Abstract
Background
Patients with essential tremor (ET) may develop Parkinson’s disease (PD); however, few studies have examined the clinical features of this combination syndrome.
Methods
53 ET-PD patients were studied and compared to 53 PD and 150 ET patients.
Results
Although the latency from onset of ET to PD was brief (<5 years) in 38.5%, in a sizable proportion (30.8%), it was very long (>20 years). The gender distribution of ET-PD (67.9% male) was identical to that of PD (67.9% male) yet differed from that of ET (50.0% male)(p =0.02). The initial cardinal sign of PD was rest tremor in 100% of patients. In ET-PD, the side of greatest initial ET severity usually matched that of greatest PD severity (p <0.05).
Conclusions
In ET-PD, male gender predominated and the sidedness of the ET and PD usually matched. The co-occurrence of the two diagnoses in the same patient may be mechanistically related.
Keywords: essential tremor, Parkinson’s Disease, clinical, epidemiology
INTRODUCTION
A longstanding clinical literature points to an association between essential tremor (ET) and Parkinson’s disease (PD); indeed, anecdotally and in small retrospective series, it has been shown that ET patients may eventually develop PD,1–6 and in a prospective follow-up study, the risk of incident PD was approximately four times higher in ET patients than in their counterparts without ET.7 In recent postmortem studies, Lewy bodies, mainly in the locus ceruleus, have been observed in some ET brains, suggesting that there are links between ET and Lewy body disease.8–12
One way to further explore the ET-PD relationship is to study patients that transition from isolated ET to the ET-PD combination. To date, few studies have looked at the latency from onset of ET to onset of PD and the clinical features that are associated with this combination syndrome.5,6
We developed the following working hypotheses. First, the latency from onset of ET to PD would be characterized by a high prevalence of both short latencies (i.e., early ET rapidly advancing to PD) and longer latencies (i.e., longstanding ET advancing to PD). Second, the predominant side of ET tremor would be the predominant side of PD signs, which would support the hypothesis that one pathological process (i.e., Lewy bodies) underlies both disorders rather than two pathological processes (e.g., cerebellar degenerative changes in ET and Lewy bodies in PD). Third, the initial sign of PD would be rest tremor. Fourth, in contrast to ET but similar to PD, we would expect a male predominance among ET-PD patients. This hypothesis was based on knowledge of the male predominance in Lewy body disease13 as well as the hypothesis that one pathological process (i.e., Lewy bodies) might underlie both disorders.
METHODS
Patient Identification
Patients with ET-PD were identified from a computerized billing database at the Center for Parkinson’s Disease and Other Movement Disorders at the Neurological Institute of New York, Columbia University Medical Center. All patients who had visited the Center in the past five years who received both an International Classification of Diseases, Version 9 (ICD-9) code of 332.0 (idiopathic PD) and 333.1 (ET) were selected. This search yielded 187 patients. Eighteen clinical records could not be located, leaving 169 clinical records, which were reviewed by a medical student (M.M.) trained by a movement disorder neurologist (E.D.L.).
During clinical record review, the ET diagnosis was confirmed if the patient’s treating movement disorder neurologist at the Center had assigned this diagnosis based on the presence of moderate to severe action tremor in the limbs or head. The PD diagnosis was confirmed when the patient’s treating neurologist at the Center had assigned this diagnosis based on the presence during their neurological examination of at least two cardinal signs of Parkinsonism. Based on clinical review, 116 patients either did not have ET or PD (e.g., incorrect use of the ICD-9 code 333.1 in patients with dystonic tremor, parkinsonian tremor or other non-ET forms of tremor; incorrect assignment of the ICD-9 code 332.0 to patients with Parkinson-plus syndrome or drug-induced parkinsonism). Fifty-three ET-PD patients remained, each of whom had had ET prior to PD and whose neurologist at our center continued to co-assign the ET diagnosis after the diagnosis of PD (i.e., both ET and PD).
To address our fourth hypothesis, we compared the gender distribution of our 53 ET-PD patients to 53 PD patients (mean age at most recent assessment = 70.1 ± 11.8 years, range = 40–92) and 150 ET patients (mean age at most recent assessment = 68.0 ± 15.0 years, range = 18–95) from the same database whose diagnoses had been confirmed and who were selected using a random digit table.
Data Abstraction and Analyses
The retrospective clinical record reviewer used an electronic data abstraction form designed for the study (SPSS version 13.0). The neurologist reviewed every fifth record to ensure high quality data abstraction. Abstracted items were: age (years) at most recent assessment, gender, use of medication at any point to treat ET, first-degree relative with ET or PD, age of reported onset of ET, age at time of initial sign of PD, age at PD diagnosis, predominant side (right, left, equal) of action tremor ET (based on patient history and also separately based on neurological examination), initial PD sign (rest tremor, rigidity, limb bradykinesia, axial bradykinesia, hypomimia, and postural instability) based on neurological examination, and predominant side of each cardinal sign of PD based on neurological examination. In some analyses, we calculated odds ratios (OR) with 95% confidence intervals (CI).
RESULTS
ET-PD Patients
There were 53 ET-PD patients (mean age at most recent assessment = 70.3 ± 12.9 years, range = 35–88; mean age at reported onset of ET = 51.8 ± 21.3, range = 13–84; mean age at ET diagnosis = 65.1 ± 13.9, range = 34–86; mean age at initial sign on neurological examination of PD = 65.8 ± 12.7, range = 34–86; mean age at PD diagnosis = 66.4 ± 13.0, range = 35–87). In 48 (90.4%), the ET action tremor was bilateral. Forty (75.5%) had at some point taken medication to treat their ET; 17 (32.1%) reported a first-degree relative with ET and 4 (7.5%) reported a first-degree relative with PD.
Latency from onset of ET to PD
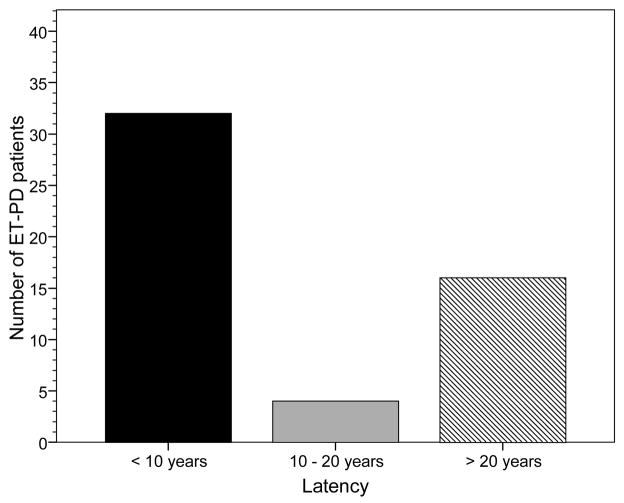
Latency data were available on 52 (98.1%) patients. The mean latency from reported onset of ET to initial sign on neurological examination of PD was 14.0 ± 15.0 years (median 6.0 years, range = 0.5–52.0 years), and from reported onset of ET to diagnosis of PD was 14.6 ± 15.0 years (median 7.2 years, range = 0.6–54.7 years). In nearly two-thirds (n = 32 or 61.5%), the latency from reported onset of ET to initial sign of PD was less than ten years; in 20 (38.5%), it was less than 5 years. In eighteen (90.0%) of these 20, the ET was bilateral. In addition, a sizable proportion (n = 16, 30.8%) also had a long latency (> 20 years). The shorter (<10 years) and longer (>20 years) latency categories, combined, comprised 48 (92.3%) of 52 ET-PD patients (Figure 1).
Figure 1.
Latency from reported onset of ET to initial sign of PD. The shorter latency category and longer latency categories comprised 48 (92.3%) of ET-PD patients.
Predominant side of ET tremor and predominant side of PD signs
The side (right vs. left) of greatest initial ET action tremor severity (by patient history) generally matched that side of greatest severity of each PD sign (based on neurological examination): diminished arm swing (concordance = 78.6%, p = 0.009), limb bradykinesia (concordance = 71.4%, p = 0.003), rigidity (concordance = 56.3%, p = 0.03), and rest tremor (concordance = 73.3%, p < 0.001). Results were similar when the side of greatest initial ET action tremor severity was determined by neurological examination as opposed to by patient history.
Initial sign of PD as rest tremor
Sufficiently detailed data were available on 47 ET-PD patients to assess the initial cardinal sign(s) of PD on neurological examination (Table 1). All had rest tremor and most also had rigidity and limb bradykinesia. Smaller proportions had hypomimia and axial bradykinesia. Postural instability was rare. Upon developing PD, 11 (23.4%) began exhibiting tremor types that were atypical of ET (e.g., lip tremor, chin tremor at rest with mouth closed).
Table 1.
Initial sign of PD in ET-PD patients
| Initial Sign of PD | Number (proportion) of ET-PD patients* |
|---|---|
| Rest tremor | 47 (100%) |
| Rigidity | 45 (95.7%) |
| Limb bradykinesia | 44 (93.6%) |
| Axial bradykinesia | 32 (68.1%) |
| Hypomimia | 32 (68.1%) |
| Postural instability | 12 (25.5%) |
47 of 53 ET-PD cases had sufficiently detailed data.
Gender distribution of ET-PD
Among the 53 ET-PD patients, there was a male predominance, with 36 (67.9%) males; the male:female ratio was 2.1:1. The gender distribution of the ET-PD group was identical to that of our PD group (36 [67.9%] males), however, ET-PD patients were twice more likely to be male than were ET patients (36 [67.9%] male vs. 75 of 150 [50.0%] male, OR = 2.1, 95% CI = 1.1–4.1, p = 0.02).
DISCUSSION
While ET patients seem to be at increased risk of developing PD, the clinical features associated with this combination syndrome have been the subject of a small number of studies. In one previous study, 13 patients were studied 6 and in another study, 11 patients with childhood-onset ET were studied.5 To our knowledge, there have been no other studies.
In our series of 53 ET-PD patients, the predominant side of ET tremor was generally the predominant side of PD signs. This finding was previously noted by Shahed and Jankovic.5 Along with epidemiological data showing a four-fold increased risk of PD in ET,7 these data suggest that the co-occurrence of these two diseases is not merely the result of chance. These data raise the possibility that one pathological process underlies both the ET and then the PD.
Most ET-PD patients developed PD either after a relatively short latency or after a longer latency of several decades. Chaudhuri et al.6 also demonstrated a long latency (up to 50 years) before there is a phenotypic alteration to PD. The preponderance in this study of either short or very long latencies suggests that the underlying pathogenic mechanisms for ET-PD could be heterogeneous. Previous postmortem work demonstrates the presence of pathological heterogeneity in ET (i.e., ET cases with locus ceruleus Lewy bodies vs. those without).11,12 Whether the ET-PD cases with the short latencies are those who as ET cases already had Lewy bodies in the locus ceruleus is not known and deserves additional exploration.
The most common initial sign of PD was rest tremor, suggesting among ET-PD patients, that tremor is a prominent feature initially of ET and then later of PD. This finding was previously noted by Shahed and Jankovic.5 Whether this means that action and rest tremors share similar mechanisms is not clear, and this is worthy of additional study.
A previous study suggested that unilateral action tremor was a feature of the ET in ET-PD patients;6 however, in our series, fewer than 10% of patients had this phenotype. In patients with ET who developed PD, there was a male preponderance, which is a pattern that occurs in PD and not in ET.13,14
The study had limitations. This was a retrospective chart review and some data (e.g., response to particular medications) were incomplete. Furthermore, UPDRS motor examinations were performed by several rather than one movement disorder neurologist and this may have constituted an additional source of variance. Finally, it is possible that bilateral action tremor without any cardinal manifestations of parkinsonism could be an early phenotypic expression of PD rather than ET. Despite this, 38.5% of our cases had had longstanding (≥ 10 years) ET prior to PD. This study had several strengths, including the sizable number of patients, the fact that most patients had visited our center multiple times, and the use of two comparison groups for some analyses.
Acknowledgments
R01 NS42859 from the National Institutes of Health (Bethesda, MD); the Parkinson’s Disease Foundation (New York, NY); the Arlene Bronstein Fund (Columbia University); and the Claire M. O’Neil Fund (Columbia University).
Footnotes
Disclosure: The authors report no conflicts of interest.
Statistical Analyses: The statistical analyses were conducted by Mia Minen MPH and Elan D. Louis MD, MSc.
AUTHOR CONTRIBUTIONS
Mia Minen: Research project organization and execution; statistical analysis execution; manuscript writing (writing the first draft and making subsequent revisions).
Elan D. Louis: Research project conception, organization and execution; statistical analyses design and execution, manuscript writing (making subsequent revisions).
References
- 1.Yahr MD, Orosz D, Purohit DP. Co-occurrence of essential tremor and Parkinson’s disease: a clinical study of a large kindred with autopsy findings. Parkinsonism Relat Disord. 2003;9:225–231. doi: 10.1016/s1353-8020(02)00057-3. [DOI] [PubMed] [Google Scholar]
- 2.Louis ED, Levy G, Meja-Santana H, et al. Risk of action tremor in relatives of tremor dominant PD and postural instability gait disorder PD. Neurology. 2003;61:931–936. doi: 10.1212/wnl.61.7.931. [DOI] [PubMed] [Google Scholar]
- 3.Ondo WG, Lai D. Olfaction testing in patients with tremor-dominant Parkinson’s disease: is this a distinct condition? Mov Disord. 2005;20:471–475. doi: 10.1002/mds.20365. [DOI] [PubMed] [Google Scholar]
- 4.Geraghty JJ, Jankovic J, Zetusky WJ. Association between essential tremor and Parkinson’s disease. Ann Neurol. 1985;17:329–333. doi: 10.1002/ana.410170404. [DOI] [PubMed] [Google Scholar]
- 5.Shahed J, Jankovic J. Exploring the relationship between essential tremor and Parkinson’s disease. Parkinsonism Relat Disord. 2006;13:67–76. doi: 10.1016/j.parkreldis.2006.05.033. [DOI] [PubMed] [Google Scholar]
- 6.Chaudhuri KR, Buxton-Thomas M, Dhawan V, Peng R, Meilak C, Brooks DJ. Long Duration asymmetrical postural tremor is likely to predict development of Parkinson’s disease and not essential tremor: clinical follow up study of 13 cases. J Neurol Neurosurg Psychiatry. 2005;76:115–117. doi: 10.1136/jnnp.2004.046292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Benito-Leon J, Louis ED, Permejo-Pareja F. Risk of incident Parkinson’s disease and parkinsonism in essential tremor: A population-based study (Abstract) Neurology. 2008 doi: 10.1136/jnnp.2008.147223. In Press. [DOI] [PubMed] [Google Scholar]
- 8.Louis ED, Honig LS, Vonsattel JPG, Maraganore DM, Borden S, Moskowitz CB. Essential tremor associated with focal non-nigral Lewy bodies: a clinical-pathological study. Arch Neurol. 2005;62:1004–1107. doi: 10.1001/archneur.62.6.1004. [DOI] [PubMed] [Google Scholar]
- 9.Ross GW, Dickson DW, Cersosimo M, et al. Pathological investigation of essential tremor. Neurology. 2004;62(Suppl 5):A537–538. (abstract) [Google Scholar]
- 10.Louis ED, Vonsattel JPG, Honig LS, Ross GW, Lyos KE, Pahwa R. Neuropathological findings in essential tremor. Neurology. 2006;66:1756–1759. doi: 10.1212/01.wnl.0000218162.80315.b9. [DOI] [PubMed] [Google Scholar]
- 11.Louis ED, Faust PL, Vonsattel JPG, et al. Neuropathological changes in essential tremor: 33 cases compared with 21 controls. Brain. 2007;130:3297–3307. doi: 10.1093/brain/awm266. [DOI] [PubMed] [Google Scholar]
- 12.Louis ED, Vonsattel JP. The emerging neuropathology of essential tremor. Mov Disord. 2007;23:174–182. doi: 10.1002/mds.21731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mayeux R, Marder K, Cote LJ, et al. The frequency of idiopathic Parkinson’s disease by age, ethnic group, and sex in northern Manhattan, 1988–1993. Am J Epidemiol. 1995;142:820–827. doi: 10.1093/oxfordjournals.aje.a117721. [DOI] [PubMed] [Google Scholar]
- 14.Benito-León J, Bermejo-Pareja F, Morales JM, Vega S, Molina JA. Prevalence of essential tremor in three elderly populations of central Spain. Mov Disord. 2003;18:389–394. doi: 10.1002/mds.10376. [DOI] [PubMed] [Google Scholar]