Abstract
CD8 T cells are necessary for costimulation blockade-resistant rejection. However, the mechanism by which CD8 T cells mediate rejection in the absence of major costimulatory signals is poorly understood. IFNγ promotes CD8 T cell-mediated immune responses, but IFNγ-deficient mice show early graft loss despite costimulation blockade. In contrast, we found that IFNγ receptor knockout mice show dramatically prolonged graft survival under costimulation blockade. To investigate this paradox, we addressed the effects of IFNγ on T cell alloresponses in vivo independent of the effects of IFNγ on graft survival. We identified a donor-specific CD8 T cell breakthrough response temporally correlated with costimulation blockade-resistant rejection. Neither IFNγ receptor knockout recipients nor IFNγ-deficient recipients showed a CD8 breakthrough response. Graft death on IFNγ-deficient recipients despite costimulation blockade could be explained by the lack of IFNγ available to act on the graft. Indeed, the presence of IFNγ was necessary for graft survival on IFNγ receptor knockout recipients, as either IFNγ neutralization or the lack of the IFNγ receptor on the graft precipitated early graft loss. Thus, IFNγ is required both for the recipient to mount a donor-specific CD8 T cell response under costimulation blockade as well as for the graft to survive after allotransplantation.
Keywords: Rodent, Transplantation, Costimulation, T cells, Cytokine Receptors
Introduction
During the last fifty years, organ transplantation has become a favored treatment option for patients with otherwise incurable conditions. Nevertheless, the immune response to donor tissue remains the major obstacle to long-term graft survival. Prolongation of allograft survival through T cell costimulation blockade (CoB) is based on the principle that T cell receptor stimulation in the absence of costimulation leads to abortive activation or deletion of T cells (1, 2). CD28 and CD40 are two major costimulatory receptors important to the initiation of effective T cell responses, and blocking the ligands for these receptors with CTLA4-Ig and monoclonal antibodies specific for CD154, respectively, induce profound suppression of primary immune responses (3, 4).
Long-term survival of skin allografts, a very stringent model for transplantation, can be achieved in some strains of mice by interrupting CD28/B7 and CD40/CD154 interactions with CTLA-4:Ig and the anti-CD154 mAb, MR1 (5). However, the C57BL/6 strain (B6) is less susceptible to this mode of immunomodulation and displays costimulation blockade-resistant rejection (6). Studies using B6 mice deficient in CD8 T cells, either by CD8 gene deletion or by CD8-depleting monoclonal antibody treatment, showed that costimulation blockade-resistant rejection depends on the presence of CD8 T cells, though the mechanisms by which these CD8 T cells are able to mediate graft rejection in the absence of major costimulatory signals have not been completely defined (7-9). Furthermore, in B6 recipients but not in C3H recipients, which accept BALB/c grafts long-term under CoB, the frequency of IFNγ-secreting cells in the spleen increases around the time of graft loss (6). However, the nature of the IFNγ-secreting cell-type could not be discerned by the ELISPOT assay used in the aforementioned study.
IFNγ produced by T cells, NK cells, macrophages and dendritic cells, was initially described as an anti-viral cytokine and potent inducer of cellular immunity and has the potential to act on virtually all hematopoietic and non-hematopoietic cell types (10, 11). As a homodimer, IFNγ binds to a heterodimeric receptor composed of two molecules of a ligand-binding subunit, IFNγR1, which then recruits two molecules of a second receptor subunit, IFNγR2, necessary for signal transduction (12). A plethora of genes are up-regulated after IFNγ stimulation, and some of the classical effects attributed to IFNγ are increased efficiency of antigen processing, upregulated MHC expression, induced expression of microbicidal molecules in macrophages, and activation of endothelium (11).
Because of the multitude of IFNγ actions, the effects ascribed to this cytokine have been controversial and often contradictory. In particular, the results from studies describing the influence of IFNγ on CD8 T cell responses are often confounded by the possibility of indirect actions of IFNγ via other cell types within the system (13-15). Indeed, IFNγ is known to skew CD4 T helper cell responses toward the Th1 phenotype, thereby indirectly boosting CD8-mediated immune responses (16). More recently, IFNγ was shown in several infection models to promote CD8 T cell responses directly (17-19). In transplantation, however, IFNγ knockout (GKO) or IFNγ-neutralized mice treated with CoB unexpectedly show not increased but decreased survival of allografts as compared to wild type (WT) recipients, and it was suggested that accelerated graft loss on CoB-treated recipients lacking IFNγ might be due to uncontrolled proliferation of activated T cell populations in the absence of IFNγ (20-23). However, results from previous studies of graft survival in IFNγ-deficient recipients treated with CoB may have been confounded by the lack of available IFNγ to act on the graft to promote its survival. As has been shown in several untreated vascularized allograft models, the lack of IFNγ in the recipient or the lack of the IFNγR on the graft itself leads to early graft necrosis associated with microvascular thromboses, hemorrhage, and excessive neutrophil invasion (24-27). Also, IFNγ has been shown to be necessary for regulatory T cells to exert their suppressive effects on T cell alloresponses, and IFNγ is known to upregulate molecules associated with counterregulation of immune responses, molecules such as PD-L1 and IDO that are important in maintaining allograft tolerance (28-32). From these studies, IFNγ could be said to promote tolerance, though this statement would be in stark contrast to the classical understanding of the role of IFNγ as well as the conclusions from infection models where IFNγ was shown to promote CD8 T cell-mediated immunity.
In this report, we provide data that help to reconcile the seemingly contradictory effects of IFNγ in models of transplantation. We illustrate the impact of CoB on in vivo T cell responses to skin allografts as the immune response unfolds. Using polychromatic flow cytometry, intracellular cytokine staining and refined cell-counting techniques, we identified a population of donor-specific effector CD8 T cells and found that this population expanded after graft placement and peaked around the time of graft loss, whether or not CoB was present. As costimulation blockade-resistant rejection is dependent on CD8 T cells, and as IFNγ is known to promote CD8 T cell responses, we hypothesized that IFNγ may be supporting rejection in the absence of major costimulatory signals. While previous studies observed the impact of IFNγ in transplantation under CoB where the cytokine was lacking completely, we investigated the role of IFNγ in transplantation under CoB where the cytokine is present yet the recipient is unable to respond to it. Through this approach, we found that IFNγR expression in the recipient was necessary for population expansion of donor-specific effector CD8 T cells in the absence of costimulatory signals, as IFNγ receptor-knockout (GRKO) recipients treated with CoB showed no expansion of this population and exhibited dramatically prolonged graft survival. In vivo, CoB consistently impaired donor-specific T cell responses, and the impairment in population expansion of donor-specific CD8 T cells under CoB was more extensive in the absence of IFNγ or the IFNγR in the recipient, providing further evidence that the IFNγ pathway plays an important role in costimulation blockade-resistant rejection. Because CD8 responses were equally impaired in GKO or GRKO recipients treated with CoB despite early graft loss in GKO recipients, we hypothesized that IFNγ may be acting on the graft to promote its survival. We show here that skin graft survival under CoB depended on direct action of IFNγ on the skin graft itself, as either IFNγ neutralization or lack of IFNγR1 on the graft precipitated early graft loss on GRKO recipients treated with CoB. Thus, while IFNγ was required by the recipient to expand a population of donor-specific effector CD8 T cells in the absence of costimulation, IFNγ was also required by the graft to sustain its survival after allotransplantation.
Materials & Methods
Mice
Male mice [C57BL/6J (WT), B6.129S7-Ifngr1tm1Agt/J (GRKO), and B6.129S7-Ifngtm1Ts/J (GKO)] aged 6−12 weeks were purchased from The Jackson Laboratories and used as recipients. BALB/cJ (BALB/c) female mice aged 6−12 weeks, also purchased from The Jackson Laboratories, were used as donors. F2 BalbxGRKO mice were generated in-house as follows: female BALB/c mice were mated with male GRKO mice on a C57BL/6 background, then F1 pups were inter-bred. Blood samples from F2 pups were screened by flow cytometry using anti-Kd FITC, anti-Kb PE and anti-CD119 (IFNγR1) biotin followed by streptavidin APC (all BD Pharmingen). F2 mice used as graft donors were Kd homozygous and either CD119−/− or CD119+/+. Animals received humane care and treatment in accordance with Emory University Institutional Animal Care and Use Committee guidelines.
Skin Grafting
Full-thickness ear and tail skin grafts were placed bilaterally on the dorsal thorax and secured with an adhesive bandage for six days. Grafts were scored by visual inspection for signs of necrosis, and rejection was declared when less than 10% of the original graft remained viable.
Costimulation Blockade
500μg CTLA-4:Ig (abatacept, Bristol-Myers Squibb) and 500μg anti-CD154 monoclonal antibody (mAb), clone MR1 (BioXcell, New Hampshire), were delivered intra-peritoneally (i.p.) on post-operative day (POD) 0, 2, 4, and 6. Graft recipients not treated with CTLA-4:Ig and MR1 were given isotype control reagents, human Fc IgG1 and hamster IgG (both BioXCell, New Hampshire), at the same dose and schedule.
IFNγ Neutralization
mAb to murine IFNγ, clone R4−6A2 (BioXcell, New Hampshire), was delivered i.p. on POD −1 (2 mg) and weekly thereafter (1 mg) either until graft rejection (graft survival kinetics experiments) or until terminal harvest of tissues (T cell responses in vivo). In graft survival kinetics experiments, mice not receiving anti-IFNγ mAb were given isotype control mAb, rat IgG1 (BioXcell, New Hampshire), at the same dose and schedule.
Measuring in vivo-generated T cell alloresponses
At indicated time points after graft placement, recipients were euthanized, and splenocytes were isolated. CD45+, CD4+ or CD8+ T cells were enumerated by TruCount bead analysis according to manufacturer's instructions (BD Biosciences). To assess for donor-reactive T cells, 106 recipient splenocytes were incubated with 2×106 BALB/c splenocytes per well in flat-bottom 96-well plates in the presence of 1 mg/mL Brefeldin A for 5 hours at 37° C. Subsequently, cells were stained with anti-CD4 Pacific Blue, anti-CD8 Pacific Orange and anti-Kd FITC (to exclude stimulator cells), then fixed, permeabilized and stained with anti-IFNγ PE and anti-TNF APC (BD Pharmingen) according to kit instructions (eBioscience). All cells were acquired on an LSR-II flow cytometer (BD Coulter), and flow data were analyzed using FlowJo software (TreeStar).
Statistical Analyses
Skin graft survival data were plotted using Prism (Graph Pad), and significance was determined using the Mann-Whitney U test. T cell response data were plotted as the geometric mean ± SEM at each timepoint using Prism (Graph Pad), and significance was determined using the unpaired, two-tailed t-test.
Results
Donor-specific dual cytokine-producing effector CD8 T cells preferentially expand during costimulation blockade-resistant rejection
First, we sought to characterize the cellular events underlying the process of costimulation blockade-resistant rejection. Consistent with an earlier report (6), B6 mice display costimulation blockade-resistant rejection when grafted with BALB/c skin, as CoB only modestly delayed graft rejection with an MST of 24d compared with 13d for isotype control-treated recipients (p<0.0001, fig. 1A). To delineate recipient T cell responses during the course of graft survival and rejection in normal mice under CoB, we analyzed splenocytes from WT recipients at postoperative days (POD) 7, 14, 21, 25, 28, and 35, with particular interest in time points around the MST of grafts in each treatment group. Graft placement on isotype control-treated recipients led to a significant increase in the total number of activated (CD44hi) CD4 and CD8 T cells in the spleen at the time of graft loss, and though most grafts were lost by POD 24 under CoB, at no time point did CoB-treated recipients show a significant increase in the absolute number of activated T cells (data not shown). Furthermore, CoB prevented the development of anti-donor antibodies, which were evident as early as POD 14 in isotype control-treated recipients (data not shown). These results indicated that CoB significantly impaired the normal patterns of T cell activation but did not reveal insight into the mechanism of costimulation blockade-resistant rejection. Therefore, we sought to track more precisely the anti-donor T cell response by looking at the total number of donor-specific CD4 and CD8 effector T cells generated during the period of breakthrough rejection.
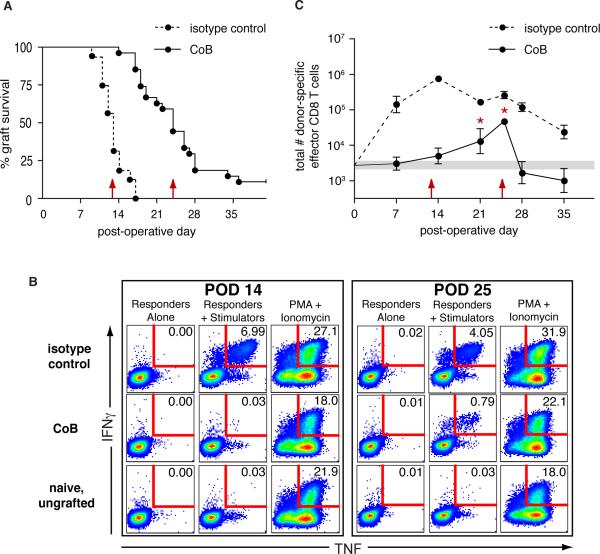
Figure 1. Donor-specific dual cytokine-producing effector CD8 T cells preferentially expand during costimulation blockade-resistant rejection.
BALB/c skin grafts were placed on B6 recipients treated either with CoB or isotype control reagents. (A) CoB-treated recipients showed costimulation blockade-resistant rejection (n=27, MST=24d) with graft survival only modestly prolonged relative to isotype control-treated recipients (n=16, MST=13d, p<0.0001). Red arrows indicate MST for each group. (B&C) Splenocytes were harvested at indicated time points from recipients of BALB/c skin grafts, stimulated in vitro with BALB/c splenocytes, then analyzed by flow cytometry for recipient CD8 T cells expressing IFNγ and TNF. (B) Representative flow plots of recipient splenic CD8 T cells showing donor-specific dual-cytokine producers expressed as a percentage of total CD8 T cells. (C) Total number of donor-specific dual-cytokine producing CD8 T cells in the spleen. Data from naïve, ungrafted, mice (n=24) are represented as a shaded horizontal bar indicating the geometric mean ± SEM. Red arrows indicate MST with isotype control or CoB treatment. * comparison of CoB-treated recipients with naive animals (POD 21, p=0.017; POD 25, p=0.0001). Data shown are from a single experiment with 3−4 recipients per group per time point. Similar results were found in four independent experiments with CoB-treated recipients.
To identify donor-specific effector T cells in graft recipients, we analyzed recipient splenocytes for T cells capable of producing cytokines in response to donor cells in an ex vivo rapid recall assay using intracellular cytokine staining for IFNγ and TNF. Single-producers of TNF in this type of assay have been shown to include naïve T cells stimulated by the short term culture conditions, so we did not consider these in our definition of effector cells generated during the graft response (33). Single-producers of IFNγ have been described as being in a state of partial exhaustion in chronic viral infection models, and in at least one transplant model under costimulation blockade CD8 T cells producing IFNγ have been shown to be tolerogenic (34, 35). Because of these findings and as dual IFNγ & TNF producers have been identified as fully-functional effector T cells (34), we restricted our definition of “donor-specific effector T cells” in our study to T cells producing both IFNγ and TNF. Though analysis of all IFNγ-producers (dual and single) yielded greater cell numbers overall than assessment of strictly dual-cytokine producers, all trends and significance of the differences between groups were the same whether the analysis is performed for all IFNγ producers or restricted to dual cytokine producers (data not shown).
Donor-specific dual cytokine producing CD4 effector T cells were evident only at POD 7 in isotype control-treated recipients, and CoB-treated recipients showed no discernable expansion of donor-specific CD4 T cells at this or any other time point during the first five weeks after graft placement (data not shown). This data is consistent with our prior observations in infection models that CD4 T cells are reliant on costimulation for acquisition of effector function (36). As shown in figure 1B, at POD 14, when grafts on isotype control-treated recipients were failing, a substantial percentage of CD8 T cells in the spleen of isotype control-treated recipients produced both IFNγ and TNF in response to donor stimulation (8.01% +/− 0.869%). In contrast, CoB-treated recipients at this time point showed 60-fold lower frequencies of antigen-specific cytokine-producing CD8 T cells (0.133% +/− 0.067 %), at a level not significantly different from naïve responses (0.085 % +/− 0.037 %, p=0.618). Importantly, at POD 25, when CoB-treated recipients were losing their grafts, the frequency of donor-specific dual cytokine-producing CD8 T cells had increased significantly over naïve responses and became readily discernable on flow plots of CD8 T cells from the spleen of CoB-treated recipients, with 0.713% +/− 0.077 % of CD8 T cells producing both IFNγ and TNF in response to donor stimulation (p<0.0001, comparison with naïve responses). Though the observed frequency of dual cytokine-producing CD8 T cells from CoB-treated recipients at the peak of the response was less than one percent, the cytokine production from these cells was donor-specific and not due simply to the inflammatory milieu in the animal nor to assay background, as un-restimulated responders alone in our assays showed 0.009% +/− 0.001% of CD8 T cells positive for IFNγ and TNF.
Because we were interested in population expansion of donor-specific effector T cells, we used the frequency of donor-specific, dual cytokine-producing T cells in each recipient to calculate the total number of donor-responsive effector T cells in the spleen of each animal through data from TruCount bead analysis (BD Biosciences) of total numbers of CD4 and CD8 T cells within the corresponding individual spleen sample. In this way, we were able to track changes in the total number of cells over time in order to visualize population expansion of donor-specific effectors in the spleen after graft placement. The total number of donor-specific effector CD4 T cells in the spleen of isotype control-treated recipients peaked at POD 7 then rapidly contracted to levels seen in naïve mice, and CoB was able to prevent the expansion of the donor-reactive CD4 T cell population such that their absolute numbers throughout the first five weeks after graft placement did not rise significantly above that observed for naïve mice (data not shown). As seen in figure 1C, the absolute number of donor-specific effector CD8 T cells in isotype control-treated recipients had increased by POD 7 and peaked at POD 14 (log10[cell number] = 5.9 ± 0.075), coinciding temporally with the MST for grafts in this group. CoB treatment prevented the expansion of the donor-specific effector CD8 T cell population at both of these early time points (log10[cell number] = 3.5 ± 0.19 and 3.7 ± 0.22), as no significant difference in absolute number of these cells was found in CoB-treated recipients compared to ungrafted, naive controls (indicated by shaded horizontal bar, log10[cell number] = 3.4 ± 0.10). However, a breakthrough population of donor-specific, dual cytokine-producing CD8 T cells did emerge in CoB-treated recipients, evident as a significant departure from naïve responses by POD 21 (p=0.017). The donor-specific effector CD8 T cell population continued to expand to a peak at POD 25 in CoB-treated recipients (log10[cell number] = 4.7 ± 0.077), temporally correlating with the MST of grafts seen with CoB treatment. Thus, as CoB was able to prevent the increase in number of activated and effector CD4 T cells responding to BALB/c skin grafts, CoB was able to blunt activated CD8 T cell numbers and to delay and diminish but not completely prevent effector CD8 T cell responses to BALB/c grafts. The expansion of a donor-specific effector CD8 T cell population seen at the time of graft loss in CoB-treated recipients (POD 24−25) we define as the CD8 breakthrough response.
Disruption of IFNγ or IFNγR in recipient mice results in divergent effects on allograft survival after treatment with costimulation blockade
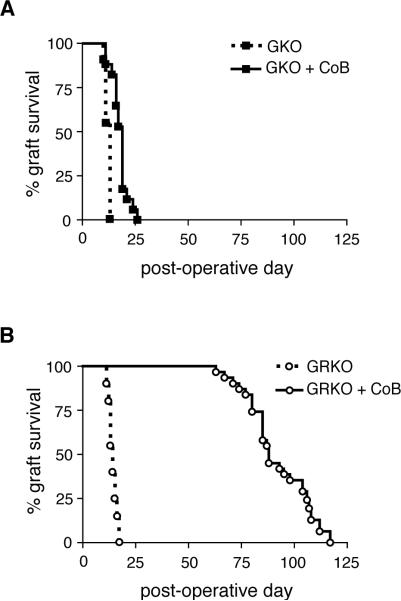
As IFNγ has long been known to promote cell-mediated immune responses and because of its potent effect on accumulation of CD8 effector T cells, we hypothesized that IFNγ contributes to the CD8 breakthrough response correlated with graft rejection under CoB (11, 19). Although previous studies had shown IFNγ-deficient recipients to be aggressive rejectors of grafts even in the presence of CoB (20-22), more recent evidence that grafts require IFNγ in order to protect against early graft necrosis suggested that conclusions about the role of IFNγ in the rejection response under CoB may have been confounded by the lack of IFNγ available to act on the graft (24-27). When we placed BALB/c skin grafts on IFNγ-deficient recipients treated with CoB, we also found graft survival time consistently shortened compared with WT recipients under CoB. As seen in figure 2A, GKO recipients treated with CoB showed a median graft survival time (MST) of only 19d, a significant but not impressive prolongation of graft survival over the MST of 13d seen on isotype control-treated GKO recipients (p<0.0001). However, we hypothesized that graft survival might be greatly prolonged when the IFNγ signal deficit could be isolated to the recipient alone, so we substituted GRKO mice for GKO mice as recipients of BALB/c skin grafts under CoB. Indeed, CoB dramatically prolonged BALB/c skin graft survival on GRKO recipients with an MST of 88d as compared to isotype control-treated GRKO recipients, which potently rejected BALB/c grafts with an MST of 14d (p<0.0001, fig. 2B). Though WT, GKO or GRKO mice are capable of rejecting BALB/c skin grafts within the same time frame (13-14d), CoB affects graft survival time on each of these recipients differently. When IFNγ was available to the graft, graft survival time was greatly prolonged in recipients lacking IFNγ signals as well as CD28 and CD40 signals, and we hypothesized that this prolongation may have been due to a decreased CD8 T cell response in the GRKO recipient treated with CoB.
Figure 2. Disruption of IFNγ or IFNγR in recipient mice results in divergent effects on allograft survival after treatment with costimulation blockade.
BALB/c skin grafts were placed on B6-background recipients treated with either CoB or isotype control reagents and monitored for graft survival. (A) GKO recipients showed normal graft rejection kinetics with isotype control treatment (n=11, MST=13d) and slightly prolonged graft survival when treated with CoB (n=17, MST=19d, p<0.0001). Data are cumulative from three independent experiments. (B) GRKO recipients showed normal graft rejection kinetics with isotype control treatment (n=20, MST=14d) and dramatically prolonged graft survival when treated with CoB (n=31, MST=88d, p<0.0001). Data are cumulative from seven independent experiments.
GRKO recipients show no CD8 breakthrough response under costimulation blockade
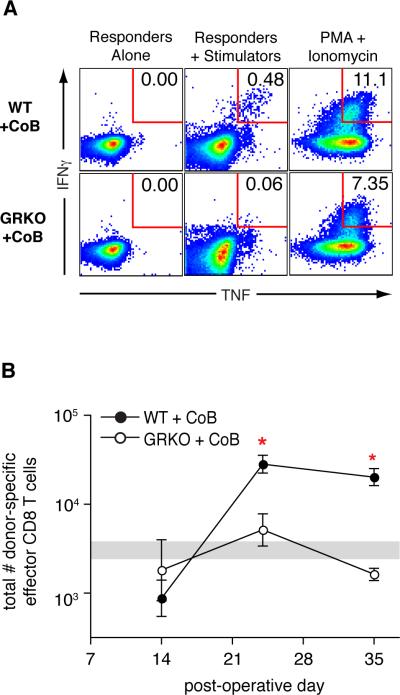
To address whether or not IFNγ affects the generation of a CD8 breakthrough response under CoB in vivo, we looked at T cells from spleens of CoB-treated WT or GRKO recipients of BALB/c skin grafts at various time points after graft placement. We analyzed these cells in the same manner as in the experiments described by figure 1 but with a focus on POD 14, 24 and 35. As observed in WT recipients, CoB-treated GRKO recipients showed no increase in total number of activated (CD44hi) CD8 T cells in the spleen at any time point (data not shown). As shown in figure 3A, WT recipients treated with CoB showed a CD8 breakthrough response of donor-specific dual-cytokine producing CD8 T cells at POD 24 (0.373% +/− 0.037 %). Importantly, this response was not seen in CoB-treated GRKO recipients (0.064 % +/− 0.026 % of CD8 T cells, p=0.0005 for difference between WT and GRKO). The lack of donor-specific dual cytokine-producing GRKO cells was not due to an inability of GRKO mice to produce IFNγ and TNF, as we detected dual cytokine production from these GRKO splenocytes after incubation with a non-specific stimulus of PMA and ionomycin (fig. 3A).
Figure 3. GRKO recipients show no CD8 breakthrough response under costimulation blockade.
Splenocytes were harvested from CoB-treated WT or GRKO recipients of BALB/c skin grafts, stimulated in vitro with BALB/c splenocytes, then analyzed by flow cytometry for CD8 T cells expressing IFNγ and TNF. (A) Representative flow plots of CD8 T cells from the spleen of recipients at POD 24 showing donor-specific dual-cytokine producers expressed as a percentage of total recipient CD8 T cells. (B) Total number of donor-specific dual-cytokine producing CD8 T cells in the spleen. Data from naïve, ungrafted, mice (n=16) are represented by a shaded horizontal bar indicating the geometric mean ± SEM.
* comparison of CoB-treated WT recipients with CoB-treated GRKO recipients (POD 24, p=0.012; POD 35, p<0.001). Data are from a single experiment with three animals per group per time point. Similar results were found in three independent experiments with CoB-treated GRKO recipients.
The frequencies of donor-specific dual cytokine-producing CD8 T cells were used to calculate the total population size of donor-specific effector CD8 T cells at three time points (fig. 3B). At POD 14, regardless of the expression of a functional IFNγR, CoB-treated recipients show naïve levels of total donor-specific effector CD8 T cells in the spleen (shaded horizontal bar, log10[cell number] = 3.5 ± 0.094). At POD 24, however, the population of donor-specific effector CD8 T cells had expanded in WT recipients treated with CoB (log10[cell number] = 4.5 ± 0.098) but remained at naïve levels in GRKO recipients treated with CoB (log10[cell number] = 3.7 ± 0.18). Untreated GRKO recipients were able to mount a donor-specific effector CD8 T cell response, as these mice did show increased numbers of dual-cytokine producing CD8 T cells at POD 14 and POD 24 (data not shown). The near-naïve levels of donor-specific effector CD8 T cells in CoB-treated GRKO recipients at time points when WT recipients showed peak numbers of donor-responsive cells suggested an impairment of antigen-specific effector CD8 T cell population expansion in recipients where critical costimulatory signals as well as IFNγ signals are lacking.
Paradoxical graft loss with no CD8 breakthrough response in IFNγ-deficient recipients treated with costimulation blockade
Previously, in experiments using GKO recipients or WT recipients treated with IFNγ-neutralizing antibody, IFNγ was found to be critical for the modest prolongation of graft survival seen with CoB (20-22). We also found that BALB/c skin grafts on GKO or IFNγ-neutralized WT recipients showed similarly shortened survival kinetics despite CoB (figs. 2A and 4A). As shown in figure 4A, BALB/c skin grafts on WT mice treated with CoB and with a neutralizing mAb to IFNγ showed a MST of 17.5d, significantly attenuated as compared to WT recipients treated with CoB alone (MST 24d, p=0.0033) but dramatically attenuated as compared to GRKO recipients treated with CoB (MST 88d, p<0.0001). Since the lack of the cytokine or the lack of the cytokine receptor should be expected to present with the same immunophenotype, we hypothesized that despite graft loss, IFNγ-deficient recipients must also harbor impaired anti-donor T cell responses under CoB.
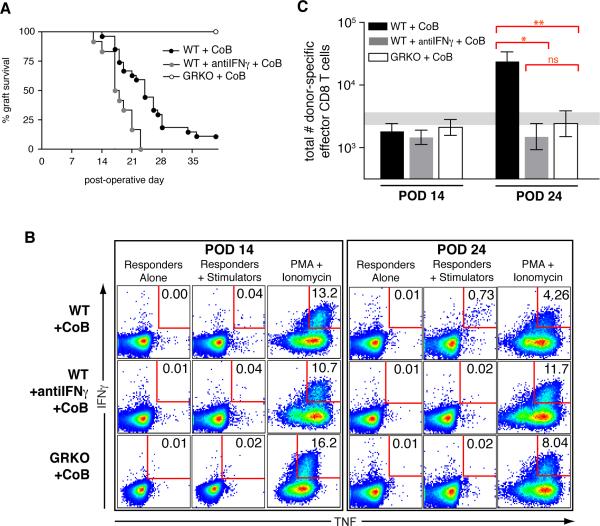
Figure 4. Paradoxical graft loss with no CD8 breakthrough response in IFNγ-deficient recipients treated with costimulation blockade.
BALB/c skin grafts were placed on B6-background recipients treated with either CoB or isotype control reagents. (A) Graft survival kinetics. Neutralization of IFNγ in WT recipients treated with CoB (WT+antiIFNγ+CoB) shorted graft survival (MST=17.5d, n=12) as compared with WT+CoB recipients (MST=24d, n=27, p=0.0033) and as compared to GRKO+CoB recipients (MST=88d, n=31, p<0.0001). Data are from three independent experiments involving each of the groups presented. (B) Representative flow plots of recipient splenic CD8 T cells showing donor-specific dual cytokine producers expressed as a percentage of total recipient CD8 T cells. (C) Total number of donor-specific dual cytokine producing CD8 T cells in the spleen at POD 14 (WT+CoB, n=13; WT+antiIFNγ+CoB, n=7, GRKO+CoB, n=8) and POD 24 (WT+CoB, n=10; WT+antiIFNγ+CoB, n=7, GRKO+CoB, n=10). * p=0.0002; ** p=0.0010. Data from naïve, ungrafted, mice (n=28) are represented by a shaded horizontal bar indicating the geometric mean ± SEM. Data are combined from independent experiments with a minimum total of seven mice per group per time point.
Because our identification of donor-specific effector T cells depended on the expression of IFNγ for intracellular cytokine staining, for the next study we used WT recipients treated with neutralizing antibody to IFNγ throughout the experiment (WT+antiIFNγ), as GKO recipients would not be able to produce the necessary IFNγ in the assay system to enumerate donor-responsive T cells. To address CD8 T cell population expansion in vivo in IFNγ-deficient mice under CoB, splenocytes from graft recipients were analyzed as in the experiments above but with a finer focus on time points relevant to the peak cytokine responses of CD8 T cells in WT recipients, namely POD 14 and POD 24. As had been seen in WT recipients, CoB blocked in vivo T cell responses in recipients where IFNγ was neutralized, in terms of activated (CD44hi) CD8 and CD4 T cell numbers as well as total numbers of donor-specific effector CD4 T cells at both time points (data not shown). As shown in figure 4B, which summarizes results from all experiments, the percent of total CD8 T cells responding to donor stimulation was negligible at POD 14 in recipients treated with CoB, whether the recipient was WT (0.055% +/− 0.024%), lacked IFNγ (0.031% +/− 0.006%) or lacked the IFNγR (0.053% +/− 0.014%). At POD 24, when WT recipients under CoB showed a breakthrough anti-donor CD8 T cell response (0.471% +/− 0.077%), donor-specific CD8 T cell responses remained at naïve levels (0.074% +/− 0.020%) in WT recipients treated with neutralizing IFNγ mAb and CoB (0.047% +/− 0.024%) as well as in GRKO recipients treated with CoB (0.040% +/− 0.015%). Thus, an intact IFNγ axis in the recipient is required for the generation of a costimulation blockade-resistant anti-donor CD8 T cell response.
When these frequencies were used to calculate total numbers of donor-specific effector CD8 T cells in the spleen at the time of the CD8 breakthrough response in WT recipients (POD 24, fig. 4C), neither IFNγ-neutralized WT recipients (log10[cell number] = 3.2 ± 0.21) nor GRKO recipients (log10[cell number] = 3.4 ± 0.21) under CoB showed a population size above that seen for naïve mice (log10[cell number] = 3.5 ± 0.089), and the responses of these two groups of recipients were not significantly different from one another (p=0.50). The lack of IFNγ signaling in recipient cells, whether by the lack of the cytokine or the cytokine receptor, led to a significant reduction in the population size of donor-specific effector CD8 T cells at POD 24 under CoB, as WT recipients (log10[cell number] = 4.4 ± 0.15) showed a significant increase in this population as compared to IFNγ-neutralized WT recipients (p=0.00020) or to GRKO recipients (p=0.0010). Despite the failure of a donor-specific effector CD8 T cell population to expand in IFNγ-deficient animals treated with CoB, skin allografts had failed on these recipients. Therefore, we hypothesized that graft death on IFNγ-deficient recipients treated with CoB may be due to lack of IFNγ available to act on the graft and that this type of death may have masked the otherwise beneficial effect of an impaired CD8 T cell response in IFNγ-deficient recipients treated with CoB.
IFNγ is required for prolonged skin graft survival on GRKO recipients treated with costimulation blockade
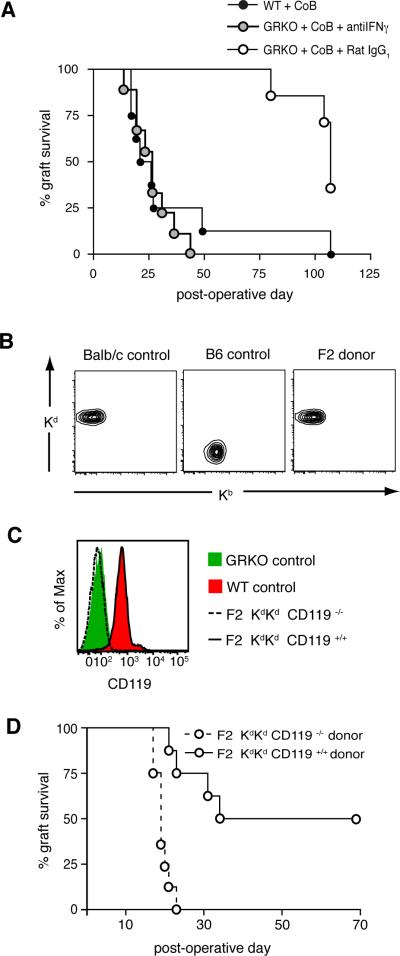
To test the hypothesis that IFNγ action on the graft is necessary for graft survival in our model of skin transplantation, we neutralized IFNγ in GRKO recipients treated with CoB (which otherwise would show long-term graft survival) such that only the cells with an intact receptor for IFNγ (the graft cells) would be affected by the absence of the cytokine. As shown in figure 5A, BALB/c skin graft survival was dramatically attenuated when IFNγ was neutralized in GRKO recipients treated with CoB, bringing graft survival from an MST of 107d in the GRKO+CoB+Rat IgG1 group down to an MST of 26d in the GRKO+CoB+antiIFNγ group (p<0.001). Neutralizing IFNγ reverted the acceptor phenotype of the CoB-treated GRKO recipient to a rejector phenotype with graft survival kinetics similar to that of the CoB-treated WT recipient (MST 23.5d).
Figure 5. IFNγ is required for prolonged skin graft survival on GRKO recipients treated with costimulation blockade.
(A) BALB/c skin grafts were placed on CoB-treated WT or GRKO recipients, which were treated with either anti-IFNγ mAb (n=9) or isotype control Rat IgG1 mAb (n=7). Neutralization of IFNγ in GRKO+CoB recipients precipitated graft loss (MST=26d) with kinetics significantly different from GRKO recipients treated with CoB and Rat IgG1 (MST=107d, p=0.00017). (B and C) F2 pups from the breeding of BALB/c mice with GRKO mice on a B6 background were typed by flow cytometry of PBMC. (B) MHC status of F2 pups used as graft donors (Kd and Kb molecules). (C) CD119 (IFNγR1 subunit) expression in F2 pups used as graft donors. (D) Skin grafts from F2 donors were placed on GRKO recipients on a B6 background treated with CoB. F2 KdKd CD119−/− grafts showed an MST of 19d, significantly different from the MST of F2 KdKd CD119+/+ grafts (51.5d, p=0.0003). Data are from two independent experiments with a total of eight mice per group.
The results from the cytokine neutralization experiments above did not rule out the possibility of an alternate, as yet undiscovered, receptor for IFNγ in the recipient that would both have tolerogenic properties as well as not use the known ligand-binding subunit, IFNγR1, mutated in GRKO mice. Therefore, to allow IFNγ to remain in the system while addressing the impact of IFNγ directly on the graft tissue, we sought to replace normal BALB/c skin in our model with fully-allogeneic donor tissue lacking IFNγR1. As GRKO mice on a BALB/c background were not commercially available, we bred BALB/c mice to B6-background GRKO mice and typed PBMC from the F2 generation by flow cytometry for class I MHC and IFNγR status. F2 pups chosen as skin donors were Kd homozygous (fig. 5B) to ensure a strong direct alloresponse and either completely lacked CD119 (IFNγR1 subunit) or had full expression of CD119 (fig. 5C). We grafted skin from these F2 donors onto B6 background GRKO mice treated with CoB, and found that F2 grafts lacking IFNγR were swiftly lost with an MST of 19d (fig. 5D), identical to that of BALB/c grafts on GKO recipients treated with CoB (fig. 2A), and significantly different from that of grafts from F2 CD119+/+ littermate controls on GRKO recipients (51.5d, p=0.0003). In sum, graft survival could not be prolonged on GRKO recipients treated with CoB when either IFNγ was absent from the system (i.e. IFNγ neutralization) or when the graft, too, could not respond to IFNγ, demonstrating for the first time in a model of costimulation blockade-based immunomodulation that IFNγ must act directly on the graft to prolong its survival.
Discussion
In light of recent evidence that IFNγ is required by the graft to avoid early necrosis after transplantation, the decade-old observation that graft survival in GKO recipients cannot be prolonged by CoB may have limited our view of the impact of IFNγ on alloresponses in the absence of T cell costimulation (24). In this study, we sought to isolate the effects of IFNγ to either the recipient or the graft in order to better understand the impact of this cytokine on costimulation blockade-resistant rejection. We found that recipients lacking the receptor for IFNγ showed greatly prolonged graft survival under CoB, bringing into question old ideas about the role of IFNγ in costimulation blockade-resistant rejection. Some previous conclusions about why grafts aggressively fail on IFNγ-deficient recipients despite CoB were based on data suggestive of “runaway” proliferation or failure of contraction of T cells in the presence of the allostimulus and the absence of IFNγ, though these studies did not address the resulting population size of the donor-specific T cell pool present in vivo during the course of graft survival and death (22, 23). To investigate the impact of IFNγ on CD8 T cells under CoB, we initially tracked the population expansion of donor-specific CD8 effector T cells during the course of the immune response to skin allografts in wild-type recipients and found that the total number of these cells in the spleen increased after graft placement and peaked around the time of graft loss. Though CoB delayed the emergence of this population of donor-specific CD8 effector T cells, a small breakthrough population did expand just prior to graft loss in treated recipients. These results unify findings from previous studies showing that CD8 T cells are necessary for rejection in the face of CoB and that the frequency of IFNγ-secreting cells of unknown origin increases around the time of graft rejection under CoB (6-9). Importantly, we found that the expansion of this donor-specific CD8 effector T cell population under CoB depended on IFNγ, for when recipients did not express the IFNγR or when IFNγ was neutralized in vivo, this population did not expand. That grafts were rapidly lost on IFNγ-neutralized recipients, in the absence of a detectable population of donor-specific effector CD8 T cells, can be explained by our finding that the grafted skin itself required IFNγ in order to survive after allotransplantation under CoB. Previous reports have demonstrated the importance of graft-specific action of IFNγ on the survival of spontaneously accepted murine liver allografts as well as on the prevention of early necrosis of heart or kidney allografts in untreated recipients (24-26, 37), though ours is the first report showing the importance of graft-directed IFNγ for graft survival in a model where the recipient T cell response is subdued by blockade of major costimulatory pathways. Evidence for a positive, direct impact of IFNγ on graft survival in this setting dictated a reappraisal of results from studies from our group and others that showed GKO recipients to be resistant to the graft-prolonging effects of CoB (20-22). Our new data indicate that accelerated graft loss on these recipients is due not to uncontrolled expansion of CoB-resistant alloreactive T cells but rather to the lack of direct IFNγ signals to the graft to support tissue survival even at times when the recipient T cell response is undetectable.
Despite a limited population size of donor-specific CD8 effector T cells in recipients treated with CoB and lacking IFNγ, graft survival time was dramatically reduced compared to recipients treated with CoB and lacking the receptor for IFNγ. The discordant outcomes in graft survival between recipients with concordant CD8 T cell responses under CoB presented a quandary initially. As transplantation by definition brings together cells derived from two separate entities, we propose a model wherein the cytokine, as a diffusible element, acts both on the recipient receptors to exert a pro-rejection effect as well as on the graft receptors to exert a pro-survival effect. Our graft survival data from IFNγ-neutralized recipients agrees with previously published studies where IFNγ-deprivation within the entire transplant system accelerates graft loss (22, 24-26). Our current data showing prolonged graft survival on recipients unable to respond to IFNγ, as well as accelerated graft loss on these recipients after IFNγ-neutralization or when the graft lacks the IFNγR, point toward a necessary action of IFNγ on the graft to activate survival mechanisms crucial for weathering the alloresponse environment.
In line with the idea of a protective effect of IFNγ in the milieu of an immune response, the presence of IFNγ has been shown to ameliorate disease in several autoimmune models such as collagen-induced arthritis, experimental autoimmune conjunctivitis, and most notably in experimental autoimmune encephalomyelitis (EAE) (38-40). Importantly, a recent study in EAE showed that IFNγ delivered specifically to the central nervous system promotes survival of oligodendrocytes after disease induction, and this survival effect was mediated by pancreatic endoplasmic reticulum kinase (PERK), a stress-response kinase activated downstream of IFNγR signals (41). The timing and location of IFNγ action may certainly be important in determining disease outcome, as intravenous delivery of IFNγ to patients with multiple sclerosis exacerbated existing disease, with an increase in activated mononuclear cells in the peripheral blood of those who relapsed under IFNγ treatment (42). However, Wang et al. found that IFNγ-treated CD4 T cells could suppress EAE upon adoptive transfer (43). Sawitzki et al. found a similar requirement for IFNγ in generating functional regulatory T cells in a transplant model where adoptively transferred CD4+CD25+ cells generated via a tolerance-inducing regimen of donor splenocyte transfusion in WT mice were able to suppress allograft rejection, but CD4+CD25+ cells generated in GKO mice did not have the same suppressive capacity (32). Though the generation of regulatory T cells may not be as important in our model as in others, our studies indicate that IFNγ signals delivered to recipient cells play a crucial role in the expansion of donor-specific CD8 effector T cells during costimulation blockade-resistant rejection.
How could IFNγ promote the population expansion of anti-donor CD8 T cells in the setting of costimulation blockade? IFNγ may partially supplant the role of CD40 stimulation in the initial activation of APCs, as IFNγ is known to upregulate MHC class I and class II as well as to effectively upregulate CD86 on murine APC (11, 44). Alternatively, or additionally, IFNγ might directly support CD8 effector T cell survival in order to amass a large enough population to reject the graft. Whitmire et al. found that the IFNγR was necessary on CD8 T cells for accumulation of CD8 effector T cells during acute LCMV infection, though division capacity and differentiation were not different from normal CD8 T cells, implying that IFNγ aids effector CD8 T cell population expansion predominantly via a pro-survival effect (19). Also, IFNγ might foster homeostasis of cross-reactive memory T cells or aid effector T cell trafficking to the graft, as direct IFNγ signals have been shown to aid trafficking of CD8 T cells to the lung during influenza A infection in mice and to be important in maintenance of CD8 memory T cell populations after LCMV infection (17, 18). Recent studies have shown that IFNγ signals in T cells can activate protein kinase C theta (PKCθ), a signaling kinase also activated downstream of TCR-mediated signals (45, 46). PKCθ has been shown to synergize with calcineurin, to activate NF-κB, and importantly, to provide an alternate pathway to activate AP-1, a transcription factor induced downstream of CD28 stimulation (45). As activation of PKCθ in CD8 T cells was shown to have a greater impact on cell survival than on proliferation (47), PKCθ activation via IFNγ signaling may be one way for donor-responding CD8 T cells to survive in the absence of critical costimulatory signals in order to amass a large enough population to mediate graft destruction. Though T cell proliferation and graft survival in the absence of IFNγ have been monitored in various transplant models (22, 23), this is the first study demonstrating the necessity of IFNγ signals to recipient cells for the population expansion of donor-specific CD8 effector T cells in the absence of major costimulatory signals.
In addition, other recipient cell types downstream of the CD8 T cell response may contribute to graft destruction and may also be impaired by the lack of IFNγ□□□□□□□. Several models have been proposed to explain the role of IFNγ in CD8 T cell-mediated tissue destruction. Schuler and Blankenstein found in a tumor model that CD8 T cells must secrete IFNγ and that the host must express the IFNγR in order for tumors to be eliminated, leading them to propose a model where CTL recognize tumor cells directly then secrete IFNγ which acts on a third cell-type in the host which is responsible for tumor cell killing (48). Diamond and Gill also found that IFNγ secretion by CD8 T cells was necessary for efficient rejection of pancreatic islet allografts, but whether the IFNγ produced by the CD8 effectors killed islets directly or promoted expansion of the CD8 effector population was not determined (49). Valujskikh et al. found that CTL kill allografts in an IFNγ-dependent manner by indirect recognition of alloantigens expressed on endothelial cells of graft-infilrating vessels and suggested that IFNγ was required for endothelial cells to process and present donor antigens (50). Ongoing experiments seek to define where in the process of the immune response to allografts IFNγ tips the balance to promote CD8 population expansion and graft rejection in the absence of CD28 and CD40 signals.
Lastly, it is possible that the pleiotropic effects of IFNγ on the graft and on host immune cells involve cell type-specific signaling pathways downstream of the IFNγ receptor (46, 51). Of particular interest is the finding that immune cells have IFNγ signaling pathways that are distinct from those used by other cells of the body (52). Exploiting the cell type-specific signaling pathways for IFNγ may be one way to target blockade of IFNγ action to just the immune cells while sparing the protective action of IFNγ on the graft. Alternately, it may be possible to expose grafts or donors to IFNγ prior to transplantation to initiate pro-survival pathways. Recipients could then be treated with anti-IFNγ antibodies to blunt T cell alloresponses. Similarly, by delaying administration of anti-IFNγ, it may be possible to allow the pro-survival effects on the graft but keep the potential mounting CD8 response in check. In addition, one could envision exploiting the species specificity of IFNγ in xenotransplantation. For example, recipients of porcine pancreatic islet grafts could be treated with porcine IFNγ to enhance specifically the survival of grafted islet cells while avoiding the pro-rejection effects of IFNγ on recipient immune cells. Though the pro-survival impact of IFNγ on the graft may not be sufficient to defend the graft from a competent T cell alloresponse, the action of IFNγ on the graft is a necessary survival force that must be considered when designing strategies to promote allograft tolerance.
The results presented in this manuscript underscore the role of IFNγ in supporting opposing sides of the struggle toward donor-specific tolerance and clarify the need for more precise manipulation of IFNγ responses when considering any new tolerance-inducing regimen. The modifying effects of IFNγ shown here have the potential to impact a broad range of immunological studies, from autoimmunity studies where the cytokine may well serve a protective role for parenchymal cells, to studies of viral infection and vaccine development where stimulation of IFNγ activity would be necessary to achieve a substantial population of effector CD8 T cells to clear virus or to generate effective memory T cell responses. In a broad sense, these results suggest what could be an elegantly efficient role for IFNγ in the normal setting of a strong immune stimulus when an important balance must be reached between mounting a CD8 T cell response of sufficient size to clear the pathogen and limiting the collateral damage to surrounding tissues in order to preserve the functionality of the organism. Often, approaches to modulate immune behavior focus on removing a problem factor completely. In considering modulating IFNγ for therapeutic benefit, more refined approaches will be necessary to achieve the desired outcome without perturbing the entire system.
Acknowledgments
We would like to acknowledge Dr. Leslie Kean of the Emory Transplant Center for helpful conversations and Dr. Robert Mittler of the Emory Vaccine Center for critical review of the manuscript.
Nonstandard Abbreviations
- CoB
costimulation blockade
- GKO
IFNγ knockout
- GRKO
IFNγR knockout
- MST
median graft survival time
- PKCθ
protein kinase C theta
- POD
postoperative day
Footnotes
This work was supported by NIH research grant R37 AI40519-11 to Dr. Christian Larsen.
References
- 1.Jenkins MK, Ashwell JD, Schwartz RH. Allogeneic non-T spleen cells restore the responsiveness of normal T cell clones stimulated with antigen and chemically modified antigen-presenting cells. J Immunol. 1988;140:3324–3330. [PubMed] [Google Scholar]
- 2.Vincenti F. Costimulation blockade in autoimmunity and transplantation. J Allergy Clin Immunol. 2008;121:299–306. doi: 10.1016/j.jaci.2008.01.002. quiz 307−308. [DOI] [PubMed] [Google Scholar]
- 3.Fox CJ, Hammerman PS, Thompson CB. Fuel feeds function: energy metabolism and the T-cell response. Nat Rev Immunol. 2005;5:844–852. doi: 10.1038/nri1710. [DOI] [PubMed] [Google Scholar]
- 4.Elster EA, Hale DA, Mannon RB, Cendales LC, Swanson SJ, Kirk AD. The road to tolerance: renal transplant tolerance induction in nonhuman primate studies and clinical trials. Transpl Immunol. 2004;13:87–99. doi: 10.1016/j.trim.2004.05.010. [DOI] [PubMed] [Google Scholar]
- 5.Larsen CP, Elwood ET, Alexander DZ, Ritchie SC, Hendrix R, Tucker-Burden C, Cho HR, Aruffo A, Hollenbaugh D, Linsley PS, Winn KJ, Pearson TC. Long-term acceptance of skin and cardiac allografts after blocking CD40 and CD28 pathways. Nature. 1996;381:434–438. doi: 10.1038/381434a0. [DOI] [PubMed] [Google Scholar]
- 6.Williams MA, Trambley J, Ha J, Adams AB, Durham MM, Rees P, Cowan SR, Pearson TC, Larsen CP. Genetic characterization of strain differences in the ability to mediate CD40/CD28-independent rejection of skin allografts. J Immunol. 2000;165:6849–6857. doi: 10.4049/jimmunol.165.12.6849. [DOI] [PubMed] [Google Scholar]
- 7.He G, Hart J, Kim OS, Szot GL, Siegel CT, Thistlethwaite JR, Newell KA. The role of CD8 and CD4 T cells in intestinal allograft rejection: a comparison of monoclonal antibody-treated and knockout mice. Transplantation. 1999;67:131–137. doi: 10.1097/00007890-199901150-00022. [DOI] [PubMed] [Google Scholar]
- 8.Trambley J, Bingaman AW, Lin A, Elwood ET, Waitze SY, Ha J, Durham MM, Corbascio M, Cowan SR, Pearson TC, Larsen CP. Asialo GM1(+) CD8(+) T cells play a critical role in costimulation blockade-resistant allograft rejection. J Clin Invest. 1999;104:1715–1722. doi: 10.1172/JCI8082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Honey K, Cobbold SP, Waldmann H. CD40 ligand blockade induces CD4+ T cell tolerance and linked suppression. J Immunol. 1999;163:4805–4810. [PubMed] [Google Scholar]
- 10.Wheelock EF, Sibley WA. Circulating Virus, Interferon and Antibody after Vaccination with the 17-D Strain of Yellow-Fever Virus. N Engl J Med. 1965;273:194–198. doi: 10.1056/NEJM196507222730404. [DOI] [PubMed] [Google Scholar]
- 11.Boehm U, Klamp T, Groot M, Howard JC. Cellular responses to interferon-gamma. Annu Rev Immunol. 1997;15:749–795. doi: 10.1146/annurev.immunol.15.1.749. [DOI] [PubMed] [Google Scholar]
- 12.Fountoulakis M, Zulauf M, Lustig A, Garotta G. Stoichiometry of interaction between interferon gamma and its receptor. Eur J Biochem. 1992;208:781–787. doi: 10.1111/j.1432-1033.1992.tb17248.x. [DOI] [PubMed] [Google Scholar]
- 13.Bucy RP, Hanto DW, Berens E, Schreiber RD. Lack of an obligate role for IFN-gamma in the primary in vitro mixed lymphocyte response. J Immunol. 1988;140:1148–1152. [PubMed] [Google Scholar]
- 14.Refaeli Y, Van Parijs L, Alexander SI, Abbas AK. Interferon gamma is required for activation-induced death of T lymphocytes. J Exp Med. 2002;196:999–1005. doi: 10.1084/jem.20020666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hidalgo LG, Urmson J, Halloran PF. IFN-gamma decreases CTL generation by limiting IL-2 production: A feedback loop controlling effector cell production. Am J Transplant. 2005;5:651–661. doi: 10.1111/j.1600-6143.2005.00761.x. [DOI] [PubMed] [Google Scholar]
- 16.Gajewski TF, Fitch FW. Anti-proliferative effect of IFN-gamma in immune regulation. I. IFN-gamma inhibits the proliferation of Th2 but not Th1 murine helper T lymphocyte clones. J Immunol. 1988;140:4245–4252. [PubMed] [Google Scholar]
- 17.Turner SJ, Olivas E, Gutierrez A, Diaz G, Doherty PC. Disregulated influenza A virus-specific CD8+ T cell homeostasis in the absence of IFN-gamma signaling. J Immunol. 2007;178:7616–7622. doi: 10.4049/jimmunol.178.12.7616. [DOI] [PubMed] [Google Scholar]
- 18.Whitmire JK, Eam B, Benning N, Whitton JL. Direct interferon-gamma signaling dramatically enhances CD4+ and CD8+ T cell memory. J Immunol. 2007;179:1190–1197. doi: 10.4049/jimmunol.179.2.1190. [DOI] [PubMed] [Google Scholar]
- 19.Whitmire JK, Tan JT, Whitton JL. Interferon-gamma acts directly on CD8+ T cells to increase their abundance during virus infection. J Exp Med. 2005;201:1053–1059. doi: 10.1084/jem.20041463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bishop DK, Chan Wood S, Eichwald EJ, Orosz CG. Immunobiology of allograft rejection in the absence of IFN-gamma: CD8+ effector cells develop independently of CD4+ cells and CD40-CD40 ligand interactions. J Immunol. 2001;166:3248–3255. doi: 10.4049/jimmunol.166.5.3248. [DOI] [PubMed] [Google Scholar]
- 21.Kishimoto K, Sandner S, Imitola J, Sho M, Li Y, Langmuir PB, Rothstein DM, Strom TB, Turka LA, Sayegh MH. Th1 cytokines, programmed cell death, and alloreactive T cell clone size in transplant tolerance. J Clin Invest. 2002;109:1471–1479. doi: 10.1172/JCI14947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Konieczny BT, Dai Z, Elwood ET, Saleem S, Linsley PS, Baddoura FK, Larsen CP, Pearson TC, Lakkis FG. IFN-gamma is critical for long-term allograft survival induced by blocking the CD28 and CD40 ligand T cell costimulation pathways. J Immunol. 1998;160:2059–2064. [PubMed] [Google Scholar]
- 23.Hassan AT, Dai Z, Konieczny BT, Ring GH, Baddoura FK, Abou-Dahab LH, El-Sayed AA, Lakkis FG. Regulation of alloantigen-mediated T-cell proliferation by endogenous interferon-gamma: implications for long-term allograft acceptance. Transplantation. 1999;68:124–129. doi: 10.1097/00007890-199907150-00023. [DOI] [PubMed] [Google Scholar]
- 24.Halloran PF, Afrouzian M, Ramassar V, Urmson J, Zhu LF, Helms LM, Solez K, Kneteman NM. Interferon-gamma acts directly on rejecting renal allografts to prevent graft necrosis. Am J Pathol. 2001;158:215–226. doi: 10.1016/s0002-9440(10)63960-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Halloran PF, Miller LW, Urmson J, Ramassar V, Zhu LF, Kneteman NM, Solez K, Afrouzian M. IFN-gamma alters the pathology of graft rejection: protection from early necrosis. J Immunol. 2001;166:7072–7081. doi: 10.4049/jimmunol.166.12.7072. [DOI] [PubMed] [Google Scholar]
- 26.Mele TS, Kneteman NM, Zhu LF, Ramassar V, Urmson J, Halloran B, Churchill TA, Jewell L, Kane K, Halloran PF. IFN-gamma is an absolute requirement for spontaneous acceptance of liver allografts. Am J Transplant. 2003;3:942–951. doi: 10.1034/j.1600-6143.2003.00153.x. [DOI] [PubMed] [Google Scholar]
- 27.Miura M, El-Sawy T, Fairchild RL. Neutrophils mediate parenchymal tissue necrosis and accelerate the rejection of complete major histocompatibility complex-disparate cardiac allografts in the absence of interferon-gamma. Am J Pathol. 2003;162:509–519. doi: 10.1016/s0002-9440(10)63845-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Thebault P, Condamine T, Heslan M, Hill M, Bernard I, Saoudi A, Josien R, Anegon I, Cuturi MC, Chiffoleau E. Role of IFNgamma in allograft tolerance mediated by CD4+CD25+ regulatory T cells by induction of IDO in endothelial cells. Am J Transplant. 2007;7:2472–2482. doi: 10.1111/j.1600-6143.2007.01960.x. [DOI] [PubMed] [Google Scholar]
- 29.Munn DH, Zhou M, Attwood JT, Bondarev I, Conway SJ, Marshall B, Brown C, Mellor AL. Prevention of allogeneic fetal rejection by tryptophan catabolism. Science. 1998;281:1191–1193. doi: 10.1126/science.281.5380.1191. [DOI] [PubMed] [Google Scholar]
- 30.Rodig N, Ryan T, Allen JA, Pang H, Grabie N, Chernova T, Greenfield EA, Liang SC, Sharpe AH, Lichtman AH, Freeman GJ. Endothelial expression of PD-L1 and PD-L2 down-regulates CD8+ T cell activation and cytolysis. Eur J Immunol. 2003;33:3117–3126. doi: 10.1002/eji.200324270. [DOI] [PubMed] [Google Scholar]
- 31.Keir ME, Liang SC, Guleria I, Latchman YE, Qipo A, Albacker LA, Koulmanda M, Freeman GJ, Sayegh MH, Sharpe AH. Tissue expression of PD-L1 mediates peripheral T cell tolerance. J Exp Med. 2006;203:883–895. doi: 10.1084/jem.20051776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sawitzki B, Kingsley CI, Oliveira V, Karim M, Herber M, Wood KJ. IFN-gamma production by alloantigen-reactive regulatory T cells is important for their regulatory function in vivo. J Exp Med. 2005;201:1925–1935. doi: 10.1084/jem.20050419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Brehm MA, Mangada J, Markees TG, Pearson T, Daniels KA, Thornley TB, Welsh RM, Rossini AA, Greiner DL. Rapid quantification of naive alloreactive T cells by TNF-alpha production and correlation with allograft rejection in mice. Blood. 2007;109:819–826. doi: 10.1182/blood-2006-03-008219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wherry EJ, Blattman JN, Murali-Krishna K, van der Most R, Ahmed R. Viral persistence alters CD8 T-cell immunodominance and tissue distribution and results in distinct stages of functional impairment. J Virol. 2003;77:4911–4927. doi: 10.1128/JVI.77.8.4911-4927.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Guillonneau C, Hill M, Hubert FX, Chiffoleau E, Herve C, Li XL, Heslan M, Usal C, Tesson L, Menoret S, Saoudi A, Le Mauff B, Josien R, Cuturi MC, Anegon I. CD40Ig treatment results in allograft acceptance mediated by CD8CD45RC T cells, IFN-gamma, and indoleamine 2,3-dioxygenase. J Clin Invest. 2007;117:1096–1106. doi: 10.1172/JCI28801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Whitmire JK, Flavell RA, Grewal IS, Larsen CP, Pearson TC, Ahmed R. CD40-CD40 ligand costimulation is required for generating antiviral CD4 T cell responses but is dispensable for CD8 T cell responses. J Immunol. 1999;163:3194–3201. [PubMed] [Google Scholar]
- 37.Famulski KS, Sis B, Billesberger L, Halloran PF. Interferon-gamma and donor MHC class I control alternative macrophage activation and activin expression in rejecting kidney allografts: a shift in the Th1-Th2 paradigm. Am J Transplant. 2008;8:547–556. doi: 10.1111/j.1600-6143.2007.02118.x. [DOI] [PubMed] [Google Scholar]
- 38.Kelchtermans H, De Klerck B, Mitera T, Van Balen M, Bullens D, Billiau A, Leclercq G, Matthys P. Defective CD4+CD25+ regulatory T cell functioning in collagen-induced arthritis: an important factor in pathogenesis, counter-regulated by endogenous IFN-gamma. Arthritis Res Ther. 2005;7:R402–415. doi: 10.1186/ar1500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Fukushima A, Fukata K, Ozaki A, Takata M, Kuroda N, Enzan H, Ueno H. Exertion of the suppressive effects of IFN-gamma on experimental immune mediated blepharoconjunctivitis in Brown Norway rats during the induction phase but not the effector phase. Br J Ophthalmol. 2002;86:1166–1171. doi: 10.1136/bjo.86.10.1166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wheeler RD, Owens T. The changing face of cytokines in the brain: perspectives from EAE. Curr Pharm Des. 2005;11:1031–1037. doi: 10.2174/1381612053381657. [DOI] [PubMed] [Google Scholar]
- 41.Lin W, Bailey SL, Ho H, Harding HP, Ron D, Miller SD, Popko B. The integrated stress response prevents demyelination by protecting oligodendrocytes against immune-mediated damage. J Clin Invest. 2007;117:448–456. doi: 10.1172/JCI29571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Panitch HS, Hirsch RL, Haley AS, Johnson KP. Exacerbations of multiple sclerosis in patients treated with gamma interferon. Lancet. 1987;1:893–895. doi: 10.1016/s0140-6736(87)92863-7. [DOI] [PubMed] [Google Scholar]
- 43.Wang Z, Hong J, Sun W, Xu G, Li N, Chen X, Liu A, Xu L, Sun B, Zhang JZ. Role of IFN-gamma in induction of Foxp3 and conversion of CD4+ CD25-T cells to CD4+ Tregs. J Clin Invest. 2006;116:2434–2441. doi: 10.1172/JCI25826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Larsen CP, Ritchie SC, Hendrix R, Linsley PS, Hathcock KS, Hodes RJ, Lowry RP, Pearson TC. Regulation of immunostimulatory function and costimulatory molecule (B7−1 and B7−2) expression on murine dendritic cells. J Immunol. 1994;152:5208–5219. [PubMed] [Google Scholar]
- 45.Isakov N, Altman A. Protein kinase C(theta) in T cell activation. Annu Rev Immunol. 2002;20:761–794. doi: 10.1146/annurev.immunol.20.100301.064807. [DOI] [PubMed] [Google Scholar]
- 46.Srivastava KK, Batra S, Sassano A, Li Y, Majchrzak B, Kiyokawa H, Altman A, Fish EN, Platanias LC. Engagement of protein kinase C-theta in interferon signaling in T-cells. J Biol Chem. 2004;279:29911–29920. doi: 10.1074/jbc.M401997200. [DOI] [PubMed] [Google Scholar]
- 47.Barouch-Bentov R, Lemmens EE, Hu J, Janssen EM, Droin NM, Song J, Schoenberger SP, Altman A. Protein kinase C-theta is an early survival factor required for differentiation of effector CD8+ T cells. J Immunol. 2005;175:5126–5134. doi: 10.4049/jimmunol.175.8.5126. [DOI] [PubMed] [Google Scholar]
- 48.Schuler T, Blankenstein T. Cutting edge: CD8+ effector T cells reject tumors by direct antigen recognition but indirect action on host cells. J Immunol. 2003;170:4427–4431. doi: 10.4049/jimmunol.170.9.4427. [DOI] [PubMed] [Google Scholar]
- 49.Diamond AS, Gill RG. An essential contribution by IFN-gamma to CD8+ T cell-mediated rejection of pancreatic islet allografts. J Immunol. 2000;165:247–255. doi: 10.4049/jimmunol.165.1.247. [DOI] [PubMed] [Google Scholar]
- 50.Valujskikh A, Lantz O, Celli S, Matzinger P, Heeger PS. Cross-primed CD8(+) T cells mediate graft rejection via a distinct effector pathway. Nat Immunol. 2002;3:844–851. doi: 10.1038/ni831. [DOI] [PubMed] [Google Scholar]
- 51.Platanias LC. Mechanisms of type-I- and type-II-interferon-mediated signalling. Nat Rev Immunol. 2005;5:375–386. doi: 10.1038/nri1604. [DOI] [PubMed] [Google Scholar]
- 52.van Boxel-Dezaire AH, Stark GR. Cell type-specific signaling in response to interferon-gamma. Curr Top Microbiol Immunol. 2007;316:119–154. doi: 10.1007/978-3-540-71329-6_7. [DOI] [PubMed] [Google Scholar]