Abstract
Unlike adult heterogeneous rats, infant rats are sensitive to ethanol's locomotor stimulating effects. Susceptibility to this ethanol effect varies as a function of baseline locomotor activity levels. Infant rats with higher baseline activity levels are more sensitive to ethanol's stimulating effects than those with lower baseline activity levels. The present study was designed to analyze susceptibility to ethanol-induced motivational learning in subpopulations of infant heterogeneous rats that differ in baseline activity in a novel environment. On postnatal day 11 (PD 11) baseline locomotor activity was registered and infants were divided into high and low responders (HR, LR). In Experiment 1, pups were trained in a procedure of conditioned taste aversion employing ethanol (0.0, 0.5 or 2.5 g/kg) as unconditioned stimulus (US) and saccharin as conditioned stimulus. In Experiment 2 the same procedure was employed with LiCl (0.0, 0.25 or 0.5 % of body weight of a 0.3 M LiCl solution) as US. HR were more resistant to the aversive effects of ethanol than LR while magnitude of LiCl-induced conditioned taste aversion was similar in HR and LR. These results suggest the possibility of early detection of subpopulations of rats with differential sensitivity to ethanol's effects.
During the first two postnatal weeks of life, preweanling rats are highly sensitive to ethanol's effects (we consider preweanling the period before the young animal is weaned, usually beginning at birth and including the neonatal period). Voluntary ethanol consumption is higher in 8- and 12-day-old infant rats than in later stages of development (Sanders & Spear, 2007;Truxell & Spear, 2004;Truxell, Molina, & Spear, 2007). In addition, during the first and second postnatal weeks infant rats are predisposed to acquire appetitive reinforcement mediated by ethanol (Arias & Chotro, 2006a;Chotro & Arias, 2007;Molina, Pautassi, Truxell, & Spear, 2007;Petrov, Varlinskaya, & Spear, 2003) and seem more resistant than older rats to aversive consequences of the drug (Arias & Chotro, 2006a;Chotro & Arias, 2007;Hunt, Spear, & Spear, 1991). Acute tolerance to motor impairment (sedation) effects of ethanol is more marked in infant than adult heterogeneous rats (Arias, Molina, Mlewski, Pautassi, & Spear, 2008;Silveri & Spear, 2001). Finally, unlike adult rats (Chuck, McLaughlin, Arizzi-LaFrance, Salamone, & Correa, 2006;Erickson & Kochhar, 1985), at least during the second postnatal week of postnatal life heterogeneous rats are sensitive to ethanol's locomotor stimulating effects (Arias, Mlewski, Molina, & Spear, 2009a; Arias, Mlewski, Molina, & Spear, 2009b;Arias et al., 2008). This effect was clearly observed when infant rats received a relatively high ethanol dose (1.25 or 2.5 g/kg) and were tested in a novel environment during the rising phase of the blood ethanol curve. The post-administration interval in which preweanling rats display ethanol-induced locomotor activation coincides with that in which ethanol induced conditioned appetitive responses (Molina et al., 2007). This temporal coincidence suggests a possible association between ethanol's activating effects and positive motivational effects of the drug during early ontogeny in the rat.
In several animal models, increased susceptibility to ethanol's reinforcing or locomotor stimulating effects is accompanied by lower sensitivity to ethanol-induced aversive effects. For example, inbred rat strains genetically selected for increased ethanol consumption, such as Alcohol-preferring (P), University of Chile B (UChB), Alko-alcohol (AA), Marchigian Sardinian (MsP) and Sardinian alcohol-preferring (sP) rats, are less sensitive to ethanol's aversive effects and more sensitive to ethanol-induced locomotor stimulation than strains selected for low ethanol intake (Bell, Rodd, Lumeng, Murphy, & McBride, 2006;Ciccocioppo et al., 2006;Colombo et al., 1998;Colombo, Lobina, Carai, & Gessa, 2006;Quintanilla, Israel, Sapag, & Tampier, 2006;Sommer, Hyytia, & Kiianmaa, 2006). Mice selectively bred for sensitivity to ethanol-induced locomotor stimulation (FAST and SLOW mice) also differ in susceptibility to ethanol-induced conditioned taste aversion, with FAST mice more resistant than SLOW mice to the aversive effects of ethanol (Risinger, Malott, Prather, Niehus, & Cunningham, 1994). Finally, adult rats with high baseline locomotor activity levels (high responders, HR) are also more sensitive to ethanol's activating effects than those with low baseline activity (low responders, LR: Cools & Gingras, 1998;Gingras & Cools, 1996;Hoshaw & Lewis, 2001). We didn't find studies analyzing sensitivity to ethanol-induced taste aversion as a function of locomotor activity in a novel environment, but it has been found that HR are more resistant to the aversive effects of amphetamine than LR (Kunin, Gaskin, Borjas, Smith, & Amit, 2001). Studies that have examined ethanol self-administration in these subpopulations of heterogeneous rats (HR and LR) have not agreed about their conclusions. Although a positive relationship between ethanol self-administration and response to novelty was reported in a few studies (Nadal, Armario, & Janak, 2002;Nowak et al., 2000), other studies failed to find this relationship (for example, Bienkowski, Koros, & Kostowski, 2001;Fahlke, Hard, Eriksson, Engel, & Hansen, 1995;Samson & Chappelle, 1995), while another reported a negative association (Cools & Gingras, 1998).
Similar to what has been observed in adult rats (Cools & Gingras, 1998;Hoshaw & Lewis, 2001), in 12-day-old rats susceptibility to ethanol's activating effects also varies as a function of baseline locomotor activity levels. Infant rats with higher baseline activity levels are more sensitive to ethanol's stimulating effects than those with lower baseline activity levels (Arias, Mlewski, Miller, Molina, & Spear, 2009). In the study by Arias et al. (2009) baseline activity did not predict other ethanol effects, such as hypothermia, motor impairment or motor suppressive effects of the drug. This result opened the possibility of early detection of subpopulations of heterogeneous rats with differential sensitivity to ethanol's effects.
The present study was designed to analyze susceptibility to ethanol-induced aversive learning among infant heterogeneous rats in accord with their baseline activity in a novel environment. If an association between response to a novel environment and susceptibility to ethanol-mediated aversive learning is observed, the present study could lead to early detection of traits that help predict differential response to ethanol. On postnatal day 11 (PD 11) baseline locomotor activity in a novel location was registered and infants were divided into HR and LR. In Experiment 1, pups were trained in a procedure of conditioned taste aversion employing ethanol as unconditioned stimulus (US) and saccharin as conditioned stimulus (CS, Experiment 1). Conditioned taste aversion is a paradigm commonly employed for assessment of motivational properties of drugs of abuse, including ethanol (Hunt and Amit, 1987;Parker, 1995). In this experimental paradigm animals learn to avoid a tastant (conditioned stimulus, CS) previously paired with ethanol's postabsorptive effects (US). Ethanol-mediated taste aversion is encountered in adult mice (Broadbent, Muccino, & Cunningham, 2002) and adult rats (Cailhol & Mormede, 2002;Eckardt, 1976) as well as infant rats (Arias & Chotro, 2006a, 2006b; P. S. Hunt, Molina, Spear, & Spear, 1990; P. S. Hunt et al., 1991). In Experiment 2 the same procedure was employed with LiCl as US to test whether differences between infant HR and LR in the acquisition of conditioned taste aversion depend on the US employed. Adult HR and LR do not differ in terms of sensitivity to the aversive effects of LiCl (Kunin et al., 2001).
Experiments 1 and 2
Material and Methods
Subjects
Sixty-five Sprague-Dawley pups (34 females and 31 males), representative of 11 litters, were utilized for Experiment 1, and sixty-six (33 males and 33 females) derived from another 11 litters were employed for Experiment 2. Animals were born and reared at the vivarium of the Center for Developmental Psychobiology (Binghamton University, NY) under conditions of constant room temperature (22 ± 1.0 °C), on a 12-hour light 12-hour dark cycle. Births were examined daily and the day of parturition was considered as postnatal day 0 (PD0). All litters were culled to 10 pups (5 females and 5 males, whenever possible) within 48 hours after birth. All procedures were in accordance with the guidelines for animal care and use established by the National Institute of Health (1986) and the Guide for Care and Use of Laboratory Animals (Institute of Laboratory Animal Resources, 1996) as indicated by the Binghamton University's institutional animal care and use committee.
Procedures
Phase 1: Baseline activity
On PD 11, pups from a given litter were separated from their mothers and placed in couples in a holding maternity cage (45 × 20 × 20 cm) partially filled with clean wood shavings. The floor of the cage was maintained at 33° C (± 1° C) through the use of a heating pad. One hour later, locomotor activity was evaluated in a novel Plexiglas container (10 × 10 × 12 cm). The floor of this environment was lined with absorbent paper. A fresh piece of paper was employed for each animal. A circuit board (2-cm in width) surrounded the four sides of each chamber. This board had six infrared photo emitters and six infrared photoreceptors. The photo beams crossed the chamber generating a matrix of nine cells that allowed measurement of overall activity. Custom-made software developed by W. Kashinsky served to analyze the number of beams crossed by each subject every 10th of a second. Each activity test continued for 5 min and data were divided into 1-min bins. The dependent variable analyzed (number of beams broken) may reflect locomottion and other kind of behaviors as well, such us stereotypy. Stereotypy was not measured in this study. In a prior pilot study, the number of beams broken was highly and positively correlated with time walking and wall climbing during the second postnatal week of life, holding experimental conditions constant (PD8: rxy = 0.89, n=15; PD12: rxy = 0.85, n=15; PD15: rxy =0.86, n=15, all ps < 0.0001; rxy represents Pearson's product-moment correlation coefficient). At these ages we rarely observe stereotypy, particularly in baseline conditions.
For the second phase of the experiment, from each litter we selected three pups with the highest and three pups with the lowest locomotor activity scores, to be composed toward detection of differences in response to ethanol as a function of baseline activity.
Phase 2: Conditioned taste aversion training
On PDs 12 and 13, those six pups (with the highest and lowest baseline activity scores) from a given litter selected in the previous phase were separated from their mothers and placed in a holding maternity cage under the same conditions as those described for Phase 1. From a given litter, the three subjects that showed highest baseline activity scores were randomly assigned to one specific ethanol (Experiment 1: 0.0, 0.5 or 2.5 g/kg) or LiCl (Experiment 2: 0.0, 0.5 or 1.0 % of body weight) condition. The same criterion of distribution was employed to assign pups that displayed lower activity levels during baseline.
Immediately after, an intraoral cannula (PE 10 polyethylene tubing, length: 5 cm, Clay Adams, Parsippany, NJ) was implanted in the right cheek of each pup, as previously described (Chotro & Alonso, 2003;Chotro & Arias, 2003). Briefly, a flanged end of the cannula was shaped by exposure to a heat source (external diameter: 1.2 mm). A dental needle (30-gauge Monoject, Sherwood Medical, Munchen, Germany) was attached to the non-flanged end of the cannula and positioned in the middle portion of the intraoral mucosa. The needle was inserted through the cheek and the cannula was pulled through the tissue until the flange end rested on the mouth's mucosa. This cannulation procedure requires no more than 20 s per subject and does not induce major stress in infant rats (Spear, Specht, Kirstein, & Kuhn, 1989). Ninety minutes after cannulation, pup's bladders were voided by gentle brushing of the anogenital area. Following this procedure body weights were recorded and subjects were placed into individual Plexiglas chambers (10 × 10 × 12 cm). Then pups received an intraoral infusion of saccharin (CS, 0.05 % w/v, duration: 10 min). Total administration volume was equivalent to 3.66 % of the subject's pre-infusion body weight. Saccharin was delivered at a constant rate by means of a infusion pump (KD Scientific, Holliston, MA) connected to the oral cannula of each pup by a polyethylene catheter (Clay Adams, PE 50 Parsippany, NJ). When employing these infusion parameters, pups are capable of either consuming or rejecting the infused solution (Arias & Chotro, 2006a;Chotro & Alonso, 1999, 2003;Dominguez, Lopez, & Molina, 1998). After the infusion procedure, subjects were weighed to estimate saccharin consumption scores. Percentage body weight gain (% BWG) was calculated as follows: 100 × [(Post-infusion weight – Pre-infusion weight) / Pre-infusion weight]. This dependent variable has been previously employed to estimate saccharin consumption in infant rats (for example, Hunt et al., 1991).
Immediately following CS exposure, pups received the corresponding ethanol (Experiment 1) or LiCl (Experiment 2) administration. Pups from Experiment 1 received an intragastric (i.g.) administration of 0.0, 0.5 or 2.5 g/kg ethanol. Ethanol was administered in a volume equivalent to 0.015 ml per gram of body weight of a 4.1 % or 21 % v/v ethanol solution. Pups given 0.0 g/kg received an equivalent volume of water. Subjects received the same dose of ethanol or LiCl on both PD12 and PD13. Within this range of ethanol dosage reliable conditioned taste aversion have been obtained during the infancy in rats (Arias, Pautassi, Molina, & Spear, submitted; P. S. Hunt et al., 1990; P. S. Hunt et al., 1991). Pups from Experiment 2 were administered an intraperitoneal, (i.p.) injection of LiCl. Dosage was 0.0, 0.25 or 0.5 % of body weight of a 0.3 M LiCl solution (Sigma Aldrich, St Louis, MO, USA). Pups from the control condition received an equivalent volume of vehicle (saline). As can be observed, we used different routes of administration for ethanol (i.g.) and LiCl (i.p.). In a recent study we observed that CTA induced by ethanol (2.5 g/kg intragastrically) or LiCl (0.5 % of body weight of a 0.3 M solution, intraperitoneally) induced similar levels of taste avoidance and disgust reactions (measured through a taste reactivity test) in preweanling rats (Arias et al., submitted). In addition, the present study is based on a recent investigation in which we observed a significant correlation between baseline locomotor activity and locomotor activity induced by ethanol (2.5 g/kg ethanol;Arias et al., 2009). In this study ethanol was administered intragastrically, so we chosew this route of administration for ethanol in Experiment 1 as well.
After drug treatment, pups were returned to their holding cage, where they remained undisturbed for three hours until being reunited with their mother.
Phase 3: Testing
On PD 14, pups were separated from their mothers, intraorally cannulated, and placed in pairs for 90 min in a heated holding cage. Then pups were tested in terms of saccharin intake for 10 min. The apparatus, parameters, and behavioral recordings of this test were similar to those described for the saccharin infusion procedure conducted on PDs 12 and 13. The intake score from one subject assigned to the 0.5 g/kg ethanol condition in Experiment 1 was not registered due to a technical problem with the infusion pump. Hence, this subject was excluded from the study.
Data analysis
Subjects from Experiments 1 and 2 came from independent litters. Hence, before the inferential analysis of the data we corroborated that baseline activity data were normally distributed and that means and variances did not differ across experiments. Descriptive statistics for baseline locomotor activity in Experiments 1 and 2 are presented in Table 1. Baseline data from each experiment were normally distributed (evaluated through the Kolmogorov-Smirnov test: Experiment 1: d=0.11, and Experiment 2: d=0.06, all ps > 0.20). Levene's test revealed that variances from samples assigned to each experiment were homogeneous [F(1,129) = 0.02, p > 0.2]. Finally, an ANOVA also revealed that baseline activity means did not differ across experiments [F (1,29) = 2.20, p > 0.1].
Table 1.
Descriptive statistics and sample size corresponding to baseline locomotor activity from subjects included in Experiments 1 and 2.
| EXPERIMENT 1 | EXPERIMENT 2 | |
|---|---|---|
| Mean | 130.94 | 146.80 |
| Standard Deviation | 60.14 | 62.33 |
| Minimum | 17 | 36 |
| Maximum | 237 | 291 |
| Median | 140 | 141 |
| N | 65 | 66 |
Pups were divided into two groups by the median of baseline locomotor activity registered on PD 11, high responders (HR) and low responders (LR). Locomotor activity during baseline (operationalized through the number of photo beams broken during the 5-minutes test) was analyzed with a 3 [Conditioning treatment (Experiment 1: 0.0, 0.5 or 2.5 g/kg ethanol; Experiment 2: 0.0, 0.25 or 0.5 % LiCl)] by 2 (Baseline activity: HR vs LR) between-factor ANOVA. Intake scores (operationalized through the percentage of body weight gained during the intake test) were analyzed by means of a mixed ANOVA. In this case, conditioning treatment (Experiment 1: 0.0, 0.5 or 2.5 g/kg ethanol; Experiment 2: 0.0, 0.25 or 0.5 % LiCl) and baseline activity (HR or LR) served as between-group factors, while day (conditioning day 1, conditioning day 2 and testing) was considered the within-group variable. Significant main effects and/or interactions were further analyzed by means of follow-up ANOVAs and post-hoc analysis [Duncan post-hoc tests]. All inferential analyses conducted in the present study employed an α level equal to 0.05. No significant effect of sex or interaction with the remaining factors was found in any of the analysis performed in the present study. Hence, for the inferential analysis and descriptive presentation of the results, data were collapsed across sex.
Results
Experiment 1
Figure 1 represents baseline activity scores as a function of subsequent ethanol treatment (0.0, 0.5 or 2.5 g/kg) and baseline activity level (HR vs LR). This analysis was conducted to corroborate that pups assigned to the different ethanol conditions before conditioning did not differ between them in terms of baseline locomotor activity. Baseline levels did not differ across conditions. This analysis also substantiated that baseline locomotor activity scores from HR were significantly higher than those from LR, F(1,59) = 153.48, p < 0.001.
Figure 1.
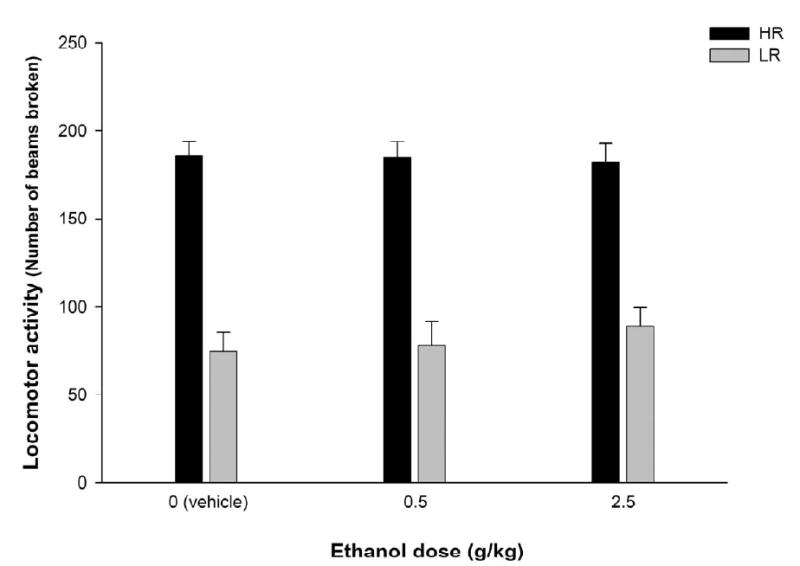
Baseline locomotor activity (operationalized through the number of beams broken) registered on PD 11. Subjects are separated as a function of: a) Ethanol treatment (0, 0.5 or 2.5 g/kg) administered during conditioning; and b) locomotor activity level during baseline (high responders, HR, and low responders, LR). Vertical lines illustrate standard errors of the means. Group HR-0 (n = 11), Group HR-0.5 (n = 12), Group HR-2.5 (n = 9), Group LR-0 (n = 11), Gropu LR-0.5 (n = 9), Group LR-2.5 (n = 13).
Figure 2 depicts intake of saccharin as a function of baseline activity, conditioning treatment and day. At testing, LR given 2.5 g/kg consumed less saccharin than LR given water, an effect that was not evident in HR. The ANOVA revealed a significant interaction involving ethanol treatment, baseline activity and day, F(4, 118) = 2.82, p < 0.05. To determine the loci of this significant interaction, follow-up two-way ANOVAs were performed considering conditioning treatment and baseline activity as independent factors, and analyzing separately intake data from each conditioning or testing day. No significant differences between groups were detected at conditioning (PDs 12 and 13). The ANOVA for testing revealed a significant interaction between conditioning treatment and baseline activity [F(2, 60) = 5.29, p < 0.01]. Post-hoc analyses indicated no differences as a function of ethanol treatment in the HR condition, but in the LR condition, pups given 2.5 g/kg ethanol as the US ingested less saccharin than pups given either 0 or 0.5 g/kg ethanol as US. Post-hoc analyses also revealed that HR given 2.5 g/kg ethanol consumed more saccharin than LR given the same ethanol dose and LR administered with 2.5 g/kg ethanol consumed less ethanol than LR treated with water.
Figure 2.
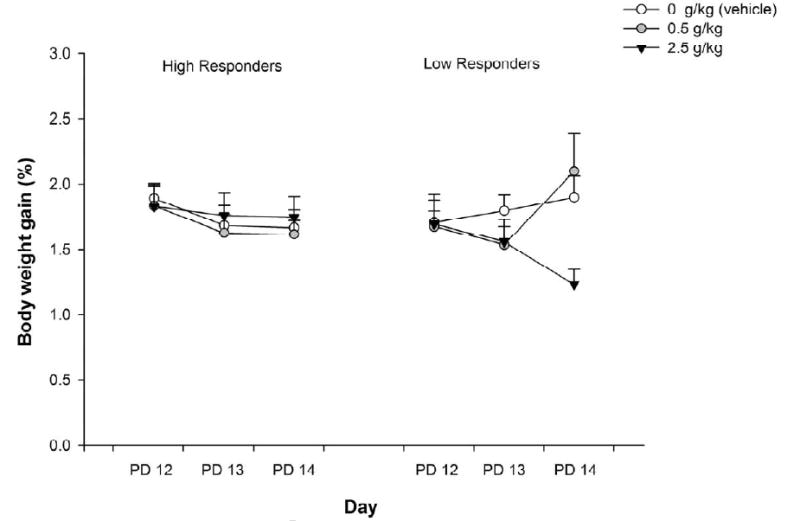
Intake of saccharin (operationalized through the percentage of body weight gained) as a function of ethanol treatment (0.0, 0.5 and 2.5 g/kg ethanol) and baseline activity level (high responders Vs low responders) during conditioning (postnatal days 12 and 13) or testing (PD14). Vertical lines illustrate standard errors of the means. Group HR-0 (n = 11), Group HR-0.5 (n = 12), Group HR-2.5 (n = 9), Group LR-0 (n = 11), Gropu LR-0.5 (n = 9), Group LR-2.5 (n = 13).
Experiment 2
According to the corresponding ANOVA, in Experiment 2 HR also had higher locomotor activity scores than LR at baseline, F(1,60 = 120.76, p < 0.0001. As expected, pups that subsequently differed in LiCl condition did not differ in baseline activity (see Figure 3).
Figure 3.
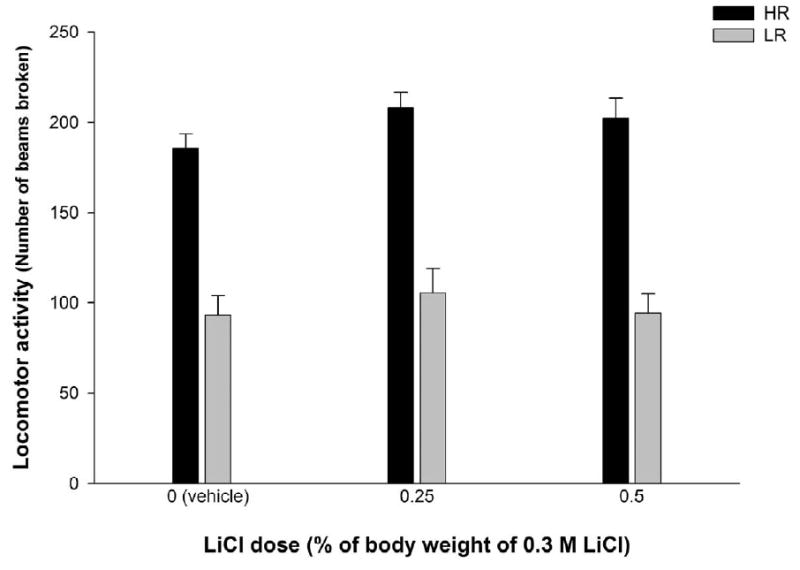
Locomotor activity scores on PD11 (operationalized through the number of beams broken) as a function of LiCl dose (0, 0.25 or 0.50 % % of body weight of 0.3 M LiCl). Subjects were divided as a function of baseline activity level in high or low responders (HR or LR). Vertical lines illustrate standard errors of the means. Group HR-0 (n = 11), Group HR-0.25 (n = 9), Group HR-0.5 (n = 12), Group LR-0 (n = 11), Gropu LR-0.25 (n = 13), Group LR-0.5 (n = 10).
Figure 4 represents intake of saccharin (CS) as a function of LiCl treatment, baseline activity level and day. The ANOVA revealed a significant main effect of day, [F(2,120) = 10.77, p < 0.001], and interaction between LiCl treatment and day, [F(4,120) = 3.83, p < 0.01]. To determine the loci of this significant interaction, follow-up one-way ANOVAs were performed considering conditioning treatment as the only independent factor, and analyzing intake data from each day separately. No significant differences between groups were detected at conditioning. The ANOVA for testing day revealed a significant effect of conditioning treatment, [F(2,60) = 11.54, p < 0.0001]. Regardless of LiCl dose, pups ingested less saccharin than controls, and unlike the results with ethanol as US, this was so whether they were HR or LR.
Figure 4.
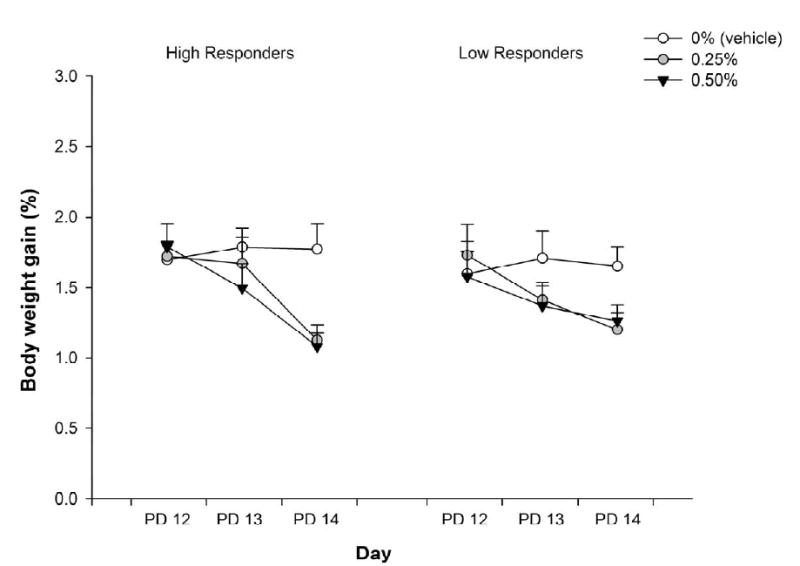
Intake of saccharin (operationalized through the percentage of body weight gained) as a function of ethanol treatment (0.0, 0.5 and 2.5 g/kg ethanol) and baseline activity level (high responders Vs low responders) during conditioning (postnatal days 12 and 13) or testing (PD14). Vertical lines illustrate standard errors of the means. Group HR-0 (n = 11), Group HR-0.25 (n = 9), Group HR-0.5 (n = 12), Group LR-0 (n = 11), Gropu LR-0.25 (n = 13), Group LR-0.5 (n = 10).
Conclusion
Recent findings from our laboratory suggest that baseline activity is a valuable predictor of ethanol's stimulating effects in infant rats: pups with high baseline activity levels are more sensitive to ethanol's locomotor stimulating effects than those with low baseline activity levels (Arias, Mlewski et al., 2009). According to the present study, high responders (HR) are more resistant to the aversive effects of ethanol than are low responders (LR). In Experiment 1, 2.5 g/kg ethanol induced clear conditioned taste aversion in LR, but not in HR. However this differential sensitivity seems to be specific to ethanol, since in Experiment 2, LiCl-induced conditioned taste aversion was similar in HR and LR. This result also indicates that differences in ethanol-mediated conditioned taste aversion between HR and LR are not due to differences in their learning capabilities.
Infant HR apparently are more sensitive to ethanol's activating effects than LR, although less sensitive to ethanol-induced conditioned taste aversion. This suggests that similar neurochemical pathways underlie ethanol-induced conditioned taste aversion and locomotor stimulation. For example, Dopamine D1 and D2 receptor antagonists attenuate ethanol's activating effects in infant (Arias et al., submitted) and adult (Pastor et al., 2005) rodents. Similarly, conditioned taste aversion induced by ethanol can be attenuated by antagonists of these dopamine receptors (for example, Risinger, Brownm, Oakes and Love, 1999). Furthermore, inhibition of NMDA receptors attenuates both ethanol-induced locomotor stimulation (Liljequist, 1991) and conditioned taste aversion (Bienkowski, Koros, Piasecki and Kostowski, 1998).
An association between ethanol-induced locomotor stimulating effects and appetitive reinforcing properties of the drug has been hypothesized (Robinson & Berridge, 1993;Wise & Bozarth, 1987). Recent results from our laboratory also suggest this association during the infantile period of the rat, since the post-administration interval in which infants rats display ethanol-induced locomotor activation coincides with that in which ethanol induces conditioned appetitive responses (Molina et al., 2007). Since infant HR are more sensitive than LR to the stimulating effects induced by ethanol (Arias et al., 2009), it is plausible that pups with high baseline locomotor activity levels are also more sensitive to ethanol-induced appetitive learning. We have not yet tested this hypothesis directly, but recently found that infant HR ingest more ethanol than LR (Miller, Arias, & Spear, 2008). However this result is not conclusive, because ethanol consumption can be modulated either by sensitivity to the rewarding or aversive effects of the drug (Broadbent et al., 2002;Green & Grahame, 2008). Greater sensitivity to the rewarding properties of ethanol among HR pups may be why HR pups are less sensitive than LR to ethanol's aversive effects. This might also explain why there were no differences between infant HR and LR in LiCl-induced taste aversion learning, since LiCl is not a drug that induces appetitive learning. Studies with adult rats have observed that HR are also less sensitive than LR to amphetamine-induced taste aversion learning but do not differ in acquisition of LiCl-mediated aversive conditioning (Kunin et al., 2001). According to Kunin et al. (2001), this result may explain why adult HR self-administer more amphetamine than LR (Kabbaj, 2004, 2006;Piazza, Deminiere, Le Moal, & Simon, 1989). We also consider relevant the fact that outbred mice sensitized to ethanol's locomotor activating effects were more resistant than non-sensitized mice to ethanol-induced, (2 g/kg), but not LiCl-induced, conditioned taste aversion (Lessov, Risinger and Phillips, 2001).
In several animal models, sensitivity to ethanol's aversive effects has been negatively related to ethanol consumption and ethanol's activating and rewarding effects. For example, mouse strains showing stronger taste aversion had lower ethanol preference (Broadbent et al., 2002). Mice selectively bred for sensitivity to ethanol-induced locomotor stimulation are more resistant to ethanol's aversive effects and also ingest more ethanol than those bred for low sensitivity to ethanol's activating effects (Risinger et al., 1994). Similarly, rat strains genetically selected for increased ethanol intake, such as P, UChB, AA, MsP and sP alcohol-preferring rats, are more resistant to ethanol-induced conditioned taste aversion and more sensitive to ethanol's activating effects than strains selected for low ethanol intake (Bell et al., 2006;Ciccocioppo et al., 2006;Colombo et al., 2006;Quintanilla et al., 2006;Sommer et al., 2006). Overall, these results suggest that these traits may be related, although there are also studies that do not support this hypothesis. For example, STDRHI mice, which were genetically selected for high ethanol consumption, showed less ethanol-induced activity and greater sensitivity to ethanol's rewarding properties than STDRLO, selected for low ethanol intake. However, these strains did not differ in terms of ethanol-induced conditioned taste aversion (Phillips et al., 2005). Mice genetically selected for high sensitivity to ethanol-induced conditioned taste aversion consumed less ethanol than mice selected for resistance to ethanol's aversive consequences, but these strains did not differ in terms of ethanol-induced locomotor stimulation (Phillips et al., 2005). The widely used strain of inbred mouse, C57BL/6J (B6), voluntarily consumes larger amounts of ethanol than DBA/2J (D2) mice (Yoneyama, Crabbe, Ford, Murillo and Finn, 2008). But the C57BL/6J (B6) strain do not typically show ethanol-induced locomotion activation (or only under very restrictive conditions, e.g., Middaugh Boggan and Randall, 1987, while DBA/2J (D2) mice are highly sensitive to ethanol's activating effects (e.g., Chester and Cunnigham, 1999). Finally, in a recent study ethanol-induced locomotor stimulation in Long-Evans rats failed to correlate with ethanol preference or ethanol intake (Chappell, and Weiner, 2008).
It is also possible that although greater sensitivity to ethanol-induced locomotor stimulation and greater resistance to ethanol-mediated conditioned taste aversion coexist in the same subpopulation of infant rats, these responses are not related functionally. In other words, HR may be more resistant than LR to ethanol-induced aversive learning independently of differences that might exist in their sensitivity to ethanol's rewarding properties. This hypothesis would also require that ethanol- and LiCl-mediated aversive learning are modulated by mechanisms that are at least partially different, since HR and LR do not differ in terms of sensitivity to LiCl-induced aversive learning.
Taste avoidance induced by emetic drugs (e.g., LiCl) and psychoactive drugs (e.g., amphetamine or cocaine) seems to be regulated by different mechanisms. Parker (1995; 2003) proposed that the conditioned taste aversion induced by psychoactive drugs may be qualitative and mechanistically distinct from that mediated by aversive emetic agents, such as LiCl. This hypothesis is supported, for example, by the fact that treatments that alleviate nausea do not affect the establishment of taste avoidance induced by psychoactive drugs, but they interfere with the establishment and expression of taste aversion induced by emetic agents (Parker, 1995, 2003). According to Parker taste avoidance induced by psychoactive drugs is mediated by fear conditioning, likely promoted by drug-related changes in homeostatic states. Parker based this hypothesis in the fact that rats cannot vomit, and, because of that, any novel homeostatic change signals danger to this rodent. Hence, a flavor associated with homeostatic change would indicate a potential risk for the rat and would result in subsequent avoidance of that taste (see Parker, 2003). An alternative hypothesis to explain taste avoidance generated by psychoactive drugs was provided by Grigson (1997). Grigson suggested that intake suppression (taste avoidance) induced by psychoactive drugs reflects the appetitive, reinforcing effects of these substances. Specifically, rats may avoid drinking the drug-paired CS because they are anticipating a more rewarding stimulus, the appetitive properties of the drug. In other words, the value of the taste CS is outweighed by the much preferred drug state, a process leading to the animal avoiding the taste CS on the basis of incentive contrast (Flaherty, 1996;Grigson, 1997).
On the other hand, it seems to be clearer that taste aversion reflects conditioned nausea (Parker, 1995, 2003). Ethanol is a complex psychoactive drug that shares with other drugs of abuse the capacity for activating the mesocorticolimbic dopaminergic system (Boehm, Piercy, Bergstrom, & Phillips, 2002;Di Chiara & Imperato, 1985;Tupala & Tiihonen, 2004;Xiao, Zhang, Krnjevic, & Ye, 2007). Adding to its complexity, there is evidence that at least high ethanol doses also can induce nausea. For example, CSs paired with high ethanol doses (3.5 g/kg) or LiCl induce similar c-Fos activity in the area postrema, the lateral parabraquial nucleus and the nucleus of the solitary tract (Thiele, Roitman, & Bernstein, 1996). These structures are located in the brainstem and participate in nausea and emesis (Yamamoto, Shimura, Sako, Yasoshima, & Sakai, 1994). In fact, there is evidence supporting the hypothesis that emetic-like effects underlie ethanol's capability to produce taste aversion (T. Hunt & Amit, 1987; Orr, Whitford-Stoddard, & Elkins, 2004). In addition 13-day-old rats exhibit similar patterns of conditioned disgust reactions when stimulated with a taste previously paired with either LiCl or ethanol, even when these drugs were titrated to induce similar levels of taste avoidance (Arias, Pautassi et al., submitted). Hence, taste aversion mediated by this drug may be associated with conditioned nausea.
Although suppression of tastant CSs previously paired with ethanol intoxication may be modulated by mechanisms that underlie taste avoidance induced by any psychoactive drugs, there are relatively unique effects of ethanol that can contribute to ethanol-induced conditioned taste aversion, such as motor impairment or hypothermia, which occur may act also during the infancy of the rat (Hunt et al., 1991). In a recent study, however, we did not find differences in ethanol-induced hypothermia or motor impairment as a function of baseline locomotor activity in 12-day-old rats (Arias, Mlewski et al., 2009).
Toward identification of ontogenetic change in ethanol's effects, it will be interesting to resolve in future studies whether neurochemical differences observed in adult HR and LR are also present in infant HR and LR, and also to analyze whether baseline activity levels during infancy are predictive of response to the stimulatory effects of ethanol in later stages of development. In adult rats, differences in response to a novel environment seem be associated with differences in sensitivity of the mesocorticolimbic dopaminergic system and HPA axis (Kabbaj et al., 2006;Piazza and Le Moal, 1997). There is not much information about these differences during early development. In one study it was reported that differences between high responders and low responders in dopaminergic and HPA-axis activity are not present in 10-day-old preweanling rats (see Cools and Gingras, 1998). These authors also indicate that by the end of the preweanling period (PD 18) ACTH plasma levels under baseline conditions are greater in high responders than in low responders, and that differences in dopaminergic activity emerge later in development. These conclusions are based on expression of m-RNA of D1 and tyrosine hydroxylase (see Cools and Gingras, 1998). However, it has been found recently that activity of the dopamine transporter, rather than tyrosine hydroxylase, may represent the critical factor in the enhanced susceptibility of high responders to locomotor activating effects of amphetamine during adulthood (Dietz, Tapocik, Gaval-Cruz, & Kabbaj, 2005). More research will be required to specifically determine the nature of the neurochemical differences between high and low responders. We recently reported that infant HRs are more sensitive to ethanol's locomotor stimulating effects than LR (Arias et al., 2009), an ethanol effect that seems to be mediated by the dopaminergic system even during infancy (see Arias et al., 2009a, Arias et al, submitted). These data suggest that differences between infants HR and LR may be associated with the dopaminergic system.
With respect to how the two major classes of ethanol reinforcement, appetitive or aversive, fit together generally in relation to ethanol's activation and sedation effects, our guess is that neither ethanol's reinforcement effect (i.e., appetitive vs aversive) nor its activating effect (i.e., increase or decrease) is absolute, even for a particular dose. Instead, the expression of ethanol reinforcement for any given dose of ethanol can be more or less appetitive (or aversive) depending on genetic (alcohol preferring or nonpreferring) or ontogenetic disposition, the CS, BEC and its previous course(increasing or decreasing) and arousal, as well as cognitive or environmental features that determine memory retrieval at the time of the test. And the same conditions, probably except those influencing memory retrieval, are likely to determine whether a given dose induces ethanol-induced increase or decrease in general activity.
In summary, the present evidence in conjunction with recent findings from our laboratory suggests that differential sensitivity to ethanol's effects may be detected in subpopulations of preweanling rats that differ in baseline activity levels in a mildly novel context. These results may help to investigate early detection of traits that could predict differential responses to drugs of abuse, as well as developmental origins of behavioral traits that predict differential response to drugs of abuse.
Acknowledgments
This work was supported by Supported by grants from the NIAAA (AA11960, AA013098, AA015992) and the NIMH (MH035219) to NES and the Agencia Nacional de Promocion Cientifica y Tecnologica (PICT 05-14024) to JCM, Postdoctoral fellowship from CONICET to CA. The authors wish to express their gratitude to Teri Tanehaus and Heather Murphy for their technical assistance.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Arias C, Chotro MG. Ethanol-induced preferences or aversions as a function of age in preweanling rats. Behav Neurosci. 2006a;120(3):710–718. doi: 10.1037/0735-7044.120.3.710. [DOI] [PubMed] [Google Scholar]
- Arias C, Chotro MG. Interactions between prenatal ethanol exposure and postnatal learning about ethanol in rat pups. Alcohol. 2006b;40(1):51–59. doi: 10.1016/j.alcohol.2006.10.002. [DOI] [PubMed] [Google Scholar]
- Arias C, Mlewski EC, Miller S, Molina JC, Spear NE. Novelty modulates the stimulating motor effects of ethanol in preweanling rats. Pharmacol Biochem and Behav. 2009 doi: 10.1016/j.pbb.2009.01.012. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arias C, Mlewski EC, Molina JC, Spear NE. Ethanol induces locomotor activating effects in preweanling Sprague-Dawley rats. Alcohol. 2009a;43:13–23. doi: 10.1016/j.alcohol.2008.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arias C, Mlewski EC, Molina JC, Spear NE. Naloxone and Baclofen attenuate ethanol's locomotor-activating effects in preweanling Sprague-Dawley rats. Behavioral Neuroscience. 2009b;123(1):172–180. doi: 10.1037/a0014049. [DOI] [PubMed] [Google Scholar]
- Arias C, Molina JC, Mlewski EC, Pautassi RM, Spear N. Acute sensitivity and acute tolerance to ethanol in preweanling rats with or without prenatal experience with the drug. Pharmacol Biochem Behav. 2008;89(4):608–622. doi: 10.1016/j.pbb.2008.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arias C, Pautassi RM, Molina JC, Spear NE. A comparison between taste avoidance and conditioned disgust reactions induced by ethanol and lithium chloride in preweanling rats. Alcohol. doi: 10.1002/dev.20460. submitted. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arias C, Mlewski EC, Molina JC, Spear NE. Dopamine receptors modulate ethanol's locomotor-activating effects in preweanling rats. Behav Pharmacol. doi: 10.1002/dev.20407. submitted. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell RL, Rodd ZA, Lumeng L, Murphy JM, McBride WJ. The alcohol-preferring P rat and animal models of excessive alcohol drinking. Addict Biol. 2006;11(34):270–288. doi: 10.1111/j.1369-1600.2005.00029.x. [DOI] [PubMed] [Google Scholar]
- Bienkowski P, Koros E, Kostowski W. Novelty-seeking behaviour and operant oral ethanol self-administration in Wistar rats. Alcohol Alcohol. 2001;36(6):525–528. doi: 10.1093/alcalc/36.6.525. [DOI] [PubMed] [Google Scholar]
- Bienkowski P, Koros E, Piasecki J, Kostowski W. Prior exposure to MK-801 sensitizes rats to ethanol-induced conditioned taste aversion. Alcohol Alcohol. 1998;33(2):116–20. doi: 10.1093/oxfordjournals.alcalc.a008366. [DOI] [PubMed] [Google Scholar]
- Boehm SL, 2nd, Piercy MM, Bergstrom HC, Phillips TJ. Ventral tegmental area region governs GABA(B) receptor modulation of ethanol-stimulated activity in mice. Neuroscience. 2002;115(1):185–200. doi: 10.1016/s0306-4522(02)00378-0. [DOI] [PubMed] [Google Scholar]
- Broadbent J, Muccino KJ, Cunningham CL. Ethanol-induced conditioned taste aversion in 15 inbred mouse strains. Behav Neurosci. 2002;116(1):138–148. [PubMed] [Google Scholar]
- Cailhol S, Mormede P. Conditioned taste aversion and alcohol drinking: strain and gender differences. J Stud Alcohol. 2002;63(1):91–99. [PubMed] [Google Scholar]
- Chappell AM, Weiner JL. Relationship between ethanol's acute locomotor effects and ethanol self-administration in male Long-Evans rats. Alcohol Clin Exp Res. 2008;32(12):2088–99. doi: 10.1111/j.1530-0277.2008.00797.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chester JA, Cunningham CL. Baclofen alters ethanol-stimulated activity but not conditioned place preference or taste aversion in mice. Pharmacol Biochem Behav. 1999;63(2):325–31. doi: 10.1016/s0091-3057(98)00253-6. [DOI] [PubMed] [Google Scholar]
- Chotro MG, Alonso G. Effects of stimulus preexposure on the generalization of conditioned taste aversions in infant rats. Dev Psychobiol. 1999;35(4):304–317. doi: 10.1002/(sici)1098-2302(199912)35:4<304::aid-dev5>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- Chotro MG, Alonso G. Stimulus preexposure reduces generalization of conditioned taste aversions between alcohol and non-alcohol flavors in infant rats. Behav Neurosci. 2003;117(1):113–122. [PubMed] [Google Scholar]
- Chotro MG, Arias C. Prenatal exposure to ethanol increases ethanol consumption: a conditioned response? Alcohol. 2003;30(1):19–28. doi: 10.1016/s0741-8329(03)00037-5. [DOI] [PubMed] [Google Scholar]
- Chotro MG, Arias C. Ontogenetic difference in ethanol reinforcing properties: the role of the opioid system. Behavioural Pharmachology. 2007;18(7):661–666. doi: 10.1097/FBP.0b013e3282f00754. [DOI] [PubMed] [Google Scholar]
- Chuck TL, McLaughlin PJ, Arizzi-LaFrance MN, Salamone JD, Correa M. Comparison between multiple behavioral effects of peripheral ethanol administration in rats: sedation, ataxia, and bradykinesia. Life Sci. 2006;79(2):154–161. doi: 10.1016/j.lfs.2005.12.045. [DOI] [PubMed] [Google Scholar]
- Ciccocioppo R, Economidou D, Cippitelli A, Cucculelli M, Ubaldi M, Soverchia L, et al. Genetically selected Marchigian Sardinian alcohol-preferring (msP) rats: an animal model to study the neurobiology of alcoholism. Addict Biol. 2006;11(34):339–355. doi: 10.1111/j.1369-1600.2006.00032.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colombo G, Agabio R, Lobina C, Reali R, Vacca G, Gessa GL. Stimulation of locomotor activity by voluntarily consumed ethanol in Sardinian alcohol-preferring rats. Eur J Pharmacol. 1998;357(23):109–113. doi: 10.1016/s0014-2999(98)00560-3. [DOI] [PubMed] [Google Scholar]
- Colombo G, Lobina C, Carai MA, Gessa GL. Phenotypic characterization of genetically selected Sardinian alcohol-preferring (sP) and - non-preferring (sNP) rats. Addict Biol. 2006;11(34):324–338. doi: 10.1111/j.1369-1600.2006.00031.x. [DOI] [PubMed] [Google Scholar]
- Cools AR, Gingras MA. Nijmegen high and low responders to novelty: a new tool in the search after the neurobiology of drug abuse liability. Pharmacol Biochem Behav. 1998;60(1):151–159. doi: 10.1016/s0091-3057(97)00586-8. [DOI] [PubMed] [Google Scholar]
- Di Chiara G, Imperato A. Ethanol preferentially stimulates dopamine release in the nucleus accumbens of freely moving rats. Eur J Pharmacol. 1985;115(1):131–132. doi: 10.1016/0014-2999(85)90598-9. [DOI] [PubMed] [Google Scholar]
- Dietz DM, Tapocik J, Gaval-Cruz M, Kabbaj M. Dopamine transporter, but not tyrosine hydroxylase, may be implicated in determining individual differences in behavioral sensitization to amphetamine. Physiol Behav. 2005;86(3):347–355. doi: 10.1016/j.physbeh.2005.08.005. [DOI] [PubMed] [Google Scholar]
- Dominguez HD, Lopez MF, Molina JC. Neonatal responsiveness to alcohol odor and infant alcohol intake as a function of alcohol experience during late gestation. Alcohol. 1998;16(2):109–117. doi: 10.1016/s0741-8329(97)00169-9. [DOI] [PubMed] [Google Scholar]
- Eckardt MJ. Alcohol-induced conditioned taste aversion in rats. Effect of concentration and prior exposure to alcohol. J Stud Alcohol. 1976;37(3):334–346. doi: 10.15288/jsa.1976.37.334. [DOI] [PubMed] [Google Scholar]
- Erickson CK, Kochhar A. An animal model for low dose ethanol-induced locomotor stimulation: behavioral characteristics. Alcohol Clin Exp Res. 1985;9(4):310–314. doi: 10.1111/j.1530-0277.1985.tb05550.x. [DOI] [PubMed] [Google Scholar]
- Fahlke C, Hard E, Eriksson CJ, Engel JA, Hansen S. Amphetamine-induced hyperactivity: differences between rats with high or low preference for alcohol. Alcohol. 1995;12(4):363–367. doi: 10.1016/0741-8329(95)00019-n. [DOI] [PubMed] [Google Scholar]
- Gingras MA, Cools AR. Analysis of the biphasic locomotor response to ethanol in high and low responders to novelty: a study in Nijmegen Wistar rats. Psychopharmacology (Berl) 1996;125(3):258–264. doi: 10.1007/BF02247337. [DOI] [PubMed] [Google Scholar]
- Green AS, Grahame NJ. Ethanol drinking in rodents: is free-choice drinking related to the reinforcing effects of ethanol? Alcohol. 2008;42(1):1–11. doi: 10.1016/j.alcohol.2007.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flaherty CF, Coppotelli C, Potaki J. Effect of chlordiazepoxide on the response to repeated reductions in sucrose concentration in free-fed rats. Physiol Behav. 1996;60(5):1291–8. doi: 10.1016/s0031-9384(96)00257-0. [DOI] [PubMed] [Google Scholar]
- Grigson PS. Conditioned taste aversions and drugs of abuse: a reinterpretation. Behav Neurosci. 1997;111(1):129–36. [PubMed] [Google Scholar]
- Hoshaw BA, Lewis MJ. Behavioral sensitization to ethanol in rats: evidence from the Sprague-Dawley strain. Pharmacol Biochem Behav. 2001;68(4):685–690. doi: 10.1016/s0091-3057(01)00489-0. [DOI] [PubMed] [Google Scholar]
- Hunt PS, Molina JC, Spear LP, Spear NE. Ethanol-mediated taste aversions and state-dependency in preweanling (16-day-old) rats. Behav Neural Biol. 1990;54(3):300–322. doi: 10.1016/0163-1047(90)90650-u. [DOI] [PubMed] [Google Scholar]
- Hunt PS, Spear LP, Spear NE. An ontogenetic comparison of ethanol-mediated taste aversion learning and ethanol-induced hypothermia in preweanling rats. Behav Neurosci. 1991;105(6):971–983. doi: 10.1037//0735-7044.105.6.971. [DOI] [PubMed] [Google Scholar]
- Hunt T, Amit Z. Conditioned taste aversion induced by self-administered drugs: paradox revisited. Neurosci Biobehav Rev. 1987;11(1):107–130. doi: 10.1016/s0149-7634(87)80005-2. [DOI] [PubMed] [Google Scholar]
- Institute of Laboratory Animal Resources. National Research Council Guide for the Care and Use of Laboratory Animals. Washington, DC: National Academic Press; 1996. [Google Scholar]
- Kabbaj M. Neurobiological bases of individual differences in emotional and stress responsiveness: high responders-low responders model. Arch Neurol. 2004;61(7):1009–1012. doi: 10.1001/archneur.61.7.1009. [DOI] [PubMed] [Google Scholar]
- Kabbaj M. Individual differences in vulnerability to drug abuse: the high responders/low responders model. CNS Neurol Disord Drug Targets. 2006;5(5):513–520. doi: 10.2174/187152706778559318. [DOI] [PubMed] [Google Scholar]
- Liljequist S. NMDA receptor antagonists inhibit ethanol-produced locomotor stimulation in NMRI mice. Alcohol. 1991;8(4):309–12. doi: 10.1016/0741-8329(91)90449-7. [DOI] [PubMed] [Google Scholar]
- Kunin D, Gaskin S, Borjas MB, Smith BR, Amit Z. Differences in locomotor response to an inescapable novel environment predict sensitivity to aversive effects of amphetamine. Behav Pharmacol. 2001;12(1):61–67. doi: 10.1097/00008877-200102000-00007. [DOI] [PubMed] [Google Scholar]
- Miller S, Arias C, Spear NE. Baseline locomotor activity and prenatal ethanol predict ethanol intake in preweanling heterogeneous rats. Paper presented at the International Society for Develpmental Psychobiology; Washington. 2008. [Google Scholar]
- Molina JC, Pautassi RM, Truxell E, Spear N. Differential motivational properties of ethanol during early ontogeny as a function of dose and postadministration time. Alcohol. 2007;41(1):41–55. doi: 10.1016/j.alcohol.2007.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Middaugh LD, Boggan WO, Randall CL. Stimulatory effects of ethanol in C57BL/6 mice. Pharmacol Biochem Behav. 1987;27(3):421–4. doi: 10.1016/0091-3057(87)90343-1. [DOI] [PubMed] [Google Scholar]
- Nadal R, Armario A, Janak PH. Positive relationship between activity in a novel environment and operant ethanol self-administration in rats. Psychopharmacology (Berl) 2002;162(3):333–338. doi: 10.1007/s00213-002-1091-5. [DOI] [PubMed] [Google Scholar]
- National Institutes of Health. Guide for the care and use of laboratory animals (DHEW Publication No 86-23) Washington, DC: Government Printing Office; 1986. [Google Scholar]
- Nowak KL, Ingraham CM, McKinzie DL, McBride WJ, Lumeng L, Li TK, et al. An assessment of novelty-seeking behavior in alcohol-preferring and nonpreferring rats. Pharmacol Biochem Behav. 2000;66(1):113–121. doi: 10.1016/s0091-3057(00)00206-9. [DOI] [PubMed] [Google Scholar]
- Pastor R, Miquel M, Aragon CM. Habituation to test procedure modulates the involvement of dopamine D2- but not D1- receptors in ethanol-induced locomotor stimulation in mice. Psychopharmacology. 2005;182:436–446. doi: 10.1007/s00213-005-0115-3. [DOI] [PubMed] [Google Scholar]
- Parker LA. Rewarding drugs produce taste avoidance, but not taste aversion. Neurosci Biobehav Rev. 1995;19(1):143–157. doi: 10.1016/0149-7634(94)00028-y. [DOI] [PubMed] [Google Scholar]
- Parker LA. Taste avoidance and taste aversion: evidence for two different processes. Learn Behav. 2003;31(2):165–172. doi: 10.3758/bf03195979. [DOI] [PubMed] [Google Scholar]
- Petrov ES, Varlinskaya EI, Spear NE. Reinforcement from pharmacological effects of ethanol in newborn rats. Alcohol Clin Exp Res. 2003;27(10):1583–1591. doi: 10.1097/01.ALC.0000089960.62640.58. [DOI] [PubMed] [Google Scholar]
- Phillips TJ, Broadbent J, Burkhart-Kasch S, Henderson C, Wenger CD, McMullin C, et al. Genetic correlational analyses of ethanol reward and aversion phenotypes in short-term selected mouse lines bred for ethanol drinking or ethanol-induced conditioned taste aversion. Behav Neurosci. 2005;119(4):892–910. doi: 10.1037/0735-7044.119.4.892. [DOI] [PubMed] [Google Scholar]
- Piazza PV, Deminiere JM, Le Moal M, Simon H. Factors that predict individual vulnerability to amphetamine self-administration. Science. 1989;245(4925):1511–1513. doi: 10.1126/science.2781295. [DOI] [PubMed] [Google Scholar]
- Piazza PV, Le Moal M. Glucocorticoids as a biological substrate of reward: physiological and pathophysiological implications. Brain Res Brain Res Rev. 1997;25(3):359–372. doi: 10.1016/s0165-0173(97)00025-8. [DOI] [PubMed] [Google Scholar]
- Quintanilla ME, Israel Y, Sapag A, Tampier L. The UChA and UChB rat lines: metabolic and genetic differences influencing ethanol intake. Addict Biol. 2006;11(34):310–323. doi: 10.1111/j.1369-1600.2006.00030.x. [DOI] [PubMed] [Google Scholar]
- Risinger FO, Malott DH, Prather LK, Niehus DR, Cunningham CL. Motivational properties of ethanol in mice selectively bred for ethanol-induced locomotor differences. Psychopharmacology (Berl) 1994;116(2):207–216. doi: 10.1007/BF02245064. [DOI] [PubMed] [Google Scholar]
- Risinger FO, Brown MM, Oakes RA, Love JA. Effects of haloperidol or SCH-23390 on ethanol-induced conditioned taste aversion. Alcohol. 1999;18(23):139–45. doi: 10.1016/s0741-8329(98)00076-7. [DOI] [PubMed] [Google Scholar]
- Robinson TE, Berridge KC. The neural basis of drug craving: an incentive-sensitization theory of addiction. Brain Res Brain Res Rev. 1993;18(3):247–291. doi: 10.1016/0165-0173(93)90013-p. [DOI] [PubMed] [Google Scholar]
- Samson HH, Chappelle AM. Home-cage ethanol consumption and motor activity: lack of relation to either initial activity or amphetamine-induced locomotion. Alcohol. 1995;12(1):37–42. doi: 10.1016/0741-8329(94)00065-l. [DOI] [PubMed] [Google Scholar]
- Sanders S, Spear NE. Ethanol acceptance is high during early infancy and becomes still higher after previous ethanol ingestion. Alcohol Clin Exp Res. 2007;31(7):1148–1158. doi: 10.1111/j.1530-0277.2007.00400.x. [DOI] [PubMed] [Google Scholar]
- Silveri MM, Spear LP. Acute, rapid, and chronic tolerance during ontogeny: observations when equating ethanol perturbation across age. Alcohol Clin Exp Res. 2001;25(9):1301–1308. [PubMed] [Google Scholar]
- Sommer W, Hyytia P, Kiianmaa K. The alcohol-preferring AA and alcoholavoiding ANA rats: neurobiology of the regulation of alcohol drinking. Addict Biol. 2006;11(34):289–309. doi: 10.1111/j.1369-1600.2006.00037.x. [DOI] [PubMed] [Google Scholar]
- Spear LP, Specht SM, Kirstein CL, Kuhn CM. Anterior and posterior, but not cheek, intraoral cannulation procedures elevate serum corticosterone levels in neonatal rat pups. Dev Psychobiol. 1989;22(4):401–411. doi: 10.1002/dev.420220407. [DOI] [PubMed] [Google Scholar]
- Thiele TE, Roitman MF, Bernstein IL. c-Fos induction in rat brainstem in response to ethanol- and lithium chloride-induced conditioned taste aversions. Alcohol Clin Exp Res. 1996;20(6):1023–1028. doi: 10.1111/j.1530-0277.1996.tb01941.x. [DOI] [PubMed] [Google Scholar]
- Truxell E, Spear NE. Immediate acceptance of ethanol in infant rats: ontogenetic differences with moderate but not high ethanol concentration. Alcohol Clin Exp Res. 2004;28(8):1200–1211. doi: 10.1097/01.alc.0000134220.34842.18. [DOI] [PubMed] [Google Scholar]
- Truxell EM, Molina JC, Spear NE. Ethanol intake in the juvenile, adolescent, and adult rat: effects of age and prior exposure to ethanol. Alcohol Clin Exp Res. 2007;31(5):755–765. doi: 10.1111/j.1530-0277.2007.00358.x. [DOI] [PubMed] [Google Scholar]
- Tupala E, Tiihonen J. Dopamine and alcoholism: neurobiological basis of ethanol abuse. Prog Neuropsychopharmacol Biol Psychiatry. 2004;28(8):1221–1247. doi: 10.1016/j.pnpbp.2004.06.022. [DOI] [PubMed] [Google Scholar]
- Wise RA, Bozarth MA. A psychomotor stimulant theory of addiction. Psychol Rev. 1987;94(4):469–492. [PubMed] [Google Scholar]
- Xiao C, Zhang J, Krnjevic K, Ye JH. Effects of ethanol on midbrain neurons: role of opioid receptors. Alcohol Clin Exp Res. 2007;31(7):1106–1113. doi: 10.1111/j.1530-0277.2007.00405.x. [DOI] [PubMed] [Google Scholar]
- Yamamoto T, Shimura T, Sako N, Yasoshima Y, Sakai N. Neural substrates for conditioned taste aversion in the rat. Behav Brain Res. 1994;65(2):123–137. doi: 10.1016/0166-4328(94)90097-3. [DOI] [PubMed] [Google Scholar]


