Abstract
“Agonist therapy” for cocaine and methamphetamine addiction involves administration of stimulant-like medications (e.g., monoamine releasers) to reduce withdrawal symptoms and prevent relapse. A significant problem with this strategy is that many candidate medications possess abuse liability because of activation of mesolimbic dopamine (DA) neurons in the brain. One way to reduce DA-mediated abuse liability of candidate drugs is to add in serotonin (5-HT) releasing properties, since substantial evidence shows that 5-HT neurons provide an inhibitory influence over mesolimbic DA neurons. This article addresses several key issues related to the development of dual DA/5-HT releasers for the treatment of substance use disorders. First, the authors briefly summarize the evidence supporting a dual deficit in DA and 5-HT function during withdrawal from chronic cocaine or alcohol abuse. Second, the authors discuss data demonstrating that 5HT release can dampen DA-mediated stimulant effects, and the “antistimulant” role of 5-HT2C receptors is considered. Next, the mechanisms underlying potential adverse effects of 5-HT releasers are described. Finally, the authors discuss recently published data with PAL-287, a novel nonamphetamine DA/5-HT releasing agent that suppresses cocaine self-administration but lacks positive reinforcing properties. It is concluded that DA/5-HT releasers could be useful therapeutic adjuncts for the treatment of cocaine and alcohol addictions, as well as for obesity, attention-deficit disorder, and depression.
Keywords: alcohol, amphetamine, cocaine, dopamine, serotonin, transporter
This article reviews data from our laboratory pertaining to the development of dual dopamine (DA)/serotonin (5-HT) releasers as medications for stimulant addiction, and possibly alcohol addiction (Baumann et al., 2000; Baumann, Ayestas, Dersch, & Rothman, 2001; Rea, Rothman, & Shippenberg, 1998; Rothman & Baumann, 2003; Rothman et al., 2005; Wee et al., 2005; Wojnicki, Rothman, Rice, & Glowa, 1999). We also integrate our findings with existing literature to provide a conceptual framework for the design of new medications for addiction disorders. The term “stimulant” refers to drugs like cocaine and amphetamines that produce a spectrum of effects in humans including mood elevation, cardiovascular stimulation, and a decreased need for sleep. At high doses, or after longer periods of use, stimulants can cause a range of adverse effects, such as disordered thoughts and psychotic episodes. In laboratory animals, stimulants increase locomotor activity and are readily self-administered because of their powerful reinforcing properties. Figure 1 shows the chemical structures of drugs discussed in this article. Many of these drugs are useful medications with long histories of efficacy and safety. Others are highly addictive substances associated with considerable morbidity and mortality (Anonymous, 1995; Das, 1993; Gonzalez Castro, Barrington, Walton, & Rawson, 2000; Musto, 1992). In some cases, as with amphetamine, the same drug can be a therapeutic entity or an abused substance, depending upon the context in which the drug is administered (Arnsten, 2006; Greenhill, 2006).
Figure 1.
Chemical structures of stimulants discussed in this article.
Most stimulant drugs interact with monoamine neurons in the central nervous system (CNS). Neurons that synthesize, store, and release monoamine transmitters—norepinephrine (NE), DA, and 5-HT—are widely distributed in the mammalian CNS. These neurons express specialized plasma membrane proteins, which function to transport previously released transmitter molecules from the extracellular space back into the cytoplasm (Amara & Kuhar, 1993; Masson, Sagne, Hamon, & el Mestikawy, 1999). It is well established that distinct transporter proteins are expressed by each type of monoamine neuron: NE transporters (NETs), DA transporters (DATs), and 5-HT transporters (SERTs) are associated with NE, DA, and 5-HT neurons, respectively. These proteins belong to a superfamily of Na+/Cl−-dependent transporters that share genetic, morphological, and functional homologies (Torres & Amara, 2007; Uhl & Johnson, 1994). Under normal circumstances, the transporter-mediated uptake of monoamine transmitters is the principal mechanism for inactivation of monoamine signaling in the brain. Consequently, drugs that interact with monoamine transporters have profound effects on CNS function, and these effects can be beneficial or detrimental depending upon the dose, route, and formulation of the drug administered (Amara & Sonders, 1998; Iversen, 2006).
Drugs that target transporter proteins can be divided into two classes based on their precise mechanism of action—reuptake inhibitors and substrate-type releasers (Rothman & Baumann, 2003). Reuptake inhibitors bind to transporter proteins but are not transported. These drugs elevate extracellular transmitter concentrations by blocking transporter-mediated recapture of transmitter molecules from the synapse. Substrate-type releasers bind to transporter proteins and are subsequently transported into the cytoplasm of nerve terminals. Thus, transmitter “releasers” are often referred to as transporter “substrates.” Releasers elevate extracellular transmitter concentrations by a two-pronged mechanism: (1) they promote efflux of transmitter by a process of transporter-mediated exchange, and (2) they increase cytoplasmic levels of transmitter by disrupting storage of transmitters in vesicles via interactions with the vesicular monoamine transporter 2 (Fleckenstein, Volz, Riddle, Gibb, & Hanson, 2007; Rudnick, 1997). The molecular mechanisms underlying transporter-mediated transmitter release are not completely understood, but ionic currents, oligomerization, and reversal of normal transport function appear to be involved (Blakely, Defelice, & Galli, 2005; Sitte & Freissmuth, 2003; Sulzer, Sonders, Poulsen, & Galli, 2005). Because substrate-type releasing agents must be transported into nerve terminals to promote transmitter release, reuptake inhibitors can block the effects of releasers.
A Dual Deficit Model of Stimulant Addiction
The use of stimulants such as cocaine and methamphetamine produces a “high” or “rush” that is likely mediated by elevations in extracellular DA levels in mesolimbic circuits (Di Chiara et al., 2004; Volkow, Fowler, & Wang, 2002), although some evidence indicates that elevations in extracellular NE may also contribute (Alexander et al., 2005; Rothman et al., 2001). Similarly, alcohol-induced increases in extracellular DA are thought to underlie the positive reinforcing effects of this commonly abused substance (Koob, 2003; Koob et al., 1998). Repeated misuse of stimulants, especially when they are self-administered via the smoked or intravenous routes, can lead to serious addiction in susceptible individuals. The chronic abuse of stimulants and alcohol, despite negative consequences, causes long-term changes in neurochemistry, including changes in the γ-aminobutyric acid and glutamate systems, and brain circuitry via processes of synaptic plasticity (Hyman, 2005; Kalivas & O’Brien, 2008; Volkow & Li, 2004).
Preclinical and human research findings demonstrate that withdrawal from stimulant and alcohol abuse is associated with deficits in DA and 5-HT function. For example, rats withdrawn from chronic cocaine or alcohol administration display decreased levels of extracellular DA and 5-HT in the nucleus accumbens (Parsons, Koob, & Weiss, 1995; Parsons, Smith, & Justice, 1991; Rossetti, Hmaidan, & Gessa, 1992; Weiss et al., 1996). Human brain imaging studies show that cocaine addicts have reductions in evoked DA release and a reduction in DA D2-binding potential in the striatum (Martinez et al., 2007; Volkow et al., 2002, 1997). Neuroendocrine responsiveness to 5-HT releasers is diminished in rats withdrawn from repeated cocaine injections (Baumann, Becketts, & Rothman, 1995; Levy, Li, & Van de Kar, 1994), and similar findings have been reported in abstinent human cocaine addicts (Ghitza, Rothman, Gorelick, Henningfield, & Baumann, 2007; Haney, Ward, Gerra, & Foltin, 2001). Taken together, these data suggest a cardinal feature of withdrawal from chronic cocaine, and possibly alcohol, is decreased synaptic levels of DA and 5-HT in critical brain circuits.
Additional clinical support for the existence of 5-HT deficits in cocaine addicts is the occurrence of symptoms resembling major depression during abstinence (Dackis & Gold, 1985; Gawin & Kleber, 1986), coupled with an increased prevalence of suicidal ideation and suicide attempts (Garlow, Purselle, & D’Orio, 2003). The well-accepted importance of 5-HT dysfunction in mediating depression and suicide (for review, see Mann, 2003) suggests a parallel role for decreased synaptic 5-HT in cocaine and alcohol withdrawal states (Lesch, 2005). Indeed, the spectrum of symptoms often reported by patients withdrawing from stimulant or alcohol use—suicidal ideations, depressed mood, obsessive thoughts, intense craving, anhedonia, increased impulsivity, and susceptibility to drug-related cues—presumably reflects long-term changes in brain function and structure. We have proposed that deficits in monoamine systems underlie at least some of the symptoms experienced during withdrawal (for review, see Baumann & Rothman, 1998b).
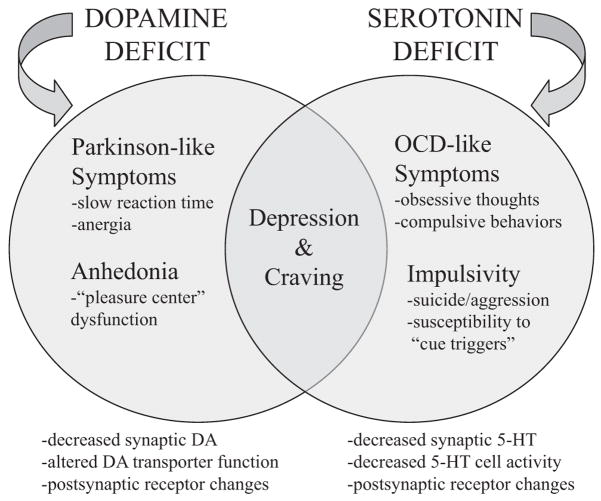
In particular, we proposed a dual deficit model of stimulant addiction in which drug-induced DA and 5-HT dysfunction contributes to withdrawal symptoms, drug craving, and relapse (Baumann, 2000; Baumann & Rothman, 1998a, 1998b; Rothman, Elmer, Shippenberg, Rea, & Baumann, 1998). Depicted diagrammatically in Figure 2, the dual deficit model postulates that decreased synaptic DA during stimulant withdrawal underlies anhedonia and psychomotor retardation, whereas decreased synaptic 5-HT gives rise to depressed mood, obsessive thoughts, and lack of impulse control. Consistent with this model, rats receiving repeated injections of abused stimulants exhibit neurobiological changes similar to those observed in human patients with major depression (Baumann, Becketts et al., 1995; Baumann & Rothman, 1998a; Lin, Koob, & Markou, 1999; Markou & Koob, 1991). If abstinent stimulant addicts exhibit DA and 5-HT deficits, medications capable of correcting abnormalities in DA and 5-HT function might be effective in treating stimulant and alcohol dependence.
Figure 2.
The dual deficit model of stimulant addiction. According to the model, withdrawal from chronic stimulant use leads to decreased synaptic availability of dopamine (DA) and serotonin (5-HT). This dual deficit contributes to withdrawal symptoms, drug craving, and relapse. DA dysfunction underlies anhedonia and psychomotor disturbances, whereas 5-HT dysfunction causes depressed mood, obsessive thoughts, and lack of impulse control. Protracted withdrawal phenomena are postulated to contribute significantly to relapse. Taken from Rothman & Baumann (2003).
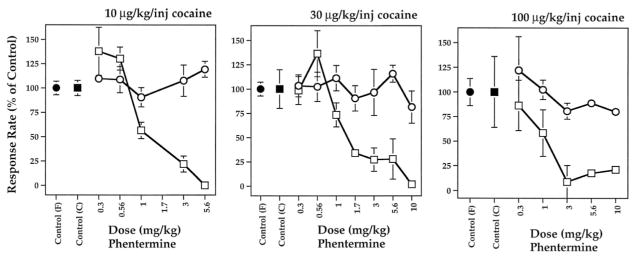
In agreement with the dual deficit hypothesis, drugs that release DA (phentermine, amphetamine) or 5-HT (fenfluramine) display properties consistent with the effective treatment of substance use disorders (Halladay, Wagner, Hsu, Sekowski, & Fisher, 1999; Rothman et al., 1998; Rothman, Gendron, & Hitzig, 1994; Yu, Fisher, Sekowski, & Wagner, 1997). For instance, acute or chronic administration of low doses of DA releasers, such as d-amphetamine, decreases cocaine self-administration behavior in rhesus monkeys (Macaca mulatta; Glowa, Wojnicki, Matecka, Rice, & Rothman, 1995; Negus & Mello, 2003a, 2003b). The data in Figure 3 demonstrate that the DA releasing agent phentermine suppresses responding for cocaine injections without affecting food-reinforced behavior, and this effect is maintained by daily administration of phentermine (Wojnicki et al., 1999). Such preclinical results provide a rationale for using DA releasers as medications for treating cocaine addiction (Grabowski, Shearer, Merrill, & Negus, 2004; Lile, 2006; Rothman, Blough, & Baumann, 2002).
Figure 3.
Acute effects of phentermine on rates of responding maintained under a fixed ratio (FR) 30 schedule of food (●) or cocaine (□) reinforcement. Different unit doses of intravenous cocaine, 10–100 μg/kg/injection, are indicated. Phentermine was administered intravenously. Effects on responding are mean ± SEM, expressed as percentage of individual control rates of responding for N = 3–4 monkeys. Control variability (filled symbols) is expressed as the average of individual coefficients of variation. Data taken from Wojnicki et al. (1999).
Under certain conditions, the 5-HT releaser fenfluramine decreases responding for cocaine in rhesus monkeys as well (Negus, Mello, Blough, Baumann, & Rothman, 2007a). Combined administration of phentermine plus fenfluramine produces a 75% decrease in cocaine self-administration in monkeys (Glowa, Rice, Matecka, & Rothman, 1997). The mixture of phentermine and d-fenfluramine reduces cocaine self-administration by 80% in rats, yet this mixture is not self-administered (Glatz et al., 2002). Important to note, 5-HT releasing agents suppress cue-elicited cocaine-seeking behavior in rats (Burmeister, Lungren, & Neisewander, 2003) and decrease cocaine craving in cocaine-dependent human patients (Buydens-Branchey et al., 1998). The collective findings suggest that combined treatment with DA and 5-HT releasers may have greater therapeutic value than treatment with either drug alone, in terms of decreasing stimulant self-administration and reducing cue-induced relapse. Moreover, a growing body of evidence shows that DA/5-HT-releasing agents may provide similar therapeutic benefits for alcohol dependence (Halladay et al., 1999; Halladay et al., 2006; Yu et al., 1997).
5-HT Release Counteracts Stimulant Effects of DA Release
The use of stimulant-like medications to treat stimulant addictions is an approach known as “agonist” therapy. This strategy involves administering medications that are less potent and less addictive than cocaine or methamphetamine, but which decrease stimulant abuse because of shared neurochemical properties with the abused drugs (Gorelick, 1998). Accordingly, we have described agonist therapy as neurochemical “normalization” therapy—that is, the stimulant medication serves to normalize or stabilize neurochemical deficits caused by chronic exposure to the abused stimulant (Rothman & Baumann, 2003; Rothman et al., 2002). Neurochemical normalization therapy has generated effective treatments for nicotine dependence (Henningfield, 1995; Rollema et al., 2007) and opioid dependence (Ling, Rawson, & Compton, 1994; White & Lopatko, 2007). This approach has been explored for the treatment of cocaine dependence as well (Alim, Rosse, Vocci, Lindquist, & Deutsch, 1995; Grabowski et al., 1997; Kampman et al., 2000; Walsh, Haberny, & Bigelow, 2000), and a number of placebo-controlled trials have shown promising results (Grabowski et al., 2001; Grabowski, Rhoades et al., 2004; Shearer, Wodak, van Beek, Mattick, & Lewis, 2003). A significant limitation of this strategy, however, is that candidate medications often exhibit inherent abuse liability due to activation of mesolimbic DA neurons in the brain (for review, see Grabowski, Shearer et al., 2004).
One feasible means to decrease the abuse liability of candidate medications is to add 5-HT-releasing properties to these drugs. Several lines of evidence support the hypothesis that elevations in synaptic 5-HT counteract the stimulant and reinforcing effects mediated by elevations in synaptic DA (Burmeister, Lungren, Kirschner, & Neisewander, 2004; Czoty, Ginsburg, & Howell, 2002; Daw, Kakade, & Dayan, 2002; Higgins & Fletcher, 2003). Administration of the 5-HT precursor L-tryptophan, which increases 5-HT synthesis and release in the CNS, decreases self-administration of cocaine and amphetamine in rats (McGregor, Lacosta, & Roberts, 1993; Smith, Yu, Smith, Leccese, & Lyness, 1986). Likewise, pretreatment with 5-HT reuptake inhibitors can reduce intravenous cocaine self-administration in rats and squirrel monkeys (Carroll, Lac, Asencio, & Kragh, 1990; Howell & Byrd, 1995). Cocaine analogs that have potent affinity at SERT support less self-administration behavior than analogs with weak affinity for SERT (Howell, Carroll, Votaw, Goodman, & Kimmel, 2007; Roberts et al., 1999). Consistent with these findings, agents that broadly activate brain 5-HT systems can reduce self-administration of stimulants and other drugs of abuse (Higgins & Fletcher, 2003). The “antistimulant” effects of increasing extracellular 5-HT are readily observed after combined administration of 5-HT releasers and DA releasers, or after administration of single agents that release both neurotransmitters.
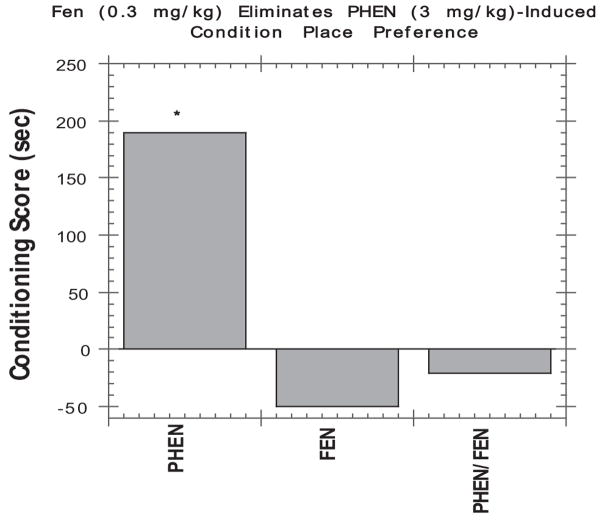
As summarized in Table 1, drugs that release [3H]DA more potently than [3H]5-HT in vitro (e.g., amphetamine, phentermine) increase endogenous extracellular DA more than extracellular 5-HT in vivo. Such indirect DA agonists are strong locomotor stimulants and support self-administration behavior. Drugs that release [3H]5-HT more potently than [3H]DA in vitro (e.g., fenfluramine, chlorphentermine) increase endogenous extracellular 5-HT more than extracellular DA. Such indirect 5-HT agonists produce minimal motor activity and do not support self-administration behavior. The antireward effect of 5-HT releasers is also seen in the conditioned place preference (CPP) assay as shown in Figure 4, where a low dose of fenfluramine greatly reduces the positive CPP induced by phentermine. Consistent with these findings, fenfluramine administration decreased the subjective effects of D-amphetamine or phentermine in humans (Brauer, Johanson, Schuster, Rothman, & de Wit, 1996).
Table 1.
Summary of Serotonergic and Dopaminergic Effects of Selected Releasing Agents
| Drug | [3H]5-HT release EC50 (nM) | [3H]DA release EC50 (nM) | Peak % increase in dialysate 5-HT (dose, mg/kg) | Peak % increase in dialysate DA (dose, mg/kg) | Self- administered | Locomotor activation |
|---|---|---|---|---|---|---|
| Amphetaminea | 1756 | 8.0 | 45 (0.3 mg/kg ip) | 224 (0.3 mg/kg ip) | Yes | Strong |
| Phenterminea | 3511 | 262 | 32 (1.0 mg/kg ip) | 156 (1.0 mg/kg ip) | Yes | Strong |
| PAL-353b | 1937 | 24.2 | 170 (1.0 mg/kg iv) | 432 (1.0 mg/kg iv) | Yes | Strong |
| Fenfluraminea | 79.3 | >10,000 | 215 (1.0 mg/kg ip) | 20 (1.0 mg/kg ip) | No | None |
| Chlorphenterminea | 30.9 | 2650 | 228 (1.0 mg/kg ip) | 86 (1.0 mg/kg ip) | No | None |
| Phentermine + fenfluraminea | NA | NA | 222 (1.0 + 1.0 mg/kg, ip) | 144 (1.0 + 1.0 mg/kg ip) | No | Weak |
| PAL-313b | 53.4 | 44.1 | 544 (1.0 mg/kg iv) | 130 (1.0 mg/kg iv) | Weak | Weak |
| PAL-287c | 3.4 | 12.6 | 464 (1.0 mg/kg iv) | 133 (1.0 mg/kg iv) | No | Weak |
Note. 5-HT = serotonin; EC50 = half maximal effective concentration; DA = dopamine; NA = not applicable. A summary of data illustrating the tendency for increasing extracellular 5-HT to reduce reinforcing and locomotor effects mediated by increases in extracellular DA.
From Baumann et al. (2000) and Rothman et al. (2001).
From Wee et al. (2005). Microdialysis data unpublished. This table originally appeared in Rothman, Blough, and Baumann (2007).
From Rothman et al. (2005).
Figure 4.
Effects of phentermine (3 mg/kg) and fenfluramine (0.3 mg/kg), given alone or in combination, on conditioned place preference. Conditioning score represents the mean difference between time (s) spent in the drug- versus vehicle-paired side of the test chamber. All drugs were administered intraperioneally. Each column represents the mean of N = 9–10 rats. *Significant place preference (Wilcoxon’s test, p < .05). Data taken from Rea et al. (1998).
It seems possible that attempts to reduce the abuse liability of a DA releaser via elevation of extracellular 5-HT could reduce therapeutic effectiveness. Some recent preclinical data support this contention (Negus, Mello, Blough, Baumann, & Rothman, 2007b). In particular, Negus and coworkers demonstrated that pretreatment of rhesus monkeys (Macaca mulatta) with selective DA releasers can suppress cocaine self-administration without affecting food-maintained responding. By contrast, mixed DA/5-HT releasers suppressed both cocaine- and food-maintained responding under identical test conditions. These data suggest that selective DA releasers might be more effective than mixed releasers in the treatment of cocaine dependence, but this hypothesis can only be addressed by carefully controlled clinical trials in human subjects.
The exact mechanisms responsible for antistimulant effects of 5-HT releasers have not been characterized, but increases in extracellular 5-HT would be expected to activate multiple 5-HT receptor subtypes known to modulate DA function (Alex & Pehek, 2007; Muller & Huston, 2006). As mentioned previously, the stimulant and reinforcing properties of drugs like cocaine and methamphetamine are mediated via activation of mesolimbic DA neurons. Cell bodies of mesolimbic DA neurons reside in the midbrain ventral tegmental area (VTA) and send axonal projections to many regions of the forebrain, most notably the nucleus accumbens (Moore & Bloom, 1978; Ungerstedt, 1971). The nucleus accumbens is a critical limbic-motor interface receiving afferent inputs from the prefrontal cortex, hippocampus and amygdala, while sending efferent outputs to the ventral pallidum and other regions known to modulate locomotor centers in the brainstem (Mogenson, Jones, & Yim, 1980; Pennartz, Groenewegen, & Lopes da Silva, 1994). 5-HT neurons in the midbrain raphe nuclei send axonal projections that densely innervate the mesolimbic system at the level of the VTA and nucleus accumbens (Molliver, 1987; Steinbusch, 1981). Furthermore, 5-HT neurons innervate various regions providing excitatory afferents to the accumbens (e.g., prefrontal cortex). In this manner, 5-HT nerve terminals are well positioned to influence the activity of mesolimbic DA neurons at multiple levels.
The serotonergic modulation of DA function is inherently complex due to the presence of at least 14 different 5-HT receptor subtypes in the CNS (Barnes & Sharp, 1999; Hoyer, Hannon, & Martin, 2002). While some 5-HT receptor subtypes enhance DA transmission (Alex & Pehek, 2007; Muller & Huston, 2006), 5-HT2C receptors provide a strong inhibitory influence on mesolimbic DA neurons (Bubar & Cunningham, 2006; Di Matteo, De Blasi, Di Giulio, & Esposito, 2001). For instance, systemic administration of the 5-HT2C agonist RO60-0175 markedly inhibits DA cell firing in the VTA and decreases extracellular levels of DA in forebrain projection areas (Di Matteo, Di Giovanni, Di Mascio, & Esposito, 1999, 2000; Gobert et al., 2000). Pretreatment with RO60-0175 reduces locomotor activity and self-administration behavior produced by cocaine, whereas pretreatment with the 5-HT2C antagonist SB242,084 has the opposite effect (Fletcher, Grottick, & Higgins, 2002; Grottick, Fletcher, & Higgins, 2000). In fact, SB242,084 given alone increases burst firing of DA cells in the VTA, suggesting 5-HT2C receptors provide tonic inhibition of mesolimbic DA activity. Collectively, these findings indicate a potential role for 5-HT2C receptors in mediating the antistimulant effects of 5-HT releasers (Higgins & Fletcher, 2003).
Recent data show that antistimulant effects of 5-HT2C receptor activation involve at least two separate mechanisms—one mechanism in the VTA and another in prefrontal cortex. Microinjection of RO60-0175 into the VTA blocks behavioral effects of cocaine (Fletcher, Chintoh, Sinyard, & Higgins, 2004), perhaps reflecting inhibition of DA cell firing and DA release as noted earlier (Di Matteo et al., 2001). Microinjection of RO60-0175 into the prefrontal cortex also markedly reduces cocaine-induced locomotor activity (Filip & Cunningham, 2003), and this action may involve suppression of excitatory glutamate outputs to the nucleus accumbens (Liu, Bubar, Lanfranco, Hillman, & Cunningham, 2007). Neuroanatomical evidence suggests that effects of 5-HT2C receptor activation in the VTA and cortex are mediated by stimulation of γ-aminobutyric acid interneurons (Bubar & Cunningham, 2006, 2007; Liu et al., 2007), and more research is needed to validate this proposal. Additional investigation is warranted to fully elucidate the role of 5-HT2C receptors in mediating antistimulant effects of 5-HT releasers. In addition, the potential of 5-HT2C agonists as medications for substance use disorders deserves to be examined (Bubar & Cunningham, 2006; Higgins & Fletcher, 2003).
Potential Adverse Effects of 5-HT Releasers
The clinical use of 5-HT-releasing agents as medications has been associated with a number of adverse effects (Rothman, Ayestas, Dersch, & Baumann, 1999; Rothman & Baumann, 2002; Zolkowska, Rothman, & Baumann, 2006). Based primarily on experience with d,l-fenfluramine and its more potent isomer d-fenfluramine, three potentially serious side effects need to be considered when 5-HT releasers are developed as treatment agents: valvular heart disease (VHD), idiopathic pulmonary arterial hypertension (IPAH), and neurotoxicity. Fenfluramines were commonly prescribed anorectics until their removal from the market in 1997 because of the occurrence of VHD in some patients (Connolly et al., 1997; Connolly & McGoon, 1999). Fenfluramine-associated VHD is characterized by thickening of valve leaflets and increased regurgitation of blood, most often detected by echocardiography. While initial findings suggested fenfluramines induce VHD in a high percentage of patients, more recent evidence shows a much smaller risk. For example, a meta-analysis of available clinical data demonstrates that the incidence of clinically significant valvular regurgitation was 12% in fenfluramine-treated patients versus 6% in untreated controls (Sachdev, Miller, Ryan, & Jollis, 2002).
Because fenfluramines are potent 5-HT releasers (Baumann et al., 2000; Rothman, Clark, Partilla, & Baumann, 2003) and 5-HT has established mitogenic effects (Nemecek, Coughlin, Handley, & Moskowitz, 1986; Seuwen, Magnaldo, & Pouyssegur, 1988), investigators initially speculated that serotonergic mechanisms might contribute to VHD (Connolly et al., 1997; Connolly & McGoon, 1999). To this end, we carried out an investigation to determine whether stereoisomers of fenfluramine, or the N-dealkylated metabolite norfenfluramine, might activate mitogenic 5-HT receptors (Rothman, Baumann et al., 2000). A number of other test drugs were included in these experiments as positive and negative controls. “Positive controls” were ergot alkaloids known to increase the risk of VHD, such as methysergide, its active metabolite methylergonovine, and ergotamine (Bana, MacNeal, LeCompte, Shah, & Graham, 1974; Bredberg, Eyjolfsdottir, Paalzow, Tfelt-Hansen, & Tfelt-Hansen, 1986; Hendrikx, Van Dorpe, Flameng, & Daenen, 1996). “Negative controls” were drugs that interact with monoamine transporters but do not cause VHD, and these drugs included phentermine, fluoxetine, and its metabolite norfluoxetine. We also tested the antidepressant trazodone and its active metabolite, m-chlorophenylpiperazine (mCPP), as negative controls (Ishida et al., 1995; Otani et al., 1997). mCPP has agonist activity at a wide range of 5-HT receptor subtypes (Hoyer et al., 1994, 2002) and is capable of releasing neuronal 5-HT via a transporter-mediated mechanism similar to fenfluramines (Baumann et al., 2001; Baumann, Rutter, & Auerbach, 1993).
Our working hypothesis was that fenfluramines, norfenfluramines, and positive control drugs would share the ability to activate a mitogenic 5-HT receptor subtype expressed in heart valves, while the negative control drugs would not. An initial receptorome screen led to a detailed evaluation of the binding of these drugs to the 5-HT2 family of receptors (Rothman, Baumann et al., 2000). Table 2 reports binding data, and Table 3 reports the functional effects of these compounds at cloned human 5-HT2A, 5-HT2B, and 5-HT2C receptors. Of note, fenfluramines have low affinity for all 5-HT2 receptor subtypes. By contrast, we found that norfenfluramines display high affinity and efficacy at the 5-HT2B receptor subtype (KI = 10–50 nM), consistent with the findings of others (Fitzgerald et al., 2000; Porter et al., 1999). Methysergide acts as a partial agonist at the 5-HT2B receptor, while the metabolite methylergonovine has even greater affinity and efficacy. Ergotamine is a potent partial agonist at the 5-HT2B receptor. Among the negative control drugs tested, only mCPP exhibits agonist activity at the 5-HT2B site. It is noteworthy that trazodone binds to the 5-HT2B receptor with moderate affinity, but functions as an antagonist. Thus, when trazodone is metabolized to mCPP in vivo (Ishida et al., 1995; Otani et al., 1997), the 5-HT2B actions of mCPP are probably blocked by antagonist actions of the parent compound.
Table 2.
Ki Values of Test Drugs at 5-HT2 Receptors
| Drug | Human 5-HT2a | Human 5-HT2B | Human 5-HT2C |
|---|---|---|---|
| (±)-Fenfluramine | 5,216 ± 423 | 4,134 ± 1,281 | 3,183 ± 637 |
| (+)-Fenfluramine | 11,107 ± 2,303 | 5,099 ± 1173 | 6,245 ± 874 |
| (−)-Fenfluramine | 5,463 ± 600 | 5,713 ± 2,285 | 3,415 ± 922 |
| (±)-Norfenfluramine | 2,316 ± 278 | 52.1 ± 21 | 557 ± 61 |
| (+)-Norfenfluramine | 1,516 ± 150 | 11.2 ± 7.3 | 324 ± 12 |
| (−)-Norfenfluramine | 3,841 ± 614 | 47.8 ± 30.6 | 814 ± 98 |
| Ergotamine | 9.0 ± 1.0 | 3.0 ± 0.4 | 12 ± 1.5 |
| Methysergide | 15.0 ± 4.0 | 9.1 ± 4.9 | 1.8 ± 0.2 |
| Methylergonovine | 12.6 ± 1.0 | 0.49 ± 0.16 | 12.4 ± 1.0 |
| Fluoxetine | 299 ± 53 | 5,030 ± 1,960 | 50 ± 10 |
| Norfluoxetine | 638 ± 108 | 5,063 ± 1,974 | 286 ± 60 |
| Trazodone | 19.8 ± 2.4 | 73.6 ± 36 | 402 ± 44 |
| m-Chlorophenylpiperazine | 391 ± 47 | 3.2 ± 1.0 | 59 ± 11 |
| 5-HT | 614 ± 74 | 4.0 ± 1.9 | 12.2 ± 1.3 |
| Phentermine | >10,000 | >10,000 | >10,000 |
Note. Values are mean ± SD for n = 3 experiments. Data taken from Rothman, Baumann et al. (2000).
Table 3.
Functional Activity of Test Drugs at 5-HT2 Receptors
| Drug | Human 5-HT2a Kact (nM ± SD) Vmax (percentage of 5-HT ± SD) | Human 5-HT2B Kact (nM ± SD) Vmax (percentage of 5-HT ± SD) | Human 5-HT2C Kact (nM ± SD) Vmax (percentage of 5-HT ± SD) |
|---|---|---|---|
| (±)-Fenfluramine | 4,131 ± 2,448 | ND | ND |
| 15 ± 4 | |||
| (+)-Fenfluramine | >10,000 | 379 ± 120 | 362 ± 109 |
| ND | 38 ± 14 | 80 ± 10 | |
| (−)-Fenfluramine | 5,279 ± 998 | 1,248 ± 430 | 360 ± 155 |
| 43 ± 7.2 | 47 ± 5 | 84 ± 15 | |
| (+)-Norfenfluramine | 630 ± 240 | 18.4 ± 9 | 13 ± 4 |
| 88 ± 9 | 73 ± 6 | 100 ± 11 | |
| (−)-Norfenfluramine | 1,565 ± 323 | 357 ± 180 | 18 ± 9 |
| 93 ± 9 | 71 ± 15 | 80 ± 17 | |
| Ergotamine | 16 ± 4 | 9.8 ± 3 | 5 ± 3 |
| 75 ± 3 | 56 ± 3 | 75 ± 15 | |
| Methysergide | 3.5 ± 1.7 | 150 ± 43 | 2.9 ± 1.5 |
| 24 ± 3 | 18 ± 4 | 33 ± 3.5 | |
| Methylergonovine | 1.3 ± 0.4 | 0.8 ± 0.5 | 2.5 ± 1.2 |
| 70 ± 7 | 40 ± 3 | 103 ± 7 | |
| Fluoxetine | ND | ND | Antagonist |
| Ki = 616 ± 172 | |||
| Norfluoxetine | ND | ND | Antagonist |
| Ki = 43 ± 17 | |||
| Trazodone | Antagonist | Antagonist | Antagonist |
| m-Chlorophenylpiperazine | 65 ± 17 | 64 ± 27 | 0.64 ± 0.3 |
| 55 ± 11 | 43 ± 14 | 79 ± 15 | |
| 5-HT | 66 ± 26 | 2.4 ± 1.5 | 0.6 ± 0.18 |
| 100 | 100 | 100 | |
| Phentermine | ND | ND | 1,394 ± 450 |
| 66 ± 10 |
Note. 5-HT = serotonin; ND = not determined. Values are mean ± SD for n = 3 experiments. Data taken from Rothman, Baumann et al. (2000).
Our results with the various positive control drugs strongly implicate the 5-HT2B receptor as a major culprit in the development of drug-induced VHD. Of note, accumulating data support this hypothesis (Fitzgerald et al., 2000; Porter et al., 1999; Roth, 2007; Setola & Roth, 2005). 5-HT2B receptors are abundantly expressed on aortic and mitral valves (Fitzgerald et al., 2000), and these receptors are known to stimulate mitogenisis (Hafizi, Taylor, Chester, Allen, & Yacoub, 2000; Lopez-Ilasaca, 1998). Additional evidence for the role of 5-HT2B receptors in drug-induced VHD is based on the effects of ergot medications such as carbergoline and pergolide. Both of these medications increase the risk of VHD in human patients and are also potent 5-HT2B receptor agonists (for review, see Roth, 2007). Setola and coworkers (2003) showed that the illicit amphetamine analog 3,4-methylenedioxymethamphetamine (MDMA) and its N-demethylated metabolite, 3,4-methylenedioxyamphetamine, are 5-HT2B receptor agonists. These drugs stimulate prolonged mitogenic responses in human valvular interstitial cells via activation of 5-HT2B receptors (Setola et al., 2003). As predicted by this study, a recent clinical report found that heavy MDMA users display a significantly higher incidence of valvular regurgitation when compared with control subjects (Droogmans et al., 2007). More clinical investigations are needed to clearly establish the link between illicit MDMA use and the risk for developing VHD.
Epidemiological evidence indicates that fenfluramines increase the risk of developing idiopathic pulmonary arterial hypertension (IPAH), a debilitating and incurable disease (Abenhaim et al., 1996; Fishman, 1999). IPAH is characterized by pulmonary arterial vasoconstriction and hyperplasia that leads to severe hypertension. The pathogenesis of IPAH is complex and difficult to study, especially given the rarity of the disorder. Nonetheless, numerous investigations from our laboratory and others implicate the involvement of 5-HT and SERT proteins in the pathogenesis of IPAH (Eddahibi et al., 2006; MacLean, Herve, Eddahibi, & Adnot, 2000; Rothman et al., 1999). We demonstrated that SERT substrate activity is a common feature of drugs associated with IPAH, but not all substrates increase the risk for the disease (Rothman et al., 1999). It is well established that blood platelets express SERT proteins identical to those expressed on neurons, and that platelet SERT accumulates nearly 99% of circulating 5-HT into platelet storage (Ni & Watts, 2006). One hypothesis—the so-called “5-HT hypothesis”—has been invoked as a potential mechanism underlying fenfluramine-induced IPAH (Fishman, 1999; MacLean et al., 2000). This hypothesis postulates that fenfluramines increase the risk for IPAH by stimulating SERT-mediated release of 5-HT from platelets, thereby elevating plasma levels of 5-HT. Persistent elevations in plasma 5-HT would then cause pulmonary dysfunction. It is noteworthy that an analogous 5-HT hypothesis has been invoked to explain VHD, but as discussed already, drug-induced activation of 5-HT2B receptors seems to be the predominant mechanism involved in this disease.
A key prediction of the 5-HT hypothesis is that fenfluramine increases plasma 5-HT to concentrations sufficient to produce vasoconstriction and mitogenesis, which then leads to serious side effects. Despite the widespread acceptance of the 5-HT hypothesis as an explanation for fenfluramine-associated IPAH, the effects of fenfluramine and related agents on plasma 5-HT have received little attention. Studies conducted in the 1990s do not support the 5-HT hypothesis because they show that acute fenfluramine does not increase plasma 5-HT in rats, and chronic fenfluramine treatment lowers blood 5-HT in humans (Martin & Artigas, 1992; Rothman, Redmon et al., 2000). Given the uncertainties regarding validity of the 5-HT hypothesis, we assessed the acute effects of fenfluramine and other amphetamines on plasma levels of 5-HT in rats (Zolkowska et al., 2006). Specifically, we developed a novel microdialysis method to measure plasma levels of 5-HT in whole blood samples obtained from conscious catheterized rats. Using this method, baseline plasma 5-HT levels in rats are 0.22 nM, or about 1 nM when corrected for dialysis probe recovery, which is similar to plasma 5-HT concentrations measured in human subjects (Herve et al., 1995). Important to note, systemic administration of fenfluramine, MDMA and other amphetamines evokes transient dose-dependent increases in plasma 5-HT ranging from 4 to 20 nM. The ability of drugs to increase plasma 5-HT is directly correlated with their ability to increase SERT-mediated 5-HT release in neurons, suggesting the involvement of platelet SERT proteins. These data show that fenfluramine and other 5-HT releasers are able to acutely increase plasma 5-HT, but the absolute levels of 5-HT are well below the concentrations required to contract pulmonary arteries or stimulate mitogenesis (Cortijo et al., 1997; Eddahibi et al., 1999).
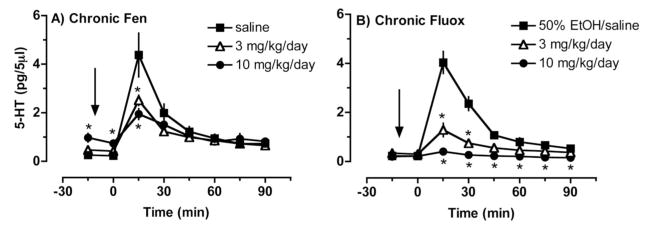
From a medication development standpoint, a more relevant issue is whether chronic administration of 5-HT releasers can persistently increases plasma 5-HT. To address this question, we used microdialysis methods to examine the effects of 2-week minipump infusions of fenfluramine (3 and 10 mg/kg/day) or the 5-HT uptake blocker fluoxetine (3 and 10 mg/kg/day) on plasma levels of 5-HT in rats (Zolkowska, Baumann, & Rothman, 2008). In this study, chronic administration of fenfluramine, but not fluoxetine, caused two- to fourfold increases in baseline dialysate 5-HT in blood. Given baseline plasma 5-HT concentrations of about 1 nM, fenfluramine-induced increases in plasma 5-HT are less than 5 nM. The data in Figure 5 show that chronic exposure to fenfluramine or fluoxetine markedly reduces the ability of acute fenfluramine to evoke increases in plasma 5-HT. Thus, chronic exposure to fenfluramine minimizes the surges in plasma 5-HT caused by acute administration of the drug. Chronic minipump infusions of fenfluramine and fluoxetine in rats give rise to steady-state blood levels of drugs and their bioactive metabolites that are similar to those measured in human patients taking prescribed doses of these medications (Lundmark, Reis, & Bengtsson, 2001; Rothman, Redmon et al., 2000). Thus, our rat model system is relevant to human patients taking fenfluramine or fluoxetine.
Figure 5.
Effects of acute fenfluramine administration on dialysate serotonin (5-HT) levels measured in blood from conscious rats previously treated with chronic administration of fenfluramine (A) or fluoxetine (B). For chronic treatments, drugs were dissolved in sterile saline (fenfluramine) or 50% ethanol/saline (fluoxetine), and administered subcutaneously via osmotic minipumps for 2 weeks. On the day of test, fenfluramine was dissolved in saline and administered intravenously at 0 min. Serial blood samples were withdrawn at 15-min intervals and immediately dialyzed. 5-HT levels are mean ± SEM for N = 9/group. * p < .05 compared with saline controls at corresponding time points (Duncans post hoc test). Data taken from Zolkowska et al. (2008).
It has been shown that 5-HT provokes contraction of human pulmonary arteries at concentrations ranging from 100 nM to 10 μmol/L (Cortijo et al., 1997). The threshold concentration of 5-HT required to stimulate mitogenic responses in cultured human pulmonary artery smooth muscle cells is about 10 nM (Eddahibi et al., 2001; Marcos et al., 2003), while much higher 5-HT concentrations are needed to stimulate mitogenic responses in rat pulmonary artery smooth muscle cells (Eddahibi et al., 1999; Pitt et al., 1994). Because we found that chronic fenfluramine elevates baseline plasma 5-HT to less than 5 nM in rats, it appears that fenfluramine-induced increases in plasma 5-HT are below the concentrations known to cause pulmonary side effects. Fenfluramine-induced elevations of plasma 5-HT are also much lower than those required to produce VHD in rats exposed to exogenous 5-HT (580–974 nM) (Gustafsson et al., 2005). Viewed collectively, these findings demonstrate that the 5-HT hypothesis cannot explain the mechanism of fenfluramine-associated IPAH or VHD. Of course, it is possible that two- to fourfold increases in plasma 5-HT could be enough to stimulate mitogenic responses in susceptible individuals and increase the risk of developing IPAH. However, this scenario seems unlikely since treatment with lithium or MAO inhibitors produces two- to fourfold increases in plasma 5-HT without increasing the risk for IPAH (Artigas et al., 1989; Celada, Dolera, Alvarez, & Artigas, 1992).
At the present time, the mechanisms underlying fenfluramine-associated IPAH remain enigmatic. One significant problem is that most animal models of IPAH require the induction of hypoxia, which is not a major factor in humans. Furthermore, the etiology of fenfluramine-associated IPAH may differ substantially from that of nondrug-related IPAH. Despite these caveats, recent findings have provided novel hypotheses to explain how fenfluramine might cause IPAH. For example, Launay and colleagues (Launay et al., 2002) have provided evidence that activation of 5-HT2B receptors in the lung is critical to the development of IPAH in a hypoxic mouse model. Since the stereoisomers of norfenfluramine are potent and selective 5-HT2B agonists (Rothman, Baumann, et al., 2000), a role for 5-HT2B receptors seems feasible. On the other hand, a number of medications that produce VHD and activate 5-HT2B receptors do not increase the risk for IPAH—these medications include methysergide, ergotamine, pergolide, and cabergoline.
Additional evidence from our laboratory disputes a role for 5-HT2B sites in fenfluramine-associated IPAH (Rothman & Baumann, 2006). Aminorex is a SERT substrate that caused an epidemic of IPAH in the 1960s (Fishman, 1999; Gurtner, 1985), and case reports implicate the related designer drug 4-methylaminorex as a cause of the disease (Gaine, Rubin, Kmetzo, Palevsky, & Traill, 2000). If 5-HT2B receptors are involved in the pathogenesis of drug-associated IPAH, then one would suspect aminorex to target 5-HT2B sites. While aminorex does interact with cloned human 5-HT2B receptors, the half maximal effective concentration (EC50) of the drug for 5-HT2B receptor activation (870 nM) is 30-fold higher than its EC50 for NE release (26.4 nM). Moreover, the activity of aminorex at 5-HT2B sites is nearly 50-fold less than that of d-norfenfluramine. It seems plausible that metabolites of aminorex may act more potently at 5-HT2B receptors, and this possibility deserves to be examined. However, the available data argue against an important role for 5-HT2B receptors in the pathogenesis of anorectic-associated IPAH.
Recent studies by Eddahibi et al. (2006) focus attention on 5-HT produced locally in the lung as a critical player in pathogenesis of IPAH. These investigators reported that endothelial cells in the pulmonary microvasculature synthesize 5-HT, which is then released as a growth factor. It has been proposed that dysregulation of 5-HT production in endothelial cells, along with overexpression of SERT by the pulmonary artery smooth muscle cells, contributes to hyperplasia observed in IPAH. Immunohistochemical studies show that pulmonary microvascular endothelium do not express SERT (Eddahibi et al., 2006), indicating that fenfluramine cannot release 5-HT from these cells. Based on the findings of Eddahibi et al., administration of the 5-HT precursor L-5-hydroxytryptophan (5-HTP) would be predicted to increase 5-HT synthesis in pulmonary endothelial cells because this compound bypasses the rate-limiting enzyme tryptophan hydroxylase. 5-HTP is a commonly used dietary supplement with a well-established history of safety (Das, Bagchi, Bagchi, & Preuss, 2004), and its use is not known to increase the risk of IPAH. Although speculative, this observation suggests that an increase in 5-HT synthesis in the pulmonary microvascular endothelial cells may be necessary, but is not sufficient, to increase the risk of IPAH.
Experiments in laboratory animals show that high-dose administration of fenfluramine or d-fenfluramine can cause long-term depletion of 5-HT and loss of SERT binding sites in the brain (McCann, Seiden, Rubin, & Ricaurte, 1997; Zaczek et al., 1990). The persistent nature of fenfluramine-induced 5-HT deficits in the CNS has been interpreted as evidence for neurotoxicity, although this hypothesis and its clinical relevance are still a matter of debate (Rose, Hunt, Collins, Hindmarsh, & Jenner, 1996; Rothman, Jayanthi, et al., 2003). The mechanisms underlying fenfluramine-induced 5-HT depletions are not well understood, but acute 5-HT release has been implicated because 5-HT uptake blockers and synthesis inhibitors can prevent long-term 5-HT depletions (Halladay, Kirschner, Hesse, Fisher, & Wagner, 2001; Steranka & Sanders-Bush, 1979). An important observation is that not all SERT subtrates deplete 5-HT (Baumann et al., 2001; Cozzi, Frescas, Marona-Lewicka, Huang, & Nichols, 1998; Nichols, Brewster, Johnson, Oberlender, & Riggs, 1990). As noted previously, mCPP interacts with SERT to release 5-HT from neurons, and mCPP is equipotent with d-fenfluramine in this regard (Baumann et al., 2001; Baumann, Mash, & Staley, 1995; Eriksson, Engberg, Bing, & Nissbrandt, 1999). The data in Figure 6 demonstrate that repeated high-dose administration of mCPP fails to affect postmortem tissue levels of 5-HT in rat brain, whereas fenfluramine causes profound loss of 5-HT. These data indicate that SERT-mediated 5-HT release is separable from long-term 5-HT depletion.
Figure 6.
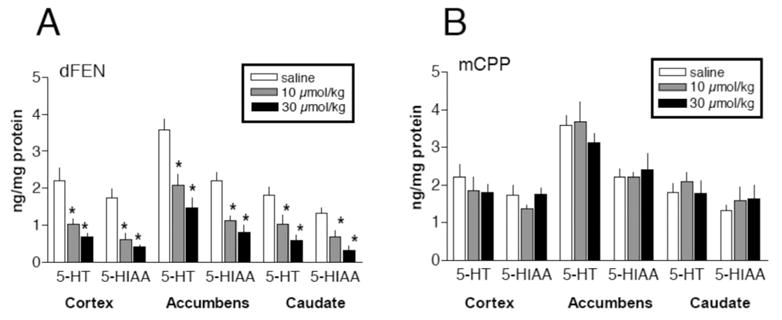
Effects of high-dose administration of d-fenfluramine (dFEN) or m-chlorophenylpiperazine (mCPP) on postmortem tissue levels of serotonin (5-HT) and its metabolite 5-hydroxyindoleacetic acid (5-HIAA) in rat brain. dFEN or mCPP was administered ip at doses of 10 or 30 μmol/kg, every 2 h, for four doses. Rats were killed two weeks after the dosing regimen. Postmortem tissue levels of 5-HT and 5-HIAA in prefrontal cortex, nucleus accumbens, and caudate nucleus were determined by high-performance liquid chromatography with electrochemical detection (HPLC-ECD). These doses of dFEN and mCPP produce equivalent increases in extracellular 5-HT. Data are mean ± SEM, expressed as ng/mg protein for N = 4–6 rats/group. * p < .05 compared with saline-treated group (Duncan’s post hoc test). Data taken from Baumann et al. (2001).
Elucidating the mechanisms responsible for adverse effects of 5-HT releasers will have important implications for the future development of SERT substrates as pharmacotherapies. The findings reviewed here provide clues for designing dual DA/5-HT releasers devoid of fenfluramine-like adverse effects. In particular, any lead drug molecule must lack 5-HT2B agonist activity to prevent the risk of VHD. In addition, candidate drugs should be chemically distinct from the phenylethylamine structure shared by amphetamine-like agents, because nonamphetamine 5-HT releasers have a reduced capacity for causing neurotoxic effects, and possibly IPAH.
PAL-287: A Nonamphetamine DA/5-HT Releaser
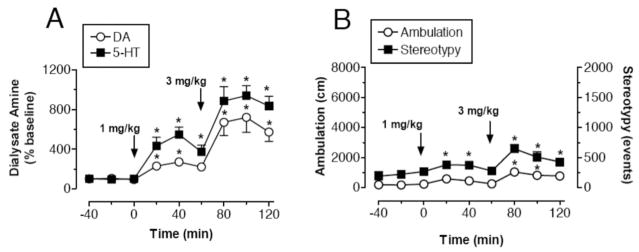
Based in part on the above rationale, we sought to identify and characterize a nonamphetamine transporter substrate that would release DA and 5-HT, without affecting release of NE. After an extensive evaluation of over 350 compounds, we found it impossible to dissociate NE- and DA-releasing properties, perhaps because of the phylogenetic similarities between NET and DAT. The first lead compound from our search was PAL-287 (1-napthyl-2-aminopropane; see structure in Figure 1), a novel nonamphetamine monoamine releaser (Rothman et al., 2005). The in vitro potency of PAL-287 at releasing radiolabeled transmitters from DAT, NET, and SERT is 12.6 ± 0.4 nM, 11.1 ± 0.9 nM, and 3.4 ± 0.2 nM, respectively (see Table 2). Figure 7 shows that administration of PAL-287 to rats increases extracellular 5-HT and DA in a dose-dependent manner, with larger effects on 5-HT compared with DA. Functional studies with cloned human 5-HT2 receptors reveal that PAL-287 is a full agonist at 5-HT2B receptors (EC50 = 40 nM) and 5-HT2A receptors (EC50 = 466 nM). The drug is a potent partial agonist at 5-HT2C receptor sites (EC50 = 2.3 nM, EMAX = 20%), an effect that suggests possible anorectic actions of PAL-287 (Nilsson, 2006; Vickers, Clifton, Dourish, & Tecott, 1999). 5-HT2C agonist activity may also contribute to the minimal reinforcing properties of PAL-287 despite potent DA releasing actions of the drug (see Czoty et al., 2002; Higgins & Fletcher, 2003). The weaker potency of PAL-287 at 5-HT2A and 5-HT2B receptors, compared with its activity at SERT, suggests the drug may not activate 5-HT2A and 5-HT2B receptors in vivo. Experiments should be conducted to test this hypothesis.
Figure 7.
Effects of PAL-287 on neurochemical and locomotor parameters in rats undergoing in vivo microdialysis in prefrontal cortex. Rats received intravenous injections of 1 mg/kg PAL-287 at time zero, followed by 3 mg/kg 60 min later. A, Concentrations of dopamine (DA) and serotonin (5-HT) in dialysate samples are mean ± SEM for N = 7 rats/group, expressed as % baseline. Baseline levels of DA and 5-HT were 0.43 ± 0.07 and 0.27 ± 0.06 pg/5 μl. B, Locomotor parameters are mean ± SEM for N = 7 rats/group, expressed as distance traveled in cm (ambulation) and number of repetitive movements (stereotypy). * p < .05 compared with preinjection control (Duncan’s post hoc test). Data taken from Rothman et al. (2005).
PAL-287 produces minimal locomotor activation despite substantial elevations in extracellular DA (see Figure 7). In particular, the amount of ambulation produced by 3 mg/kg PAL-287 is one third the amount produced by 1 mg/kg d-amphetamine, even though both drug treatments cause equivalent DA release. These data suggest that 5-HT-releasing properties of PAL-287 limit the stimulant effects of concurrent DA release. Repeated high-dose administration of PAL-287 to rats (18 mg/kg ip, q 2 hr, 3 doses) fails to affect brain tissue 5-HT levels when assessed two weeks after injections, unlike d-methamphetamine (6.0 mg/kg ip, q 2hr, 3 doses) and MDMA (7.5 mg/kg ip, q 2 hr, 3 doses) which cause significant 5-HT depletions. The data in Figure 8 show that PAL-287 does not support self-administration behavior, and chronic administration of the drug decreases cocaine self-administration in rhesus monkeys. A dose of 1.0 mg/kg/hr PAL-287 significantly reduces both cocaine- and food-maintained responding, but the suppression of cocaine self-administration is somewhat greater than the reduction in food-maintained responding.
Figure 8.
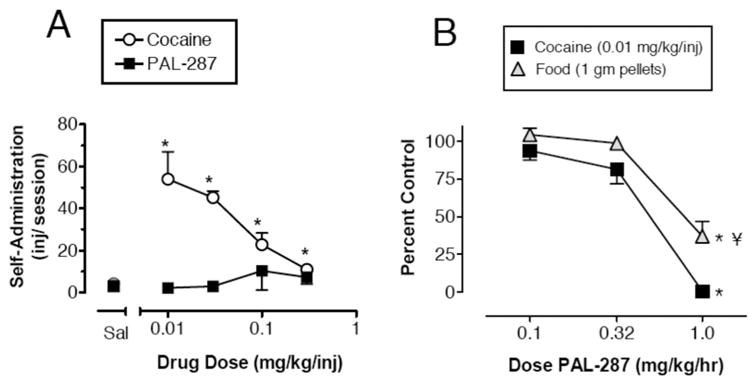
Effects of PAL-287 in the monkey self-administration assay. A, Self-administration of cocaine and PAL 287 by rhesus monkeys. Drugs were available under a fixed ratio (FR) 25 schedule of reinforcement for two hours/day. Each point is the mean of two sessions of access to each dose of the drugs. Data are mean ± SEM for N = 4 monkeys. Symbols without bars have variability smaller than the points. * p < .05 compared with saline-injected control (Newman–Keuls post hoc test). B, Effects of chronic 7-day treatment with PAL-287 on cocaine- and food-maintained responding. Control levels of responding were defined as levels of cocaine- or food-maintained responding observed during 7 days of saline treatment. Each point shows mean ± SEM for three monkeys, with data collected during the last three days of each 7-day treatment. *p < .05 compared with control for a given reinforcer (Newman–Keuls). ¥ p < .05 compared with cocaine-maintained responding at that dose of PAL-287 (Newman–Keuls). Data taken from Rothman et al. (2005).
Our results with PAL-287 confirm the hypothesis that a nonamphetamine substrate at DAT and SERT will release DA and 5-HT from neurons in vivo, be minimally reinforcing, and also suppress ongoing cocaine self-administration. PAL-287 displays a number of desirable qualities for a candidate treatment medication, including minimal locomotor activation, lack of long-term 5-HT neurotoxicity, and weak reinforcing effects. Future studies will be necessary to determine the potential of PAL-287 for increasing the risk for VHD and IPAH, especially given the 5-HT2B agonist effects of the drug. The present data with PAL-287 support the use of monoamine releasers as agonist medications for the treatment of stimulant addictions. A dose of 1.0 mg/kg/hr PAL-287 virtually eliminated cocaine self-administration in rhesus monkeys by the end of the 7-day treatment, although this effect was not entirely selective for cocaine versus food. We also note that the role of NE in the actions of PAL-287 is an important issue awaiting additional study (Rothman et al., 2001).
Conclusions
Our findings with PAL-287 in monkeys are similar to the suppression of cocaine self-administration produced by d-amphetamine, although d-amphetamine displays greater selectivity in reducing cocaine self-administration as opposed to food-maintained responding (Negus & Mello, 2003a). Grabowski et al. (Grabowski et al., 2001; Grabowski, Shearer et al., 2004) showed that a slow-release formulation of d-amphetamine is effective in maintaining cocaine addicts in treatment and reducing illicit cocaine use. We predict that agents like PAL-287, which have mixed DA/5-HT releasing activity, will possess the therapeutic effects of amphetamine-type monoamine releasers, while minimizing the adverse effects associated the phenethylamine structure. Based on observations that dual DA/5-HT releasers suppress alcohol ingestion (Halladay et al., 1999; Halladay et al., 2006; Yu et al., 1997), it seems that PAL-287 or similar agents should be tested as potential treatments for alcohol addiction. In addition, combined treatment with DA releasers and 5-HT releasers blocks alcohol withdrawal seizures (Yu et al., 1997). Although further work remains to refine PAL-287, in particular to reduce its potency at 5-HT2B receptors, we believe that PAL-287 represents the prototype for a new generation of drugs that enhance monoamine release by acting as substrates at multiple transporters.
While compounds like PAL-287 move slowly from the preclinical arena toward clinical development, it is possible to test the concept of administering dual DA/5-HT releasers in humans by implementing clinically available compounds. For example, the utility of DA/5-HT releasers as treatments for addictive disorders can be tested by administration of the DA releaser d-amphetamine along with the 5-HT precursor 5-HTP. It is noteworthy that 5-HTP must be coadministered with the peripheral decarboxylase inhibitor carbidopa to selectively increase extracellular 5-HT in CNS (see Halladay et al., 2006). Moreover, the utility of DA/5-HT releasers could also be tested using phentermine and 5-HTP/carbidopa, a drug combination with predicted efficacy as an appetite suppressant (Rothman, in press). In summary, we suggest that drugs with a mode of action similar to that of PAL-287 will provide neurochemical normalization therapy for stimulant addictions, and might also be useful for treating depression, obsessive–compulsive disorder, attention deficit hyperactivity disorder and obesity.
Acknowledgments
This research was supported in part by the Intramural Research Program of the National Institutes of Health, NIDA and NIDA R01 DA12970 to Bruce E. Blough.
Contributor Information
Richard B. Rothman, National Institute on Drug Abuse, National Institutes of Health
Bruce E. Blough, Research Triangle Institute International
Michael H. Baumann, National Institute on Drug Abuse, National Institutes of Health
References
- Abenhaim L, Moride Y, Brenot F, Rich S, Benichou J, Kurz X, et al. Appetite-suppressant drugs and the risk of primary pulmonary hypertension. International Primary Pulmonary Hypertension Study Group. New England Journal of Medicine. 1996;335:609–616. doi: 10.1056/NEJM199608293350901. [see comments] [DOI] [PubMed] [Google Scholar]
- Alex KD, Pehek EA. Pharmacologic mechanisms of serotonergic regulation of dopamine neurotransmission. Pharmacology and Therapeutics. 2007;113:296–320. doi: 10.1016/j.pharmthera.2006.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander M, Rothman RB, Baumann MH, Endres CJ, Brasic JR, Wong DF. Noradrenergic and dopaminergic effects of (+)-amphetamine-like stimulants in the baboon Papio anubis. Synapse. 2005;56:94–99. doi: 10.1002/syn.20126. [DOI] [PubMed] [Google Scholar]
- Alim TN, Rosse RB, Vocci FJ, Jr, Lindquist T, Deutsch SI. Diethylpropion pharmacotherapeutic adjuvant therapy for inpatient treatment of cocaine dependence: A test of the cocaine-agonist hypothesis. Clinical Neuropharmacology. 1995;18:183–195. doi: 10.1097/00002826-199504000-00009. [DOI] [PubMed] [Google Scholar]
- Amara SG, Kuhar MJ. Neurotransmitter transporters: Recent progress. Annual Review of Neuroscience. 1993;16:73–93. doi: 10.1146/annurev.ne.16.030193.000445. [DOI] [PubMed] [Google Scholar]
- Amara SG, Sonders MS. Neurotransmitter transporters as molecular targets for addictive drugs. Drug and Alcohol Dependence. 1998;51:87–96. doi: 10.1016/s0376-8716(98)00068-4. [DOI] [PubMed] [Google Scholar]
- Anonymous Increasing morbidity and mortality associated with abuse of methamphetamine–United States, 1991–1994. MMWR Morbidity and Mortality Weekly Report. 1995;44:882–886. [PubMed] [Google Scholar]
- Arnsten AF. Stimulants: Therapeutic actions in ADHD. Neuropsychopharmacology. 2006;31:2376–2383. doi: 10.1038/sj.npp.1301164. [DOI] [PubMed] [Google Scholar]
- Artigas F, Sarrias MJ, Martinez E, Gelpi E, Alvarez E, Udina C. Increased plasma free serotonin but unchanged platelet serotonin in bipolar patients treated chronically with lithium. Psychopharmacology (Berl) 1989;99:328–332. doi: 10.1007/BF00445552. [DOI] [PubMed] [Google Scholar]
- Bana DS, MacNeal PS, LeCompte PM, Shah Y, Graham JR. Cardiac murmurs and endocardial fibrosis associated with methysergide therapy. American Heart Journal. 1974;88:640–655. doi: 10.1016/0002-8703(74)90251-8. [DOI] [PubMed] [Google Scholar]
- Barnes NM, Sharp T. A review of central 5-HT receptors and their function. Neuropharmacology. 1999;38:1083–1152. doi: 10.1016/s0028-3908(99)00010-6. [DOI] [PubMed] [Google Scholar]
- Baumann MH, Ayestas MA, Dersch CM, Brockington A, Rice KC, Rothman RB. Effects of phentermine and fenfluramine on extracellular dopamine and serotonin in rat nucleus accumbens: Therapeutic implications. Synapse. 2000;36:102–113. doi: 10.1002/(SICI)1098-2396(200005)36:2<102::AID-SYN3>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- Baumann MH, Ayestas MA, Dersch CM, Rothman RB. 1-(m-Chlorophenyl)piperazine (mCPP) dissociates in vivo serotonin release from long-term serotonin depletion in rat brain. Neuropsychopharmacology. 2001;24:492–501. doi: 10.1016/S0893-133X(00)00221-9. [DOI] [PubMed] [Google Scholar]
- Baumann MH, Becketts KM, Rothman RB. Evidence for alterations in presynaptic serotonergic function during withdrawal from chronic cocaine in rats. European Journal of Pharmacology. 1995;282:87–93. doi: 10.1016/0014-2999(95)00280-x. [DOI] [PubMed] [Google Scholar]
- Baumann MH, Mash DC, Staley JK. The serotonin agonist m-chlorophenylpiperazine (mCPP) binds to serotonin transporter sites in human brain. Neuroreport. 1995;6:2150–2152. doi: 10.1097/00001756-199511000-00013. [DOI] [PubMed] [Google Scholar]
- Baumann MH, Rothman RB. Alterations in serotonergic responsiveness during cocaine withdrawal in rats: Similarities to major depression in humans. Biological Psychiatry. 1998a;44:578–591. doi: 10.1016/s0006-3223(98)00123-1. [DOI] [PubMed] [Google Scholar]
- Baumann MH, Rothman RB. Serotonergic dysfunction during cocaine withdrawal: Implications for cocaine-induced depression. In: Karch SB, editor. Drug abuse handbook. Boca Raton, FL: CRC Press; 1998b. pp. 463–484. [Google Scholar]
- Baumann MH, Rutter JJ, Auerbach SB. Intravenous administration of the serotonin agonist m-chlorophenylpiperazine (mCPP) increases extracellular serotonin in the diencephalon of awake rats. Neuropharmacology. 1993;32:1381–1386. doi: 10.1016/0028-3908(93)90034-z. [DOI] [PubMed] [Google Scholar]
- Blakely RD, Defelice LJ, Galli A. Biogenic amine neurotransmitter transporters: Just when you thought you knew them. Physiology (Bethesda) 2005;20:225–231. doi: 10.1152/physiol.00013.2005. [DOI] [PubMed] [Google Scholar]
- Brauer LH, Johanson CE, Schuster CR, Rothman RB, de Wit H. Evaluation of phentermine and fenfluramine, alone and in combination, in normal, healthy volunteers. Neuropsychopharmacology. 1996;14:233–241. doi: 10.1016/0893-133X(95)00113-R. [DOI] [PubMed] [Google Scholar]
- Bredberg U, Eyjolfsdottir GS, Paalzow L, Tfelt-Hansen P, Tfelt-Hansen V. Pharmacokinetics of methysergide and its metabolite methylergometrine in man. European Journal of Clinical Pharmacology. 1986;30:75–77. doi: 10.1007/BF00614199. [DOI] [PubMed] [Google Scholar]
- Bubar MJ, Cunningham KA. Serotonin 5-HT2A and 5-HT2C receptors as potential targets for modulation of psychostimulant use and dependence. Current Topics in Medicinal Chemistry. 2006;6:1971–1985. doi: 10.2174/156802606778522131. [DOI] [PubMed] [Google Scholar]
- Bubar MJ, Cunningham KA. Distribution of serotonin 5-HT2C receptors in the ventral tegmental area. Neuroscience. 2007;146:286–297. doi: 10.1016/j.neuroscience.2006.12.07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burmeister JJ, Lungren EM, Kirschner KF, Neisewander JL. Differential roles of 5-HT receptor subtypes in cue and cocaine reinstatement of cocaine-seeking behavior in rats. Neuropsychopharmacology. 2004;29:660–668. doi: 10.1038/sj.npp.1300346. [DOI] [PubMed] [Google Scholar]
- Burmeister JJ, Lungren EM, Neisewander JL. Effects of fluoxetine and d-fenfluramine on cocaine-seeking behavior in rats. Psychopharmacology. 2003;168:146–154. doi: 10.1007/s00213-002-1307-8. [DOI] [PubMed] [Google Scholar]
- Buydens-Branchey L, Branchey M, Hudson J, Rothman M, Fergeson P, McKernin C. Effect of fenfluramine challenge on cocaine craving in addicted male users. American Journal on Addictions. 1998;7:142–155. [PubMed] [Google Scholar]
- Carroll ME, Lac ST, Asencio M, Kragh R. Fluoxetine reduces intravenous cocaine self-administration in rats. Pharmacology, Biochemistry and Behavior. 1990;35:237–244. doi: 10.1016/0091-3057(90)90232-7. [DOI] [PubMed] [Google Scholar]
- Celada P, Dolera M, Alvarez E, Artigas F. Effects of acute and chronic treatment with fluvoxamine on extracellular and platelet serotonin in the blood of major depressive patients: Relationship to clinical improvement. Journal of Affective Disorders. 1992;25:243–249. doi: 10.1016/0165-0327(92)90082-h. [DOI] [PubMed] [Google Scholar]
- Connolly HM, Crary JL, McGoon MD, Hensrud DD, Edwards BS, Schaff HV. Valvular heart disease associated with fenfluramine-phentermine. New England Journal of Medicine. 1997;337:581–588. doi: 10.1056/NEJM199708283370901. [DOI] [PubMed] [Google Scholar]
- Connolly HM, McGoon MD. Obesity drugs and the heart. Current Problems in Cardiology. 1999;24:745–792. doi: 10.1016/s0146-2806(99)90013-0. [DOI] [PubMed] [Google Scholar]
- Cortijo J, Marti-Cabrera M, Bernabeu E, Domenech T, Bou J, Fernandez AG, et al. Characterization of 5-HT receptors on human pulmonary artery and vein: Functional and binding studies. British Journal of Pharmacology. 1997;122:1455–1463. doi: 10.1038/sj.bjp.0701509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cozzi NV, Frescas S, Marona-Lewicka D, Huang X, Nichols DE. Indan analogs of fenfluramine and norfenfluramine have reduced neurotoxic potential. Pharmacology, Biochemistry and Behavior. 1998;59:709–715. doi: 10.1016/s0091-3057(97)00557-1. [DOI] [PubMed] [Google Scholar]
- Czoty PW, Ginsburg BC, Howell LL. Serotonergic attenuation of the reinforcing and neurochemical effects of cocaine in squirrel monkeys. Journal of Pharmacology and Experimental Therapeutics. 2002;300(I):831–837. doi: 10.1124/jpet.300.3.831. [DOI] [PubMed] [Google Scholar]
- Dackis CA, Gold MS. New concepts in cocaine addiction: The dopamine depletion hypothesis. Neuroscience and Biobehavioral Reviews. 1985;9:469–477. doi: 10.1016/0149-7634(85)90022-3. [DOI] [PubMed] [Google Scholar]
- Das G. Cocaine abuse in North America: A milestone in history. Journal of Clinical Pharmacology. 1993;33:296–310. doi: 10.1002/j.1552-4604.1993.tb04661.x. [DOI] [PubMed] [Google Scholar]
- Das YT, Bagchi M, Bagchi D, Preuss HG. Safety of 5-hydroxy-L-tryptophan. Toxicology Letters. 2004;150:111–122. doi: 10.1016/j.toxlet.2003.12.070. [DOI] [PubMed] [Google Scholar]
- Daw ND, Kakade S, Dayan P. Opponent interactions between serotonin and dopamine. Neural Network. 2002;15:603–616. doi: 10.1016/s0893-6080(02)00052-7. [DOI] [PubMed] [Google Scholar]
- Di Chiara G, Bassareo V, Fenu S, De Luca MA, Spina L, Cadoni C, et al. Dopamine and drug addiction: The nucleus accumbens shell connection. Neuropharmacology. 2004;47(Suppl 1):227–241. doi: 10.1016/j.neuropharm.2004.06.032. [DOI] [PubMed] [Google Scholar]
- Di Matteo V, De Blasi A, Di Giulio C, Esposito E. Role of 5-HT(2C) receptors in the control of central dopamine function. Trends in Pharmacological Sciences. 2001;22:229–232. doi: 10.1016/s0165-6147(00)01688-6. [DOI] [PubMed] [Google Scholar]
- Di Matteo V, Di Giovanni G, Di Mascio M, Esposito E. SB 242084, a selective serotonin2C receptor antagonist, increases dopaminergic transmission in the mesolimbic system. Neuropharmacology. 1999;38:1195–1205. doi: 10.1016/s0028-3908(99)00047-7. [DOI] [PubMed] [Google Scholar]
- Di Matteo V, Di Giovanni G, Di Mascio M, Esposito E. Biochemical and electrophysiological evidence that RO 60–0175 inhibits mesolimbic dopaminergic function through serotonin (2C) receptors. Brain Research. 2000;865:85–90. doi: 10.1016/s0006-8993(00)02246-0. [DOI] [PubMed] [Google Scholar]
- Droogmans S, Cosyns B, D’Haenen H, Creeten E, Weytjens C, Franken PR, et al. Possible association between 3,4-Methylenedioxymethamphetamine abuse and valvular heart disease. American Journal of Cardiology. doi: 10.1016/j.amjcard.2007.06.045. (in press) [DOI] [PubMed] [Google Scholar]
- Eddahibi S, Fabre V, Boni C, Martres MP, Raffestin B, Hamon M, et al. Induction of serotonin transporter by hypoxia in pulmonary vascular smooth muscle cells: Relationship with the mitogenic action of serotonin. Circulation Research. 1999;84:329–336. doi: 10.1161/01.res.84.3.329. [DOI] [PubMed] [Google Scholar]
- Eddahibi S, Guignabert C, Barlier-Mur AM, Dewachter L, Fadel E, Dartevelle P, et al. Cross talk between endothelial and smooth muscle cells in pulmonary hypertension: Critical role for serotonin-induced smooth muscle hyperplasia. Circulation. 2006;113:1857–1864. doi: 10.1161/CIRCULATIONAHA.105.591321. [DOI] [PubMed] [Google Scholar]
- Eddahibi S, Humbert M, Fadel E, Raffestin B, Darmon M, Capron F, et al. Serotonin transporter overexpression is responsible for pulmonary artery smooth muscle hyperplasia in primary pulmonary hypertension. Journal of Clinical Investigation. 2001;108:1141–1150. doi: 10.1172/JCI12805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eriksson E, Engberg G, Bing O, Nissbrandt H. Effects of mCPP on the extracellular concentrations of serotonin and dopamine in rat brain. Neuropsychopharmacology. 1999;20:287–296. doi: 10.1016/S0893-133X(98)00070-0. [DOI] [PubMed] [Google Scholar]
- Filip M, Cunningham KA. Hyperlocomotive and discriminative stimulus effects of cocaine are under the control of serotonin(2C) (5-HT(2C)) receptors in rat prefrontal cortex. Journal of Pharmacology and Experimental Therapeutics. 2003;306:734–743. doi: 10.1124/jpet.102.045716. [DOI] [PubMed] [Google Scholar]
- Fishman AP. Aminorex to fen/phen: An epidemic foretold. Circulation. 1999;99:156–161. doi: 10.1161/01.cir.99.1.156. [DOI] [PubMed] [Google Scholar]
- Fitzgerald LW, Burn TC, Brown BS, Patterson JP, Corjay MH, Valentine PA, et al. Possible role of valvular serotonin 5-HT2B receptors in the cardiopathy associated with fenfluramine. Molecular Pharmacology. 2000;57:75–81. [PubMed] [Google Scholar]
- Fleckenstein AE, Volz TJ, Riddle EL, Gibb JW, Hanson GR. New insights into the mechanism of action of amphetamines. Annual Review of Pharmacology and Toxicology. 2007;47:681–698. doi: 10.1146/annurev.pharmtox.47.120505.105140. [DOI] [PubMed] [Google Scholar]
- Fletcher PJ, Chintoh AF, Sinyard J, Higgins GA. Injection of the 5-HT2C receptor agonist Ro60–0175 into the ventral tegmental area reduces cocaine-induced locomotor activity and cocaine self-administration. Neuropsychopharmacology. 2004;29:308–318. doi: 10.1038/sj.npp.1300319. [DOI] [PubMed] [Google Scholar]
- Fletcher PJ, Grottick AJ, Higgins GA. Differential effects of the 5-HT(2A) receptor antagonist M100907 and the 5-HT(2C) receptor antagonist SB242084 on cocaine-induced locomotor activity, cocaine self-administration and cocaine-induced reinstatement of responding. Neuropsychopharmacology. 2002;27:576–586. doi: 10.1016/S0893-133X(02)00342-1. [DOI] [PubMed] [Google Scholar]
- Gaine SP, Rubin LJ, Kmetzo JJ, Palevsky HI, Traill TA. Recreational use of aminorex and pulmonary hypertension. Chest. 2000;118:1496–1497. doi: 10.1378/chest.118.5.1496. [DOI] [PubMed] [Google Scholar]
- Garlow SJ, Purselle D, D’Orio B. Cocaine use disorders and suicidal ideation. Drug and Alcohol Dependence. 2003;70:101–104. doi: 10.1016/s0376-8716(02)00337-x. [DOI] [PubMed] [Google Scholar]
- Gawin FH, Kleber HD. Abstinence symptomatology and psychiatric diagnosis in cocaine abusers. Archives of General Psychiatry. 1986;43:107–113. doi: 10.1001/archpsyc.1986.01800020013003. [DOI] [PubMed] [Google Scholar]
- Ghitza UE, Rothman RB, Gorelick DA, Henningfield JE, Baumann MH. Serotonergic responsiveness in human cocaine users. Drug and Alcohol Dependence. 2007;86:207–213. doi: 10.1016/j.drugalcdep.2006.06.007. [DOI] [PubMed] [Google Scholar]
- Glatz AC, Ehrlich M, Bae RS, Clarke MJ, Quinlan PA, Brown EC, et al. Inhibition of cocaine self-administration by fluoxetine or D-fenfluramine combined with phentermine. Pharmacology, Biochemistry and Behavior. 2002;71:197–204. doi: 10.1016/s0091-3057(01)00657-8. [DOI] [PubMed] [Google Scholar]
- Glowa JR, Rice KC, Matecka D, Rothman RB. Phentermine/fenfluramine decreases cocaine self-administration in rhesus monkeys. Neuroreport. 1997;8:1347–1351. doi: 10.1097/00001756-199704140-00006. [DOI] [PubMed] [Google Scholar]
- Glowa JR, Wojnicki FHE, Matecka D, Rice KC, Rothman RB. Effects of dopamine reuptake inhibitors on food- and cocaine-maintained responding: II. Comparisons with other drugs and repeated administrations. Experimental and Clinical Psychopharmacology. 1995;3:232–239. [Google Scholar]
- Gobert A, Rivet JM, Lejeune F, Newman-Tancredi A, Adhumeau-Auclair A, Nicolas JP, et al. Serotonin(2C) receptors tonically suppress the activity of mesocortical dopaminergic and adrenergic, but not serotonergic, pathways: A combined dialysis and electrophysiological analysis in the rat. Synapse. 2000;36:205–221. doi: 10.1002/(SICI)1098-2396(20000601)36:3<205::AID-SYN5>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- Gonzalez Castro F, Barrington EH, Walton MA, Rawson RA. Cocaine and methamphetamine: Differential addiction rates. Psychology of Addictive Behaviors. 2000;14:390–396. [PubMed] [Google Scholar]
- Gorelick DA. The rate hypothesis and agonist substitution approaches to cocaine abuse treatment. Advances in Pharmacology. 1998;42:995–997. doi: 10.1016/s1054-3589(08)60914-x. [DOI] [PubMed] [Google Scholar]
- Grabowski J, Rhoades H, Schmitz J, Stotts A, Daruzska LA, Creson D, et al. Dextroamphetamine for cocaine-dependence treatment: A double-blind randomized clinical trial. Journal of Clinical Psychopharmacology. 2001;21:522–526. doi: 10.1097/00004714-200110000-00010. [DOI] [PubMed] [Google Scholar]
- Grabowski J, Rhoades H, Stotts A, Cowan K, Kopecky C, Dougherty A, et al. Agonist-like or antagonist-like treatment for cocaine dependence with methadone for heroin dependence: Two double-blind randomized clinical trials. Neuropsychopharmacology. 2004;29:969–981. doi: 10.1038/sj.npp.1300392. [DOI] [PubMed] [Google Scholar]
- Grabowski J, Roache JD, Schmitz JM, Rhoades H, Creson D, Korszun A. Replacement medication for cocaine dependence: Methylphenidate. Journal of Clinical Psychopharmacology. 1997;17:485–488. doi: 10.1097/00004714-199712000-00008. [DOI] [PubMed] [Google Scholar]
- Grabowski J, Shearer J, Merrill J, Negus SS. Agonist-like, replacement pharmacotherapy for stimulant abuse and dependence. Addictive Behaviors. 2004;29:1439–1464. doi: 10.1016/j.addbeh.2004.06.018. [DOI] [PubMed] [Google Scholar]
- Greenhill LL. The science of stimulant abuse. Pediatric Annals. 2006;35:552–556. doi: 10.3928/0090-4481-20060801-07. [DOI] [PubMed] [Google Scholar]
- Grottick AJ, Fletcher PJ, Higgins GA. Studies to investigate the role of 5-HT(2C) receptors on cocaine- and food-maintained behavior. Journal of Pharmacology and Experimental Therapeutics. 2000;295:1183–1191. [PubMed] [Google Scholar]
- Gurtner HP. Aminorex and pulmonary hypertension. Cor et Vasa. 1985;27:160–171. [PubMed] [Google Scholar]
- Gustafsson BI, Tommeras K, Nordrum I, Loennechen JP, Brunsvik A, Solligard E, et al. Long-term serotonin administration induces heart valve disease in rats. Circulation. 2005;111:1517–1522. doi: 10.1161/01.CIR.0000159356.42064.48. [DOI] [PubMed] [Google Scholar]
- Hafizi S, Taylor PM, Chester AH, Allen SP, Yacoub MH. Mitogenic and secretory responses of human valve interstitial cells to vasoactive agents. Journal of Heart Valve Disease. 2000;9:454–458. [PubMed] [Google Scholar]
- Halladay AK, Kirschner E, Hesse K, Fisher H, Wagner GC. Role of monoamine oxidase inhibition and monoamine depletion in fenfluramine-induced neurotoxicity and serotonin release. Pharmacology and Toxicology. 2001;89:237–248. doi: 10.1034/j.1600-0773.2001.d01-154.x. [DOI] [PubMed] [Google Scholar]
- Halladay AK, Wagner GC, Hsu T, Sekowski A, Fisher H. Differential effects of monoaminergic agonists on alcohol intake in rats fed a tryptophan-enhanced diet. Alcohol. 1999;18:55–64. doi: 10.1016/s0741-8329(98)00068-8. [DOI] [PubMed] [Google Scholar]
- Halladay AK, Wagner GC, Sekowski A, Rothman RB, Baumann MH, Fisher H. Alterations in alcohol consumption, withdrawal seizures, and monoamine transmission in rats treated with phentermine and 5-hydroxy-L-tryptophan. Synapse. 2006;59:277–289. doi: 10.1002/syn.20239. [DOI] [PubMed] [Google Scholar]
- Haney M, Ward AS, Gerra G, Foltin RW. Neuroendocrine effects of d-fenfluramine and bromocriptine following repeated smoked cocaine in humans. Drug and Alcohol Dependence. 2001;64:63–73. doi: 10.1016/s0376-8716(00)00232-5. [DOI] [PubMed] [Google Scholar]
- Hendrikx M, Van Dorpe J, Flameng W, Daenen W. Aortic and mitral valve disease induced by ergotamine therapy for migraine: A case report and review of the literature. Journal of Heart Valve Disease. 1996;5:235–237. [PubMed] [Google Scholar]
- Henningfield JE. Nicotine medications for smoking cessation. New England Journal of Medicine. 1995;333:1196–1203. doi: 10.1056/NEJM199511023331807. [DOI] [PubMed] [Google Scholar]
- Herve P, Launay JM, Scrobohaci ML, Brenot F, Simonneau G, Petitpretz P, et al. Increased plasma serotonin in primary pulmonary hypertension. American Journal of Medicine. 1995;99:249–254. doi: 10.1016/s0002-9343(99)80156-9. [DOI] [PubMed] [Google Scholar]
- Higgins GA, Fletcher PJ. Serotonin and drug reward: Focus on 5-HT2C receptors. European Journal of Pharmacology. 2003;480:151–162. doi: 10.1016/j.ejphar.2003.08.102. [DOI] [PubMed] [Google Scholar]
- Howell LL, Byrd LD. Serotonergic modulation of the behavioral effects of cocaine in the squirrel monkey. Journal of Pharmacology and Experimental Therapeutics. 1995;275:1551–1559. [PubMed] [Google Scholar]
- Howell LL, Carroll FI, Votaw JR, Goodman MM, Kimmel HL. Effects of combined dopamine and serotonin transporter inhibitors on cocaine self-administration in rhesus monkeys. Journal of Pharmacology and Experimental Therapeutics. 2007;320:757–765. doi: 10.1124/jpet.106.108324. [DOI] [PubMed] [Google Scholar]
- Hoyer D, Clarke DE, Fozard JR, Hartig PR, Martin GR, Mylecharane EJ, et al. International Union of Pharmacology classification of receptors for 5-hydroxytryptamine (serotonin) Pharmacological Reviews. 1994;46:157–203. [PubMed] [Google Scholar]
- Hoyer D, Hannon JP, Martin GR. Molecular, pharmacological and functional diversity of 5-HT receptors. Pharmacology, Biochemistry and Behavior. 2002;71:533–554. doi: 10.1016/s0091-3057(01)00746-8. [DOI] [PubMed] [Google Scholar]
- Hyman SE. Addiction: A disease of learning and memory. American Journal of Psychiatry. 2005;162:1414–1422. doi: 10.1176/appi.ajp.162.8.1414. [DOI] [PubMed] [Google Scholar]
- Ishida M, Otani K, Kaneko S, Ohkubo T, Osanai T, Yasui N, et al. Effects of various factors on steady state plasma concentrations of trazodone and its active metabolite m-chlorophenylpiperazine. International Clinical Psychopharmacology. 1995;10:143–146. doi: 10.1097/00004850-199510030-00002. [DOI] [PubMed] [Google Scholar]
- Iversen L. Neurotransmitter transporters and their impact on the development of psychopharmacology. British Journal of Pharmacology. 2006;147(Suppl 1):S82–88. doi: 10.1038/sj.bjp.0706428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalivas PW, O’Brien C. Drug addiction as a pathology of staged neuroplasticity. Neuropsychopharmacology. 2008;33:166–180. doi: 10.1038/sj.npp.1301564. [DOI] [PubMed] [Google Scholar]
- Kampman KM, Rukstalis M, Pettinati H, Muller E, Acosta T, Gariti P, et al. The combination of phentermine and fenfluramine reduced cocaine withdrawal symptoms in an open trial. Journal of Substance Abuse Treat. 2000;19:77–79. doi: 10.1016/s0740-5472(99)00076-8. [DOI] [PubMed] [Google Scholar]
- Koob GF. Alcoholism: Allostasis and beyond. Alcoholism, Clinical and Experimental Research. 2003;27:232–243. doi: 10.1097/01.ALC.0000057122.36127.C2. [DOI] [PubMed] [Google Scholar]
- Koob GF, Roberts AJ, Schulteis G, Parsons LH, Heyser CJ, Hyytia P, et al. Neurocircuitry targets in ethanol reward and dependence. Alcoholism, Clinical and Experimental Research. 1998;22:3–9. [PubMed] [Google Scholar]
- Launay JM, Herve P, Peoc’h K, Tournois C, Callebert J, Nebigil CG, et al. Function of the serotonin 5-hydroxytryptamine 2B receptor in pulmonary hypertension. Nature Medicine. 2002;8:1129–1135. doi: 10.1038/nm764. [DOI] [PubMed] [Google Scholar]
- Lesch KP. Alcohol dependence and gene × environment interaction in emotion regulation: Is serotonin the link? European Journal of Pharmacology. 2005;526:113–124. doi: 10.1016/j.ejphar.2005.09.027. [DOI] [PubMed] [Google Scholar]
- Levy AD, Li Q, Van de Kar LD. Repeated cocaine exposure inhibits the adrenocorticotropic hormone response to the serotonin releaser d-fenfluramine and the 5-HT1A agonist, 8-OH-DPAT. Neuropharmacology. 1994;33:335–342. doi: 10.1016/0028-3908(94)90063-9. [DOI] [PubMed] [Google Scholar]
- Lile JA. Pharmacological determinants of the reinforcing effects of psychostimulants: Relation to agonist substitution treatment. Experimental and Clinical Psychopharmacology. 2006;14:20–33. doi: 10.1037/1064-1297.14.1.20. [DOI] [PubMed] [Google Scholar]
- Lin D, Koob GF, Markou A. Differential effects of withdrawal from chronic amphetamine or fluoxetine administration on brain stimulation reward in the rat–interactions between the two drugs. Psychopharmacology. 1999;145:283–294. doi: 10.1007/s002130051060. [DOI] [PubMed] [Google Scholar]
- Ling W, Rawson RA, Compton MA. Substitution pharmacotherapies for opioid addiction: From methadone to LAAM and buprenorphine. Journal of Psychoactive Drugs. 1994;26:119–128. doi: 10.1080/02791072.1994.10472259. [DOI] [PubMed] [Google Scholar]
- Liu S, Bubar MJ, Lanfranco MF, Hillman GR, Cunningham KA. Serotonin2C receptor localization in GABA neurons of the rat medial prefrontal cortex: Implications for understanding the neurobiology of addiction. Neuroscience. 2007;146:1677–1688. doi: 10.1016/j.neuroscience.2007.02.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez-Ilasaca M. Signaling from G-protein-coupled receptors to mitogen-activated protein (MAP)-kinase cascades. Biochemical Pharmacology. 1998;56:269–277. doi: 10.1016/s0006-2952(98)00059-8. [DOI] [PubMed] [Google Scholar]
- Lundmark J, Reis M, Bengtsson F. Serum concentrations of fluoxetine in the clinical treatment setting. Therapeutic Drug Monitoring. 2001;23:139–147. doi: 10.1097/00007691-200104000-00008. [DOI] [PubMed] [Google Scholar]
- MacLean MR, Herve P, Eddahibi S, Adnot S. 5-hydroxytryptamine and the pulmonary circulation: Receptors, transporters and relevance to pulmonary arterial hypertension. British Journal of Pharmacology. 2000;131:161–168. doi: 10.1038/sj.bjp.0703570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mann JJ. Neurobiology of suicidal behaviour. Nature Reviews: Neuroscience. 2003;4:819–828. doi: 10.1038/nrn1220. [DOI] [PubMed] [Google Scholar]
- Marcos E, Adnot S, Pham MH, Nosjean A, Raffestin B, Hamon M, et al. Serotonin transporter inhibitors protect against hypoxic pulmonary hypertension. American Journal of Respiratory and Critical Care Medicine. 2003;168:487–493. doi: 10.1164/rccm.200210-1212OC. [DOI] [PubMed] [Google Scholar]
- Markou A, Koob GF. Postcocaine anhedonia: An animal model of cocaine withdrawal. Neuropsychopharmacology. 1991;4:17–26. [PubMed] [Google Scholar]
- Martin F, Artigas F. Simultaneous effects of p-chloroamphetamine, d-fenfluramine, and reserpine on free and stored 5-hydroxytryptamine in brain and blood. Journal of Neurochemistry. 1992;59:1138–1144. doi: 10.1111/j.1471-4159.1992.tb08356.x. [DOI] [PubMed] [Google Scholar]
- Martinez D, Narendran R, Foltin RW, Slifstein M, Hwang DR, Broft A, et al. Amphetamine-induced dopamine release: Markedly blunted in cocaine dependence and predictive of the choice to self-administer cocaine. American Journal of Psychiatry. 2007;164:622–629. doi: 10.1176/ajp.2007.164.4.622. [DOI] [PubMed] [Google Scholar]
- Masson J, Sagne C, Hamon M, el Mestikawy S. Neurotransmitter transporters in the central nervous system. Pharmacological Reviews. 1999;51:439–464. [PubMed] [Google Scholar]
- McCann UD, Seiden LS, Rubin LJ, Ricaurte GA. Brain serotonin neurotoxicity and primary pulmonary hypertension from fenfluramine and dexfenfluramine: A systematic review of the evidence. Journal of the American Medical Association. 1997;278:666–672. [PubMed] [Google Scholar]
- McGregor A, Lacosta S, Roberts DC. L-tryptophan decreases the breaking point under a progressive ratio schedule of intravenous cocaine reinforcement in the rat. Pharmacology, Biochemistry and Behavior. 1993;44:651–655. doi: 10.1016/0091-3057(93)90181-r. [DOI] [PubMed] [Google Scholar]
- Mogenson GJ, Jones DL, Yim CY. From motivation to action: Functional interface between the limbic system and the motor system. Progress in Neurobiology. 1980;14:69–97. doi: 10.1016/0301-0082(80)90018-0. [DOI] [PubMed] [Google Scholar]
- Molliver ME. Serotonergic neuronal systems: What their anatomic organization tells us about function. Journal of Clinical Psychopharmacology. 1987;7(Suppl 6):3S–23S. [PubMed] [Google Scholar]
- Moore RY, Bloom FE. Central catecholamine neuron systems: Anatomy and physiology of the dopamine systems. Annual Review of Neuroscience. 1978;1:129–169. doi: 10.1146/annurev.ne.01.030178.001021. [DOI] [PubMed] [Google Scholar]
- Muller CP, Huston JP. Determining the region-specific contributions of 5-HT receptors to the psychostimulant effects of cocaine. Trends in Pharmacological Sciences. 2006;27:105–112. doi: 10.1016/j.tips.2005.12.003. [DOI] [PubMed] [Google Scholar]
- Musto DF. Cocaine’s history, especially the American experience. Ciba Foundation Symposium. 1992;166:7–14. doi: 10.1002/9780470514245.ch2. [DOI] [PubMed] [Google Scholar]
- Negus SS, Mello NK. Effects of chronic d-amphetamine treatment on cocaine- and food-maintained responding under a progressive-ratio schedule in rhesus monkeys. Psychopharmacology. 2003a;167:324–332. doi: 10.1007/s00213-003-1409-y. [DOI] [PubMed] [Google Scholar]
- Negus SS, Mello NK. Effects of chronic d-amphetamine treatment on cocaine- and food-maintained responding under a second-order schedule in rhesus monkeys. Drug and Alcohol Dependence. 2003b;70:39–52. doi: 10.1016/s0376-8716(02)00339-3. [DOI] [PubMed] [Google Scholar]
- Negus SS, Mello NK, Blough BE, Baumann MH, Rothman RB. Monoamine releasers with varying selectivity for dopamine/norepinephrine versus serotonin release as candidate “agonist” medications for cocaine dependence: Studies in assays of cocaine discrimination and cocaine self-administration in rhesus monkeys. Journal of Pharmacology and Experimental Therapeutics. 2007a;320:627–636. doi: 10.1124/jpet.106.107383. [DOI] [PubMed] [Google Scholar]
- Negus SS, Mello NK, Blough BE, Baumann MH, Rothman RB. Monoamine releasers with varying selectivity for dopamine/norepinephrine versus serotonin release as candidate “agonist” medications for cocaine dependence: Studies in assays of cocaine discrimination and cocaine self-administration in rhesus monkeys. Journal of Pharmacology and Experimental Therapeutics. 2007b;320:627–636. doi: 10.1124/jpet.106.107383. [DOI] [PubMed] [Google Scholar]
- Nemecek GM, Coughlin SR, Handley DA, Moskowitz MA. Stimulation of aortic smooth muscle cell mitogenesis by serotonin. Proceedings of the National Academy of Sciences of the United States of America. 1986;83:674–678. doi: 10.1073/pnas.83.3.674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ni W, Watts SW. 5-hydroxytryptamine in the cardiovascular system: Focus on the serotonin transporter (SERT) Clinical and Experimental Pharmacology and Physiology. 2006;33:575–583. doi: 10.1111/j.1440-1681.2006.04410.x. [DOI] [PubMed] [Google Scholar]
- Nichols DE, Brewster WK, Johnson MP, Oberlender R, Riggs RM. Nonneurotoxic tetralin and indan analogues of 3,4-(methylenedioxy)amphetamine (MDA) Journal of Medicinal Chemistry. 1990;33:703–710. doi: 10.1021/jm00164a037. [DOI] [PubMed] [Google Scholar]
- Nilsson BM. 5-Hydroxytryptamine 2C (5-HT2C) receptor agonists as potential antiobesity agents. Journal of Medicinal Chemistry. 2006;49:4023–4034. doi: 10.1021/jm058240i. [DOI] [PubMed] [Google Scholar]
- Otani K, Mihara K, Yasui N, Ishida M, Kondo T, Tokinaga N, et al. Plasma concentrations of trazodone and m-chlorophenylpiperazine at steady state can be predicted from those after an initial dose of trazodone. Progress in NeuroPsychopharmacology and Biological Psychiatry. 1997;21:239–244. doi: 10.1016/s0278-5846(96)00140-6. [DOI] [PubMed] [Google Scholar]
- Parsons LH, Koob GF, Weiss F. Serotonin dysfunction in the nucleus accumbens of rats during withdrawal after unlimited access to intravenous cocaine. Journal of Pharmacology and Experimental Therapeutics. 1995;274:1182–1191. [PubMed] [Google Scholar]
- Parsons LH, Smith AD, Justice JB., Jr Basal extracellular dopamine is decreased in the rat nucleus accumbens during abstinence from chronic cocaine. Synapse. 1991;9:60–65. doi: 10.1002/syn.890090109. [DOI] [PubMed] [Google Scholar]
- Pennartz CM, Groenewegen HJ, Lopes da Silva FH. The nucleus accumbens as a complex of functionally distinct neuronal ensembles: An integration of behavioural, electrophysiological and anatomical data. Progress in Neurobiology. 1994;42:719–761. doi: 10.1016/0301-0082(94)90025-6. [DOI] [PubMed] [Google Scholar]
- Pitt BR, Weng W, Steve AR, Blakely RD, Reynolds I, Davies P. Serotonin increases DNA synthesis in rat proximal and distal pulmonary vascular smooth muscle cells in culture. American Journal of Physiology. 1994;266:L178–L186. doi: 10.1152/ajplung.1994.266.2.L178. [DOI] [PubMed] [Google Scholar]
- Porter RH, Benwell KR, Lamb H, Malcolm CS, Allen NH, Revell DF, et al. Functional characterization of agonists at recombinant human 5-HT2A, 5- HT2B and 5-HT2C receptors in CHO-K1 cells. British Journal of Pharmacology. 1999;128:13–20. doi: 10.1038/sj.bjp.0702751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rea WP, Rothman RB, Shippenberg TS. Evaluation of the conditioned reinforcing effects of phentermine and fenfluramine in the rat: Concordance with clinical studies. Synapse. 1998;30:107–111. doi: 10.1002/(SICI)1098-2396(199809)30:1<107::AID-SYN13>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- Roberts DC, Phelan R, Hodges LM, Hodges MM, Bennett B, Childers S, et al. Self-administration of cocaine analogs by rats. Psychopharmacology. 1999;144:389–397. doi: 10.1007/s002130051022. [DOI] [PubMed] [Google Scholar]
- Rollema H, Coe JW, Chambers LK, Hurst RS, Stahl SM, Williams KE. Rationale, pharmacology and clinical efficacy of partial agonists of alpha4beta2 nACh receptors for smoking cessation. Trends in Pharmacological Sciences. 2007;28:316–325. doi: 10.1016/j.tips.2007.05.003. [DOI] [PubMed] [Google Scholar]
- Rose S, Hunt S, Collins P, Hindmarsh JG, Jenner P. Repeated administration of escalating high doses of dexfenfluramine does not produce morphological evidence for neurotoxicity in the cortex of rats. Neurodegeneration. 1996;5:145–152. doi: 10.1006/neur.1996.0021. [DOI] [PubMed] [Google Scholar]
- Rossetti ZL, Hmaidan Y, Gessa GL. Marked inhibition of mesolimbic dopamine release: A common feature of ethanol, morphine, cocaine and amphetamine abstinence in rats. European Journal of Pharmacology. 1992;221:227–234. doi: 10.1016/0014-2999(92)90706-a. [DOI] [PubMed] [Google Scholar]
- Roth BL. Drugs and valvular heart disease. New England Journal of Medicine. 2007;356:6–9. doi: 10.1056/NEJMp068265. [DOI] [PubMed] [Google Scholar]
- Rothman RB. Treatment of obesity with “combination” pharmacotherapy. American Journal of Therapeutics. doi: 10.1097/MJT.0b013e31818e30da. (in press) [DOI] [PubMed] [Google Scholar]
- Rothman RB, Ayestas MA, Dersch CM, Baumann MH. Aminorex, fenfluramine, and chlorphentermine are serotonin transporter substrates: Implications for primary pulmonary hypertension. Circulation. 1999;100:869–875. doi: 10.1161/01.cir.100.8.869. [DOI] [PubMed] [Google Scholar]
- Rothman RB, Baumann M. Therapeutic and adverse actions of serotonin transporter substrates. Pharmacology and Therapeutics. 2002;95:73–88. doi: 10.1016/s0163-7258(02)00234-6. [DOI] [PubMed] [Google Scholar]
- Rothman RB, Baumann MH. Monoamine transporters and psychostimulant drugs. European Journal of Pharmacology. 2003;479:23–40. doi: 10.1016/j.ejphar.2003.08.054. [DOI] [PubMed] [Google Scholar]
- Rothman RB, Baumann MH. Therapeutic potential of monoamine transporter substrates. Current Topics in Medicinal Chemistry. 2006;6:1845–1859. doi: 10.2174/156802606778249766. [DOI] [PubMed] [Google Scholar]
- Rothman RB, Baumann MH, Dersch CM, Romero DV, Rice KC, Carroll FI, et al. Amphetamine-type central nervous system stimulants release norepinephrine more potently than they release dopamine and serotonin. Synapse. 2001;39:32–41. doi: 10.1002/1098-2396(20010101)39:1<32::AID-SYN5>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- Rothman RB, Baumann MH, Savage JE, Rauser L, McBride A, Hufisein S, et al. Evidence for possible involvement of 5-HT2B receptors in the cardiac valvulopathy associated with fenfluramine and other serotonergic medications. Circulation. 2000;102:2836–2841. doi: 10.1161/01.cir.102.23.2836. [DOI] [PubMed] [Google Scholar]
- Rothman RB, Blough BE, Baumann MH. Appetite suppressants as agonist substitution therapies for stimulant dependence. Annals of the New York Academy of Sciences. 2002;965:109–126. doi: 10.1111/j.1749-6632.2002.tb04155.x. [DOI] [PubMed] [Google Scholar]
- Rothman RB, Blough BE, Baumann MH. Dual dopamine/serotonin releasers as potential medications for stimulant and alcohol addictions. The AAPS Journal. 2007;9:E1–E10. doi: 10.1208/aapsj0901001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothman RB, Blough BE, Woolverton WL, Anderson KG, Negus SS, Mello NK, et al. Development of a rationally-designed, low abuse potential, biogenic amine releaser that suppresses cocaine self-administration. Journal of Pharmacology and Experimental Therapeutics. 2005;313:1361–1369. doi: 10.1124/jpet.104.082503. [DOI] [PubMed] [Google Scholar]
- Rothman RB, Clark RD, Partilla JS, Baumann MH. (+)-Fenfluramine and its major metabolite, (+)-norfenfluramine, are potent substrates for norepinephrine transporters. Journal of Pharmacology and Experimental Therapeutics. 2003;305:1191–1199. doi: 10.1124/jpet.103.049684. [DOI] [PubMed] [Google Scholar]
- Rothman RB, Elmer GI, Shippenberg TS, Rea W, Baumann MH. Phentermine and fenfluramine: Pre-clinical studies in animal models of cocaine addiction. Annals of the New York Academy of Sciences. 1998;844:59–74. [PubMed] [Google Scholar]
- Rothman RB, Gendron TM, Hitzig P. Combined use of fenfluramine and phentermine in the treatment of cocaine addiction: A pilot case series. Journal of Substance Abuse Treatment. 1994;11:273–275. doi: 10.1016/0740-5472(94)90086-8. [DOI] [PubMed] [Google Scholar]
- Rothman RB, Jayanthi S, Wang X, Dersch CM, Cadet JL, Prisinzano T, et al. High-dose fenfluramine administration decreases serotonin transporter binding, but not serotonin transporter protein levels, in rat forebrain. Synapse. 2003;50:233–239. doi: 10.1002/syn.10266. [DOI] [PubMed] [Google Scholar]
- Rothman RB, Redmon JB, Raatz SK, Kwong CA, Swanson JE, Bantle JP. Chronic treatment with the phentermine combined with fenfluramine lowers plasma serotonin. American Journal of Cardiology. 2000;85:913–915. doi: 10.1016/s0002-9149(99)00896-6. [DOI] [PubMed] [Google Scholar]
- Rudnick G. Mechanisms of biogenic amine transporters. In: Reith MEA, editor. Neurotransmitter transporters: Structure, function and regulation. Totowa, NJ: Humana Press; 1997. pp. 73–100. [Google Scholar]
- Sachdev M, Miller WC, Ryan T, Jollis JG. Effect of fenfluramine-derivative diet pills on cardiac valves: A meta-analysis of observational studies. American Heart Journal. 2002;144:1065–1073. doi: 10.1067/mhj.2002.126733. [DOI] [PubMed] [Google Scholar]
- Setola V, Hufeisen SJ, Grande-Allen KJ, Vesely I, Glennon RA, Blough B, et al. 3,4-methylenedioxymethamphetamine (MDMA, “Ecstasy”) induces fenfluramine-like proliferative actions on human cardiac valvular interstitial cells in vitro. Molecular Pharmacology. 2003;63:1223–1229. doi: 10.1124/mol.63.6.1223. [DOI] [PubMed] [Google Scholar]
- Setola V, Roth BL. Screening the receptorome reveals molecular targets responsible for drug-induced side effects: Focus on “fen-phen“. Expert Opinion on Drug Metabolism and Toxicology. 2005;1:377–387. doi: 10.1517/17425255.1.3.377. [DOI] [PubMed] [Google Scholar]
- Seuwen K, Magnaldo I, Pouyssegur J. Serotonin stimulates DNA synthesis in fibroblasts acting through 5-HT1B receptors coupled to a Gi-protein. Nature. 1988;335:254–256. doi: 10.1038/335254a0. [DOI] [PubMed] [Google Scholar]
- Shearer J, Wodak A, van Beek I, Mattick RP, Lewis J. Pilot randomized double blind placebo-controlled study of dexamphetamine for cocaine dependence. Addiction. 2003;98:1137–1141. doi: 10.1046/j.1360-0443.2003.00447.x. [DOI] [PubMed] [Google Scholar]
- Sitte HH, Freissmuth M. Oligomer formation by Na+-Cl–coupled neurotransmitter transporters. European Journal of Pharmacology. 2003;479:229–236. doi: 10.1016/j.ejphar.2003.08.072. [DOI] [PubMed] [Google Scholar]
- Smith FL, Yu DS, Smith DG, Leccese AP, Lyness WH. Dietary tryptophan supplements attenuate amphetamine self-administration in the rat. Pharmacology, Biochemistry and Behavior. 1986;25:849–855. doi: 10.1016/0091-3057(86)90397-7. [DOI] [PubMed] [Google Scholar]
- Steinbusch HW. Distribution of serotonin-immunoreactivity in the central nervous system of the rat-cell bodies and terminals. Neuroscience. 1981;6:557–618. doi: 10.1016/0306-4522(81)90146-9. [DOI] [PubMed] [Google Scholar]
- Steranka LR, Sanders-Bush E. Long-term effects of fenfluramine on central serotonergic mechanisms. Neuropharmacology. 1979;18:895–903. doi: 10.1016/0028-3908(79)90088-1. [DOI] [PubMed] [Google Scholar]
- Sulzer D, Sonders MS, Poulsen NW, Galli A. Mechanisms of neurotransmitter release by amphetamines: A review. Progress in Neurobiology. 2005;75:406–433. doi: 10.1016/j.pneurobio.2005.04.003. [DOI] [PubMed] [Google Scholar]
- Torres GE, Amara SG. Glutamate and monoamine transporters: New visions of form and function. Current Opinion in Neurobiology. 2007;17:304–312. doi: 10.1016/j.conb.2007.05.002. [DOI] [PubMed] [Google Scholar]
- Uhl GR, Johnson PS. Neurotransmitter transporters: Three important gene families for neuronal function. Journal of Experimental Biology. 1994;196:229–236. doi: 10.1242/jeb.196.1.229. [DOI] [PubMed] [Google Scholar]
- Ungerstedt U. Stereotaxic mapping of the monoamine pathways in the rat brain. Acta Physiologica Scandinavica Supplement. 1971;367:1–48. doi: 10.1111/j.1365-201x.1971.tb10998.x. [DOI] [PubMed] [Google Scholar]
- Vickers SP, Clifton PG, Dourish CT, Tecott LH. Reduced satiating effect of d-fenfluramine in serotonin 5-HT(2C) receptor mutant mice. Psychopharmacology. 1999;143:309–314. doi: 10.1007/s002130050952. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Fowler JS, Wang GJ. Role of dopamine in drug reinforcement and addiction in humans: Results from imaging studies. Behavioural Pharmacology. 2002;13:355–366. doi: 10.1097/00008877-200209000-00008. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Li TK. Drug addiction: The neurobiology of behaviour gone awry. Nature Reviews: Neuroscience. 2004;5:963–970. doi: 10.1038/nrn1539. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Wang GJ, Fowler JS, Logan J, Gatley SJ, Hitzemann R, et al. Decreased striatal dopaminergic responsiveness in detoxified cocaine-dependent subjects. Nature. 1997;386:830–833. doi: 10.1038/386830a0. [DOI] [PubMed] [Google Scholar]
- Walsh SL, Haberny KA, Bigelow GE. Modulation of intravenous cocaine effects by chronic oral cocaine in humans. Psychopharmacology. 2000;150:361–373. doi: 10.1007/s002130000439. [DOI] [PubMed] [Google Scholar]
- Wee S, Anderson KG, Baumann MH, Rothman RB, Blough BE, Woolverton WL. Relationship between the serotonergic activity and reinforcing effects of a series of amphetamine analogs. Journal of Pharmacology and Experimental Therapeutics. 2005;313:848–854. doi: 10.1124/jpet.104.080101. [DOI] [PubMed] [Google Scholar]
- Weiss F, Parsons LH, Schulteis G, Hyytia P, Lorang MT, Bloom FE, et al. Ethanol self-administration restores withdrawal-associated deficiencies in accumbal dopamine and 5-hydroxytryptamine release in dependent rats. Journal of Neuroscience. 1996;16:3474–3485. doi: 10.1523/JNEUROSCI.16-10-03474.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White JM, Lopatko OV. Opioid maintenance: A comparative review of pharmacological strategies. Expert Opin Pharmacother. 2007;8:1–11. doi: 10.1517/14656566.8.1.1. [DOI] [PubMed] [Google Scholar]
- Wojnicki FHE, Rothman RB, Rice KC, Glowa JR. Effects of phentermine on responding maintained under multiple fixed-ratio schedules of food-presentation and cocaine-delivery in the rhesus monkey. Journal of Pharmacology and Experimental Therapeutics. 1999;288:550–560. [PubMed] [Google Scholar]
- Yu YL, Fisher H, Sekowski A, Wagner GC. Amphetamine and fenfluramine suppress ethanol intake in ethanol-dependent rats. Alcohol. 1997;14:45–48. doi: 10.1016/s0741-8329(96)00110-3. [DOI] [PubMed] [Google Scholar]
- Zaczek R, Battaglia G, Culp S, Appel NM, Contrera JF, De Souza EB. Effects of repeated fenfluramine administration on indices of monoamine function in rat brain: Pharmacokinetic, dose response, regional specificity and time course data. Journal of Pharmacology and Experimental Therapeutics. 1990;253:104–112. [PubMed] [Google Scholar]
- Zolkowska D, Rothman RB, Baumann MH. Amphetamine analogs increase plasma serotonin: Implications for cardiac and pulmonary disease. Journal of Pharmacology and Experimental Therapeutics. 2006;318:604–610. doi: 10.1124/jpet.106.101618. [DOI] [PubMed] [Google Scholar]
- Zolkowska D, Baumann MH, Rothman RB. Chronic fenfluramine administration increases plasma serotonin (5-HT) to non-toxic levels. Journal of Pharmacology and Experimental Therapeutics. 2008;324:791–797. doi: 10.1124/jpet.107.132654. [DOI] [PubMed] [Google Scholar]