Abstract
Although elevated activity of the tryptophan-degrading enzyme indoleamine 2,3-dioxygenase(IDO) has been proposed to mediate comorbid depression in inflammatory disorders, its causative role has never been tested. We report that peripheral administration of lipopolysaccharide (LPS) activates IDO and culminates in a distinct depressive-like behavioral syndrome, measured by increased duration of immobility in both the forced swim and tail suspension tests. Blockade of IDO activation either indirectly with the anti-inflammatory tetracycline derivative minocycline, that attenuates LPS-induced expression of proinflammatory cytokines, or directly with the IDO antagonist 1-methyltryptophan (1-MT), prevents development of depressive-like behavior. Both minocycline and 1-MT normalize the kynurenine/tryptophan ratio in the plasma and brain of LPS-treated mice without changing the LPS-induced increase in turnover of brain serotonin. Administration of L-kynurenine, a metabolite of tryptophan that is generated by IDO, to naïve mice dose-dependently induces depressive-like behavior. These results implicate IDO as a critical molecular mediator of inflammation-induced depressive-like behavior, probably through the catabolism of tryptophan along the kynurenine pathway.
Keywords: 1-methyltryptophan, minocycline, kynurenine, tryptophan, serotonin, interleukin-1β, tumor necrosis factor-α, interferon-γ, tail suspension test, forced swim test, depression
Introduction
Increased prevalence of comorbid major depressive disorders occurs in a number of conditions (e.g., aging, obesity) and diseases (e.g., atherosclerosis, congestive heart failure, rheumatoid arthritis), all of which have a common chronic inflammatory component (1). Depressive symptoms frequently develop in patients undergoing cytokine immunotherapy for the treatment of viral diseases and certain cancers (2, 3). In most of these conditions, clinical reports have revealed an increase in the ratio of plasma kynurenine to tryptophan. This increase in the kynurenine/tryptophan ratio is associated with increased plasma levels of neopterin, a marker of macrophage activation, which points to activation of the tryptophan catabolizing enzyme indoleamine 2,3-dioxygenase (IDO)(4). IDO is an extrahepatic enzyme that is present in macrophages and other cells that degrades the essential amino acid tryptophan along the kynurenine pathway. This enzyme is induced by proinflammatory cytokines, mainly interferon-gamma (IFN-γ) (5) and tumor necrosis factor-alpha (TNF-α) (6, 7). When IDO is activated in conditions of chronic inflammation, its degree of activation is correlated to the intensity of depressive symptoms, as observed in cancer patients chronically treated with IFNα (8).
Acute activation of the peripheral innate immune system in laboratory animals, through the administration of the cytokine inducer lipopolysaccharide (LPS), induces depressive-like behavior, as measured by increased immobility in the forced swim test and tail suspension test, decreased consumption of a sweetened solution and a suppression of sexual behavior (9, 10), that can be attenuated by chronic antidepressant administration (9). LPS-induced depressive-like behavior can be observed even after the acute behavioral response and reduction in food intake that are characteristic of sickness in LPS-treated mice have normalized (10). Chronic activation of the immune system via inoculation of Bacillus Calmette-Guerin, an attenuated form of Mycobacterium bovis, has the same effect (11). In both cases, depressive-like behavior is associated with increased IDO activity.
Activation of IDO by proinflammatory cytokines alters serotoninergic and glutamatergic neurotransmission. Since tryptophan is the limiting factor for the synthesis of serotonin, decreased circulating tryptophan concentrations, as occurs in patients undergoing immunotherapy, have the potential to negatively impact serotoninergic neurotransmission (8, 12). IDO is also expressed in brain endothelial cells, perivascular macrophages, astrocytes and microglia (13), so that fluctuations in its enzymatic activity can alter brain tryptophan metabolism. Alternatively, the major product of peripheral tryptophan degradation by IDO, kynurenine, is readily transported across the blood brain barrier (14) into the brain where it can be further metabolized by perivascular macrophages, microglia and astrocytes to generate neuroactive glutamatergic compounds (13). In fact, heightened glutamate receptor activity may play an important role in major depression (15, 16).
In order to test the possibility that activation of IDO during inflammation is responsible for development of major depressive disorders, we determined whether inhibition of IDO indirectly (by a treatment that targets proinflammatory cytokines that induce IDO) or directly (by a treatment that blocks IDO activation) abrogates depressive-like behavior in the LPS model of acute immune stimulation. The semi-synthetic tetracycline derivative, minocycline, was chosen for the first approach. Minocycline has potent anti-inflammatory effects independent from its microbicidal properties, as it is well known to inhibit macrophage and microglial activation. Of all the tetracyclines, it has the greatest permeability through the blood brain barrier (17), and it confers therapeutic benefits in many CNS disease models, including ischemia (18), Parkinson disease (19), amyotrophic lateral sclerosis (20) and multiple sclerosis (17). The tryptophan analog 1-methyltryptophan (1-MT) was chosen for the second approach. 1-MT, a competitive inhibitor of IDO, blocks human dendritic cell regulatory function in vitro (21) and functions in vivo to block IDO-mediated immune events in animal models of rheumatoid arthritis (22), type 1 diabetes (23) and multiple sclerosis (24). We show here that inhibition of IDO activation with either approach fully abrogates LPS-induced depressive like behavior.
Methods
Animals and treatments
All animal care and use were conducted in accordance with the Guide for the Care and Use of Laboratory Animals (NRC) and approved by the Institutional Animal Care and Use Committee. Experiments were performed on 10–14 week-old male Crl:CD1 (ICR) mice obtained from Charles River Laboratories (Wilmington, MA), whose average body weights were 35–40 g at the beginning of the experiments. Mice were individually housed in standard shoebox cages, with wood shavings litter, in a temperature (23°C) and humidity (45–55%) controlled environment with a 12/12-h modified dark-light cycle (light on 11:00 PM–11:00 AM). Food and water were available ad libitum. Mice were handled individually everyday for 10 days before the experiments.
LPS (L-3129, serotype 0127:B8) and minocycline-HCl (M-9511) were purchased from Sigma (St. Louis, MO). On the day of injection, fresh solutions were prepared by dissolving compounds in sterile endotoxin-free isotonic saline and administered intraperitoneally (i.p.). The dose of LPS (0.83 mg/kg) was selected on the basis of its ability to induce the full spectrum of the acute sickness response (25) and a reliable increase of brain IDO activity, a putative mechanism in the depressive-like behavior induced by LPS (26). Minocycline was administered at a dose of 50 mg/kg once daily for two days prior to and on the same day as LPS injection. 1-methyltryptophan (1-MT), or placebo, slow release pellets designed to continuously release 5 mg/d of drug for 21 days were purchased from Innovative Research of America (Sarasota, FL). Pellets were implanted subcutaneously beneath the dorsal skin surface, according to the manufacturer’s instructions, one week prior to i.p. injection. 1-MT was only present in the plasma (8.05 ± 0.58 μmol/L) and brain tissue (4.51 ± 0.28 pmol/mg tissue) of mice implanted with the drug-containing pellet, not placebo.
Behavioral experiments
All behavioral experiments were performed during the first 4 h of the dark phase of the light cycle.
Locomotor activity
The effects of LPS on locomotor activity (LMA) were assessed in mice individually placed into a clean, novel cage similar to the home cage, but devoid of bedding or litter. The cage was divided into four virtual quadrants, and LMA was measured by counting the number of line crossings and rearings over a five-min period. Counting was done by a well-trained observer who was blind to the treatments.
Forced swim test
The forced swim test (FST) was conducted essentially as described previously (27). Briefly, each mouse was placed individually in a cylinder (diameter: 23 cm; height: 31 cm) containing 15 cm of water maintained at 23 ± 1°C. The water was changed between testing sessions. Mice were placed into the water for 6 min. and then returned to their home cage. During the test, the mice were video recorded from above, and the duration of immobility was determined over the last five min. of the test using the mobility function of the “Observer Basic” software (Noldus, Netherlands). Briefly, mice are recognized in contrast from their background and tracked in two dimensions as the surface area of the detectable object (mouse) moves within the predefined arena. Mobility is defined as the displacement of detectable surface area (mouse) over time and is averaged over 3 sample intervals to reduce error generated by sharp movements or missing frames in the digital record. Program analysis settings were: Sampling rate = 3/s; detection method = subtration with low threshold of 20 and high threshold of 255 and minimum detectable object size of 200 pixels; image filtering = 2 pixel erosion and dilation; mobility threshold of 20% with 3 interval averaging.
Tail suspension test
The mice were taken from their home cage and a small piece of adhesive tape was placed approximately two cm from the tip of the tail. A single hole was punched in the tape and the mice were hung individually for a period of 10 min. on a hook connected to a strain gauge. A computerized system for processing the force exerted on the gauge (Mouse Tail Suspension Package, MED-TSS-MS, Med Associates, St. Albans, VT) automatically collected and analyzed the movements of each individual mouse. The time of immobility was determined after establishing a threshold level for each individual mouse that was set precisely at the activity level that would exclude all movements and only encompass immobility. Time below this threshold indicated the time of immobility. Program analysis settings were integration = on; resolution = 0.1 s; gain = 4; start trigger = 20.
RNA Extraction and Reverse Transcription
Total RNA from whole brain samples was extracted in TRIzol reagent. All reverse transcriptase reactions were carried out in a Stratagene Robocycler Gradient 96, using an Ambion (cat # 1710) reverse transcriptase kit according to manufacturer instructions, using 125 ng total RNA and random decamer primers for each reaction. All RNA samples were reverse transcribed simultaneously to minimize inter-assay variation associated with the reverse transcription reaction.
Real-Time PCR
Real-time RT-PCR was performed on an Applied Biosystems Prism 7900 using Taqman® gene expression assays for TNF-α (cat # Mm00443258_m1), IL-1β (cat # Mm00434228_m1), IFNγ (cat # Mm00801778_m1), IDO (cat # Mm00492586_m1) and GAPDH (cat # Mm999999_g1) were purchased from Applied Biosystems (Foster City, CA). Reactions were performed in duplicate according to manufacturer instructions, using 125 ng cDNA template for each reaction. Amplifications without reverse transcription or template were included as negative controls. Relative quantitative measurement of target gene levels was performed using the ΔΔCt method, where Ct is the threshold concentration. GAPDH was used as the endogenous housekeeping control gene.
HPLC
Plasma tryptophan and kynurenine were analyzed by HPLC using an ESA Coulochem II detector with a 5041 Enhanced Analytical cell containing a glassy carbon electrode (+600 mV). Mobile phase (pH = 4.6) consisted of 75 mM NaH2PO4, 25 μM EDTA (disodium salt), and 100μl/L triethylamine in acetonitrile:water (6:94 v:v). Brain monoamines were analyzed at +320 mV. The monoamine mobile phase (pH = 3.0) consisted of 75 mM NaH2PO4, 25 μM EDTA (disodium salt), 1.7 mM octanesulfonic acid, and 100μl/L triethylamine in acetonitrile:water (7:93 v:v). The chromatograms were integrated and quantified using Dynamax MacIntegrator II software (Rainin Instruments, Woburn, MA).
Plasma (50 μl) was mixed with a solution of 10% sulfosalicylic acid solution (10 μl) and allowed to precipitate proteins on ice for at least 30 minutes. After the precipitation, samples were centrifuged at 12,000 × g for 10 minutes at 4° C. The supernatant was extracted and loaded into a Costar Spin-X centrifuge tube filter (0.22μM Nylon Part #8169 Corning Incorporated) and centrifuged at 12,000 × g for 6 minutes at 4° C. For the current study, plasma extracts were diluted at 1:50 following the extraction steps described above.
Mouse brains were first weighed and then homogenized in 500 μl of a 0.1 N HClO4/10μM ascorbate solution using a 1.5 ml centrifuge tube and disposable pestle. Upon complete homogenization, the brain samples were centrifuged at 12,000 × g for 5 min. at 4° C. After homogenization, the supernatant was extracted and loaded into a Costar Spin-X tube filter and centrifuged at 12,000 × g for 5 minutes at 4° C. For the current study, the sample extracts were then diluted at 1:10 (v:v) in 0.02 N HClO4 and analyzed for kynurenine, tryptophan and 1-methyl-tryptophan. The brain extracts which were analyzed for 5-HIAA and serotonin were diluted at 1:100 (w:v) and injected into the HPLC system. Data from plasma samples are expressed as μmol/L, while data from brain samples are expressed as pmol/mg tissue.
A standard curve was generated on each day from concentrated (2 μM) tryptophan, 1-methyl-tryptophan, and kynurenine standards made up in 0.02 N HClO4 and held at 4° C until a 20 μL volume was injected into the system. Standards were made using a serial dilution technique that made the standards to levels that would encompass expected levels in the plasma samples. The standard curve was created using the system software and samples were not analyzed unless a linear standard curve with r2 greater than 0.995 was achieved.
Statistical Analysis
Behavioral data (mean ± SEM) were analyzed using a one-way (treatment), two-way (pretreatment × treatment) or a three-way (pretreatment × treatment × time) ANOVA with repeated measurement on the time factor where appropriate, followed by a post-hoc pairwise multiple comparison procedure using the Fisher’s LSD method, if the interaction was significant.
Results
Minocycline blocks LPS-induced depressive-like behavior
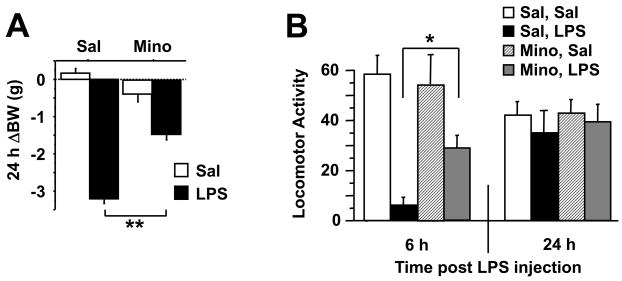
Mice were pretreated with minocycline (50 mg/kg) or physiological saline once daily for three days followed by a single i.p. injection of LPS (0.83 mg/kg) or saline in a 2 × 2 factorial arrangement of treatments. Mice were then weighed and submitted to tests of sickness or depressive-like behaviors before being euthanized for collection of brain samples. The LPS-induced sickness response was measured at 6 and 24 h post-injection by assessing changes in body weight loss (Fig. 1A), core body temperature, food consumption and locomotor activity in a new cage (Fig. 1B). As expected, there was a significant minocycline × LPS effect on the change in body weight 24 h after LPS injection (F1,46=49.39, P<0.01), and the sickness behavior-inducing properties of LPS were more marked at 6 than at 24 h and attenuated by pretreatment with minocycline (P<0.01).
Fig. 1.
Minocycline inhibits LPS-induced depressive-like behavior that is associated with blockade of brain proinflammatory cytokine and IDO upregulation. Mice were injected i.p. with saline or minocycline (50 mg/kg) for 3 successive days. Immediately following the final injection, mice also received either an i.p. injection of non-pyrogenic saline or LPS (0.83 mg/kg). (A) The 24 h change in body weight following LPS administration was measured. The general locomotor activity was measured either 6 or 24 h following LPS injection (B). The duration of immobility during the forced swim test was recorded 24 h following administration of LPS (C). The duration of immobility during the tail suspension test 28 h post-LPS was recorded cumulatively over each 10 min. test (D) or in one min. increments over 10 min (E). Immediately following behavioral testing at 28 h post-LPS, mice were sacrificed, perfused with ice-cold PBS and tissues were collected. Steady-state expression of mRNA transcripts in the brain were measured by real-time RT-PCR for (F) TNF-α (G) IL-1β (H) IFN-γ and (I) IDO. Data represent means ± SEM (n=11–14 mice/group). Bars indicate statistical differences among groups. * P<0.05, ** P<0.01. Average Ct values for saline + LPS treated mice were, IFNγ=35 ± 0.8, TNF-α=26 ± 0.2, IL-1β=26 ± 0.5, and IDO=33 ± 1.1.
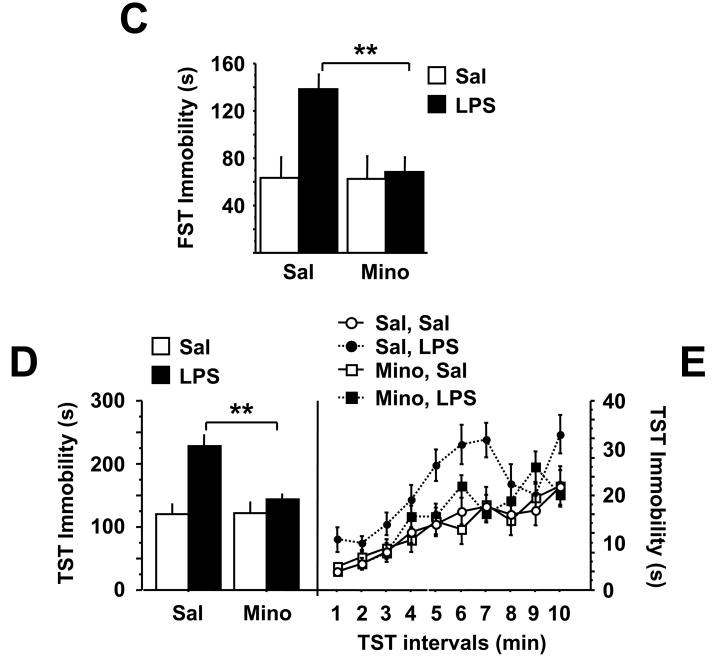
There was a significant minocycline × LPS interaction (F1,46=5.06, P<0.05) for duration of immobility in the forced swim test carried out at 24 h post-LPS injection (Fig. 1C). This is a time point when typical acute sickness behavior was no longer apparent. LPS increased duration of immobility in saline-pretreated mice (P<0.01), and this effect was totally blocked by minocyline pretreatment (P<.01). Similar results were obtained in the tail suspension test carried out 4 hours later. The minocycline × LPS interaction (F1,46=6.80, P<0.05) for the duration of immobility was significant (Fig. 1D). LPS increased the duration of immobility in the tail suspension test, and this effect was totally blocked by minocyline pretreatment. There was no significant minocycline × LPS × time interaction when the duration of immobility was measured in 1 min. intervals throughout the entire 10 min test (Fig. 1E). Collectively, these data strongly suggest that acute activation of the peripheral innate immune system with LPS induces a depressive-like syndrome in mice that is not biased by acute sickness behaviors. Moreover, an anti-inflammatory treatment is able to prevent the onset of these depressive-like behaviors.
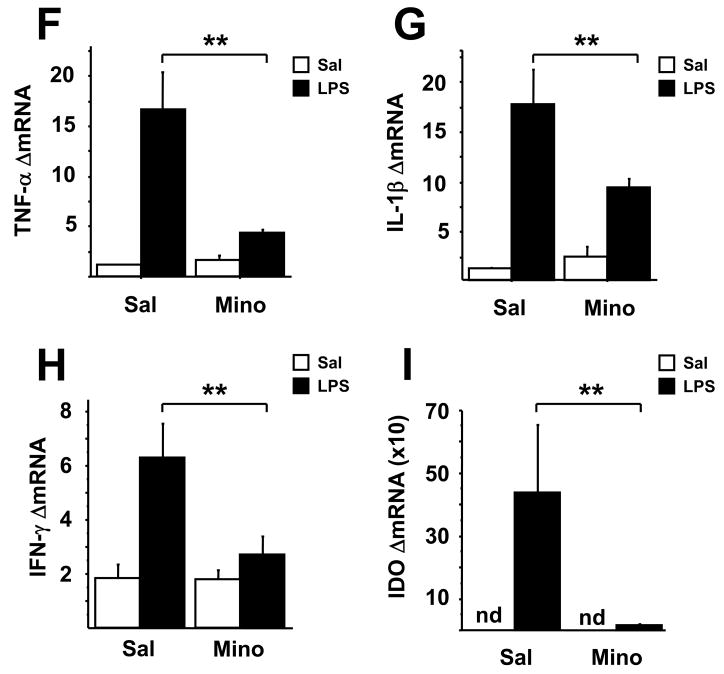
Minocycline blocks central LPS-induced cytokine and IDO expression
To confirm the anti-inflammatory effects of minocycline, we analyzed proinflammatory cytokine and IDO mRNA expression in brain samples that were collected 28 h after administration of LPS. There was a significant minocycline × LPS interaction for all variables of interest (TNFα: F1,46=14.06, P<0.001; IL-1β: F1,46=7.62, P<0.01; IFNγ: F1,46=5.98, P<0.01; IDO: F1,46=5.46, P<0.05). LPS increased expression of TNFα, IL-1β, and IFNγ steady state mRNAs (Figs. 1F, G, and H, respectively), all of which were inhibited by pretreatment with minocycline (P<0.01). Brain expression of IDO, which was undetectable in non-LPS treated mice, was markedly increased as a result of LPS treatment (P<0.01) (Fig. 1I), and this effect was completely abolished by minocycline (P<0.01). Measurement of cytokine and IDO mRNA expression in discrete brain regions (brain stem, hypothalamus, hippocampus, striatum and frontal cortex) revealed a significant, ubiquitous upregulation in all regions in response to LPS, which precluded further analysis by brain region in these studies (data not shown).
1-MT specifically inhibits IDO and depressive-like behavior
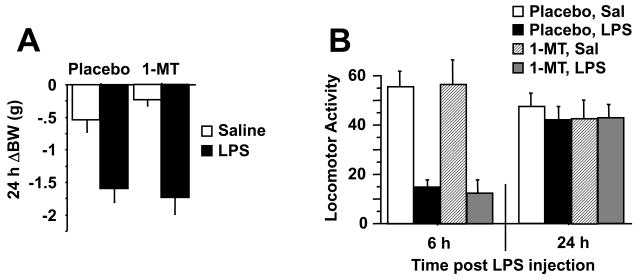
To directly target IDO in vivo, mice were implanted with slow release pellets containing either 1-MT (5 mg/d) or placebo. One week later, mice were injected i.p. with LPS (0.83 mg/kg) or physiologic saline. As opposed to the mice that had been pretreated with minocycline, 1-MT pretreament had no effect on the LPS-induced reduction in body weight (Fig. 2A), acute reduction in locomotor activity (Fig. 2B), febrile or anorectic properties of LPS (P>0.05). Indeed, the robust sickness response elicited by LPS was wholly unaffected by 1-MT.
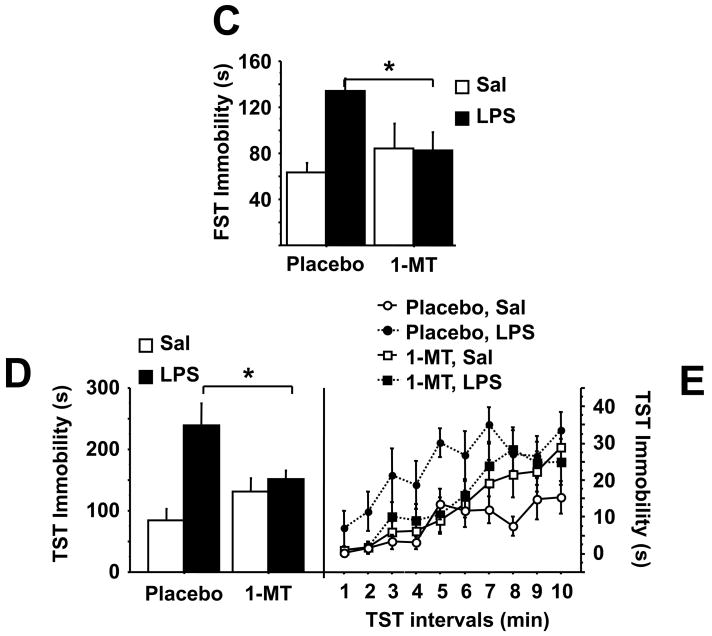
Fig. 2.
1-methyltryptophan abrogates depressive-like behavior in response to LPS without reducing expression of proinflammatory cytokines. Mice were implanted s.c. with a slow release pellet of the IDO competitive inhibitor, 1-MT or placebo. One week later, mice were injected i.p. with either non-pyrogenic saline or LPS (0.83 mg/kg). As in figure 1, the 24 h change in body weight (A) and the reduction in locomotor activity 6 or 24 h after LPS injection (B) were measured. The duration of immobility during the forced swim test was measured 24 h following administration of LPS (C). The duration of immobility during the tail suspension test was recorded 28 h post-LPS and measured either cumulatively (D) or in one min. increments over the 10 min. test (E). Immediately following behavioral testing, mice were sacrificed, perfused with ice-cold PBS and tissue collected. Steady-state expression of mRNA transcripts in the brain was measured by real-time RT-PCR for (F) TNF-α (G) IL-1β (H) IFN-γ and (I) IDO. Data represent means ± SEM (n=8 mice/group). Bars indicate statistical differences among groups. * P<0.05, ** P<0.01. Average Ct values for saline + LPS treated mice were, IFNγ=36 ± 1.7, TNF-α=27 ± 0.4, IL-1β=27 ± 0.3, and IDO=32 ± 1.6.
Depressive-like behaviors were then measured at 24 h and 28 h post-LPS injection. There was a significant 1-MT × LPS interaction for duration of immobility in both the forced swim (F1,28=5.51, P<0.05) and tail suspension tests (F1,28=6.63, P<0.05). LPS increased the duration of immobility in the forced swim test (P<0.01) and tail suspension test (P<0.01), both of which were completely ablated in 1-MT pretreated mice (P<0.05) (Fig. 2C and D). Similar to figure 1D, there was no significant minocycline × LPS × time interaction when the duration of immobility was measured in 1 min. blocks throughout the 10 min. test (Fig. 2E). There was, however, a marked increase in the cumulative duration of immobility during in LPS-treated mice, an effect that was inhibited by pretreatment with 1-MT (P<0.05) (Fig. 2D). Together, these data establish IDO as a causative factor in the precipitation of depressive-like behaviors induced by peripheral inflammation.
To determine whether 1-MT also had an anti-inflammatory effect, TNF-α, IL-1β and IFNγ (Figs. 2F, G, and H, respectively) mRNA expression was measured in the brains of mice 28 h following LPS-treatment. Cytokine expression was markedly increased in response to LPS treatment (TNFα: F1,28=16.64, P<0.01; IL-1β: F1,28=25.47, P<0.01; IFNγ: F1,28=5.87, P<0.05), but in contrast to the results with minocycline (Fig. 1F,G,H), this effect of LPS was not modified by 1-MT. However, the 1-MT × LPS interaction was significant for IDO mRNA expression (F1,28=7.62, P<0.01). Pretreatment with 1-MT significantly reduced expression of brain IDO mRNA following LPS administration (Fig. 2I).
IDO inhibition normalizes kynurenine/tryptophan, but not serotonin turnover
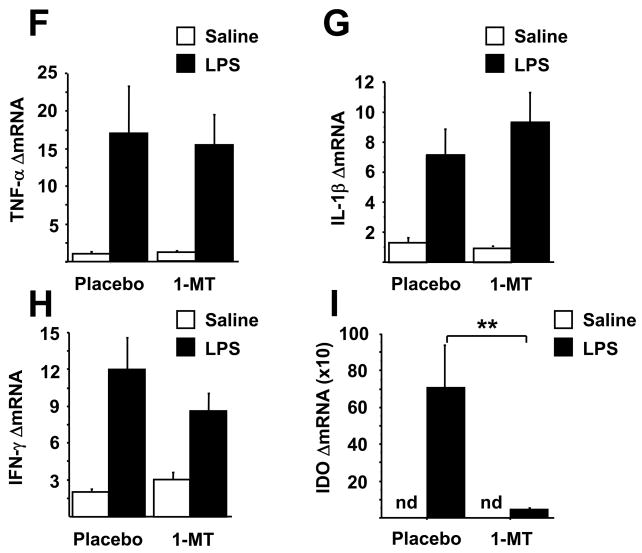
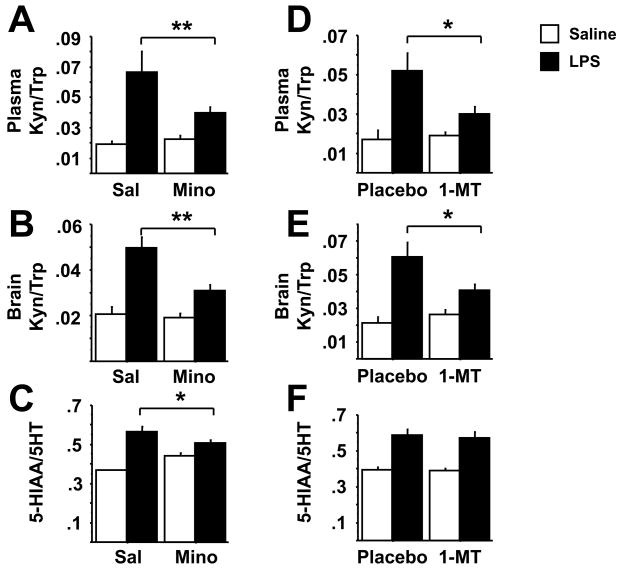
To determine the mechanism by which alterations in tryptophan degradation contribute to depressive-like behavior, we measured tryptophan, serotonin and their metabolites in the brains of mice that were collected at the end of behavioral experiments. Consistent with existing literature, we found that plasma tryptophan levels were significantly reduced and kynurenine increased in LPS-treated mice (P<0.01) (raw data not shown). There was a significant minocycline × LPS interaction (F1,46=4.56, P<0.05) and a significant 1-MT × LPS interaction (F1,28=4.27, P>0.05) for the plasma kynurenine/tryptophan ratio. Pretreatment with minocycline or 1-MT attenuated the LPS-induced increase in the plasma kynurenine/tryptophan ratio (Fig. 3A, D). Neither pretreatment restored LPS-induced depletion of tryptophan levels (data not shown; P>0.05), although they reduced kynurenine accumulation (data not shown; P<0.05). Even though tryptophan concentrations were reduced in the plasma of LPS-treated mice, brain tryptophan and serotonin concentrations were modestly but significantly higher in LPS-treated mice (tables 1A and B), with no pretreatment × treatment interactions (P>0.05). There was a significant minocycline × LPS interaction (F1,46=6.15, P<0.05) and a significant 1-MT × LPS interaction (F1,28=5.16, P<0.05) for the brain kynurenine/tryptophan ratio (Fig 3B and E). LPS increased the kynurenine/tryptophan ratio and this effect was attenuated by both minocyline (P<0.01) and 1-MT (P<0.05).
Fig. 3.
Minocycline and 1-MT attenuate the LPS-induced increases in the kynurenine/tryptophan ratio in both the plasma and the brain without affecting 5-HT turnover. Mice were treated as in figures 1 & 2 with minocycline or 1-MT prior to i.p. LPS injection. Kynurenine and tryptophan concentrations were determined by HPLC in both the plasma (A, D) and brain (B, E), and the ratio of 5-HIAA/5-HT was measured in brain tissue (C, F). Data represent means ± SEM (n=8–14 mice/group). Bars indicate statistical differences among groups. * P<0.05, ** P<0.01. Saline vs. LPS groups in 1-MT pretreated mice (D and E) approached significance (P<0.1)
Table 1.
Tryptophan, serotonin, and their metabolites were measured by HPLC in brain tissue from mice treated with LPS and pretreated with either minocycline (Table A) or 1-MT (Table B), separate experiments. Data represent mean (μmol/mg tissue) ± SEM, (n=8–14 mice/group). Means within a row with different letters are significantly different from each other at P<0.05. 5-HIAA=5-hydroxyindoleacetic acid; 5-HT=5-hydroxytryptamine or serotoni
| Table 1A. | ||||
|---|---|---|---|---|
| μmol/mg brain tissue | Saline, Saline | Saline, LPS | Minocycline, Saline | Minocycline, LPS |
| Kynurenine | 0.19 ± 0.1 a | 0.60 ± 0.1 b | 0.19 ± 0.4 a | 0.39 ± 0.1 c |
| Tryptophan | 9.37 ± 0.2 a | 12.18 ± 0.4 b | 10.20 ± 0.3 a | 12.56 ± 0.3 b |
| 5-HIAA | 1.07 ± .1 a | 1.78 ± 0.1 b | 1.27 ± 0.1 a | 1.64 ± .1 b |
| 5-HT | 2.94 ± .1 a | 3.20 ± 0.1 ab | 2.91 ± 0.1 a | 3.26 ± 0.1 b |
| Table 1B. | ||||
|---|---|---|---|---|
| μmol/mg brain tissue | Placebo, Saline | Placebo, LPS | 1-MT, Saline | 1-MT, LPS |
| Kynurenine | 0.20 ± 0.1 a | 0.66 ± 0.1 b | 0.24 ± 0.1 a | 0.47 ± 0.1 c |
| Tryptophan | 8.91 ± 0.5 a | 10.79 ± 0.8 b | 8.59 ± 0.2 a | 10.70 ± 0.4 b |
| 5-HIAA | 1.22 ± 0.1 a | 2.04 ± 0.1 b | 1.22 ± 0.1 a | 2.00 ± 0.1 b |
| 5-HT | 2.74 ± 0.1 a | 3.10 ± 0.1 b | 2.80 ± 0.1 a | 3.10 ± 0.1 b |
To further investigate the effects of minocycline and 1-MT pretreatments on LPS-induced changes in serotoninergic neurotransmission, 5-HT and its major metabolite, 5-HIAA, were measured in the brains of LPS-treated mice that were pretreated with either minocycline or 1-MT, as previously described. Turnover of serotonin was measured by comparing the ratio of 5-HIAA/5-HT. In each case, there was an LPS effect but no pretreatment × LPS interaction, except that for minocycline × LPS (F1,46=11.43, P<0.01). LPS treatment resulted in a significant 30% increase in the 5-HIAA/5-HT ratio (Figs. 3C and F) (P<0.01), and minocyline alone increased serotonin turnover. However, direct inhibition of IDO, using 1-MT pretreatment, had no effect on LPS-induced serotonin turnover (Fig. 3F) (P>0.05). Together, these results argue against any direct involvement of LPS-induced changes in 5-HT turnover rates in the depressive-like behavior that develops in LPS-treated mice. Rather, they indicate that degradation of tryptophan along the kynurenine pathway might generate neuroactive metabolites that contribute to induction of depressive-like behaviors.
L-kynurenine induces depressive-like behavior
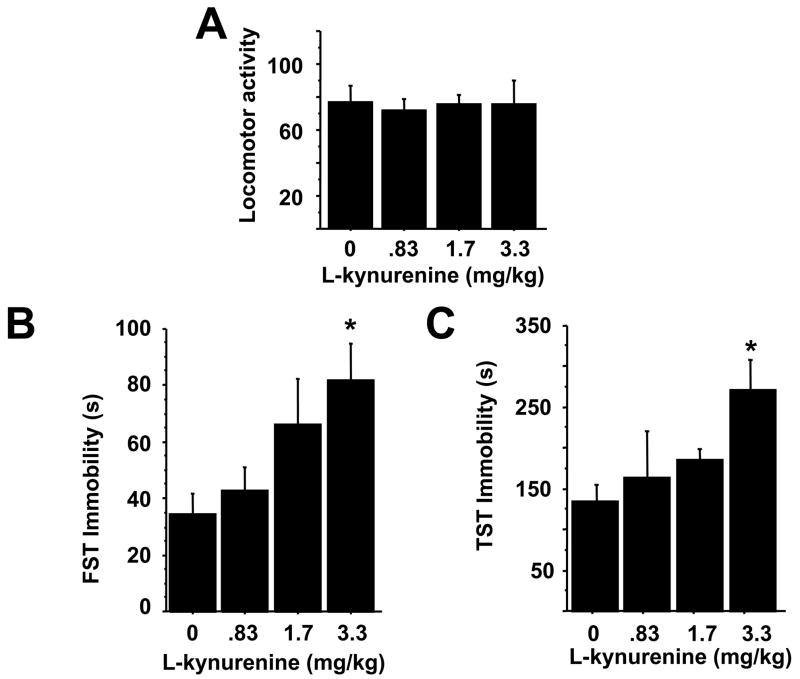
To determine if increased levels of kynurenine are capable of inducing depressive-like behaviors, independent of immune or IDO activation, we administered increasing doses of exogenous L-kynurenine i.p. to mice. Two hours later, locomotor activity was unchanged in response to L-kynurenine (Fig. 4A), but the duration of immobility in the FST and TST was significantly increased (p<0.05) at a dose of 3.3 mg/kg (Figs. 4B and C). Doses 5–10 times higher tended to increase locomotor activity, but no further change in FST or TST immobility was observed (data not shown).
Fig. 4.
Kynurenine dose-dependently induces depressive-like behavior. Mice were injected i.p. with saline or increasing amounts of L-kynurenine. Two hours later, mice were tested for changes in locomotor activity (A), immobility in the forced swim (B) or tail suspension tests (C), and the total duration of immobility was measured. Data represent means ± SEM (n=4–8 mice/group). Bars indicate statistical differences among groups. * P<0.05, ** P<0.01.
Discussion
Understanding the pathophysiological mechanisms by which inflammation and depression are linked has wide ranging implications that spans both disciplines and clinical specialties. Although IDO activation has often been proposed to mediate the relationship between inflammation and depression, its causative role had never been tested. Results of the present experiments demonstrate that inhibition of IDO by targeting proinflammatory cytokine expression (via minocycline) or IDO itself (via 1-MT) blocks development of depressive-like behaviors in mice in response to LPS. This effect appears to be independent of LPS-induced changes in serotoninergic neurotransmission and could be due to IDO-mediated generation of tryptophan neuroactive tryptophan metabolites.
Minocycline acts very early in the chain of events that leads from Toll-like receptor 4 activation by lipopolysaccharide to IDO activation and depressive-like behavior. Its ability to block macrophage and microglial activation in various in vivo animal models of peripheral or brain inflammation has already been amply documented. For instance, minocycline is neuroprotective and reduces damage to the blood brain barrier in models of ischemic stroke by attenuating microglial activation(18). This effect could be mediated by inhibition of TNFα production since a similar protective effect was observed in TNFα knock-out mice or in wild-type mice that were treated with minocycline prior to the intraparenchymal injection of a dopamine neurotoxin(28). Minocycline may also have therapeutic benefit for the treatment of HIV-induced neuroinflammation, as minocycline reduced the severity of encephalitis, suppressed viral load in the brain and decreased the expression of brain inflammatory markers in a Simian immunodeficiency model of HIV (29). Further, inhibition of spinal cord microglial activation and cytokine expression by intrathecal administration of minocycline attenuated low-threshold mechanical allodynia in two rat models of pain facilitation (30). The anti-inflammatory effects of minocycline extend to the clinic. In a 48-week long double-blinded placebo controlled study, minocycline effectively relieved joint swelling and tenderness in patients with moderate rheumatoid arthritis (31). The observation in the present study that minocycline attenuated the acute sickness response induced by LPS is therefore not surprising although the level (peripheral versus central) at which this activity occurs has not been established. Inhibition of proinflammatory cytokine expression in the brain, including TNFα, could be secondary to a peripheral anti-inflammatory activity of minocycline, or it could be due to the ability of this tetracycline to down-regulate microglial activation and therefore the increased brain projnflammatory cytokine expression that ultimately mediates the sickness-inducing effects of peripherally administered LPS (32–34). Although the ability of minocycline to inhibit expression of the inducible form of nitric oxide synthase during neuroinflammation is well documented (20), its ability to block activation of IDO had not yet been tested. IFNγ and TNF-α are the main inducers of IDO activation (5, 6). Minocycline has already been shown to block IFNγ-mediated protein kinase C (alpha/betaII) phosphorylation and nuclear translocation of both PKC (alpha/betaII) and IRF-1(17) which is necessary for IDO activation. It also inhibits nuclear factor kappa-B and MAP kinase activation (35), which are both necessary for the synergistic effects of TNFα and IFNγ on IDO activation (36).
In contrast to minocycline, the competitive antagonist 1-MT blocks only IDO without altering LPS-induced proinflammatory cytokine expression. This effect is associated with an abrogation of LPS-induced depressive-like behavior despite the absence of any alteration in LPS-induced sickness. This finding is in accord with previous published data that demonstrate independence between sickness behavior and depressive-like behavior in response to LPS (10). Since its first characterization as a competitive inhibitor of IDO (37), 1-MT has become the compound of reference for IDO blocking studies (21, 22, 38–40). Its ability to block IDO activation in response to LPS is demonstrated in the present study by the decreased kynurenine/tryptophan ratio that is observed in both the periphery and the brain of 1-MT treated mice. Since the main consequence of IDO activation is development of immunotolerance because of an inability of tryptophan-deprived T cells to proliferate and induce cytotoxicity, blockade of IDO has mainly been studied in the context of reproductive physiology, and tumor immune surveillance (41). In the brain, IDO activation has been proposed to induce neurotoxic metabolites of tryptophan that contribute to the pathogenesis of cerebral malaria (42), experimental allergic encephalomyelitis (17, 39) and Alzheimer’s disease (43). Although the implication of IDO activation in development of inflammation-associated depressive disorders has been suspected for many years based on increased peripheral concentrations of kynurenine, its causative role has never been tested.
Increases in IDO activity have the potential to negatively impact serotoninergic neurotransmission by decreasing bioavailability of tryptophan. Although a decrease in peripheral concentrations of tryptophan was observed in response to LPS, it did not translate into decreased brain tryptophan levels and was not associated with reduced serotoninergic neurotransmission. Instead, LPS induced an increased turnover of serotonin, as already described (44, 45). This alteration in serotoninergic neurotransmission was not responsible for the development of LPS-induced depressive-like behavior since it was not blocked by 1-MT. It could be argued that such an effect was not detected because we did not measure serotonin turnover in discrete brain areas. However, the fact that cytokine-induced increased serotonin turnover is dependent on diffuse microglial activation does not favor such a possibility since there is usually no difference in turnover rates between different brain areas following immune stimulation (46). The lack of an effect on serotonin turnover does not preclude the possibility of more subtle cytokine-induced alterations in serotoninergic neurotransmission, as might be caused by increases in serotonin transporter expression (47) or modulation of specific serotonin receptor subtype (47) or sensitivity. Studies designed to specifically examine the effects of LPS and IDO activation on the temporal and spatial patterns of 5-HT related processes in discrete brain regions still need to be carried out before being able to dismiss a possible serotoninergic component in the papthophysiology of LPS-induced depressive-like behavior.
An alternative interpretation for the involvement of IDO in cytokine-induced depressive-like behavior is the generation of neuroactive tryptophan metabolites. This possibility is favored by the observation that in the present study, IDO blockade normalized kynurenine levels, and reciprocally, kynurenine administration induced depressive-like behavior. This interpretation is consistent with a previous study by Wichers et. al. that used circulating levels of tryptophan vs. competing amino acids to predict that the availability of tryptophan to the brain is unchanged by immunotherapy (16). A potential role of the kynurenine pathway in depression was speculatively proposed in the early 1970’s (48, 49). While kynurenine itself is not neuroactive, it readily crosses the blood brain barrier via the large neutral amino acid transporter (14). The kynurenine pathway results in the generation of three neuroactive compounds, all of which are derived from L-kynurenine. 3-hydroxykynurenine (3-HK) and quinolinic acid (QA) are generated in route to NAD production, while kynurenic acid (KA) is formed in a ‘dead end’ branch of the pathway. 3-HK and QA generate free radicals, and QA also acts as an NMDA receptor agonist. Therefore, both these kynurenine metabolites are excitotoxic. KA, on the other hand, is an NMDA receptor antagonist and is considered to be neuroprotective(50). Intracerebroventricular administration of a high dose QA or L-kynurenine sulfate induced excitotoxic lesions or seizures, whereas administration of KA induced an anti-seizure anxiolytic response (51). However, several more recent studies indicate an important role of glutamatergic activity in depression (see review (15)). Whether kynurenine induces depressive-like behavior by itself or after further generation of its downstream neuroactive metabolites remains to be determined by experiments that block kynurenine metabolizing enzymes, e.g., kynurenine 3-hydroxylase or kynurenine aminotransferase.
It is important to note that direct or indirect inhibition of IDO in the present study had no effect on baseline duration of immobility, in the absence of immune activation. This is not surprising because IDO expression during non-inflammatory states is very low. In fact, we were unable to detect IDO mRNA in the brains of any non-LPS stimulated mouse. Only upon upregulation of IDO by LPS did we observe an increase in the kynurenine/tryptophan ratio and a concomitant induction of depressive-like behaviors. Further, our data confirm a previous study showing that minocyline (20 or 40 mg/kg) did not change the behavioral response in the FST (52). This same study indicated that LPS at doses of 10 and 100 μg/kg/mouse injected i.p. did not increase immobility in the FST (52). In our study, the dose of LPS administered was about 8-fold higher, and the behavioral response to exogenously administered L-kynurenine was dose dependent, which speaks to a possible threshold requirement for the activation of IDO, generation of neuroactive substrates and subsequent induction of depressive-like behaviors caused by peripheral immune signals.
In conclusion, the present study identifies IDO as a critical molecular mediator of inflammation-induced depressive-like behaviors. Moreover, the depressive-like behaviors are precipitated via an increase in degradation of tryptophan along the kynurenine pathway. These findings indicate that targeting the inflammatory pathways that lead to the activation of IDO, or targeting IDO itself, may provide novel therapeutic treatment strategies in patients suffering from inflammation-associated depression. In fact, minocycline, which has been used for its antibiotic activity for decades, has more recently been proposed as a potential novel treatment for various neurologic injuries. This is due to its anti-inflammatory activity that extends to the brain since this drug can easily pass the blood brain barrier (53).1-MT appears to have little, if any, toxicity issues and is currently being developed fro use as a vaccine adjuvant and combination chemotherapeutic agent (54). However, further insight into the role of downstream kynurenine pathway metabolites in this pathophysiological process is still needed to fully understand this long-standing and very complex process.
Acknowledgments
Supported by NIH grants to RD (R01 MH 71349 and R01 MH 079829), KWK (R01 AG 029573) and post-doctoral training grant to JCO (T32 DK59802-01)
References
- 1.Evans DL, Charney DS, Lewis L, Golden RN, Gorman JM, Krishnan KR, et al. Mood disorders in the medically ill: scientific review and recommendations. Biol Psychiatry. 2005;58(3):175–89. doi: 10.1016/j.biopsych.2005.05.001. [DOI] [PubMed] [Google Scholar]
- 2.Capuron L, Dantzer R. Cytokines and depression: the need for a new paradigm. Brain Behav Immun. 2003;17(Suppl 1):S119–24. doi: 10.1016/s0889-1591(02)00078-8. [DOI] [PubMed] [Google Scholar]
- 3.Capuron L, Gumnick JF, Musselman DL, Lawson DH, Reemsnyder A, Nemeroff CB, et al. Neurobehavioral effects of interferon-alpha in cancer patients: phenomenology and paroxetine responsiveness of symptom dimensions. Neuropsychopharmacology. 2002;26(5):643–52. doi: 10.1016/S0893-133X(01)00407-9. [DOI] [PubMed] [Google Scholar]
- 4.Widner B, Laich A, Sperner-Unterweger B, Ledochowski M, Fuchs D. Neopterin production, tryptophan degradation, and mental depression--what is the link? Brain Behav Immun. 2002;16(5):590–5. doi: 10.1016/s0889-1591(02)00006-5. [DOI] [PubMed] [Google Scholar]
- 5.Takikawa O, Tagawa Y, Iwakura Y, Yoshida R, Truscott RJ. Interferon-gamma-dependent/independent expression of indoleamine 2,3-dioxygenase. Studies with interferon-gamma-knockout mice. Adv Exp Med Biol. 1999;467:553–7. doi: 10.1007/978-1-4615-4709-9_68. [DOI] [PubMed] [Google Scholar]
- 6.Popov A, Abdullah Z, Wickenhauser C, Saric T, Driesen J, Hanisch FG, et al. Indoleamine 2,3-dioxygenase-expressing dendritic cells form suppurative granulomas following Listeria monocytogenes infection. J Clin Invest. 2006;116(12):3160–70. doi: 10.1172/JCI28996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fujigaki H, Saito K, Fujigaki S, Takemura M, Sudo K, Ishiguro H, et al. The signal transducer and activator of transcription 1alpha and interferon regulatory factor 1 are not essential for the induction of indoleamine 2,3-dioxygenase by lipopolysaccharide: involvement of p38 mitogen-activated protein kinase and nuclear factor-kappaB pathways, and synergistic effect of several proinflammatory cytokines. J Biochem (Tokyo) 2006;139(4):655–62. doi: 10.1093/jb/mvj072. [DOI] [PubMed] [Google Scholar]
- 8.Capuron L, Ravaud A, Neveu PJ, Miller AH, Maes M, Dantzer R. Association between decreased serum tryptophan concentrations and depressive symptoms in cancer patients undergoing cytokine therapy. Mol Psychiatry. 2002;7(5):468–73. doi: 10.1038/sj.mp.4000995. [DOI] [PubMed] [Google Scholar]
- 9.Yirmiya R. Endotoxin produces a depressive-like episode in rats. Brain Res. 1996;711(1–2):163–74. doi: 10.1016/0006-8993(95)01415-2. [DOI] [PubMed] [Google Scholar]
- 10.Frenois F, Moreau M, O’Connor J, Lawson M, Micon C, Lestage J, et al. Lipopolysaccharide induces delayed FosB/DeltaFosB immunostaining within the mouse extended amygdala, hippocampus and hypothalamus, that parallel the expression of depressive-like behavior. Psychoneuroendocrinology. 2007;32(5):516–31. doi: 10.1016/j.psyneuen.2007.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Moreau M, Lestage J, Verrier D, Mormede C, Kelley KW, Dantzer R, et al. Bacille Calmette-Guerin inoculation induces chronic activation of peripheral and brain indoleamine 2,3-dioxygenase in mice. J Infect Dis. 2005;192(3):537–44. doi: 10.1086/431603. [DOI] [PubMed] [Google Scholar]
- 12.Booij L, Van der Does AJ, Riedel WJ. Monoamine depletion in psychiatric and healthy populations: review. Mol Psychiatry. 2003;8(12):951–73. doi: 10.1038/sj.mp.4001423. [DOI] [PubMed] [Google Scholar]
- 13.Guillemin GJ, Smythe G, Takikawa O, Brew BJ. Expression of indoleamine 2,3-dioxygenase and production of quinolinic acid by human microglia, astrocytes, and neurons. Glia. 2005;49(1):15–23. doi: 10.1002/glia.20090. [DOI] [PubMed] [Google Scholar]
- 14.Fukui S, Schwarcz R, Rapoport SI, Takada Y, Smith QR. Blood-brain barrier transport of kynurenines: implications for brain synthesis and metabolism. J Neurochem. 1991;56(6):2007–17. doi: 10.1111/j.1471-4159.1991.tb03460.x. [DOI] [PubMed] [Google Scholar]
- 15.Muller N, Schwarz MJ. The immune-mediated alteration of serotonin and glutamate: towards an integrated view of depression. Mol Psychiatry. 2007;12(11):988–1000. doi: 10.1038/sj.mp.4002006. [DOI] [PubMed] [Google Scholar]
- 16.Wichers MC, Koek GH, Robaeys G, Verkerk R, Scharpe S, Maes M. IDO and interferon-alpha-induced depressive symptoms: a shift in hypothesis from tryptophan depletion to neurotoxicity. Mol Psychiatry. 2005;10(6):538–44. doi: 10.1038/sj.mp.4001600. [DOI] [PubMed] [Google Scholar]
- 17.Nikodemova M, Watters JJ, Jackson SJ, Yang SK, Duncan ID. Minocycline down-regulates MHC II expression in microglia and macrophages through inhibition of IRF-1 and protein kinase C (PKC)alpha/betaII. J Biol Chem. 2007;282(20):15208–16. doi: 10.1074/jbc.M611907200. [DOI] [PubMed] [Google Scholar]
- 18.Yrjanheikki J, Keinanen R, Pellikka M, Hokfelt T, Koistinaho J. Tetracyclines inhibit microglial activation and are neuroprotective in global brain ischemia. Proc Natl Acad Sci U S A. 1998;95(26):15769–74. doi: 10.1073/pnas.95.26.15769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Du Y, Ma Z, Lin S, Dodel RC, Gao F, Bales KR, et al. Minocycline prevents nigrostriatal dopaminergic neurodegeneration in the MPTP model of Parkinson’s disease. Proc Natl Acad Sci U S A. 2001;98(25):14669–74. doi: 10.1073/pnas.251341998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhu S, Stavrovskaya IG, Drozda M, Kim BY, Ona V, Li M, et al. Minocycline inhibits cytochrome c release and delays progression of amyotrophic lateral sclerosis in mice. Nature. 2002;417(6884):74–8. doi: 10.1038/417074a. [DOI] [PubMed] [Google Scholar]
- 21.Munn DH, Sharma MD, Lee JR, Jhaver KG, Johnson TS, Keskin DB, et al. Potential regulatory function of human dendritic cells expressing indoleamine 2,3-dioxygenase. Science. 2002;297(5588):1867–70. doi: 10.1126/science.1073514. [DOI] [PubMed] [Google Scholar]
- 22.Seo SK, Choi JH, Kim YH, Kang WJ, Park HY, Suh JH, et al. 4-1BB-mediated immunotherapy of rheumatoid arthritis. Nat Med. 2004;10(10):1088–94. doi: 10.1038/nm1107. [DOI] [PubMed] [Google Scholar]
- 23.Ueno A, Cho S, Cheng L, Wang J, Hou S, Nakano H, et al. Transient upregulation of indoleamine 2,3-dioxygenase in dendritic cells by human chorionic gonadotropin downregulates autoimmune diabetes. Diabetes. 2007;56(6):1686–93. doi: 10.2337/db06-1727. [DOI] [PubMed] [Google Scholar]
- 24.Kwidzinski E, Bunse J, Aktas O, Richter D, Mutlu L, Zipp F, et al. Indolamine 2,3-dioxygenase is expressed in the CNS and down-regulates autoimmune inflammation. Faseb J. 2005;19(10):1347–9. doi: 10.1096/fj.04-3228fje. [DOI] [PubMed] [Google Scholar]
- 25.Mormede C, Palin K, Kelley KW, Castanon N, Dantzer R. Conditioned taste aversion with lipopolysaccharide and peptidoglycan does not activate cytokine gene expression in the spleen and hypothalamus of mice. Brain Behav Immun. 2004;18(2):186–200. doi: 10.1016/S0889-1591(03)00133-8. [DOI] [PubMed] [Google Scholar]
- 26.Lestage J, Verrier D, Palin K, Dantzer R. The enzyme indoleamine 2,3-dioxygenase is induced in the mouse brain in response to peripheral administration of lipopolysaccharide and superantigen. Brain Behav Immun. 2002;16(5):596–601. doi: 10.1016/s0889-1591(02)00014-4. [DOI] [PubMed] [Google Scholar]
- 27.Porsolt RD. Animal models of depression: utility for transgenic research. Rev Neurosci. 2000;11(1):53–8. doi: 10.1515/revneuro.2000.11.1.53. [DOI] [PubMed] [Google Scholar]
- 28.Zhao C, Ling Z, Newman MB, Bhatia A, Carvey PM. TNF-alpha knockout and minocycline treatment attenuates blood-brain barrier leakage in MPTP-treated mice. Neurobiol Dis. 2007;26(1):36–46. doi: 10.1016/j.nbd.2006.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zink MC, Uhrlaub J, DeWitt J, Voelker T, Bullock B, Mankowski J, et al. Neuroprotective and anti-human immunodeficiency virus activity of minocycline. Jama. 2005;293(16):2003–11. doi: 10.1001/jama.293.16.2003. [DOI] [PubMed] [Google Scholar]
- 30.Ledeboer A, Sloane EM, Milligan ED, Frank MG, Mahony JH, Maier SF, et al. Minocycline attenuates mechanical allodynia and proinflammatory cytokine expression in rat models of pain facilitation. Pain. 2005;115(1–2):71–83. doi: 10.1016/j.pain.2005.02.009. [DOI] [PubMed] [Google Scholar]
- 31.Tilley BC, Alarcon GS, Heyse SP, Trentham DE, Neuner R, Kaplan DA, et al. Minocycline in rheumatoid arthritis. A 48-week, double-blind, placebo-controlled trial. MIRA Trial Group. Ann Intern Med. 1995;122(2):81–9. doi: 10.7326/0003-4819-122-2-199501150-00001. [DOI] [PubMed] [Google Scholar]
- 32.Kelley KW, Bluthe RM, Dantzer R, Zhou JH, Shen WH, Johnson RW, et al. Cytokine-induced sickness behavior. Brain Behav Immun. 2003;17(Suppl 1):S112–8. doi: 10.1016/s0889-1591(02)00077-6. [DOI] [PubMed] [Google Scholar]
- 33.Dantzer R, O’Connor JC, Freund GG, Johnson RW, Kelley KW. From Inflammation to Sickness and Depression: When the Immune System Subjugates the Brain. Nat Rev Neurosci. 2007 doi: 10.1038/nrn2297. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Konsman JP, Parnet P, Dantzer R. Cytokine-induced sickness behaviour: mechanisms and implications. Trends Neurosci. 2002;25(3):154–9. doi: 10.1016/s0166-2236(00)02088-9. [DOI] [PubMed] [Google Scholar]
- 35.Nikodemova M, Duncan ID, Watters JJ. Minocycline exerts inhibitory effects on multiple mitogen-activated protein kinases and IkappaBalpha degradation in a stimulus-specific manner in microglia. J Neurochem. 2006;96(2):314–23. doi: 10.1111/j.1471-4159.2005.03520.x. [DOI] [PubMed] [Google Scholar]
- 36.Robinson CM, Hale PT, Carlin JM. NF-kappa B activation contributes to indoleamine dioxygenase transcriptional synergy induced by IFN-gamma and tumor necrosis factor-alpha. Cytokine. 2006;35(1–2):53–61. doi: 10.1016/j.cyto.2006.07.007. [DOI] [PubMed] [Google Scholar]
- 37.Cady SG, Sono M. 1-Methyl-DL-tryptophan, beta-(3-benzofuranyl)-DL-alanine (the oxygen analog of tryptophan), and beta-[3-benzo(b)thienyl]-DL-alanine (the sulfur analog of tryptophan) are competitive inhibitors for indoleamine 2,3-dioxygenase. Arch Biochem Biophys. 1991;291(2):326–33. doi: 10.1016/0003-9861(91)90142-6. [DOI] [PubMed] [Google Scholar]
- 38.Munn DH, Zhou M, Attwood JT, Bondarev I, Conway SJ, Marshall B, et al. Prevention of allogeneic fetal rejection by tryptophan catabolism. Science. 1998;281(5380):1191–3. doi: 10.1126/science.281.5380.1191. [DOI] [PubMed] [Google Scholar]
- 39.Sakurai K, Zou JP, Tschetter JR, Ward JM, Shearer GM. Effect of indoleamine 2,3-dioxygenase on induction of experimental autoimmune encephalomyelitis. J Neuroimmunol. 2002;129(1–2):186–96. doi: 10.1016/s0165-5728(02)00176-5. [DOI] [PubMed] [Google Scholar]
- 40.Uyttenhove C, Pilotte L, Theate I, Stroobant V, Colau D, Parmentier N, et al. Evidence for a tumoral immune resistance mechanism based on tryptophan degradation by indoleamine 2,3-dioxygenase. Nat Med. 2003;9(10):1269–74. doi: 10.1038/nm934. [DOI] [PubMed] [Google Scholar]
- 41.Mellor AL, Munn DH. IDO expression by dendritic cells: tolerance and tryptophan catabolism. Nat Rev Immunol. 2004;4(10):762–74. doi: 10.1038/nri1457. [DOI] [PubMed] [Google Scholar]
- 42.Sanni LA, Thomas SR, Tattam BN, Moore DE, Chaudhri G, Stocker R, et al. Dramatic changes in oxidative tryptophan metabolism along the kynurenine pathway in experimental cerebral and noncerebral malaria. Am J Pathol. 1998;152(2):611–9. [PMC free article] [PubMed] [Google Scholar]
- 43.Guillemin GJ, Williams KR, Smith DG, Smythe GA, Croitoru-Lamoury J, Brew BJ. Quinolinic acid in the pathogenesis of Alzheimer’s disease. Adv Exp Med Biol. 2003;527:167–76. doi: 10.1007/978-1-4615-0135-0_19. [DOI] [PubMed] [Google Scholar]
- 44.Dunn AJ, Chuluyan HE. Endotoxin elicits normal tryptophan and indolamine responses but impaired catecholamine and pituitary-adrenal responses in endotoxin-resistant mice. Life Sci. 1994;54(13):847–53. doi: 10.1016/0024-3205(94)00621-0. [DOI] [PubMed] [Google Scholar]
- 45.Lacosta S, Merali Z, Anisman H. Behavioral and neurochemical consequences of lipopolysaccharide in mice: anxiogenic-like effects. Brain Res. 1999;818(2):291–303. doi: 10.1016/s0006-8993(98)01288-8. [DOI] [PubMed] [Google Scholar]
- 46.Swiergiel AH, Dunn AJ. Feeding, exploratory, anxiety- and depression-related behaviors are not altered in interleukin-6-deficient male mice. Behav Brain Res. 2006;171(1):94–108. doi: 10.1016/j.bbr.2006.03.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zhu CB, Blakely RD, Hewlett WA. The proinflammatory cytokines interleukin-1beta and tumor necrosis factor-alpha activate serotonin transporters. Neuropsychopharmacology. 2006;31(10):2121–31. doi: 10.1038/sj.npp.1301029. [DOI] [PubMed] [Google Scholar]
- 48.Lapin IP. Kynurenines as probable participants of depression. Pharmakopsychiatr Neuropsychopharmakol. 1973;6(6):273–9. doi: 10.1055/s-0028-1094391. [DOI] [PubMed] [Google Scholar]
- 49.Mangoni A. The “kynurenine shunt” and depression. Adv Biochem Psychopharmacol. 1974;11(0):293–8. [PubMed] [Google Scholar]
- 50.Schwarcz R. The kynurenine pathway of tryptophan degradation as a drug target. Curr Opin Pharmacol. 2004;4(1):12–7. doi: 10.1016/j.coph.2003.10.006. [DOI] [PubMed] [Google Scholar]
- 51.Nemeth H, Toldi J, Vecsei L. Role of kynurenines in the central and peripheral nervous systems. Curr Neurovasc Res. 2005;2(3):249–60. doi: 10.2174/1567202054368326. [DOI] [PubMed] [Google Scholar]
- 52.Deak T, Bellamy C, D’Agostino LG, Rosanoff M, McElderry NK, Bordner KA. Behavioral responses during the forced swim test are not affected by anti-inflammatory agents or acute illness induced by lipopolysaccharide. Behav Brain Res. 2005;160(1):125–34. doi: 10.1016/j.bbr.2004.11.024. [DOI] [PubMed] [Google Scholar]
- 53.Elewa HF, Hilali H, Hess DC, Machado LS, Fagan SC. Minocycline for short-term neuroprotection. Pharmacotherapy. 2006;26(4):515–21. doi: 10.1592/phco.26.4.515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Jia L, Schweikart K, Tomaszewski J, Page JG, Noker PE, Buhrow SA, et al. Toxicology and pharmacokinetics of 1-methyl-d-tryptophan: Absence of toxicity due to saturating absorption. Food Chem Toxicol. 2007 doi: 10.1016/j.fct.2007.07.017. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]