Abstract
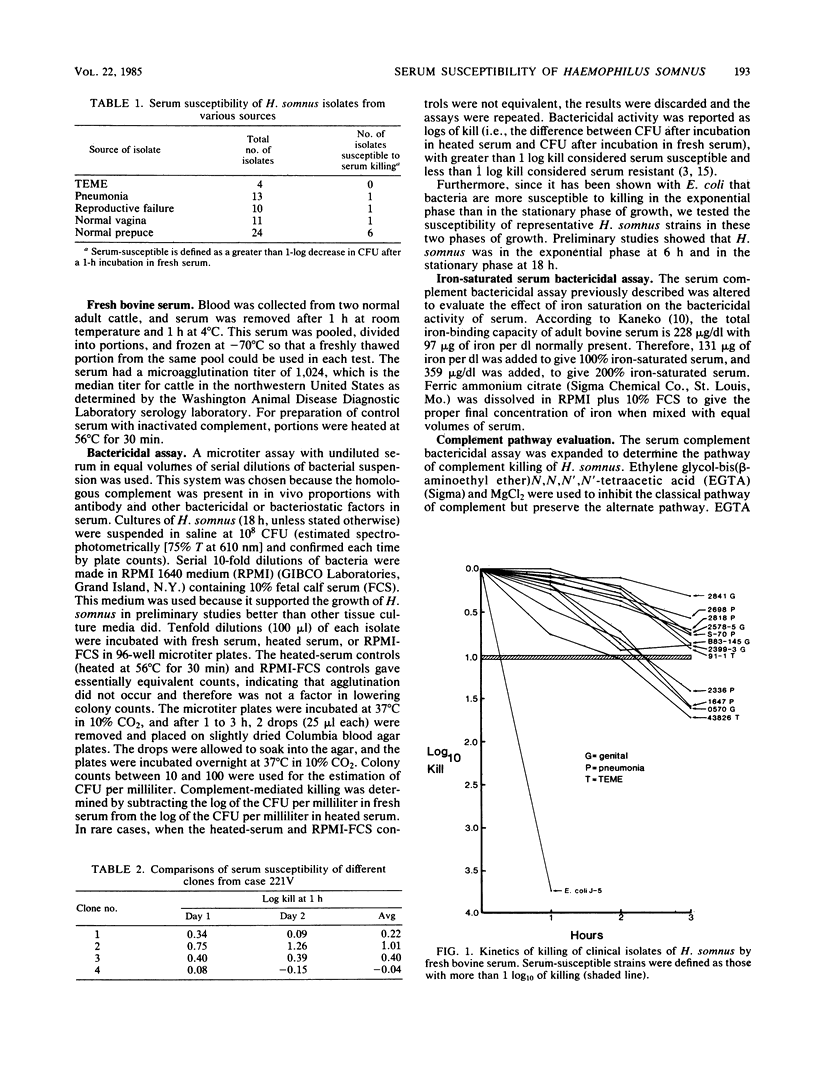
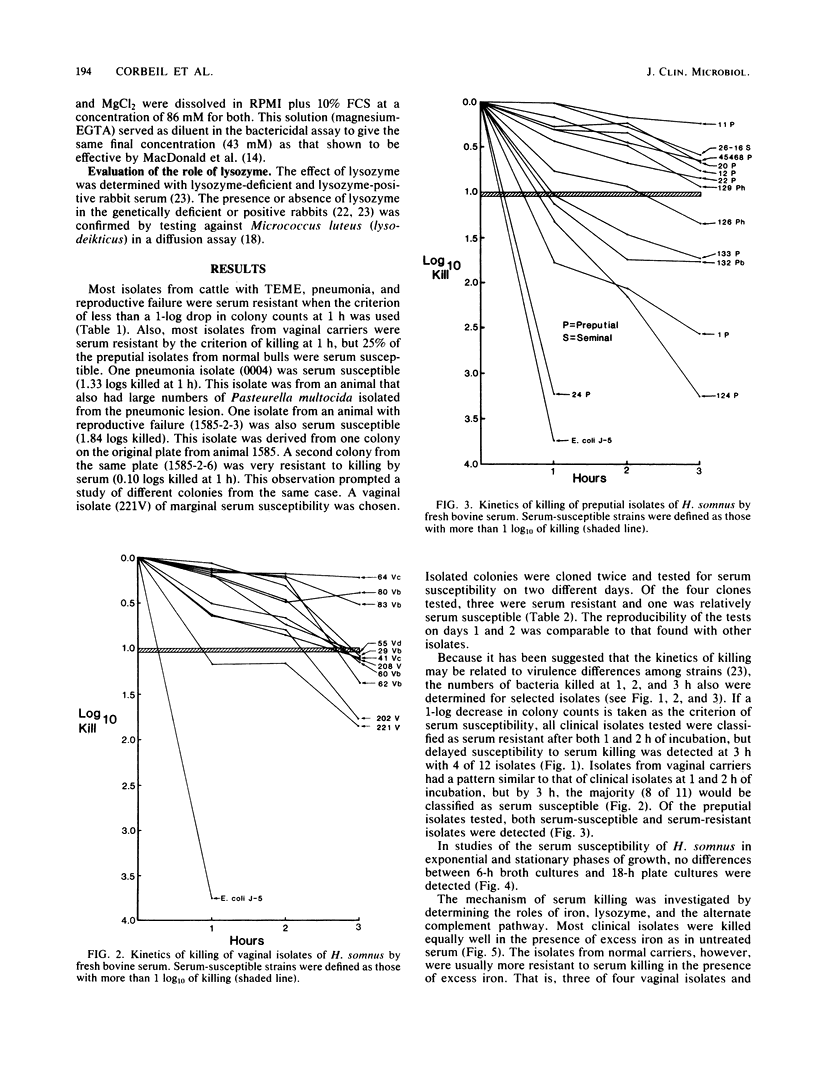
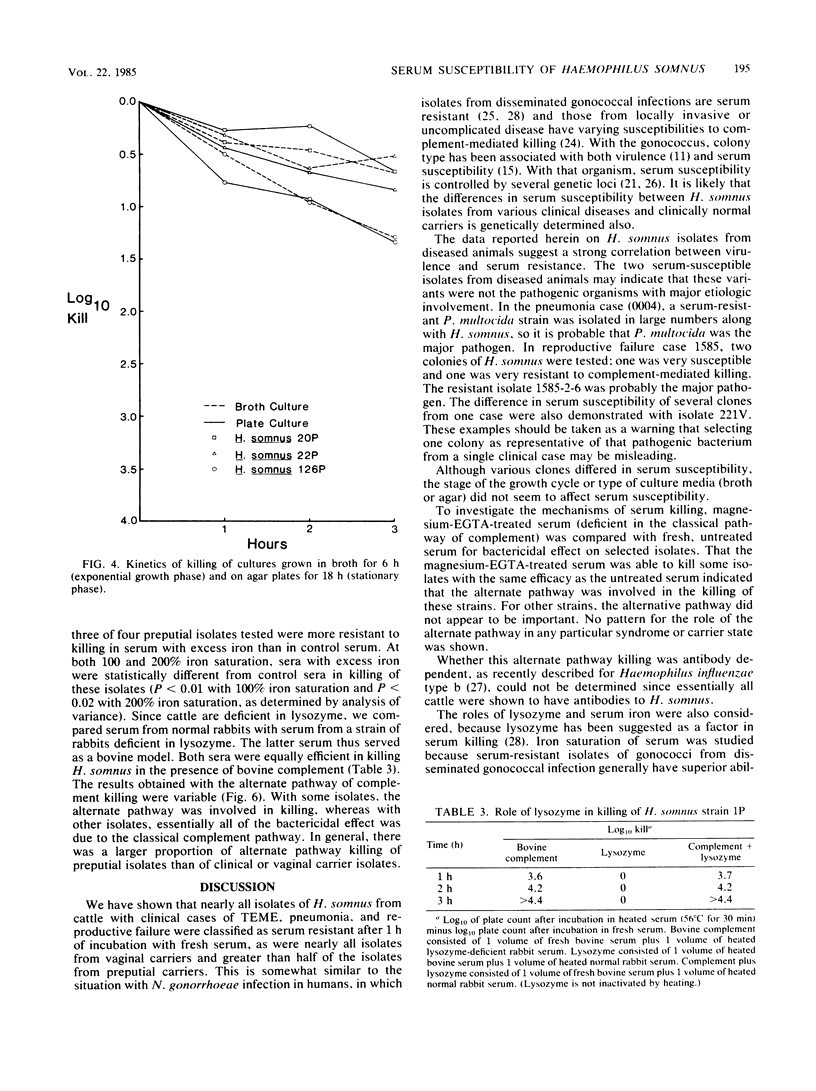
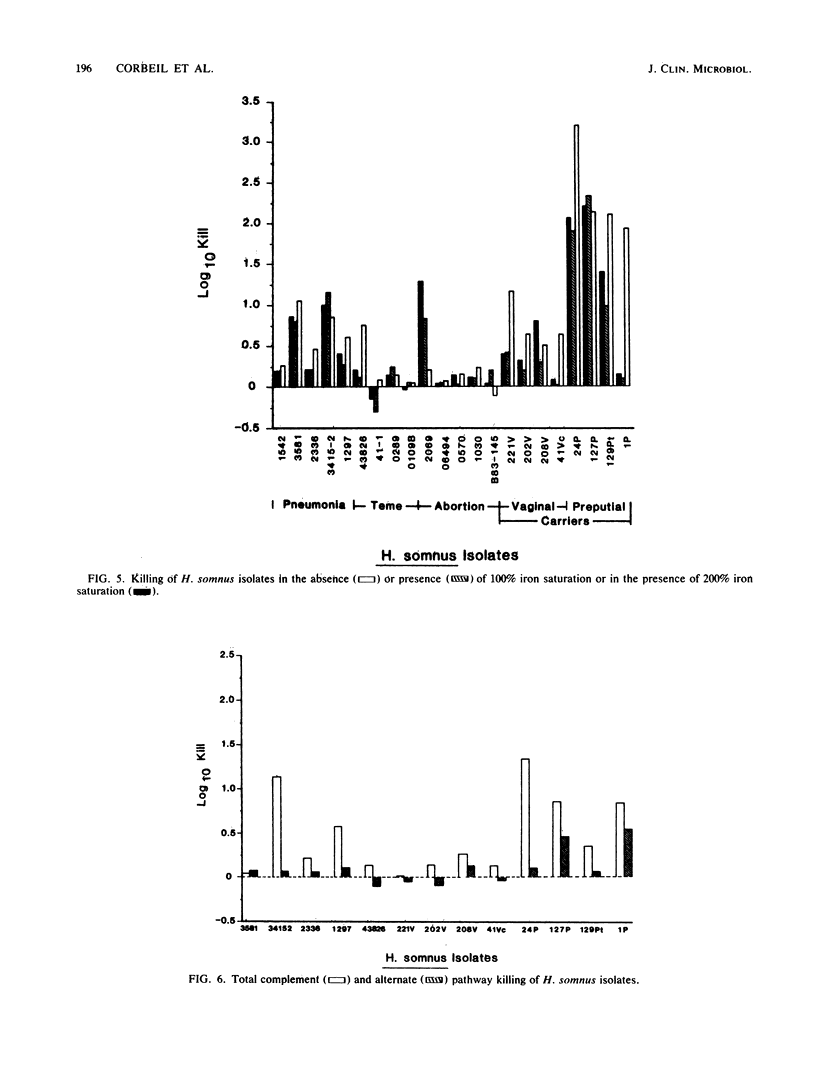
The serum susceptibility of 64 isolates of Haemophilus somnus from cattle was determined in a bactericidal assay with undiluted fresh or inactivated bovine serum with serial dilutions of bacterial suspension in RPMI 1640 medium. A total of 27 strains isolated from cattle with clinical disease (4 with thromboembolic meningoencephalitis, 13 with pneumonia, and 10 with reproductive failure) were compared with 35 strains from asymptomatic carriers (11 from the vagina and 24 from the prepuce). Essentially, all clinical isolates were serum resistant, whereas approximately 25% of preputial isolates were serum susceptible, as judged after 1 h of incubation in serum; a majority of vaginal isolates showed delayed serum susceptibility. Lysozyme played no role in serum killing, and the alternative complement pathway played only a minor role. Iron saturation, however, appeared to impart greater serum resistance to serum-susceptible strains from the vagina and prepuce. Perhaps the serum-susceptible strains from carriers would be useful vaccine candidates, but resistant strains from carriers may be pathogenic.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Binns M. M., Mayden J., Levine R. P. Further characterization of complement resistance conferred on Escherichia coli by the plasmid genes traT of R100 and iss of ColV,I-K94. Infect Immun. 1982 Feb;35(2):654–659. doi: 10.1128/iai.35.2.654-659.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brynger H., Brunner F. P., Jacobs C., Kramer P., Selwood N. H., Wing A. J. Graft survival and policies regarding blood transfusion and cold ischemia time in renal transplantation in Europe. Transplant Proc. 1984 Oct;16(5):1342–1343. [PubMed] [Google Scholar]
- Bullen J. J., Rogers H. J., Lewin J. E. The bacteriostatic effect of serum on Pasteurella septica and its abolition by iron compounds. Immunology. 1971 Mar;20(3):391–406. [PMC free article] [PubMed] [Google Scholar]
- Corbeil L. B., Wunderlich A. C., Ito J. I., McCutchan J. A. Plaque assay for measuring serum bactericidal activity against gonococci. J Clin Microbiol. 1978 Nov;8(5):618–620. doi: 10.1128/jcm.8.5.618-620.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crandell R. A., Smith A. R., Kissil M. Colonization and transmission of Haemophilus somnus in cattle. Am J Vet Res. 1977 Nov;38(11):1749–1751. [PubMed] [Google Scholar]
- Humphrey J. D., Little P. B., Stephens L. R., Barnum D. A., Doig P. A., Thorsen J. Prevalence and distribution of Haemophilus somnus in the male bovine reproductive tract. Am J Vet Res. 1982 May;43(5):791–795. [PubMed] [Google Scholar]
- Joiner K. A., Schmetz M. A., Goldman R. C., Leive L., Frank M. M. Mechanism of bacterial resistance to complement-mediated killing: inserted C5b-9 correlates with killing for Escherichia coli O111B4 varying in O-antigen capsule and O-polysaccharide coverage of lipid A core oligosaccharide. Infect Immun. 1984 Jul;45(1):113–117. doi: 10.1128/iai.45.1.113-117.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KELLOGG D. S., Jr, PEACOCK W. L., Jr, DEACON W. E., BROWN L., PIRKLE D. I. NEISSERIA GONORRHOEAE. I. VIRULENCE GENETICALLY LINKED TO CLONAL VARIATION. J Bacteriol. 1963 Jun;85:1274–1279. doi: 10.1128/jb.85.6.1274-1279.1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kochan I., Kvach J. T., Wiles T. I. Virulence-associated acquisition of iron in mammalian serum by Escherichia coli. J Infect Dis. 1977 Apr;135(4):623–632. doi: 10.1093/infdis/135.4.623. [DOI] [PubMed] [Google Scholar]
- Lambris J., Papamichail M., Ioannidis C., Dimitracopoulos G. Activation of the alternative pathway of human complement by the extracellular slime glycolipoprotein of Pseudomonas aeruginosa. J Infect Dis. 1982 Jan;145(1):78–82. doi: 10.1093/infdis/145.1.78. [DOI] [PubMed] [Google Scholar]
- MacDonald J. T., Maheswaran S. K., Opuda-Asibo J., Townsend E. L., Thies E. S. Susceptibility of Pasteurella haemolytica to the bactericidal effects of serum, nasal secretions and bronchoalveolar washings from cattle. Vet Microbiol. 1983 Nov;8(6):585–599. doi: 10.1016/0378-1135(83)90007-x. [DOI] [PubMed] [Google Scholar]
- McCutchan J. A., Levine S., Braude A. I. Influence of colony type on susceptibility of gonococci to killing by human serum. J Immunol. 1976 Jun;116(6):1652–1655. [PubMed] [Google Scholar]
- Miller R. B., Lein D. H., McEntee K. E., Hall C. E., Shin S. Haemophilus somnus infection of the reproductive tract of cattle: a review. J Am Vet Med Assoc. 1983 Jun 15;182(12):1390–1392. [PubMed] [Google Scholar]
- Nielsen K., Duncan J. R., Stemshorn B., Ruckerbauer G. Relationship of humoral factors (antibody and complement) to immune responsiveness, resistance and diagnostic serology. Adv Exp Med Biol. 1981;137:367–389. [PubMed] [Google Scholar]
- Osserman E. F., Lawlor D. P. Serum and urinary lysozyme (muramidase) in monocytic and monomyelocytic leukemia. J Exp Med. 1966 Nov 1;124(5):921–952. doi: 10.1084/jem.124.5.921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Padgett G. A., Hirsch J. G. Lysozyme: its absence in tears and leukocytes of cattle. Aust J Exp Biol Med Sci. 1967 Oct;45(5):569–570. doi: 10.1038/icb.1967.56. [DOI] [PubMed] [Google Scholar]
- Payne S. M., Holmes K. K., Finkelstein R. A. Role of iron in disseminated gonococcal infections. Infect Immun. 1978 May;20(2):573–574. doi: 10.1128/iai.20.2.573-574.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pluschke G., Achtman M. Degree of antibody-independent activation of the classical complement pathway by K1 Escherichia coli differs with O antigen type and correlates with virulence of meningitis in newborns. Infect Immun. 1984 Feb;43(2):684–692. doi: 10.1128/iai.43.2.684-692.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prieur D. J., Cámara V. M. Inheritance of lysozyme deficiency in rabbits. J Hered. 1979 May-Jun;70(3):181–184. doi: 10.1093/oxfordjournals.jhered.a109230. [DOI] [PubMed] [Google Scholar]
- Prieur D. J., Olson H. M., Young D. M. Lysozyme deficiency-an inherited disorder of rabbits. Am J Pathol. 1974 Nov;77(2):283–298. [PMC free article] [PubMed] [Google Scholar]
- Rice P. A., McCormack W. M., Kasper D. L. Natural serum bactericidal activity against Neisseria gonorrhoeae isolates from disseminated, locally invasive, and uncomplicated disease. J Immunol. 1980 May;124(5):2105–2109. [PubMed] [Google Scholar]
- Schoolnik G. K., Buchanan T. M., Holmes K. K. Gonococci causing disseminated gonococcal infection are resistant to the bactericidal action of normal human sera. J Clin Invest. 1976 Nov;58(5):1163–1173. doi: 10.1172/JCI108569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shafer W. M., Guymon L. F., Sparling P. F. Identification of a new genetic site (sac-3+) in Neisseria gonorrhoeae that affects sensitivity to normal human serum. Infect Immun. 1982 Mar;35(3):764–769. doi: 10.1128/iai.35.3.764-769.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steele N. P., Munson R. S., Jr, Granoff D. M., Cummins J. E., Levine R. P. Antibody-dependent alternative pathway killing of Haemophilus influenzae type b. Infect Immun. 1984 May;44(2):452–458. doi: 10.1128/iai.44.2.452-458.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor P. W. Bactericidal and bacteriolytic activity of serum against gram-negative bacteria. Microbiol Rev. 1983 Mar;47(1):46–83. doi: 10.1128/mr.47.1.46-83.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]



