Abstract
Pharmacological activation of the constitutive androstane receptor (CAR) protects the liver during cholestasis. The current study evaluates how activation of CAR influences genes involved in bile acid biosynthesis as a mechanism of hepatoprotection during bile acid-induced liver injury. CAR activators phenobarbital (PB) and 1,4-bis[2-(3,5-dichloropyridyloxy)]benzene (TCPOBOP) or corn oil (CO) were administered to C57BL/6 wild-type (WT) and CAR knockout (CAR-null) mice before and during induction of intrahepatic cholestasis using the secondary bile acid, lithocholic acid (LCA). In LCA-treated WT and all the CAR-null groups (excluding controls), histology revealed severe multifocal necrosis. This pathology was absent in WT mice pretreated with PB and TCPOBOP, indicating CAR-dependent hepatoprotection. Decreases in total hepatic bile acids and hepatic monohydroxy, dihydroxy, and trihydroxy bile acids in PB- and TCPOBOP-pretreated WT mice correlated with hepatoprotection. In comparison, concentrations of monohydroxylated and dihydroxylated bile acids were increased in all the treated CAR-null mice compared with CO controls. Along with several other enzymes (Cyp7b1, Cyp27a1, Cyp39a1), Cyp8b1 expression was increased in hepatoprotected mice, which could be suggestive of a shift in the bile acid biosynthesis pathway toward the formation of less toxic bile acids. In CAR-null mice, these changes in gene expression were not different among treatment groups. These results suggest CAR mediates a shift in bile acid biosynthesis toward the formation of less toxic bile acids, as well as a decrease in hepatic bile acid concentrations. We propose that these combined CAR-mediated effects may contribute to the hepatoprotection observed during LCA-induced liver injury.
Bile acids are amphipathic steroid molecules important for lipid metabolism. The first step in the conversion of cholesterol to bile acids is hydroxylation of the steroid structure by cholesterol 7α-hydroxylase (CYP7A1). There are several steps and multiple intermediates involved in the formation of primary bile acids, which are cholic acid (CA) and chenodeoxycholic acid (CDCA) in humans and CA and β-muricholic acid in mice (Russell, 2003). Human primary bile acids, CA and CDCA, can be dehydroxylated by intestinal bacteria to form secondary bile acids, deoxycholic acid (DCA) and lithocholic acid (LCA), respectively (Norlin and Wikvall, 2007), and/or they can undergo amidation reactions to form tauroconjugates or glycoconjugates, such as taurocholic acid (TCA). It has been proposed that after entering the phospholipid bilayer, bile acids interfere with cholesterol and phospholipids and compromise membrane integrity, which leads to cell death (Fickert et al., 2006). Individual bile acids differ in structure according to the position and number of hydroxyl groups attached to the steroid backbone. In humans, hydroxylation commonly occurs at positions 3, 7, and 12, whereas rodent bile acids are hydroxylated primarily at the 3, 6, and 7 positions (Radominska et al., 1993). In general, the addition of hydroxyl groups reduces the toxicity of bile acids such that trihydroxylated bile acids are the least toxic (Schölmerich et al., 1984; Stedman et al., 2004). As one of the most toxic bile acids, the monohydroxy bile acid LCA was selected to produce intrahepatic cholestasis in this study (Kitada et al., 2003; Zhang et al., 2004).
Bile acid concentrations are regulated by negative feedback control under normal conditions. When present in excess, bile acids bind to the nuclear receptor farnesoid X receptor (FXR), and a cascade of events results in the down-regulation of the rate-limiting enzyme CYP7A1. Lower levels of CYP7A1 result in reduced bile acid production via a decrease the conversion of cholesterol into bile acids. When the excretion of bile acids is disrupted by disease, bile acids accumulate in hepatocytes, resulting in cholestasis. Once bile acid concentrations exceed their critical micellar concentration, they no longer aggregate with phospholipids as micelles. At that point the hydrophobic properties of bile acids are cytotoxic, leading to apoptotic or necrotic cell death. Excess concentrations of bile acids also cause adaptive changes in the liver, such as decreased hepatobiliary transport (Zollner et al., 2003). For example, Fickert et al. (2006) have shown that administration of LCA for 4 days in mice can result in hepatocellular necrosis with significant reductions in basolateral bile acid uptake (Ntcp, Oatp1) and increased expression of sinusoidal bile acid efflux transporters (Mrp3). These adaptive changes in the liver represent an attempt to protect cells from the inherent toxicity of accumulating bile acids. Interestingly, Yu et al. (2002) report that LCA is an FXR antagonist. This finding contrasts the effects of CDCA, which activates FXR to increase bile salt export pump expression and facilitate bile acid excretion. This down-regulation of a bile acid efflux transporter, such as bile salt export pump, by LCA may also help explain why this monohydroxylated bile acid is considered one of the most toxic bile acid species.
Phase I cytochrome P450 enzymes (P450s) have broad substrate specificity and catalyze the oxidation of a diverse array of structurally dissimilar compounds, including bile acids. In addition to CYP7A1, other P450s participate in bile acid biosynthesis. CYP8B1 forms the primary bile acid CA, whereas CYP27A1, CYP7B1, and CYP39A1 are involved in bile acid synthesis through the alternative pathway, derived from oxysterols. Expression of bile acid synthesis and detoxication enzymes is tightly regulated by nuclear hormone receptors and other transcription factors. One such nuclear receptor is constitutive androstane receptor (CAR). CAR assists in the regulation of bile acid metabolism by inducing phase I and II enzymes, as well as bile acid transport proteins. For example, CYP3A4 (Cyp3a11 rodent homolog) participates in bile acid detoxication via 6α-hydroxylation of LCA (Araya and Wikvall, 1999). The addition of this hydroxyl group makes the molecule more hydrophilic, which promotes elimination. In addition, phase II sulfotransferase enzyme (SULT2A1) adds a sulfate moiety to LCA to increase its water solubility and subsequent excretion (Kitada et al., 2003). Both CYP3A4 and SULT2A are downstream targets of CAR.
Previous studies have shown that pretreatment of mice with CAR activators phenobarbital (PB) or 1,4-bis[2,5-dichloropyridyloxy)]benzene (TCPOBOP) protects against the hepatotoxicity of LCA-induced cholestasis (Saini et al., 2004; Zhang et al., 2004; Beilke et al., 2008). The hepatoprotective effect of these chemicals during cholestasis is hypothesized to occur via an increase in the expression of bile acid-metabolizing enzymes, such as CYP3A, sulfotransferases, and glucuronosyltransferases (Saini et al., 2004; Zhang et al., 2004; Wagner et al., 2005), as well as up-regulation of hepatic efflux transporters, such as the multidrug resistance-associated proteins 3 and 4 (Bohan et al., 2003; Assem et al., 2004; Teng and Piquette-Miller, 2007). Unlike its adverse effects on the metabolism of acetaminophen whereby several P450 enzymes are up-regulated that convert acetaminophen to reactive metabolites (N-acetyl-p-benzoquinone imine), CAR activation during cholestasis positively regulates bile acid metabolism and bilirubin clearance via enhanced solubility and efflux transport (Kakizaki et al., 2008). Whereas changes in bile acid metabolism seem to be important in protecting against cholestatic liver injury, the up-regulation of efflux transporter expression is not consistently found in models of hepatoprotection from cholestasis (Beilke et al., 2008), indicating that other mechanisms probably contribute to the hepatoprotection. CAR as well as pregnane X receptor have been shown to repress Cyp7A in response to LCA, suggesting a protective role by decreasing bile acid production (Staudinger et al., 2001; Stedman et al., 2005). As such, a decrease in total liver bile acids in protected PB- and TCPOBOP-pretreated mice that was observed in our previous studies on LCA-induced hepatotoxicity led us to further explore the effects of CAR activation on individual bile acid concentrations and the genes that regulate bile acid biosynthesis.
Materials and Methods
Materials. PB, TCPOBOP, LCA, taurolithocholate 3-sulfate, taurochenodeoxycholic acid, and glycocholic acid were purchased from Sigma-Aldrich (St. Louis, MO). Tauro-β-muricholic acid, α-muricholic acid, and β-muricholic acid were purchased from Steraloids (Newport, RI). The remaining bile acids were a gift from Dr. Jesse Martinez (University of Arizona, Tucson, AZ).
Animals. Ten-week-old adult male C57BL/6 (Charles River Laboratories, Inc., Wilmington, MA) wild-type (WT) or CAR knockout (CAR-null) mice were weight-matched into treatment groups (n = 4–6 mice/group). Breeding pairs of CAR-null mice in the C57BL/6 background were obtained from Dr. Ivan Rusyn (University of North Carolina, Chapel Hill, NC), which were engineered by Tularik, Inc. (South San Francisco, CA) as described previously (Ueda et al., 2002). Animals received 3 days of CAR activator pretreatment (PB 80 mg/kg) or corn oil (CO) in the control groups via intraperitoneal injection in a volume of 2.5 ml/kg. On the 4th day, LCA administration was started (125 mg/kg twice daily, i.p.), and the CAR activator treatment in combination with LCA was continued for another 3 days. TCPOBOP (3 mg/kg, i.p.) pretreatment was begun on day 3 and continued during LCA treatment. Animals were euthanized approximately 12 h after the last LCA treatment, and livers were removed and stored at –80°C. Urine and serum were collected at necropsy and then stored at –80°C until needed for bile acid analysis. Animals were maintained in a 12-h light/dark cycle with access to food and water ad libitum. The experimental protocol was approved by the University of Kansas Medical Center Institutional Animal Care and Use Committee (IACUC), and humane care of the animals was in accordance with the criteria outlined in the Guide for the Care and Use of Laboratory Animals (National Research Council, 1996).
Liver Histology. Midsections of the left liver lobe from each mouse were fixed in 10% neutral buffered formalin. Tissues from two mice per treatment group were embedded in paraffin, and 5-μm sections were stained with hematoxylin and eosin according to a standard staining protocol. Under treatment-blinded conditions, the tissues were evaluated for liver injury by a board-certified veterinary pathologist.
Bile Acid Extraction. Liver samples were homogenized in a 1:1 solution of t-butanol/water (approximately 200 mg of liver/ml) and extracted overnight as described previously (Mennone et al., 2006). Samples were centrifuged at 10,000g for 20 min, and the supernatant was removed and placed in a SpeedVac (Thermo Fisher Scientific, Waltham, MA) until dry. Samples were then reconstituted with saline in a volume equal to the liver weight (milligrams per microliters) and stored at –80°C. Serum samples were mixed with chilled acetone (1:4), shaken vigorously, and left on ice for 5 min before centrifugation at 10,000g for 3 min (Daykin et al., 2002). The supernatant was removed and stored at –80°C. No extraction procedure was conducted on urine samples.
Total Bile Acid Analysis. Extracted liver samples were analyzed for total bile acid concentrations in a reaction catalyzed by 3α-hydroxysteroid dehydrogenase using the total bile acids assay kit by Diazyme Laboratories (Poway, CA). Total bile acid concentrations were quantified in triplicate according to the manufacturer's instructions.
Individual Bile Acid Analysis by High-Performance Liquid Chromatography/Electrospray Ionization/Tandem Mass Spectrometry. Urine and extracted liver and serum samples were removed from –80°C and allowed to thaw at room temperature. The chromatography was conducted with a capillary high-performance liquid chromatography (HPLC) system from Packings-Dionex (Amsterdam, The Netherlands) and comprised an UltiMate quaternary pump and a FAMOS autosampler. An Aquasil C18 reversed-phase column (3-μm particle size, 1 × 150 mm) was used for chromatography with a precolumn (1 × 10 mm) containing C18 reversed-phase resin (Thermo Fisher Scientific). HPLC mobile phase A consisted of 3 mM ammonium formate in H2O, pH 5.2, and B was 3 mM ammonium formate in 10% H2O and 90% CH3CN. The separation of bile acids was accomplished with a linear gradient starting from 95% A and 5% B held for 1 min going to 70% A and 30% B for 1:10 min, and then increasing to 100% B over 10 min at a flow rate of 50 μl/min using a 2-μl injection of extract and standards. Samples were diluted to 200 μl with the 70:30 solution of mobile phases A/B. Bile acid detection and quantification were done by electrospray ionization (ESI)/tandem mass spectrometry with a Waters (Milford, MA) Micromass Quattro Ultima triple quadrupole mass spectrometer. Quantitative analysis was performed in negative ionization mode using the selected reaction monitoring transitions specific for each bile acid. The specific selected reaction monitoring transitions were determined by direct infusion of standards. Separation of DCA and CDCA under the HPLC conditions used for this study was not possible; therefore, these bile acids are presented together as DCA/CDCA.
Development of Specific Oligonucleotide Probe Sets for Branched DNA Analysis. Probe sets for mouse Cyp3a11, Cyp7a1, Cyp7b1, Cyp8b1, Cyp27a1, Cyp39a1, Sult2a1/2, Ugt1a1, and bile acid-CoA amino acid N-acyltransferase (BAT) and FXR were used for quantification of mRNA expression. Mouse gene sequences were acquired from GenBank (National Center for Biotechnology Information, Bethesda, MD). Multiple oligonucleotide probe sets (capture extender, label extender, and blocker probes) were designed using Probe Designer software version 1.0 (Bayer Corp., Emeryville, CA) to be highly specific to a single mRNA transcript. The development of several probe sets for mice has been previously published: Cyp3a11 (Maher et al., 2005), Ugt1a1 (Buckley and Klaassen, 2007), and Sult2a1/2 (Alnouti and Klaassen, 2006). The probe set sequences for mouse Cyp7a1, Cyp7b1, Cyp8b1, Cyp27a1, Cyp39a1, FXR, and BAT are described in Supplemental Table 1. All of the oligonucleotide probes were designed with a Tm of approximately 63°C, enabling optimal hybridization conditions to be held constant (i.e., 53°C). Each probe designed in Probe Designer was submitted to the National Center for Biotechnology Information for nucleotide comparison by the basic local alignment search tool (BLASTn) to ensure minimal cross-reactivity with other mouse sequences.
RNA Isolation and mRNA Expression (Branched DNA Assay). Total RNA was isolated from liver using RNA Bee reagent (Tel-Test Inc., Friends-wood, TX) according to the manufacturer's instructions. The concentration of total RNA in each sample was quantified spectrophotometrically at 260 nm. RNA integrity was confirmed by visualization of intact 18S and 28S rRNA under UV light. The branched DNA (bDNA) assay was used to quantify mRNA expression as described previously (Beilke et al., 2008).
Statistical Analysis. For all the quantitative data, the mean and S.E.M. were calculated. Statistical differences were determined using one-way analysis of variance followed by Duncan's multiple range post hoc test using Statistica software, version 4.5 (StatSoft, Tulsa, OK). Asterisks (*) represent statistical differences (p ≤ 0.05) between the control and treated groups, whereas daggers (†) represent a statistical difference (p ≤ 0.05) between the LCA-only treated group and other treated groups.
Results
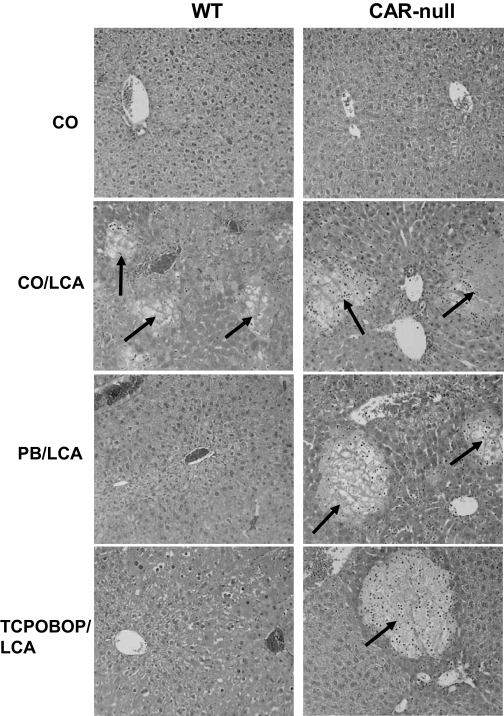
Evaluation of Liver Histopathology. After chemical pretreatment with CAR activators and induction of intrahepatic cholestasis, mouse livers were evaluated for hepatoprotection using histopathology (Fig. 1). Control WT and CAR-null mice exhibited normal histopathology with minimal hepatocellular vacuolization, whereas both genotypes of LCA-treated mice displayed a similar degree of severe multifocal hepatic necrosis, diffuse vacuolization, and infiltrating neutrophils consistent with cholestasis. Pretreatment of WT mice with the CAR activators PB and TCPOBOP protected against LCA-induced injury, and histopathology in these groups was similar to that observed in WT CO control mice. None of the CAR activator pretreatments were hepatoprotective in CAR-null mice, in which multifocal hepatocellular necrosis was observed, showing the importance of CAR in hepatoprotection.
Fig. 1.
CAR activators protect the liver against bile acid-induced toxicity. A midsection of the left liver lobe was removed and fixed from each animal. Tissues were stained with hematoxylin and eosin, and histopathology was determined under treatment-blinded conditions by a board-certified veterinary pathologist. Two animals per treatment group were evaluated, and pictures are representative of treatment group pathology. Multifocal hepatic necrosis (arrows) is easily distinguished from surrounding parenchyma and is more extensive in mice treated with LCA; 50× magnification.
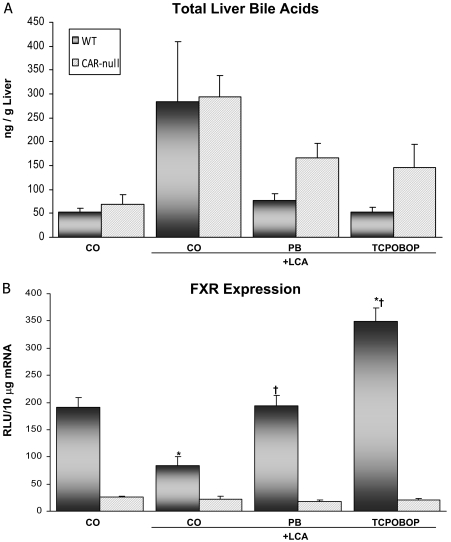
Total Liver Bile Acid Concentrations and FXR Expression. Total bile acid concentrations were quantified as depicted in Fig. 2A. Basal concentrations of total bile acids were similar between WT and CAR-null mice. Bile acid concentrations in LCA-treated WT mice alone were increased 5.4-fold above CO controls, and this seems to correlate with the necrosis observed histologically. Likewise, bile acid concentrations in LCA-treated CAR-null mice were increased 4.2-fold above control levels in CAR-null mice. LCA in combination with PB or TCPOBOP pretreatment in WT mice prevented the increase in total bile acid concentrations caused by LCA treatment alone. Bile acid concentrations in the hepatoprotected PB and TCPOBOP WT mice were similar to basal levels observed in CO control mice.
Fig. 2.
Total hepatic bile acid concentrations and FXR expression. Animals (n = 4–6/group) were dosed with activators and LCA (125 mg/kg twice daily) as described under Materials and Methods. A, bile acids were extracted from the liver, and the concentration was determined using 3α-hydroxysteroid dehydrogenase. Liver bile acid concentrations are presented as nanograms per gram of liver tissue. B, total RNA was isolated from livers of WT and CAR-null mice. The data are presented as mean relative light units ± S.E.M. *, indicates p ≤ 0.05 compared with respective CO; †, indicates p ≤ 0.05 compared with respective LCA only.
Expression of FXR, the main nuclear receptor involved in bile acid regulation, was significantly reduced by LCA treatment (56%) compared with expression in CO controls (Fig. 2B). It is interesting to note that FXR expression was up-regulated 1.8-fold in TCPOBOP-pretreated WT mice but not by PB pretreatment. Across treatment groups, FXR expression was clearly reduced in CAR-null mice compared with those with CAR (WT), suggesting that hepatic bile acid concentrations do not correlate with FXR expression.
Hepatic Concentrations of Individual Bile Acids. Data on individual bile acids in livers of WT mice analyzed by HPLC/ESI/tandem mass spectrometry are presented in Table 1. To ascertain overall changes in the types of individual bile acids, the concentrations of monohydroxylated, dihydroxylated, and trihydroxylated bile acids were grouped and averaged, and are presented in Fig. 3. Basal expression of monohydroxylated bile acids was reduced 87% in CAR-null mice compared with WT controls. As expected, LCA treatment increased monohydroxylated bile acid concentrations (LCA and taurolithocholic acid sulfate) above CO control mice in both genotypes (WT 4-fold, CAR-null 94-fold). Pretreatment of WT mice with PB and TCPOBOP prevented the increase in monohydroxy, dihydroxy, and trihydroxy bile acids caused by LCA treatment alone. Likewise, in CAR-null mice, pretreatment with PB (77%) and TCPOBOP (70%) reduced the increases in monohydroxy bile acid concentrations caused by LCA.
TABLE 1.
Individual bile acid concentrations in WT mice
Individual bile acid concentrations in liver, serum, and urine in WT mice. Results are presented as mean concentration ± S.E.M. Units in liver expressed as nanograms per gram liver; units in serum and urine expressed as nanogram per microliter.
| Liver | Serum | Urine | |
|---|---|---|---|
| Monohydroxy bile acids | |||
| LCA | |||
| CO | 2.40 ± 0.64 | 0.021 ± 0.003 | 0.000 ± 0.000 |
| CO + LCA | 19.1 ± 11.6 | 0.345 ± 0.121 | 0.334 ± 0.214 |
| PB + LCA | 10.6 ± 4.30 | 0.203 ± 0.40 | 0.094 ± 0.042 |
| TCPOBOP + LCA | 4.95 ± 1.07 | 0.171 ± 0.021 | 0.345 ± 0.215 |
| TLCA-S | |||
| CO | 0.027 ± 0.02 | 0.002 ± 0.001 | 0.027 ± 0.008 |
| CO + LCA | 0.022 ± 0.01 | 0.004 ± 0.002 | 0.012 ± 0.006 |
| PB + LCA | 0.006 ± 0.003 | 0.003 ± 0.001 | 0.021 ± 0.017 |
| TCPOBOP + LCA | 0.017 ± 0.017 | 0.001 ± 0.001 | 0.004 ± 0.002 |
| Dihydroxy bile acids | |||
| DCA/CDCA | |||
| CO | 0.000 ± 0.000 | 0.048 ± 0.023 | 0.046 ± 0.026 |
| CO + LCA | 0.200 ± 0.090 | 0.142 ± 0.117 | 0.042 ± 0.015 |
| PB + LCA | 0.028 ± 0.007 | 0.013 ± 0.003 | 0.040 ± 0.038 |
| TCPOBOP + LCA | 0.122 ± 0.025 | 0.010 ± 0.004 | 0.380 ± 0.147 |
| GCDCA | |||
| CO | None detected | 0.002 ± 0.001 | 0.008 ± 0.001 |
| CO + LCA | None detected | 0.016 ± 0.008 | 0.006 ± 0.005 |
| PB + LCA | None detected | 0.000 ± 0.000 | 0.005 ± 0.005 |
| TCPOBOP + LCA | None detected | 0.000 ± 0.000 | 0.013 ± 0.008 |
| TCDCA | |||
| CO | 3.68 ± 0.429 | 0.035 ± 0.005 | 0.016 ± 0.005 |
| CO + LCA | 138.7 ± 63.9 | 18.38 ± 8.314 | 0.153 ± 0.086 |
| PB + LCA | 14.4 ± 2.42 | 0.633 ± 0.336 | 0.021 ± 0.008 |
| TCPOBOP + LCA | 58.1 ± 28.2 | 0.057 ± 0.024 | 0.174 ± 0.048 |
| UDCA | |||
| CO | 0.00 ± 0.00 | 0.077 ± 0.032 | 0.026 ± 0.012 |
| CO + LCA | 3.09 ± 1.0 | 0.857 ± 0.385 | 1.650 ± 0.935 |
| PB + LCA | 0.68 ± 0.10 | 0.207 ± 0.035 | 0.031 ± 0.029 |
| TCPOBOP + LCA | 3.16 ± 0.95 | 0.159 ± 0.046 | 0.240 ± 0.109 |
| Trihydroxy bile acids | |||
| αMCA | |||
| CO | 0.21 ± 0.03 | 0.030 ± 0.005 | 2.068 ± 1.252 |
| CO + LCA | 1.01 ± 0.59 | 0.279 ± 0.157 | 3.148 ± 2.225 |
| PB + LCA | 0.28 ± 0.03 | 0.012 ± 0.001 | 0.112 ± 0.065 |
| TCPOBOP + LCA | 0.58 ± 0.16 | 0.011 ± 0.002 | 1.102 ± 0.461 |
| βMCA | |||
| CO | 0.37 ± 0.05 | 0.012 ± 0.000 | 0.124 ± 0.102 |
| CO + LCA | 1.00 ± 0.37 | 0.045 ± 0.029 | 0.979 ± 0.551 |
| PB + LCA | 0.35 ± 0.03 | 0.007 ± 0.001 | 0.045 ± 0.029 |
| TCPOBOP + LCA | 0.86 ± 0.21 | 0.007 ± 0.001 | 0.269 ± 0.094 |
| TβMCA | |||
| CO | 15.76 ± 2.1 | 0.020 ± 0.005 | 0.016 ± 0.006 |
| CO + LCA | 80.19 ± 30.1 | 13.37 ± 6.251 | 0.176 ± 0.143 |
| PB + LCA | 28.51 ± 4.1 | 0.580 ± 0.357 | 0.133 ± 0.099 |
| TCPOBOP + LCA | 37.11 ± 13.6 | 0.017 ± 0.003 | 0.335 ± 0.123 |
| CA | |||
| CO | 0.36 ± 0.04 | 0.049 ± 0.019 | 0.123 ± 0.103 |
| CO + LCA | 0.08 ± 0.08 | 0.033 ± 0.025 | 0.383 ± 0.0210 |
| PB + LCA | 0.03 ± 0.01 | 0.003 ± 0.002 | 0.104 ± 0.050 |
| TCPOBOP + LCA | 0.17 ± 0.06 | 0.010 ± 0.005 | 0.427 ± 0.197 |
| GCA | |||
| CO | 0.082 ± 0.02 | 0.0041 ± 0.001 | 0.009 ± 0.002 |
| CO + LCA | 0.032 ± 0.02 | 0.016 ± 0.006 | 0.022 ± 0.002 |
| PB + LCA | 0.022 ± 0.01 | 0.002 ± 0.001 | 0.008 ± 0.003 |
| TCPOBOP + LCA | 0.022 ± 0.01 | 0.001 ± 0.001 | 0.016 ± 0.001 |
| TCA | |||
| CO | 29.5 ± 5.14 | 0.084 ± 0.026 | 0.023 ± 0.006 |
| CO + LCA | 41.3 ± 16.8 | 11.65 ± 7.154 | 0.256 ± 0.008* |
| PB + LCA | 21.9 ± 4.01 | 0.671 ± 0.409 | 0.136 ± 0.047* |
| TCPOBOP + LCA | 30.4 ± 10.7 | 0.038 ± 0.009 | 0.266 ± 0.038* |
, indicates p ≤ 0.05 compared with CO.
Fig. 3.
Hydroxylated bile acid concentrations in the liver. Animals (n = 4–6/group) were pretreated with activators and LCA (125 mg/kg twice daily) as described under Materials and Methods. Results are presented as mean concentration ± S.E.M. *, indicates p ≤ 0.05 compared with respective CO; †, indicates p ≤ 0.05 compared with respective LCA only.
Basal concentrations of dihydroxylated bile acids (DCA/CDCA, taurochenodeoxycholic acid, ursodeoxycholic acid) were similar between WT and CAR-null mice. LCA treatment increased the concentrations of dihydroxylated bile acids in both genotypes (WT 39-fold, CAR-null 32-fold). Pretreatment with PB or TCPOBOP in WT mice prevented the increase caused by LCA. In addition, the increase was not significantly reduced by PB or TCPOBOP pretreatment in CAR-null mice.
Basal concentrations of trihydroxylated bile acids (α-muricholic acid, β-muricholic acid, tauro-β-muricholic acid, TCA, glycocholic acid, CA) tended to be slightly higher in CAR-null mice compared with WT controls. Concentrations of trihydroxylated bile acids were highest in LCA-treated mice of both genotypes and reduced by pretreatments in WT and CAR-null mice.
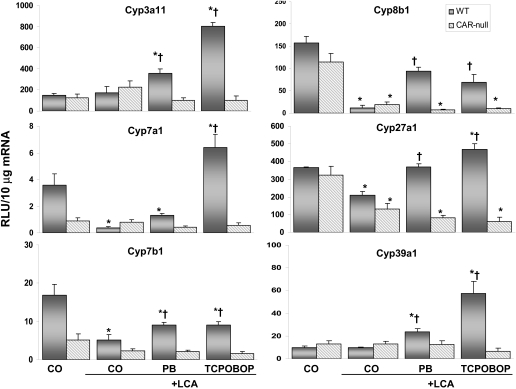
Hepatic mRNA Expression: Phase I Enzymes. Expression of several enzymes involved in bile acid biosynthesis is shown in Fig. 4. No changes in basal Cyp3a11 expression were observed between genotypes. Likewise, LCA did not alter the expression of Cyp3a11 in mice of either genotype. However, pretreatment with PB or TCPOBOP increased Cyp3a11 expression by 2.1- and 4.7-fold, respectively, in WT mice. The mRNA levels of Cyp3a11 were unchanged in all the groups of CAR-null mice. Cyp2b10, the hallmark gene product of CAR activation, was increased in WT mice pretreated with PB (11-fold) and TCPOBOP (116-fold) compared with both CO control and LCA-only groups (data not shown).
Fig. 4.
Expression of phase I bile acid biosynthesis and metabolizing genes. Hepatic Cyp3a11, Cyp7a1, Cyp7a1, Cyp8a1, Cyp27a1, and Cyp39a1 mRNA levels in each treatment group were quantified by the bDNA signal amplification assay, as described under Materials and Methods. Data are expressed as relative light units (RLU) ± S.E.M. *, indicates p ≤ 0.05 compared with respective CO; †, indicates p ≤ 0.05 compared with respective LCA only.
Basal expression of Cyp7a1 (rate-limiting enzyme involved in conversion of cholesterol into bile acids) was reduced 75% in CAR-null mice compared with WT controls. Expression was also reduced 89% in LCA-treated WT mice but was increased 1.8-fold with TCPOBOP pretreatment. Expression of Cyp7a1 was similar between treatment groups of CAR-null mice. In WT mice, LCA reduced or maintained the expression of Cyp7b1, Cyp8b1, Cyp27a1, and Cyp39a1, but all were significantly up-regulated by both PB and TCPOBOP pretreatments. Basal expression of Cyp7b1, which is involved in the formation of dihydroxylated CDCA, was reduced 69% in CAR-null mice compared with WT controls. In WT mice, Cyp7b1 expression was also reduced 69% by LCA and partially restored by PB and TCPOBOP pretreatments. LCA alone or in combination with CAR activators did not alter Cyp7b1 expression in CAR-null mice.
Basal expression of Cyp8b1 was reduced 27% in CAR-null mice compared with WT controls. The amount of Cyp8b1 present determines the ratio of primary bile acids, CA and CDCA. With that in mind, the expression of Cyp8b1 was reduced 93% by LCA treatment in WT mice, indicating a shift toward CDCA formation and the downstream synthesis of highly toxic LCA. In contrast, pretreatment with PB and TCPOBOP was able to maintain Cyp8b1 expression near control levels, thus preserving the formation of trihydroxylated CA. Cyp8b1 mRNA in CAR-null mice was decreased by LCA (83%) and was similarly low in mice pretreated with CAR activators.
Basal levels of Cyp27a1, which is involved in the formation of oxysterols via the alternative bile acid pathway, were unchanged between genotypes. Cyp27a1 expression was reduced 43% by LCA in WT mice. Conversely, expression was maintained by PB and elevated by TCPOBOP pretreatments (1.3-fold). In CAR-null mice, Cyp27a1 mRNA was lowered by LCA (59%) treatment. This decrease in CYP27a1 in CAR-null mice was not prevented by PB or TCPOBOP pretreatments. Basal expression of Cyp39a1, another enzyme involved in the alternative pathway, was also unchanged between genotypes. Cyp39a1 expression was maintained at basal levels by LCA treatment but increased by PB (2.5-fold) and TCPOBOP (6.0-fold) pretreatments, whereas expression was maintained at basal levels in CAR-null mice.
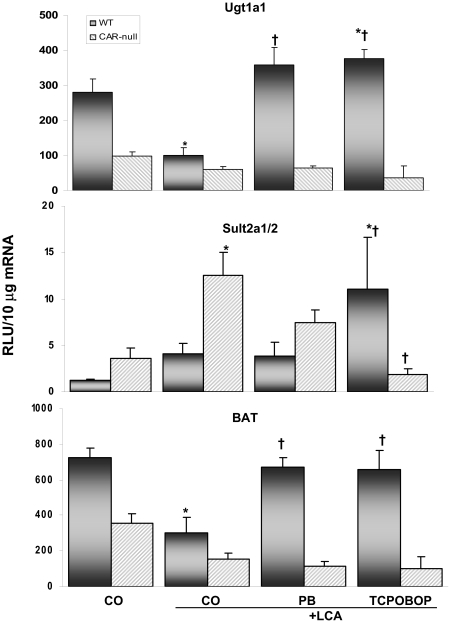
Hepatic mRNA Expression: Phase II Enzymes. Figure 5 shows the expression of three phase II enzymes involved in bile acid metabolism. Glucuronidation of bile acids increases their water solubility and enhances excretion. In WT mice, Ugt1a1 expression was reduced 65% by LCA, indicating a reduction in the ability to detoxify bile acids. However, pretreatment with PB and TCPOBOP in combination with LCA increased Ugt1a1 expression 3.6- and 3.8-fold, respectively, above LCA alone. Expression of Ugt1a1 was similarly low between groups of CAR-null mice. Expression of Sult2a1/2, the enzyme responsible for adding a sulfate moiety to LCA to render it less toxic, was up-regulated 3.5-fold by LCA in CAR-null mice but not significantly changed in WT mice. Sult2a1/2 is regulated by CAR activators, and, not surprisingly, expression was elevated 9.4-fold above basal levels by TCPOBOP pretreatment in WT mice (Assem et al., 2004), whereas expression of Sult2a1/2 by CAR activators in CAR-null mice was not increased. Expression of BAT, the enzyme responsible for adding an amino acid (glycine or taurine) to bile acids to increase their aqueous solubility and excretion, was reduced 58% by LCA, whereas the ability to reduce the toxicity of bile acids via conjugation with BAT was maintained in mice pretreated with PB and TCPOBOP as expression remained near basal levels. Expression of BAT was lower in CAR-null mice and was not significantly different among groups.
Fig. 5.
Expression of phase II bile acid-conjugating genes. Hepatic Sult2A1/2, Ugt1A1, and BAT mRNA levels in each treatment group were quantified by the bDNA signal amplification assay, as described under Materials and Methods. Data are expressed as relative light units (RLU) ± S.E.M. *, indicates p ≤ 0.05 compared with respective CO; †, indicates p ≤ 0.05 compared with respective LCA-only.
Discussion
The ability of CAR to regulate bile acid metabolism highlights its importance in mitigating cholestasis. In this study, the role of CAR in regulating genes involved in bile acid biosynthesis during intrahepatic cholestasis was examined as a possible mechanism for hepatoprotection. In addition, this is the first study to comprehensively characterize the changes in total and individual liver bile acids in WT and CAR-null mice during LCA-induced liver injury. These data show CAR-mediated hepatoprotection in PB- and TCPOBOP-pretreated WT mice. Histopathology in the hepatoprotected groups correlates with decreased serum alanine aminotransferase levels as observed in previous studies (Beilke et al., 2008). In contrast, the absence of CAR during LCA treatment results in severe liver damage, thus underscoring the necessity of CAR during hepatoprotection.
Liver histology evaluated in the CAR activators used in the present study (PB, TCPOBOP) has been similarly reported in previous studies to be similar to controls (Annapurna et al., 1989; Davies et al., 1991; Huang et al., 2005; Baskin-Bey et al., 2007). The hepatoprotection observed in WT mice in the current study is consistent with findings from Zhang et al. (2004), who showed that pretreatment with TCPOBOP protects mice from 5 days of LCA administration (250 mg/kg). Likewise, activation of CAR by TCPOBOP conferred protection against LCA-induced hepatotoxicity in mice (Saini et al., 2004). Further support of the hepatoprotective effects of CAR activation during cholestasis is suggested in the present study by the decrease in individual serum bile acids quantified in the hepatoprotected PB- and TCPOBOP-pretreated WT mice compared with those receiving LCA only. In addition, observed was a decrease in total hepatic bile acid concentrations in PB- and TCPOBOP-pretreated mice compared with LCA alone. These findings are consistent with previous findings where total hepatic bile acid concentrations were significantly lower in PB- and TCPOBOP-hepatoprotected mice compared with those treated with LCA alone (Beilke et al., 2008). As mentioned, several studies have shown the hepatoprotective properties of CAR during cholestasis; however, the mechanism underlying the protection has not been fully elucidated.
The increase in total liver bile acids in both genotypes of LCA-treated mice correlates with reduced expression of Cyp7a1, as would be expected from the negative feedback regulation of bile acids. As bile acid concentrations increase, FXR is activated to decrease the production of bile acids via decreased Cyp7a1. Following this logic, one would expect to see an increase in FXR expression; however, expression of FXR was not increased in either genotype of LCA-treated mice. Possible explanations for this lack of effect include the idea that expression of FXR many not directly translate into protein activity and/or the degree of expression present was sufficient to regulate Cyp7a1. It is interesting to note that FXR expression was significantly reduced in all the groups of CAR-null mice, suggesting a role for CAR in the regulation of FXR. This also indicates that hepatic bile acid concentrations are not the only factors involved in activation of FXR. Considering the complexity of bile acid regulation with the involvement of multiple nuclear receptors (e.g., LXR, pregnane X receptor, vitamin D receptor), it is not surprising that CAR may work coordinately with FXR to regulate bile acids.
Early research has suggested that the toxicity of bile acids is inversely proportional to the number of hydroxyl groups on the steroid nucleus (Schölmerich et al., 1984). With that in mind, we speculated that hepatoprotection via activation of CAR might occur as a result of altered bile acid composition in the form of reduced individual bile acids. Indeed, the increase in monohydroxylated and dihydroxylated bile acid concentrations caused by LCA was reduced in the protected PB- and TCPOBOP-pretreated mice. Monohydroxylated bile acids are considered the most toxic bile acids; thus, the 4-fold increase above basal levels in LCA-treated WT mice most likely contributes to the multifocal hepatic necrosis that was observed in these mice (Fig. 1). The significant increase in monohydroxylated bile acids observed in LCA-only treated mice of both genotypes is also consistent with an increase in total bile acid concentrations. However, WT mice pretreated with PB or TCPOBOP were able to maintain basal concentrations of individual bile acids to near basal levels, whereas in CAR-null mice, both monohydroxylated and dihydroxylated bile acids were increased above control levels. Individual bile acid concentrations were also measured in the serum and urine of WT mice; however, minimal changes were observed. There was a general trend for increased taurine-conjugated bile acids in the serum of LCA-treated mice compared with control, PB, or TCPOBOP groups. In contrast to humans, conjugation of bile acids occurs more predominantly with taurine rather than glycine in mice. An increase in serum or urine bile acid concentrations may be expected as an indicator of elimination; however, bile acid concentrations were already decreased in the livers of protected mice, thus alleviating the need to excrete bile acids into serum or urine.
Individual bile acids are regulated by several P450s. The most biologically significant change in gene expression produced by the CAR activators was that of Cyp8b1, which is responsible for producing the primary bile acid, CA. Trihydroxylated bile acids such as CA are less toxic to cell membranes than other bile acid species with fewer hydroxyl groups. Cyp8b1 expression was significantly reduced by LCA treatment in both genotypes compared with CO controls. However, with the addition of PB and TCPOBOP pretreatment, the reduction caused by LCA treatment was absent, and expression was maintained near basal levels. The maintenance of Cyp8b1 expression in PB- and TCPOBOP-pretreated mice correlated with histologic hepatoprotection in these groups and implies a shift in the bile acid biosynthesis pathway toward the formation of CA and other less toxic dihydroxylated and trihydroxylated bile acids (Fig. 6). The fact that Cyp8b1 expression was not completely restored to basal levels in PB- and TCPOBOP-pretreated mice may explain why dihydroxylated and trihydroxylated bile acid concentrations were not increased further.
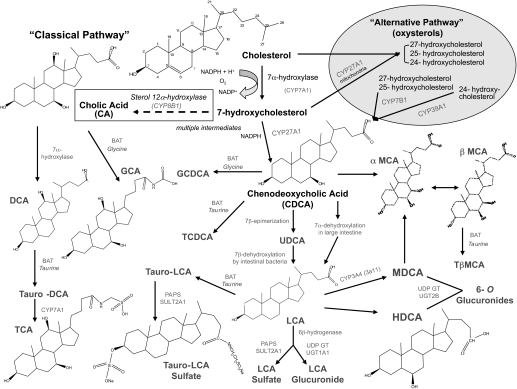
Fig. 6.
Simplified overview of the bile acid biosynthesis pathway derived from cholesterol. The neutral (classic) and acidic (alternative) routes are shown with the main enzymes involved. The 7α-hydroxylation of cholesterol by CYP7A1 is the rate-limiting enzyme in the neutral pathway. The neutral pathway is considered the most important pathway for bile acid formation in humans, whereas the acidic pathway is important for the removal of cholesterol from extrahepatic tissues, and it seems to be able to compensate for the neutral pathway when it is repressed to maintain bile acid formation. Boxed area highlights the shift in biosynthesis via up-regulation of Cyp8b1 in hepatoprotected mice. (Note: not all the bile acids, enzymes, or intermediate steps shown.) Chemical structure sources: http://sigmaaldrich.com, http://steraloids.com.
It is interesting to note that the hepatic concentration of CDCA was also decreased in the two hepatoprotected groups compared with LCA alone. This may be the result of increased or maintained expression of Cyp7b1 and Cyp27a1, which would shift the bile acid synthesis route toward the oxysterol route, thereby indirectly decreasing the amount of CDCA produced. Cyp7b1 and Cyp27a1, along with Cyp3a11 and Cyp39a1, were maintained and/or increased in the hepatoprotected PB- and TCPOBOP-pretreated WT mice compared with LCA-only mice, whereas these changes were not observed in CAR-null mice. Thus, CAR may regulate the expression of selected P450 isoforms involved in the formation of primary bile acids. Further examination of this effect is required to definitively determine whether CAR has a direct role in the regulation of these CYP genes, or whether CAR indirectly alters other transcriptional mechanisms by affecting their signaling cascades. The lack of relevant changes in bile acid biosynthesis genes along with the significant hepatocellular necrosis in CAR-null mice further supports the importance of bile acid composition in the hepatoprotection from bile acid-induced toxicity.
The ability to conjugate bile acids was generally maintained or increased in hepatoprotected mice. Ugt1a1 expression was increased in hepatoprotected mice, and it is important for the glucuronidation of bilirubin, as evidenced by Crigler-Najjar syndrome, in which there is a complete loss of Ugt1a1 function that results in severe hyperbilirubinemia that can be fatal if left untreated (Jansen, 1999). Expression of Sult2a1/2 was up-regulated in WT mice pretreated with TCPOBOP, which could contribute to the decreased hepatotoxicity observed in this group. However, expression of Sult2a1/2 was not similarly up-regulated in mice pretreated with PB. This indicates that sulfation of bile acids may aid, but not cause, the overall protection from LCA-induced toxicity. Sult2a1/2 was also increased in LCA-treated CAR-null mice, which is consistent with hydroxysteroid sulfotransferase-mediated LCA sulfation as a major pathway for protection against LCA-induced liver damage (Kitada et al., 2003). Because LXR has been associated with increased expression of Sult2a, it is likely that the increase observed in CAR-null mice was a result of LXR (Uppal et al., 2007).
Expression of BAT, the enzyme involved in the amidation of bile acids, was maintained at control levels by PB and TCPOBOP pretreatments compared with LCA. In mice, BAT primarily conjugates bile acids with taurine (Shonsey et al., 2005), as evidenced in Table 1 by the higher individual concentrations of taurine-conjugated bile acids compared with glycine (e.g., taurochenodeoxycholic acid versus glycochenodeoxycholic acid and TCA versus glycocholic acid). Up-regulation of bile acid-conjugating enzymes also correlates with increased urinary excretion of dihydroxylated and trihydroxylated bile acids in the TCPOBOP-pretreated mice. These data are consistent with previous reports on the importance of CAR on conjugation enzymes during hepatoprotection (Guo et al., 2003; Huang et al., 2003; Wagner et al., 2005).
Taken together, these data show a correlation between hepatoprotection and decreased total and individual bile acid concentrations, effects not observed in CAR-null mice. Several bile acid biosynthesis genes were up-regulated by strong CAR activators in hepatoprotected mice, and the lack of these expression changes in CAR-null mice suggests potential downstream control by CAR. The maintenance of Cyp8b1 in hepatoprotected mice indicates a possible shift in the bile acid biosynthesis pathway to the formation of CA and other less toxic bile acids, which may be a contributing factor to the protection afforded by CAR. These novel findings add to the body of knowledge surrounding bile acid-induced toxicity during cholestasis and indicate that the regulation of bile acid biosynthesis by CAR may contribute as a mechanism of hepatoprotection from bile acid-induced toxicity.
Supplementary Material
Acknowledgments
The authors would like to sincerely thank Dr. Katerina Dvorak, Hana Holubec, and Lisa Augustine for technical assistance with portions of the manuscript. The views presented in this article do not necessarily reflect those of the U.S. Food and Drug Administration.
This work was supported in part by the National Institutes of Health National Institute of Diabetes and Digestive and Kidney Diseases [Grant DK068039]; and the National Institutes of Health National Institute of Environmental Health Sciences [Grant ES011646].
Parts of this work were previously presented at the following meeting: Beilke L, Holland R, Besselsen D, Beger R, and Cherrington N (2007) Induction of drug-metabolizing genes during lithocholic acid-induced intrahepatic cholestasis alters individual bile acid concentrations. Mountain West Regional Chapter of the Society of Toxicology Annual Meeting; 2007 Sept 6–7; Breckenridge, CO. Society of Toxicology, Reston, VA.
Article, publication date, and citation information can be found at http://dmd.aspetjournals.org.
doi:10.1124/dmd.108.023317.
ABBREVIATIONS: CA, cholic acid; CDCA, chenodeoxycholic acid; DCA, deoxycholic acid; LCA, lithocholic acid; TCA, taurocholic acid; FXR, farnesoid X receptor; P450, cytochrome P450; CAR, constitutive androstane receptor; PB, phenobarbital; TCPOBOP, 1,4-bis[2,5-dichloropyridyloxy)]benzene; WT, wild-type; CAR-null, CAR knockout; CO, corn oil; HPLC, high-performance liquid chromatography; ESI, electrospray ionization; BAT, bile acid-CoA amino acid N-acyltransferase; bDNA, branched DNA.
The online version of this article (available at http://dmd.aspetjournals.org) contains supplemental material.
References
- Alnouti Y and Klaassen CD (2006) Tissue distribution and ontogeny of sulfotransferase enzymes in mice. Toxicol Sci 93 242–255. [DOI] [PubMed] [Google Scholar]
- Annapurna V, Mukundan M, Sesikeran B, and Bamji M (1989) Effects of ovulen-50, diethylnitrosamine and phenobarbital on liver regeneration in female rats. J Biosci 14 1–7. [Google Scholar]
- Araya Z and Wikvall K (1999) 6alpha-Hydroxylation of taurochenodeoxycholic acid and lithocholic acid by CYP3A4 in human liver microsomes. Biochim Biophys Acta 1438 47–54. [DOI] [PubMed] [Google Scholar]
- Assem M, Schuetz EG, Leggas M, Sun D, Yasuda K, Reid G, Zelcer N, Adachi M, Strom S, Evans RM, et al. (2004) Interactions between hepatic Mrp4 and SULT2a1/2 as revealed by the constitutive androstane receptor and Mrp4 knockout mice. J Biol Chem 279 22250–22257. [DOI] [PubMed] [Google Scholar]
- Baskin-Bey E, Anan A, Isomoto H, Bronk S, and Gores G (2007) Constitutive androstane receptor agonist, TCPOBOP, attenuates steatohepatitis in the methionine choline-deficient diet-fed mouse. World J Gastroenterol 13 5635–5641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beilke LD, Besselsen DG, Cheng Q, Kulkarni S, Slitt AL, and Cherrington NJ (2008) Minimal role of hepatic transporters in the hepatoprotection against LCA-induced intrahepatic cholestasis. Toxicol Sci 102 196–204. [DOI] [PubMed] [Google Scholar]
- Bohan A, Chen WS, Denson LA, Held MA, and Boyer JL (2003) Tumor necrosis factor a-dependent up-regulation of Lrh-1 and Mrp3 (Abcc3) reduces liver injury in obstructive cholestasis. J Biol Chem 278 36688–36698. [DOI] [PubMed] [Google Scholar]
- Buckley DB and Klaassen CD (2007) Tissue- and gender-specific mRNA expression of UDP-glucuronosyltransferases (UGTs) in mice. Drug Metab Dispos 35 121–127. [DOI] [PubMed] [Google Scholar]
- Davies M, Schamber G, and Schnell R (1991) Oltipraz-induced amelioration of acetaminophen hepatotoxicity in hamsters. I. Lack of dependence on glutathione. Toxicol Appl Pharmacol 109 17–28. [DOI] [PubMed] [Google Scholar]
- Daykin CA, Foxall PJ, Connor SC, Lindon JC, and Nicholson JK (2002) The comparison of plasma deproteinization methods for the detection of low-molecular weight metabolites by 1H nuclear magnetic resonance spectroscopy. Anal Biochem 304 220–230. [DOI] [PubMed] [Google Scholar]
- Fickert P, Fuchsbichler A, Marschall HU, Wagner M, Zollner G, Krause R, Zatloukal K, Jaeschke H, Denk H, and Trauner M (2006) Lithocholic acid feeding induces segmental bile duct obstruction and destructive cholangitis in mice. Am J Pathol 168 410–422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo GL, Lambert G, Negishi M, Ward JM, Brewer HB Jr, Kliewer SA, Gonzalez FJ, and Sinal CJ (2003) Complementary roles of farnesoid X receptor, pregnane X receptor, and constitutive androstane receptor in protection against bile acid toxicity. J Biol Chem 278 45062–45071. [DOI] [PubMed] [Google Scholar]
- Huang W, Zhang J, Chua SS, Qatanani M, Han Y, Granata R, and Moore DD (2003) Induction of bilirubin clearance by the constitutive androstane receptor (CAR). Proc Natl Acad Sci U S A 100 4156–4161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang W, Zhang J, Washington M, Liu J, Parant JM, Lozano G, and Moore DD (2005) Xenobiotic stress induces hepatomegaly and liver tumors via the nuclear receptor constitutive androstane receptor. Mol Endocrinol 19 1646–1653. [DOI] [PubMed] [Google Scholar]
- Jansen PL (1999) Diagnosis and management of Crigler-Najjar syndrome. Eur J Pediatr 158 S89–S94. [DOI] [PubMed] [Google Scholar]
- Kakizaki S, Yamazaki Y, Takizawa D, and Negishi M (2008) New insights on the xenobiotic-sensing nuclear receptors in liver disease–CAR and PXR–. Curr Drug Metab 9 614–621. [DOI] [PubMed] [Google Scholar]
- Kitada H, Miyata M, Nakamura T, Tozawa A, Honma W, Shimada M, Nagata K, Sinal CJ, Guo GL, Gonzalez FJ, et al. (2003) Protective role of hydroxysteroid sulfotransferase in lithocholic acid-induced liver toxicity. J Biol Chem 278 17838–17844. [DOI] [PubMed] [Google Scholar]
- Maher JM, Cheng X, Slitt AL, Dieter MZ, and Klaassen CD (2005) Induction of the multidrug resistance-associated protein family of transporters by chemical activators of receptor-mediated pathways in mouse liver. Drug Metab Dispos 33 956–962. [DOI] [PubMed] [Google Scholar]
- Mennone A, Soroka CJ, Cai SY, Harry K, Adachi M, Hagey L, Schuetz JD, and Boyer JL (2006) Mrp4–/– mice have an impaired cytoprotective response in obstructive cholestasis. Hepatology 43 1013–1021. [DOI] [PubMed] [Google Scholar]
- National Research Council (1996) Guide for the Care and Use of Laboratory Animals, National Academy Press, Washington, DC.
- Norlin M and Wikvall K (2007) Enzymes in the conversion of cholesterol into bile acids. Curr Mol Med 7 199–218. [DOI] [PubMed] [Google Scholar]
- Radominska A, Treat S, and Little J (1993) Bile acid metabolism and the pathophysiology of cholestasis. Semin Liver Dis 13 219–234. [DOI] [PubMed] [Google Scholar]
- Russell DW (2003) The enzymes, regulation, and genetics of bile acid synthesis. Annu Rev Biochem 72 137–174. [DOI] [PubMed] [Google Scholar]
- Saini SP, Sonoda J, Xu L, Toma D, Uppal H, Mu Y, Ren S, Moore DD, Evans RM, and Xie W (2004) A novel constitutive androstane receptor-mediated and CYP3A-independent pathway of bile acid detoxification. Mol Pharmacol 65 292–300. [DOI] [PubMed] [Google Scholar]
- Schölmerich J, Becher MS, Schmidt K, Schubert R, Kremer B, Feldhaus S, and Gerok W (1984) Influence of hydroxylation and conjugation of bile salts on their membrane-damaging properties—studies on isolated hepatocytes and lipid membrane vesicles. Hepatology 4 661–666. [DOI] [PubMed] [Google Scholar]
- Shonsey EM, Sfakianos M, Johnson M, He D, Falany CN, Falany J, Merkler DJ, and Barnes S (2005) Bile acid coenzyme A: amino acid N-acyltransferase in the amino acid conjugation of bile acids. Methods Enzymol 400 374–394. [DOI] [PubMed] [Google Scholar]
- Staudinger J, Goodwin B, Jones S, Hawkins-Brown D, MacKenzie K, LaTour A, Liu Y, Klaassen C, Brown K, Reinhard J, et al. (2001) The nuclear receptor PXR is a lithocholic acid sensor that protects against liver toxicity. Proc Natl Acad Sci U S A 98 3369–3374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stedman C, Liddle C, Coulter S, Sonoda J, Alvarez J, Moore D, Evans R, and Downes M (2005) Nuclear receptors constitutive androstane receptor and pregnane X receptor ameliorate cholestatic liver injury. Proc Natl Acad Sci U S A 102 2063–2068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stedman C, Robertson G, Coulter S, and Liddle C (2004) Feed-forward regulation of bile acid detoxification by CYP3A4: studies in humanized transgenic mice. J Biol Chem 279 11336–11343. [DOI] [PubMed] [Google Scholar]
- Teng S and Piquette-Miller M (2007) Hepatoprotective role of PXR activation and MRP3 in cholic acid-induced cholestasis. Br J Pharmacol 151 367–376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ueda A, Hamadeh HK, Webb HK, Yamamoto Y, Sueyoshi T, Afshari CA, Lehmann JM, and Negishi M (2002) Diverse roles of the nuclear orphan receptor CAR in regulating hepatic genes in response to phenobarbital. Mol Pharmacol 61 1–6. [DOI] [PubMed] [Google Scholar]
- Uppal H, Saini S, Moschetta A, Mu Y, Zhou J, Gong H, Zhai Y, Ren S, Michalopoulos G, Mangelsdorf D, et al. (2007) Activation of LXRs prevents bile acid toxicity and cholestasis in female mice. Hepatology 45 422–432. [DOI] [PubMed] [Google Scholar]
- Wagner M, Halilbasic E, Marschall HU, Zollner G, Fickert P, Langner C, Zatloukal K, Denk H, and Trauner M (2005) CAR and PXR agonists stimulate hepatic bile acid and bilirubin detoxification and elimination pathways in mice. Hepatology 42 420–430. [DOI] [PubMed] [Google Scholar]
- Yu J, Lo JL, Huang L, Zhao A, Metzger E, Adams A, Meinke PT, Wright SD, and Cui J (2002) Lithocholic acid decreases expression of bile salt export pump through farnesoid X receptor antagonist activity. J Biol Chem 277 31441–31447. [DOI] [PubMed] [Google Scholar]
- Zhang J, Huang W, Qatanani M, Evans RM, and Moore DD (2004) The constitutive androstane receptor and pregnane X receptor function coordinately to prevent bile acid-induced hepatotoxicity. J Biol Chem 279 49517–49522. [DOI] [PubMed] [Google Scholar]
- Zollner G, Fickert P, Fuchsbichler A, Silbert D, Wagner M, Arbeiter S, Gonzalez FJ, Marschall HU, Zatloukal K, Denk H, et al. (2003) Role of nuclear bile acid receptor, FXR, in adaptive ABC transporter regulation by cholic and ursodeoxycholic acid in mouse liver, kidney and intestine. J Hepatol 39 480–488. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.