translocation, and moreover, cotreatment of OA with PB did not inhibit the PB-mediated hCAR nuclear accumulation in HPHs (Supplemental Fig. 1). This observation is in contrast with an early report from Negishi and colleagues, in which OA treatment inhibited PB-mediated mCAR translocation in mouse primary hepatocytes (Kawamoto et al., 1999). Although the species specificity of hCAR versus mCAR might be one of the reasons for the controversial effects of OA between human and mouse, detailed explanations are beyond the scope of the current studies. Given the antagonistic nature of CLZ and PK11195, along with the agonistic property of CITCO, it is reasonable to speculate that ligand binding itself may trigger CAR nuclear accumulation regardless of its agonistic or antagonistic nature.
Although hCAR shares some common characteristics with its rodent counterparts, apparent species-specific activation between human and rodent CARs renders the necessity for evaluating hCAR activation in the physiologically relevant in vitro system—HPHs. In the current study, TCPOBOP, the most effective mCAR agonist identified thus far, exhibited negative nuclear translocation of hCAR in HPHs. Notably, our results also showed that Wy-14,643 failed to reallocate hCAR to the nucleus of HPHs (Fig. 3B) and are in contrast with the observation made by Guo et al. (2007) in adenoviral-mCAR-infected mouse liver, emphasizing the species selectivity of hCAR activation. To date, accumulating evidence has revealed xenobiotic receptors that coordinately regulate the expression of their target genes through cross-talking are abundant in liver. For instance, PB activates both hCAR and hPXR, whereas RIF selectively actives hPXR. Activation of hCAR and hPXR induces the expression of a broad spectrum of DMEs and transporters in the liver. To gain insight into the chemical specificity of Ad/EYFP-hCAR nuclear translocation, five selective activators of other xenobiotic receptors have been evaluated in Ad/EYFP-hCAR-infected HPHs. Without exception, typical activators of PXR (RIF), AhR (3MC), liver X receptor (HOC), farnesoid X receptor (CDCA), and PPARα (Wy-14, 643) all failed to significantly relocate Ad/EYFP-hCAR from the cytoplasm to the nucleus in HPHs. Overall, current evidence suggests that the Ad/EYFP-hCAR translocation assay in HPHs exhibits both species-specific and chemical-specific selectivity in hCAR activation.
Because of the apparent difficulties associated with identifying hCAR activators, particularly in a higher throughput fashion, the numbers of known hCAR activators thus far, are relatively small. In the current study, we also evaluated the hCAR nuclear translocation by four reported rodent CYP2B inducers (FLU, BHA, DZP, and MCB) that have no available information regarding hCAR activation. It is noteworthy that three of the four compounds exhibited significant nuclear translocation of the Ad/EYFP-hCAR in HPHs. Through a combination of direct and indirect experimental approaches, our results showed that BHA-, DZP-, and MCB-mediated hCAR nuclear translocation is closely associated with the actual induction of their target gene CYP2B6 and CYP3A4. To our knowledge, this is the first report of use of a hCAR translocation assay in HPHs to successfully identify novel CYP2B6 and CYP3A4 inducers. Note that, although FLU is a potent inducer of CYP2B1 in rat (Sun et al., 2006), induction of either CYP2B6 or CYP3A4 is barely visible, and FLU did not enhance the nuclear translocation of Ad/EYFP-hCAR in HPHs. These data further highlight the species specificity and the value of the Ad/EYFP-hCAR translocation assay in HPHs.
In summary, our data suggest that Ad/EYFP-hCAR infection of HPHs provides a valuable tool for efficiently identifying hCAR activators in vitro. Because of the unique characteristics of hCAR activation, the Ad/EYFP-hCAR translocation assay in HPHs exhibits several apparent advantages over the cell-based reporter assays in cell lines and in vitro ligand binding assays, regarding the sensitivity to chemical stimulation and responsiveness to both direct and indirect activators. Meanwhile, we do realize that one limitation of this screening test is the incapability of discerning the agonist and antagonist of hCAR. Nonetheless, we have demonstrated recently that the constitutive activation of hCAR in HepG2 cells was efficiently repressed by the hCAR antagonist PK11195 and was reactivated by the hCAR agonist CITCO but not by indirect activators such as PB and PHN (Li et al., 2008). With the use of this reactivation assay, our results indicated that all three novel hCAR activators identified in the current study are most likely indirect activators (Fig. 6D). Overall, the combination of the Ad/EYFP-hCAR translocation assay in HPHs with the hCAR reactivation assay in HepG2 cells offers a valuable avenue for the identification of hCAR activators in vitro.
Supplementary Material
Acknowledgments
We thank Dr. Masahiko Negishi (National Institute of Environmental and Health Sciences, National Institutes of Health, Research Triangle Park, NC) for providing hCAR expression plasmids. We also gratefully acknowledge the technical support by Dr. Xilin Niu (University of North Carolina at Chapel Hill, Chapel Hill, NC) in our preparation of the Ad/EYFP-hCAR. Human liver tissues were obtained from The University of Maryland Medical Center (Baltimore, MD).
This work was supported by the National Institutes of Health National Institute of Diabetes and Digestive and Kidney Diseases [Grant DK061652].
Article, publication date, and citation information can be found at http://dmd.aspetjournals.org.
doi:10.1124/dmd.108.026005.
ABBREVIATIONS: CAR, constitutive androstane receptor; PXR, pregnane X receptor; DME, drug-metabolizing enzyme; h, human; CITCO, 6-(4-chlorophenyl) imidazo[2,1-b][1,3]thiazole-5-carbaldehyde-O-(3,4-dichlorobenzyl) oxime; ART, artemisinin; PB, phenobarbital; m, mouse; TCPOBOP, 1,4-bis[2-(3,5-dichlorpyridyloxy)]benzene; PK11195, 1-(2-chlorophenyl-N-methylpropyl)-3-isoquinoline-carboxamide; PHN, phenytoin; Ad/EYFP-hCAR, adenoviral-enhanced yellow fluorescent protein-human constitutive androstane receptor; HPH, human primary hepatocyte; RIF, rifampicin; CMZ, carbamezapine; Wy-14643, pirinixic acid; 4-chloro-6-(2,3-xylidino)-2-pyrimidinyl)thioacetic acid; CDCA, chenodeoxycholic acid; HOC, 22(R)-hydroxycholesterol; 3MC, 3-methylcholanthrene; BHA, butylated hydroxyanisole; CLZ, clotrimazole; DZP, diazepam; CPZ, chlorpromazine; OA, okadaic acid; EFV, efavirenz; NVP, nevirapine; FLU, fluconazole; MCB, myclobutanil; kb, kilobase(s); bp, base pair(s); DMSO, dimethyl sulfoxide; PCR, polymerase chain reaction; GAPDH, glyceraldehyde-3-phosphate dehydrogenase; Ct, cycle threshold; AhR, aryl hydrocarbon receptor; PPARα, peroxisome proliferator-activated receptor α.
The online version of this article (available at http://dmd.aspetjournals.org) contains supplemental material.
References
- Baes M, Gulick T, Choi HS, Martinoli MG, Simha D, and Moore DD (1994) A new orphan member of the nuclear hormone receptor superfamily that interacts with a subset of retinoic acid response elements. Mol Cell Biol 14 1544–1552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faucette SR, Zhang TC, Moore R, Sueyoshi T, Omiecinski CJ, LeCluyse EL, Negishi M, and Wang H (2007) Relative activation of human pregnane X receptor versus constitutive androstane receptor defines distinct classes of CYP2B6 and CYP3A4 inducers. J Pharmacol Exp Ther 320 72–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo D, Sarkar J, Suino-Powell K, Xu Y, Matsumoto K, Jia Y, Yu S, Khare S, Haldar K, Rao MS, et al. (2007) Induction of nuclear translocation of constitutive androstane receptor by peroxisome proliferator-activated receptor alpha synthetic ligands in mouse liver. J Biol Chem 282 36766–36776. [DOI] [PubMed] [Google Scholar]
- Honkakoski P, Sueyoshi T, and Negishi M (2003) Drug-activated nuclear receptors CAR and PXR. Ann Med 35 172–182. [DOI] [PubMed] [Google Scholar]
- Huang W, Zhang J, Chua SS, Qatanani M, Han Y, Granata R, and Moore DD (2003) Induction of bilirubin clearance by the constitutive androstane receptor (CAR). Proc Natl Acad Sci U S A 100 4156–4161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang W, Zhang J, Washington M, Liu J, Parant JM, Lozano G, and Moore DD (2005) Xenobiotic stress induces hepatomegaly and liver tumors via the nuclear receptor constitutive androstane receptor. Mol Endocrinol 19 1646–1653. [DOI] [PubMed] [Google Scholar]
- Huang W, Zhang J, Wei P, Schrader WT, and Moore DD (2004) Meclizine is an agonist ligand for mouse constitutive androstane receptor (CAR) and an inverse agonist for human CAR. Mol Endocrinol 18 2402–2408. [DOI] [PubMed] [Google Scholar]
- Kanno Y, Aoki S, Mochizuki M, Mori E, Nakahama T, and Inouye Y (2005) Expression of constitutive androstane receptor splice variants in rat liver and lung and their functional properties. Biol Pharm Bull 28 2058–2062. [DOI] [PubMed] [Google Scholar]
- Kawamoto T, Sueyoshi T, Zelko I, Moore R, Washburn K, and Negishi M (1999) Phenobarbital-responsive nuclear translocation of the receptor CAR in induction of the CYP2B gene. Mol Cell Biol 19 6318–6322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi K, Sueyoshi T, Inoue K, Moore R, and Negishi M (2003) Cytoplasmic accumulation of the nuclear receptor CAR by a tetratricopeptide repeat protein in HepG2 cells. Mol Pharmacol 64 1069–1075. [DOI] [PubMed] [Google Scholar]
- Kodama S, Koike C, Negishi M, and Yamamoto Y (2004) Nuclear receptors CAR and PXR cross talk with FOXO1 to regulate genes that encode drug-metabolizing and gluconeogenic enzymes. Mol Cell Biol 24 7931–7940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeCluyse EL, Alexandre E, Hamilton GA, Viollon-Abadie C, Coon DJ, Jolley S, and Richert L (2005) Isolation and culture of primary human hepatocytes. Methods Mol Biol 290 207–229. [DOI] [PubMed] [Google Scholar]
- Li L, Chen T, Stanton JD, Sueyoshi T, Negishi M, and Wang H (2008) The peripheral benzodiazepine receptor ligand 1-(2-chlorophenyl-methylpropyl)-3-isoquinoline-carboxamide is a novel antagonist of human constitutive androstane receptor. Mol Pharmacol 74 443–453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maglich JM, Parks DJ, Moore LB, Collins JL, Goodwin B, Billin AN, Stoltz CA, Kliewer SA, Lambert MH, Willson TM, et al. (2003) Identification of a novel human constitutive androstane receptor (CAR) agonist and its use in the identification of CAR target genes. J Biol Chem 278 17277–17283. [DOI] [PubMed] [Google Scholar]
- Merrell MD, Jackson JP, Augustine LM, Fisher CD, Slitt AL, Maher JM, Huang W, Moore DD, Zhang Y, Klaassen CD, et al. (2008) The Nrf2 activator oltipraz also activates the constitutive androstane receptor. Drug Metab Dispos 36 1716–1721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore LB, Parks DJ, Jones SA, Bledsoe RK, Consler TG, Stimmel JB, Goodwin B, Liddle C, Blanchard SG, Willson TM, et al. (2000) Orphan nuclear receptors constitutive androstane receptor and pregnane X receptor share xenobiotic and steroid ligands. J Biol Chem 275 15122–15127. [DOI] [PubMed] [Google Scholar]
- Parkinson A, Leonard N, Draper A, and Ogilvie BW (2006) On the mechanism of hepatocarcinogenesis of benzodiazepines: evidence that diazepam and oxazepam are CYP2B inducers in rats, and both CYP2B and CYP4A inducers in mice. Drug Metab Rev 38 235–259. [DOI] [PubMed] [Google Scholar]
- Qatanani M and Moore DD (2005) CAR, the continuously advancing receptor, in drug metabolism and disease. Curr Drug Metab 6 329–339. [DOI] [PubMed] [Google Scholar]
- Simonsson US, Lindell M, Raffalli-Mathieu F, Lannerbro A, Honkakoski P, and Lang MA (2006) In vivo and mechanistic evidence of nuclear receptor CAR induction by artemisinin. Eur J Clin Invest 36 647–653. [DOI] [PubMed] [Google Scholar]
- Stanley LA, Horsburgh BC, Ross J, Scheer N, and Wolf CR (2006) PXR and CAR: nuclear receptors which play a pivotal role in drug disposition and chemical toxicity. Drug Metab Rev 38 515–597. [DOI] [PubMed] [Google Scholar]
- Sueyoshi T, Kawamoto T, Zelko I, Honkakoski P, and Negishi M (1999) The repressed nuclear receptor CAR responds to phenobarbital in activating the human CYP2B6 gene. J Biol Chem 274 6043–6046. [DOI] [PubMed] [Google Scholar]
- Sueyoshi T, Moore R, Pascussi JM, and Negishi M (2002) Direct expression of fluorescent protein-tagged nuclear receptor CAR in mouse liver. Methods Enzymol 357 205–213. [DOI] [PubMed] [Google Scholar]
- Sueyoshi T, Moore R, Sugatani J, Matsumura Y, and Negishi M (2008) PPP1R16A, the membrane subunit of protein phosphatase 1β, signals nuclear translocation of the nuclear receptor constitutive active/androstane receptor. Mol Pharmacol 73 1113–1121. [DOI] [PubMed] [Google Scholar]
- Sugatani J, Kojima H, Ueda A, Kakizaki S, Yoshinari K, Gong QH, Owens IS, Negishi M, and Sueyoshi T (2001) The phenobarbital response enhancer module in the human bilirubin UDP-glucuronosyltransferase UGT1A1 gene and regulation by the nuclear receptor CAR. Hepatology 33 1232–1238. [DOI] [PubMed] [Google Scholar]
- Sun B and Fukuhara M (1997) Effects of co-administration of butylated hydroxytoluene, butylated hydroxyanisole and flavonoids on the activation of mutagens and drug-metabolizing enzymes in mice. Toxicology 122 61–72. [DOI] [PubMed] [Google Scholar]
- Sun G, Grindstaff RD, Thai SF, Lambert GR, Tully DB, Dix DJ, and Nesnow S (2007) Induction of cytochrome P450 enzymes in rat liver by two conazoles, myclobutanil and triadimefon. Xenobiotica 37 180–193. [DOI] [PubMed] [Google Scholar]
- Sun G, Thai SF, Lambert GR, Wolf DC, Tully DB, Goetz AK, George MH, Grindstaff RD, Dix DJ, and Nesnow S (2006) Fluconazole-induced hepatic cytochrome P450 gene expression and enzymatic activities in rats and mice. Toxicol Lett 164 44–53. [DOI] [PubMed] [Google Scholar]
- Tien ES and Negishi M (2006) Nuclear receptors CAR and PXR in the regulation of hepatic metabolism. Xenobiotica 36 1152–1163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tirona RG and Kim RB (2005) Nuclear receptors and drug disposition gene regulation. J Pharm Sci 94 1169–1186. [DOI] [PubMed] [Google Scholar]
- Tzameli I, Pissios P, Schuetz EG, and Moore DD (2000) The xenobiotic compound 1,4-bis[2-(3,5-dichloropyridyloxy)]benzene is an agonist ligand for the nuclear receptor CAR. Mol Cell Biol 20 2951–2958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ueda A, Hamadeh HK, Webb HK, Yamamoto Y, Sueyoshi T, Afshari CA, Lehmann JM, and Negishi M (2002) Diverse roles of the nuclear orphan receptor CAR in regulating hepatic genes in response to phenobarbital. Mol Pharmacol 61 1–6. [DOI] [PubMed] [Google Scholar]
- Wang H, Faucette S, Moore R, Sueyoshi T, Negishi M, and LeCluyse E (2004) Human constitutive androstane receptor mediates induction of CYP2B6 gene expression by phenytoin. J Biol Chem 279 29295–29301. [DOI] [PubMed] [Google Scholar]
- Wang H, Faucette S, Sueyoshi T, Moore R, Ferguson S, Negishi M, and LeCluyse EL (2003) A novel distal enhancer module regulated by pregnane X receptor/constitutive androstane receptor is essential for the maximal induction of CYP2B6 gene expression. J Biol Chem 278 14146–14152. [DOI] [PubMed] [Google Scholar]
- Wang H and Negishi M (2003) Transcriptional regulation of cytochrome p450 2B genes by nuclear receptors. Curr Drug Metab 4 515–525. [DOI] [PubMed] [Google Scholar]
- Wang H and Tompkins LM (2008) CYP2B6: new insights into a historically overlooked cytochrome P450 isozyme. Curr Drug Metab 9 598–610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto Y, Kawamoto T, and Negishi M (2003) The role of the nuclear receptor CAR as a coordinate regulator of hepatic gene expression in defense against chemical toxicity. Arch Biochem Biophys 409 207–211. [DOI] [PubMed] [Google Scholar]
- Yamamoto Y, Moore R, Goldsworthy TL, Negishi M, and Maronpot RR (2004) The orphan nuclear receptor constitutive active/androstane receptor is essential for liver tumor promotion by phenobarbital in mice. Cancer Res 64 7197–7200. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.