Summary
The present study examined whether or not neuronal hemoglobin (Hb) is present in rats. It then examined whether cerebral ischemia or ischemic preconditioning (IPC) affects neuronal Hb levels in vivo and in vitro. In vivo, male Sprague-Dawley rats were subjected to either 15 minutes of transient middle cerebral artery occlusion with 24 hours of reperfusion, an IPC stimulus, or 24 hours of permanent middle cerebral artery occlusion (pMCAO), or IPC followed three days later by 24 hours of pMCAO. In vitro, primary cultured neurons were exposed to 2 hours of oxygen-glucose deprivation with 22 hours of reoxygenation. Results showed that Hb is widely expressed in rat cerebral neurons but not astrocytes. Hb expression was significantly upregulated in the ipsilateral caudate and the cortical core of the middle cerebral artery territory after IPC. Hb levels also increased in more penumbral cortex and the contralateral hemisphere 24 hours after pMCAO, but expression in the ipsilateral caudate and cortical core area were decreased. Ischemic preconditioning modified pMCAO-induced brain Hb changes. Neuronal Hb levels in vitro were increased by 2 hours of oxygen-glucose deprivation and 22 hours of reoxygenation. These results indicate that Hb is synthesized in neurons and can be upregulated by ischemia.
Keywords: cerebral ischemia, hemoglobin, ischemic preconditioning, oxygen glucose deprivation
INTRODUCTION
Hemoglobins are heme proteins widely distributed in diverse organisms, including bacteria, fungi, protists, plants and animals. They carry out many different functions, including transport of oxygen between tissues, intracellular oxygen transport and catalysis of redox reactions (Hardison 1998). In vertebrates, hemoglobin (Hb) is a heterotetramer of two α-globin and two β-globin subunits. Each subunit contains a hydrophobic pocket in which a heme molecule binds tightly and allows for the reversible binding of oxygen (Poyart et al 1992). Vertebrate Hb is usually thought of as an important oxygen transport protein mainly present in erythrocytes circulating in the blood. However, recent studies have showed Hb is expressed in neurons of embryonic and adult brain (Ohyagi et al 1994). Hb expression has also been found in the hippocampus and in cerebellum (Blalock et al 2003; Slemmon et al 1994; Wu et al 2004).
The presence of neuronal Hb and its high affinity for oxygen indicate it may have an important role in regulating oxygen hemostasis in neurons, which may be important under conditions of cerebral ischemia or neuronal hypoxia. On the other hand, it has been known that free Hb is deleterious to the brain after intracerebral hemorrhage and brain trauma(Xi et al 2006). Exogenous Hb can induce cell death in rat cerebral cortical neurons (Wang et al 2002). Our previous studies have demonstrated that intracerebral infusion of hemoglobin and its degradation products cause brain damage (Hua et al 2003; Huang et al 2002). To date, little is known about the response of endogenous neuronal Hb to cerebral ischemia.
In the present study, Hb expression was examined after 15 minutes of transient middle cerebral artery occlusion, an ischemic preconditioning (IPC) stimulus (Masada et al 2001), permanent middle cerebral artery occlusion (pMCAO) and IPC followed by pMCAO in the rat. The effects of oxygen-glucose deprivation (OGD) on neuronal Hb levels were also examined in vitro.
MATERIALS AND METHODS
Animal preparation and middle cerebral artery occlusion
The protocols for these animal studies were approved by the Committee of University Michigan in the Animals Use and Care. Adult male Sprague-Dawley rats (275–325g; Charles River Laboratories, MI, U.S.A.) were used. Rats were fasted overnight before surgery with free access of water. Anesthesia was induced with 5% isoflurane. After the animals were intubated, anesthesia was maintained by 2.25% isoflurane through mechanical ventilation with a rodent ventilator model 683 (Harvard Apparatus, Inc, S. Natick, MA, U.S.A). A polyethylene catheter (PE-50) was then inserted into the femoral artery to monitor arterial blood pressure and to obtain blood samples for analysis of pH, PaO2, PaCO2, hematocrit and blood glucose concentration before cerebral ischemia. Body temperature was maintained at 37.5 °C by using a feedback-controlled heating pad.
Middle cerebral artery occlusion was induced by insertion of a 3-0 monofilament nylon suture via the left common carotid artery as described previously (Karabiyikoglu et al 2004). The suture was gently advanced into the internal carotid approximately 19 or 20 mm from the bifurcation to occlude the middle cerebral artery. Rat cortical blood flow was measured in the core area of middle cerebral artery territory by a laser-doppler flowmeter, Laserflo BMP2 (Vasamedics, Inc, Little Canada, MN, U.S.A) before the onset of ischemia and during the ischemia to confirm that the blood flow reached the ischemic level. The sham operation was identical except that the intraluminal suture was not inserted.
Experimental groups
There were three parts to the in vivo study. In the first part, rats were subjected to 15 minutes of transient middle cerebral artery occlusion, an IPC stimulus, or sham operation on the left side (n=6, each group) and were sacrificed 24 hours later for Western blot analysis and real-time polymerase chain reaction (PCR). This duration of transient middle cerebral artery occlusion has been shown to induce ischemic preconditioning in the rat (Masada et al 2001). In the second part, rats underwent a pMCAO to induce brain injury or a sham operation and were killed 24 hours later for immunohistochemistry, Western blot analysis and real-time PCR (n=10, each group). In the third part, rats underwent a further pMCAO three days after IPC or sham operation (n=7, each group). The rats were killed 24 hours later for Western blotting and real-time PCR. In addition, three rats were used for brain Hb determination.
Cell Culture
Primary neuronal cultures were obtained from embryonic day-17 Sprague-Dawley rats (Charles River Laboratories, MI, U.S.A.). Cultures were prepared according to a previously described procedure with some modifications (Jiang et al 2002). Briefly, cerebral cortex was dissected, stripped of meninges, and dissociated by a combination of 0.5% trypsin digestion and mechanical trituration. The dissociated cell suspensions were seeded into poly-L-lysine precoated 6-well plates in a density of 6 × 100,000/cm2. The cells were grown in neurobasal medium with 2% B27, 0.5mM glutamine and 1% Antibiotic-Antimycotic and maintained in a humidified incubator at 37°C with 5% CO2. Half of the cultured mediums were changed 3–4 days for each. The neurons were used for experiments after 7 days.
Astrocyte cultures were prepared from the brains of 1–3 days old Sprague-Dawley rat pups (McCarthy and de Vellis 1980) with some modifications. Cerebral cortex was isolated, meninges were removed and the tissue was incubated in 0.5% trypsin for 20 minutes at 37°C. After digestion the tissue was rinsed twice in HBSS, followed by a mechanical dissociation in Dulbecco’s modified Eagle medium (DMEM, 10% fetal calf serum, 0.5 mM glutamine and 2% Antibiotic-Antimycotic). The cells were plated at a density of 6 × 100,000/cm2 on precoated poly-L-lysine 6-well plates with DMEM. The cells were kept at 37°C with 5% CO2 and growth medium was changed twice a week. Astrocytes were used for the experiments at 7–10 days.
Oxygen glucose deprivation
Primary cultured neurons were deprived of oxygen-glucose for 2 hours for ischemic injury in vitro as previously described (Andjelkovic et al 2003). The culture medium was removed. Cells were washed twice with phosphate-buffered saline (pH 7.4), and then placed in OGD medium (DMEM without glucose, gassed with 85% N2, 10% H 2and 5% CO2 for 30 minutes). Cells were maintained in an anaerobic chamber (Coy Laboratory, MI, U.S.A) containing a gas mixture composed of 85% N2, 10% H2 and 5%CO2 at 37°C for 2 hours. Anaerobic conditions in the chamber were monitored using an oxygen sensor (Teledyne Analytical Instruments, CA, U.S.A). OGD was terminated by removing cells from the chamber, followed by replenishing with serum-free DMEM solution and placing them back into the normoxic incubator for 22 hours. Control cell cultures were kept in the normoxic incubator for 24 hours with serum-free DMEM solution.
Western blot analysis
Rats were anesthetized with pentobarbital (60 mg/kg, i.p.) and underwent intracardiac perfusion with 0.1 M phosphate-buffered saline (pH 7.4) until the outflow fluid from the right atrium was colorless. The brains were removed quickly from the skull and divide into two hemispheres. The caudate was sampled from the ipsi- and contralateral hemispheres. The cortices were then sampled using 5- and 7-mm cork borers (Masada et al 2001). A cortical core sample was taken from tissue underlying the middle cerebral artery immediately distal to the region of occlusion using the 5-mm borer. Next a ring of tissue surrounding the core was taken with the 7-mm borer. The tissue was called the ‘intermediate’ sample and it represents, in the ipsilateral hemisphere, less densely ischemic tissue than the ‘core’ sample. Samples were frozen in liquid nitrogen.
In vitro, cell medium was removed and the plates were washed three times with chilled phosphate-buffered saline. The cells were quickly scraped and collected by centrifugation at 4°C, then stored at −80°C.
Western blot analysis was performed as described previously (Xi et al 1999). Brain and cell samples were sonicated with Western blot lysis buffer. Protein concentration was determined using a Bio-Rad protein assay kit (Hercules, CA, USA). Equal amounts of protein from each sample was separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis and transferred to a hybond-C-pure nitrocellulose membrane (Amersham, Piscataway, NJ, USA). Membranes were blocked in Carnation nonfat milk and probed with the primary and secondary antibodies. The primary antibodies were polyclonal rabbit anti-hemoglobin antibody at a 1:1000 dilution (Cappel, MP Biomedics Inc., OH, U.S.A). The second antibody was goat anti-rabbit IgG (Bio-Rad, Hercules, CA, USA; 1:2500 dilution). The antigen-antibody complexes were observed with the ECL chemiluminescence system (Amersham, Piscataway, NJ, USA) and exposed to Kodak X-OMAT film. After developed, the membrane were stripped and reprobed with antibody against β-actin (Sigma, St Louis, MO, USA; 1:6000 dilution). The relative densities of bands were analyzed with the NIH Image J.
Immunohistochemistry
For the in vivo models, rats were reanesthetized with pentobarbital (60 mg/kg, i.p.) and underwent intracardiac perfusion with 4% paraformaldehyde in 0.1 M phosphate-buffered saline (pH 7.4). The brains were removed and kept in 4% paraformaldehyde overnight, and then immersed in 30% sucrose for 3 to 4 days at 4°C. Specimens were then placed in embedding compound and sectioned (18 µm thickness) on a cryostat. For the in vitro studies, the culture medium was removed and cells were washed three times with chilled PBS. The cells were fixed with 4% paraformaldehyde in 0.1 M phosphate-buffered saline (pH 7.4) for 30 minutes. Immunostaining for brain sections and cultured cells was performed according to a previously described method (Xi et al 1999). The primary antibody was polyclonal rabbit anti-rat hemoglobin antibody (1:400 dilution, Cappel, MP Biomedics Inc., OH, U.S.A). Rhodamine-conjugated goat anti-rabbit antibody (Chemicon Interantional Inc., Temecula, CA, USA; 1:600 dilution) was used as second antibody. Normal rabbit immunoglobulin G was used as a negative control.
For immunofluorescent double labeling, the primary antibodies were rabbit anti-hemoglobin (Cappel, MP Biomedics Inc., OH, U.S.A; 1:100 dilution), mouse anti-glial fibrillary acid protein (GFAP) (Chemicon International Inc. Temecula, CA, USA; 1:100 dilution) and mouse anti-rat neuronal nuclei (NeuN) (Chemicon International Inc., Temecula, CA, USA; 1:100 dilution). Rhodamine-conjugated goat anti-rabbit antibody (Chemicon International Inc., Temecula, CA, USA; 1:100 dilution) and fluorescein isothiocyanate (FITC)-labeled horse anti-mouse antibody (Vector Laboratories, Burlingame, CA, USA; 1:100 dilution) were used as second antibodies. The double labeling was analyzed using fluorescence microscope.
Real-time quantitative polymerase chain reaction
For the in vivo studies, rats were anesthetized and decapitated. The brain tissue was sampled as described for Western blot analysis. For the cells, the medium was removed and plates were washed three times with chilled PBS. The cells were quickly scraped and collected by centrifugation at 4°C, then stored at −80°C. Total RNA was extracted from the frozen brain tissue and cultured cells with Trizol reagent (Gibco BRL, Grand Island, NY, USA), and 1µg RNA was digested with deoxyribonuclease I (DNaseI, Amplification-grade, Gibco BRL Grand Island, NY, USA). Complimentary DNA was synthesized by reverse transcription using the digested 1-µg RNA (11µL) with 14 µL reaction buffer (Perkin Elmer, Foster City, CA, U.S.A.) containing dNTP (dATP, dCTP, dGTP, and dTTP), 25 mmol/L MgCl2, 10× polymerase chain reaction buffer II, Random Hexamer Primer, Rnase inhibitor, and MuL V reverse transcriptase. The reaction was performed at 42°C for 30 minutes and terminated at 99°C after 5 minutes. Diethyl pyrocarbonate water (75 µL) was added to dilute the complimentary DNA to 100 µL and stored at −20°C for later use.
Real-time quantitative PCR was performed with SYBR green as a double strand DNA specific dye in Eppendorf Mastercycler ep realplex (Eppendorf North America Inc., NY, USA). The primers for rat hemoglobin α chain (HbA) and β chain (HbB) were designed from known sequences of rat HbA-mRNA (GeneBank No. U29528) and HbB-mRNA (GeneBank No. NM_033234) searched by PrimerQuest (Integrated DNA Technologies Inc., Coralville, IA, USA). The primers were: rat HbA 5′-TGATCCACTTCCTTCTCTGCCCAA-3′(forward primer) and 5′-ATCAGTTGCCCAAGTGCTTCTTGC-3′ (reverse primer) and HbB 5′-ATGGCCTGAAACACTTGGACAACC-3′ (forward primer) and 5′-TGGTGGCCCAACACAATCACAATC-3′ (reverse primer). Rat GAPDH primers were 5′-CCGTGCCAAGATGAAATTGGCTGT-3′ (forward) and 5′-TGTGCATATGTGCGTGTGTGTGTG-3′ (reverse) as a housekeeping gene served as a control. PCR reaction was run in triplicate on 96-well plate with a total volume of 20 µL per well using 2.5 × SYBR® Green universal master mix. Cycling condition were 2 minutes at 95°C, 30 seconds at 95°C, 30 seconds at 60°C, 1 minute at 72°C, 40 cycles, and a melting-curve program (60°C to 95°C with warming of 1.75°C per minute). The relative quantification analysis module was used to compare expression levels of a target gene. The expression levels were calculated by using the ΔΔCT method (Livak and Schmittgen 2001). With this method, we had value equal to 1 when no change in relative expression occurred between untreated and treated samples. We defined over-expression when 2−ΔΔCT >1 and under-expression when 2−ΔΔCT < 1
Sequencing of the PCR products
PCR products were isolated by 2% agarose gel electrophoresis. Gels were observed with ethidium bromide staining and ultraviolet transillumination. The DNA fragments of interest were excised and then purified with an Agarose Gel DNA Extraction kit (Roche, Diagnostics GmbH, Mannheim, Germany). Purified DNA fragments were sequenced. All sequencing was performed on Applied Biosystems DNA Sequencers (Model 3730 XL). Sequence data was analyzed using the nucleotide blast at National Center for Biotechnology Information.
Spectrophotometric assay of hemoglobin
Brain hemoglobin content was quantified by spectrophotometric assay (Choudhri et al 1997). Normal rats were perfused transcardially with 0.1 M PBS under deep anesthesia until the outflow fluid from the right atrium was colorless. Brains were rapidly removed and dissected into two parts, the cortex and the basal ganglia. The tissues were weighed on an electronic balance and then homogenized in 0.1M PBS, followed by 30-minute centrifugation (13,000g). Then 200 µl reagent (BioAssay Systems, QuantiChrom ™ Hemoglobin Assay Kit) was added to 50 µl supernatant. After 15 minutes, optical density was measured at 400 nm with a spectrophotometer. Hemoglobin content was expressed as µg per gram wet brain tissue.
Statistical analysis
All data in this study is presented as mean ± SD. Data were analyzed with Student’s t-test and analysis of variance, or Mann-Whitney U-rank test. Statistical significance was set at 0.05.
RESULTS
Physiological variables including blood pH, PaO2, PaCO2, glucose, hematocrit and blood pressure were measured before the onset of MCAO and controlled in normal range. The combined mean physiologic variable are shown in Table 1. Hemoglobin levels in normal rat brains were 161±16 µg/g in the cortex and 240±19 µg/g in the basal ganglia.
Table 1.
Physiological Parameters
| Group (n) | pH | PaCO2 (mm Hg) | PaO2 (mm Hg) | Hematocrit (%) | Glucose (mg/dL) | MABP (mm Hg) |
|---|---|---|---|---|---|---|
| Sham (16) | 7.42±0.05 | 41±6 | 90±10 | 43±3 | 128±37 | 90±7 |
| IPC (8) | 7.42±0.04 | 41±4 | 89±13 | 45±2 | 129±37 | 88±11 |
| MCAO (10) | 7.41±0.02 | 41±5 | 91±8 | 42±2 | 138±32 | 87±22 |
| Sham+MCAO (7) | 7.42±0.03 | 43±3 | 101±12 | 45±3 | 130±21 | 102±16 |
| IPC+MCAO (7) | 7.42±0.03 | 40±4 | 96±7 | 45±2 | 130±24 | 101±18 |
Values are mean ± SD.
Hb expression in neurons
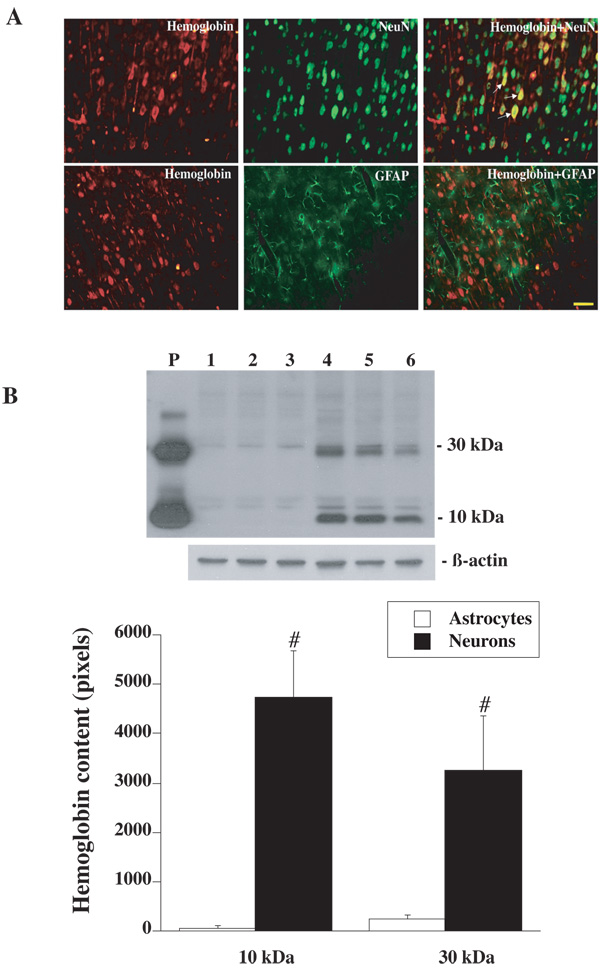
Immunohistochemistry revealed that Hb was widely expressed in cerebral cortex, with lower levels in the caudate. Most Hb positive cells appeared to be neurons and Hb immunoreactivity was primarily localized within the cytoplasm and dendrites. Negative control sections showed no staining. Double labeling demonstrated that Hb positive cells were co-localized with NeuN positive cells, but not GFAP positive cells (Figure 1A). Western blot analysis of primary cultured cerebral neurons showed one Hb band around 10 kDa and another band around 30 kDa. Hb protein levels were significantly higher in primary cultured neurons than that in the primary cultured astrocytes (Figure 1B).
Figure 1.
(A) Double labeling of rhodamine-labeled hemoglobin with FITC-labeled NeuN (a neuronal marker) or GFAP (an astrocyte marker). Scale bar=50µm. (B) Western blot analysis showing Hb levels in primary cultured cerebral astrocytes (lanes 1 to 3) and neurons (lanes 4 to 6). Rat Hb was used as a positive control (P). Value are expressed as mean ± SD, n=3. #p<0.05 versus astrocytes.
IPC-induced Hb mRNA and protein upregulation
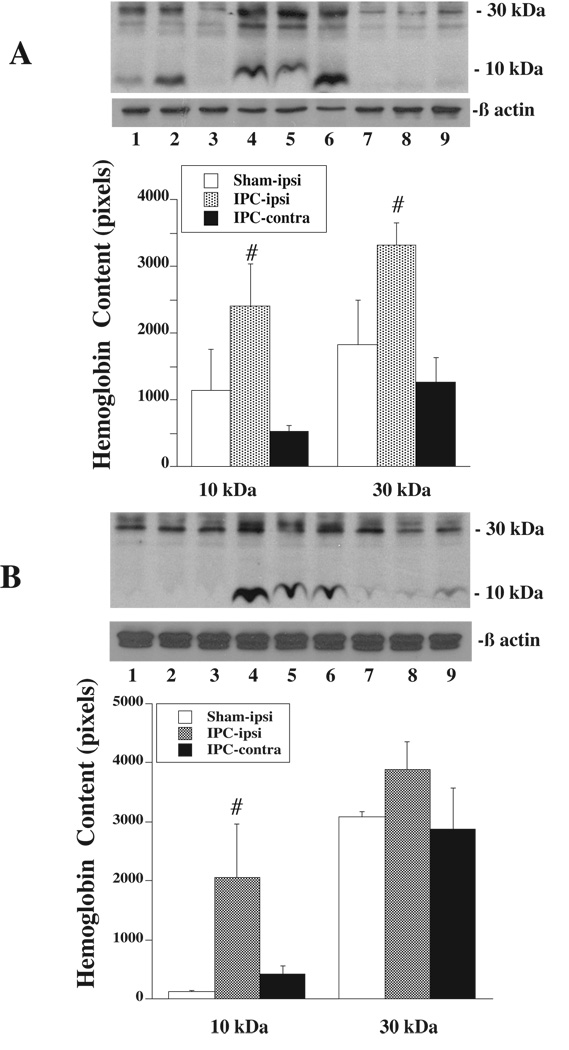
Western blot analysis showed that the Hb protein levels were higher in the ipsilateral caudate and the ipsilateral cortical core sample 24 hours after 15 minutes of IPC compared to both the contralateral side and the ipsilateral side of sham operated rats (Figure 2). To further confirm the synthesis of Hb within the brain, quantitative real-time PCR was performed on rat brains subjected to IPC or sham operation. There was a 1.8±0.9 fold increase in HbA mRNA in the ipsilateral caudate and 2.6±1.2 fold increase in the ipsilateral cortical core sample in IPC-treated rats compared to shams (p<0.05). HbB mRNA was also upregulated by 27.9±8.4 times in the ipsilateral caudate and 4.8±2.3 times in the ipsilateral cortical core compared to shams (p<0.05).
Figure 2.
Western blot analysis showing Hb levels in the caudate (A) and cortical core sample (B) 24 h after sham operation or 15 min of IPC. Lanes 1 to 3: the ipsilateral caudate or cortical core of shams; Lanes 4 to 6: the ipsilateral caudate or cortical core sample of IPC-treated rats; Lanes 7 to 9: the contralateral caudate or cortical core sample of IPC-treated rats. Value are expressed as mean ± SD, n=3. #p<0.05 versus the other groups.
pMCAO-induced Hb expression
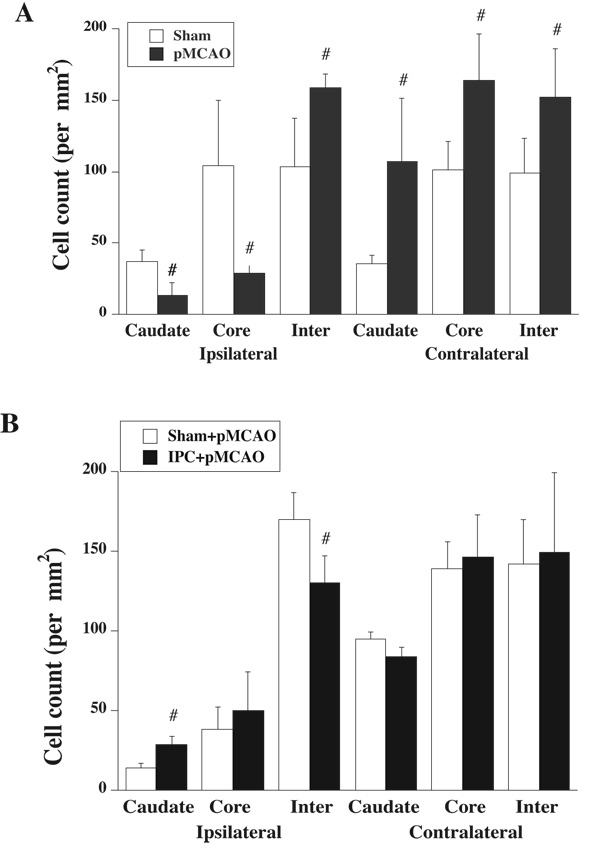
To test the effect of ischemic injury on Hb expression, rats were subjected to 24 hours of pMCAO. Immunohistochemistry staining was performed on brain sections and Hb positive cells were counted in six sites, the ipsi- and contralateral caudate, and cortical core and intermediate zones. The number of Hb positive cells in the ipsilateral intermediate zone of rats that underwent pMCAO was increased compared to shams (p<0.05, Figure 3A). There were more Hb positive cells in the contralateral caudate, and cortical core and intermediate zones than in shams. However, Hb positive cells in the ipsilateral caudate and cortical core were less in pMCAO rats than in shams (Figure 3A).
Figure 3.
(A) Hb positive cells in the caudate, cortical core (core) and intermediate (inter) zones of ipsilateral and contralateral hemisphere 24 h after sham or permanent MCAO. Value are expressed as mean ± SD, n=4. #p<0.05 versus sham. (B) Hb positive cells in the caudate, and cortex core (core) and intermediate (inter) zones of rats that underwent 24 h of permanent MCAO 3 days after IPC or sham operation. Values are expressed as mean ± SD, n=3. #p<0.05 versus sham.
By quantitative real-time PCR, there was a 3.2±2.4 fold increase in HbA mRNA in the ipsilateral intermediate zone of MCAO-subjected rats compared to shams (p<0.05, Table 2). In contrast, a decreased expression of HbA mRNA was observed in the ipsilateral caudate and cortical core 24 hours after MCAO (p<0.05, Table 2). There were no significant differences in HbA mRNA levels in the contralateral caudate, and cortical core and intermediate zones between MCAO-subjected and shams. Changes in HbB mRNA expression 24 hours after permanent MCAO were similar to that for HbA (Table 2). To confirm the PCR products were HbA and HbB, the products were sequenced. PCR products were HbA and HbB rather than neuroglobin.
Table 2.
Fold changes in HbA and HbB mRNA after 24 hours of permanent MCAO
| Ipsilateral |
Contralateral |
|||||
|---|---|---|---|---|---|---|
| Caudate | Core | Intermediate | Caudate | Core | Intermediate | |
| HbA | 0.38 ± 0.11* | 0.35 ± 0.14* | 3.23 ± 2.43* | 1.15 ± 0.41 | 0.85 ± 0.44 | 1.23 ± 0.26 |
| HbB | 0.43 ± 0.28* | 0.23 ± 0.39* | 1.82 ± 1.36 | 0.57 ± 0.09* | 0.33 ± 0.09* | 0.49 ± 0.13* |
Values are mean ± SD, n=3 per group
p <0.05 versus the shams (value=1). Mann-Whitney U-rank test.
Effects of IPC on pMCAO-induced Hb expression
To investigate the effect of IPC on Hb expression after pMCAO, rats underwent pMCAO three days after 15 minutes of IPC or sham operation and were killed 24 hours after pMCAO. The rat brains were used for immunohistochemistry analysis and quantitative real-time PCR. The number of Hb positive cells in the ipsilateral caudate was significantly increased in IPC-treated rats (p<0.05, Figure 3B). In contrast, IPC-treated rat had fewer Hb positive cells in the ipsilateral intermediate zone than that in the shams (p<0.05, Figure 3B). There was similar number of Hb positive cells in the contralateral side in these two groups (Figure 3B).
HbA mRNA levels in the ipsilateral caudate in IPC-treated rat were 2.4 ± 1.4 folds higher than in sham operated rats undergoing pMCAO (p<0.05, Table 3). HbB mRNA expression in the ipsilateral caudate and cortical intermediate zone were also increased by 1.9 ± 0.6 and 47.3 ± 2.9 fold, respectively (p<0.05, Table 3). However, IPC-treated rats had lower HbB mRNA levels in the contralateral caudate and cortex core than in shams (p<0.05, Table 3).
Table 3.
Fold changes of HbA and HbB mRNA after 24 hours of permanent MCAO in IPC-treated rats
| Ipsilateral |
Contralateral |
|||||
|---|---|---|---|---|---|---|
| Caudate | Core | Intermediate | Caudate | Core | Intermediate | |
| HbA | 2.39 ± 1.17* | 0.99 ± 0.22 | 0.77 ± 0.33 | 1.49 ± 1.45 | 1.57 ± 1.78 | 1.73 ± 1.37 |
| HbB | 1.86 ± 0.59* | 1.38 ± 0.62 | 47.3 ± 2.94* | 0.33 ± 0.04* | 0.55 ± 0.39* | 0.88 ± 0.34 |
Values are mean ± SD, n=3 per group
p <0.05 versus the sham+MCAO (value=1). Mann-Whitney U-rank test.
OGD-induced Hb expression in cultured neurons
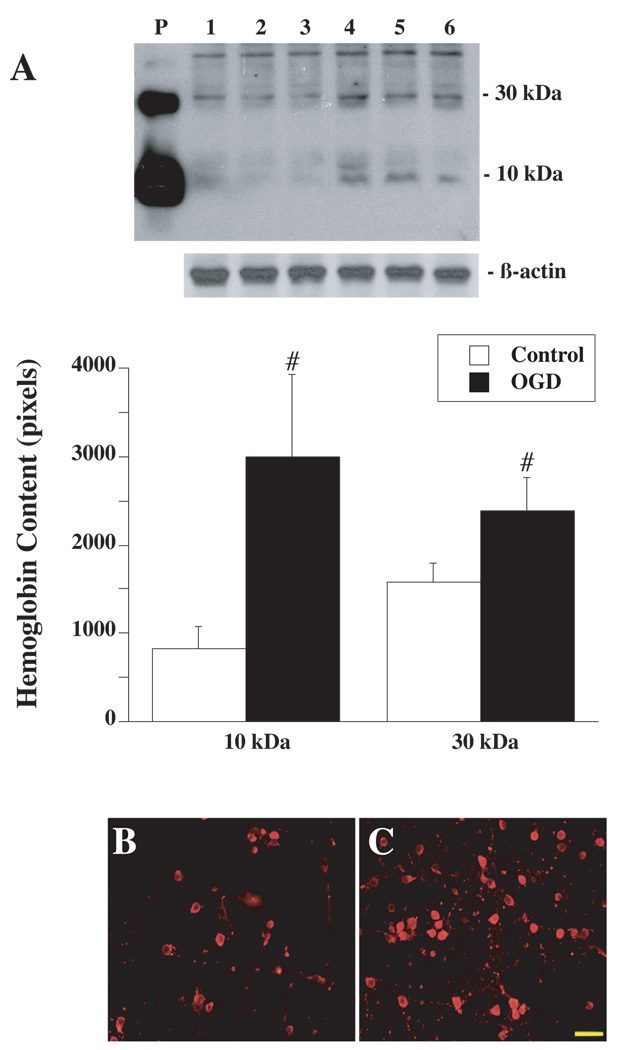
To investigate whether Hb expression is induced by ischemic-like conditions in vitro, primary cultured neurons were deprived of oxygen-glucose for 2 hours followed by 22 hours reoxygenation. Western blot analysis showed that OGD-treated cells had markedly higher Hb protein levels compared to the control (Figure 4A). Immunohistochemistry demonstrated that Hb immunoreactivity was localized in the cytoplasm and dendrites of cultured neurons (Figure 4B and Figure 4C). Quantitative real-time PCR showed a 1.9±0.4 folds increase in HbA mRNA in OGD-treated cells compared to controls (p<0.05), whereas HbB mRNA expression was decreased to 0.3±0.1 times to the control (p<0.05).
Figure 4.
(A) Western blot analysis showing Hb levels in primary cultured neurons not exposed to OGD (control, lanes 1 to 3) or exposed to 2 h of OGD with 22 h of reoxygenation (lanes 4 to 6). Rat Hb was used as a positive control (P). Values are expressed as mean ± SD, n=3. #p< 0.05 versus control. Hb immunoreactivities in primary cultured neurons not exposed to OGD (B) or exposed to 2 h of OGD (C) with 22 h of reoxygenation. Scale bar=50µm.
DISCUSSION
The main findings of our study were that: (1) Hb was mainly expressed in cerebral neurons in vivo and in vitro; (2) Hb protein and mRNA levels were significantly upregulated in the ipsilateral caudate and cortical core samples after IPC; (3) Hb was increased in the ischemic penumbra (intermediate zone) and nonischemic hemisphere after 24 hours of pMCAO, but it was decreased in ischemic core areas; (4) Hb was increased in neurons after exposed to OGD for 2 hours with 22 hours of reoxygenation; (5) IPC reduced pMCAO-induced Hb downregulation. These results indicate that neurons can synthesize Hb, which can be upregulated by ischemia. IPC-induced Hb upregulation may contribute to IPC-induced neuroprotection.
Hb has been studied for over a century and it has become clear that hemoglobins are widely distributed in diverse organisms including those which do not possess a bloodstream (Bogusz et al 1988; Poyart et al 1992; Wakabayashi et al 1986; Zhu and Riggs 1992). In vertebrates, hemoglobin is usually thought of as an important oxygen transport protein mainly present in erythrocytes circulating in the blood. In the present study, we provide the evidence that Hb is also present in neurons. Immunohistochemistry of adult rat brains revealed that Hb is preferentially expressed in cerebral neurons, with very low expression in glia. Western blotting of primary cultured cerebral neurons and astrocytes demonstrated that neurons have much higher levels of Hb than astrocytes, consistent with the in vivo results. Ohyagi et al. also found Hb in neurons of embryonic and adult mice brain, with low or no expression in the glia cells (Ohyagi et al 1994). The expression of Hb and its mRNA have also been found in neurons in hippocampus and granule cells in cerebellum (Blalock et al 2003; Slemmon et al 1994; Wu et al 2004). Taken together, these data suggest that Hb is widely expressed in brain and is primarily neuronal.
The function of neuronal Hb is still unclear. The concentration of Hb in brain is only about one thousandth of that in blood indicating that it does not represent a major repository of oxygen. It may be that neuronal Hb acts to facilitate the movement of O2 from the cell membrane to mitochondria, in a manner analogous to myoglobin in slow-twitch muscle fibers that rely on oxidative phosphorylation to produce ATP. It is also possible that Hb has functions in brain that are not directly related to O2, for example Hb could be a substrate for degradation by heme oxygenases resulting in the production of carbon monoxide, Fe and bilirubin.
As Hb is an oxygen-binding protein preferentially expressed in neurons and it is of interest to understand if cerebral ischemia can regulate neuronal Hb levels. In the present study, we found that Hb protein is significantly increased in the ipsilateral caudate and cortical core after IPC which can induce ischemic tolerance in the rat (Masada et al 2001). Quantitative real-time PCR also demonstrated that such IPC also significantly upregulates the expression of HbA and HbB mRNA. An important point to be discussed here involves the opposite results obtained by a previous study in mice, where HbB mRNA was significantly decreased in the ischemic cortex compared to nonischemic cortex 24 hours after IPC (Stenzel-Poore et al 2003). Several factors including species, brain region and severity of ischemia may account for such discrepancies.
Upregulation of Hb after IPC may be related to the activation of hypoxia inducible factor-1 (HIF-1α). HIF-1α is an important regulator of the response to hypoxia and oxygen homeostasis (Sharp and Bernaudin 2004). HIF-1 modulates β-globin gene expression during vertebrate development (Bichet et al 1999). There is evidence that HIF-1α is activated in response to ischemic preconditioning (Bergeron et al 2000; Gidday et al 1994). Further studies will be needed to investigate the mechanisms of induction of Hb by IPC.
In present study, we found that the number of Hb positive cells in the cortical intermediate (penumbral) zone and nonischemic hemisphere of rats undergoing 24 hours of pMCAO were markedly increased compared to those in the shams, whereas Hb positive cells in the ischemic core were significantly less than in shams. To confirm the expression of Hb in the brain, we performed quantitative real-time PCR after 24 hours of permanent MCAO. Real-time PCR revealed that Hb mRNA expression was increased in the cortex intermediate zone compared to shams, with markedly lower levels in the ischemic core. The reductions in Hb protein and mRNA within the core of the infarct probably reflect the severe reductions in blood flow within that region with concomitant declines in mRNA levels and protein synthesis.
Oxygen glucose deprivation is used as in vitro model as ischemia/reperfusion injury (Goldberg and Choi 1993). In the present study, we demonstrated that 2 hours of OGD followed by 22 hours of recovery significantly increased Hb protein levels in neurons similar to the effects of IPC in vivo. However, while PCR revealed that HbA mRNA expression was upregulated by 2 hours of OGD, HbB mRNA expression was downregulated, suggesting that α-globin and β-globin may have a different expression pattern.
There is growing evidence showing that IPC provides robust protection or tolerance against a subsequent prolonged ischemic event (Dirnagl et al 2003; Kirino 2002; Kitagawa et al 1990; Murry et al 1986). Our previous studies have also demonstrated that IPC attenuates brain edema formation and cerebrovascular injury after pMCAO (Masada et al 2001). The precise mechanisms of neuroprotection that lead to ischemic tolerance are not well understood. As mentioned above, Hb is upregulated after IPC in rats. We then tested the effect of IPC on Hb expression after pMCAO. Immunostaining showed that the number of Hb positive cells in the ipsilateral caudate was increased after pMCAO in IPC-treated rats compared to the sham-treated rats, whereas the number of Hb positive cells in the ipsilateral intermediate zone following pMCAO was less in IPC-treated rats. Quantitative real-time PCR revealed that IPC-treated rats had higher Hb mRNA levels in the ipsilateral caudate compared to the shams. An increased of expression of Hb in the ischemic core area may help neurons to survive under cerebral ischemia. There is evidence showing that enhanced expression of neuroglobin, a respiratory protein of the nervous system, promotes neuronal survival under brain hypoxia and ischemia (Khan et al 2006; Sun et al 2001; Sun et al 2003).
It is well known that exogenous hemoglobin released from red blood cells after intracerebral hemorrhage is neurotoxic (Xi et al 2006). Recent studies have shown that exogenous Hb can induce cell death in the rat cerebral cortical neurons through the activation of caspase cascades and oxidative stress (Wang et al 2002). However, the function of endogenous neuronal hemoglobin, which are present in much lower concentrations than found in blood, is still not clear. Our previous study found ICH-induced brain injury in the iron-deficient rats is greater than normal control rats (Shao et al 2005). It is not clear if iron deficiency results in lower neuronal hemoglobin levels as well as anemia. Future studies should determine if endogenous neuronal hemoglobin is neuroprotective.
In conclusion, our present study demonstrates that Hb is expressed in cerebral neurons, and that neuronal Hb is inducible after cerebral ischemia. An understanding of the mechanisms of induction of neuronal Hb after ischemia and the function of neuronal Hb should be helpful in seeking effective new treatment for stroke.
Acknowledgements
This study was supported by grants NS-017760, NS-039866, NS-047245, and NS-052510 from the National Institutes of Health (NIH). The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
REFERENCES
- Andjelkovic AV, Stamatovic SM, Keep RF. The protective effects of preconditioning on cerebral endothelial cells in vitro. J Cereb Blood Flow Metab. 2003;23:1348–1355. doi: 10.1097/01.WCB.0000091762.61714.FE. [DOI] [PubMed] [Google Scholar]
- Bergeron M, Gidday JM, Yu AY, Semenza GL, Ferriero DM, Sharp FR. Role of hypoxia-inducible factor-1 in hypoxia-induced ischemic tolerance in neonatal rat brain. Ann Neurol. 2000;48:285–296. [PubMed] [Google Scholar]
- Bichet S, Wenger RH, Camenisch G, Rolfs A, Ehleben W, Porwol T, Acker H, Fandrey J, Bauer C, Gassmann M. Oxygen tension modulates beta-globin switching in embryoid bodies. Faseb J. 1999;13:285–295. doi: 10.1096/fasebj.13.2.285. [DOI] [PubMed] [Google Scholar]
- Blalock EM, Chen KC, Sharrow K, Herman JP, Porter NM, Foster TC, Landfield PW. Gene microarrays in hippocampal aging: statistical profiling identifies novel processes correlated with cognitive impairment. J Neurosci. 2003;23:3807–3819. doi: 10.1523/JNEUROSCI.23-09-03807.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bogusz D, Appleby CA, Landsmann J, Dennis ES, Trinick MJ, Peacock WJ. Functioning haemoglobin genes in non-nodulating plants. Nature. 1988;331:178–180. doi: 10.1038/331178a0. [DOI] [PubMed] [Google Scholar]
- Choudhri TF, Hoh BL, Solomon RA, Connolly ES, Jr, Pinsky DJ. Use of a spectrophotometric hemoglobin assay to objectively quantify intracerebral hemorrhage in mice. Stroke. 1997;28:2296–2302. doi: 10.1161/01.str.28.11.2296. [DOI] [PubMed] [Google Scholar]
- Dirnagl U, Simon RP, Hallenbeck JM. Ischemic tolerance and endogenous neuroprotection. Trends Neurosci. 2003;26:248–254. doi: 10.1016/S0166-2236(03)00071-7. [DOI] [PubMed] [Google Scholar]
- Gidday JM, Fitzgibbons JC, Shah AR, Park TS. Neuroprotection from ischemic brain injury by hypoxic preconditioning in the neonatal rat. Neurosci Lett. 1994;168:221–224. doi: 10.1016/0304-3940(94)90455-3. [DOI] [PubMed] [Google Scholar]
- Goldberg MP, Choi DW. Combined oxygen and glucose deprivation in cortical cell culture: calcium-dependent and calcium-independent mechanisms of neuronal injury. J Neurosci. 1993;13:3510–3524. doi: 10.1523/JNEUROSCI.13-08-03510.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardison R. Hemoglobins from bacteria to man: evolution of different patterns of gene expression. J Exp Biol. 1998;201:1099–1117. doi: 10.1242/jeb.201.8.1099. [DOI] [PubMed] [Google Scholar]
- Hua Y, Keep RF, Hoff JT, Xi G. Thrombin preconditioning attenuates brain edema induced by erythrocytes and iron. J Cereb Blood Flow Metab. 2003;23:1448–1454. doi: 10.1097/01.WCB.0000090621.86921.D5. [DOI] [PubMed] [Google Scholar]
- Huang FP, Xi G, Keep RF, Hua Y, Nemoianu A, Hoff JT. Brain edema after experimental intracerebral hemorrhage: role of hemoglobin degradation products. J Neurosurg. 2002;96:287–293. doi: 10.3171/jns.2002.96.2.0287. [DOI] [PubMed] [Google Scholar]
- Jiang Y, Wu J, Hua Y, Keep RF, Xiang J, Hoff JT, Xi G. Thrombin-receptor activation and thrombin-induced brain tolerance. J Cereb Blood Flow Metab. 2002;22:404–410. doi: 10.1097/00004647-200204000-00004. [DOI] [PubMed] [Google Scholar]
- Karabiyikoglu M, Hua Y, Keep RF, Ennis SR, Xi G. Intracerebral hirudin injection attenuates ischemic damage and neurologic deficits without altering local cerebral blood flow. J Cereb Blood Flow Metab. 2004;24:159–166. doi: 10.1097/01.WCB.0000100062.36077.84. [DOI] [PubMed] [Google Scholar]
- Khan AA, Wang Y, Sun Y, Mao XO, Xie L, Miles E, Graboski J, Chen S, Ellerby LM, Jin K, Greenberg DA. Neuroglobin-overexpressing transgenic mice are resistant to cerebral and myocardial ischemia. Proc Natl Acad Sci U S A. 2006;103:17944–17948. doi: 10.1073/pnas.0607497103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirino T. Ischemic tolerance. J Cereb Blood Flow Metab. 2002;22:1283–1296. doi: 10.1097/01.WCB.0000040942.89393.88. [DOI] [PubMed] [Google Scholar]
- Kitagawa K, Matsumoto M, Tagaya M, Hata R, Ueda H, Niinobe M, Handa N, Fukunaga R, Kimura K, Mikoshiba K, et al. 'Ischemic tolerance' phenomenon found in the brain. Brain Res. 1990;528:21–24. doi: 10.1016/0006-8993(90)90189-i. [DOI] [PubMed] [Google Scholar]
- Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- Masada T, Hua Y, Xi G, Ennis SR, Keep RF. Attenuation of ischemic brain edema and cerebrovascular injury after ischemic preconditioning in the rat. J Cereb Blood Flow Metab. 2001;21:22–33. doi: 10.1097/00004647-200101000-00004. [DOI] [PubMed] [Google Scholar]
- McCarthy KD, de Vellis J. Preparation of separate astroglial and oligodendroglial cell cultures from rat cerebral tissue. J Cell Biol. 1980;85:890–902. doi: 10.1083/jcb.85.3.890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murry CE, Jennings RB, Reimer KA. Preconditioning with ischemia: a delay of lethal cell injury in ischemic myocardium. Circulation. 1986;74:1124–1136. doi: 10.1161/01.cir.74.5.1124. [DOI] [PubMed] [Google Scholar]
- Ohyagi Y, Yamada T, Goto I. Hemoglobin as a novel protein developmentally regulated in neurons. Brain Res. 1994;635:323–327. doi: 10.1016/0006-8993(94)91455-9. [DOI] [PubMed] [Google Scholar]
- Poyart C, Wajcman H, Kister J. Molecular adaptation of hemoglobin function in mammals. Respir Physiol. 1992;90:3–17. doi: 10.1016/0034-5687(92)90130-o. [DOI] [PubMed] [Google Scholar]
- Shao J, Xi G, Hua Y, Schallert T, Felt B. Intracerebral hemorrhage in the iron-deficient rat. Stroke. 2005;36:660–664. doi: 10.1161/01.STR.0000155744.90689.78. [DOI] [PubMed] [Google Scholar]
- Sharp FR, Bernaudin M. HIF1 and oxygen sensing in the brain. Nat Rev Neurosci. 2004;5:437–448. doi: 10.1038/nrn1408. [DOI] [PubMed] [Google Scholar]
- Slemmon JR, Hughes CM, Campbell GA, Flood DG. Increased levels of hemoglobin-derived and other peptides in Alzheimer's disease cerebellum. J Neurosci. 1994;14:2225–2235. doi: 10.1523/JNEUROSCI.14-04-02225.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stenzel-Poore MP, Stevens SL, Xiong Z, Lessov NS, Harrington CA, Mori M, Meller R, Rosenzweig HL, Tobar E, Shaw TE, Chu X, Simon RP. Effect of ischaemic preconditioning on genomic response to cerebral ischaemia: similarity to neuroprotective strategies in hibernation and hypoxia-tolerant states. Lancet. 2003;362:1028–1037. doi: 10.1016/S0140-6736(03)14412-1. [DOI] [PubMed] [Google Scholar]
- Sun Y, Jin K, Mao XO, Zhu Y, Greenberg DA. Neuroglobin is up-regulated by and protects neurons from hypoxic-ischemic injury. Proc Natl Acad Sci U S A. 2001;98:15306–15311. doi: 10.1073/pnas.251466698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun Y, Jin K, Peel A, Mao XO, Xie L, Greenberg DA. Neuroglobin protects the brain from experimental stroke in vivo. Proc Natl Acad Sci U S A. 2003;100:3497–3500. doi: 10.1073/pnas.0637726100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wakabayashi S, Matsubara H, Webster DA. Primary sequence of a dimeric bacterial haemoglobin from Vitreoscilla. Nature. 1986;322:481–483. doi: 10.1038/322481a0. [DOI] [PubMed] [Google Scholar]
- Wang X, Mori T, Sumii T, Lo EH. Hemoglobin-induced cytotoxicity in rat cerebral cortical neurons: caspase activation and oxidative stress. Stroke. 2002;33:1882–1888. doi: 10.1161/01.str.0000020121.41527.5d. [DOI] [PubMed] [Google Scholar]
- Wu CW, Liao PC, Yu L, Wang ST, Chen ST, Wu CM, Kuo YM. Hemoglobin promotes Abeta oligomer formation and localizes in neurons and amyloid deposits. Neurobiol Dis. 2004;17:367–377. doi: 10.1016/j.nbd.2004.08.014. [DOI] [PubMed] [Google Scholar]
- Xi G, Keep RF, Hua Y, Xiang J, Hoff JT. Attenuation of thrombin-induced brain edema by cerebral thrombin preconditioning. Stroke. 1999;30:1247–1255. doi: 10.1161/01.str.30.6.1247. [DOI] [PubMed] [Google Scholar]
- Xi G, Keep RF, Hoff JT. Mechanisms of brain injury after intracerebral haemorrhage. Lancet Neurol. 2006;5:53–63. doi: 10.1016/S1474-4422(05)70283-0. [DOI] [PubMed] [Google Scholar]
- Zhu H, Riggs AF. Yeast flavohemoglobin is an ancient protein related to globins and a reductase family. Proc Natl Acad Sci U S A. 1992;89:5015–5019. doi: 10.1073/pnas.89.11.5015. [DOI] [PMC free article] [PubMed] [Google Scholar]