Abstract
Objective
To investigate the rate and mechanism of oxygen consumption by the vitreous.
Methods
Oxygen consumption was measured with a microrespirometer. Vitreous ascorbate was measured spectrophotometrically and by gas chromatography–mass spectrometry. Vitreous degeneration was related to the rate of oxygen consumption and ascorbate concentration in samples obtained during vitrectomy.
Results
Prolonged exposure to oxygen or treatment with ascorbate oxidase eliminated oxygen consumption by the vitreous. Adding ascorbate restored oxygen consumption. Oxygen consumption persisted after boiling or treating the vitreous with the chelating agents EDTA and deferoxamine. In patients undergoing retinal surgery, liquefaction of the vitreous and previous vitrectomy were associated with decreased ascorbate concentration and lower oxygen consumption.
Conclusions
Ascorbate in the vitreous decreases exposure of the lens to oxygen. The catalyst for this reaction is not known, although free iron may contribute. The gel state of the vitreous preserves ascorbate levels, thereby sustaining oxygen consumption. Vitrectomy or advanced vitreous degeneration may increase exposure of the lens to oxygen, promoting the progression of nuclear cataracts.
Clinical Relevance
Determining how the eye is protected from nuclear cataracts should suggest treatments to reduce their incidence.
Age-related cataract is the leading cause of blindness worldwide, with almost 20 million individuals bilaterally affected. In developing countries, 50% to 90% of blindness is caused by cataracts.1 In the United States, surgical removal of cataracts is the most frequently performed ophthalmic surgery, at an annual cost to the US Medicare system of $3 billion.2 Preventing cataracts requires an understanding of the pathogenic mechanisms of lens opacification.
Increased exposure of the lens to molecular oxygen is implicated in the pathogenesis of nuclear sclerotic cataract, the most common type of age-related cataract. It is well established that oxidative damage to proteins in the center of the lens, the lens nucleus, is a hallmark of the disease and plays a central role in opacification.3,4 Under normal physiologic conditions, the human lens exists in an environment with very low oxygen partial pressure.5–7 Nonphysiologic conditions demonstrate the potential effect of increased oxygen exposure on the lens. Nearly 50% of patients undergoing long-term hyperbaric oxygen therapy developed nuclear cataract in 1 to 3 years.8 Shorter-term hyperbaric oxygen exposure caused a myopic shift, an early symptom of nuclear sclerosis.8–11 Vitrectomy surgery, a procedure in which the vitreous gel is removed from the eye and replaced with saline, usually during surgery for retinal disease, causes rapid progression of nuclear cataracts, resulting in the need for cataract surgery in 60% to 95% of patients within 2 years.12–15
The distribution of oxygen in the eye is tightly regulated. Under physiologic conditions, oxygen diffuses from the retinal vasculature into the vitreous gel near the surface of the retina.16–20 Simultaneously, the lens is maintained under hypoxic conditions.6,7,21 Consequently, there is a gradient of oxygen concentration in the vitreous body between the retina and the lens.16,18,19,22,23 After vitrectomy surgery, the oxygen gradient is reduced or absent and the lens is exposed to increased oxygen.7,22 Oxygen levels are lowest in the center of the vitreous gel, suggesting that the vitreous may consume oxygen.7
We hypothesized that the intraocular oxygen concentration gradients in the posterior segment of the eye are actively maintained by the vitreous body.24 An intact gel structure is critical to this process. Loss of the structure of the vitreous body, as a consequence of either agerelated degeneration24 or vitrectomy,12–14 would lead to increased exposure of the lens to oxygen from the retina and increased risk of nuclear cataracts.
The experiments reported herein demonstrate that human vitreous gel consumes oxygen by an ascorbatedependent mechanism. More intact gel vitreous had a higher concentration of ascorbate and consumed oxygen more rapidly than the liquefied vitreous resulting from age-related vitreous degeneration or previous vitrectomy. Therefore, loss of the vitreous gel structure correlated with loss of vitreous function. This may account for the increase in Po2 near the lens and the rapid progression of nuclear cataracts that occurs after vitrectomy or advanced vitreous liquefaction.
METHODS
OXYGEN CONSUMPTION BY VITREOUS FROM CADAVER EYES
Vitreous was extracted from cadaver eyes within 12 to 48 hours after death24 or obtained at vitreous surgery (see “Oxygen Consumption by Vitreous Obtained at Surgery” section). Cadaver vitreous was frozen at −80°C for up to 1 week. Liquid vitreous was transferred to a custom-designed glass tube. Care was taken to exclude air bubbles. The tube was sealed, and a modified Po2 optical oxygen sensor was inserted through a septum in one end (Oxylab; Oxford Optronics, Oxford, England). Transfer of the sample to the tube exposed the vitreous to room air, increasing the oxygen level from its normal low level in the eye of approximately 10 mm Hg7 to greater than 100 mm Hg. The tube was placed on a heating block at 35°C in the dark. Oxygen consumption was measured for at least 2 hours. Aliquots of vitreous were immersed in boiling water for 10 minutes. The vitreous was cooled and tested for its ascorbate concentration (3 samples) and ability to consume oxygen (5 samples). Oxygen consumption was also measured after adding EDTA, a chelating agent for divalent and trivalent cations, to a final concentration of 10mM (7 samples), or deferoxamine mesylate, an iron chelator, to a final concentration of 100µM or 500µM (3 samples). Cadaver vitreous was also pretreated with 1 to 2 U of ascorbate oxidase (Sigma-Aldrich Corp, St Louis, Missouri) to test the dependence of oxygen consumption on ascorbate (12 samples). Samples of cadaver vitreous were stirred for 48 hours at room temperature while being exposed to a stream of moist 5% oxygen and 95% nitrogen (5 samples). Untreated vitreous, oxygen-treated vitreous, and aliquots of oxygen-treated vitreous to which ascorbate had been added were assayed to determine the rate of oxygen and ascorbate consumption. Ascorbate was also dissolved in water that had been purified by ion exchange and reverse osmosis (Milli-Q; Millipore Corp, Billerica, Massachusetts) and tested for oxygen consumption and ascorbate degradation (5 samples).
ASCORBATE MEASUREMENTS
Ascorbate concentration was quantified in triplicate, based on the ability of ascorbate to reduce Fe3+ to Fe2+ and the resulting change in the A525 of complexes of Fe2+ with 2,2′-dipyridyl.25 This assay was modified to permit the analysis of 15-µL samples. A freshly prepared standard curve was used for all measurements. The specificity of this assay for ascorbate in the vitreous was assessed by means of gas chromatography– mass spectrometry (GC-MS) on a subset of vitreous samples and on the ascorbate standard used for the colorimetric assay. Samples (5 µL) of vitreous humor from donor eyes or ascorbate standards were mixed with a known amount of uniformly carbon 13–labeled ascorbic acid (13C6-ascorbic acid [Omicron Biochemicals, South Bend, Indiana]), dried and reacted with N,O-bis(trimethylsily)trifluoroacetamide. The sample was separated on a gas chromatograph (Varian Inc, Palo Alto, California) using a 30-m, 0.25-mm–internal diameter GC column with a 0.25-µm film (DB-5ms column; P.J. Cobert Associates Inc, St Louis, Missouri). The sample was maintained at 80°C for 1 minute, then eluted with a temperature gradient of 80°C to 300°C at 15°C/min. The injection port and transfer line were at 250°C and the source temperature at 200°C of a mass spectrometer (Finnigan MS SSQ7000; Thermo Electron Corp, Waltham, Massachusetts) operated in the electron ionization mode at 70 eV. The concentration of ascorbate in vitreous humor was calculated from the ratio of carbon 13– to carbon 12– labeled ascorbate.
OXYGEN CONSUMPTION BY VITREOUS OBTAINED AT SURGERY
Approval for the studies of oxygen consumption and ascorbate concentration in vitreous from patients and human cadaver material was obtained from the Human Studies Committee at Washington University School of Medicine and conformed to the Declaration of Helsinki for the Protection of Human Subjects. Patients undergoing vitrectomy surgery for a variety of retinal conditions between June 1, 2005, and January 31, 2007, were asked to participate in the research study. All patients signed informed consent. Patient age, sex, presence of diabetes mellitus, surgical eye diagnosis, presence of partial posterior vitreous detachment, previous ocular surgery history, type of anesthesia, procedure performed, adverse events, and any deviations from the study protocol were recorded.
A standard 3-port pars plana vitrectomy was performed on each study eye. Before the infusion was turned on, a 1-mL syringe was connected to the vitrectomy probe and 0.25 to 0.3 mL of vitreous was slowly drawn from the center of the vitreous cavity. In the operating room, a 50-µL sample of vitreous was removed and stored on dry ice for ascorbate assay. The remainder of the undiluted vitreous sample was immediately transferred to a microrespirometer, as described in the “Oxygen Consumption by Vitreous From Cadaver Eyes” section. Oxygen consumption (microliters per milliliter per hour) was recorded for at least 2 hours, although to provide the most sensitive and accurate measures we reported the rate of consumption during the first 30 minutes of measurement. Samples that consumed oxygen most rapidly during the first 30 minutes also consumed oxygen most rapidly during the final 30 minutes (data not shown).
SCORING VITREOUS LIQUEFACTION
A grading method for human vitreous liquefaction was established for the purposes of this study. Before the starting measurement of oxygen consumption was determined by the method described earlier, the elasticity and viscosity of a sample of vitreous was assessed on a 5-point scale from 1 (firm gel) to 5 (most fluid) by the investigator handling the samples, an experienced ocular surgeon (Y.-B.S.). The retinal surgeon (N.M.H.) independently scored the condition of the vitreous on the basis of its physical appearance during vitrectomy, using the same 5-point scale. In most cases, the same score was assigned by the 2 independent evaluators and grades never differed by more than 1 point. In the 6 cases in which grades differed, they were averaged to provide a final grade for each patient before the results of oxygen consumption and ascorbate levels were known.
STATISTICS
Samples were compared by 1-way analysis of variance and linear regression (Instat; GraphPad Software Co, La Jolla, California). Statistical significance was considered to be P <.05.
RESULTS
OXYGEN CONSUMPTION IN HUMAN VITREOUS
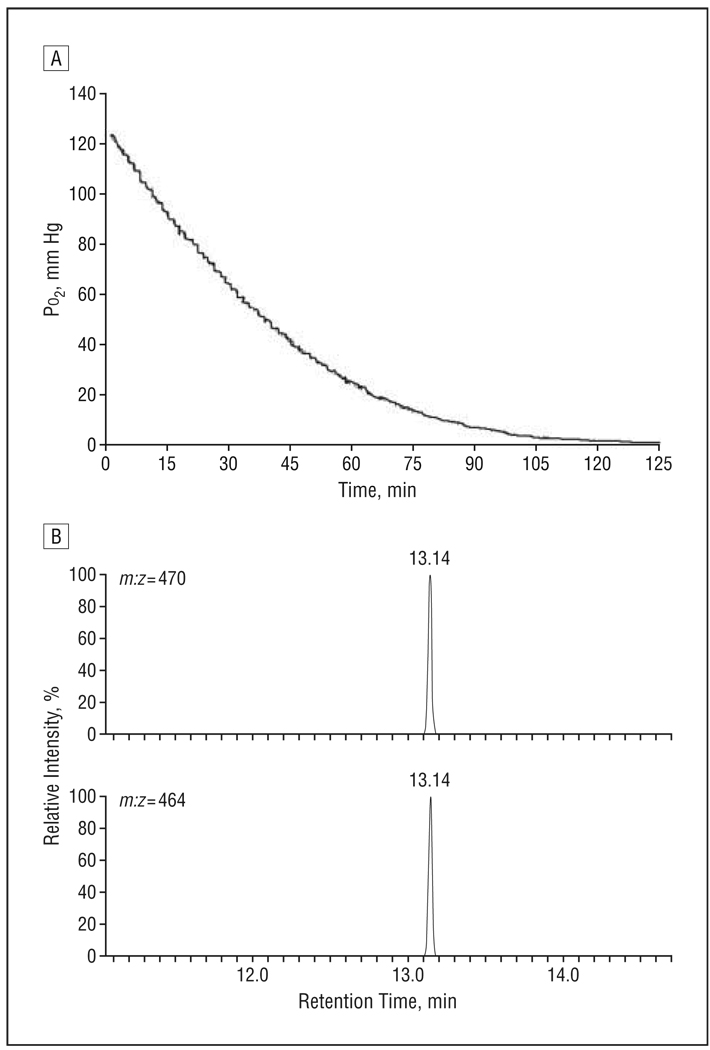
In vitreous from cadaver eyes, Po2 rapidly decreased from approximately 100 mm Hg, usually reaching undetectable levels within 2 hours. A representative oxygen consumption curve from a donor eye is shown in Figure 1A.
Figure 1.
Measurement of oxygen consumption and verification of ascorbate assay. A, Representative graph showing the change in oxygen consumption with time in vitreous obtained from a donor eye. B, Reconstructed ion chromatogram showing the results of gas chromatography–mass spectrometry analysis using selected ion monitoring of a sample of uniformly carbon 13–labeled ascorbic acid (13C6-ascorbic acid) (upper trace) and ascorbate in human vitreous (lower trace). m:z indicates mass to charge ratio.
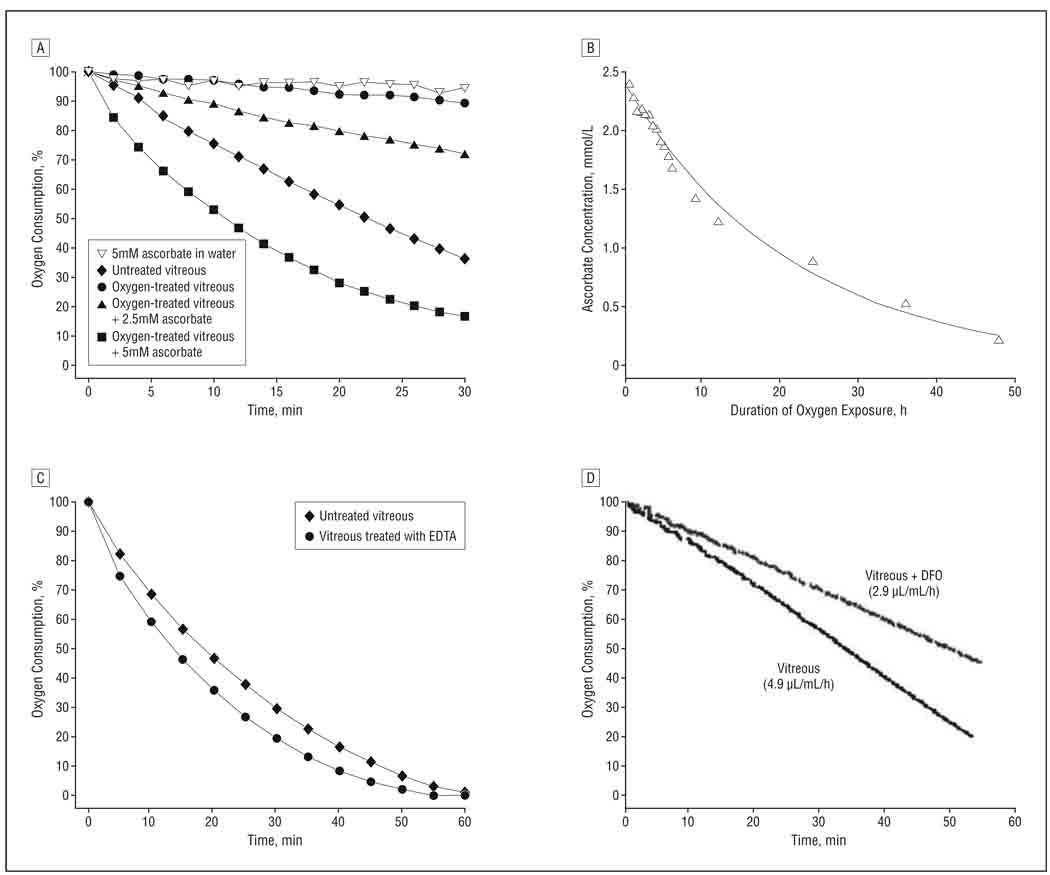
Ascorbate (vitamin C), which is reported to be found at a high concentration in the human eye, has been postulated to react with oxygen in the ocular fluids.26–28 We measured ascorbate levels in vitreous by colorimetric assay and confirmed the presence of ascorbate and the accuracy of the ascorbate assay by GC-MS (Figure 1B; Table 1). Depletion of ascorbate by treating the vitreous with ascorbate oxidase eliminated its ability to consume oxygen (not shown). Pretreating human vitreous with a stream of 5% oxygen for 48 hours greatly slowed its subsequent ability to consume oxygen (Figure 2A, solid circles), compared with untreated vitreous (Figure 2A, solid diamonds). Supplementing oxygen-treated vitreous with ascorbate restored its ability to consume oxygen in a dose-dependent manner (Figure 2A, solid triangles and solid squares). Ascorbate dissolved in water purified by ion exchange chromatography and reverse osmosis (Milli-Q water purifier; Millipore Corp) did not consume detectable quantities of oxygen (Figure 2A, solid diamonds). Ascorbate levels decreased substantially during exposure of vitreous to oxygen, indicating that ascorbate was consumed during exposure (Figure 2B).
Table 1.
Ascorbate Levels in Vitreous Measured by Colorimetric and GC-MS Assay
| Ascorbate Level, mmol/L | |||||
|---|---|---|---|---|---|
| Sample | |||||
| No. | Assay | No Treatment | AOa | Boil 10 min | AO + Boil + Ascorbateb |
| 1 | Colorimetric | 1.9 | 0.1 | 1.1 | 1.7 |
| GC-MS | 2.2 | 0.0 | 1.2 | 1.7 | |
| 2 | Colorimetric | 1.2 | 0.1 | 0.5 | 1.7 |
| GC-MS | 1.7 | 0.0 | 0.7 | 1.7 | |
| 3 | Colorimetric | 0.4 | 0.0 | 0.0 | 1.7 |
| GC-MS | 0.5 | 0.0 | 0.0 | 1.7 | |
Abbreviations: AO, ascorbate oxidase; GC-MS, gas chromatography–mass spectrometry.
Pretreated with AO.
Vitreous that was treated with AO and boiled, then supplemented with a constant amount of ascorbate.
Figure 2.
Results of oxygen consumption studies. A, Oxygen consumption in untreated donor vitreous or vitreous exposed to 5% oxygen for 48 hours. B, Changes in ascorbate concentration during exposure of donor vitreous to 5% oxygen. The line is the best fit of the data to a first-order reaction. C, Oxygen consumption by untreated vitreous or vitreous brought to a concentration of 10mM with EDTA. D, Oxygen consumption by human vitreous (4.9 µL/mL/h) and vitreous supplemented with deferoxamine mesylate (DFO) to a final concentration of 100µM DFO (2.9 µL/mL/h).
To test whether cellular mechanisms or endogenous enzymes might be responsible for the reaction between ascorbate and oxygen, samples of vitreous were immersed in boiling water for 10 minutes. Although heating decreased ascorbate levels by approximately onehalf (Table 1), the vitreous still consumed oxygen at rates that were similar to those of untreated cadaver vitreous with similar levels of ascorbate (not shown). Addition of EDTA to vitreous samples to a final concentration of 10mM did not reduce the rate of oxygen consumption and may have slightly stimulated oxygen consumption (Figure 2C). As reported previously, cupric ions (10–100µM) catalyzed the reaction of ascorbate with oxygen in a model reaction.28,29 Addition of 1 to 10mM EDTA prevented this reaction (not shown). Addition to human vitreous of 100µM deferoxamine, an iron chelator that inhibits the ability of iron to catalyze oxidation reactions,30 reduced oxygen consumption by 30% to 50% (Figure 2D). Increasing the concentration of deferoxamine to 500µM did not further decrease oxygen consumption (not shown).
EFFECT OF LIQUEFACTION OF THE VITREOUS
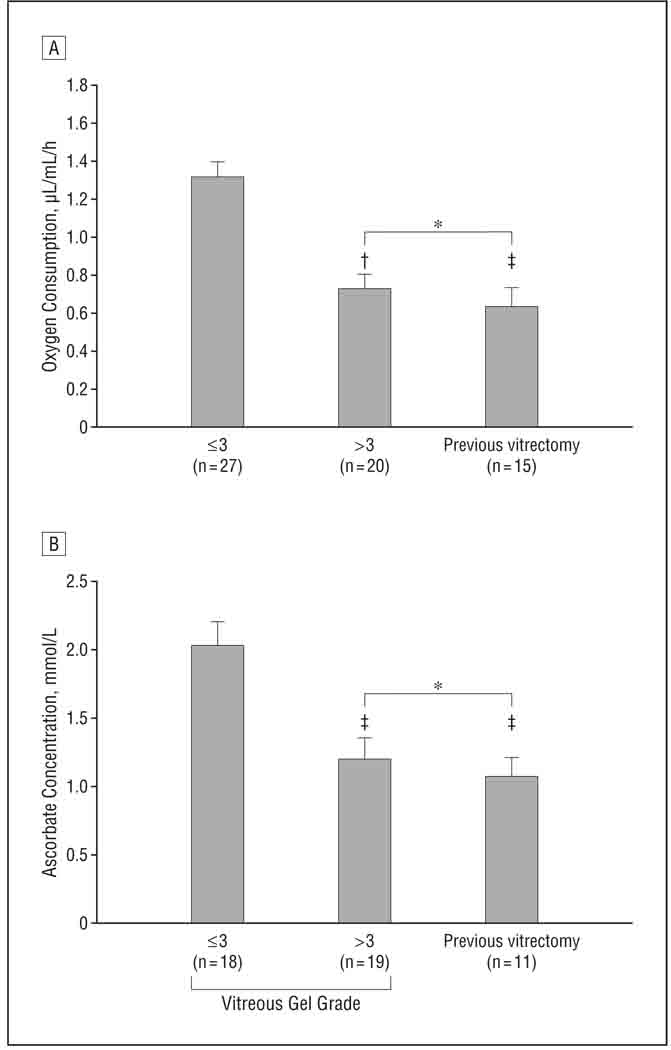
To determine whether oxygen consumption by cadaver vitreous might be the result of postmortem changes in the eye, undiluted samples of vitreous gel were obtained from consenting patients at the initiation of vitrectomy surgery. Fresh vitreous consumed oxygen at rates comparable to that of samples from postmortem eyes (Figure 3A). Because loss of the gel structure of the vitreous body is a risk factor for nuclear cataract formation, we examined the effect of vitreous liquefaction on the ability of the vitreous to consume oxygen.
Figure 3.
Mean rate of oxygen consumption (A) and mean ascorbate concentration (B) in vitreous samples obtained at the time of surgery. In patients who were undergoing their first vitrectomy, the gel status of the vitreous was scored on a 5-point scale. Vitreous that received a score of 3 or less had firmer gel; greater than 3, more liquid. *P>.05. †P<.001. ‡P<.01.
The rate of oxygen consumption was measured in 62 patients undergoing vitrectomy surgery for common retinal diseases (Table 2). Sixty-six patients were recruited for the study. Four patients were excluded, 2 because of malfunction of the oxygen probe and 2 because the eye became too soft intraoperatively to safely collect vitreous samples. Of the patients included in the study, 15 had had previous vitrectomy surgery and, therefore, had little or no vitreous gel. In the remaining 47 patients undergoing their first vitrectomy, the gel state of the vitreous body was assessed on a 5-step scale, with a score of 1 denoting firm gel vitreous, and 5, fully liquid vitreous.
Table 2.
Reasons for Surgery
| Reason | No. of Eyes |
|---|---|
| First vitrectomy | 47 |
| Macular hole | 11 |
| Diabetic retinopathy | 14 |
| Macular pucker | 8 |
| Retinal detachment | 6 |
| Premacular fibroplasia | 3 |
| Vitreous opacity | 3 |
| Vitreoretinal traction syndrome | 2 |
| Previous vitrectomy | 15 |
| Recurring macular hole | 5 |
| Recurring retinal detachment | 5 |
| Recurring premacular fibroplasia | 3 |
| Recurring macular pucker | 2 |
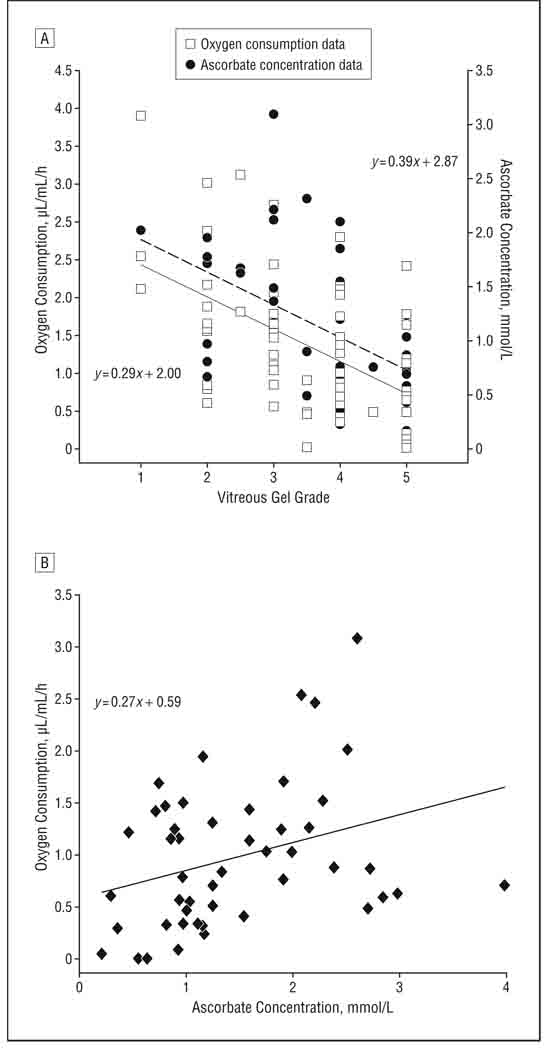
The rate of oxygen consumption for individuals with vitreous of different gel grades is shown in Figure 3A. Samples with more gelled vitreous (score ≤3) had the highest rate of oxygen consumption. Samples with a more liquefied vitreous (score >3) and samples from individuals who had had previous vitrectomy surgery consumed oxygen at significantly lower rates (P< .001 and P=.002, respectively). The rates of oxygen consumption in eyes with more liquefied vitreous and previous vitrectomy were not significantly different from each other. For eyes that had not undergone previous vitrectomy surgery, regression analysis showed that the initial rate of oxygen consumption and the gel state of the vitreous were linearly related, with a slope that was significantly different from 0 (P < .001; Figure 4A, dashed line).
Figure 4.
Scatterplots of oxygen consumption and ascorbate concentration. A, Oxygen consumption and ascorbate concentration relative to the vitreous liquefaction score of samples obtained from patients with no previous vitrectomy. Lower liquefaction score indicates a firmer gel; higher numbers are more liquid. The best linear fit to the oxygen consumption data is shown by the dashed line and that to the ascorbate concentration by the solid line. B, Relationship between oxygen consumption and ascorbate concentration for the 48 samples in which both were measured.
Ascorbate concentration was determined in 48 of the 62 samples in which oxygen consumption was measured. The mean (SD) ascorbate level for all subjects tested was 1.46 (0.82) mmol/L. Ascorbate concentration showed a relationship similar to that of oxygen to the gel state of the vitreous (Figure 3B). Samples with more gel vitreous had higher levels of ascorbate, while samples with more liquefied vitreous or samples from eyes with previous vitrectomy had ascorbate levels that were approximately 40% lower (P = .002 and <.001, respectively, for each). There was no significant difference between the ascorbate concentration in the vitreous of individuals with advanced vitreous liquefaction and those with previous vitrectomy. For individuals with no previous vitrectomy, the relationship between ascorbate concentration and vitreous liquefaction could be described by a straight line with a slope that was significantly different from zero (P=.02; Figure 4A, solid line). Comparison of the ascorbate concentration and oxygen consumption for each of the 48 samples in which both were measured showed that ascorbate concentration was linearly associated with oxygen consumption (P=.03; correlation coefficient, 0.32; Figure 4B). Despite this association, it appeared that at least 1 unknown factor influenced the relationship between oxygen consumption and ascorbate concentration because some samples with high ascorbate levels had unexpectedly low oxygen consumption and some samples with lower levels of ascorbate showed relatively high oxygen consumption (Figure 4B).
Multiple regression analysis examining the effects of age, sex, diabetes mellitus, panretinal photocoagulation, posterior vitreous detachment, previous cataract surgery, degree of vitreous liquefaction, rate of oxygen consumption, and ascorbate concentration showed that age was significantly associated only with a lower chance of being diabetic (P=.002) and greater likelihood of having had cataract surgery (P=.001). Greater vitreous liquefaction was associated with previous cataract surgery (P=.02), lower oxygen consumption (P <.001), and lower ascorbate concentration (P=.04).
COMMENT
It has been suggested that the main functions of the vitreous gel are to maintain the volume of the globe and provide optically clear media. Consumption of oxygen in an ascorbate-dependent manner identifies a novel biochemical property of human vitreous and links it to an important physiologic function. Oxygen consumption by the vitreous may serve to maintain lower levels of molecular oxygen near the lens, protecting the lens from nuclear cataract.
Identification of the relationship between vitreous liquefaction, ascorbate concentration, and oxygen consumption required a method to estimate the gel state of the vitreous. We evaluated several methods to determine the extent of vitreous liquefaction, including magnetic resonance imaging, high-resolution ultrasonography, optical coherence tomography, rheology, and dynamic light scattering. Our pilot studies indicated that none was sufficiently sensitive or suited to assess the degeneration of the vitreous and also readily applicable to clinical specimens obtained during surgery (data not shown). We developed a subjective scoring system to assess liquefaction, performed by 2 experienced investigators in a masked fashion. The agreement between the evaluators using this method suggested that it provided an adequate scoring system until an objective, clinically feasible method is identified and validated.
The concentration of ascorbate in human vitreous is remarkably high. In this study, the mean concentration of ascorbate in the vitreous from eyes with a more intact gel (score ≤3) was approximately 2 mmol/L, whereas blood levels are only 50 to 60 µmol/L, a 33- to 40-fold difference.31 The specificity and accuracy of the colorimetric assay for vitreous ascorbate used for all clinical specimens was confirmed by GC-MS analysis using an internal standard of uniformly labeled 13C-ascorbic acid. A previous study,27 which used high-performance liquid chromatography with UV detection to measure ascorbate in vitreous humor from patients with a variety of retinal diseases, detected levels of ascorbate between 0.7 and 2.5 mmol/L, a range similar to that found in this study.
The function of the high level of ascorbate found in human vitreous has received surprisingly little experimental evaluation. The ability of ascorbate to scavenge free radicals and the high level of ascorbate in the eyes of diurnal animals suggested that the vitamin might protect against oxidative or photo-oxidative damage.32,33 Pirie26 noted that ascorbic acid in aqueous humor was degraded more rapidly in light and concluded that light stimulated the reaction of ascorbate with oxygen to produce hydrogen peroxide, although oxygen was not measured and the method used was later shown to report excessive levels of hydrogen peroxide in the presence of ascorbate.34 Eaton28 speculated that the reaction of ascorbate with oxygen might protect the lens from oxidative damage and showed that addition of porcine aqueous or vitreous humor to solutions of ascorbate and copper sulfate stimulated oxygen consumption. However, Eaton did not indicate whether the intraocular fluids consumed oxygen without added copper. Our study demonstrated that ascorbate metabolizes molecular oxygen in undiluted human vitreous without an exogenous catalyst and in a manner that is not dependent on light. Together with previous measurement of oxygen gradients in the human eye,7 this observation demonstrates, as Eaton predicted, that oxygen is continually removed from the vitreous by reaction with ascorbate.
The reaction between ascorbate and oxygen produces dehydroascorbate and hydrogen peroxide. Hydrogen peroxide reacts with another molecule of ascorbate to produce dehydroascorbate and water or may be directly converted to water by catalase in the ocular fluids.35 Unless reduced to ascorbate by cellular enzymatic activity or extracellular glutathione, dehydroascorbate rapidly hydrolyzes to 2,3-diketogulonic acid.36 The concentration of glutathione in human cadaver vitreous is reported to be 0.23 mmol/L, nearly 10 times lower than that of ascorbate.37 Glutathione is, therefore, unlikely to regenerate a significant amount of ascorbate. Although both ascorbate and oxygen are consumed in the vitreous, ascorbate is replenished by active transport from the blood by the ciliary epithelium.38,39 The concentration of ascorbate in the vitreous is maintained by a sodiumdependent ascorbate transporter (SLC23A2) in the pigmented layer of the ciliary epithelium.40 The rat ortholog of this transporter shows half-maximal saturation at an ascorbate concentration of approximately 10 µmol/L, well below the concentration in human blood.40 Unless ascorbate transport by human ciliary epithelial cells is markedly less efficient than that in rats, dietary supplementation with ascorbate is unlikely to significantly increase the concentration of ascorbate in the vitreous. This is consistent with the results of recent interventional trials, which found that dietary supplementation with vitamin C did not protect against nuclear cataract.41,42
The heat-stable catalyst in vitreous that promotes the reaction between ascorbate and oxygen is not known. The oxidation of ascorbate by oxygen can be catalyzed by metal ions, such as copper or iron, and trace concentrations of metals have been detected in human vitreous.28,43,44 However, although 1mM to 10mM EDTA completely prevented copper from catalyzing the oxidation of ascorbate by oxygen in a model reaction, treatment of vitreous with 10mM EDTA slightly enhanced the consumption of oxygen by vitreous humor, as described previously for Fe3+-EDTA complexes.29 Deferoxamine, which binds iron and inhibits its ability to catalyze oxidation reactions,30 only partially inhibited the ascorbate-dependent consumption of oxygen. This suggests that free iron contributes to this reaction, but that at least 1 other catalyst is involved. Variations in the rate of vitreous oxygen consumption that were not accounted for by the concentration of ascorbate might be explained by variations in the level of 1 or more catalysts in human vitreous. The identification of these agents is required before this possibility can be directly tested.
This study found that gel vitreous has a higher concentration of ascorbate and consumes oxygen at a faster rate than does liquid vitreous (ie, vitreous gel that has undergone age-related liquefaction or surgical removal). A standing concentration gradient of oxygen is found near the retinal vessels, providing a major source of oxygen to the vitreous.16,19,23 We propose that, when the vitreous is mostly in the gel state, oxygen diffusing into the gel from these vessels is taken up and metabolized by nearby retinal tissue, as shown by oxygen microelectrode studies in experimental animals.23 However, when the vitreous liquefies, oxygen diffusing from the retinal vessels is readily carried away and distributed throughout the eye by fluid currents generated by convection and movement of the eyes or head.24,45 Therefore, as suggested previously, the critical difference between gel and liquid vitreous may be the extent of mixing that occurs in each.45 The more that oxygen is mixed with the vitreous fluid, the more opportunity it will have to react with ascorbate. If the rate of transport of ascorbate into the eye is constant, the net result of increased mixing would be a lower concentration of ascorbate in the vitreous fluid, slowing the consumption of oxygen and permitting more oxygen to reach the lens. This hypothesis is consistent with previous studies,7,22,46 in which vitreous oxygen tension was higher in eyes that had undergone vitrectomy or experimental destruction of the vitreous gel than in eyes with undisturbed vitreous.
There is ample clinical evidence that destruction of the vitreous gel contributes to nuclear cataract formation. Stickler syndrome, which results from mutations in COL2A1, the major collagen forming the vitreous gel, is a constellation of pathological severe myopia, retinal detachment, early vitreous liquefaction, and nuclear cataract.47 Several studies have reported that eyes with severe myopia, a condition associated with early-onset vitreous liquefaction, develop nuclear cataract more frequently or at an earlier age.48–50 We previously found that liquefaction of the vitreous gel is a risk factor for nuclear opacities.24 Finally, in individuals older than 50 years, vitrectomy surgery is associated with a 2-year incidence of nuclear cataract formation of up to 95%.12–14 Importantly, the increased risk of nuclear cataract is avoided when retinal surgery is performed in a manner that preserves the gel structure of the vitreous body.51 These clinical scenarios suggest that liquefaction of the vitreous gel may be a unifying step leading to nuclear cataract.
On the basis of interactions between the gel state of the vitreous, ascorbate concentration, intraocular oxygen levels, and the formation of nuclear opacities found in this and previous studies, it should be feasible to formulate strategies to delay or prevent nuclear cataracts. These could include restoring the gel vitreous after vitrectomy or supplementing ascorbate to the vitreous cavity. If these or similar treatments reduced the progression of nuclear sclerotic cataract, they would provide experimental confirmation of the importance of the gel vitreous in preventing nuclear cataract and make a significant step toward preventing this costly and debilitating disease.
Acknowledgments
Funding/Support: This study was supported by the Washington University Department of Ophthalmology and Visual Sciences, an unrestricted grant from Research to Prevent Blindness, a Research to Prevent Blindness Senior Scientist award (to Dr Beebe), and grants EY04853 and EY15863 from the National Eye Institute (to Dr Beebe). Mr Wilkins was supported by a National Glaucoma Research grant from the American Health Assistance Foundation (to Dr Siegfried). The mass spectrometry facility of the Clinical Nutrition Research Unit at Washington University is supported by grants P41 RR00954, P60 DK20579, and P30 DK56341 from the National Institute of Diabetes and Digestive and Kidney Diseases, National Institutes of Health.
Role of the Sponsors: The sponsors of this research had no role in the study design; collection, analysis, and interpretation of the data; and preparation, review, or approval of the manuscript; or in the decision to submit the manuscript for publication.
Additional Contributions: We are indebted to one of the reviewers of the manuscript for pointing out the possibility that iron-EDTA complexes may enhance ascorbate oxidation. Jessie Hardges, BS, Byron Henderson, BA, and Orlando Crisp, BA, of the Anatomic and Molecular Pathology Department, Washington University, contributed samples used in this work.
Footnotes
Financial Disclosure: None reported.
REFERENCES
- 1.Murray CJL, Lopez AD. Global Burden of Disease: A Comprehensive Assessment of Mortality and Disability From Diseases, Injuries, and Risk Factors in 1990 and Projected to 2020. Cambridge, MA: Harvard School of Public Health; 1996. [Google Scholar]
- 2.Salm M, Belsky D, Sloan FA. Trends in cost of major eye diseases to Medicare, 1991 to 2000. Am J Ophthalmol. 2006;142(6):976–982. doi: 10.1016/j.ajo.2006.07.057. [DOI] [PubMed] [Google Scholar]
- 3.Spector A. Oxidative stress-induced cataract: mechanism of action. FASEB J. 1995;9(12):1173–1182. [PubMed] [Google Scholar]
- 4.Truscott RJ, Augusteyn RC. Oxidative changes in human lens proteins during senile nuclear cataract formation. Biochim Biophys Acta. 1977;492(1):43–52. doi: 10.1016/0005-2795(77)90212-4. [DOI] [PubMed] [Google Scholar]
- 5.Holekamp NM, Shui Y-B, Beebe D. Lower intraocular oxygen tension in diabetic patients: possible contribution to decreased incidence of nuclear sclerotic cataract. Am J Ophthalmol. 2006;141(6):1027–1032. doi: 10.1016/j.ajo.2006.01.016. [DOI] [PubMed] [Google Scholar]
- 6.Helbig H, Hinz JP, Kellner U, Foerster MH. Oxygen in the anterior chamber of the human eye. Ger J Ophthalmol. 1993;2(3):161–164. [PubMed] [Google Scholar]
- 7.Holekamp NM, Shui YB, Beebe DC. Vitrectomy surgery increases oxygen exposure to the lens: a possible mechanism for nuclear cataract formation. Am J Ophthalmol. 2005;139(2):302–310. doi: 10.1016/j.ajo.2004.09.046. [DOI] [PubMed] [Google Scholar]
- 8.Palmquist BM, Philipson B, Barr PO. Nuclear cataract and myopia during hyperbaric oxygen therapy. Br J Ophthalmol. 1984;68(2):113–117. doi: 10.1136/bjo.68.2.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lyne AJ. Ocular effects of hyperbaric oxygen. Trans Ophthalmol Soc U K. 1978;98(1):66–68. [PubMed] [Google Scholar]
- 10.Fischer BH, Marks M, Reich T. Hyperbaric-oxygen treatment of multiple sclerosis: a randomized, placebo-controlled, double-blind study. N Engl J Med. 1983;308(4):181–186. doi: 10.1056/NEJM198301273080402. [DOI] [PubMed] [Google Scholar]
- 11.Fledelius HC, Jansen EC, Thorn J. Refractive change during hyperbaric oxygen therapy: a clinical trial including ultrasound oculometry. Acta Ophthalmol Scand. 2002;80(2):188–190. doi: 10.1034/j.1600-0420.2002.800213.x. [DOI] [PubMed] [Google Scholar]
- 12.Novak MA, Rice TA, Michels RG, Auer C. The crystalline lens after vitrectomy for diabetic retinopathy. Ophthalmology. 1984;91(12):1480–1484. doi: 10.1016/s0161-6420(84)34100-8. [DOI] [PubMed] [Google Scholar]
- 13.Melberg NS, Thomas MA. Nuclear sclerotic cataract after vitrectomy in patients younger than 50 years of age. Ophthalmology. 1995;102(10):1466–1471. doi: 10.1016/s0161-6420(95)30844-5. [DOI] [PubMed] [Google Scholar]
- 14.de Bustros S, Thompson JT, Michels RG, Enger C, Rice TA, Glaser BM. Nuclear sclerosis after vitrectomy for idiopathic epiretinal membranes. Am J Ophthalmol. 1988;105(2):160–164. doi: 10.1016/0002-9394(88)90180-8. [DOI] [PubMed] [Google Scholar]
- 15.Thompson JT, Glaser BM, Sjaarda RN, Murphy RP. Progression of nuclear sclerosis and long-term visual results of vitrectomy with transforming growth factor beta-2 for macular holes. Am J Ophthalmol. 1995;119(1):48–54. doi: 10.1016/s0002-9394(14)73812-7. [DOI] [PubMed] [Google Scholar]
- 16.Wilson CA, Berkowitz BA, McCuen BW, II, Charles HC. Measurement of preretinal oxygen tension in the vitrectomized human eye using fluorine-19 magnetic resonance spectroscopy. Arch Ophthalmol. 1992;110(8):1098–1100. doi: 10.1001/archopht.1992.01080200078028. [DOI] [PubMed] [Google Scholar]
- 17.Berkowitz BA, Kowluru RA, Frank RN, Kern TS, Hohman TC, Prakash M. Subnormal retinal oxygenation response precedes diabetic-like retinopathy. Invest Ophthalmol Vis Sci. 1999;40(9):2100–2105. [PubMed] [Google Scholar]
- 18.Linsenmeier RA, Goldstick TK, Blum RS, Enroth-Cugell C. Estimation of retinal oxygen transients from measurements made in the vitreous humor. Exp Eye Res. 1981;32(4):369–379. doi: 10.1016/s0014-4835(81)80016-4. [DOI] [PubMed] [Google Scholar]
- 19.Alder VA, Niemeyer G, Cringle SJ, Brown MJ. Vitreal oxygen tension gradients in the isolated perfused cat eye. Curr Eye Res. 1986;5(4):249–256. doi: 10.3109/02713688609020050. [DOI] [PubMed] [Google Scholar]
- 20.Cringle SJ, Yu D-Y. Intraretinal oxygenation and oxygen consumption in the rabbit during systemic hyperoxia. Invest Ophthalmol Vis Sci. 2004;45(9):3223–3228. doi: 10.1167/iovs.03-1364. [DOI] [PubMed] [Google Scholar]
- 21.McNulty R, Wang H, Mathias RT, Ortwerth BJ, Truscott RJW, Bassnett S. Regulation of tissue oxygen levels in the mammalian lens. J Physiol. 2004;559(pt 3):883–898. doi: 10.1113/jphysiol.2004.068619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Barbazetto IA, Liang J, Chang S, Zheng L, Spector A, Dillon JP. Oxygen tension in the rabbit lens and vitreous before and after vitrectomy. Exp Eye Res. 2004;78(5):917–924. doi: 10.1016/j.exer.2004.01.003. [DOI] [PubMed] [Google Scholar]
- 23.Buerk DG, Shonat RD, Riva CE, Cranstoun SD. O2 gradients and countercurrent exchange in the cat vitreous humor near retinal arterioles and venules. Microvasc Res. 1993;45(2):134–148. doi: 10.1006/mvre.1993.1013. [DOI] [PubMed] [Google Scholar]
- 24.Harocopos GJ, Shui Y-B, McKinnon M, Holekamp NM, Gordon MO, Beebe DC. Importance of vitreous liquefaction in age-related cataract. Invest Ophthalmol Vis Sci. 2004;45(1):77–85. doi: 10.1167/iovs.03-0820. [DOI] [PubMed] [Google Scholar]
- 25.Kampfenkel K, Vanmontagu M, Inze D. Extraction and determination of ascorbate and dehydroascorbate from plant tissue. Anal Biochem. 1995;225(1):165–167. doi: 10.1006/abio.1995.1127. [DOI] [PubMed] [Google Scholar]
- 26.Pirie A. A light-catalysed reaction in the aqueous humor of the eye. Nature. 1965;205:500–501. doi: 10.1038/205500a0. [DOI] [PubMed] [Google Scholar]
- 27.Takano S, Ishiwata S, Nakazawa M, Mizugaki M, Tamai M. Determination of ascorbic acid in human vitreous humor by high-performance liquid chromatography with UV detection. Curr Eye Res. 1997;16(6):589–594. doi: 10.1076/ceyr.16.6.589.5080. [DOI] [PubMed] [Google Scholar]
- 28.Eaton JW. Is the lens canned? Free Radic Biol Med. 1991;11(2):207–213. doi: 10.1016/0891-5849(91)90173-z. [DOI] [PubMed] [Google Scholar]
- 29.Buettner GR. In the absence of catalytic metals ascorbate does not autoxidize at pH 7: ascorbate as a test for catalytic metals. J Biochem Biophys Methods. 1988;16(1):27–40. doi: 10.1016/0165-022x(88)90100-5. [DOI] [PubMed] [Google Scholar]
- 30.Yoshida Y, Furuta S, Niki E. Effects of metal chelating agents on the oxidation of lipids induced by copper and iron. Biochim Biophys Acta. 1993;1210(1):81–88. doi: 10.1016/0005-2760(93)90052-b. [DOI] [PubMed] [Google Scholar]
- 31.Duarte TL, Lunec J. Review: when is an antioxidant not an antioxidant? a review of novel actions and reactions of vitamin C. Free Radic Res. 2005;39(7):671–686. doi: 10.1080/10715760500104025. [DOI] [PubMed] [Google Scholar]
- 32.Rose RC, Richer SP, Bode AM. Ocular oxidants and antioxidant protection. Proc Soc Exp Biol Med. 1998;217(4):397–407. doi: 10.3181/00379727-217-44250. [DOI] [PubMed] [Google Scholar]
- 33.Garland DL. Ascorbic acid and the eye. Am J Clin Nutr. 1991;54(6 suppl) doi: 10.1093/ajcn/54.6.1198s. 1198S–1202S. [DOI] [PubMed] [Google Scholar]
- 34.García-Castiñeiras S, Velázquez S, Martínez P, Torres N. Aqueous humor hydrogen peroxide analysis with dichlorophenol-indophenol. Exp Eye Res. 1992;55(1):9–19. doi: 10.1016/0014-4835(92)90086-8. [DOI] [PubMed] [Google Scholar]
- 35.Spector A, Ma W, Wang RR. The aqueous humor is capable of generating and degrading H2O2. Invest Ophthalmol Vis Sci. 1998;39(7):1188–1197. [PubMed] [Google Scholar]
- 36.Linster CL, Van Schaftingen E. Vitamin C biosynthesis, recycling and degradation in mammals. FEBS J. 2007;274(1):1–22. doi: 10.1111/j.1742-4658.2006.05607.x. [DOI] [PubMed] [Google Scholar]
- 37.Cicik E, Tekin H, Akar S, et al. Interleukin-8, nitric oxide and glutathione status in proliferative vitreoretinopathy and proliferative diabetic retinopathy. Ophthalmic Res. 2003;35(5):251–255. doi: 10.1159/000072145. [DOI] [PubMed] [Google Scholar]
- 38.Rittenhouse KD, Peiffer RL, Jr, Pollack GM. Assessment of ascorbate ocular disposition in the conscious rabbit: an approach using the microdialysis technique. CurrEyeRes. 2000;20(5):351–360. [PubMed] [Google Scholar]
- 39.DiMattio J. A comparative study of ascorbic acid entry into aqueous and vitreous humors of the rat and guinea pig. Invest Ophthalmol Vis Sci. 1989;30(11):2320–2331. [PubMed] [Google Scholar]
- 40.Tsukaguchi H, Tokui T, Mackenzie B, et al. A family of mammalian Na+-dependent L-ascorbic acid transporters. Nature. 1999;399(6731):70–75. doi: 10.1038/19986. [DOI] [PubMed] [Google Scholar]
- 41.Age-Related Eye Disease Study Research Group. A randomized, placebocontrolled, clinical trial of high-dose supplementation with vitamins C and E and beta carotene for age-related cataract and vision loss: AREDS report No. 9. Arch Ophthalmol. 2001;119(10):1439–1452. doi: 10.1001/archopht.119.10.1439. [pubished correction appears in Arch Ophthalmol. 2008;126(9)1251]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gritz DC, Srinivasan M, Smith SD, et al. The Antioxidants in Prevention of Cataracts Study: effects of antioxidant supplements on cataract progression in South India. Br J Ophthalmol. 2006;90(7):847–851. doi: 10.1136/bjo.2005.088104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Davies MB, Austin J, Partridge DA. Vitamin C: Its Chemistry and Biochemistry. Cambridge, England: Royal Society of Chemistry; 1991. [Google Scholar]
- 44.Erie JC, Butz JA, Good JA, Erie EA, Burritt MF, Cameron JD. Heavy metal concentrations in human eyes. Am J Ophthalmol. 2005;139(5):888–893. doi: 10.1016/j.ajo.2004.12.007. [DOI] [PubMed] [Google Scholar]
- 45.Stefánsson E, Novack RL, Hatchell DL. Vitrectomy prevents retinal hypoxia in branch retinal vein occlusion. Invest Ophthalmol Vis Sci. 1990;31(2):284–289. [PubMed] [Google Scholar]
- 46.Quiram PA, Leverenz VR, Baker RM, Dang L, Giblin FJ, Trese MT. Microplasmininduced posteriorvitreous detachmentaffects vitreous oxygen levels. Retina. 2007;27(8):1090–1096. doi: 10.1097/IAE.0b013e3180654229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Brown DM, Nichols BE, Weingeist TA, Sheffield VC, Kimura AE, Stone EM. Procollagen II gene mutation in Stickler syndrome. Arch Ophthalmol. 1992;110(11):1589–1593. doi: 10.1001/archopht.1992.01080230089027. [DOI] [PubMed] [Google Scholar]
- 48.Kubo E, Kumamoto Y, Tsuzuki S, Akagi Y. Axial length, myopia, and the severity of lens opacity at the time of cataract surgery. Arch Ophthalmol. 2006;124(11):1586–1590. doi: 10.1001/archopht.124.11.1586. [DOI] [PubMed] [Google Scholar]
- 49.Wong TY, Klein BE, Klein R, Tomany SC, Lee KE. Refractive errors and incident cataracts: the Beaver Dam Eye Study. Invest Ophthalmol Vis Sci. 2001;42(7):1449–1454. [PubMed] [Google Scholar]
- 50.Chen S-N, Lin K-K, Chao A-N, Kuo Y-H, Ho J-D. Nuclear sclerotic cataract in young patients in Taiwan. J Cataract Refract Surg. 2003;29(5):983–988. doi: 10.1016/s0886-3350(02)01700-5. [DOI] [PubMed] [Google Scholar]
- 51.Sawa M, Ohji M, Kusaka S, et al. Nonvitrectomizing vitreous surgery for epiretinal membrane: long-term follow-up. Ophthalmology. 2005;112(8):1402–1408. doi: 10.1016/j.ophtha.2005.02.014. [DOI] [PubMed] [Google Scholar]