Abstract
During the period January 1982 to January 1985, 2,234 specimens were cultured for isolation of herpes simplex virus (HSV). HSV was isolated from 23% of these, Trichomonas vaginalis was isolated from 1.6%, and 75.3% were negative. In 0.2% of these, HSV and T. vaginalis were isolated from the same specimen. Cytopathic effects produced by HSV were identified by their sensitivity to arabinosylthymine, whereas those produced by T. vaginalis were identified by their lack of sensitivity to arabinosylthymine and by observation of motility. Cytopathic effects produced by T. vaginalis were reproduced by trophozoites from axenic cultures of T. vaginalis as well as by lysates of T. vaginalis added to serum-free BHK cells.
Full text
PDF
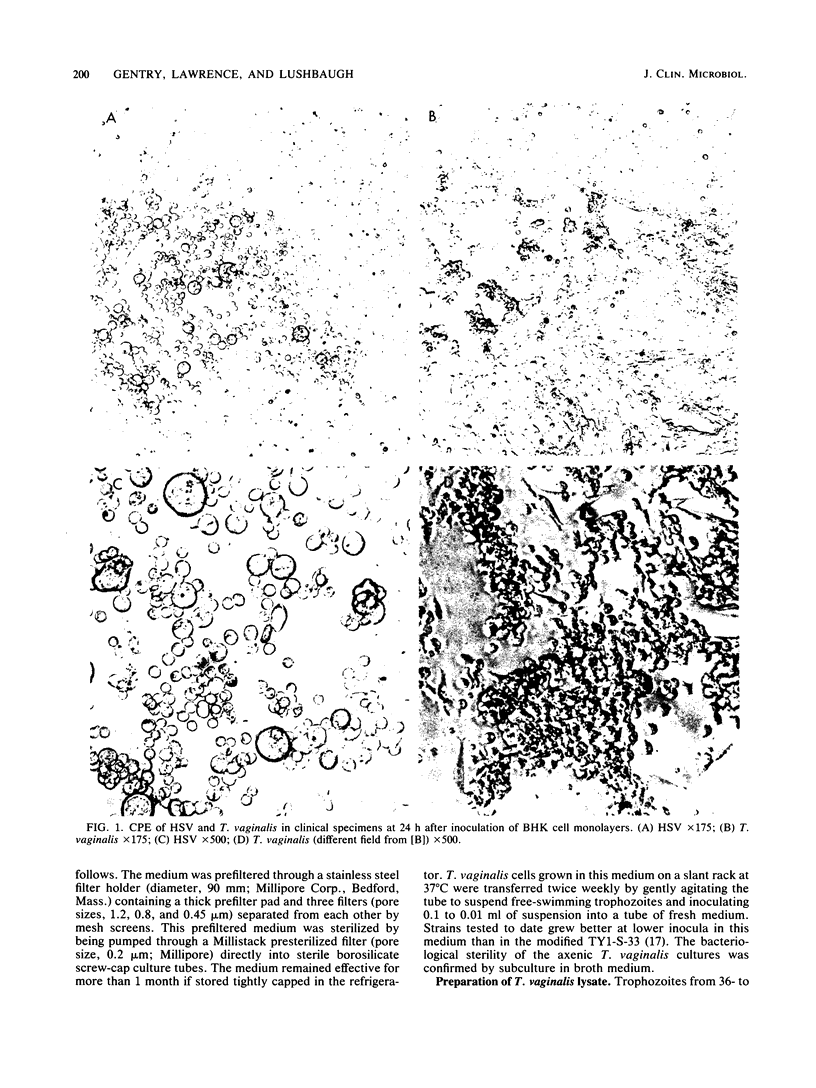
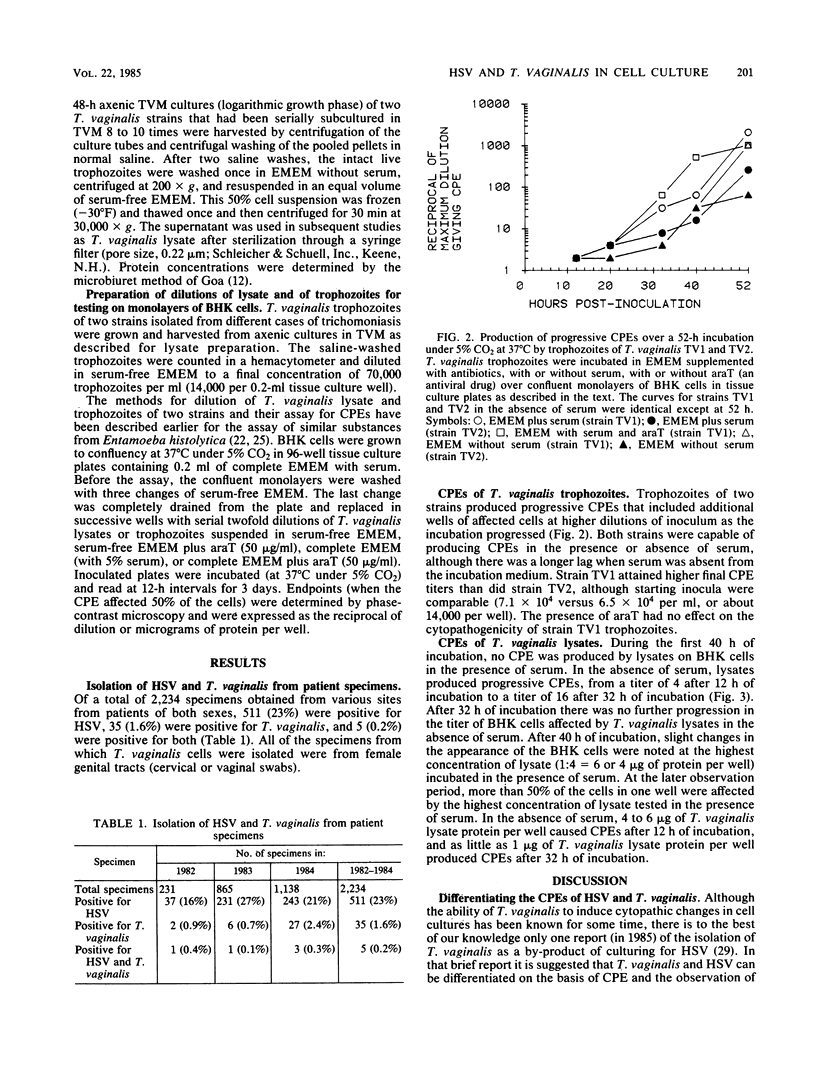
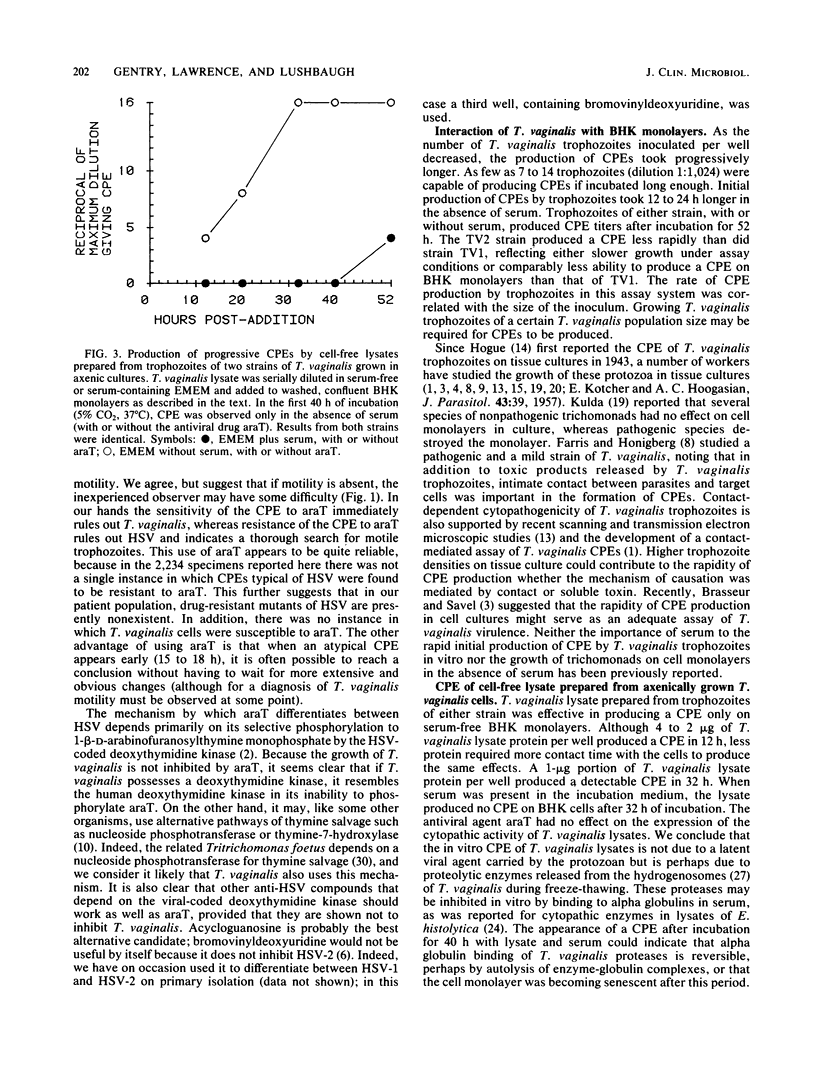


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alderete J. F., Pearlman E. Pathogenic Trichomonas vaginalis cytotoxicity to cell culture monolayers. Br J Vener Dis. 1984 Apr;60(2):99–105. doi: 10.1136/sti.60.2.99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aswell J. F., Allen G. P., Jamieson A. T., Campbell D. E., Gentry G. A. Antiviral activity of arabinosylthymine in herpesviral replication: mechanism of action in vivo and in vitro. Antimicrob Agents Chemother. 1977 Aug;12(2):243–254. doi: 10.1128/aac.12.2.243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brasseur P., Savel J. Evaluation de la virulence des souches de Trichomonas vaginalis par l'étude de l'effet cytopathogène sur culture de cellules. C R Seances Soc Biol Fil. 1982;176(6):849–860. [PubMed] [Google Scholar]
- CHRISTIAN R. T., MILLER N. F., LUDOVICI P. P., RILEY G. M. A study of Trichomonas vaginalis in human cell culture. Am J Obstet Gynecol. 1963 Apr 1;85:947–954. doi: 10.1016/s0002-9378(16)35599-5. [DOI] [PubMed] [Google Scholar]
- Coombs G. H., North M. J. An analysis of the proteinases of Trichomonas vaginalis by polyacrylamide gel electrophoresis. Parasitology. 1983 Feb;86(Pt 1):1–6. doi: 10.1017/s0031182000057103. [DOI] [PubMed] [Google Scholar]
- De Clercq E., Descamps J., Verhelst G., Walker R. T., Jones A. S., Torrence P. F., Shugar D. Comparative efficacy of antiherpes drugs against different strains of herpes simplex virus. J Infect Dis. 1980 May;141(5):563–574. doi: 10.1093/infdis/141.5.563. [DOI] [PubMed] [Google Scholar]
- FROST J. K. Trichomonas vaginalis and cervical epithelial changes. Ann N Y Acad Sci. 1962 Sep 29;97:792–799. doi: 10.1111/j.1749-6632.1962.tb34689.x. [DOI] [PubMed] [Google Scholar]
- GOA J. A micro biuret method for protein determination; determination of total protein in cerebrospinal fluid. Scand J Clin Lab Invest. 1953;5(3):218–222. doi: 10.3109/00365515309094189. [DOI] [PubMed] [Google Scholar]
- Gentry G. A., Allen G. P., Holton R., Nevins R. B., McGowan J. J., Veerisetty V. Thymine salvage, mitochondria, and the evolution of the herpesviruses. Intervirology. 1983;19(2):67–76. doi: 10.1159/000149340. [DOI] [PubMed] [Google Scholar]
- Gentry G. A., Aswell J. F. Inhibition of herpes simplex virus replication by araT. Virology. 1975 May;65(1):294–296. doi: 10.1016/0042-6822(75)90034-3. [DOI] [PubMed] [Google Scholar]
- Heath J. P. Behaviour and pathogenicity of Trichomonas vaginalis in epithelial cell cultures: a study by light and scanning electron microscopy. Br J Vener Dis. 1981 Apr;57(2):106–117. doi: 10.1136/sti.57.2.106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honigberg B. M., Gupta P. K., Spence M. R., Frost J. K., Kuczyńska K., Choromański L., Wartoń A. Pathogenicity of Trichomonas vaginalis: cytopathologic and histopathologic changes of the cervical epithelium. Obstet Gynecol. 1984 Aug;64(2):179–184. [PubMed] [Google Scholar]
- Keister D. B. Axenic culture of Giardia lamblia in TYI-S-33 medium supplemented with bile. Trans R Soc Trop Med Hyg. 1983;77(4):487–488. doi: 10.1016/0035-9203(83)90120-7. [DOI] [PubMed] [Google Scholar]
- Krieger J. N., Poisson M. A., Rein M. F. Beta-hemolytic activity of Trichomonas vaginalis correlates with virulence. Infect Immun. 1983 Sep;41(3):1291–1295. doi: 10.1128/iai.41.3.1291-1295.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kulda J., Honigberg B. M. Behavior and pathogenicity of Tritrichomonas foetus in chick liver cell cultures. J Protozool. 1969 Aug;16(3):479–495. doi: 10.1111/j.1550-7408.1969.tb02304.x. [DOI] [PubMed] [Google Scholar]
- Lockwood B. C., North M. J., Coombs G. H. Trichomonas vaginalis, Tritrichomonas foetus, and Trichomitus batrachorum: comparative proteolytic activity. Exp Parasitol. 1984 Dec;58(3):245–253. doi: 10.1016/0014-4894(84)90041-9. [DOI] [PubMed] [Google Scholar]
- Lushbaugh W. B., Hofbauer A. F., Kairalla A. A., Cantey J. R., Pittman F. E. Relationship of cytotoxins of axenically cultivated Entamoeba histolytica to virulence. Gastroenterology. 1984 Jun;86(6):1488–1495. [PubMed] [Google Scholar]
- Lushbaugh W. B., Hofbauer A. F., Pittman F. E. Proteinase activities of Entamoeba histolytica cytotoxin. Gastroenterology. 1984 Jul;87(1):17–27. [PubMed] [Google Scholar]
- Lushbaugh W. B., Kairalla A. B., Hofbauer A. F., Arnaud P., Cantey J. R., Pittman F. E. Inhibition of Entamoeba histolytica cytotoxin by alpha 1 antiprotease and alpha 2 macroglobulin. Am J Trop Med Hyg. 1981 May;30(3):575–585. doi: 10.4269/ajtmh.1981.30.575. [DOI] [PubMed] [Google Scholar]
- McLaughlin J., Müller M. Purification and characterization of a low molecular weight thiol proteinase from the flagellate protozoon Tritrichomonas foetus. J Biol Chem. 1979 Mar 10;254(5):1526–1533. [PubMed] [Google Scholar]
- Ravdin J. I., Guerrant R. L. A review of the parasite cellular mechanisms involved in the pathogenesis of amebiasis. Rev Infect Dis. 1982 Nov-Dec;4(6):1185–1207. doi: 10.1093/clinids/4.6.1185. [DOI] [PubMed] [Google Scholar]
- Wang C. C., Verham R., Tzeng S. F., Aldritt S., Cheng H. W. Pyrimidine metabolism in Tritrichomonas foetus. Proc Natl Acad Sci U S A. 1983 May;80(9):2564–2568. doi: 10.1073/pnas.80.9.2564. [DOI] [PMC free article] [PubMed] [Google Scholar]