Abstract
We report the case of a 17-year-old girl with toxic adenoma scheduled for surgery right lobectomy and isthmectomy of thyroid gland. During the examination before surgery, patient was diagnosed for the first time as having with Wolff – Parkinson – White (WPW) syndrome. In the operating room, after the induction of anesthesia, the electrocardiogram showed wide QRS complex tachycardia with a rate of 180 beats/min, which was diagnosed as paroxysmal supraventricular tachycardia. The patient was treated immediately with antiarrhythmic drugs: adenosine iv three times (at doses of 6 mg, 12mg, 12mg bolus) and esmolol iv twice (at doses 28.5 mg). This approach resulted in disappearance of the delta wave and tachycardia for the whole surgery period. In this case report we discuss the role of induction of anesthesia and presence of toxic adenoma in a patient with WPW.
Keywords: Wolff-Parkinson-White syndrome, accessory pathway, preexcitation, congenital heart defect, toxic adenoma, hyper functioning solitary nodule, radio-frequency ablation, surgical ablation
Pre-excitation or the Wolff – Parkinson – White (WPW) electrocardiogram (ECG) abnormality affects 1 - 3 per 1000 persons1,2. About 2/3 of these patients have marked arrhythmias1,2. These patients have ECG findings suggesting that, in addition to atria-ventricular conduction over the His pathway, there is also conduction through an accessory pathway3. These congenital accessory atria – ventricular connections (bundle of Kent) are composed of strands of atria-like muscle, which may occur almost anywhere around the atria – ventricular rings. As accessory pathways often have a more rapid rate of conduction compared to the normal atria - ventricular node, ventricular depolarization occurs sooner than expected. The patient with WPW is often symptomatic because of cardiac arrhythmias.
Of patients with WPW syndrome 3.4% have first-degree relatives with pre-excitation4. The familial form is inherited as a mendelian autosomal dominant trait5. The WPW syndrome may also be inherited with other cardiac or non-cardiac disorders, such as familial atrial septal defects, familial hypopothasemic periodic paralysis, tuberous sclerosis.
Common ECG abnormalities of the usual form of WPW syndrome (ante grade) include: (1) a short PR interval – PR < 120 ms during sinus rhythm; (2) a wide QRS complex – QRS complex duration > 120 ms with a slurred, slowly rising onset of the QRS in some leads (delta wave) and usually a normal terminal QRS portion and (3) secondary ST-T wave changes that are generally directed in an opposite direction to the major delta and QRS vectors.
In the past, the patients with WPW were treated with antiarrhythmic drugs and surgical ablation was performed only in those with refractory to drug treatment or life-threatening arrhythmias. Nowadays, radio-frequency ablation has greatly deteriorated the need for both surgical ablation and antiarrhythmic-drug therapy9.
Case report
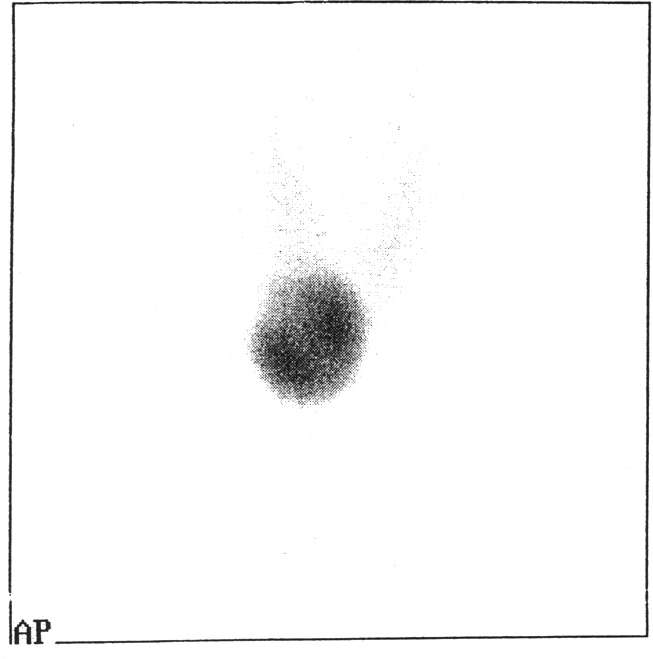
V.K. a 17 – year old girl ( body weight 56kg, height 162cm, ASA II) with a nodule at the isthmus of thyroid gland, diagnosed as "hot" by radionuclide scanning (Figure 1) was referred to our hospital for surgery. Antithyroid drugs were used before surgery. Clinical assessment, chest x-ray and laboratory findings were normal.
Figure 1: Radionuclide scanning of thyroid gland showing a hot nodule.
The patient was for the first time in her life diagnosed as having WPW syndrome during the preoperative cardiac examination (Figure 2, 3). The patient and her family were informed about this diagnosis and the possible complications during surgery.
Figure 2: ECG of Wolff-Parkinson-White syndrome showing a short PR interval and delta wave fused with normal QRS complex in I and Avl.
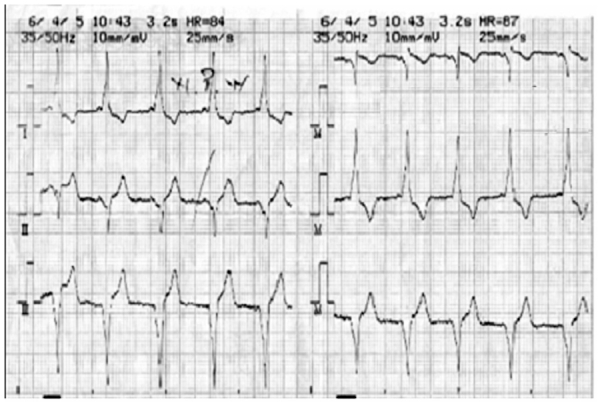
Figure 3: ECG of Wolff-Parkinson-White syndrome showing pre-exitation.
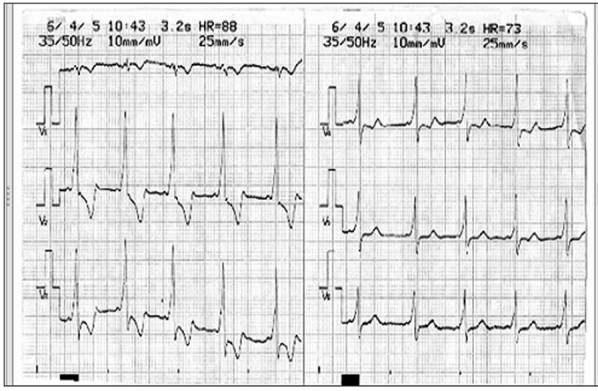
Midazolam 15mg per os was used as premedication, 40 min before surgery. After standard monitoring for operation and continuous ECG monitoring, general anesthesia was induced with propofol 4 mg/kg, fentanyl 3 µg/kg, and pancuronium 1 mg/kg and maintained with 1.2-1.5% halothane and fentanyl 1 µg/kg. After intubation using an endo-tracheal tube (ETT) 7.0 the ECG showed wide QRS complex tachycardia with a rate of 180 180 beats/min which was diagnosed as paroxysmal supraventricular tachycardia and a delta wave. Blood pressure was 111/71 mm Hg. Our choice was treatment with antiarrhythmic drugs: adenosine i.v three times (at doses 6 mg, 12 mg, 12 mg bolus) and esmolol i.v. twice (at doses 28.5 mg, injected slowly for about 4min). These drugs lead to the delta wave and tachycardia disappearance. The continuous ECG monitoring showed a sinus rhythm until the end of surgery. Serum electrolyte concentration and arterial gases were within normal limits.
Halothane was discontinued at the end of operation (isthmusectomy) which lasted 110 min. Because of the increasing ventricular response through the normal pathway, atropine was not used with neostigmine (1mg) at the recovery room.
The patient was extubated without complications at the end of thyroid surgery. She made an uneventful recovery and ECG was the same as before the operation. The patient was discharged from the hospital two days later.
Discussion
Solitary thyroid adenomas that cause hyperthyroidism (toxic adenoma, hyper functioning solitary nodule, toxic nodule) are benign monoclonal tumors characterized by their capacity to grow and produce T4 and T3 autonomously, i.e., in the absence of thyrotropin (TSH)11.
The manifestations of toxic adenoma are generally milder than those of Graves' disease and are notable for the absence of the autoimmune signs (infiltrative orbitopathy, myopathy); cardiovascular manifestations may be prominent12,13.
Patients who require thyroidectomy may have other unrelated diseases that affect anesthetic management. WPW syndrome is one of those situations. Patients with WPW syndrome have an accessory pathway that bypasses the atrio-ventricular node. The two most common types of arrhythmia in the WPW syndrome are: (1) a circus movement tachycardia, also called an atrio-ventricular reentrant tachycardia, (2) atrial fibrillation7. Atrial fibrillation is a life-threatening arrhythmia in patients with WPW syndrome if the atrio ventricular accessory pathway has a short anterograde refractory period that allows many atrial impulses to be conducted to the ventricle. That will result in very high ventricular rates with possible deterioration into ventricular fibrillation and sudden death8. A circus movement tachycardia is well tolerated in general by the patient when additional heart disease is absent, but may deteriorate into atrial fibrillation, and the ventricular rate and risk for ventricular fibrillation will depend on the anterograde refractory period of the accessory pathway. Patients with the WPW syndrome can have intermittent pre-excitation with variable ECG patterns. Conduction by way of an accessory pathway frequently occurs in a retrograde fashion, from ventricles to atria, and the pathway can thus be concealed and the electrocardiogram normal. Analysis of the ECG pattern can be used to localize the accessory pathway6.
Anesthetic management is aimed at avoiding the tachyarrhythmia in patients with WPW. In our case paroxysmal supra-ventricular tachycardia happened after the application of pancuronium and halothane anesthetic in a patient with a WPW syndrome. Other published adverse effects include elevation of heart rate and blood pressure that can be manifested as tachycardia14. The supra-ventricular tachycardia was precipitated from electrophysiological effect of anesthetic drugs on accessory pathway conduction (pancuronium and halothane).
This case illustrated tachyarrhythmia produced by pancuronium ,which has a tendency to cause increased pulse rate, blood pressure, and cardiac output via interactions with muscarinic receptors in the autonomic nervous system and by inhibiting catecholamine reuptake at sympathetic nerve terminals15.
We started treatment with adenosine and esmolol. We negated the role of toxic adenoma in induction of paroxysmal supraventricular tachycardia, as the patient was euthyroid before the operation and at the moment that tachyarrhythmia occurred, we had not started the manipulations of the thyroid gland.
References
- 1.Glob MH, Green MS, Tang AS-L, et al. Identification of a gene responsible for familial Wolff-Parkinson-White syndrome. N Engl J Med. 2001;344:1823–1831. doi: 10.1056/NEJM200106143442403. [DOI] [PubMed] [Google Scholar]
- 2.Deal BJ, Keane JF, Gillette PC, Garson AJr. Wolff-Parkinson-White syndrome and supraventricular tachycardia during infancy: management and follow-up. J Am Coll Cardiol. 1985;5:130–135. doi: 10.1016/s0735-1097(85)80095-4. [DOI] [PubMed] [Google Scholar]
- 3.Klein GJ, Yee R, Sharma AD. Longitudinal electrophysiological assessment of asymptomatic patient with the Wolff-Parkinson-White electrocardiographic pattern. N Eng J Med. 1989;320:1229–1233. doi: 10.1056/NEJM198905113201901. [DOI] [PubMed] [Google Scholar]
- 4.Massumi RA. Familial Wolff-Parkinson-White syndrome with cardiomyopathy. Is J Med. 1967;43:951–955. doi: 10.1016/0002-9343(67)90254-9. [DOI] [PubMed] [Google Scholar]
- 5.MacRae CA, Ghaisas N, Kass S, et al. Familial hypertrophic cardiomyopathy with Wolff-Parkinson-White syndrome maps to a locus on chromosome 7q3. J Clin Invest. 1995;96:1216–1220. doi: 10.1172/JCI118154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fitzpatrick AP, Gonzales RP, Lesh MD, et al. New algorithm for the localization of accessory atrioventricular connections using a baseline electrocardiogram. J Am Coll Cardiol. 1994;23:107–116. doi: 10.1016/0735-1097(94)90508-8. [DOI] [PubMed] [Google Scholar]
- 7.Gallagher JJ, Pritchett ELC, Sealy WC, Kassell J, Wallace AG. The preexcitation syndromes. Prog Cardiovasc Dis. 1978;20:285–327. doi: 10.1016/0033-0620(78)90015-4. [DOI] [PubMed] [Google Scholar]
- 8.Dreyfus LS, Haiat R, Watanabe Y. Ventricular fibrillation: a possible mechanism of sudden death in patients with Wolff-Parkinson-White syndrome. Circulation. 1971;43:520–527. doi: 10.1161/01.cir.43.4.520. [DOI] [PubMed] [Google Scholar]
- 9.Jackman WM, Wang X, Friday KJ, et al. Catheter ablation of accessory atrioventricular pathways (Wolff-Parkinson-White syndrome) by radiofrequency current. N Engl J Med. 1991;324:1605–1611. doi: 10.1056/NEJM199106063242301. [DOI] [PubMed] [Google Scholar]
- 10.Pappone C, Manguso F, Santinelli R, et al. Radiofrequency ablation in children with asymptomatic Wolff-Parkinson-White syndrome. N Eng J Med. 2004;351:1197–1205. doi: 10.1056/NEJMoa040625. [DOI] [PubMed] [Google Scholar]
- 11.Van Sande J, Lamy F, Lecocq R, et al. Pathogenesis of autonomous thyroid nodules: in vitro study of iodine and adenosine 38, 58-monophosphate metabolism. J Clin Endocrinol Metab. 1988;66:570–579. doi: 10.1210/jcem-66-3-570. [DOI] [PubMed] [Google Scholar]
- 12.Trivalle C, Doucet J, Chassagne P, et al. Differences in the signs and symptoms of hyperthyroidism in older and younger patients. J Am Geriatr Soc. 1996:44–50. doi: 10.1111/j.1532-5415.1996.tb05637.x. [DOI] [PubMed] [Google Scholar]
- 13.Ross DS, Ridgway EC, Daniels GH. Successful treatment of solitary toxic thyroid nodules with relatively low-dose iodine-131, with low prevalence of hypothyroidism. Ann Intern Med. 1984;101:488–490. doi: 10.7326/0003-4819-101-4-488. [DOI] [PubMed] [Google Scholar]
- 14.Murray MJ, Cowen J, DeBlock H, et al. Clinical Practice Guidelines for Sustained Neuromuscular Blockade in the Adult Critically Ill Patient. Task Force of the American College of Critical Care Medicine (ACCM) of the Society of Critical Care Medicine (SCCM), American Society of Health-System Pharmacists, American College of Chest Physicians. Crit Care Med. 2002;30:142–156. doi: 10.1097/00003246-200201000-00021. [DOI] [PubMed] [Google Scholar]
- 15.Miller RD, Agoston S, Booij LH, et al. The comparative potency and pharmacokinetics of pancuronium and its metabolites in anesthetized man. J Pharmacol Exp Ther. 1978;207:532. [PubMed] [Google Scholar]
- 16.Allman KG, Iain HW. Oxford handbook of anaesthesia. 2nd ed. New York: Oxford university press; 2006. Oxford handbook of anaesthesia; pp. 868–1106. [Google Scholar]


