Abstract
Gene transfer to the dorsal root ganglion using replication defective herpes simplex virus (HSV)-based vectors reduces pain related behaviors in rodent models of inflammatory pain, neuropathic pain, and pain caused by cancer in bone. HSV vectors engineered to produce inhibitory neurotransmitters including the delta opioid agonist peptide enkephalin, the mu opioid agonist peptide endomorphin-2 and glutamic acid decarboxylase (GAD) to effect the release of gamma amino butyric acid (GABA) act to inhibit nociceptive neurotransmission at the first synapse between primary nociceptive and second-order neuron in the dorsal horn of spinal cord. HSV vectors engineered to release anti-inflammatory peptides including interleukin (IL)-4, IL-10 and the p55 soluble tumor necrosis factor α (TNFα) receptor reduce neuroimmune activation in the spinal dorsal horn. The path leading from preclinical animal studies to the ongoing phase 1 human trial of the enkephalin-producing vector in patients with pain from cancer, and plans for an efficacy trial with an opioid producing vector in inflammatory pain and an efficacy trial with a GAD producing vector in diabetic neuropathic pain are outlined.
The development of novel effective treatments for chronic pain has been disappointingly slow. In part this is because the conservative use of a limited repertoire of neurotransmitters, receptors and ion channels at multiple sites and in many converging and diverging pathways serving different functions in the nervous system that limits the ability to use systemically administered small molecules to selectively interrupt nociceptive neurotransmission 1. To overcome this limitation we have constructed a series of non-replicating herpes simplex virus (HSV)-based vectors that efficiently target gene delivery to dorsal root ganglia (DRG) neurons from skin inoculation 2,3 to effect the release of antinociceptive neurotransmitters from afferent DRG terminals in a regionally restricted manner in the dorsal horn of spinal cord.
The neuroanatomic pathways involved in the complex process of pain perception are well established. Primary nociceptors are pseudounipolar neurons with cell bodies in the dorsal root ganglion and axons that terminate peripherally in the skin or organs and project centrally to terminate in the dorsal horn of spinal cord in an anatomically defined (dermatomal or radicular) pattern. Second order neurons project from the spinal cord principally to sensory nuclei in the thalamus. Third order neurons in the thalamus project to sensory cortex to subserve the discriminative aspects of pain perception, and to limbic cortex to subserve the hedonic aspects of the pain experience. Many disparate regions of brain are subsequently recruited. In addition to the ascending pathways, descending pathways that ultimately project from the brainstem back to the spinal dorsal horn act to modulate primary nociceptive neurotransmission at the first synapse in the dorsal horn.
Gene transfer methods may be used to achieve continuous focal expression of short-lived analgesic peptides at several sites in the ascending pain pathway, with the aim of selectively interrupting nociceptive neurotransmission while limiting the occurrence of off-target effects. In rodent models, alteration in nociception has been achieved by gene transfer into brain nuclei (rostral agranular insular cortex 4, amygdala 5), transduction of the meninges to result in release into cerebrospinal fluid 6-8, direct injection of vector into the dorsal horn of spinal cord 9, and transduction of DRG either by injection of vector directly into DRG 10 or into the intrathecal space 11. The first synapse between the primary nociceptor and the second order projection neuron in the spinal cord is a particularly attractive target because of the ability to selectively block nociception at that level thereby preventing the perception of pain 12. This site has been exploited using conventional analgesic drugs (e.g. morphine, baclofen) by delivery through chronic intrathecal infusion. Intrathecal administration results in a 10-fold reduction in dose requirement and a reduction in off-target adverse events. But even with this device-directed approach, problems remain in effectiveness and tolerance.
The first HSV vector tested with analgesic properties was a replication competent vector that expressed preproenkephalin to effect the release of enkephalin peptides from transduced neurons. Pohl and colleagues demonstrated that inoculation of the vector into the footpad results in strong expression of preproenkephalin mRNA in the dorsal root ganglion and a substantial increase in radio immunoassay detected met enkephalin like material in the DRG and in dorsal horn of spinal cord 13. Wilson, Yeomans and coworkers demonstrated that subcutaneous inoculation of the vector interferes with the hyperalgesic response of Aδ and C fibers sensitized with dimethylsulfoxide or capsaicin respectively 14. This was followed by the demonstration that inoculation of the vector into the foot reduces pain related behaviors in adjuvant induced polyarthritis in the rat 15. Our initial studies of the properties of a replication defective HSV vector expressing preproenkephalin demonstrated efficacy in the formalin test 16. We found that inoculation of the vector subcutaneously into the plantar surface of the hind foot reduces pain-related behaviors in the delayed phase of the formalin test. The vector mediated effect was maximal in animals inoculated with the vector one week prior to formalin test and diminished in magnitude over the course of the subsequent 3 weeks 16. Two lines of indirect evidence support the interpretation that the reduction in analgesic effectiveness of the vector is not subject to the development of tolerance to vector-mediated enkephalin released from the nerve terminals. First, reinoculation of the enkephalin expressing vector into the foot 4 weeks after the initial inoculation reestablished a substantial analgesic effect in animals tested one week after the reinoculation. Second, the time course of the effect produced by this vector, in which enkephalin expression is driven by the human cytomegalovirus immediate early promoter (HCMV IEp), is similar to the time course that we have observed in other experiments using the HCMV IEp to drive expression of other transgenes from non-replicating HSV vectors in vivo 17,18.
In the selective L5 spinal nerve ligation model of neuropathic pain, inoculation of the replication defective enkephalin expressing HSV vector into the foot one week after spinal nerve ligation results in a statistically significant attenuation in mechanical allodynia that persisted for several weeks 19. During that time, anti-allodynic effect of the vector is continuous throughout the day. As in the formalin model, the effect of vector-mediated enkephalin release persists for several weeks and is reestablished by reinoculation. Pharmacologically, the effect of vector mediated enkephalin is additive with systemically administered morphine, reducing the ED50 of morphine from 1.8 mg/kg in vehicle- or control-treated animals with spinal nerve ligation to 0.15 mg/kg in animals with spinal nerve ligation and inoculated with the enkephalin-expressing vector. We found that the vector mediated enkephalin effect persists in animals rendered tolerant to morphine by repeated twice daily administration of the drug 19. Finally, in a model of cancer related pain created by implantation of sarcoma cells into the medullary space of the femur, subcutaneous inoculation of the enkephalin-expressing HSV vector results in a significant naltrexone reversible decrease in pain related behavior assessed by open field motor activity 20.
In order to target the spinal mu opioid receptor we constructed an HSV vector to express endomorphin-2, a carboxy amidated tetrapeptide identified from bovine brain extracts as a highly selective mu opioid receptor ligand 21. The engineered expression cassette consists of the 99 N-terminal amino acid residues of the human preproenkephalin gene to direct the polypeptides into the secretory pathway for proteolytic processing, followed by a pair of endomorphin-2 coding elements each flanked by dibasic cleavage sites to provide for processing and peptide liberation by cellular proteases. Each endomomorphin-2 moiety contains a C-terminal glycine residue extension to direct processing by the ubiquitous peptidyl-glycine α amidating monooxygenase to yield C-terminal amidation of the cleaved peptide 22. Subcutaneous inoculation of the endomorphin expressing HSV vector into the footpad results in a substantial reduction in nocisponsive behaviors in response to mechanical and thermal stimuli in the complete Freund’s adjuvant (CFA) model of inflammatory pain (Figure 1) and reduced spontaneous pain behaviors in the delayed phase of the formalin test 23. In the selective spinal nerve ligation model of neuropathic pain, subcutaneous inoculation of the endomorphin-expressing HSV vector reduces both mechanical allodynia and thermal hyperalgesia 22, an effect that is similar in magnitude to the effect produced by the enkephalin expressing vector in the same model.
Figure 1.
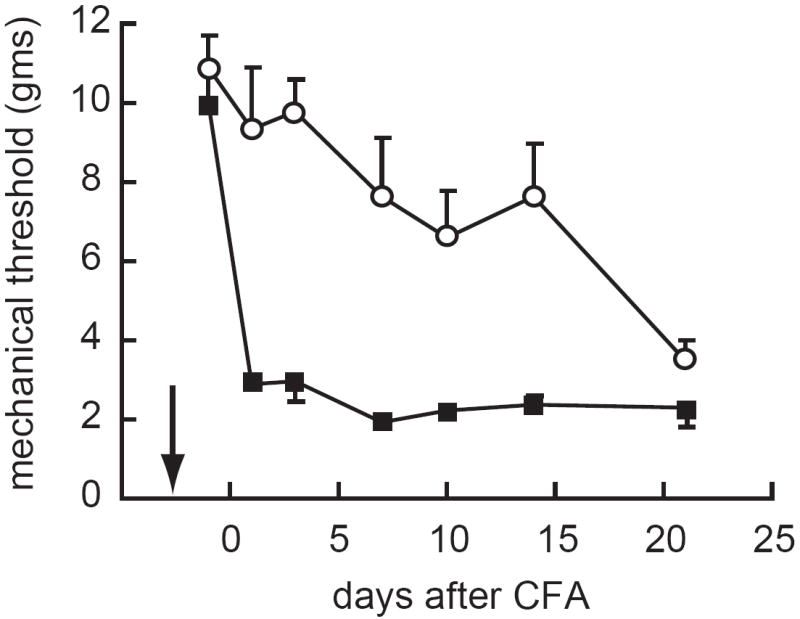
Subcutaneous inoculation of an HSV vector expressing endomorphin-2 reduces mechanical allodynia in the CFA model of chronic inflammatory pain. Arrow indicates time of vector inoculation. Reprinted from 23 with permission.
The anti-allodynic effects of the enkephalin- and endomorphin- expressing vectors in neuropathic pain, while statistically significant, are incomplete; an outcome that is not entirely unexpected given the limited effectiveness of opiate drugs in the treatment of neuropathic pain. Because of substantial evidence suggesting a selective loss of GABAergic inhibition in the dorsal horn of spinal cord in the setting of peripheral nerve injury we constructed an HSV vector to express glutamic acid decarboxylase, the enzyme that catalyzes the conversion of glutamate to GABA 24. For this purpose we used GAD67, the “glial” isoform of the enzyme that localizes in the cytoplasm and whose activity is relatively independent of regulatory controls. Infection of primary dorsal root ganglion neurons in culture with the GAD-expressing HSV vector results in the production of GAD67 protein and the constitutive release of GABA from the cells through the GABA transporter (GAT1) functioning in “reverse” to mediate release of GABA directly from the cytoplasm into the extracellular space 25. Subcutaneous inoculation of the GAD-expressing vector into rodent footpad resulted in gene delivery to DRG neurons, transport of GAD protein to the afferent nerve terminals in the dorsal horn of spinal cord, and constitutive release of GABA into the dorsal horn 24. In the selective L5 spinal nerve ligation model of neuropathic pain inoculation of the GAD- expressing vector produces an almost complete reversal of mechanical allodynia and a substantial reduction in thermal hyperalgesia 26 (Figure 2). The time course of the GAD-mediated effect is slightly longer than that produced by either the enkephalin or endomorphin expressing HSV vectors. This time course likely reflects the underlying mechanisms of inhibitory neurotransmitter production from the respective vectors. In the case of the opioid peptides, once gene expression ceases the peptides are no longer expressed; in the case of the GABA vector GAD protein produced from the vector will continue to generate GABA from glutamate for a time (related to the protein half-life) after gene expression is shut off. The GAD-expressing vector also produced a statistically significant reduction in mechanical allodynia and thermal hyperalgesia in the spinal cord hemisection model of below-level pain after spinal cord injury 24. The antiallodynic and anti-hyperalgesia effects of the vector were both reversed in part by intrathecal administration of either the GABAA receptor antagonist bicuculline and the GABAB receptor antagonist phaclofen, indicating that the effects of GABA released from the central terminals of the primary afferents are mediated in part by both GABAA and GABAB receptors 24.
Figure 2.
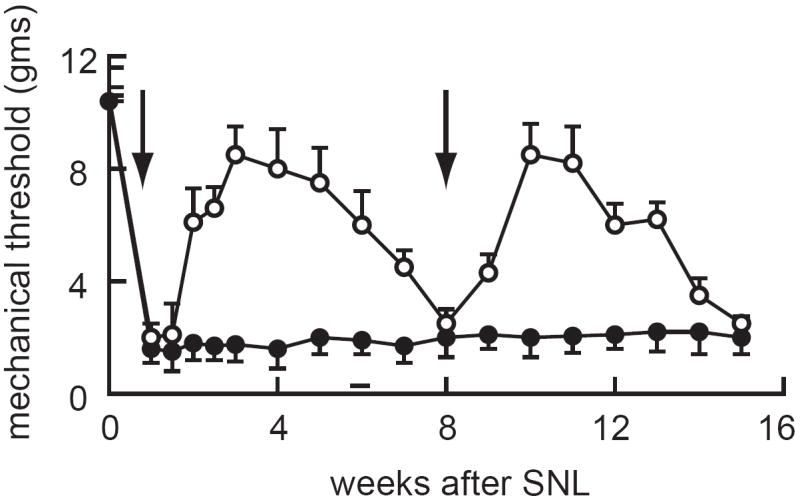
Subcutaneous inoculation of an HSV vector expressing GAD attenuates mechanical allodynia in the selective L5 spinal nerve ligation model of chronic neuropathic pain. Arrow indicates time of vector inoculation. Reprinted from 26 with permission.
Expression of inhibitory neurotransmitters represents an important avenue by which to interrupt nociceptive neurotransmission at the spinal level. An alternative approach involves the expression of peptides with anti-inflammatory activity. An emerging body of evidence suggests that chronic pain is associated with activation of microglia and astrocytes within the dorsal horn of spinal cord, and that this neuroimmune activation plays an important role in the biology of the chronic pain state 27 and may interact in a direct fashion with the therapeutic effect of opiate drugs 28. Like inhibitory neurotransmitters though, the systemic administration of potent anti-inflammatory peptides for the treatment of pain is likely to be problematic in a fashion that could be overcome by the local delivery of these anti-inflammatory peptides to the dorsal horn of spinal cord. We have examined this possibility with a series of vectors expressing peptides with anti-inflammatory activity.
Subcutaneous inoculation of a non-replicating HSV vector engineered to express the interleukin IL-4 has no effect on tactile threshold or thermal latency in normal animals, but inoculation one week after selective L5 spinal nerve ligation reduces mechanical allodynia and reversed thermal hyperalgesia resulting from the nerve injury 29. Vector mediated IL-4 expression prevents the increase in IL-1β, PGE2 and phosphorylated p38 MAP kinase (pP38) in the dorsal horn of spinal cord that is characteristic of neuropathic pain in this model 29. Inoculation of a nonreplicating HSV vector engineered to express IL-10 reduces pain related behaviors in the formalin model of inflammatory pain concomitant with a reduction in pP38 and decreased expression of the full length membrane spanning precursor of tumor necrosis factor α (mTNFα) in spinal microglia 30. Direct interference with TNFα signaling by expression of a truncated form of the p55 TNFα receptor (p55 sTNFR) from a nonreplicating herpes vector inoculated into the skin results in a significant reduction in mechanical allodynia and thermal hyperalgesia in the selective L5 spinal nerve ligation model of neuropathic pain, concomitant with a reduction in pP38 and IL-1β in the dorsal horn, as well as a reduction in the expression of mTNFα in the spinal cord 31. Similar results were observed in the T13 lateral hemisection model of below-level spinal cord injury pain where subcutaneous inoculation of the p55 sTNFR- expressing vector attenuated mechanical allodynia, reduced phosphorylation of p38 and decreased expression of mTNFα in the dorsal horn 32. In the immune system mTNFα is capable of mediating bidirectional cell-cell interactions through binding directly to the membrane receptor complex (p55 or p75 TNFR) with both “forward” signaling through the receptor complex and “reverse” signaling in the ligand-expressing cell activated by the receptor. Downregulation of spinal mTNFα by vector-mediated expression of p55 sTNFR suggested that microglia mTNFα may play a similar reverse signaling role in the pathogenesis of neuropathic pain.
HSV vectors inoculated into the skin for the treatment of pain are likely to be safe. There is substantial clinical evidence from human studies involving injection of replication-competent oncolytic HSV delivered into tumors of brain (glioblastoma), breast, liver, or skin in which the virus has been shown to be well tolerated and safe with no serious adverse events (SAEs) reported even with intracerebral inoculation 33. These viruses did not reactivate endogenous HSV 34,35, did not cause encephalitis 34,36 nor spread appreciably from the site of inoculation 34,37. Generation of anti-HSV antibodies in patients is routinely reported but these responses are not robust. In addition several clinical studies have been completed that used recombinant replication-competent HSV as a potential vaccine. The HSV-1 recombinant R7020 (now known as NV1020) was found safe and well tolerated in a Phase 1 vaccine study 38, and the HSV-2 based vector ICP10DPK was found safe and well tolerated in a Phase 1/2 trial carried out in Mexico 39. Since no previous studies have been performed using nonreplicating HSV vectors carrying therapeutic transgenes, we elected to move forward with a phase 1 trial in patients with pain from terminal cancer. We judged that this group of patients represents an appropriate population for a true phase 1 trial of this novel agent to establish the safety profile of the vector platform. Of the several different HSV-based vectors that we have tested in the osteolytic sarcoma model of pain from cancer, the analgesic effect of the enkephalin-expressing vector is as robust as that produced by any other vector and we proceeded to develop the enkephalin-expressing vector for the human trial.
The first HSV vectors to be used in models of pain were deleted for accessory viral functions (e.g. thymidine kinase, tk) that are important to virus replication in neurons and thus contribute to neurovirulence. Recombinant HSV defective in tk can be propagated in culture and will replicate in skin, but are unable to replicate in the DRG and are thus forced into a pseudo-latent state. The vectors employed in our rodent studies were rendered replication defective by removal of essential virus genes (coding infected cell polypeptides (ICP) ICP4, ICP4 and ICP27, or ICP4, ICP27, ICP22 and ICP47 2. These recombinants are propagated in cells that provide the missing gene product(s) in-trans 2,40. While these vectors are completely incapable of replication in vivo, the recombinant particles retain the targeting properties of the wild-type virus and can be used to effectively deliver genes from the skin to the sensory ganglion where the vector establishes a persistent state in which no lytic viral genes are expressed.
To produce a vector appropriate for human use, the genomic HSV backbone and the complementing cell lines had to be re-engineered. The critical deletions in the essential immediate early HSV genes ICP4 and ICP27 in the vectors employed in the original preclinical animal studies do not delete the entire ICP4 or ICP27 open reading frames, but require a complementing cell line that contains the complete ICP4 and ICP27 coding regions in order to support virus growth. The resultant residual homology between the vector and cell line leads to very low frequency rescue of the deletion(s) as the virus is propagated. To produce a high titer vector stock while eliminating the potential of homologous recombination producing replication competent recombinants that would be appropriate for human use the replication defective HSV backbone was reengineered to completely remove homology between the cell line and vector genomes (Figure 3). The expanded ICP4 deletion in the clinically viable vector backbone also removes the upstream promoter elements of immediate early genes ICP22 and ICP47 that restricts the expression of those genes to complementing cells. The complementing cell line was also re-engineered by separately introducing the HSV genes ICP4 and ICP27 stably into the genome of ATCC Vero cells. The clinical-grade replication defective ICP4 and ICP27 deleted HSV vector engineered to express human preproenkephalin was termed NP2.
Figure 3.
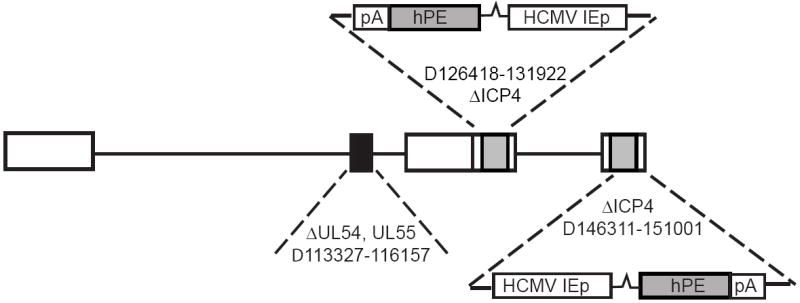
Schematic of the nonreplicating HSV vector backbone approved by the FDA for human use.
Toxicology and biodistribution studies of the NP2 enkephalin-expressing vector were carried out according to FDA guidance. Four groups of 80 mice (40 male, 40 female) were dosed subcutaneously on study day 0. Group 1 received a single dose of PBS. Groups 2-4 were injected with NP2 at (respectively) 1 × 103, 1 × 105, and 1 × 107pfu/animal. Animals (10 mice/sex/group/time point) were sacrificed on days 1, 7, 28, and 91 (one day, one week, one month, and three months) post-dosing. There was no evidence of adverse treatment-related effects of the test article as judged by clinical observations, body weight, or food consumption. Clinical pathology revealed no significant changes in hematology or clinical chemistry parameters at each time point. There were no treatment-related abnormalities in organ weights and macroscopic pathology. Histopathology examination revealed no test article treatment-related microscopic changes. Incidental and or spontaneous histological changes were noted in, heart, sciatic nerve, spinal cord, injection site dorsal root ganglia, and optic nerve in both control and test article-treated groups; therefore, these findings were judged not to be test article-related. Based on the predefined parameters of the toxicology study, administration of the test article according to the conditions of this study was well tolerated with no significant toxicity.
A total of 2400 tissues harvested from the in-life phase of the biodistribution study were examined for vector distribution. Using vector-specific primers a GLP qualified QPCR assay was developed and DNA extraction efficiencies of target tissues performed. This assay has a limit of quantification of 20 copies and a limit of detection of between 1 and 5 copies. All tissues from the day 1 and day 7 cohorts were examined, as well as tissues from 10 animals of each sex from the control (PBS) group. In agreement with FDA guidance, because there was evidence of vector only at the injection site, innervating DRG, and underlying muscle in some day 1 and day 7 samples, only these tissues were analyzed in the day 28 and day 90 cohorts. A GAPDH normalization assay was included in separate reaction in order to calculate the total number of rat genomes present in the sample. All HSV and GAPDH target assays were run in duplicate. Each test included a negative control (no template) and positive control (target plasmid spike) to test for non-target amplification and PCR inhibition, respectively. All samples were amplified using the Applied Biosystems Prism 7900 Sequence Detection System with standard cycling conditions for 45 cycles in a 50 μl reaction volume. No PCR inhibition was observed in any samples. No amplification was observed in negative control reactions. Control (PBS) injected animals did not show positive amplification in any case. A total of 1180 tissues were evaluated by QPCR including all tissues from NP2 injected day 1 and 7 cohorts. A total of 27 tissues were found to have quantifiable vector sequences. These samples were the injection site, underlying muscle, and associated DRG; there was no quantifiable dissemination to any other tissue.
The human clinical trial was initially presented to the Recombinant DNA Advisory Committee (NIH). Following completion of the contract toxicology and biodistribution studies an IND application for the phase 1 trial of the replication incompetent HSV vector in patients with pain due to malignancy was approved by the FDA on February 9, 2008, and approved by the Institutional Review Board at the University of Michigan in July, 2008. The study was opened for enrollment September 15, 2008. The trial is a single-center, open-label, escalating dose trial designed to evaluate the safety of intradermal injection of the enkephalin-expressing vector in patients who have intractable pain due to malignant disease anatomically located below the angle of the jaw. Patients with intractable pain from malignant disease that is moderate to severe (≥40 on a 100 mm VAS at Screening) despite: (a) stable analgesic treatment with at least 200mg/day morphine or equivalent; or (b) having reached a stable maximum tolerable dose of narcotic or other analgesics that is less that 200mg/day morphine or equivalent will be eligible to participate. The vector will be administered in 10 injections of 0.1 ml each within the dermatome(s) corresponding to the radicular distribution of the pain. Three escalating dose levels with 3 to 6 patients at each dose level will be administered until i) the maximum tolerated dose (MTD), defined as the highest dose with <= 1/6 patients having DLTs or ii) highest dose level is attained. The trial also contains an optional extension to recruit an additional 12 patients in order to investigate efficacy potential. The primary outcome of this phase 1 safety/dose-finding trial will be adverse events, defined by history, physical examination and laboratory assessment. Efficacy will be assessed as a secondary outcome by a numeric rating scale of pain intensity, the short form of the McGill Pain Questionnaire, performance status measured by the Eastern Cooperative oncology Group Performance Status measure, a 12 item short form health survey (SF-12) and the concurrent use of pain medications.
Based on the preclinical testing animal toxicology and biodistribution studies of NP2 and the extensive data from attenuated oncolytic herpes viruses that have been tested in patients we are relatively confident that NP2 will prove to be safe in the ongoing Phase 1 study, but the design of this study with only 3 patients in each group and no placebo-control will make it difficult to realistically assess treatment efficacy. Efficacy will need to be tested in a subsequent phase 2 trial of inflammatory pain; chronic intractable focal pain from arthritis would appear to represent a reasonable target population for this study. Beyond inflammatory pain, chronic neuropathic pain represents a large unmet need for which there are few available effective therapies. In the case of painful diabetic neuropathy for example, available medical treatments rarely reduce pain below a rating of 4 on a 0-10 analog pain scale, and only 30-40% of patients achieve a 50% reduction in pain beyond placebo effect 41. Because the GAD-expressing HSV vector is substantially more effective than the opioid peptide- (enkephalin or endomorphin) expressing vectors in the spinal nerve ligation model of neuropathic pain we have engineered and begun testing of a clinical-grade GAD-expressing HSV vector that, contingent on successful completion of the ongoing phase 1 study of NP2, could be tested in a phase 2 trial of patients with painful diabetic neuropathy. The outcome of these two phase 2 trials will represent an important decision point in the development of HSV vectors as a treatment for pain.
Acknowledgments
We acknowledge the substantial contributions of many collaborators in the work reviewed including: Joseph Glorioso, Bill Goins and James Goss (University of Pittsburgh), Jim Wechuck and David Krisky (Diamyd Incorporated), Shuanglin Hao, Munmun Chattopadhyay, Zhigang Zhou and Xiangmin Peng (University of Michigan) and Jun Liu (deceased). This work was supported by NIH grants NS044507, NS038850, DK044935, grants from the Department of Veterans Affairs and the Juvenile Diabetes Research Foundation. Darren Wolfe is an employee of Diamyd Incorporated, a wholly owned subsidiary of Diamyd Medical, which is sponsoring the human trial of HSV-mediated gene transfer for pain. David Fink receives research funding from Diamyd in support of that trial; he has no equity interest in or other financial relationship with Diamyd.
Footnotes
Dr. Mata has no conflict of interest to declare.
References
- 1.Mata M, Hao S, Fink DJ. Applications of gene therapy to the treatment of chronic pain. Curr Gene Ther. 2008;8:42–48. doi: 10.2174/156652308783688527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wolfe D, Goins WF, Yamada M, Moriuchi S, Krisky DM, Oligino TJ, et al. Engineering herpes simplex virus vectors for CNS applications. Exp Neurol. 1999;159:34–46. doi: 10.1006/exnr.1999.7158. [DOI] [PubMed] [Google Scholar]
- 3.Glorioso JC, Fink DJ. Herpes vector-mediated gene transfer in treatment of diseases of the nervous system. Annu Rev Microbiol. 2004;58:253–271. doi: 10.1146/annurev.micro.58.030603.123709. [DOI] [PubMed] [Google Scholar]
- 4.Jasmin L, Rabkin SD, Granato A, Boudah A, Ohara PT. Analgesia and hyperalgesia from GABA-mediated modulation of the cerebral cortex. Nature. 2003;424:316–320. doi: 10.1038/nature01808. [DOI] [PubMed] [Google Scholar]
- 5.Kang W, Wilson MA, Bender MA, Glorioso JC, Wilson SP. Herpes virus-mediated preproenkephalin gene transfer to the amygdala is antinociceptive. Brain Res. 1998;792:133–135. doi: 10.1016/s0006-8993(98)00194-2. [DOI] [PubMed] [Google Scholar]
- 6.Finegold AA, Mannes AJ, Iadarola MJ. A paracrine paradigm for in vivo gene therapy in the central nervous system: treatment of chronic pain. Hum Gene Ther. 1999;10:1251–1257. doi: 10.1089/10430349950018238. [DOI] [PubMed] [Google Scholar]
- 7.Ledeboer A, Jekich BM, Sloane EM, Mahoney JH, Langer SJ, Milligan ED, et al. Intrathecal interleukin-10 gene therapy attenuates paclitaxel-induced mechanical allodynia and proinflammatory cytokine expression in dorsal root ganglia in rats. Brain Behav Immun. 2007;21:686–698. doi: 10.1016/j.bbi.2006.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Milligan ED, Soderquist RG, Malone SM, Mahoney JH, Hughes TS, Langer SJ, et al. Intrathecal polymer-based interleukin-10 gene delivery for neuropathic pain. Neuron Glia Biol. 2006;2:293–308. doi: 10.1017/S1740925X07000488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Meunier A, Latremoliere A, Dominguez E, Mauborgne A, Philippe S, Hamon M, et al. Lentiviral-mediated targeted NF-kappaB blockade in dorsal spinal cord glia attenuates sciatic nerve injury-induced neuropathic pain in the rat. Mol Ther. 2007;15:687–697. doi: 10.1038/sj.mt.6300107. [DOI] [PubMed] [Google Scholar]
- 10.Xu Y, Gu Y, Xu GY, Wu P, Li GW, Huang LY. Adeno-associated viral transfer of opioid receptor gene to primary sensory neurons: A strategy to increase opioid antinociception. Proc Natl Acad Sci U S A. 2003;100:6204–6209. doi: 10.1073/pnas.0930324100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Storek B, Reinhardt M, Wang C, Janssen WG, Harder NM, Banck MS, et al. Sensory neuron targeting by self-complementary AAV8 via lumbar puncture for chronic pain. Proc Natl Acad Sci U S A. 2008;105:1055–1060. doi: 10.1073/pnas.0708003105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yaksh TL, Rudy TA. Analgesia mediated by a direct spinal action of narcotics. Science. 1976;192:1357–1358. doi: 10.1126/science.1273597. [DOI] [PubMed] [Google Scholar]
- 13.Antunes Bras JM, Epstein AL, Bourgoin S, Hamon M, Cesselin F, Pohl M. Herpes simplex virus 1-mediated transfer of preproenkephalin A in rat dorsal root ganglia. J Neurochem. 1998;70:1299–1303. doi: 10.1046/j.1471-4159.1998.70031299.x. [DOI] [PubMed] [Google Scholar]
- 14.Wilson SP, Yeomans DC, Bender MA, Lu Y, Goins WF, Glorioso JC. Antihyperalgesic effects of infection with a preproenkephalin-encoding herpes virus. Proc Natl Acad Sci U S A. 1999;96:3211–3216. doi: 10.1073/pnas.96.6.3211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Braz J, Beaufour C, Coutaux A, Epstein AL, Cesselin F, Hamon M, et al. Therapeutic efficacy in experimental polyarthritis of viral-driven enkephalin overproduction in sensory neurons. J Neurosci. 2001;21:7881–7888. doi: 10.1523/JNEUROSCI.21-20-07881.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Goss JR, Mata M, Goins WF, Wu HH, Glorioso JC, Fink DJ. Antinociceptive effect of a genomic herpes simplex virus-based vector expressing human proenkephalin in rat dorsal root ganglion. Gene Ther. 2001;8:551–556. doi: 10.1038/sj.gt.3301430. [DOI] [PubMed] [Google Scholar]
- 17.Puskovic V, Wolfe D, Goss J, Huang S, Mata M, Glorioso JC, et al. Prolonged biologically active transgene expression driven by HSV LAP2 in brain in vivo. Mol Ther. 2004;10:67–75. doi: 10.1016/j.ymthe.2004.04.004. [DOI] [PubMed] [Google Scholar]
- 18.Chattopadhyay M, Wolfe D, Mata M, Huang S, Glorioso JC, Fink DJ. Long-term neuroprotection achieved with latency-associated promoter-driven herpes simplex virus gene transfer to the peripheral nervous system. Mol Ther. 2005;12:307–313. doi: 10.1016/j.ymthe.2005.04.009. [DOI] [PubMed] [Google Scholar]
- 19.Hao S, Mata M, Goins W, Glorioso JC, Fink DJ. Transgene-mediated enkephalin release enhances the effect of morphine and evades tolerance to produce a sustained antiallodynic effect. Pain. 2003;102:135–142. doi: 10.1016/s0304-3959(02)00346-9. [DOI] [PubMed] [Google Scholar]
- 20.Goss JR, Harley CF, Mata M, O’Malley ME, Goins WF, Hu X-P, et al. Herpes vector-mediated expression of proenkephalin reduces pain-related behavior in a model of bone cancer pain. Ann Neurol. 2002;52:662–665. doi: 10.1002/ana.10343. [DOI] [PubMed] [Google Scholar]
- 21.Zadina JE, Hackler L, Ge LJ, Kastin AJ. A potent and selective endogenous agonist for the mu-opiate receptor. Nature. 1997;386:499–502. doi: 10.1038/386499a0. [DOI] [PubMed] [Google Scholar]
- 22.Wolfe D, Hao S, Hu J, Srinivasan R, Goss J, Mata M, et al. Engineering an endomorphin-2 gene for use in neuropathic pain therapy. Pain. 2007;133:29–38. doi: 10.1016/j.pain.2007.02.003. [DOI] [PubMed] [Google Scholar]
- 23.Hao S, Wolfe D, Glorioso JC, Mata M, Fink DJ. Effects of transgene-mediated endomorphin-2 in inflammatory pain. Eur J Pain. 2008 doi: 10.1016/j.ejpain.2008.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Liu J, Wolfe D, Hao S, Huang S, Glorioso JC, Mata M, et al. Peripherally delivered glutamic acid decarboxylase gene therapy for spinal cord injury pain. Mol Ther. 2004;10:57–66. doi: 10.1016/j.ymthe.2004.04.017. [DOI] [PubMed] [Google Scholar]
- 25.Liu J, Tai C, de Groat WC, Peng XM, Mata M, Fink DJ. Release of GABA from sensory neurons transduced with a GAD67-expressing vector occurs by non-vesicular mechanisms. Brain Res. 2006;1073-1074:297–304. doi: 10.1016/j.brainres.2005.12.091. [DOI] [PubMed] [Google Scholar]
- 26.Hao S, Mata M, Wolfe D, Glorioso JC, Fink DJ. Gene transfer of glutamic acid decarboxylase reduces neuropathic pain. Ann Neurol. 2005;57:914–918. doi: 10.1002/ana.20483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.DeLeo JA, Tawfik VL, Lacroix-Fralish ML. The tetrapartite synapse: Path to CNS sensitization and chronic pain. Pain. 2006;112:17–21. doi: 10.1016/j.pain.2006.02.034. [DOI] [PubMed] [Google Scholar]
- 28.Watkins LR, Hutchinson MR, Milligan ED, Maier SF. “Listening” and “talking” to neurons: implications of immune activation for pain control and increasing the efficacy of opioids. Brain Res Rev. 2007;56:148–169. doi: 10.1016/j.brainresrev.2007.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hao S, Mata M, Glorioso JC, Fink DJ. HSV-mediated expression of interleukin-4 in dorsal root ganglion neurons reduces neuropathic pain. Mol Pain. 2006;2:6. doi: 10.1186/1744-8069-2-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhou Z, Peng X, Hao S, Fink DJ, Mata M. HSV-mediated transfer of interleukin-10 reduces inflammatory pain through modulation of membrane tumor necrosis factor alpha in spinal cord microglia. Gene Ther. 2008;15:183–190. doi: 10.1038/sj.gt.3303054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hao S, Mata M, Glorioso JC, Fink DJ. Gene transfer to interfere with TNFalpha signaling in neuropathic pain. Gene Ther. 2007;14:1010–1016. doi: 10.1038/sj.gt.3302950. [DOI] [PubMed] [Google Scholar]
- 32.Peng X, Zhou Z, Glorioso JC, Fink DJ, Mata M. Tumor necrosis factor alpha contributes to below-level neuropathic pain after spinal cord injury. Ann Neurol. 2006;59:843–851. doi: 10.1002/ana.20855. [DOI] [PubMed] [Google Scholar]
- 33.Harrow S, Papanastassiou V, Harland J, Mabbs R, Petty R, Fraser M, et al. HSV1716 injection into the brain adjacent to tumour following surgical resection of high-grade glioma: safety data and long-term survival. Gene Ther. 2004;11:1648–1658. doi: 10.1038/sj.gt.3302289. [DOI] [PubMed] [Google Scholar]
- 34.Rampling R, Cruickshank G, Papanastassiou V, Nicoll J, Hadley D, Brennan D, et al. Toxicity evaluation of replication-competent herpes simplex virus (ICP 34.5 null mutant 1716) in patients with recurrent malignant glioma. Gene Ther. 2000;7:859–866. doi: 10.1038/sj.gt.3301184. [DOI] [PubMed] [Google Scholar]
- 35.Nakao A, Kimata H, Imai T, Kikumori T, Teshigahara O, Nagasaka T, et al. Intratumoral injection of herpes simplex virus HF10 in recurrent breast cancer. Ann Oncol. 2004;15:988–989. doi: 10.1093/annonc/mdh225. [DOI] [PubMed] [Google Scholar]
- 36.Markert JM, Medlock MD, Rabkin SD, Gillespie GY, Todo T, Hunter WD, et al. Conditionally replicating herpes simplex virus mutant, G207 for the treatment of malignant glioma: results of a phase I trial. Gene Ther. 2000;7:867–874. doi: 10.1038/sj.gt.3301205. [DOI] [PubMed] [Google Scholar]
- 37.Papanastassiou V, Rampling R, Fraser M, Petty R, Hadley D, Nicoll J, et al. The potential for efficacy of the modified (ICP 34.5(-)) herpes simplex virus HSV1716 following intratumoural injection into human malignant glioma: a proof of principle study. Gene Ther. 2002;9:398–406. doi: 10.1038/sj.gt.3301664. [DOI] [PubMed] [Google Scholar]
- 38.Ramachandran S, Kinchington PR. Potential prophylactic and therapeutic vaccines for HSV infections. Curr Pharm Des. 2007;13:1965–1973. doi: 10.2174/138161207781039760. [DOI] [PubMed] [Google Scholar]
- 39.Casanova G, Cancela R, Alonzo L, Benuto R, Magana Mdel C, Hurley DR, et al. A double-blind study of the efficacy and safety of the ICP10deltaPK vaccine against recurrent genital HSV-2 infections. Cutis. 2002;70:235–239. [PubMed] [Google Scholar]
- 40.DeLuca NA, McCarthy AM, Schaffer PA. Isolation and characterization of deletion mutants of herpes simplex virus type 1 in the gene encoding immediate-early regulatory protein ICP4. J Virol. 1985;56:558–570. doi: 10.1128/jvi.56.2.558-570.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Turk DC. Clinical effectiveness and cost-effectiveness of treatments for patients with chronic pain. Clin J Pain. 2002;18:355–365. doi: 10.1097/00002508-200211000-00003. [DOI] [PubMed] [Google Scholar]


