Abstract
The association of CYP2C9 and VKORC1 1173C/T genotype and risk of hemorrhage among African Americans and European Americans is presented. This association was evaluated using Cox proportional hazard regression with adjustment for demographics, comorbidity, and time-varying covariates. Forty-four major and 203 minor hemorrhages occurred over 555 person-years among 446 patients (60.67±15.6 years, 50% men, 227 African Americans). The variant CYP2C9 genotype conferred an increased risk for major (hazard ratio (HR) 3.0; 95% confidence interval (CI): 1.1–8.0) but not minor (HR 1.3; 95% CI: 0.8–2.1) hemorrhage. The risk of major hemorrhage was 5.3-fold (95% CI: 0.4–64.0) higher before stabilization of therapy, 2.2-fold (95% CI: 0.7–6.5) after stabilization, and 2.4-fold (95% CI: 0.8–7.4) during all periods when anticoagulation was not stable. The variant VKORC1 1173C/T genotype did not confer a significant increase in risk for major (HR 1.7; 95% CI: 0.7–4.4) or minor (HR 0.8; 95% CI: 0.5–1.3) hemorrhage. The variant CYP2C9 genotype is associated with an increased risk of major hemorrhage, which persists even after stabilization of therapy.
Thromboembolic disorders are significant contributors to morbidity and mortality.1 Although the efficacy of warfarin in the treatment and prevention of thromboembolic disorders is proven,2-4 it is vastly underutilized,5,6 with difficulties in management5,7 and risk of complications being the main deterrents.7,8 Recognition of genetic regulation of warfarin response has fueled efforts aimed at quantifying this influence, but past efforts have focused on a few cytochrome P450 2C9 (CYP2C9) alleles in largely white populations.9-13 Outcome definitions varied significantly, with several studies addressing risk of over-anticoagulation,12,13 whereas others analyzed hemorrhagic complications retrospectively.9-11 Exclusion of underrepresented ethnic groups and inability to address factors such as concurrent medications and comorbid conditions9-13 limit the generalizability of study results. Although these studies have enhanced our understanding of the association of CYP2C9 and hemorrhagic complications, a prospective study in racially representative population is lacking. A recent report indicates the significance of 1173C/T polymorphism in the vitamin K epoxide reductase complex 1 (VKORC1) gene in explaining variability in warfarin dose requirements among both European-American and African-American patients.14 However, the influence of VKORC1 polymorphisms in risk of hemorrhagic complications is lacking.
Herein we present the association of CYP2C9 and VKORC1 1173C/T (rs9934438) genotypes and risk of hemorrhagic complications among African Americans and European Americans on warfarin therapy.
RESULTS
All patients meeting eligibility criteria (n=526) were asked to participate. The age, gender, and racial makeup of patients declining participation (n=36, 17 African Americans, 21 women) did not differ from that of patients enrolled. Four hundred and ninety patients (mean age, 60.6 years; SD, 15.6 years) were recruited between August 2003 and August 2006. African-American patients comprise 48% of the cohort and men 51.3% with an average of 14.9 (±10.7) months of follow-up accrued.
CYP2C9 genotype was determined for 446 patients. Two patients, one Hispanic and one African-American man with a previously unreported polymorphism (G1078A) in the same region as the CYP2C9*3 mutation, were excluded from the analysis assessing the Hardy—Weinberg equilibrium (HWE). The distribution of CYP2C9 alleles differed by race (P<0.0001) but not by gender (P=0.13, data not shown). The frequency of variant CYP2C9 genotype (Table 1) is significantly higher in European Americans than African Americans (29.8 vs 9.7%, P<0.0001). CYP2C9 genotype frequencies were found to be in HWE among European Americans (P>0.45) and African Americans (P>0.75).
Table 1.
CYP2C9 and VKORC1 1173C/T genotype distribution in participants of the POAT cohorta
| All participants | African Americans |
European Americans |
|
|---|---|---|---|
| Genotype CYP2C9 |
N=444 No. of positive (%) |
N=226 No. of positive (%) |
N=218 No. of positive (%) |
| *1/*1 | 357 (80.405) | 204 (90.265) | 153 (70.183) |
| *1/*2 | 45 (10.135) | 5 (2.212) | 40 (18.349) |
| *1/*3 | 26 (5.855) | 7 (3.097) | 19 (8.715) |
| *1/*5 | 3 (0.676) | 3 (1.327) | 0 (0.000) |
| *1/*6 | 0 (0.000) | 0 (0.000) | 0 (0.000) |
| *1/*10 | 0 (0.000) | 0 (0.000) | 0 (0.000) |
| *1/*11 | 5 (1.126) | 5 (2.212) | 0 (0.000) |
| *2/*2 | 3 (0.676) | 0 (0.000) | 3 (1.376) |
| *2/*3 | 3 (0.676) | 0 (0.000) | 3 (1.376) |
| *3/*6 | 1 (0.225) | 1 (0.442) | 0 (0.000) |
| *5/*6 | 1 (0.225) | 1 (0.442) | 0 (0.000) |
| VKORC1 | N=441 | N=225 | N=216 |
|---|---|---|---|
| CC | 262 (59.4) | 181 (80.4) | 81 (37.5) |
| CT | 150 (34.0) | 42 (18.7) | 108 (50.0) |
| TT | 29 (6.6) | 2 (0.9) | 27 (12.5) |
*V denotes variant allele (2, *3, *5, *6, *10, or *11).
Subjects recruited during the interval August 2003 to August 2006. Analysis excludes one Hispanic patient and one African-American patient with the G1078A mutation. VKORC1 1173C/T genotype determined for 441 patients.
VKORC1 1173C/T was determined for 441 patients (Table 1). The variant VKORC1 genotype is more frequent among European Americans compared to African Americans (and T allele, 62.5 vs 19.6%, P<0.0001). VKORC1 1173C/T frequencies were found to be in HWE among both European Americans (exact statistic P=0.69) and African Americans (exact statistic, P=0.99).
There were no significant differences in gender, body mass index, indication for therapy, comorbidity, concomitant medications, level of physical activity, or smoking status by CYP2C9 genotype among study participants (Table 2). Patients with the variant CYP2C9 genotype consumed more alcohol (P=0.018) and vitamin K-rich foods (P=0.008) and had a higher frequency of VKORC1 1173C/T variant. The occurrence of minor or major hemorrhagic complications did not differ by race (P=0.66 and 0.5, respectively), gender (P=0.16 and 0.39, respectively), or across VKORC1 genotype (P=0.38 and 0.75, respectively). Patients with variant CYP2C9 genotype experienced major (P=0.0024) but not minor (P=0.15) complications more frequently than the wild-type genotype (Table 3).
Table 2.
Demographic and clinical characteristics of patients participating in the POAT cohort by variant CYP2C9 genotype
|
CYP2C9 genotype |
|||
|---|---|---|---|
| Variable | Wild type (N=358) |
Variant (N=88) |
P-values |
| Age (years) | 60.1 (±16.0) | 62.9 (±14.0) | 0.14 |
| BMI | 29.8 (±7.5) | 28.4 (±7.0) | 0.11 |
| Follow-up time | |||
| Mean (±SD) | 14.5 (10.7) | 16.4 (10.5) | 0.15 |
| Median (range) | 12.0 (0.0–47.0) | 15.0 (1.0–38.0) | - |
| Average alcohol intakea | 1.3 (3.2) | 2.3 (3.7) | 0.018 |
| Average level of physical activityb |
3.37 (0.9) | 3.5 (0.8) | 0.30 |
| Average vitamin K intakec | 1.7 (1.0) | 2.0 (1.2) | 0.008 |
| Average warfarin dose (mg/day) | |||
| Mean (±SD) | 5.8 (±2.53) | 4.4 (±1.92) | <0.0001 |
| Median (range) | 5.3 (1.3–20.9) | 4.3 (1.1–10.4) | - |
|
CYP2C9 genotype |
|||
|---|---|---|---|
| Variable | N(%) | N(%) | P-values |
| Race | |||
| African American | 204 (57.0) | 23 (26.1) | <0.0001 |
| European American | 153 (42.7) | 65 (73.9) | - |
| Hispanic | 1 (0.3) | 0 (0.0) | - |
| Gender | |||
| Female | 186 (51.0) | 37 (42.0) | 0.10 |
| Male | 172 (48.0) | 51 (58.0) | - |
| Indicationd,e | |||
| Arterial thromboembolism | 152 (42.5) | 44 (50.0) | 0.20 |
| Venous thromboembolism | 148 (41.3) | 29 (32.9) | 0.15 |
| None | 62 (17.3) | 16 (18.2) | 0.85 |
| Comorbidityf | |||
| Low (<2 comorbid conditions) |
119 (33.2) | 23 (26.1) | 0.11 |
| Moderate (2–4 comorbid conditions) |
159 (44.4) | 50 (56.8) | - |
| High (>44 comorbid conditions) |
80 (22.4) | 15 (17.1) | - |
| Medicationsd,g | |||
| CYP2C9 inducers | 5 (2.0) | 0 (0.0) | 0.26 |
| CYP2C9 inhibitors | 124 (34.6) | 28 (31.8) | 0.61 |
| CYP2C9 substrates | 139 (38.8) | 27 (30.7) | 0.16 |
| NSAID | 51 (14.2) | 11 (12.5) | 0.67 |
| Antiplatelet agents | 197 (55.0) | 58 (65.9) | 0.06 |
| Smoking statush | |||
| Current | 53 (14.8) | 11 (12.5) | 0.82 |
| Past | 134 (37.4) | 31 (35.2) | - |
| Never | 163 (45.5) | 43 (48.9) | - |
| - | |||
| VKORC1 1173C/Ti | |||
| CC | 219 (62.0) | 43 (48.9) | 0.024 |
| Any T | 134 (38.0) | 45 (51.1) | - |
BMI, body mass index; NSAID, non-steroidal anti-inflammatory drug. CYP2C9 genotype (wild type denotes *1/*1, V denotes at least one variant allele (*2, *3, *5, *6, *10, or *11)). One patient with a previously unreported polymorphism (G1078A) in the same region as the CYP2C9*3 mutation was categorized as variant.
Average alcohol intake is defined as number of alcoholic beverages (beer, wine, whiskey, liquor) consumed per week.
Average level of physical activity: we created a simple five-point physical activity scale: wheelchair bound (1), uses walker/cane (2), ambulates without assistance (3), physically active (4), and consistent exercise (5). The level of physical activity is then averaged over the study period.
Average vitamin K intake is defined as the equivalent number of servings of foods rich in vitamin K consumed per week.
Individual patients can have more than one indication for warfarin therapy listed and can be on multiple medications.
Arterial thromboembolism includes patients with myocardial infarction, stroke, or transient ischemic attack. Venous thromboembolism includes patients with deep vein thrombosis or pulmonary embolism. None includes patients with no prior thromboembolic events (e.g., atrial fibrillation, cardiomyopathy, low ejection fraction).
Level of comorbidity is calculated based on number of comorbid diseases in each individual patient. Comorbid conditions include hypertension, hyperlipidemia, atrial fibrillation, congestive heart failure, coronary artery disease, diabetes mellitus, malignancy, renal insufficiency, renal failure, peptic ulcer disease, and history of hemorrhage.
Medications: CYP2C9 inhibitors include amiodarone, fluconazole, fluvastatin, isoniazid, episodic use of antibiotics such as metronidazole and sulfamethoxazole. CYP2C9 inducers include phenobarbital, carbamazepine, and rifampin. CYP2C9 substrates include ibuprofen, diclofenac, losartan, irbesartan, glipizide, tolbutamide, fluvastatin, fluoxetine, and so on. NSAIDs include ibuprofen, naproxen, piroxicam, diclofenac, and so on. Antiplatelet agents include aspirin, clopidogrel, and dipyridamole.
Missing in 11 patients.
VKORC1 1173C/T assessed in 441 patients. All significant P-values (P<0.05) are in bold.
Table 3.
Incidence rate of hemorrhagic complications stratified by CYP2C9 and VKORC1 1173C/T genotype (per 100 patient-years)
|
CYP2C9 genotype |
||||
|---|---|---|---|---|
| Total (N=446) | Wild type (N=358) | Variant (N=88) | P-values | |
| Follow-up, person-years | 554.84 | 434.16 | 120.68 | — |
| Major hemorrhage | ||||
| Patients (n, %) | 37 (8.3%) | 22 (6.1%) | 15 (17.0%) | 0.0024 |
| Number of events | 44 | 25 | 19 | |
| Incidence rate | 7.93 (5.8–10.6) | 5.67 (3.8, 8.4) | 15.74 (9.8, 24.1) | 0.0015 |
| Minor hemorrhage | ||||
| Patients (n, %) | 115 (25.8%) | 87 (24.3%) | 28 (31.8%) | 0.15 |
| Number of events | 203 | 151 | 52 | |
| Incidence rate | 36.6 (31.8, 41.9) | 34.8 (29.6, 40.7) | 43.1 (32.5, 56.1) | 0.182 |
|
VKORC1 1173C/T genotypea |
||||
|---|---|---|---|---|
| Total (N=441) | “CC” (N=262) | Any “T” (N=179) | P-values | |
| Follow-up, person-years | 551.9 | 339.4 | 212.5 | — |
| Major hemorrhage | ||||
| Patients (n, %) | 37 (8.4%) | 21 (8.0%) | 16 (8.4%) | 0.75 |
| Number of events | 44 | 25 | 19 | |
| Incidence rate | 8.0 (5.8, 10.7) | 7.4 (4.8, 10.9) | 8.9 (5.4, 13.9) | 0.52 |
| Minor hemorrhage | ||||
| Patients (n, %) | 113 (25.6%) | 65 (24.8%) | 48 (26.8%) | 0.38 |
| Number of events | 201 | 125 | 76 | |
| Incidence rate | 36.5 (31.6, 41.8) | 36.8 (30.7, 43.9) | 35.8 (28.2, 44.8) | 0.84 |
CYP2C9 genotype (wild type denotes *1/*1, V denotes at least one variant allele (*2, *3, *5, *6, *10, or *11)). Minor hemorrhagic complications included mild nosebleeds (lasting less than 30 min), microscopic hematuria, mild bruising, and mild hemorrhoidal bleeding. Major hemorrhagic complications include serious, life-threatening and fatal bleeding episodes as defined by Fihn et al.47 Among patients with variant CYP2C9 genotype, nine major hemorrhages occurred in patients with *1/*3, eight in *1/*2, one in *2/*3, and one in *5/*6.
VKORC1 1173C/T determined for 441 patients. All significant P-values (P<0.05) are in bold.
Forty-four major (in 37 patients) and 203 (in 115 patients) minor hemorrhages were encountered during 554.84 years of follow-up resulting in an overall incidence rate of 7.93 (95% confidence interval (CI): 5.8–10.6) per 100 patient-years for major and 36.6 (95% CI: 31.8–41.9) per 100 patient-years for minor hemorrhage, respectively.
The incidence rate for minor hemorrhage was not significantly higher for patients with variant CYP2C9 genotype than wild-type genotype, as indicated by the unadjusted incidence rate ratio (IRR: 1.24, 95% CI: 0.9–1.7). The incidence rate of major hemorrhage was significantly higher among patients with variant CYP2C9 genotype than those with the wild-type genotype (15.7 vs 5.8 per 100 patient-years, P=0.0015, Table 3), resulting in an unadjusted IRR of 2.73 (95% CI: 1.5–5.0). The incidence rate for minor or major hemorrhage was not significantly different for patients with variant VKORC1 1173C/T compared to wild-type genotype (IRR: 0.98, 95% CI: 0.7–1.3 and 1.2, 95% CI: 0.7–2.1, respectively).
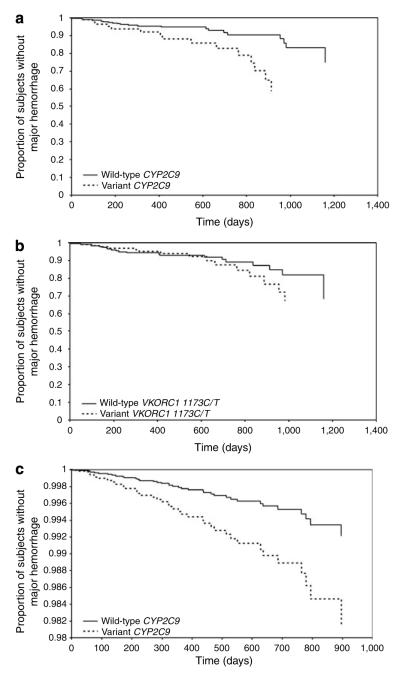
Major hemorrhage, fatal in four patients, most commonly involved the gastrointestinal, genitourinary, or dermatologic systems. Major hemorrhage occurred over the duration of follow-up; with two of the 47 (4.3%) events occurring within 1 month, nine (17.0%) within 3 months, 17 (36.2%) within 6 months, and 28 (59.6%) within 1 year of therapy initiation. The mean international normalized ratio (INR) at the time of event was 4.5±3.8 (median 3.4, range: 1.3–21.0) with 20 events (42.5%) occurring at INR of <3. At the time of the major hemorrhage, patients with the variant genotype had lower median INRs (2.9 (range: 1.1–8.2) vs 4.1 (range: 1.3–21.6), P=0.31) and lower median warfarin dose (22.0 mg/week (range: 7–47.5) vs 35 mg/week (range: 10–77.5), P=0.0009). There was no difference in the use of medications that interact with warfarin by genotype (P>0.4) at the time of the event. Time to first major hemorrhage was significantly shorter for patients with variant CYP2C9 genotype than wild-type genotype (log-rank P=0.004, Figure 1a) but not for patients with variant VKORC1 genotype (P=0.5, Figure 1b).
Figure 1.
Time to major hemorrhage by CYP2C9 and VKORC1 genotype. Kaplan—Meier curves for time to major hemorrhagic complication analyses plotted for (a) CYP2C9 wild-type vs variant genotype and (b) VKORC1 1173C/T wild-type vs variant genotype. (c) Estimated survival curve from Cox PH model adjusted for VKORC1 1173C/T genotype, age, warfarin dose, gender, body mass index, INR, alcohol intake, vitamin K intake, number of comorbid conditions, and drug interactions (note: y axis starts at 0.98).
Hazard ratios (HRs) from covariate-adjusted Cox proportional hazard (PH) models are presented in Table 4. The variant CYP2C9 genotype confers an increased risk of major (HR 3.0; 95% CI: 1.1–8.3, Figure 1c) but not minor (HR 1.3; 95% CI: 0.8–2.1) hemorrhage. Variant VKORC1 1173C/T genotype did not confer a significant increase in risk for major (HR 1.7; 95% CI: 0.7–4.4) or minor (HR 0.8; 95% CI: 0.5–1.3) hemorrhage. We recognize that European American and African Americans have distinct genetic ancestry. The genetic heterogeneity introduced by combining these groups in the analyses could potentially result in a spurious gene—hemorrhage association.15,16 Therefore, we also conducted analysis stratified by race. The risk conferred by the variant CYP2C9 genotype did not differ across race for minor hemorrhage. For major hemorrhage, the risk was higher among European-American (HR 3.9; 95% CI: 1.1–13.7) than African-American patients (HR 2.8; 95% CI: 0.5–17.3, Table 4), although not significantly different (P=0.21 for CYP2C9* race interaction). The interaction of VKORC1 and CYP2C9 assessed in an epistatic model was found to be statistically nonsignificant (P=0.48). Therefore, further analyses assessed the association of CYP2C9 genotype and hemorrhage with adjustment for VKORC1 genotype and race.
Table 4.
Adjusted hazard ratios and 95% CI for the association of variant CYP2C9 genotype and hemorrhagic complication among POAT participantsa
| Adjusted HRb |
Adjusted HR with robust variance estimationc |
|||
|---|---|---|---|---|
| Hemorrhagic event | HR (95% CI) | P-values | HR (95% CI) | P-values |
| Minor hemorrhage | 1.3 (0.89, 1.88) | 0.16 | 1.3 (0.79, 2.14) | 0.3 |
| African Americans | 1.5 (0.81, 2.76) | 0.21 | 1.5 (0.75, 2.93) | 0.26 |
| European Americans | 1.2 (0.73, 1.82) | 0.55 | 1.2 (0.65, 2.04) | 0.63 |
| Major hemorrhagec | 3.0 (1.22, 7.54) | 0.017 | 3.0 (1.12, 8.03) | 0.032 |
| Stratified by race | ||||
| African Americans | 2.8 (0.56, 14.79) | 0.21 | 2.8 (0.37, 15.95) | 0.25 |
| European Americans | 3.9 (1.1, 14.26) | 0.039 | 3.9 (1.16, 13.67) | 0.03 |
| Stratified by stage of anticoagulation therapy | ||||
| Before attaining first target INR | Undefined | — | Undefined | — |
| Before first stabilization of therapy | 5.3 (0.47, 58.70) | 0.17 | 5.3 (0.43, 64.67) | 0.19 |
| After first stabilization of therapy | 2.2 (0.72, 6.57) | 0.16 | 2.2 (0.73, 6.50) | 0.16 |
| During all periods of unstable therapy | 2.4 (0.91, 6.39) | 0.08 | 2.4 (0.77, 7.38) | 0.13 |
| During all periods of stable therapy | Undefined | — | Undefined | — |
| Stratified by INR at time of eventd | ||||
| INR<2 | 3.6 (0.47, 27.76) | 0.21 | 3.6 (0.46, 27.9) | 0.22 |
| INR 2–3 | 5.6 (1.8, 16.9) | 0.02 | 5.6 (1.19, 26.28) | 0.03 |
| INR>3 | 2.6 (0.50, 13.81) | 0.26 | 2.6 (0.41, 16.79) | 0.31 |
| Stratified by concurrent pathology±drug interactions at the time of the event | ||||
| Without pathology±drug interactions | 4.7 (0.93, 23.53) | 0.06 | 4.7 (0.91, 24.07) | 0.065 |
| With pathology±drug interactions | 3.4 (1.06, 11.08) | 0.039 | 3.4 (0.71, 16.52) | 0.12 |
BMI, body mass index; CI, confidence interval. Minor hemorrhagic complications included mild nosebleeds (lasting less than 30 min), microscopic hematuria, mild bruising.
Target INR range for all patients in the study was 2.0–3.0. Only one event occurred before first attainment of INR of 2.0. Five events occurred before first stabilization of anticoagulation therapy. Thirty-six events occurred after initial stabilization of therapy. Thirty-nine events occurred during all periods where anticoagulation therapy was not stable. Four events occurred during all periods of stable anticoagulation therapy. All significant P-values (P<0.05) are in bold.
Adjusted for age, gender, race, BMI, VKORC1 1173C/T, vitamin K and alcohol intake, warfarin dose, interacting drugs, number of comorbid conditions, and INR at the time of the event.
All major hemorrhagic complications were adjudicated by the Director of the Anticoagulation Clinic blinded to genotype. Major hemorrhagic complications include serious, life-threatening and fatal bleeding episodes as defined by Fihn et al.47 Major hemorrhagic complications were further classified as those occurring in the absence (major without pathology) or presence (major with pathology) of other contributing factors (medical comorbidity and concomitant drugs).
INR at the time of event was <2 in seven patients, between 2 and 3 in 13 patients, and >3 in 24 patients with major hemorrhage.
The effect of variant CYP2C9 genotype on risk before attaining first INR in the target range could not be evaluated, as only one event occurred in this time period. The variant CYP2C9 genotype conferred a fivefold increased risk of major hemorrhage before first stabilization and continued to confer a 2.2-fold risk even after anticoagulation is first stabilized. As only four events occurred during periods of stable therapy, the risk conferred by the variant CYP2C9 genotype could not be assessed. During periods where anticoagulation was not stable, patients with variant CYP2C9 genotype were at a 2.4-fold higher, although statistically nonsignificant, risk of major hemorrhage. The variant CYP2C9 genotype conferred an increased risk at INRs below (HR 3.6; 95% CI: 0.5–27.9), within (HR 5.6; 95% CI: 1.8–16.9), and above (HR 2.6; 95% CI: 0.5–13.8) the target range. However, a statistically significant increase was observed only at INRs within the target range.
The risk conferred by the variant CYP2C9 genotype was greater in patients experiencing major hemorrhage without pathology (e.g., active ulcer) and drug interactions (HR 4.7; 95% CI: 0.9–24.1) than those with significant pathology (HR 3.4; 95% CI: 0.7–16.5). Among patients experiencing major hemorrhage, six patients also had end-stage renal disease. These patients have a high level of comorbidity and multiple factors increasing the risk of hemorrhage. Therefore, the association of variant CYP2C9 genotype with major hemorrhage was reassessed after their exclusion. In patients without end-stage renal disease, the variant CYP2C9 genotype confers a higher risk (HR 3.7; 95% CI: 1.4–9.6, P=0.007) unchanged by correction for dependence (HR 3.7;=95% CI: 1.3–10.7, P=0.016).
DISCUSSION
This study provides strong evidence of the increased risk of major hemorrhage among patients with a variant CYP2C9 genotype throughout the duration of warfarin therapy after accounting for VKORC1 1173C/T genotype, demographics, and clinical covariates. To our knowledge, this is the largest prospective study aiming to define the association of variant CYP2C9 genotype with major hemorrhage among both European Americans and African Americans.
Our study is the first to examine the effect of the CYP2C9*5, CYP2C9*6, and CYP2C9*11 allele in a large African-American population in addition to CYP2C9*2 and CYP2C9*3. CYP2C9*2 and CYP2C9*3 were the most common CYP2C9 variant alleles in both racial groups, with observed frequencies consistent with previous reports.17,18 On the basis of variant allele frequencies, the combined poor-metabolizer genotypes are significantly more frequent among European Americans than African Americans (29.8 vs 9.73%, P<0.0001). Inclusion of the *5, *6, and *11 alleles in determining CYP2C9 genotype resulted in a higher poor-metabolizer genotype frequency (9.7%) among African Americans than that reported previously.19 The frequency of VKORC1 1173C/T variant was similar for European Americans (60%) but slightly higher for African Americans (19.6 vs 15.4%) than that reported by Schelleman et al.14
Among European Americans, the risk conferred by the variant CYP2C9 genotype is consistent with previous reports.9,10 To our knowledge, this is the first report of CYP2C9 genotype—major hemorrhage association in African Americans. Population stratification is often cited as a potential confounder in genetic association studies.15 For it to introduce confounding bias, three conditions must be met.20 First, the allele frequencies must differ across race; second, the occurrence of disease (hemorrhage) must vary by race; third, the genetic marker should not be in the casual pathway. In our study, although CYP2C9 allele frequencies show substantial differences across race (P<0.0001), the occurrence of major or minor hemorrhage does not. Therefore, population stratification does not bias the association of CYP2C9 genotype and risk of hemorrhage in our study. This is further emphasized by the lack of significant differences in the race-specific HRs (P-value for gene×race interaction=0.27). The lower frequency of the variant genotype in African Americans suggests that the population-attributable risk of this allele may be lower among African Americans than European Americans.
The incidence rate for major hemorrhagic complications in this study was similar to that reported previously.10,21 However, the incidence rate for minor hemorrhagic complications in our population was higher than that reported previously (14.5 per 100 patient-years; 95% CI: 13.7–16.22),21 perhaps because of inclusion of patients with minor hemorrhage who required additional testing or clinic visits.
Several studies have reported an increased risk of hemorrhage among patients with a variant CYP2C9 genotype. However, most have defined the event as “over-anticoagulation” rather than a hemorrhagic event.13,22-28 Four studies measured the occurrence of hemorrhagic events.9-11,29 Of these, only three reported bleeding risk by genotype.10,11,29 Aithal et al.9 reported bleeding risk for patients in a predefined low-dose category (<1.5 mg/day) rather than by genotype. Ogg et al.29 reported the influence of a sole variant, CYP2C9*3 variant, on risk of bleeding. A recent meta-analysis30 summarized the bleeding risk by genotype. Patients with at least one copy of a variant allele had an increased risk of bleeding compared with non-carriers for CYP2C9*2 (relative risk (RR) 1.9, 95% CI: 1.2–3.2), CYP2C9*3 (RR 1.8, 95% CI: 1.1–2.9), and CYP2C9*2 or *3 (RR 2.3, 95% CI: 1.4–3.7). Our results of increased risk (HR 3.0, 95% CI: 1.1, 8.0) are consistent with these reports.
Previous studies have not been able to compare the influence of individual variant alleles on the risk of major hemorrhage because of the rarity of the event and limited sample size. In our study, patients with the CYP2C9*3 variant had a higher, although not statistically significant, risk of major hemorrhage than those with CYP2C9*2 variant (RR 1.7, 95% CI: 0.47–5.8, P=0.42). The small number of events prevented the determination of the risk of major hemorrhage conferred by the other variant alleles.
As reported by Higashi et al.,10 patients with the variant genotype had a higher risk of major hemorrhage before stabilization of anticoagulation. However, this association could not be assessed in a multivariable model because of the small number (n=5) of events. The availability of longitudinal data enabled us to define periods of “stable and unstable” anticoagulation for each patient. Patients with the variant genotype continue to have a higher risk throughout the duration of therapy, including periods of “unstable” anticoagulation across the INR range after accounting for the effects of covariates. These findings highlight that the risk conferred by the variant genotype does not dissipate after stabilization of therapy.
This study has several strengths including a 2-year follow-up in a clinically well-characterized cohort, adjustment for time-varying covariates, drug interactions and comorbidity, objective documentation of incident complications, separation of the genotype—phenotype data until time of analyses, and event adjudication by an expert without knowledge of patients’ genotype. Besides the large African-American subgroup, the ability to model time-varying covariates is a major strength. The inclusion of patients from initiation of therapy in this prospective study and the high participation rate (93.1%) minimize selection bias. The generalizability of our findings is further improved by the representativeness of the study cohort as indicated by the similarity in age, gender, and racial distribution as compared to patients treated at University of Alabama at Birmingham (UAB) from the Birmingham metropolitan area.
We also recognize limitations of this study. Documentation of vitamin K intake was based on patients’ report using vitamin K inventory,31 not quantified by assay/measurements. However, all measurements were used consistently among all participants; therefore, bias if any should be non-differential. We also recognize that drug response is influenced by multiple genes. At least one other gene, VKORC1, has been shown to influence significantly warfarin dose in European Americans.25,32-36 Although it has been hypothesized that VKORC1 may influence the risk of hemorrhage, all studies have aimed at quantifying its influence on risk of severe over-anticoagulation (defined as INR of 6 or higher).25-27,37 Schalekamp et al.37 found that among acenocoumarol users the possession of CYP2C9*3 allele was associated with an increased risk of over-anticoagulation compared to the CYP2C9*2 and *1 alleles. However, among phenprocoumon users both CYP2C9*2 and CYP2C9*3 were associated with an increased risk of over-anticoagulation.27 Accounting for the effects of VKORC1, the risk of over-anticoagulation was increased by a combination of VKORC1 and CYP2C9 genotypes. Carriers of a combination of CYP2C9 and VKORC1 polymorphism had an increased risk of severe over-anticoagulation compared with subjects with no polymorphism or only one polymorphism among both acenocoumarol (HR 3.8, 95% CI: 1.6–9.0)26 and phenprocoumon (HR 7.2, 95% CI: 2.1–24.7) users.25
One study by Reitsma et al.38 suggests an increased risk of bleeding in the phenprocoumon users (crude odds ratio 2.6, 95% CI: 1.2–5.7), but not in acenocoumarol users (crude odds ratio 1.2, 95% CI: 0.6–2.3). Of note, this association is not consistent across the coumarin anticoagulants and did not account for other factors known to increase the risk of hemorrhage, such as CYP2C9 genotype, INR, drug interactions, and so on. Our results indicate a higher (but not statistically significant) hemorrhagic risk of VKORC1 1173C/T variants after adjusting for CYP2C9 genotype, clinical and demographic variables. Results of ongoing investigations will help better define the influence of 1173C/T and other VKORC1 variants (which may differ by race) on the risk of hemorrhagic events among patients on chronic warfarin therapy.
Results of this prospective study provide evidence of an association of variant CYP2C9 genotype and risk of major hemorrhage in both African-American and European-American patients after accounting for the effects of VKORC1 1173C/T, age, gender, warfarin dose, INR, drug interactions, vitamin K intake, and comorbidity. Compared to patients with the wild-type genotype, patients with the variant CYP2C9 genotype exhibit an increased risk of major hemorrhage, which persists even after stabilization of anticoagulation therapy.
METHODS
Patients ≥20 years of age, initiated on warfarin with a target INR range of 2–3 and ≥2 years anticipated treatment duration were eligible. The study is being conducted at the UAB enrolling patients from the anticoagulation clinic at The Kirklin Clinics (TKC-AC) and the Jefferson Clinic PC, Jefferson County Health System (CGH-JC) under the approval of the respective Institutional Review Boards. At both clinics, patient care is managed via a physician-approved prescriptive authority protocol that provides a standardized approach to warfarin dose adjustments based on INR results, management of over-anticoagulation and under-anticoagulation, and frequency of follow-up.39 A detailed medical, lifestyle, and medication history was documented. Information collected included indication for therapy, comorbid conditions, medications, smoking, alcohol use, education, income, medical insurance, and physical activity. Number of servings of foods rich in vitamin K consumed per week was used to document vitamin K intake.31
Time-dependent covariates
The risk of hemorrhage is influenced by factors that are likely to change over time including warfarin dose, INR, medications, vitamin K31 and alcohol intake,40 and level of physical activity.41 As patients initiated on warfarin tend to be older and may have physical limitations, we created a simple five-point scale: wheelchair bound, uses walker/cane, ambulatory without assistance, physically active (moderate exercise 30 min 3 times/week), and moderately physically active (moderate to intense exercise 4–5 times/week).
Drug interactions can influence warfarin dose and risk of hemorrhage.7,42 The increase in risk can result from concomitant use of drugs such as non-steroidal anti-inflammatory drugs or antiplatelet agents,43 or drugs that alter warfarin pharmacokinetics, including CYP2C9 inhibitors (e.g., amiodarone), CYP2C9 inducers (e.g., rifampin), or CYP2C9 substrates (e.g., losartan).44,45 Changes in these time-dependent covariates were documented at each visit.
Patient follow-up, event documentation, verification, and adjudication
During the 2-year follow-up, all complications were captured and verified through review of admissions and emergency department visits, and patient self-report. Only medically documented events were included in the analyses. The Alabama Center for Health Statistics was queried to verify cause of death for patients known to have expired to ensure inclusion of deaths due to hemorrhagic complications. All complications were documented by the study nurse followed by an independent review by the principal investigator. Finally, all major hemorrhagic complications were reviewed independently by the Medical Director of the Anticoagulation Clinic. All genotype information was maintained separately until data analysis.
CYP2C9 genotype determination
Blood was collected in a Qaigen PAX gene tube and DNA was extracted using the PAX gene blood DNA extraction kits. CYP2C9*2 genotyping was performed by polymerase chain reaction (PCR)-restriction fragment length polymorphism methodology,46 and genotyping for *2, *3, *5, *6, and *11 variants was performed using new pyrosequencing methodology.
Genomic DNA (10–30 ng) was amplified with 1 U Ampli Taq Gold (Applied Biosystems, Foster City, CA) in 40 μl reaction mixture containing 1× PCR buffer, 0.2 pmol/μl biotinylated forward primer, 0.2 pmol/μl reverse primer (Eurogentec, San Diego, CA) (Table 5), 2 mM final MgCl2, and 0.5 mM final dNTPs. An initial denaturation of 95°C for 8 min is used followed by 95°C for 15s, 561C for 30s, and 72°C for 15s for 45 cycles. This reaction is followed by a final extension at 72°C for 5 min (GeneAmp PCR 9700 System, Applied Biosystems).
Table 5.
PCR sequencing primers for pyrosequencing CYP2C9 alleles
| PCR primers | bp sizea |
2C9 allele |
Sequencing primer |
|---|---|---|---|
| Exon 3 | |||
| F: B-5′-AAACAGAGACTTACAG AGCTC-3′ |
381 | *2 | 5′-GGGCTTCCT CTTGAAC-3′ |
| R: 5′-CTAACAACCAGACTCAT AATG-3′ |
|||
| Exon 5 | |||
| F: B-5′-CAGAGCTTGGTATATGGT ATG-3′ |
323 | *6, *10 | 5′-AAGCTTTTGT TTACATTTT-3′ |
| R: 5′-TCGTAAACACAGAACTAGT CAAC-3′ |
|||
| Exon 7 | |||
| F: B-5′-CTGAATTGCTACAACAAA TGTG-3′ |
314 | *11 | 5′-TTGCATGC AGGGGCT-3′ |
| R: 5′-GATACTATGAATTTGGGAC TTC-3′ |
155 | *3, *5 | 5′-GCTGGTGG GGAGAAG-3′ |
| F: B-5′-TGCACGAGGTCCAGA GAT-3′ |
|||
| R: 5′-GATACTATGAATTTGGG ACTTC-3′ |
B, biotin-labeled primer; F, forward primer; PCR, polymerase chain reaction; R, reverse primer.
bp indicates PCR fragment base pair size. 2C9 allele: cytochrome 02C9 single-nucleotide polymorphism.
The entire biotinylated PCR product was mixed with 40 μl of 2×binding-washing buffer II (10 mM Tris—HCl, 2 M NaCl, 1 mM EDTA, and 0.1% Tween-20, pH 7.6) and immobilized with 3 μl (10 μg/μl) streptavidin-coated polystyrene beads (Amersham Biosciences, Piscataway, NJ). Samples were mixed at room temperature for 10 min. To achieve DNA strand separation, a Vacuum Prep Tool (Biotage, Foxboro, MA) was used; the PCR products were isolated through alkaline denaturation and wash steps (Biotage). Beads are released into wells of a PSQ sequencing plate (Biotage) containing 20 pmol of the appropriate sequencing primer (Table 5). The reactions annealed at 90°C for 5 min and cooled at room temperature for 10 min. Substrates and enzymes as well as dNTP from the manufacturer’s kit (Biotage) were placed in a cartridge (supplied by Biotage) and then placed into the PSQ 96 MA Pyrosequencer (Biotage) for sequence determination. These methods allow the determination of the six alleles with four PCRs (Table 5).
VKORC1 1173C/T (rs9934438) genotyping was conducted (at The Broad Institute Center for Genotyping and Analysis) using the Sequenom iPLEX technology, which relies on matrix assisted laser desorption/ionization-time-of-flight mass spectrometry. In brief, PCR is performed across the region of the single-nucleotide polymorphism with a high-fidelity polymerase followed by a primer extension that generates allele-specific products with a unique mass that can be separated on mass spectrometry. Primers and the multiplex assay are designed with the Sequenom SPECTRO-DESIGNER and IPLEX software, respectively.
Classification of hemorrhagic outcomes
The review of hemorrhagic complications included objective documentation (e.g., endoscopy) of complication site (e.g., gastrointestinal), gravity of the event (requiring transfusion, surgical intervention, and so on), and laboratory findings (INR, hemoglobin/hematocrit, and so on) at the time of the event. In the absence of evidence of bleeding, isolated elevated INRs were not classified as hemorrhagic events.
Hemorrhagic complications were classified as minor or major using the scheme detailed by Fihn et al.47 Minor hemorrhages included mild nosebleeds, microscopic hematuria, mild bruising, and mild hemorrhoidal bleeding. The “no additional testing/visit” criteria were relaxed, as these patients have multiple medical problems, heightened health concerns, and frequently access medical care. This modification was afforded by the prospective nature of the study, ascertainment of incident complications, and adjusting for comorbid conditions and changes in time-dependent covariates.
Serious, life-threatening and fatal bleeding episodes as defined by Fihn et al.47 were combined into one end point; “major hemorrhage” as these events are infrequent, demand medical/surgical intervention, and may carry grave prognosis. Any hemorrhage resulting in discontinuation of warfarin was classified as a major hemorrhage. To explore the influence of underlying comorbidity (e.g., ulcers) and drug interactions (e.g., non-steroidal anti-inflammatory drugs, CYP2C9 inhibitors),8 major hemorrhagic complications were further classified as those occurring in the absence or presence of concurrent pathology or drug interactions.
Analysis
Patients were categorized as wild type (CYP2C9*1/*1 homozygotes) and variant (≥1 variant alleles) with the former serving as the referent group. The HR and 95% CI for the association between CYP2C9 genotype and hemorrhage was evaluated using the counting process format in the Cox PH model.48,49 This format allows individuals to contribute more than one event for the hemorrhage outcome. Valid CIs were obtained by correction of dependence using robust variance estimation.50 Departures from the PH assumption were assessed by examination of the log—log survival curves, statistical testing of Schoenfeld residuals, and using linear regression to evaluate the relationship between time and covariate-specific Schoenfeld residuals.
The PH models included CYP2C9 genotype, age, race, gender, body mass index, VKORC1 1173C/T, and comorbid conditions. Changes in medications, vitamin K and alcohol intake, and level of physical activity, warfarin dose, and INRs were included as time-varying covariates. Models with interaction terms were fit to assess the potential effect modification of the genotype by each covariate. All interaction terms were statistically nonsignificant (full model, all P>0.05) and therefore excluded. Both statistically significant and clinically relevant covariates were included in the models.
Patients were followed from initiation of therapy until the date of event (if warfarin was discontinued) or the end of the follow-up, when all data were right censored. All patients withdrawn from the study were censored at the time of the last clinic visit. To understand the effect of recent initiation of therapy on risk of hemorrhage, analyses restricted by time to attainment of first target INR, time to first stabilization of anticoagulation therapy, and duration of therapy after first attainment of stable anticoagulation (three INRs in target range without dose change) were conducted. The HWE assumption was assessed with the χ2 test of independence and exact test.51 All analyses were conducted at a non-directional α-level of 0.05.
ACKNOWLEDGMENTS
We thank Joyce Blaisdell for her work with CYP2C9 genotyping. NA Limdi wishes to acknowledge Dr Edward Faught for his support and mentorship and Dr Melissa Baird (Medical Director of the Anticoagulation Clinic) for event adjudication. We are grateful to all the patients who participated in the study. We thank Janice Ware for her untiring efforts with patient recruitment and the staff of the Anticoagulation Clinic at The Kirklin Clinic, the Cooper Green Hospital, and Jefferson Clinic PC for their help with identification of potential participants. We also thank the physicians, especially Drs Mark Wilson and Melissa Baird, at the University of Alabama at Birmingham and the Health Service Foundation for their support for this research. We thank Steve Duncan and Darlene Green and the Office of Data Resources for their work with the POAT database and quality assurance. This work was supported in part by a grant from the National Institute of Neurological Disorders and Stroke (Grant no. K23NS45598-01) and in part by the Intramural Research Program of the NIH, National Institute of Environmental Health Sciences. This study has contributed samples to the NINDS Human Genetics Resource Center DNA and Cell Line Repository (http://ccr.coriell.org/ninds). NINDS Repository sample numbers corresponding to the samples used are ND04466, ND04556, ND04604, ND04605, ND04626, ND04869, ND04907, ND04934, ND04951, ND05036, ND05108, ND05175, ND05176, ND05239, ND05605, ND05606, ND05701, ND05702, ND05735, ND06147, ND06207, ND06385, ND06424, ND06480, ND06706, ND06814, ND06871, ND06983, ND07057, ND07234, ND07304, ND07494, ND07602, ND07711, ND07712, ND08065, ND08596, ND08864, ND08932, ND09079, ND09172, ND09760, ND09761, ND09809. The Broad Institute Center for Genotyping and Analysis is supported by Grant U54 RR020278-01 from the National Center for Research Resources.
Footnotes
CONFLICT OF INTEREST The authors declared no conflict of interest.
References
- 1.Thom T, et al. Heart disease and stroke statistics—2006 update: a report from the American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Circulation. 2006;113:e85–e151. doi: 10.1161/CIRCULATIONAHA.105.171600. [DOI] [PubMed] [Google Scholar]
- 2.Albers GW, Amarenco P, Easton JD, Sacco RL, Teal P. Antithrombotic and thrombolytic therapy for ischemic stroke: the Seventh ACCP Conference on Antithrombotic and Thrombolytic Therapy. Chest. 2004;126:483S–512S. doi: 10.1378/chest.126.3_suppl.483S. [DOI] [PubMed] [Google Scholar]
- 3.Buller HR, et al. Antithrombotic therapy for venous thromboembolic disease: the Seventh ACCP Conference on Antithrombotic and Thrombolytic Therapy. Chest. 2004;126:401S–428S. doi: 10.1378/chest.126.3_suppl.401S. [DOI] [PubMed] [Google Scholar]
- 4.Harrington RA, et al. Antithrombotic therapy for coronary artery disease: the Seventh ACCP Conference on Antithrombotic and Thrombolytic Therapy. Chest. 2004;126:513S–548S. doi: 10.1378/chest.126.3_suppl.513S. [DOI] [PubMed] [Google Scholar]
- 5.Hylek EM, et al. Translating the results of randomized trials into clinical practice: the challenge of warfarin candidacy among hospitalized elderly patients with atrial fibrillation. Stroke. 2006;37:1075–1080. doi: 10.1161/01.STR.0000209239.71702.ce. [DOI] [PubMed] [Google Scholar]
- 6.Wittkowsky AK. Effective anticoagulation therapy: defining the gap between clinical studies and clinical practice. Am. J. Manag. Care. 2004;10:S297–S306. discussion S312–S317. [PubMed] [Google Scholar]
- 7.Wittkowsky AK, Devine EB. Frequency and causes of overanticoagulation and underanticoagulation in patients treated with warfarin. Pharmacotherapy. 2004;24:1311–1316. doi: 10.1592/phco.24.14.1311.43144. [DOI] [PubMed] [Google Scholar]
- 8.Hylek EM. Complications of oral anticoagulant therapy: bleeding and nonbleeding, rates and risk factors. Semin. Vasc. Med. 2003;3:271–278. doi: 10.1055/s-2003-44463. [DOI] [PubMed] [Google Scholar]
- 9.Aithal GP, Day CP, Kesteven PJ, Daly AK. Association of polymorphisms in the cytochrome P450 CYP2C9 with warfarin dose requirement and risk of bleeding complications. Lancet. 1999;353:717–719. doi: 10.1016/S0140-6736(98)04474-2. [DOI] [PubMed] [Google Scholar]
- 10.Higashi MK, et al. Association between CYP2C9 genetic variants and anticoagulation-related outcomes during warfarin therapy. JAMA. 2002;287:1690–1698. doi: 10.1001/jama.287.13.1690. [DOI] [PubMed] [Google Scholar]
- 11.Margaglione M, et al. Genetic modulation of oral anticoagulation with warfarin. Thromb. Haemost. 2000;84:775–778. [PubMed] [Google Scholar]
- 12.Tassies D, et al. Pharmacogenetics of acenocoumarol: cytochrome P450 CYP2C9 polymorphisms influence dose requirements and stability of anticoagulation. Haematologica. 2002;87:1185–1191. [PubMed] [Google Scholar]
- 13.Taube J, Halsall D, Baglin T. Influence of cytochrome P-450 CYP2C9 polymorphisms on warfarin sensitivity and risk of overanticoagulation in patients on long-term treatment. Blood. 2000;96:1816–1819. [PubMed] [Google Scholar]
- 14.Schelleman H, et al. Warfarin response and vitamin K epoxide reductase complex 1 in African Americans and Caucasians. Clin. Pharmacol. Ther. 2007;81:742–747. doi: 10.1038/sj.clpt.6100144. [DOI] [PubMed] [Google Scholar]
- 15.Little J, et al. Reporting, appraising, and integrating data on genotype prevalence and gene-disease associations. Am. J. Epidemiol. 2002;156:300–310. doi: 10.1093/oxfordjournals.aje.a000179. [DOI] [PubMed] [Google Scholar]
- 16.Thomas DC, Witte JS. Point: population stratification: a problem for case—control studies of candidate-gene associations? Cancer Epidemiol. Biomarkers Prev. 2002;11:505–512. [PubMed] [Google Scholar]
- 17.Lee CR, Goldstein JA, Pieper JA. Cytochrome P450 2C9 polymorphisms: a comprehensive review of the in-vitro and human data. Pharmacogenetics. 2002;12:251–263. doi: 10.1097/00008571-200204000-00010. [DOI] [PubMed] [Google Scholar]
- 18.Xie HG, Kim RB, Wood AJ, Stein CM. Molecular basis of ethnic differences in drug disposition and response. Annu. Rev. Pharmacol. Toxicol. 2001;41:815–850. doi: 10.1146/annurev.pharmtox.41.1.815. [DOI] [PubMed] [Google Scholar]
- 19.Xie HG, Prasad HC, Kim RB, Stein CM. CYP2C9 allelic variants: ethnic distribution and functional significance. Adv. Drug Deliv. Rev. 2002;54:1257–1270. doi: 10.1016/s0169-409x(02)00076-5. [DOI] [PubMed] [Google Scholar]
- 20.Wacholder S, Rothman N, Caporaso N. Counterpoint: bias from population stratification is not a major threat to the validity of conclusions from epidemiological studies of common polymorphisms and cancer. Cancer Epidemiol. Biomarkers Prev. 2002;11:513–520. [PubMed] [Google Scholar]
- 21.Fihn SD, et al. The risk for and severity of bleeding complications in elderly patients treated with warfarin. The National Consortium of Anticoagulation Clinics. Ann. Intern. Med. 1996;124:970–979. doi: 10.7326/0003-4819-124-11-199606010-00004. [DOI] [PubMed] [Google Scholar]
- 22.Joffe HV, et al. Warfarin dosing and cytochrome P450 2C9 polymorphisms. Thromb. Haemost. 2004;91:1123–1128. doi: 10.1160/TH04-02-0083. [DOI] [PubMed] [Google Scholar]
- 23.Lindh JD, Lundgren S, Holm L, Alfredsson L, Rane A. Several-fold increase in risk of overanticoagulation by CYP2C9 mutations. Clin. Pharmacol. Ther. 2005;78:540–550. doi: 10.1016/j.clpt.2005.08.006. [DOI] [PubMed] [Google Scholar]
- 24.Peyvandi F, Spreafico M, Siboni SM, Moia M, Mannucci PM. CYP2C9 genotypes and dose requirements during the induction phase of oral anticoagulant therapy. Clin. Pharmacol. Ther. 2004;75:198–203. doi: 10.1016/j.clpt.2003.09.015. [DOI] [PubMed] [Google Scholar]
- 25.Schalekamp T, et al. VKORC1 and CYP2C9 genotypes and phenprocoumon anticoagulation status: interaction between both genotypes affects dose requirement. Clin. Pharmacol. Ther. 2007;81:185–193. doi: 10.1038/sj.clpt.6100036. [DOI] [PubMed] [Google Scholar]
- 26.Schalekamp T, et al. VKORC1 and CYP2C9 genotypes and acenocoumarol anticoagulation status: interaction between both genotypes affects overanticoagulation. Clin. Pharmacol. Ther. 2006;80:13–22. doi: 10.1016/j.clpt.2006.04.006. [DOI] [PubMed] [Google Scholar]
- 27.Schalekamp T, et al. Effects of cytochrome P450 2C9 polymorphisms on phenprocoumon anticoagulation status. Clin. Pharmacol. Ther. 2004;76:409–417. doi: 10.1016/j.clpt.2004.08.006. [DOI] [PubMed] [Google Scholar]
- 28.Steward DJ, et al. Genetic association between sensitivity to warfarin and expression of CYP2C9*3. Pharmacogenetics. 1997;7:361–367. doi: 10.1097/00008571-199710000-00004. [DOI] [PubMed] [Google Scholar]
- 29.Ogg MS, Brennan P, Meade T, Humphries SE. CYP2C9*3 allelic variant and bleeding complications. Lancet. 1999;354:1124. doi: 10.1016/S0140-6736(05)76918-X. [DOI] [PubMed] [Google Scholar]
- 30.Sanderson S, Emery J, Higgins J. CYP2C9 gene variants, drug dose, and bleeding risk in warfarin-treated patients: a HuGEnet systematic review and meta-analysis. Genet. Med. 2005;7:97–104. doi: 10.1097/01.gim.0000153664.65759.cf. [DOI] [PubMed] [Google Scholar]
- 31.Booth SL, Saowski JA, Weihrauch JL, Ferland G. Vitamin K (phylloquinone) content of foods. J. Food Consump. Anal. 1993;6:109–120. [Google Scholar]
- 32.Bodin L, et al. Cytochrome P450 2C9 (CYP2C9) and vitamin K epoxide reductase (VKORC1) genotypes as determinants of acenocoumarol sensitivity. Blood. 2005;106:135–140. doi: 10.1182/blood-2005-01-0341. [DOI] [PubMed] [Google Scholar]
- 33.D’Andrea G, et al. A polymorphism in the VKORC1 gene is associated with an interindividual variability in the dose-anticoagulant effect of warfarin. Blood. 2005;105:645–649. doi: 10.1182/blood-2004-06-2111. [DOI] [PubMed] [Google Scholar]
- 34.Rieder MJ, et al. Effect of VKORC1 haplotypes on transcriptional regulation and warfarin dose. N. Engl. J. Med. 2005;352:2285–2293. doi: 10.1056/NEJMoa044503. [DOI] [PubMed] [Google Scholar]
- 35.Vecsler M, et al. Combined genetic profiles of components and regulators of the vitamin K-dependent gamma-carboxylation system affect individual sensitivity to warfarin. Thromb. Haemost. 2006;95:205–211. doi: 10.1160/TH05-06-0446. [DOI] [PubMed] [Google Scholar]
- 36.Yuan HY, et al. A novel functional VKORC1 promoter polymorphism is associated with inter-individual and inter-ethnic differences in warfarin sensitivity. Hum. Mol. Genet. 2005;14:1745–1751. doi: 10.1093/hmg/ddi180. [DOI] [PubMed] [Google Scholar]
- 37.Schalekamp T, et al. Acenocoumarol stabilization is delayed in CYP2C93 carriers. Clin. Pharmacol. Ther. 2004;75:394–402. doi: 10.1016/j.clpt.2003.12.017. [DOI] [PubMed] [Google Scholar]
- 38.Reitsma PH, van der Heijden JF, Groot AP, Rosendaal FR, Buller HRA. C1173T dimorphism in the VKORC1 gene determines coumarin sensitivity and bleeding risk. PLoS Med. 2005;10:e312. doi: 10.1371/journal.pmed.0020312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ansell JE, Oertel LB, Wittkowsky AK. Managing Oral Anticoagulation Therapy: Clinical and Operational Guidelines. Lippincott Williams & Wilkins; St Louis, MO: 2005. [Google Scholar]
- 40.Havrda DE, Mai T, Chonlahan J. Enhanced antithrombotic effect of warfarin associated with low-dose alcohol consumption. Pharmacotherapy. 2005;25:303–307. doi: 10.1592/phco.25.2.303.56955. [DOI] [PubMed] [Google Scholar]
- 41.Shibata Y, et al. Influence of physical activity on warfarin therapy. Thromb. Haemost. 1998;80:203–204. [PubMed] [Google Scholar]
- 42.McGriff-Lee NJ, et al. Search for predictors of nontherapeutic INR results with warfarin therapy. Ann. Pharmacother. 2005;39:1996–2002. doi: 10.1345/aph.1E381. [DOI] [PubMed] [Google Scholar]
- 43.Gasse C, Hollowell J, Meier CR, Haefeli WE. Drug interactions and risk of acute bleeding leading to hospitalisation or death in patients with chronic atrial fibrillation treated with warfarin. Thromb. Haemost. 2005;94:537–543. doi: 10.1160/TH05-03-0166. [DOI] [PubMed] [Google Scholar]
- 44.Holbrook AM, et al. Systematic overview of warfarin and its drug and food interactions. Arch. Intern. Med. 2005;165:1095–1106. doi: 10.1001/archinte.165.10.1095. [DOI] [PubMed] [Google Scholar]
- 45.Rettie AE, Jones JP. Clinical and toxicological relevance of CYP2C9: drug—drug interactions and pharmacogenetics. Annu. Rev. Pharmacol. Toxicol. 2005;45:477–494. doi: 10.1146/annurev.pharmtox.45.120403.095821. [DOI] [PubMed] [Google Scholar]
- 46.Sullivan-Klose TH, et al. The role of the CYP2C9-Leu359 allelic variant in the tolbutamide polymorphism. Pharmacogenetics. 1996;6:341–349. doi: 10.1097/00008571-199608000-00007. [DOI] [PubMed] [Google Scholar]
- 47.Fihn SD, et al. Risk factors for complications of chronic anticoagulation. A multicenter study. Warfarin Optimized Outpatient Follow-up Study Group. Ann. Intern. Med. 1993;118:511–520. doi: 10.7326/0003-4819-118-7-199304010-00005. [DOI] [PubMed] [Google Scholar]
- 48.Ake CF, Carpenter AL. Proc. 11th Annu. Western Users of SAS; Cary, NC: SAS Institute; 2003. [Google Scholar]
- 49.Therneau TM, Grambsch PM. The Cox Model. In: Therneau TM, Grambsch PM, editors. Modeling Survival Data: Extending the Cox Model (Statistics for Biology and Health) Springer; New York, NY: 2001. pp. 39–79. [Google Scholar]
- 50.Therneau TM, Grambsch PM. Multiple events per subject. In: Therneau TM, Grambsch PM, editors. Modeling Survival Data: Extending the Cox Model (Statistics for Biology and Health) Springer; New York, NY: 2001. pp. 169–229. [Google Scholar]
- 51.Guo SW, Thompson EA. Performing the exact test of Hardy—Weinberg proportion for multiple alleles. Biometrics. 1992;48:361–372. [PubMed] [Google Scholar]