Abstract
Dysfunction in the cardiovascular system can lead to the progression of a number of disease entities that can involve cancer, diabetes, cardiac ischaemia, neurodegeneration and immune system dysfunction. In order for new therapeutic avenues to overcome some of the limitations of present clinical treatments for these disorders, future investigations must focus upon novel cellular processes that control cellular development, proliferation, metabolism and inflammation. In this respect, members of the mammalian forkhead transcription factors of the O class (FoxOs) have increasingly become recognized as important and exciting targets for disorders of the cardiovascular system. In the present review, we describe the role of these transcription factors in the cardiovascular system during processes that involve angiogenesis, cardiovascular development, hypertension, cellular metabolism, oxidative stress, stem cell proliferation, immune system regulation and cancer. Current knowledge of FoxO protein function combined with future studies should continue to lay the foundation for the successful translation of these transcription factors into novel and robust clinical therapies.
Keywords: cancer, cardiovascular disease, diabetes, forkhead box (Fox), immune system, oxidative stress, stem cell
INTRODUCTION
Disorders that involve conditions such as cardiovascular disease, diabetes or cancer affect a wide spectrum of the world’s population, with estimated annual healthcare costs consuming a significant proportion of the gross domestic product in the United States alone [1]. Successful care and treatment for multiple disease entities can therefore rely heavily upon new therapeutic avenues that focus upon the vascular system and address novel cellular pathways of proliferation, metabolism, inflammation and longevity. In this respect, members of the mammalian forkhead transcription factors of the O class (FoxOs), FoxO1, FoxO3, FoxO4 and FoxO6, have emerged as important targets for the cardiovascular system as they can modulate processes associated with angiogenesis, stem cell proliferation, cardiovascular injury, tumorigenesis and vascular cell longevity.
More than 100 forkhead genes and 19 human subgroups that range from FOXA to FOXS are now known to exist since the initial discovery of the fly Drosophila melanogaster gene forkhead (fkh) [2]. The nomenclature for human Fox proteins places all letters in upper case, otherwise only the initial letter is listed as upper case for the mouse, and for all other chordates the initial and subclass letters are in uppercase [3]. As forkhead proteins function as transcription factors to either inhibit or activate target gene expression, these proteins bind to DNA through the forkhead domain that relies upon 14 protein–DNA contacts. The forkhead domain in Fox proteins consists of three α-helices, three β-sheets and two loops that are referred to as the wings [4], but not all winged helix domains are considered to be Fox proteins [5]. High sequence homology is present in the α-helices and β-sheets, with variations described in either absent β-sheets and loops or additional α-helices. On X-ray crystallography [4] or nuclear magnetic resonance [6], the forkhead domain is described as a ‘winged helix’ as a result of a butterfly-like appearance. It is believed that post-translational modification of forkhead proteins, such as phosphorylation or acetylation, alters the binding to DNA to prevent transcriptional activity. However, other mechanisms may influence DNA binding of forkhead proteins, such as variations in the N-terminal region of the DNA recognition helix, changes in electrostatic distribution and the ability of forkhead proteins to be shuttled to the cell nucleus [7,8].
FoxO proteins modulate normal cellular function as well as pathological mechanisms of disease. Initial work with FoxO proteins has shown that metabolic signalling with these transcription factors is conserved among multiple species including Caenorhabditis elegans, D. melanogaster and mammals. For example, FoxO proteins are homologous with the transcription factor DAF-16 (dauer formation-16) in the worm C. elegans that can determine metabolic insulin signalling and lead to lifespan extension [9,10], suggesting a significant role for FoxO proteins in relation to mammalian cell function [7,11].
FoxO proteins are expressed throughout the body and are found in the ovary, prostate, skeletal muscle, brain, heart, lung, liver, pancreas, spleen, thymus, testis and cardiovascular system. FoxO expression occurs in both cardiac and vascular tissue, and studies in mice have shown that the mRNA distribution of Foxo1, Foxo3a and Foxo4 is similar in the embryo and adult [12]. In addition, Foxo1 expression was highest in adipose tissue, Foxo3a expression was greatest in the liver and Foxo4 expression was strongest in muscle [12]. Subsequent work in mice has described Foxo1 expression in all tissues with high levels in the ovaries [13]. Foxo3a also was found to be expressed in all tissues, and Foxo4 expression was considered to be more tissue specific in skeletal muscle [13]. As FoxO proteins are not equally expressed in all tissues, it is possible that individual FoxO proteins may have specificity with regard to cellular function. For example, FoxO6 expression is found in several regions of the brain that play a significant role in cognitive function and emotion, such as the hippocampus, the amygdala and the nucleus accumbens [14]. In contrast, FoxO1 may be more suited for the control of motor function and memory formation, since the expression of this protein is primarily in the striatum and subregions of the hippocampus [14]. In addition, FoxO3 is more diffusely represented in the hippocampus, cortex and cerebellum, suggesting a complementary role for this FoxO protein to control cognitive and motor function.
FOXO PROTEINS AND POST-TRANSLATIONAL MODIFICATION
The function of FoxO proteins are controlled by different post-translational modifications that include phosphorylation, acetylation and ubiquitylation [11,15–17]. The serine/threonine kinase Akt [also known as PKB (protein kinase B)] is one mediator of phosphorylation of FoxO1, FoxO3a and FoxO4 [7,11]. Activation of Akt is usually cytoprotective, such as during endothelial cell hypoxia [18], β-amyloid toxicity [19,20] and cardiomyopathy [21]. Akt can prevent cellular apoptosis through the phosphorylation of FoxO proteins [22,23]. Post-translational phosphorylation of FoxO proteins will maintain FoxO transcription factors in the cytoplasm [24] by association with 14-3-3 proteins and prevent the transcription of pro-apoptotic target genes [25,26] (Figure 1). An exception with regard to the subcellular trafficking of FoxO proteins involves FoxO6. This FoxO protein usually resides in the nucleus of cells and is phosphorylated by Akt in the nucleus [7].
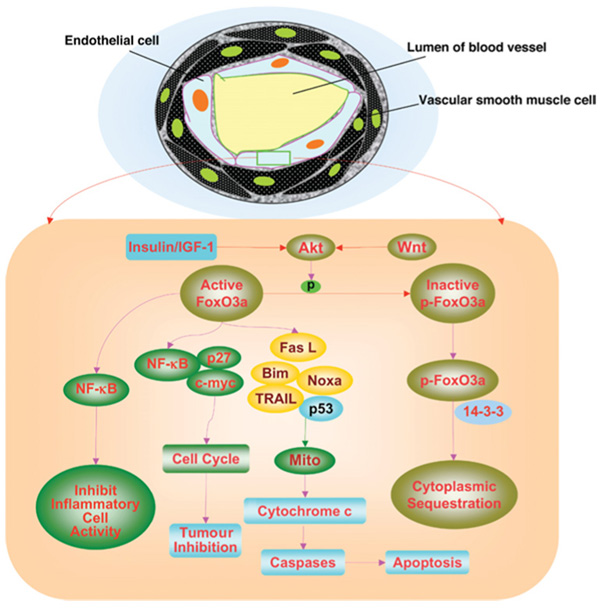
Figure 1. Inhibitory pathways for FoxO proteins involve insulin, IGF-1 and Akt, while downstream pathways of active FoxO proteins modulate immune system function, tumour inhibition and apoptosis.
In either blood vessels or vascular smooth muscle cells, FoxO protein activity can be blocked by insulin, IGF-1 or Wnt through the activation of Akt and the subsequent phosphorylation (p) of FoxO proteins. which maintain FoxO transcription factors in the cytoplasm by association with 14-3-3 proteins. During FoxO protein activation, FoxOs can prevent inflammatory cell activation through the inhibition of NF-κB. FoxO proteins can lead to apoptotic death pathways that involve mitochondrial (Mito) release of cytochrome c and caspase activation through a FasL (Fas-mediated ligand) death pathway, TRAIL, BH3-only proteins Noxa and Bim or p53. Cell-cycle inhibition that blocks tumour growth through FoxO protein activation can rely upon c-myc, p27 and NF-κB.
Activation of Akt ultimately during oxidative stress controls the apoptotic pathways of the caspase family that may offer an alternative mechanism to regulate FoxO proteins [27,28]. The caspases 1 and 3 have each been linked to the apoptotic pathways of genomic DNA cleavage, cellular membrane PS (phosphatidylserine) exposure and activation of inflammatory cells [29–31]. Caspase pathways may be tied to the forkhead transcription factor FoxO3a since increased activity of FoxO3a can result in cytochrome c release and caspase-induced apoptotic death [25,32–34] (Figure 1). Pathways that can inhibit caspase 3 activity appear to offer a unique regulatory mechanism. For example, caspase 3 cleavage of FoxO3a can lead to pro-apoptotic N-terminal fragments that can lead to cell death; however, during caspase 3 inhibition, inactive phosphorylated FoxO3a remains intact and does not lead to apoptotic cell injury during oxidative stress [25,32,33].
Additional post-translational mechanisms for FoxO proteins depend upon ubiquitylation and acetylation. Akt phosphorylation of FoxO proteins not only retains these transcription factors in the cytoplasm, but also leads to ubiquitination and degradation through the 26S proteasome [15,35]. In the absence of Akt, IKK {IκB [inhibitor of NF-κB (nuclear factor κB)] kinase} can also directly phosphorylate and block the activity of FoxO proteins, such as FoxO3a. This leads to the proteolysis of FoxO3a via the ubiquitin-dependent proteasome pathway [11,15–17]. Sgk (serum- and glucocorticoid-inducible protein kinase), a member of a family of kinases termed AGC (protein kinase A/protein kinase G/protein kinase C) kinases, which includes Akt, can also phosphorylate and retain FoxO3a in the cytoplasm [7,11]. Knowledge that Sgk and Akt can phosphorylate FoxO3a at different sites may offer new opportunities to more effectively prevent apoptotic cell injury that may be mediated by FoxO3a activity. Yet, phosphorylation of FoxO proteins does not always lead to negative regulation. Interestingly, JNK (c-Jun N-terminal kinase) phosphorylates 14-3-3 protein leading to the nuclear localization of FoxO proteins, such as FoxO3a [7], suggesting that JNK promotes apoptosis through increased FoxO protein activity. The protein kinase mammalian sterile 20-like kinase-1 also can phosphorylate FoxO proteins directly and lead to their activation [36]. The ability of sterile 20-like kinase-1 to activate FoxO proteins may be linked to JNK, as the kinase can increase JNK activation [37]. FoxO proteins also are acetylated by histone acetyltransferases that include p300, CBP [CREB (cAMP-response-element-binding protein)-binding protein] and the CBP-associated factor and are deacetylated by histone deacetylases, such as SIRT1 [the mammalian orthologue of the Sir2 (silent information regulator 2) protein] [11,15–17]. Acetylation of FoxO proteins provides another avenue for the control of these proteins. Once acetylated, such as by CBP, FoxO proteins may translocate to the cell nucleus but have diminished activity, since acetylation of lysine residues on FoxO proteins has been shown to limit the ability of the proteins to bind to DNA [7]. In addition, acetylation can increase phosphorylation of FoxO proteins by Akt [7].
FOXO PROTEINS, OXIDATIVE STRESS AND NOVEL INTRACELLULAR PATHWAYS
Throughout the body, cell survival and lifespan is tied to the presence of oxidative stress and the subsequent induction of apoptotic cell injury. Although several different mechanisms can account for the degeneration of cardiac and vascular cells in the body, the generation of cellular oxidative stress represents a significant component of the pathological complications that can ensue in the vascular system. Oxidative stress is a result of the release of ROS (reactive oxygen species) that consist of oxygen free radicals and other chemical entities. Oxygen free radicals and mitochondrial DNA mutations have become associated with tissue injury, aging and accumulated toxicity for an organism [38]. ROS include superoxide free radicals, H2O2, singlet oxygen, NO and peroxynitrite [28]. Most reactive species are produced at low levels during normal physiological conditions and are scavenged by endogenous antioxidant systems that include SOD (superoxide dismutase), glutathione peroxidase, catalase and small molecules, such as vitamins C, E, D3 and nicotinamide, the amide form of niacin or vitamin B3 [39–41].
During periods of oxidative stress, FoxO transcription factors can lead to cell injury and apoptosis [42], since forkhead transcription factors such as FoxO1 and FoxO3a must be present for oxidative stress to result in apoptotic cell injury [43]. In addition, FoxO3a in conjunction with JNK has been shown to modulate an apoptotic ligand, activating a Fas-mediated death pathway in cultured motoneurons [44], to lead to apoptosis through TRAIL (tumour-necrosis-factor-related apoptosis-inducing ligand) and the BH3-only proteins Noxa and Bim in neuroblastoma cells [34], and to promote pro-apoptotic activity of p53 [45] (Figure 1). These studies correlate well with experimental work demonstrating that protein inhibition or gene knockdown of FoxO1 or FoxO3a can result in stroke reduction [46], mediate protection of metabotropic glutamate receptors during vascular injury [32], enhance pancreatic β-cell or neuronal survival through NAD+ precursors during oxidative stress [33] and provide trophic factor protection in the cardiovascular system with EPO (erythropoietin) [25] and neurotrophins [47]. However, further work is required since complete knockdown of FoxO proteins may be too simplistic of an approach in light of some investigations suggesting that FoxO1 is necessary to control insulin signalling for the normalization of cellular metabolism [48] and that the loss of FoxO1, FoxO3a and FoxO4 protein expression may actually lead to an increase in free radical release that can be responsible for oxidative stress [49]. In addition, FoxO proteins may be protective during aging and exercise, since FoxO3a activity may enhance vascular smooth muscle antioxidant properties in aged animals and be beneficial to the cardiovascular system during physical exertion [50].
Interestingly, FoxO proteins are linked to novel signal transduction pathways involved in both cell development and cell death. One pathway in particular involves proteins that are derived from the Drosophila Wingless (Wg) and the mouse Wnt-1 (previously known as Int-1) genes (Figure 1 and Figure 2). The Wnt proteins are secreted cysteine-rich glycosylated proteins that can control cell proliferation, differentiation, survival and tumorigenesis [51,52]. One Wnt pathway controls target gene transcription through β-catenin, generally referred to as the canonical pathway [51,52]. It is the β-catenin pathway that appears to tie FoxO proteins and Wnt signalling together. For example, in relation to Alzheimer’s disease, amyloid is toxic to cells [19,20] and is associated with the phosphorylation of FoxO1 and FoxO3a that can be blocked with ROS scavengers [53]. A common denominator in the pathways linked to amyloid toxicity involves Wnt signalling through β-catenin. β-Catenin may increase FoxO transcriptional activity and competitively limit β-catenin interaction with members of the LEF/Tcf (lymphoid enhancer factor/T-cell factor) family [54], and β-catenin also has been demonstrated to be necessary for protection against amyloid toxicity in neuronal cells [20].
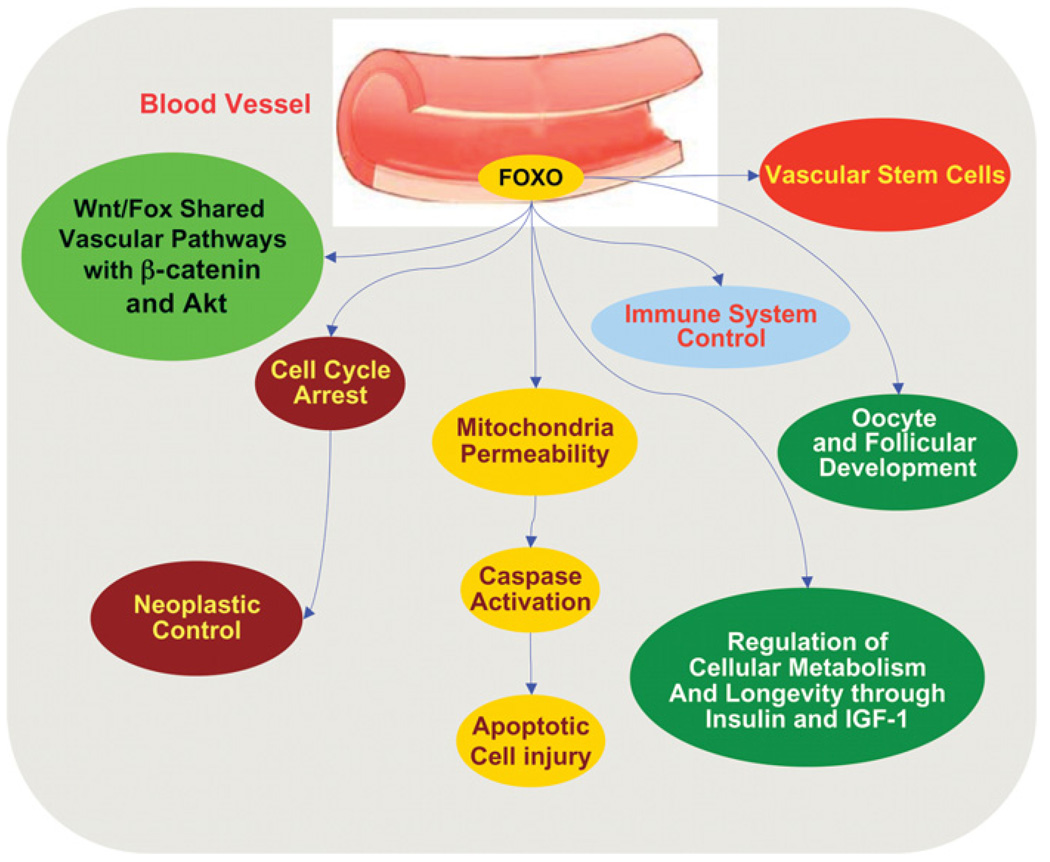
Figure 2. In the vascular system, FoxO proteins provide critical oversight of a broad range of cellular functions that span from stem cell development, modulation of cellular metabolism to apoptotic cell loss.
FoxO proteins are vital to processes that involve apoptotic cell injury following increased mitochondrial membrane permeability with subsequent caspase activation. In addition, FoxO proteins can block neoplastic growth through cellular mechanisms that require cell-cycle arrest, regulate oocyte and follicular development, and modulate inflammatory cell activation. FoxO proteins also are involved in pathways that control cellular metabolism and longevity. For example, FoxO proteins are homologous with DAF-16 that can regulate cellular metabolism and lifespan. Furthermore, FoxO proteins can stimulate the IGFBP-1 promoter by binding to the IRS and both insulin as well as IGF-1 can suppress FoxO activity through activation of Akt. In relation to the integration with novel signal transduction pathways, FoxO proteins interface with Wnt and Akt to control cellular proliferation and survival with common mechanisms that can impact upon pathways such as those associated with β-catenin.
Another shared signal transduction pathway between Wnt and FoxO proteins involves Akt (Figure 2). Processes that involve cellular proliferation, injury and immune system modulation with Akt and FoxO proteins also have parallel cellular pathways with Wnt. For example, Wnt relies upon Akt for the proliferation and differentiation of cardiomyocytes [55]. In addition, reduction in tissue injury during pressure overload cardiac hypertrophy and the cytoprotective benefits of cardiac ischaemic preconditioning also appear to depend upon Akt [51,52]. Furthermore, Wnt overexpression can independently increase the phosphorylation and activation of Akt to promote cellular protection and control microglial activation [20].
FOXO PROTEINS, ANGIOGENESIS AND CARDIOVASCULAR FUNCTION
Critical to both the cardiac and vascular systems in relation to their integrated function is the ability of FoxO proteins to govern cardiovascular cell development, new vessel growth and smooth muscle cell proliferation. New capillary formation from pre-existing vessels into an avascular area is a process known as angiogenesis, which is present during embryogenesis, menstruation and pathological processes that involve wound healing, chronic inflammation and tumour growth [52,56]. FoxO proteins are intimately involved in endothelial cell development and angiogenesis (Figure 2). For example, Foxo3a−/− and Foxo4−/− mice develop without incidence and are indistinguishable from control littermates; however, mice that are singly deficient in Foxo1 die by embryonic day 11 and lack development of the vascular system [57]. Other work illustrates that endothelial cell colonies in Foxo1-deficient mice fail to respond to VEGF (vascular endothelial growth factor) in a manner similar to wildtype endothelial cells [58], suggesting that FoxOs are necessary not only for the development of vascular cells, but also for the biological response to cellular mediators.
During cardiac development, FoxO proteins also appear to be necessary to modulate cardiomyocyte proliferation. Both FoxO1 and FoxO3 are expressed during embryonic through prenatal stages in the developing myocardium. The expression of these FoxO proteins is believed to negatively regulate cardiomyocyte growth, since overexpression of FoxO1 blocks cardiomyocyte proliferation but expression of dominantnegative FoxO1 leads to enhanced cardiomyocyte growth [59]. These observations may provide clues into the roles of FoxO proteins during cardiac hypertrophy. Atrogin-1, a protein that can block cardiac hypertrophy, may rely upon the up-regulation of Foxo1 and Foxo3a to disrupt cardiac hypertrophy, since mice lacking atrogin-1 are susceptible to cardiac hypertrophy and do not yield increased expression of Foxo1 and Foxo3a [60].
With regard to smooth muscle cell growth, Foxo3a has been demonstrated to block vascular smooth muscle proliferation and may lessen the effects from disorders such as atherosclerosis and hypertension. In a rat balloon carotid arterial injury model, gene transfer of FoxO3a can inhibit neointimal hyperplasia through the prevention of vascular smooth muscle growth [61]. However, not all FoxO proteins may exert an inhibitory effect upon vascular smooth muscle cells. FoxO4 may inhibit smooth muscle cell differentiation through the repression of the transcriptional coactivator of smooth muscle genes myocardin [62], but other work suggests that FoxO4 also can increase MMP-9 (matrix metalloproteinase-9) expression to promote vascular smooth muscle migration and foster neointimal hyperplasia [63].
In light of the ability of FoxO proteins to regulate vascular smooth muscle cell proliferation, these transcription factors may have a significant clinical role with regard to disorders that involve hypertension and cardiac failure. Vascular smooth muscle cells are vital for the regulation of vascular tone and systemic arterial blood pressure. For example, high flow states in vessels can reduce FoxO1 activity, resulting in the potential proliferation of vascular smooth muscle cells, vascular neointimal hyperplasia and subsequent pathological states such as hypertension [64]. In fact, α1-adrenergic agonists that increase systemic blood pressure can have the reverse effect and stimulate the expression of FoxO1 and its nuclear translocation that ultimately may lead to apoptotic endothelial cell injury [65]. In addition, more than moderate levels of vessel cyclic stretch that can occur during hypertension may lead to the phosphorylation and inhibition of FoxO1 and FoxO3a in smooth muscle cells to contribute further to pathological smooth muscle cell proliferation [66]. Furthermore, in human as well as murine models of cardiac failure, increased expression of Fox transcription factors, such as FoxO1a, have been observed to suggest a potential association of FoxO proteins with imminent cardiac failure [67].
FOXO PROTEINS, CANCER PATHWAYS AND STEM CELLS
One of the most significant clinical applications for FoxO proteins involves treatments designed to control human cancer progression. FoxO proteins appear to be ideal in regulating tumour growth since they are pro-apoptotic, inhibit cell-cycle progression and can modulate vascular cell proliferation (Figure 2). For example, FoxO3a and FoxO4 can promote cell-cycle arrest in mouse myoblastic cell lines through modulation of GADD45 (growtharrest and DNA-damage-response protein 45) [7,68]. Treatment of chronic myelogenous leukaemia cell lines with the Bcr-Abl tyrosine kinase inhibitor imatinib requires FoxO3a activation to antagonize cell proliferation and promote apoptotic cell death through increased TRAIL production [69]. In addition, the transcription factor E2F-1 that controls the induction of the cell cycle has been reported in cell lines to increase the endogenous expression of FoxO1 and FoxO3a to lead to cell-cycle arrest [70]. However, the loss of FoxO3a activity in association with c-myc, p27 and NF-κB can result in cell-cycle induction and malignant transformation of mouse cells in the presence of oncogene activation [7,11] (Figure 1). It should be noted that FoxO protein inhibition of cell-cycle progression might not consistently lead to apoptotic cell death. Some investigations suggest that during oxidative stress, FoxO3a activation in association with the Sir2 homologue SIRT1 can lead to cell-cycle arrest, but not result in apoptosis [71].
Early clinical studies of breast cancer in relation to FOXO3a suggested that activation of FOXO3a was associated with lymph nodal metastasis and a poor prognosis [7,11]. However, other studies reported that FOXO3a was confined to the cytoplasm of human tumour cells, inactivated by IKK and that this inactivation of FOXO3a was associated with a poor prognosis in breast cancer [7,11], suggesting that FOXO3a subcellular localization and pathways that enhance its activity could be used not only as prognostic assays, but also as therapeutic targets. In animal studies, somatic deletion in mice of Foxo1, Foxo3a and Foxo4 results in the growth of thymic lymphomas and haemangiomas, illustrating further the potential of FoxO proteins to control vascular proliferation and function as repressors of tumour growth [72]. Studies in breast cancer cells parallel this work and show that increased activity of FOXO3a in association with JNK in breast cancer cell lines [73] or in association with CDK (cyclin-dependent kinase) inhibitor p27 in isolated human breast cancer cells can suppress breast cancer progression [74].
Studies with prostate cancer have shown that the tumour suppressor PTEN (phosphatase and tensin homologue deleted on chromosome 10) was mutated in almost 80% of tumours with the loss of FOXO1 and FOXO3a activity. In cell culture work, overexpression of FOXO1 and FOXO3a in prostrate tumour cell lines could result in apoptosis, suggesting that FOXO1 and FOXO3a were necessary for limiting prostate cell tumour growth [7,11]. In further support of this work, inhibition of FoxO3a activity can result in enhanced prostate tumour cell growth [7,11], while agents that increase FOXO3a activity in both androgen-sensitive and androgen-insensitive prostate cell lines prevent prostate cancer cell progression [75].
In addition to neoplasms in the breast and prostate, FoxO proteins also may represent a viable option to control tumour growth in tissues throughout the body. FOXO3a activation in colon carcinoma cell lines prevents tumour proliferation through Myc target genes that involve the Mad/Mxd family of transcriptional repressors [76]. Other investigations, such as with haemopoietic cancers, illustrate that the loss of FOXO3a activity in primary leukaemic cells may participate in oncogenic transformation in B-chronic lymphocytic leukaemia [77] and in the progression of chronic myelogenous leukaemia cell lines [69]. However, FoxO proteins may have a complex role during tumour growth. FOXO3a is a positive regulator of androgen receptor expression and therefore may also assist with prostate cancer cell proliferation [78]. In addition, loss of functional FOXO3a in human ovarian cancer cell lines can limit the sensitivity of ovarian cancer cells to chemotherapy [79], suggesting that FoxO proteins may be responsible for altered treatment outcomes in the presence of combined therapeutic approaches.
Interestingly, the ability of FoxOs to block tumour growth may have far more reaching clinical implications, such as in relation to vascular stem cell viability and regeneration (Figure 2). FoxO proteins were initially identified in fusion genes in human soft-tissue tumours and leukaemias, neoplasms now believed to harbour cancer stem cells for tumour self-renewal [80]. For example, FOXO1, termed FKHR (forkhead in rhabdomyosarcoma), and FOXO3a, also known as FKHRL-1 (forkhead in rhabdomyosarcoma like protein-1), and their genes were initially identified through chromosomal translocations in alveolar rhabdomyosarcoma tumours [7,15]. AFX (acute leukaemia fusion gene located in chromosome X), also known as the FOXO4 gene, was described as a gene that fused to the MLL transcription factor as a result of the t(X;11) chromosomal translocation in acute lymphoblastic leukaemia [7,15]. A fusion between FOXO2 and MLL also occurs in some cases of acute myeloid leukaemia that is believed to be identical with FOXO3a [7,15]. As a result of subsequent investigations from these early studies, it is now evident that FoxO proteins also have a significant role during progenitor cell development and reproduction. With regard to stem cell development, either simultaneous deletion of Foxo1, Foxo3a and Foxo4 or single deletion of Foxo3a in mice prevents the repopulation of haemopoietic stem cells and leads to apoptosis in these stem cell populations [49,81]. A number of cardiovascular cytoprotective agents, such as the growth factor EPO [26,82,83], also may be required to modulate FoxO protein activity during erythroid progenitor cell development [56,68], suggesting that current clinical use of agents, such as EPO during anaemia or cancer, may have less defined treatment implications for patients than originally anticipated [26,56]. In cell culture and animal studies, EPO is cytoprotective in vascular cells and can stimulate postnatal neovascularization by increasing EPC (endothelial progenitor cell) mobilization from the bone marrow [56,68,84]. Interestingly, the ability of EPO to foster eythroid progenitor cell development is dependent upon the inhibition of FoxO3a activity [26,56], but may also require regulation of specific gene expression through an EPO–FoxO3a association to promote erythropoiesis in cultured cells [85]. In relation to the reproductive potential of an organism, deletion of the FoxO3a gene results in the depletion of oocytes and subsequent infertility [86]. Other work using a mouse model of FoxO3a overexpression in oocytes suggests further that FoxO3a retards oocyte growth and follicular development and leads to anovulation and luteinization of unruptured follicles [87]. These studies may suggest a role for FoxO proteins, and specifically FoxO3a, in relation to not only the development of cancer stem cell niches, but also with regard to oocyte and follicular cell maturation. For example, in a small percentage of women who suffer from premature ovarian failure, mutations in FOXO3a and FOXO1a have been observed [88].
FOXO PROTEINS, DIABETES, STROKE AND AGING
Clinical studies also suggest that FoxO proteins play a significant role during diabetes, stroke and aging (Figure 2). When one considers diabetes, this disorder is a significant health concern for both young and older populations [89,90]. Approx. 16 million individuals in the United States and more than 165 million individuals worldwide suffer from diabetes. By the year 2030, it is predicted that more than 360 million individuals will be afflicted with diabetes and its debilitating conditions. Type 2 diabetes represents at least 80 % of all diabetics and is dramatically increasing in incidence as a result of changes in human behaviour and increased BMI (body mass index) [39,89]. Type 1 insulin-dependent diabetes is present in 5–10 % of all diabetics, but is increasing in adolescent minority groups [39,89]. Furthermore, the incidence of undiagnosed diabetes, impaired glucose tolerance and fluctuations in serum glucose in the young raises additional concerns.
Patients with diabetes can develop significant cardiovascular disease [39,91]. Interestingly, the development of insulin resistance and the complications of diabetes in the vascular system can be the result of cellular oxidative stress [39,89]. Hyperglycaemia can lead to increased production of ROS in endothelial cells, liver cells and pancreatic β-cells [39,89,90]. Recent clinical correlates support these experimental studies to show that elevated levels of ceruloplasmin are suggestive of increased ROS [39,89,90]. Furthermore, acute glucose swings in addition to chronic hyperglycaemia can trigger oxidative stress mechanisms, illustrating the importance for therapeutic interventions during acute and sustained hyperglycaemic episodes [39,89].
FoxO proteins are involved in several pathways responsible for cellular metabolism and diabetes. As noted previously, FoxO proteins are homologous with DAF-16, which can regulate cellular metabolism and lifespan [9,10]. FoxO proteins can stimulate the IGFBP-1 [IGF (insulin-like growth factor)-binding protein-1] promoter by binding to the IRS (insulin-responsive sequence) [92]. Both insulin as well as IGF-1 can suppress this activity through activation of Akt [92,93] (Figure 1 and Figure 2). Clinical studies provide further support for the role of FoxO proteins during metabolic disorders. The c.-343-1582C>T polymorphism of FOXO3a has been shown to display a significant association with BMI, such that the highest BMI was present in individuals homozygous for this allele [94]. Analysis of the genetic variance in FOXO1a and FOXO3a on metabolic profiles, age-related diseases, fertility, fecundity and mortality have observed higher HbA1c (glycated haemoglobin) levels and increased mortality risk associated with specific haplotypes of FOXO1a. Furthermore, there is an increased risk of stroke in two haplotypes of FOXO3a block-A [95], suggesting an association with FOXO1a and FOXO3a genes during oxidative stress disorders such as diabetes and stroke. These clinical observations may coincide with the demonstration in human EPCs that elevated glucose levels can reduce post-translational phosphorylation of FOXO1, FOXO3a and FOXO4 and allow for the nuclear translocation of these proteins to initiate an apoptotic programme in EPCs [96].
In some animal and cell culture studies, FoxOs may counteract the detrimental effects of high serum glucose levels. For example, IFN-γ (interferon-γ)-driven expression of tryptophan catabolism by CTLA-4 (cytotoxic T-lymphocyte antigen-4) may activate Foxo3a to protect dendritic cells from injury in non-obese diabetic mice [97]. Additional investigations have associated diabetic nephropathy with post-translational changes in FoxO3a by demonstrating that inhibitory phosphorylation of FoxO3a increases in rat and mouse renal cortical tissues 2 weeks after the induction of diabetes by streptozotocin [98], suggesting that the loss of FoxO3a activity can lead to renal disease. Recent work has demonstrated further that adipose-tissue-specific expression of Foxo1 in mice improved glucose tolerance and sensitivity to insulin during an elevated fat diet [99].
The preservation of cellular energy reserves and mitochondrial function also may be a critical factor for FoxO proteins to regulate cellular metabolism during diabetes (Figure 2). Chronic exposure to elevated levels of NEFAs (non-esterified fatty acids; ‘free fatty acids’) can increase ROS production in cells and has been shown to lead to mitochondrial DNA damage and impaired pancreatic β-cell function [39,89,90]. Furthermore, insulin resistance in the elderly has been associated with elevation in fat accumulation and altered mitochondrial oxidative and phosphorylation activity [39,89,90]. Interestingly, both animal and cell culture studies suggest that FoxO proteins may protect against mitochondrial injury to modulate cellular metabolism. In caloric-restricted mice that have decreased energy reserves, mRNA expression is progressively increased for Foxo1, Foxo3a and Foxo4 over a 2-year course [100]. This work is complementary to investigations in Drosophila and mammalian cells that demonstrate an increase in insulin signalling to regulate cellular metabolism during the up-regulation of FoxO1 expression [48].
However, additional work is required since other studies have indicated that inactivation of FoxOs is necessary for cytoprotection during diabetes. For example, studies in cardiomyocytes suggest detrimental results with enhanced FoxO activity. Increased transcriptional activity of FoxO1, such as by the Sirt1 activator resveratrol, can diminish insulin-mediated glucose uptake and result in insulin resistance [101]. In addition, other studies have shown that overexpression of Foxo1 in skeletal muscles of mice can lead to reduced skeletal muscle mass and poor glycaemic control [102], illustrating that activation of FoxO proteins may also impair cellular energy reserves. As a result, one potential agent to consider for the maintenance of cellular metabolism in diabetes is nicotinamide [40,103], an agent that also can inhibit FoxO protein activity [33]. In patients with diabetes, oral nicotinamide protects β-cell function, prevents clinical disease in islet-cell antibody-positive first-degree relatives of Type 1 diabetes and can reduce HbA1c levels [40,89,103]. It is of interest to note that nicotinamide may derive its protective capacity through two separate mechanisms of post-translational modification of FoxO3a. Nicotinamide not only can maintain phosphorylation of FoxO3a and inhibit its activity, but can also preserve the integrity of the FoxO3a protein to block FoxO3a proteolysis that can yield pro-apoptotic N-terminal fragments [33].
As an extension of the studies examining FoxOs and cellular metabolism, FoxO proteins have been linked to cell longevity and aging as shown by early studies linking DAF-16 in C. elegans to increased longevity [11,15–17] (Figure 2). However, the relationship between FoxO transcription factors and proteins that increased cellular lifespan has been met with controversy. SIRT1 is a NAD+-dependent deacetylase and the mammalian orthologue of the Sir2 protein associated with increased lifespan in yeast. Some studies suggest that stimulation of SIRT1 during starvation is dependent upon FoxO3a activity as well as p53 [104]. In contrast, other reports have shown in cell culture that SIRT1 may repress the activity of FoxO1, FoxO3a and FoxO4, suggesting that cellular longevity may benefit from reduction in FoxO-protein-generated apoptosis [105]. Additional studies offer alternative views to illustrate that SIRT1 binds to FoxO proteins, such as FoxO4, to catalyse its deacetylation and enhance FoxO4 activity, while acetylation of FoxO4 by CBP serves to inhibit FoxO4 transcriptional activity [11,15–17].
FoxO proteins may also be protective during aging, cell senescence and exercise. In cultured human dermal fibroblasts, gene silencing of FoxO3a protein results in cell morphology consistent with cell senescence, cell population doubling times and the generation of ROS, suggesting that FoxO protein activity may be required to extend cell longevity and limit oxidative stress [106]. Additional work in animal models of aging demonstrates a reduction in SIRT1 in the heart, but no significant change in FoxO3a expression with advanced age. However, during exercise training, an up-regulation of FoxO3a and SIRT1 activity is observed in the heart [50], suggesting that the benefits of physical activity for the cardiovascular system may be associated with FoxO proteins. Interestingly, increased levels of SIRT1 of < 7.5-fold can be associated with the expression of catalase, an antioxidant that is controlled by FoxO1a, to possibly reduce cell injury during oxidative stress. However, elevated levels of SIRT1 at 12.5-fold can result in cardiomyocyte apoptosis and decreased cardiac function [107]. In addition, FoxO proteins may be protective during aging, since loss of FoxO3a activity in explanted vascular smooth muscle of aged animals may limit tissue antioxidant properties through decreased MnSOD (manganese-SOD) and lead to enhanced cell injury with aging [108]. Extension of cellular lifespan that depends upon the prevention of cell senescence, at least in primary human cultured vascular cells, may also require the negative regulation of Akt to allow for the activation of FoxO3a [109].
FOXO PROTEINS AND IMMUNE SYSTEM REGULATION
Given that inflammatory cell activation may significantly influence vascular integrity [38], it may come as no surprise to learn that FoxO proteins also function as critical components for modulation of the immune system (Figure 2). The ability to regulate early apoptotic membrane PS externalization [30] and subsequent inflammatory cell activity [110] can ultimately impact upon cell survival and longevity, since activated immune cells can lead to the phagocytic removal of both neurons and vascular cells [28,111]. Inflammatory cells, such as macrophages or microglia, require the activation of intracellular cytoprotective pathways to proliferate and remove injured cells [112,113]. This can be a beneficial process and form a barrier for the removal of foreign micro-organisms and promote tissue repair during cell injury [39,68]. However, inflammatory cells may also lead to cellular damage through the generation of ROS and the production of cytokines [68].
Studies in patients with rheumatoid arthritis and osteoarthritis following synovial biopsy have demonstrated the phosphorylation of FOXO1 in macrophages, FOXO3a in lymphocytes and FOXO4 in macrophages, suggesting that loss of functional FOXO family members may lead to inflammatory cell activation in these disorders [114]. Additional work has shown that FOXO1 gene transcript levels are down-regulated in peripheral blood mononuclear cells of patients with systemic lupus erythematosus and rheumatoid arthritis [115], illustrating a potential aetiology through the loss of functional FOXO proteins for these disorders and possibly providing a biomarker of disease activity. Clinical work also suggests a relationship between the regulation of immune system activity and the induction of apoptotic pathways that are dependent upon FoxO proteins. FoxO proteins may work in concert with Fas signalling to clear activated T-cells following a decrease in cytokine stimulation in patients with autoimmune lymphoproliferative syndromes, suggesting that specific FoxO proteins may be targeted for treatment of autoimmune disorders [7]. In mice deficient for Foxo3a, lymphoproliferation, organ inflammation of the salivary glands, lung and kidney, and increased activity of helper T-cells results, supporting an important role for FoxO3a in preventing T-cell hyperactivity [116]. FoxO3a also appears to be necessary for neutrophil activity, since Foxo3a-null mice are resistant to models of neutrophilic inflammation that involve immune-complex-mediated inflammatory arthritis [117]. Prevention of inflammatory activation and cellular apoptosis, such as in systemic lupus erythematosus in animal models, may require the up-regulation of different Fox proteins, such as FoxJ1 and FoxO3a, that can block NF-κB activation and IFN-γ secretion [118] (Figure 1). Animal studies using experimental autoimmune encephalomyelitis to mimic multiple sclerosis and myelin injury also have shown that osteopontin, a protein expressed in multiple sclerosis lesions, leads to the prolonged survival of myelin-reactive T-cells and disease progression through a combination of events that involve FoxO3a inhibition, NF-κB activation and the expression of the pro-apoptotic proteins Bim, Bak and Bax [119].
CLINICAL CONCEPTS AND CONSIDERATIONS FOR THE FUTURE
It is extremely exciting to consider the almost limitless potential to target FoxO proteins for new clinical treatments in cardiovascular and related systems. These transcription factors modulate a number of critical cellular functions in relation to cell metabolism, cell survival and immune system function. For example, the ability of FoxO proteins to control cell-cycle progression and promote apoptotic cell death suggests that FoxO transcription factors may be developed for new advances against tumorigenesis. As an example, use of triple mutant FoxO3a expression, in which three phosphorylation sites have been altered to prevent inactivation of this protein, has been proposed as a potential therapeutic agent against melanoma tumours [120]. In addition, the known mutations in FoxO proteins that exist in several disease entities may provide novel insights for therapeutic strategies that can address a broad range of disorders. Further analysis in larger populations of patients with premature ovarian failure, diabetes or stroke could enhance our understanding of the role of FoxO proteins in these disorders. When one considers the role of FoxO proteins at the cellular level in cardiac and endothelial cells, targeting the activity of FoxO1, FoxO3a or FoxO4 may prevent the onset of pathological cardiac hypertrophy and neointimal hyperplasia that may result in atherosclerosis. Interestingly, new findings suggest that the utilization and combination of multiple biomarkers may improve risk assessment for patients suffering from cardiovascular disorders [121]. These studies support further the premise that FoxO proteins can serve as biomarkers of disease activity such as in individuals with imminent cardiac failure [67].
However, it must be realized that protocols to modulate FoxO proteins may have both beneficial and detrimental clinical outcomes. For example, the common pathways shared between Wnt and forkhead proteins may have unexpected side effects that relate to tumorigenesis [52]. In some scenarios, FoxO proteins may prevent tumorigenesis during Wnt deregulation, but, in other examples, FoxO proteins may assist with β-catenin activation and lead to tumour cell proliferation. Some forkhead transcription factors can activate the Wnt/β-catenin pathway [52]. In the presence of Wnt deregulation and increased β-catenin activity, tumorigenesis may ensue, such as with the proliferation of medulloblastoma tumours [80]. Investigations are required to clarify both independent and shared signal transduction pathways of FoxO proteins to understand further the role of these transcription factors during cardiovascular disease. For example, independent of Wnt signalling, FoxO proteins can sometimes prevent cell-cycle progression in cells without leading to apoptotic injury. Although this result may be considered beneficial to block hyper-proliferative processes in blood vessels that could lead to atherosclerosis and hypertension, in the setting of cancer these results would severely limit clinical utility. Prediction of biological outcomes during FoxO protein involvement may be uncertain and dependent upon a host of factors, such as tissue characteristics, cellular metabolic state and the age of an individual, advocating a much wider investigation of the complex biological roles FoxO proteins play during normal physiology and disease. Through further clinical and basic studies, we should be able to establish the immense potential as well as the possible contra-indications for the targeting of FoxO proteins into novel and robust clinical therapies for cardiovascular disorders.
ACKNOWLEDGEMENTS
We apologize to our colleagues whose work we were unable to cite as a result of space limitations.
FUNDING
This research was supported for K.M. by the American Diabetes Association; the American Heart Association; a Bugher Foundation Award; a Janssen Neuroscience Award; a LEARN Foundation Award; an MI Life Sciences Challenge Award; a Nelson Foundation Award; the National Institutes of Health National Institute of Environmental Health Sciences [grant number P30 ES06639]; and the National Institutes of Health National Institute of Neurological Disorders and Stroke/National Institute of Aging.
Abbreviations
- BMI
body mass index
- CBP
CREB (cAMP-response-element-binding protein)-binding protein
- DAF-16
dauer formation-16
- EPC
endothelial progenitor cell
- EPO
erythropoietin
- FKHR
forkhead in rhabdomyosarcoma
- FKHRL-1
forkhead in rhabdomyosarcoma like protein-1
- Fox
forkhead box
- HbA1c
glycated haemoglobin
- IFN-γ
interferon-γ
- IGF
insulin-like growth factor
- IGFBP-1
IGF-binding protein-1
- IRS
insulin-responsive sequence
- JNK
c-Jun N-terminal kinase
- NF-κB
nuclear factor κB
- IκB
inhibitor of NF-κB
- IKK
IκB kinase
- PS
phosphatidylserine
- PTEN
phosphatase and tensin homologue deleted on chromosome 10
- ROS
reactive oxygen species
- Sgk
serum- and glucocorticoid-inducible protein kinase
- Sir2
silent information regulator 2
- SOD
superoxide dismutase
- TRAIL
tumour-necrosis-factor-related apoptosis-inducing ligand.
REFERENCES
- 1.Maiese K. Triple play: promoting neurovascular longevity with nicotinamide, WNT, and erythropoietin in diabetes mellitus. Biomed. Pharmacother. 2008;62:218–232. doi: 10.1016/j.biopha.2008.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Weigel D, Jurgens G, Kuttner F, Seifert E, Jackle H. The homeotic gene fork head encodes a nuclear protein and is expressed in the terminal regions of the Drosophila embryo. Cell. 1989;57:645–658. doi: 10.1016/0092-8674(89)90133-5. [DOI] [PubMed] [Google Scholar]
- 3.Kaestner KH, Knochel W, Martinez DE. Unified nomenclature for the winged helix/forkhead transcription factors. Genes Dev. 2000;14:142–146. [PubMed] [Google Scholar]
- 4.Clark KL, Halay ED, Lai E, Burley SK. Co-crystal structure of the HNF-3/fork head DNA-recognition motif resembles histone H5. Nature. 1993;364:412–420. doi: 10.1038/364412a0. [DOI] [PubMed] [Google Scholar]
- 5.Larson ET, Eilers B, Menon S, et al. A winged-helix protein from Sulfolobus turreted icosahedral virus points toward stabilizing disulfide bonds in the intracellular proteins of a hyperthermophilic virus. Virology. 2007;368:249–261. doi: 10.1016/j.virol.2007.06.040. [DOI] [PubMed] [Google Scholar]
- 6.Jin C, Marsden I, Chen X, Liao X. Sequence specific collective motions in a winged helix DNA binding domain detected by 15N relaxation NMR. Biochemistry. 1998;37:6179–6187. doi: 10.1021/bi980031v. [DOI] [PubMed] [Google Scholar]
- 7.Maiese K, Chong ZZ, Shang YC. OutFOXOing disease and disability: the therapeutic potential of targeting FoxO proteins. Trends Mol. Med. 2008;14:219–227. doi: 10.1016/j.molmed.2008.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wijchers PJ, Burbach JP, Smidt MP. In control of biology: of mice, men and Foxes. Biochem. J. 2006;397:233–246. doi: 10.1042/BJ20060387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lin K, Dorman JB, Rodan A, Kenyon C. Daf-16: an HNF-3/forkhead family member that can function to double the life-span of Caenorhabditis elegans. Science. 1997;278:1319–1322. doi: 10.1126/science.278.5341.1319. [DOI] [PubMed] [Google Scholar]
- 10.Ogg S, Paradis S, Gottlieb S, et al. The Fork head transcription factor DAF-16 transduces insulin-like metabolic and longevity signals in C. elegans. Nature. 1997;389:994–999. doi: 10.1038/40194. [DOI] [PubMed] [Google Scholar]
- 11.Maiese K, Chong ZZ, Shang YC. ‘Sly as a FOXO’: new paths with Forkhead signaling in the brain. Curr. Neurovasc. Res. 2007;4:295–302. doi: 10.2174/156720207782446306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Furuyama T, Nakazawa T, Nakano I, Mori N. Identification of the differential distribution patterns of mRNAs and consensus binding sequences for mouse DAF-16 homologues. Biochem. J. 2000;349:629–634. doi: 10.1042/0264-6021:3490629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Biggs WH, III, Cavenee WK, Arden KC. Identification and characterization of members of the FKHR (FOX O) subclass of winged-helix transcription factors in the mouse. Mamm. Genome. 2001;12:416–425. doi: 10.1007/s003350020002. [DOI] [PubMed] [Google Scholar]
- 14.Hoekman MF, Jacobs FM, Smidt MP, Burbach JP. Spatial and temporal expression of FoxO transcription factors in the developing and adult murine brain. Gene Expr. Patterns. 2006;6:134–140. doi: 10.1016/j.modgep.2005.07.003. [DOI] [PubMed] [Google Scholar]
- 15.Jagani Z, Singh A, Khosravi-Far R. FoxO tumor suppressors and BCR-ABL-induced leukemia: a matter of evasion of apoptosis. Biochim. Biophys. Acta. 2008;1785:63–84. doi: 10.1016/j.bbcan.2007.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Myatt SS, Lam EW. The emerging roles of forkhead box (Fox) proteins in cancer. Nat. Rev. Cancer. 2007;7:847–859. doi: 10.1038/nrc2223. [DOI] [PubMed] [Google Scholar]
- 17.van der Horst A, Burgering BM. Stressing the role of FoxO proteins in lifespan and disease. Nat. Rev. Mol. Cell. Biol. 2007;8:440–450. doi: 10.1038/nrm2190. [DOI] [PubMed] [Google Scholar]
- 18.Chong ZZ, Kang JQ, Maiese K. Erythropoietin is a novel vascular protectant through activation of Akt1 and mitochondrial modulation of cysteine proteases. Circulation. 2002;106:2973–2979. doi: 10.1161/01.cir.0000039103.58920.1f. [DOI] [PubMed] [Google Scholar]
- 19.Chong ZZ, Li F, Maiese K. Erythropoietin requires NF-κB and its nuclear translocation to prevent early and late apoptotic neuronal injury during β-amyloid toxicity. Curr. Neurovasc. Res. 2005;2:387–399. doi: 10.2174/156720205774962683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chong ZZ, Li F, Maiese K. Cellular demise and inflammatory microglial activation during β-amyloid toxicity are governed by Wnt1 and canonical signaling pathways. Cell. Signalling. 2007;19:1150–1162. doi: 10.1016/j.cellsig.2006.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kim KH, Oudit GY, Backx PH. Erythropoietin protects against doxorubicin-induced cardiomyopathy via a phosphatidylinositol 3-kinase-dependent pathway. J. Pharmacol. Exp. Ther. 2008;324:160–169. doi: 10.1124/jpet.107.125773. [DOI] [PubMed] [Google Scholar]
- 22.Kops GJ, de Ruiter ND, De Vries-Smits AM, Powell DR, Bos JL, Burgering BM. Direct control of the Forkhead transcription factor AFX by protein kinase B. Nature. 1999;398:630–634. doi: 10.1038/19328. [DOI] [PubMed] [Google Scholar]
- 23.Tang ED, Nunez G, Barr FG, Guan KL. Negative regulation of the forkhead transcription factor FKHR by Akt. J. Biol. Chem. 1999;274:16741–16746. doi: 10.1074/jbc.274.24.16741. [DOI] [PubMed] [Google Scholar]
- 24.Biggs WH, III, Meisenhelder J, Hunter T, Cavenee WK, Arden KC. Protein kinase B/Akt-mediated phosphorylation promotes nuclear exclusion of the winged helix transcription factor FKHR1. Proc. Natl. Acad. Sci. U.S.A. 1999;96:7421–7426. doi: 10.1073/pnas.96.13.7421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chong ZZ, Maiese K. Erythropoietin involves the phosphatidylinositol 3-kinase pathway, 14-13-3 protein and FOXO3a nuclear trafficking to preserve endothelial cell integrity. Br. J. Pharmacol. 2007;150:839–850. doi: 10.1038/sj.bjp.0707161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Maiese K, Li F, Chong ZZ. New avenues of exploration for erythropoietin. JAMA, J. Am. Med. Assoc. 2005;293:90–95. doi: 10.1001/jama.293.1.90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chong ZZ, Kang JQ, Maiese K. Akt1 drives endothelial cell membrane asymmetry and microglial activation through Bcl-xL and caspase 1, 3, and 9. Exp. Cell Res. 2004;296:196–207. doi: 10.1016/j.yexcr.2004.01.021. [DOI] [PubMed] [Google Scholar]
- 28.Chong ZZ, Maiese K. The Src homology 2 domain tyrosine phosphatases SHP-1 and SHP-2: diversified control of cell growth, inflammation, and injury. Histol. Histopathol. 2007;22:1251–1267. doi: 10.14670/hh-22.1251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chong ZZ, Kang JQ, Maiese K. Apaf-1, Bcl-xL, cytochrome c, and caspase-9 form the critical elements for cerebral vascular protection by erythropoietin. J. Cereb. Blood Flow Metab. 2003;23:320–330. doi: 10.1097/01.WCB.0000050061.57184.AE. [DOI] [PubMed] [Google Scholar]
- 30.Chong ZZ, Kang JQ, Maiese K. Erythropoietin fosters both intrinsic and extrinsic neuronal protection through modulation of microglia, Akt1, Bad, and caspase-mediated pathways. Br. J. Pharmacol. 2003;138:1107–1118. doi: 10.1038/sj.bjp.0705161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chong ZZ, Kang JQ, Maiese K. Essential cellular regulatory elements of oxidative stress in early and late phases of apoptosis in the central nervous system. Antioxid. Redox Signaling. 2004;6:277–287. doi: 10.1089/152308604322899341. [DOI] [PubMed] [Google Scholar]
- 32.Chong ZZ, Li F, Maiese K. Group I metabotropic receptor neuroprotection requires Akt and its substrates that govern FOXO3a, Bim, and β-catenin during oxidative stress. Curr. Neurovasc. Res. 2006;3:107–117. doi: 10.2174/156720206776875830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chong ZZ, Lin SH, Maiese K. The NAD+ precursor nicotinamide governs neuronal survival during oxidative stress through protein kinase B coupled to FOXO3a and mitochondrial membrane potential. J. Cereb. Blood Flow Metab. 2004;24:728–743. doi: 10.1097/01.WCB.0000122746.72175.0E. [DOI] [PubMed] [Google Scholar]
- 34.Obexer P, Geiger K, Ambros PF, Meister B, Ausserlechner MJ. FKHRL1-mediated expression of Noxa and Bim induces apoptosis via the mitochondria in neuroblastoma cells. Cell Death Differ. 2007;14:534–547. doi: 10.1038/sj.cdd.4402017. [DOI] [PubMed] [Google Scholar]
- 35.Plas DR, Thompson CB. Akt activation promotes degradation of tuberin and FOXO3a via the proteasome. J. Biol. Chem. 2003;278:12361–12366. doi: 10.1074/jbc.M213069200. [DOI] [PubMed] [Google Scholar]
- 36.Lehtinen MK, Yuan Z, Boag PR, et al. A conserved MST-FOXO signaling pathway mediates oxidative-stress responses and extends life span. Cell. 2006;125:987–1001. doi: 10.1016/j.cell.2006.03.046. [DOI] [PubMed] [Google Scholar]
- 37.Song JJ, Lee YJ. Differential cleavage of Mst1 by caspase-7/-3 is responsible for TRAIL-induced activation of the MAPK superfamily. Cell. Signalling. 2008;20:892–906. doi: 10.1016/j.cellsig.2008.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chong ZZ, Li F, Maiese K. Oxidative stress in the brain: novel cellular targets that govern survival during neurodegenerative disease. Prog. Neurobiol. 2005;75:207–246. doi: 10.1016/j.pneurobio.2005.02.004. [DOI] [PubMed] [Google Scholar]
- 39.Maiese K. Diabetic stress: new triumphs and challenges to maintain vascular longevity. Expert Rev. Cardiovasc. Ther. 2008;6:281–284. doi: 10.1586/14779072.6.3.281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Maiese K, Chong ZZ. Nicotinamide: necessary nutrient emerges as a novel cytoprotectant for the brain. Trends Pharmacol. Sci. 2003;24:228–232. doi: 10.1016/S0165-6147(03)00078-6. [DOI] [PubMed] [Google Scholar]
- 41.Slomka M, Zieminska E, Lazarewicz J. Nicotinamide and 1-methylnicotinamide reduce homocysteine neurotoxicity in primary cultures of rat cerebellar granule cells. Acta Neurobiol. Exp. 2008;68:1–9. doi: 10.55782/ane-2008-1666. [DOI] [PubMed] [Google Scholar]
- 42.Maiese K, Chong ZZ, Hou J, Shang YC. Erythropoietin and oxidative stress. Curr. Neurovasc. Res. 2008;5:125–142. doi: 10.2174/156720208784310231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Nakamura T, Sakamoto K. Forkhead transcription factor FOXO subfamily is essential for reactive oxygen species-induced apoptosis. Mol. Cell. Endocrinol. 2007;281:47–55. doi: 10.1016/j.mce.2007.10.007. [DOI] [PubMed] [Google Scholar]
- 44.Barthelemy C, Henderson CE, Pettmann B. Foxo3a induces motoneuron death through the Fas pathway in cooperation with JNK. BMC Neurosci. 2004;5:48. doi: 10.1186/1471-2202-5-48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.You H, Yamamoto K, Mak TW. Regulation of transactivation-independent proapoptotic activity of p53 by FOXO3a. Proc. Natl. Acad. Sci. U.S.A. 2006;103:9051–9056. doi: 10.1073/pnas.0600889103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Won CK, Ji HH, Koh PO. Estradiol prevents the focal cerebral ischemic injury-induced decrease of forkhead transcription factors phosphorylation. Neurosci. Lett. 2006;398:39–43. doi: 10.1016/j.neulet.2005.12.060. [DOI] [PubMed] [Google Scholar]
- 47.Caporali A, Sala-Newby GB, Meloni M, et al. Identification of the prosurvival activity of nerve growth factor on cardiac myocytes. Cell Death Differ. 2008;15:299–311. doi: 10.1038/sj.cdd.4402263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Puig O, Tjian R. Transcriptional feedback control of insulin receptor by dFOXO/FOXO1. Genes Dev. 2005;19:2435–2446. doi: 10.1101/gad.1340505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Tothova Z, Kollipara R, Huntly BJ, et al. FoxOs are critical mediators of hematopoietic stem cell resistance to physiologic oxidative stress. Cell. 2007;128:325–339. doi: 10.1016/j.cell.2007.01.003. [DOI] [PubMed] [Google Scholar]
- 50.Ferrara N, Rinaldi B, Corbi G, et al. Exercise training promotes SIRT1 activity in aged rats. Rejuvenation Res. 2007;11:139–150. doi: 10.1089/rej.2007.0576. [DOI] [PubMed] [Google Scholar]
- 51.Li F, Chong ZZ, Maiese K. Winding through the WNT pathway during cellular development and demise. Histol. Histopathol. 2006;21:103–124. doi: 10.14670/hh-21.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Maiese K, Li F, Chong ZZ, Shang YC. The Wnt signaling pathway: aging gracefully as a protectionist? Pharmacol Ther. 2008;118:58–81. doi: 10.1016/j.pharmthera.2008.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Smith WW, Norton DD, Gorospe M, et al. Phosphorylation of p66Shc and forkhead proteins mediates Aβ toxicity. J. Cell Biol. 2005;169:331–339. doi: 10.1083/jcb.200410041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hoogeboom D, Essers MA, Polderman PE, Voets E, Smits LM, Burgering BM. Interaction of FOXO with β-catenin inhibits β-catenin/t cell factor activity. J. Biol. Chem. 2008;283:9224–9230. doi: 10.1074/jbc.M706638200. [DOI] [PubMed] [Google Scholar]
- 55.Naito AT, Akazawa H, Takano H, et al. Phosphatidylinositol 3-kinase-Akt pathway plays a critical role in early cardiomyogenesis by regulating canonical Wnt signaling. Circ. Res. 2005;97:144–151. doi: 10.1161/01.RES.0000175241.92285.f8. [DOI] [PubMed] [Google Scholar]
- 56.Maiese K, Chong ZZ, Shang YC. Raves and risks for erythropoietin. Cytokine Growth Factor Rev. 2008;19:145–155. doi: 10.1016/j.cytogfr.2008.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Hosaka T, Biggs WH, III, Tieu D, et al. Disruption of forkhead transcription factor (FOXO) family members in mice reveals their functional diversification. Proc. Natl. Acad. Sci. U.S.A. 2004;101:2975–2980. doi: 10.1073/pnas.0400093101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Furuyama T, Kitayama K, Shimoda Y, et al. Abnormal angiogenesis in Foxo1 (Fkhr)-deficient mice. J. Biol. Chem. 2004;279:34741–34749. doi: 10.1074/jbc.M314214200. [DOI] [PubMed] [Google Scholar]
- 59.Evans-Anderson HJ, Alfieri CM, Yutzey KE. Regulation of cardiomyocyte proliferation and myocardial growth during development by FOXO transcription factors. Circ. Res. 2008;102:686–694. doi: 10.1161/CIRCRESAHA.107.163428. [DOI] [PubMed] [Google Scholar]
- 60.Li HH, Willis MS, Lockyer P, et al. Atrogin-1 inhibits Akt-dependent cardiac hypertrophy in mice via ubiquitin-dependent coactivation of Forkhead proteins. J. Clin. Invest. 2007;117:3211–3223. doi: 10.1172/JCI31757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Abid MR, Yano K, Guo S, et al. Forkhead transcription factors inhibit vascular smooth muscle cell proliferation and neointimal hyperplasia. J. Biol. Chem. 2005;280:29864–29873. doi: 10.1074/jbc.M502149200. [DOI] [PubMed] [Google Scholar]
- 62.Liu ZP, Wang Z, Yanagisawa H, Olson EN. Phenotypic modulation of smooth muscle cells through interaction of Foxo4 and myocardin. Dev. Cell. 2005;9:261–270. doi: 10.1016/j.devcel.2005.05.017. [DOI] [PubMed] [Google Scholar]
- 63.Li H, Liang J, Castrillon DH, DePinho RA, Olson EN, Liu ZP. FoxO4 regulates tumor necrosis factor α-directed smooth muscle cell migration by activating matrix metalloproteinase 9 gene transcription. Mol. Cell. Biol. 2007;27:2676–2686. doi: 10.1128/MCB.01748-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Goettsch W, Gryczka C, Korff T, et al. Flow-dependent regulation of angiopoietin-2. J. Cell. Physiol. 2008;214:491–503. doi: 10.1002/jcp.21229. [DOI] [PubMed] [Google Scholar]
- 65.Morris JB, Kenney B, Huynh H, Woodcock EA. Regulation of the proapoptotic factor FOXO1 (FKHR) in cardiomyocytes by growth factors and α1-adrenergic agonists. Endocrinology. 2005;146:4370–4376. doi: 10.1210/en.2005-0162. [DOI] [PubMed] [Google Scholar]
- 66.Sedding DG, Seay U, Fink L, et al. Mechanosensitive p27Kip1 regulation and cell cycle entry in vascular smooth muscle cells. Circulation. 2003;108:616–622. doi: 10.1161/01.CIR.0000079102.08464.E2. [DOI] [PubMed] [Google Scholar]
- 67.Hannenhalli S, Putt ME, Gilmore JM, et al. Transcriptional genomics associates FOX transcription factors with human heart failure. Circulation. 2006;114:1269–1276. doi: 10.1161/CIRCULATIONAHA.106.632430. [DOI] [PubMed] [Google Scholar]
- 68.Maiese K, Chong ZZ, Li F, Shang YC. Erythropoietin: elucidating new cellular targets that broaden therapeutic strategies. Prog. Neurobiol. 2008;85:194–213. doi: 10.1016/j.pneurobio.2008.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Kikuchi S, Nagai T, Kunitama M, Kirito K, Ozawa K, Komatsu N. Active FKHRL1 overcomes imatinib resistance in chronic myelogenous leukemia-derived cell lines via the production of tumor necrosis factor-related apoptosis-inducing ligand. Cancer Sci. 2007;98:1949–1958. doi: 10.1111/j.1349-7006.2007.00623.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Nowak K, Killmer K, Gessner C, Lutz W. E2F-1 regulates expression of FOXO1 and FOXO3a. Biochim. Biophys. Acta. 2007;1769:244–252. doi: 10.1016/j.bbaexp.2007.04.001. [DOI] [PubMed] [Google Scholar]
- 71.Brunet A, Sweeney LB, Sturgill JF, et al. Stress-dependent regulation of FOXO transcription factors by the SIRT1 deacetylase. Science. 2004;303:2011–2015. doi: 10.1126/science.1094637. [DOI] [PubMed] [Google Scholar]
- 72.Paik JH, Kollipara R, Chu G, et al. FoxOs are lineage-restricted redundant tumor suppressors and regulate endothelial cell homeostasis. Cell. 2007;128:309–323. doi: 10.1016/j.cell.2006.12.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Sunters A, Madureira PA, Pomeranz KM, et al. Paclitaxel-induced nuclear translocation of FOXO3a in breast cancer cells is mediated by c-Jun NH2-terminal kinase and Akt. Cancer Res. 2006;66:212–220. doi: 10.1158/0008-5472.CAN-05-1997. [DOI] [PubMed] [Google Scholar]
- 74.Eddy SF, Kane SE, Sonenshein GE. Trastuzumab-resistant HER2-driven breast cancer cells are sensitive to epigallocatechin-3 gallate. Cancer Res. 2007;67:9018–9023. doi: 10.1158/0008-5472.CAN-07-1691. [DOI] [PubMed] [Google Scholar]
- 75.Li Y, Wang Z, Kong D, et al. Regulation of FOXO3a/β-catenin/GSK-3β signaling by 3,3′-diindolylmethane contributes to inhibition of cell proliferation and induction of apoptosis in prostate cancer cells. J. Biol. Chem. 2007;282:21542–21550. doi: 10.1074/jbc.M701978200. [DOI] [PubMed] [Google Scholar]
- 76.Delpuech O, Griffiths B, East P, et al. Induction of Mxi1-SRα by FOXO3a contributes to repression of Myc-dependent gene expression. Mol. Cell. Biol. 2007;27:4917–4930. doi: 10.1128/MCB.01789-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Ticchioni M, Essafi M, Jeandel PY, et al. Homeostatic chemokines increase survival of B-chronic lymphocytic leukemia cells through inactivation of transcription factor FOXO3a. Oncogene. 2007;26:7081–7091. doi: 10.1038/sj.onc.1210519. [DOI] [PubMed] [Google Scholar]
- 78.Yang L, Xie S, Jamaluddin MS, et al. Induction of androgen receptor expression by phosphatidylinositol 3-kinase/Akt downstream substrate, FOXO3a, and their roles in apoptosis of LNCaP prostate cancer cells. J. Biol. Chem. 2005;280:33558–33565. doi: 10.1074/jbc.M504461200. [DOI] [PubMed] [Google Scholar]
- 79.Arimoto-Ishida E, Ohmichi M, Mabuchi S, et al. Inhibition of phosphorylation of a forkhead transcription factor sensitizes human ovarian cancer cells to cisplatin. Endocrinology. 2004;145:2014–2022. doi: 10.1210/en.2003-1199. [DOI] [PubMed] [Google Scholar]
- 80.Sauvageot CM, Kesari S, Stiles CD. Molecular pathogenesis of adult brain tumors and the role of stem cells. Neurol. Clin. 2007;25:891–924. doi: 10.1016/j.ncl.2007.07.014. [DOI] [PubMed] [Google Scholar]
- 81.Miyamoto K, Araki KY, Naka K, et al. Foxo3a is essential for maintenance of the hematopoietic stem cell pool. Cell Stem Cells. 2007;1:101–112. doi: 10.1016/j.stem.2007.02.001. [DOI] [PubMed] [Google Scholar]
- 82.Arcasoy MO. The non-haematopoietic biological effects of erythropoietin. Br. J. Haematol. 2008;141:14–31. doi: 10.1111/j.1365-2141.2008.07014.x. [DOI] [PubMed] [Google Scholar]
- 83.Cariou A, Claessens YE, Pene F, et al. Early high-dose erythropoietin therapy and hypothermia after out-of-hospital cardiac arrest: a matched control study. Resuscitation. 2008;76:397–404. doi: 10.1016/j.resuscitation.2007.10.003. [DOI] [PubMed] [Google Scholar]
- 84.Chong ZZ, Shang YC, Maiese K. Vascular injury during elevated glucose can be mitigated by erythropoietin and Wnt signaling. Curr. Neurovasc. Res. 2007;4:194–204. doi: 10.2174/156720207781387150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Bakker WJ, van Dijk TB, Parren-van Amelsvoort M, et al. Differential regulation of Foxo3a target genes in erythropoiesis. Mol. Cell. Biol. 2007;27:3839–3854. doi: 10.1128/MCB.01662-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Furukawa-Hibi Y, Yoshida-Araki K, Ohta T, Ikeda K, Motoyama N. FOXO forkhead transcription factors induce G-M checkpoint in response to oxidative stress. J. Biol. Chem. 2002;277:26729–26732. doi: 10.1074/jbc.C200256200. [DOI] [PubMed] [Google Scholar]
- 87.Liu L, Rajareddy S, Reddy P, et al. Infertility caused by retardation of follicular development in mice with oocyte-specific expression of Foxo3a. Development. 2007;134:199–209. doi: 10.1242/dev.02667. [DOI] [PubMed] [Google Scholar]
- 88.Watkins WJ, Umbers AJ, Woad KJ, et al. Mutational screening of FOXO3A and FOXO1A in women with premature ovarian failure. Fertil. Steril. 2006;86:1518–1521. doi: 10.1016/j.fertnstert.2006.03.054. [DOI] [PubMed] [Google Scholar]
- 89.Maiese K, Chong ZZ, Shang YC. Mechanistic insights into diabetes mellitus and oxidative stress. Curr. Med. Chem. 2007;14:1729–1738. doi: 10.2174/092986707781058968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Maiese K, Morhan SD, Chong ZZ. Oxidative stress biology and cell injury during type 1 and type 2 diabetes mellitus. Curr. Neurovasc. Res. 2007;4:63–71. doi: 10.2174/156720207779940653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Donahoe SM, Stewart GC, McCabe CH, et al. Diabetes and mortality following acute coronary syndromes. JAMA, J. Am Med. Assoc. 2007;298:765–775. doi: 10.1001/jama.298.7.765. [DOI] [PubMed] [Google Scholar]
- 92.Guo S, Rena G, Cichy S, He X, Cohen P, Unterman T. Phosphorylation of serine 256 by protein kinase B disrupts transactivation by FKHR and mediates effects of insulin on insulin-like growth factor-binding protein-1 promoter activity through a conserved insulin response sequence. J. Biol. Chem. 1999;274:17184–17192. doi: 10.1074/jbc.274.24.17184. [DOI] [PubMed] [Google Scholar]
- 93.Nakae J, Park BC, Accili D. Insulin stimulates phosphorylation of the forkhead transcription factor FKHR on serine 253 through a wortmannin-sensitive pathway. J. Biol. Chem. 1999;274:15982–15985. doi: 10.1074/jbc.274.23.15982. [DOI] [PubMed] [Google Scholar]
- 94.Kim JR, Jung HS, Bae SW, et al. Polymorphisms in FOXO gene family and association analysis with BMI. Obesity. 2006;14:188–193. doi: 10.1038/oby.2006.24. [DOI] [PubMed] [Google Scholar]
- 95.Kuningas M, Magi R, Westendorp RG, Slagboom PE, Remm M, van Heemst D. Haplotypes in the human Foxo1a and Foxo3a genes; impact on disease and mortality at old age. Eur. J. Hum. Genet. 2007;15:294–301. doi: 10.1038/sj.ejhg.5201766. [DOI] [PubMed] [Google Scholar]
- 96.Marchetti V, Menghini R, Rizza S, et al. Benfotiamine counteracts glucose toxicity effects on endothelial progenitor cell differentiation via Akt/FoxO signaling. Diabetes. 2006;55:2231–2237. doi: 10.2337/db06-0369. [DOI] [PubMed] [Google Scholar]
- 97.Fallarino F, Bianchi R, Orabona C, et al. CTLA-4-Ig activates forkhead transcription factors and protects dendritic cells from oxidative stress in nonobese diabetic mice. J. Exp. Med. 2004;200:1051–1062. doi: 10.1084/jem.20040942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Kato M, Yuan H, Xu ZG, et al. Role of the Akt/FoxO3a pathway in TGF-β1-mediated mesangial cell dysfunction: a novel mechanism related to diabetic kidney disease. J. Am. Soc. Nephrol. 2006;17:3325–3335. doi: 10.1681/ASN.2006070754. [DOI] [PubMed] [Google Scholar]
- 99.Nakae J, Cao Y, Oki M, et al. Forkhead transcription factor FoxO1 in adipose tissue regulates energy storage and expenditure. Diabetes. 2008;57:563–576. doi: 10.2337/db07-0698. [DOI] [PubMed] [Google Scholar]
- 100.Furuyama T, Yamashita H, Kitayama K, Higami Y, Shimokawa I, Mori N. Effects of aging and caloric restriction on the gene expression of Foxo1, 3, and 4 (FKHR, FKHRL1, and AFX) in the rat skeletal muscles. Microsc. Res. Tech. 2002;59:331–334. doi: 10.1002/jemt.10213. [DOI] [PubMed] [Google Scholar]
- 101.Ni YG, Wang N, Cao DJ, et al. FoxO transcription factors activate Akt and attenuate insulin signaling in heart by inhibiting protein phosphatases. Proc. Natl. Acad. Sci. U.S.A. 2007;104:20517–20522. doi: 10.1073/pnas.0610290104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Kamei Y, Miura S, Suzuki M, et al. Skeletal muscle FOXO1 (FKHR) transgenic mice have less skeletal muscle mass, down-regulated Type I (slow twitch/red muscle) fiber genes, and impaired glycemic control. J. Biol. Chem. 2004;279:41114–41123. doi: 10.1074/jbc.M400674200. [DOI] [PubMed] [Google Scholar]
- 103.Li F, Chong ZZ, Maiese K. Cell life versus cell longevity: the mysteries surrounding the NAD+ precursor nicotinamide. Curr. Med. Chem. 2006;13:883–895. doi: 10.2174/092986706776361058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Nemoto S, Fergusson MM, Finkel T. Nutrient availability regulates SIRT1 through a forkhead-dependent pathway. Science. 2004;306:2105–2108. doi: 10.1126/science.1101731. [DOI] [PubMed] [Google Scholar]
- 105.Motta MC, Divecha N, Lemieux M, et al. Mammalian SIRT1 represses forkhead transcription factors. Cell. 2004;116:551–563. doi: 10.1016/s0092-8674(04)00126-6. [DOI] [PubMed] [Google Scholar]
- 106.Kyoung Kim H, Kyoung Kim Y, Song IH, et al. Down-regulation of a forkhead transcription factor, FOXO3a, accelerates cellular senescence in human dermal fibroblasts. J. Gerontol. A. Biol. Sci. Med. Sci. 2005;60:4–9. doi: 10.1093/gerona/60.1.4. [DOI] [PubMed] [Google Scholar]
- 107.Alcendor RR, Gao S, Zhai P, et al. Sirt1 regulates aging and resistance to oxidative stress in the heart. Circ. Res. 2007;100:1512–1521. doi: 10.1161/01.RES.0000267723.65696.4a. [DOI] [PubMed] [Google Scholar]
- 108.Li M, Chiu JF, Mossman BT, Fukagawa NK. Down-regulation of manganese-superoxide dismutase through phosphorylation of FOXO3a by Akt in explanted vascular smooth muscle cells from old rats. J. Biol. Chem. 2006;281:40429–40439. doi: 10.1074/jbc.M606596200. [DOI] [PubMed] [Google Scholar]
- 109.Miyauchi H, Minamino T, Tateno K, Kunieda T, Toko H, Komuro I. Akt negatively regulates the in vitro lifespan of human endothelial cells via a p53/p21-dependent pathway. EMBO J. 2004;23:212–220. doi: 10.1038/sj.emboj.7600045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Kang JQ, Chong ZZ, Maiese K. Critical role for Akt1 in the modulation of apoptotic phosphatidylserine exposure and microglial activation. Mol. Pharmacol. 2003;64:557–569. doi: 10.1124/mol.64.3.557. [DOI] [PubMed] [Google Scholar]
- 111.Chong ZZ, Kang J, Li F, Maiese K. mGluRI targets microglial activation and selectively prevents neuronal cell engulfment through Akt and caspase dependent pathways. Curr. Neurovasc. Res. 2005;2:197–211. doi: 10.2174/1567202054368317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Chong ZZ, Li F, Maiese K. The pro-survival pathways of mTOR and protein kinase B target glycogen synthase kinase-3β and nuclear factor-κB to foster endogenous microglial cell protection. Int. J. Mol. Med. 2007;19:263–272. [PMC free article] [PubMed] [Google Scholar]
- 113.Li F, Chong ZZ, Maiese K. Microglial integrity is maintained by erythropoietin through integration of Akt and its substrates of glycogen synthase kinase-3β, β-catenin, and nuclear factor-κB. Curr. Neurovasc. Res. 2006;3:187–201. doi: 10.2174/156720206778018758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Ludikhuize J, de Launay D, Groot D, et al. Inhibition of forkhead box class O family member transcription factors in rheumatoid synovial tissue. Arthritis Rheum. 2007;56:2180–2191. doi: 10.1002/art.22653. [DOI] [PubMed] [Google Scholar]
- 115.Kuo CC, Lin SC. Altered FOXO1 transcript levels in peripheral blood mononuclear cells of systemic lupus erythematosus and rheumatoid arthritis patients. Mol. Med. 2007;13:561–566. doi: 10.2119/2007-00021.Kuo. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Lin L, Hron JD, Peng SL. Regulation of NF-κB, Th activation, and autoinflammation by the forkhead transcription factor Foxo3a. Immunity. 2004;21:203–213. doi: 10.1016/j.immuni.2004.06.016. [DOI] [PubMed] [Google Scholar]
- 117.Jonsson H, Allen P, Peng SL. Inflammatory arthritis requires Foxo3a to prevent Fas ligand-induced neutrophil apoptosis. Nat. Med. 2005;11:666–671. doi: 10.1038/nm1248. [DOI] [PubMed] [Google Scholar]
- 118.Sela U, Dayan M, Hershkoviz R, Cahalon L, Lider O, Mozes E. The negative regulators Foxj1 and Foxo3a are up-regulated by a peptide that inhibits systemic lupus erythematosus-associated T cell responses. Eur. J. Immunol. 2006;36:2971–2980. doi: 10.1002/eji.200636137. [DOI] [PubMed] [Google Scholar]
- 119.Hur EM, Youssef S, Haws ME, Zhang SY, Sobel RA, Steinman L. Osteopontin-induced relapse and progression of autoimmune brain disease through enhanced survival of activated T cells. Nat. Immunol. 2007;8:74–83. doi: 10.1038/ni1415. [DOI] [PubMed] [Google Scholar]
- 120.Gomez-Gutierrez JG, Souza V, Hao HY, et al. Adenovirus-mediated gene transfer of FKHRL1 triple mutant efficiently induces apoptosis in melanoma cells. Cancer Biol. Ther. 2006;5:875–883. doi: 10.4161/cbt.5.7.2911. [DOI] [PubMed] [Google Scholar]
- 121.Zethelius B, Berglund L, Sundstrom J, et al. Use of multiple biomarkers to improve the prediction of death from cardiovascular causes. N. Engl. J. Med. 2008;358:2107–2116. doi: 10.1056/NEJMoa0707064. [DOI] [PubMed] [Google Scholar]