Abstract
The elasticity of a given arterial segment of the aorta and of big elastic arteries is not constant but depends on its distending pressure. As distending pressure increases, there is greater recruitment of inelastic collagen fibers and thereby a reduction in elasticity. It also depends on structural changes in the medial layer of the elastic arteries (mainly aorta and major arterial conduits), and is largely the result of progressive elastic fibre degeneration.
Aortic Pulse Wave Velocity (PWV), is the most robust marker of arterial stiffness, however additional useful information can also be provided by the Central Augmentation Index (AIx C), and pulse pressure. The presence of systemic inflammation in cardiovascular disease and in particular in essential hypertension affects arterial stiffness and increases PWV. Some pharmacological and non-pharmacological interventions may improve arterial stiffness and thereby decrease PWV.
Keywords: pulse wave velocity, arterial stiffness, augmentation index, pulse pressure
Arterial stiffness
Arteries are conduits that deliver blood at high pressure to peripheral vascular beds. They are separated into two anatomic regions with distinct functions: (1) Large elastic arteries (e.g. aorta, carotid, iliac), which store the blood during systole and expel it to the peripheral circulation during diastole, so that the capillaries receive a steady blood flow through the whole cardiac cycle. (2) Muscular arteries, especially those in the lower body (e.g. femoral, popliteal, posterior tibial), which alter their tone and thus can modify the speed of travel of the pressure wave along their length, thereby determining the extent to which and timing at which, the reflected wave arrives back at the heart1.
The arterial wall consists of three layers: intima, media, and adventitia. The intima consists of a single layer of endothelial cells, supported by smooth muscle cells and is separated from the media by the internal elastic lamina, which is composed largely of elastic fibres. The medial layer represents the main determinant of the mechanical properties of the elastic arteries, and comprises of elastic laminae in concentric layers interspersed with collagen and smooth muscle cells. The third layer is the adventitia, consisting mainly of fibroblasts and collagen. The elasticity of the large arteries is the result of the high elastin to collagen ratio in their walls, which progressively declines toward the periphery2. Moreover, the elasticity of a given arterial segment is not constant but depends also on its distending pressure. As distending pressure increases, there is greater recruitment of inelastic collagen fibers and thereby a reduction in elasticity.
Increased arterial stiffness parallels structural changes in the medial layer of the elastic arteries (mainly aorta and major arterial conduits), and is largely the result of progressive elastic fiber degeneration. An increase in stiffness related to arterial wall composition occurs with aging, and is accelerated in patients with hypertension3,4. It is also seen in patients with end-stage renal disease and diabetes5,6. Recently, increased arterial stiffness has been reported in women but not in men with type II diabetes mellitus7. With ageing, the orderly structure of the elastic lamina becomes deranged due to its thinning and fracturing. Furthermore, there is secondary accumulation of collagen in the arterial wall and increased collagen crosslinking. The most obvious clinical consequences of arterial stiffening are increased pulse pressure (PP), caused by higher SBP and lower DBP, with a resulting increase in left ventricular afterload and reduction in coronary perfusion8.
Measurement of wave reflections
Applanation tonometry is used to record the pulse pressure waveform in the radial artery. This waveform can be then analysed by applying a transfer function9, and the central pulse pressure waveform in the aorta can be inferred. However, in a recent study it was shown that transfer function, although required to determine central SBP from the radial artery, is not necessary and that similar information about the central pressure wave can be derived directly from the radial pulse10.
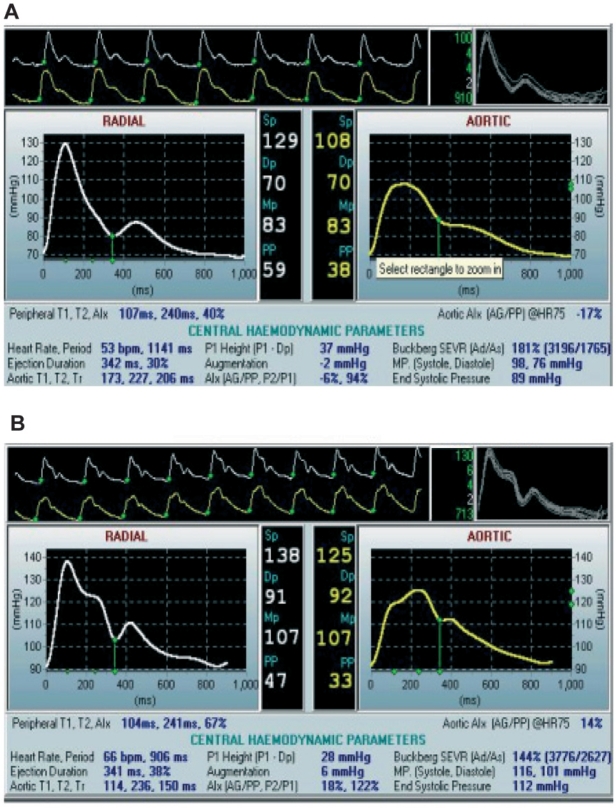
The pulse pressure (PP) wave is formed by the combination of the incident wave and waves reflected back from the periphery. The incident wave is generated by the left ventricle during systole and travels along the arterial system towards the periphery through a low resistance pathway which keeps the mean pressure almost unchanged. However, close to capillaries, mean pressure falls in a short distance within the high resistance arterioles. At the junction between high-conductance arteries and high-resistance arterioles, wave reflection occurs. Under normal circumstances, almost 80% of the incident wave is reflected from arterioles11. The PP wave is a combination of incident and reflected waves at any point along the arterial system. When the large elastic arteries are compliant, the incident wave travelling from the heart to the periphery is responsible for peak SBP. The wave velocity is slow and therefore the reflected pressure wave arrives from the periphery in diastole augmenting the DBP and preserving coronary perfusion. As large elastic arteries stiffen the wave velocity is increased and the reflected wave returns earlier and merges with the systolic part of the incident wave1. As a consequence, an increase in systolic and a decrease in diastolic BP occurs, thereby increasing PP and decreasing coronary perfusion12. The shape of PP waveform varies throughout the arterial tree due to differences in elastic qualities and wave reflection. In young healthy subjects, the SBP and PP are amplified in the peripheral circulation, whereas at older ages this amplification is reduced, as a result of both the increase in pulse wave velocity (PWV) with age, and the earlier return of the reflected wave due to the involvement of reflection sites closer to the heart. PP waveform is different in health and disease, with characteristic shapes in elderly or hypertensive patients. Characteristic PP waveforms from the radial artery and the central aorta, from a younger (20 years old, A) and from an older (64 years old, B) healthy man, are shown for comparison in Figure 1.
Figure 1: Pulse pressure waveforms from the radial artery and the derived waveform in the central aorta. Peripheral and central pressure waveforms, acquired with the Sphygmocor device, in a young (A) and an older (B) healthy man.
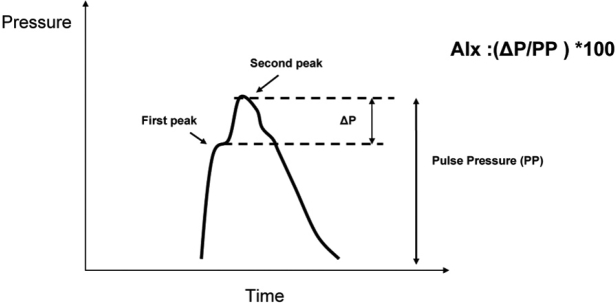
Augmentation index (AIx) is calculated as the increment in pressure from the first shoulder in the ascending aortic pressure wave to the peak of this wave, expressed as a percentage of the peak ascending aortic pressure wave (Figure 2). AIx depends on the duration of the cardiac cycle (and consequently on heart rate), on the velocity of the pulse wave, and on the amplitude of the reflected pulse wave. Therefore DBP and height may influence AIx as they may influence the reflection sites12. In healthy elastic arteries, AIx is related mainly to the magnitude of the reflected wave rather than to its velocity, whereas in stiff arteries the relationship between PWV and AIx is stronger13. By the measurement of the pulse wave one can obtain information about central systolic pressure and PP, and also central AIx (AIx C). However, AIx C is not considered a direct index of arterial stiffness and needs to be combined with the measurement of PWV. In a recent study performed in 4,001 healthy subjects, it was shown that both AIx C and PWV increase with age but AIx C increases more in young individuals and PWV increases more in older ones, suggesting that the former is a more sensitive marker of arterial stiffness in young and the latter is more sensitive in older subjects14.
Figure 2: Illustration of a central aortic pressure waveform in a middle-aged subject. The second systolic peak becomes more prominent with age or as arteries stiffen, and is caused by wave reflection. The AIx C is defined as the difference between the second and first systolic peaks (ΔP) expressed as a percentage of the PP.
Measurement of pulse wave velocity
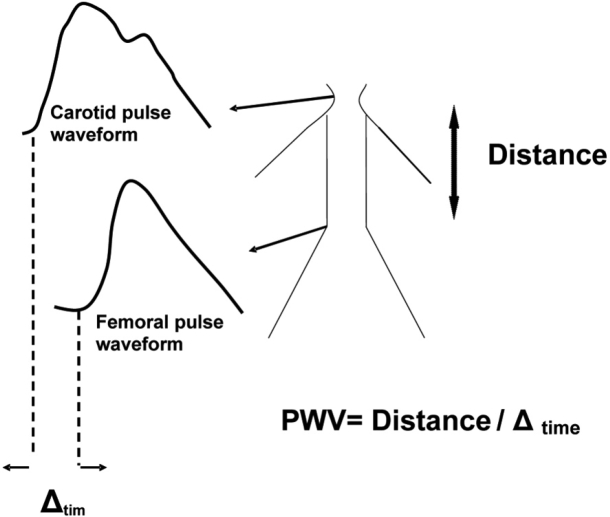
The aortic PWV is the "gold standard" marker for measuring arterial stiffness, and is widely used to estimate vascular stiffness and "vascular health". The velocity of the arterial wave is predicted by the Moens-Korteweg equation, PWV=√ (Eh/2ρR), where E is Young's modulus of the arterial wall, h is wall thickness, R is arterial radius at the end of diastole, and ρ is blood density1. The velocity of the wave is measured between two predefined sites of the arterial system. The arterial pulse wave is recorded at a proximal artery such as the common carotid, as well as at a distal artery such as the femoral. These two arteries are the most widely used, because they are superficial and the recording of the pulse waveforms can be done non-invasively using tonometry. Furthermore, between those two sites, the pulse wave has to travel through most of the aorta, an artery particularly prone to atherosclerosis. The distance travelled by the pulse wave is measured over the body surface. The time delay of the arrival of the foot of the pulse wave at these two sites is obtained by gating to the R wave of the ECG12 (Figure 3).
Figure 3: Measurement of aortic PWV. The time delay (Δtime) is measured between the foot of the wave at the two arterial sites, and the distance is measured over the body surface. PWV is calculated as Distance/Δtime.
The measurement of aortic PWV has been recognized as the most simple and reproducible for estimating arterial stiffness12. Therefore it has been widely used in a variety of patient groups and has been established as a strong marker of cardiovascular events. Data from the Framingham study showed that men have slightly higher PWV compared to women and, although significant, this difference is small15.
Heart rate has been reported to influence PWV16,17 but this has been attributed to methodological considerations15. Recently a study was performed comparing the effects of increased heart rate, induced either by pacing or by infusing a β2-adrenergic agonist, and measuring PWV using two different devices (SphygmoCor and Complior); it was shown that there is indeed a true increase in PWV with heart rate, and that this is not attributable to changes in BP or to different algorithms used by the two devices18. However, the authors concluded that the influence of heart rate on PWV is likely to vary according to age, sex and degree of arterial stiffening. Indeed, another study demonstrated an increase in aortic PWV after an increase in heart rate in males but not in females19. However, Zambanini and colleagues have proposed that, because the heart-pacing rates which were used in most of these studies may increase central mean and diastolic pressure, it is likely that this contributes to the increase in PWV rather than the increase of the heart rate itself20.
The role of NO in regulating arterial elasticity has been controversial. By investigating the effect of L-NMMA (NG-monomethyl-L-arginine, a non-specific inhibitor of Nitric Oxide Synthase) on the elastic properties of different arteries (e.g. radial and brachial), the results from studies have been conflicting. Some researchers showed that infusion of L-NMMA decreases the elastic properties of the brachial artery21, while others showed no effect on radial artery stiffness22. Similarly the effect of endothelium- derived NO on aortic PWV is also controversial. PWV measured in the ovine iliac artery, a more muscular artery than the aorta, was found to be significantly increased after infusion of L-NMMA23. These investigators applied the same technique to humans, and showed that L-NMMA increased and GTN (glyceryl trinitrate, an exogenous Nitric Oxide donor) reduced iliac arterial stiffness, when infused locally into the artery, without any change in the mean arterial pressure24. In contrast to these studies, Stewart et al showed that systemic infusion of L-NMMA increased mean arterial pressure and carotidfemoral PWV in humans; however, they found that this effect was similar to that produced by two other vasoactive drugs (norepinephrine and dobutamine) used as controls25. In accordance with this, a previous study from the same group showed that systemic infusion of GTN, in the absence of a change in BP, has no effect on aortic stiffness13. The authors suggested that mean arterial pressure is the most important determinant of short-term changes in carotid-femoral PWV25.
Αrterial stiffness and inflammatory markers
Arterial stiffness is largely dependent on the structural properties of the large artery wall. Elastin, as the main component of the large artery wall, is susceptible to degradation by enzymes such as metalloproteinases (MMP), mainly MMP-9 and MMP-2, and also by serum elastase. Increased activity of the above enzymes has been related to atherosclerosis and also to generation of aneurysms in humans through destruction of elastic laminae26,27. Recently, it has been demonstrated that individuals with isolated systolic hypertension have increased activity of MMP-9, MMP-2 and serum elastase. MMP-9 was also correlated linearly to aortic PWV in hypertensives, and both MMP-9 and serum elastase activity levels were linearly correlated to PWV in young healthy individuals28. Levels of MMP-9 were associated with C-reactive protein (CRP) levels also in young individuals, indicating a possible link between inflammation and increase in arterial stiffness28. Indeed it has been shown that inflammation is associated with increased arterial stiffness, and PWV is independently correlated to CRP levels in patients with inflammatory diseases such as systemic vasculitis29 and rheumatoid arthritis30. Treatment of the inflammatory disease may result in significant reduction of PWV30. Furthermore acute systemic inflammation, after Salmonella typhi vaccination, leads to increase in aortic stiffness31. A strong correlation between CRP levels and PWV was also found in healthy individuals, suggesting a role of inflammation in the process of arterial stiffening even in health32. In essential hypertension, the contribution of systemic inflammation to arterial stiffening was investigated by Mahmud and colleagues. They showed that levels of CRP, tumor necrosis factor (TNF-α) and interleukin-6 (IL-6) were independently correlated to PWV and to AIx C33. Other investigators also have shown independent correlations between CRP and PWV in essential hypertension34. Apart from the relation between inflammation and arterial stiffening, evidence also exists for a genetic contribution. It has been shown that polymorphisms of the MMP-9 gene may affect arterial stiffening and levels of elastase activity in healthy individuals35.
The usefulness of measuring AIx and PWV in cardiovascular diseases, including essential hypertension
AIx C and central PP are independent predictors of cardiovascular and all-cause mortality in patients with end-stage renal disease36,37, and of cardiovascular events in hypertensive patients38. In the latter study patients that were on antihypertensive treatment with amlodipine/ perindopril had lower central aortic pressure and PP compared with patients taking atenolol/thiazide, despite similar brachial SBP and no difference in PWV. Central PP was significantly associated with total cardiovascular events and procedures and with the development of renal impairment in this cohort of hypertensive patients. This was attributed mainly to the prolonged systolic ejection time in the atenolol/thiazide group, which delayed the outgoing pressure wave and increased the possibility that the reflected wave will augment it during systole. An earlier return of the wave reflection due to relative vasoconstriction in the atenolol/thiazide group may also have contributed to the findings38. This study was the first powered sufficiently to investigate if different antihypertensive drug treatments may have differential effects on central aortic pressure and if this is associated with cardiovascular outcomes. It provides also a reasonable explanation why β-blockers (and specifically atenolol), according to a recent meta-analysis of the early BP trials, were not found to be better than placebo in preventing cardiovascular events39. These findings imply that brachial BP may not always be the best measure of severity of hypertension, and that in future the measurement of central aortic BP may provide more useful information about prognosis and the effectiveness of treatment.
Aortic PWV has been found to be a strong predictor of cardiovascular mortality in healthy subjects older than 70 years old40, in end-stage renal disease5, diabetes6, and hypertension41. Aortic PWV is an independent predictor of cardiovascular and all-cause mortality42, of coronary events43, and of fatal strokes44 in patients with essential hypertension. It also correlates strongly with coronary atherosclerosis45, and predicts coronary heart disease and stroke in a population of older individuals without a previous history of either46.
Since aortic stiffness predicts cardiovascular events, it is important to investigate factors that may worsen or improve it. For example, it has been shown that caffeine, smoking, black tea and acute mental stress all have detrimental effects on arterial stiffness47–50. Non-pharmacological measures such as exercise51 and dietary changes, in particular weight loss52, low salt diet53 and moderate alcohol consumption54, can all reduce arterial stiffness. Pharmacological studies in hypertensive populations have shown improvement of arterial stiffness after initiation of antihypertensive treatment. These studies include treatment with diuretics55, ACE inhibitors55,56, ARBs57, Ca2+- blockers58, and aldosterone antagonists59. Other pharmacological interventions that decrease arterial stiffening are hormone replacement therapy60, antidiabetic agents61, and advanced glycation end product breakers62, and antitumor necrosis factor-alpha (anti-TNFa) treatment30 and statin therapy63 in patients with rheumatoid arthritis
Conclusion
In summary, measurement of AIx C, PP, and PWV are useful markers for estimating vascular function in patients with cardiovascular diseases and in particular essential hypertension. The "gold standard" marker of arterial stiffness remains PWV, however in the light of the most recent studies additional important information may be obtained also by measuring AIx C and PP.
References
- 1.O'Rourke MF, Kelly RP, Avolio AP. The arterial pulse Philadelphia. London: Lea & Febiger; 1992. [Google Scholar]
- 2.Nichols WW, Singh BM. Augmentation index as a measure of peripheral vascular disease state. Curr Opin Cardiol. 2002;17:543–551. doi: 10.1097/00001573-200209000-00016. [DOI] [PubMed] [Google Scholar]
- 3.Avolio AP, Deng FQ, Li WQ, et al. Effects of aging on arterial distensibility in populations with high and low prevalence of hypertension: comparison between urban and rural communities in China. Circulation. 1985;71:202–210. doi: 10.1161/01.cir.71.2.202. [DOI] [PubMed] [Google Scholar]
- 4.Avolio AP, Chen SG, Wang RP, Zhang CL, Li MF, O'Rourke MF. Effects of aging on changing arterial compliance and left ventricular load in a northern Chinese urban community. Circulation. 1983;68:50–58. doi: 10.1161/01.cir.68.1.50. [DOI] [PubMed] [Google Scholar]
- 5.Blacher J, Guerin AP, Pannier B, Marchais SJ, Safar ME, London GM. Impact of aortic stiffness on survival in end-stage renal disease. Circulation. 1999;99:2434–2439. doi: 10.1161/01.cir.99.18.2434. [DOI] [PubMed] [Google Scholar]
- 6.Cruickshank K, Riste L, Anderson SG, Wright JS, Dunn G, Gosling RG. Aortic pulse-wave velocity and its relationship to mortality in diabetes and glucose intolerance: an integrated index of vascular function? Circulation. 2002;106:2085–2090. doi: 10.1161/01.cir.0000033824.02722.f7. [DOI] [PubMed] [Google Scholar]
- 7.De Angelis L, Millasseau SC, Smith A, et al. Sex differences in age-related stiffening of the aorta in subjects with type 2 diabetes. Hypertension. 2004;44:67–71. doi: 10.1161/01.HYP.0000130482.81883.fd. [DOI] [PubMed] [Google Scholar]
- 8.O'Rourke MF, Staessen JA, Vlachopoulos C, Duprez D, Plante GE. Clinical applications of arterial stiffness; definitions and reference values. Am J Hypertens. 2002;15:426–444. doi: 10.1016/s0895-7061(01)02319-6. [DOI] [PubMed] [Google Scholar]
- 9.Chen CH, Nevo E, Fetics B, et al. Estimation of central aortic pressure waveform by mathematical transformation of radial tonometry pressure. Validation of generalized transfer function. Circulation. 1997;95:1827–1836. doi: 10.1161/01.cir.95.7.1827. [DOI] [PubMed] [Google Scholar]
- 10.Millasseau SC, Patel SJ, Redwood SR, Ritter JM, Chowienczyk PJ. Pressure wave reflection assessed from the peripheral pulse: is a transfer function necessary? Hypertension. 2003;41:1016–1020. doi: 10.1161/01.HYP.0000057574.64076.A5. [DOI] [PubMed] [Google Scholar]
- 11.Vlachopoulos C, O'Rourke M. Genesis of the normal and abnormal arterial pulse. Curr Probl Cardiol. 2000;25:303–367. doi: 10.1067/mcd.2000.104057. [DOI] [PubMed] [Google Scholar]
- 12.Laurent S, Cockcroft J, Van Bortel L, et al. Expert consensus document on arterial stiffness: methodological issues and clinical applications. Eur Heart J. 2006;27:2588–2605. doi: 10.1093/eurheartj/ehl254. [DOI] [PubMed] [Google Scholar]
- 13.Kelly RP, Millasseau SC, Ritter JM, Chowienczyk PJ. Vasoactive drugs influence aortic augmentation index independently of pulse-wave velocity in healthy men. Hypertension. 2001;37:1429–1433. doi: 10.1161/01.hyp.37.6.1429. [DOI] [PubMed] [Google Scholar]
- 14.McEniery CM, Yasmin, Hall IR, Qasem A, Wilkinson IB, Cockcroft JR. Normal vascular aging: differential effects on wave reflection and aortic pulse wave velocity: the Anglo-Cardiff Collaborative Trial (ACCT) J Am Coll Cardiol. 2005;46:1753–1760. doi: 10.1016/j.jacc.2005.07.037. [DOI] [PubMed] [Google Scholar]
- 15.O'Rourke MF, Hayward CS. Arterial stiffness, gender and heart rate. J Hypertens. 2003;21:487–490. doi: 10.1097/00004872-200303000-00009. [DOI] [PubMed] [Google Scholar]
- 16.Lantelme P, Mestre C, Lievre M, Gressard A, Milon H. Heart rate: an important confounder of pulse wave velocity assessment. Hypertension. 2002;39:1083–1087. doi: 10.1161/01.hyp.0000019132.41066.95. [DOI] [PubMed] [Google Scholar]
- 17.Haesler E, Lyon X, Pruvot E, Kappenberger L, Hayoz D. Confounding effects of heart rate on pulse wave velocity in paced patients with a low degree of atherosclerosis. J Hypertens. 2004;22:1317–1322. doi: 10.1097/01.hjh.0000125447.28861.18. [DOI] [PubMed] [Google Scholar]
- 18.Millasseau SC, Stewart AD, Patel SJ, Redwood SR, Chowienczyk PJ. Evaluation of carotid-femoral pulse wave velocity: influence of timing algorithm and heart rate. Hypertension. 2005;45:222–226. doi: 10.1161/01.HYP.0000154229.97341.d2. [DOI] [PubMed] [Google Scholar]
- 19.Albaladejo P, Laurent P, Pannier B, Achimastos A, Safar M, Benetos A. Influence of sex on the relation between heart rate and aortic stiffness. J Hypertens. 2003;21:555–562. doi: 10.1097/00004872-200303000-00021. [DOI] [PubMed] [Google Scholar]
- 20.Zambanini A, Mc GTSA, Hughes AD, Parker KH. Central aortic pressure influences pulse wave velocity. ypertension. 2002;40:e10–11. doi: 10.1161/01.hyp.0000041883.60004.7b. [DOI] [PubMed] [Google Scholar]
- 21.Kinlay S, Creager MA, Fukumoto M, et al. Endothelium-derived nitric oxide regulates arterial elasticity in human arteries in vivo. Hypertension. 2001;38:1049–1053. doi: 10.1161/hy1101.095329. [DOI] [PubMed] [Google Scholar]
- 22.Joannides R, Richard V, Haefeli WE, et al. Role of nitric oxide in the regulation of the mechanical properties of peripheral conduit arteries in humans. Hypertension. 1997;30:1465–1470. doi: 10.1161/01.hyp.30.6.1465. [DOI] [PubMed] [Google Scholar]
- 23.Wilkinson IB, Qasem A, McEniery CM, Webb DJ, Avolio AP, Cockcroft JR. Nitric oxide regulates local arterial distensibility in vivo. Circulation. 2002;105:213–217. doi: 10.1161/hc0202.101970. [DOI] [PubMed] [Google Scholar]
- 24.Schmitt M, Avolio A, Qasem A, et al. Basal NO locally modulates human iliac artery function in vivo. Hypertension. 2005;46:227–231. doi: 10.1161/01.HYP.0000164581.39811.bd. [DOI] [PubMed] [Google Scholar]
- 25.Stewart AD, Millasseau SC, Kearney MT, Ritter JM, Chowienczyk PJ. Effects of inhibition of basal nitric oxide synthesis on carotid-femoral pulse wave velocity and augmentation index in humans. Hypertension. 2003;42:915–918. doi: 10.1161/01.HYP.0000092882.65699.19. [DOI] [PubMed] [Google Scholar]
- 26.Longo GM, Xiong W, Greiner TC, Zhao Y, Fiotti N, Baxter BT. Matrix metalloproteinases 2 and 9 work in concert to produce aortic aneurysms. J Clin Invest. 2002;110:625–632. doi: 10.1172/JCI15334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gibbons GH, Dzau VJ. The emerging concept of vascular remodeling. N Engl J Med. 1994;330:1431–1438. doi: 10.1056/NEJM199405193302008. [DOI] [PubMed] [Google Scholar]
- 28.Yasmin, McEniery CM, Wallace S, et al. Matrix metalloproteinase-9 (MMP-9), MMP-2, and serum elastase activity are associated with systolic hypertension and arterial stiffness. Arterioscler Thromb Vasc Biol. 2005;25:372. doi: 10.1161/01.ATV.0000151373.33830.41. [DOI] [PubMed] [Google Scholar]
- 29.Booth AD, Wallace S, McEniery CM, et al. Inflammation and arterial stiffness in systemic vasculitis: a model of vascular inflammation. Arthritis Rheum. 2004;50:581–588. doi: 10.1002/art.20002. [DOI] [PubMed] [Google Scholar]
- 30.Maki-Petaja KM, Hall FC, Booth AD, et al. Rheumatoid arthritis is associated with increased aortic pulse-wave velocity, which is reduced by anti-tumor necrosis factor-alpha therapy. Circulation. 2006;114:1185–1192. doi: 10.1161/CIRCULATIONAHA.105.601641. [DOI] [PubMed] [Google Scholar]
- 31.Vlachopoulos C, Dima I, Aznaouridis K, et al. Acute systemic inflammation increases arterial stiffness and decreases wave reflections in healthy individuals. Circulation. 2005;112:2193–2200. doi: 10.1161/CIRCULATIONAHA.105.535435. [DOI] [PubMed] [Google Scholar]
- 32.Yasmin, McEniery CM, Wallace S, Mackenzie IS, Cockcroft JR, Wilkinson IB. C-reactive protein is associated with arterial stiffness in apparently healthy individuals. Arterioscler Thromb Vasc Biol. 2004;24:969–974. doi: 10.1161/01.ATV.zhq0504.0173. [DOI] [PubMed] [Google Scholar]
- 33.Mahmud A, Feely J. Arterial stiffness is related to systemic inflammation in essential hypertension. Hypertension. 2005;46:1118–1122. doi: 10.1161/01.HYP.0000185463.27209.b0. [DOI] [PubMed] [Google Scholar]
- 34.Pietri P, Vyssoulis G, Vlachopoulos C, et al. Relationship between low-grade inflammation and arterial stiffness in patients with essential hypertension. J Hypertens. 2006;24:2231–2238. doi: 10.1097/01.hjh.0000249701.49854.21. [DOI] [PubMed] [Google Scholar]
- 35.Yasmin, McEniery CM, O'Shaughnessy KM, et al. Variation in the human matrix metalloproteinase-9 gene is associated with arterial stiffness in healthy individuals. Arterioscler Thromb Vasc Biol. 2006;26:1799–1805. doi: 10.1161/01.ATV.0000227717.46157.32. [DOI] [PubMed] [Google Scholar]
- 36.London GM, Blacher J, Pannier B, Guerin AP, Marchais SJ, Safar ME. Arterial wave reflections and survival in end-stage renal failure. Hypertension. 2001;38:434–438. doi: 10.1161/01.hyp.38.3.434. [DOI] [PubMed] [Google Scholar]
- 37.Safar ME, Blacher J, Pannier B, et al. Central pulse pressure and mortality in end-stage renal disease. Hypertension. 2002;39:735–738. doi: 10.1161/hy0202.098325. [DOI] [PubMed] [Google Scholar]
- 38.Williams B, Lacy PS, Thom SM, et al. Differential impact of blood pressure-lowering drugs on Central Aortic Pressure and clinical outcomes: principal results of the Conduit Artery Function Evaluation (CAFE) Study. Circulation. 2006;113:1213–1225. doi: 10.1161/CIRCULATIONAHA.105.595496. [DOI] [PubMed] [Google Scholar]
- 39.Carlberg B, Samuelsson O, Lindholm LH. Atenolol in hypertension: is it a wise choice? Lancet. 2004;364:1684–1689. doi: 10.1016/S0140-6736(04)17355-8. [DOI] [PubMed] [Google Scholar]
- 40.Meaume S, Benetos A, Henry OF, Rudnichi A, Safar ME. Aortic pulse wave velocity predicts cardiovascular mortality in subjects >70 years of age. Arterioscler Thromb Vasc Biol. 2001;21:2046–2050. doi: 10.1161/hq1201.100226. [DOI] [PubMed] [Google Scholar]
- 41.Blacher J, Asmar R, Djane S, London GM, Safar ME. Aortic pulse wave velocity as a marker of cardiovascular risk in hypertensive patients. Hypertension. 1999;33:1111–1117. doi: 10.1161/01.hyp.33.5.1111. [DOI] [PubMed] [Google Scholar]
- 42.Laurent S, Boutouyrie P, Asmar R, et al. Aortic stiffness is an independent predictor of all-cause and cardiovascular mortality in hypertensive patients. Hypertension. 2001;37:1236–1241. doi: 10.1161/01.hyp.37.5.1236. [DOI] [PubMed] [Google Scholar]
- 43.Boutouyrie P, Tropeano AI, Asmar R, et al. Aortic stiffness is an independent predictor of primary coronary events in hypertensive patients: a longitudinal study. Hypertension. 2002;39:10–15. doi: 10.1161/hy0102.099031. [DOI] [PubMed] [Google Scholar]
- 44.Laurent S, Katsahian S, Fassot C, et al. Aortic stiffness is an independent predictor of fatal stroke in essential hypertension. Stroke. 2003;34:1203–1206. doi: 10.1161/01.STR.0000065428.03209.64. [DOI] [PubMed] [Google Scholar]
- 45.van Popele NM, Mattace-Raso FU, Vliegenthart R, et al. Aortic stiffness is associated with atherosclerosis of the coronary arteries in older adults: the Rotterdam Study. J Hypertens. 2006;24:2371–2376. doi: 10.1097/01.hjh.0000251896.62873.c4. [DOI] [PubMed] [Google Scholar]
- 46.Mattace-Raso FU, van der Cammen TJ, Hofman A, et al. Arterial stiffness and risk of coronary heart disease and stroke: the Rotterdam Study. Circulation. 2006;113:657–663. doi: 10.1161/CIRCULATIONAHA.105.555235. [DOI] [PubMed] [Google Scholar]
- 47.Vlachopoulos C, Panagiotakos D, Ioakeimidis N, Dima I, Stefanadis C. Chronic coffee consumption has a detrimental effect on aortic stiffness and wave reflections. Am J Clin Nutr. 2005;81:1307–1312. doi: 10.1093/ajcn/81.6.1307. [DOI] [PubMed] [Google Scholar]
- 48.Vlachopoulos C, Kosmopoulou F, Panagiotakos D, et al. Smoking and caffeine have a synergistic detrimental effect on aortic stiffness and wave reflections. J Am Coll Cardiol. 2004;44:1911–1917. doi: 10.1016/j.jacc.2004.07.049. [DOI] [PubMed] [Google Scholar]
- 49.Vlachopoulos C, Alexopoulos N, Dima I, Aznaouridis K, Andreadou I, Stefanadis C. Acute effect of black and green tea on aortic stiffness and wave reflections. J Am Coll Nutr. 2006;25:216–223. doi: 10.1080/07315724.2006.10719535. [DOI] [PubMed] [Google Scholar]
- 50.Vlachopoulos C, Kosmopoulou F, Alexopoulos N, Ioakeimidis N, Siasos G, Stefanadis C. Acute mental stress has a prolonged unfavorable effect on arterial stiffness and wave reflections. Psychosom Med. 2006;68:231–237. doi: 10.1097/01.psy.0000203171.33348.72. [DOI] [PubMed] [Google Scholar]
- 51.Kingwell BA, Berry KL, Cameron JD, Jennings GL, Dart AM. Arterial compliance increases after moderate-intensity cycling. Am J Physiol. 1997;273:H2186–H2191. doi: 10.1152/ajpheart.1997.273.5.H2186. [DOI] [PubMed] [Google Scholar]
- 52.Balkestein EJ, van Aggel-Leijssen DP, van Baak MA, Struijker-Boudier HA, Van Bortel LM. The effect of weight loss with or without exercise training on large artery compliance in healthy obese men. J Hypertens. 1999;17:1831–1835. doi: 10.1097/00004872-199917121-00008. [DOI] [PubMed] [Google Scholar]
- 53.Avolio AP, Clyde KM, Beard TC, Cooke HM, Ho KK, O'Rourke MF. Improved arterial distensibility in normotensive subjects on a low salt diet. Arteriosclerosis. 1986;6:166–169. doi: 10.1161/01.atv.6.2.166. [DOI] [PubMed] [Google Scholar]
- 54.Sierksma A, Muller M, van der Schouw YT, Grobbee DE, Hendriks HF, Bots ML. Alcohol consumption and arterial stiffness in men. J Hypertens. 2004;22:357–362. doi: 10.1097/00004872-200402000-00020. [DOI] [PubMed] [Google Scholar]
- 55.Girerd X, Giannattasio C, Moulin C, Safar M, Mancia G, Laurent S. Regression of radial artery wall hypertrophy and improvement of carotid artery compliance after long-term antihypertensive treatment in elderly patients. J Am Coll Cardiol. 1998;31:1064–1073. doi: 10.1016/s0735-1097(98)00043-6. [DOI] [PubMed] [Google Scholar]
- 56.Kool MJ, Lustermans FA, Breed JG, et al. The influence of perindopril and the diuretic combination amiloride+hydrochlorothi azide on the vessel wall properties of large arteries in hypertensive patients. J Hypertens. 1995;13:839–848. doi: 10.1097/00004872-199508000-00004. [DOI] [PubMed] [Google Scholar]
- 57.Mahmud A, Feely J. Effect of angiotensin ii receptor blockade on arterial stiffness: beyond blood pressure reduction. Am J Hypertens. 2002;15:1092–1095. doi: 10.1016/s0895-7061(02)02982-5. [DOI] [PubMed] [Google Scholar]
- 58.Topouchian J, Asmar R, Sayegh F, et al. Changes in arterial structure and function under trandolapril-verapamil combination in hypertension. Stroke. 1999;30:1056–1064. doi: 10.1161/01.str.30.5.1056. [DOI] [PubMed] [Google Scholar]
- 59.Mahmud A, Feely J. Aldosterone-to-renin ratio, arterial stiffness, and the response to aldosterone antagonism in essential hypertension. Am J Hypertens. 2005;18:50–55. doi: 10.1016/j.amjhyper.2004.08.026. [DOI] [PubMed] [Google Scholar]
- 60.Rajkumar C, Kingwell BA, Cameron JD, et al. Hormonal therapy increases arterial compliance in postmenopausal women. J Am Coll Cardiol. 1997;30:350–356. doi: 10.1016/s0735-1097(97)00191-5. [DOI] [PubMed] [Google Scholar]
- 61.Nakamura T, Matsuda T, Kawagoe Y, Ogawa H, Takahashi Y, Sekizuka KHK. Effect of pioglitazone on carotid intima-media thickness and arterial stiffness in type 2 diabetic nephropathy patients. Metabolism. 2004;53:1382–1386. doi: 10.1016/j.metabol.2004.05.013. [DOI] [PubMed] [Google Scholar]
- 62.Kass DA, Shapiro EP, Kawaguchi M, et al. Improved arterial compliance by a novel advanced glycation end-product crosslink breaker. Circulation. 2001;104:1464–1470. doi: 10.1161/hc3801.097806. [DOI] [PubMed] [Google Scholar]
- 63.Mäki-Petäjä KM, Booth AD, Hall FC, et al. Ezetimibe and simvastatin reduce inflammation, disease activity, and aortic stiffness and improve endothelial function in rheumatoid arthritis. J Am Coll Cardiol. 2007;50:852–858. doi: 10.1016/j.jacc.2007.04.076. [DOI] [PubMed] [Google Scholar]