Abstract
The hormonal control of cell death is currently the best-established mechanism for creating sex differences in cell number in the brain and spinal cord. For example, males have more cells than do females in the principal nucleus of the bed nucleus of the stria terminalis (BNSTp) and spinal nucleus of the bulbocavernosus (SNB), whereas females have a cell number advantage in the anteroventral periventricular nucleus (AVPV). In each case, the difference in cell number in adulthood correlates with a sex difference in the number of dying cells at some point in development. Mice with over- or under-expression of cell death genes have recently been used to test more directly the contribution of cell death to neural sex differences, to identify molecular mechanisms involved, and to determine the behavioural consequences of suppressing developmental cell death. Bax is a pro-death gene of the Bcl-2 family that is singularly important for apoptosis in neural development. In mice lacking bax, the number of cells in the BNSTp, SNB, and AVPV are significantly increased and sex differences in total cell number in each of these regions are eliminated. Cells rescued by bax gene deletion in the BNSTp express markers of differentiated neurones and the androgen receptor. On the other hand, sex differences in other phenotypically identified populations, such as vasopressin-expressing neurones in the BNSTp or dopaminergic neurones in AVPV, are not affected by either bax deletion or bcl-2 over-expression. Possible mechanisms by which testosterone may regulate cell death in the nervous system are discussed, as are the behavioural effects of eliminating sex differences in neuronal cell number.
Keywords: sex difference, cell death, bax, bcl-2, androgen, oestrogen
Introduction
Many of the best-studied sex differences in vertebrate brains relate to cell number. These may be either sex differences in the total number of cells in a given brain region, as identified in a Nissl stain, or in the number of cells of a particular phenotype. In most cases, sex differences in cell number have been attributed to gonadal steroid hormones, in particular testosterone, or a metabolite of testosterone, acting early in development. In theory, testosterone could cause sex differences in cell number by regulating any of the major neurodevelopmental events: neurogenesis, migration, the differentiation of phenotype, or cell death. Of these, the hormonal control of cell death is the best-established mechanism for generating sex differences in cell number in the brain, and will be the main focus of this review. It is likely that neurogenesis, cell migration, and the differentiation of cellular phenotype also contribute to sexual differentiation of the brain, although evidence is more preliminary for these cellular mechanisms (see ref. 1 for a recent review).
For some neural sex differences, the birth of cells is complete before the testes differentiate (2), making it very unlikely that gonadal steroids control neurogenesis to establish sex differences. In other regions, cell birth overlaps with perinatal testicular steroid production (3). At present there is no sex difference in cell number in the adult brain that has been definitively linked to a sex difference in perinatal neurogenesis, although gonadal steroids may cause sex differences in neurogenesis in the developing CA1 region of the hippocampus (4). Neurogenesis continues throughout life in some neural areas, and a very recent report argues that the addition of cells around the time of puberty could serve to maintain or establish sex differences in the brain (5).
Evidence for sex differences in migration comes from the study of organotypic slices of the embryonic mouse brain. Neurones in the medial preoptic area and anterior hypothalamus show different migratory pathways, depending upon whether the brain slice is from a male or female embryo (6). In addition, the movement of neurones in slice cultures is affected by oestradiol and by GABA receptor antagonists (7–9) raising the possibility that sex differences in hormone levels or in neural activity could lead to different numbers of cells within a given region. Estrogen receptor βcells are more laterally located in male rats than in females in the anteroventral periventricular nucleus of the hypothalamus (AVPV) (10); although the mechanism underlying this sex difference is not known, it could be the result of sex differences in migration.
The third possible mechanism for establishing sex differences in cell number, the hormonal control of cellular differentiation, includes both morphological differentiation (e.g., cell size or the complexity of dendritic trees) and chemical differentiation (e.g., expression of receptors, peptides or neurotransmitters). Although sex differences of this type abound in adult brains, it is actually difficult to determine whether a difference in the number of cells of a particular phenotype is due to the control of cell phenotype, per se, or to some other mechanism (for additional discussion see ref. 1). For example males might have larger cells in a given region because testosterone increases cell size (hormonal control of morphological phenotype), or because testosterone increases the birth or survival of large cells. In at least one case, the sex difference in the number of vasopressin neurones in the forebrain, a good case can be made that testosterone controls cell phenotype (11).
Neuronal Cell Death and Sexual Differentiation
A role for cell death in sexual differentiation of the central nervous system was proposed almost 30 years ago, and differential cell death in males and females underlies several of the most prominent neural sex differences (reviewed in ref. 12). For example, adult males have more neurones than do females in the spinal nucleus of the bulbocavernosus and sexually dimorphic nucleus of the preoptic area, and females have more dying cells in these regions than do males during perinatal life (13,14). The opposite pattern a greater number of cells in adulthood in females and more dying cells during development in males is seen for the AVPV (15). These inverse correlations between cell number in adulthood and rate of cell death perinatally provide circumstantial evidence that cell death contributes to the sex difference, but it is difficult to rule out other mechanisms. We have taken advantage of genetically altered mice to determine whether cell death alone can account for given neural sex differences, and to test the involvement of specific cell death genes.
Naturally occurring cell death is a widespread phenomenon of the developing nervous system, and over 50% of neurones initially generated undergo apoptosis during a restricted, developmental time window that varies from region to region (16). Tremendous strides have been made in understanding the molecular bases of apoptosis over the past 15 years. A breakthrough in understanding neuronal cell death in particular came with the discovery of the Bcl-2 protein (17). Bcl-2 is now known to belong to a family of apoptosis-regulating proteins that fall into three subgroups: 1) anti-apoptotic members (e.g., Bcl-2 and Bcl-xL), which typically contain four conserved motifs termed Bcl-2 homology (BH) domains; 2) full-length pro-death members (e.g., Bax and Bak), which include two of the BH domains; and 3) BH3-only proteins (e.g., Bim, Bad and Bid), which contain only the third BH domain. BH-3 members are almost always pro-death and act by binding to and inactivating the antiapoptotic Bcl-2 proteins that reside in the mitochondrial membrane, or in some cases by directly activating Bax or Bak (18).
Despite substantial redundancy in the Bcl-2 family, the decision of whether or not a cell undergoes apoptosis really comes down to the full-length pro-death proteins, Bax and Bak, which normally reside in the cytoplasm but translocate to the mitochondrial membrane upon receiving a death signal (19). If not opposed by pro-survival members, they are capable of permeabilizing the membrane, leading to the release of factors such as cytochrome c which trigger activation of the caspases cascade and apoptotic cell death (Figure 1). This forms the basis of the “Bax/Bak checkpoint” within each cell. Interestingly, neurones are unusual in that they appear to lack Bak, or produce an alternately spliced Bak with altered function (20, 21), leaving Bax as a singularly important protein for neuronal cell death.
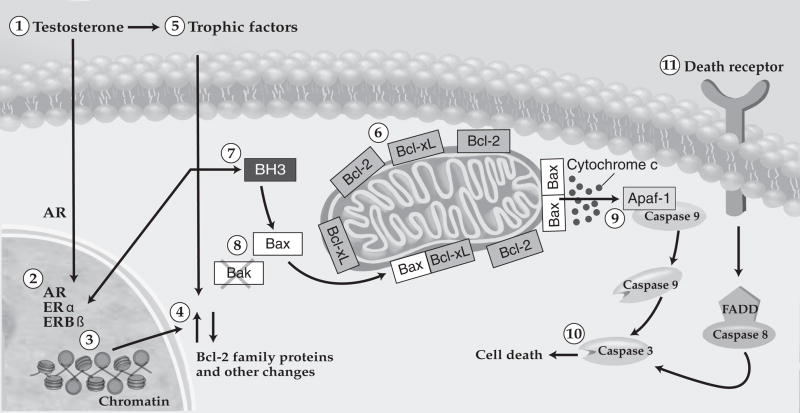
Figure 1. The regulation of neuronal cell death by hormones and Bcl-2 family members.
Testosterone, produced by the perinatal testes, regulates cell number in several sexually dimorphic areas. In this simplified model, (1) testosterone binds to (2) androgen receptors (AR), or is aromatized to an estrogen before binding estrogen receptors (ER), such as ERα and ERβ. (3,4) The activation of ARs or ERs leads to changes in the expression of proteins involved in cell death, including those of the Bcl-2 family; this may involve epigenetic changes in chromatin associated with cell death genes. (5) In other instances, testosterone regulates the availability of neurotrophic factors which may also lead, directly or indirectly, to changes in the expression or activity of Bcl-2 family proteins. (6) Pro-survival members of the family, such as Bcl-2 and Bcl-xL, are primarily localized to the outer mitochondrial membrane. (7) Most cell types have two full-length pro-death members, Bax and Bak, but developing neurons lack Bak. Bax normally resides in the cytoplasm, and translocates to the mitochondria in response to a death signal. (8) BH3-only proteins are pro-apoptotic; they bind to and inactivate the pro-survival proteins, or directly activate Bax. (9) If not opposed by pro-survival family members such as Bcl-2 or Bcl-xL, Bax forms oligomers at the mitochondrial membrane capable of releasing of cytochrome c and other factors. Cytochrome c interacts with apoptotic protease activating factor 1 (Apaf-1) to trigger ‘initiation caspases’ such as caspase 9. (10) These in turn activate ‘executioner caspases’ such as caspase 3, which degrade cellular proteins and kill the cell. (11) A second cell death mechanism involves death receptors embedded in the plasma membrane (e.g., fas or the tumor necrosis factor receptor). Although not thought to be the predominant pathway for controlling cell number in the developing nervous system, some sub-populations of neurons may depend on this mechanism. Like the pathway regulated by Bcl-2 family members, the death receptor-mediated apoptosis also converges on executioner caspases.
In mice with a targeted deletion of the bax gene, neuronal cell death is profoundly decreased in many, though not all, regions (22). We reasoned that if a given neural sex difference was due to cell death, it might be reduced or eliminated in bax knockout mice. In the sections that follow, I will first describe the effects of bax deletion on total cell number and cell number in several populations of phenotypically identified cells in the BNSTp, AVPV and SNB of mice. I then consider possible molecular mechanisms tying testosterone to changes in neuronal cell death and, finally, raise the question of how the elimination of sexually dimorphic cell death, or for that matter neuronal cell death in general, affects behavior.
Principal nucleus of the BNST
The BNST is a limbic forebrain structure involved in the control of sexual behavior, gonadotrophic release, stress and anxiety (23–27). The principal nucleus of the BNST (BNSTp; also known as the encapsulated region of the BNST, or the medial part of the posteromedial subdivision of the BNST) is larger in males than in females of several species, including rats, mice, guinea pigs, and humans (28–31). In rats and mice, the sex difference in volume is due to an increased number of cells in males, and treatment of females with testosterone on the day of birth completely masculinizes cell number (28, 32). Female rats have more dying cells in the BNSTp than do males during postnatal life and testosterone treatment of females or castration of males reverses this sex difference (33). The effect of testosterone 0on cell survival in the BNSTp is likely due to oestrogenic metabolites, as we find that oestradiol, but not the non-aromatizable androgen dihydrotestosterone, completely masculinizes BNSTp cell number of mice (32). Moreover, both oestrogen receptors αand βappear to be involved as treatment of females with agonists that specifically target each receptor increases BNSTp cell number relative to controls (unpublished observations).
We tested whether Bax is required for the sex difference in total cell number in the BNSTp by counting thionin-labeled cells in bax −/− mice and their wild-type, bax +/+, siblings. Bax gene deletion increased BNSTp cell number in both sexes and, more important, eliminated the sex difference in cell number in adulthood (29). At birth, wild-type males and females have equal numbers of cells in the BNSTp, with a significant sex different (male > female) developing between postnatal day 7 and 9 (34). Females have about twice as many dying cells in the BNSTp [as identified by terminal deoxynucleotidyl nick end labeling (TUNEL)] than do males on postnatal days 5 and 6, whereas bax −/− mice of both sexes have virtually no TUNEL-positive cells in the BNSTp (34). Taken together, the sex difference in total cell number in the BNSTp can be accounted for by bax-dependent cell death during postnatal life.
Like many brain regions, the BNSTp is heterogeneous, and sex differences have been reported for several cell types (e.g., vasopressinergic neurones and androgen receptor positive cells) (35, 36). Cell counts based on a thionin stain, as described above, do not reveal what cells are affected by bax gene deletion; it is possible that some populations are affected very little and others a great deal. Phenotyping of BNSTp cells in wild-type and bax −/− mice indicates that there are significant sex differences in neurones (NeuN-immunoreactive) and androgen-receptor expressing cells, but not in astrocytes (GFAP-immunoreactive cells). Bax deletion increases both NeuN+ and AR+ cell counts and eliminates sex differences in these populations (37).
In contrast, Bax-dependent cell death is not responsible for the sex difference in vasopressin cell number in the BNSTp. A deletion of the bax gene increases the number of vasopressin cells in the BNSTp of both sexes but does not reduce the size of the sex difference (11). This same pattern is observed in mice over-expressing Bcl-2 (11). Thus, the pool of potential vasopressinergic neurones is subject to the mitochondrial cell death pathway regulated by Bcl-2 and Bax, but this is not the mechanism underlying the sex difference in cell number. Instead, testosterone likely acts on a pluripotent set of cells in the BNSTp to direct them to become vasopressinergic.
Anteroventral Periventricular Nucleus
The AVPV sits at the rostral extreme of the third ventricle and is critically involved in control of the luteinizing hormone surge at the time of ovulation (38, 39). In contrast to most other sexually dimorphic nuclei, AVPV is larger in volume larger and more “cell dense” in females of several rodent species (15, 40, 41). In addition, females have fewer dying cells than do males in the perinatal AVPV, and treatment with either testosterone or oestradiol increases cell death in females (15, 42, 43). Sex differences in cell density and total cell number are eliminated in AVPV of mice over-expressing Bcl-2 (44) or with a deletion of bax (29), respectively. Thus, for a region where testosterone increases cell death (AVPV), as well as one where the same hormone decreases cell death (BNSTp), bax is required for the sex difference in cell number. In both nuclei the actions of testosterone are likely mediated by oestrogen receptors (32, 45), suggesting that there is a molecular switch someplace between the binding of hormone receptors and activation of bax that determines whether the hormonal signal will favor life or death.
In addition to the sex difference in total cell number, there is also a large sex difference in the subset of AVPV neurones that are dopaminergic, as defined by tyrosine hydroxylase (TH) immunoreactivity. Female rats and mice have 2–3 times as many TH cells as do males (46, 47). Interestingly, this sex difference is not reduced at all by over-expressing Bcl-2 in neurones or by deleting the bax gene (29, 44). In contrast to the vasopressin story described above, there also is no overall increase in TH cell number in AVPV of Bcl-2 over-expressing or bax −/− mice. One possible interpretation of this finding is that cell death is not the mechanism determining the sex difference in dopaminergic cell number in AVPV. Alternatively, cell death may be involved, but controlled by a pathway independent of Bcl-2 or Bax. A recent report demonstrates that trophic factor deprivation triggers a novel cell death pathway in cultured dopaminergic neurones from the mouse midbrain (48). Instead of triggering the mitochondrial pathway that is critically regulated by Bcl-2 family proteins, the death of these cells occurs via the activation of a cell surface death receptor (see Figure 1, right). It would be interesting to know whether this mechanism also controls dopaminergic cell number in AVPV.
Taken together, the TH cells in AVPV and vasopressinergic neurones in the BNSTp remind us that the control of cell number may differ not only from region to region, but also for subtypes of neurones within a region.
Spinal Nucleus of the Bulbocavernosus
The SNB is a cluster of motoneurones that reside in the lumbar spinal cord and innervate striated muscles involved in copulation. Male mice and rats have more SNB motoneurones than do females (49, 50), and a similar sex difference is seen in the homologous cell group of other mammalian species. SNB motoneurones innervate striated muscles of the perineum that control copulation, including the bulbocavernosus (BC) and levator ani (LA). These muscles are also markedly dimorphic, with the BC completely absent and the LA severely reduced in females. The SNB motoneurone number and BC/LA muscle morphology can be masculinized by treating females with testosterone around the time of birth (51). In this case, effects of testosterone do not involve conversion to oestrogens, but instead require functional intracellular androgen receptors (52; for review see ref. 53).
We used retrograde labeling to identify SNB motoneurones in wild-type and bax −/− mice of both sexes and find that, as for total cell number in the BNSTp and AVPV, deletion of the bax gene eliminates the sex difference in SNB motoneurone number (54). Interestingly, however, the BC and LA muscles remained highly sexually dimorphic in bax −/− mice (large in males and virtually absent in females), despite the complete masculinization of their innervating motoneurones (54). This might suggest that differentiation of the perineal muscles does not involve cell death, as suggested previously for the developing LA (55). However, most cell types other than neurones express both full-length pro-death Bcl-2 family members, Bax and Bak. In such cases, either protein may be capable of triggering cytochrome c release and other downstream events of apoptosis (Figure 1), and deletion of both the bax and bak genes may be required to effectively block cell death (56). This possibility was tested for the BC and LA by comparing the perineal muscles in wild-type mice with mice bearing single deletions of either the bax or bak gene, or a deletion of both genes (bax/bak double knockouts). The BC muscle was absent and the LA was rudimentary in all wild-type females and in females with at least one copy of either bax or bak. In sharp contrast, bax/bak double knockout females possessed a BC muscle and the LA was markedly increased: female double knockouts had over 800 LA muscle fibers, in contrast to the ~30 LA fibers in controls (57). In addition, TUNEL-positive cells were virtually eliminated in the perineal muscles of newborn female bax/bak double knockouts (57). Thus, sexual differentiation of both SNB motoneurones and the perineal muscles is dependent on cell death, and particularly on the mitochondrial pathway regulated by Bcl-2 family members; however muscle cell death can be triggered by either Bax or Bak.
How Do Gonadal Steroids Control Sexually Dimorphic Cell Death?
The findings discussed above present cases in which cell death converges on bax whether testosterone increases or decreases cell survival, and whether androgen or oestrogen receptors mediate effects of the hormone. What are the molecular links between testosterone, androgen or oestrogen receptors, and Bcl-2 family members? One possibility is that testosterone or its metabolites might control the expression of Bcl-2 family members. Androgens and estrogens regulate the expression of Bcl-2 family members in peripheral tissues and cancer cell lines, and estrogen response elements have been described in the Bcl-2 and Bcl-xL genes (58, 59). In addition, newborn male rats have higher levels of Bcl-2 and lower levels of Bax protein in the SDN-POA than do females, and the opposite pattern of protein expression is seen in AVPV (60). Moreover, oestradiol treatment increases Bcl-2 and decreases Bax in the preoptic area (61). Thus, directly or indirectly, testosterone may regulate the expression of Bcl-2 family proteins to control cell number.
Interestingly, however, sexual differentiation often involves a delay between hormone exposure and some cellular response. For example, a single injection of testosterone or oestradiol on the day of birth results in sex differences in cell death in the BNSTp 5 or 6 days later (33). Effects of neonatal testosterone on AVPV volume are not evident for several weeks (41), long after the hormone is cleared from circulation. Lag times such as these suggest a cellular memory for the hormone signal that is consistent with epigenetic changes. That is, testosterone present during a critical developmental period may cause covalent modifications of chromatin that lead to lasting changes in gene expression, without an alteration in underlying DNA sequences.
Chromatin consists of DNA wrapped around a core of histone proteins. Covalent modifications of the DNA (as in methylation of cytosine residues) or of histone protein tails can have lasting effects on gene transcription (62). The best understood of the histone modifications is acetylation, which is often associated with active gene expression (63). Interestingly, steroid hormone receptors recruit cofactors with histone-acetylating activity to target genes (64, 65), and blocking these co-factors can prevent effects of testosterone on sexual differentiation (66, 67). Thus, we hypothesized that a disruption in the balance of histone acetylation and deacetylation might interfere with effects of testosterone on sexual differentiation. Preliminary findings support this prediction. When the histone deacetylase inhibitor, valproic acid, is administered to newborn mice, masculinization of cell number in the BNSTp is blocked (68). The deacetylase inhibitor does not affect cell number in the BNSTp of females or in nonsexually dimorphic areas, suggesting that it does not cause a generalized suppression of cell survival, but specifically prevents the sparing actions of testosterone. The genes or promoter regions that are affected in mice treated with valproic acid are not known, and there are many chromatin modifications in addition to histone acetylation that may play a role in sexual differentiation. However, it is noteworthy that histone deacetylase inhibitors preferentially target the expression of genes linked to cell cycle or cell death (69), and that valproic acid regulates the expression of Bcl-2 family members in the brain and in cultured neurons (70, 71). Thus, one testable hypothesis is that perinatal exposure to testosterone (or a metabolite) may alter histone acetylation in promoter regions of Bcl-2 family genes, making it more or less likely that the gene is expressed several days later, during the cell death period.
What are the Consequences of Eliminating Neuronal Cell Death?
Cell death during neural development is so ubiquitous that it is noteworthy to identify an area in the mammalian nervous system that does not undergo a significant pruning of cell numbers during embryonic or early postnatal life. In addition, Bcl-2 related proteins regulate neuronal cell death in species from roundworms to humans (72). This would suggest that the process of naturally occurring cell death during neural development serves an extremely important function. What that function is, however, is not well understood. In contrast to the explosion of information on the molecular mechanisms of apoptosis, the role(s) of cell death in neural development has received comparatively little attention (for a review of this issue see ref. 73). The most common textbook explanation is that developmental neuronal cell death serves to “match” afferent populations to their targets. Most tests of this idea were performed decades ago and involved neurones projecting to targets outside of the nervous system (16). It should give us pause that bax knockout mice, which exhibit a profound reduction of neuronal cell death in many if not most neural areas, are not only viable, but are behaviorally and physically indistinguishable from wild-type littermates based on simple observation (74; and our own observations). In mice lacking the Bax protein, or over-expressing Bcl-2 under a neurone-specific promoter, performance on basic sensory and motor tests is normal (75–78). Bax knockouts and Bcl-2 over-expressors show some impairment on complex tasks, such as maintaining balance on a high speed RotaRod (76, 79), but bax −/− mice are actually superior to controls in a test of motor strength (78).
Given that bax gene deletion eliminates sex differences in cell number in several neuronal populations, we asked whether sexually dimorphic functions might be altered in the knockout mice. In a test of feminine sexual behavior, wildtype and bax −/− animals of both sexes were gonadectomized in adulthood and treated with oestradiol and progesterone. As expected, wild-type females showed high levels of lordosis over six weekly tests whereas feminine sexual behavior was low in males (79). Lordosis quotients were low in bax −/− mice of both sexes; the knockout males and females did not differ from each other and were equivalent to wild-type males on this measure (79). Preventing neuronal cell death also eliminates a sex difference in olfactory preference. Wild-type males prefer to sniff female-soiled bedding over male-soiled bedding, whereas females show the opposite preference (80). This sex difference is eliminated in bax −/− animals (81). In addition, bax −/− mice of both sexes are “like males” in time spent sniffing a female stimulus animal in a social recognition test. Not all sexually dimorphic behaviors are absent in bax −/− mice, however, as both wild-type and bax−/− males show more aggression than do females in a resident-intruder test (81).
Bax gene deletion affects many neural regions, and it is not known whether the elimination of sex differences in cell number in the BNSTp, AVPV, or SNB (29, 54) specifically contributes to the elimination of sex differences in feminine sexual behavior or olfactory preference in bax −/− animals. More generally, this work highlights the fact that it really is not known how “extra” cells in one sex in any sexually dimorphic area affect neural function or behavior. Based on projection patterns of BNSTp neurones, Segovia and Guillamon (82) proposed that additional cells in the BNSTp of males serve to suppress feminine sexual behavior, and our results are consistent with that idea. In addition, the BNSTp is part of a well-defined neural circuit for processing sexually relevant olfactory cues that shows differential activation of the immediate early gene product, Fos, in response to male soiled bedding (83–85). It will be of interest to determine whether such sex differences in neural activation are eliminated in bax −/− mice and, if so, where in the circuit this occurs. The relatively large effects of bax deletion on sexual and olfactory behaviors suggests that sex differences in cell number are important for generating sex differences in behavior. By studying animals with alterations in neuronal cell death it may be possible to address long-standing questions about the function of specific sex differences in cell number in the mammalian brain.
References
- 1.McCarthy MM, De Vries GJ, Forger NG. Sexual differentiation of the brain: Mode, mechanisms and meaning. In: Pfaff D, editor. Hormones, Brain and Behavior. Elsevier; 2009. p. 37. [Google Scholar]
- 2.Breedlove SM, Jordan CL, Arnold AP. Neurogenesis of motoneurons in the sexually dimorphic spinal nucleus of the bulbocavernosus in rats. Brain Res. 1983;285:39–43. doi: 10.1016/0165-3806(83)90106-2. [DOI] [PubMed] [Google Scholar]
- 3.Jacobson CD, Gorski RA. Neurogenesis of the sexually dimorphic nucleus of the preoptic area in the rat. J Comp Neurol. 1981;196:519–529. doi: 10.1002/cne.901960313. [DOI] [PubMed] [Google Scholar]
- 4.Zhang JM, Konkle AT, Zup SL, McCarthy MM. Impact of sex and hormones on new cells in the developing rat hippocampus: a novel source of sex dimorphism? Eur J Neurosci. 2008;27:791–800. doi: 10.1111/j.1460-9568.2008.06073.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ahmed EI, Zehr JL, Schulz KM, Lorenz BH, Doncarlos LL, Sisk CL. Pubertal hormones modulate the addition of new cells to sexually dimorphic brain regions. Nat Neurosci. 2008 Aug 17; doi: 10.1038/nn.2178. Epup ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Henderson RG, Brown AE, Tobet SA. Sex differences in cell migration in the preoptic area/anterior hypothalamus of mice. J Neurobiol. 1999;41:252–266. doi: 10.1002/(sici)1097-4695(19991105)41:2<252::aid-neu8>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- 7.Knoll JG, Wolfe CA, Tobet SA. Estrogen modulates neuronal movements within the developing preoptic area-anterior hypothalamus. Eur J Neurosci. 2007;26:1091–1099. doi: 10.1111/j.1460-9568.2007.05751.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.McClellan KM, Calver AR, Tobet SA. GABAB receptors role in cell migration and positioning within the ventromedial nucleus of the hypothalamus. Neurosci. 2008;151:1119–1131. doi: 10.1016/j.neuroscience.2007.11.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tobet SA. J Neuroendocrinol. 2009 this issue. [Google Scholar]
- 10.Orikasa C, Kondo Y, Hayashi S, McEwen BS, Sakuma Y. Sexually dimorphic expression of estrogen receptor beta in the anteroventral periventricular nucleus of the rat preoptic area: implication in luteinizing hormone surge. Proc Natl Acad Sci U S A. 2002;99:3306–3311. doi: 10.1073/pnas.052707299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.De Vries GJ, Jardon M, Reza M, Rosen GJ, Immerman E, Forger NG. Sexual differentiation of vasopressin innervation of the brain: cell death versus phenotypic differentiation. Endocrinology. 2008;149:4632–4637. doi: 10.1210/en.2008-0448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Forger NG. Cell death and sexual differentiation of the nervous system. Neuroscience. 2006;138:929–938. doi: 10.1016/j.neuroscience.2005.07.006. [DOI] [PubMed] [Google Scholar]
- 13.Nordeen EJ, Nordeen KW, Sengelaub DR, Arnold AP. Androgens prevent normally occurring cell death in a sexually dimorphic spinal nucleus. Science. 1985;229:671–673. doi: 10.1126/science.4023706. [DOI] [PubMed] [Google Scholar]
- 14.Davis EC, Popper P, Gorski RA. The role of apoptosis in sexual differentiation of the rat sexually dimorphic nucleus of the preoptic area. Brain Res. 1996;734:10–18. [PubMed] [Google Scholar]
- 15.Sumida H, Nishizuka M, Kano Y, Arai Y. Sex differences in the anteroventral periventricular nucleus of the preoptic area and in the related effects of androgen in prenatal rats. Neurosci Lett. 1993;151:41–44. doi: 10.1016/0304-3940(93)90040-r. [DOI] [PubMed] [Google Scholar]
- 16.Oppenheim RW. Cell death during development of the nervous system. Annu Rev Neurosci. 1991;14:453–501. doi: 10.1146/annurev.ne.14.030191.002321. [DOI] [PubMed] [Google Scholar]
- 17.Merry DE, Korsmeyer SJ. Bcl-2 gene family in the nervous system. Annu Rev Neurosci. 1997;20:245–267. doi: 10.1146/annurev.neuro.20.1.245. [DOI] [PubMed] [Google Scholar]
- 18.Bouillet P, Strasser A. BH3-only proteins - evolutionarily conserved proapoptotic Bcl-2 family members essential for initiating programmed cell death. J Cell Sci. 2002;115:1567–1574. doi: 10.1242/jcs.115.8.1567. [DOI] [PubMed] [Google Scholar]
- 19.Putcha GV, Deshmukh M, Johnson EM., Jr BAX translocation is a critical event in neuronal apoptosis: regulation by neuroprotectants, BCL-2, and caspases. J Neurosci. 1999;19:7476–85. doi: 10.1523/JNEUROSCI.19-17-07476.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sun YF, Yu LY, Saarma M, Timmusk T, Arumae U. Neuron-specific Bcl-2 homology 3 domain-only splice variant of Bak is anti-apoptotic in neurons, but pro-apoptotic in non-neuronal cells. J Biol Chem. 2001;276:16240–16247. doi: 10.1074/jbc.M010419200. [DOI] [PubMed] [Google Scholar]
- 21.Uo T, Kinoshita Y, Morrison RS. Neurons exclusively express N-Bak, a BH3 domain-only Bak isoform that promotes neuronal apoptosis. J Biol Chem. 2005;280:9065–9073. doi: 10.1074/jbc.M413030200. [DOI] [PubMed] [Google Scholar]
- 22.White FA, Keller-Peck CR, Knudson CM, Korsmeyer SJ, Snider WD. Widespread elimination of naturally occurring neuronal death in Bax-deficient mice. J Neurosci. 1998;18:1428–1439. doi: 10.1523/JNEUROSCI.18-04-01428.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Emery DE, Sachs BD. Copulatory behavior in male rats with lesions in the bed nucleus of the stria terminalis. Physiol Behav. 1976;17:803–806. doi: 10.1016/0031-9384(76)90044-5. [DOI] [PubMed] [Google Scholar]
- 24.Beltramino C, Taleisnik S. Dual action of electrochemical stimulation of the bed nucleus of the stria terminalis on the release of LH. Neuroendocrinol. 1980;30:238–242. doi: 10.1159/000123007. [DOI] [PubMed] [Google Scholar]
- 25.Walker DL, Toufexis DJ, Davis M. Role of the bed nucleus of the stria terminalis versus the amygdala in fear, stress, and anxiety. Eur J Pharmacol. 2003;463:199–216. doi: 10.1016/s0014-2999(03)01282-2. [DOI] [PubMed] [Google Scholar]
- 26.Hammack SE, Richey KJ, Watkins LR, Maier SF. Chemical lesion of the bed nucleus of the stria terminalis blocks the behavioral consequences of uncontrollable stress. Behav Neurosci. 2004;118:443–448. doi: 10.1037/0735-7044.118.2.443. [DOI] [PubMed] [Google Scholar]
- 27.Bangasser DA, Santollo J, Shors TJ. The bed nucleus of the stria terminalis is critically involved in enhancing associative learning after stressful experience. Behav Neurosci. 2005;119:1459–1466. doi: 10.1037/0735-7044.119.6.1459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Guillamon A, Segovia S, del Abril A. Early effects of gonadal steroids on the neuron number in the medial posterior region and the lateral division of the bed nucleus of the stria terminalis in the rat. Brain Res Dev Brain Res. 1988;44:281–90. doi: 10.1016/0165-3806(88)90226-x. [DOI] [PubMed] [Google Scholar]
- 29.Forger NG, Rosen GJ, Waters EM, Jacob D, Simerly RB, de Vries GJ. Deletion of Bax eliminates sex differences in the mouse forebrain. Proc Natl Acad Sci U S A. 2004;101:13666–13671. doi: 10.1073/pnas.0404644101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hines M, Davis FC, Coquelin A, Goy RW, Gorski RA. Sexually dimorphic regions in the medial preoptic area and the bed nucleus of the stria terminalis of the guinea pig brain: a description and an investigation of their relationship to gonadal steroids in adulthood. J Neurosci. 1985;5:40–47. doi: 10.1523/JNEUROSCI.05-01-00040.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Allen LS, Gorski RA. Sex difference in the bed nucleus of the stria terminalis of the human brain. J Comp Neurol. 1990;302:697–706. doi: 10.1002/cne.903020402. [DOI] [PubMed] [Google Scholar]
- 32.Seney ML, Hisasue S, Gotsiridze T, Forger NG. Cell death and the development of sex differences in the bed nucleus of the stria terminalis of mice. Society for Neuroscience Meeting Planner. 2007 Abstract 294.23. [Google Scholar]
- 33.Chung WC, Swaab DF, De Vries GJ. Apoptosis during sexual differentiation of the bed nucleus of the stria terminalis in the rat brain. J Neurobiol. 2000;43:234–243. [PubMed] [Google Scholar]
- 34.Gotsiridze T, Kang N, Jacob D, Forger NG. Development of sex differences in the principal nucleus of the bed nucleus of the stria terminalis of mice: role of Bax-dependent cell death. Dev Neurobiol. 2007;67:355–362. doi: 10.1002/dneu.20353. [DOI] [PubMed] [Google Scholar]
- 35.DeVries GJ, Buijs RM, Van Leeuwen FW, Caffe AR, Swaab DF. The vasopressinergic innervation of the brain in normal and castrated rats. J Comp Neurol. 1985;233:236–254. doi: 10.1002/cne.902330206. [DOI] [PubMed] [Google Scholar]
- 36.Shah NM, Pisapia DJ, Maniatis S, Mendelsohn MM, Nemes A, Axel R. Visualizing sexual dimorphism in the brain. Neuron. 2004;43:313–319. doi: 10.1016/j.neuron.2004.07.008. [DOI] [PubMed] [Google Scholar]
- 37.Holmes MM, McCutcheon J, Forger NG. Bax Gene Deletion Increases NeuN- and Androgen Receptor-Positive Cells in the Bed Nucleus of the Stria Terminalis. Neuroscience. 2009 doi: 10.1016/j.neuroscience.2008.11.020. (in revision) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wiegand SJ, Terasawa E. Discrete lesions reveal functional heterogeneity of suprachiasmatic structures in regulation of gonadotropin secretion in the female rat. Neuroendocrinology. 1982;34:395–404. doi: 10.1159/000123335. [DOI] [PubMed] [Google Scholar]
- 39.Gu GB, Simerly RB. Projections of the sexually dimorphic anteroventral periventricular nucleus in the female rat. J Comp Neurol. 1997;384:142–164. [PubMed] [Google Scholar]
- 40.Bleier R, Byne W, Siggelkow I. Cytoarchitectonic sexual dimorphisms of the medial preoptic and anterior hypothalamic areas in guinea pig, rat, hamster, and mouse. J Comp Neurol. 1982;212:118–130. doi: 10.1002/cne.902120203. [DOI] [PubMed] [Google Scholar]
- 41.Davis EC, Shryne JE, Gorski RA. Structural sexual dimorphisms in the anteroventral periventricular nucleus of the rat hypothalamus are sensitive to gonadal steroids perinatally, but develop peripubertally. Neuroendocrinol. 1996;63:142–148. doi: 10.1159/000126950. [DOI] [PubMed] [Google Scholar]
- 42.Murakami S, Arai Y. Neuronal death in the developing sexually dimorphic periventricular nucleus of the preoptic area in the female rat: effect of neonatal androgen treatment. Neurosci Lett. 1989;102:185–90. doi: 10.1016/0304-3940(89)90076-1. [DOI] [PubMed] [Google Scholar]
- 43.Arai Y, Murakami S, Nishizuka M. Androgen enhances neuronal degeneration in the developing preoptic area: apoptosis in the anteroventral periventricular nucleus (AVPvN-POA) Horm Behav. 1994;28:313–319. doi: 10.1006/hbeh.1994.1027. [DOI] [PubMed] [Google Scholar]
- 44.Zup SL, Carrier H, Waters EM, Tabor A, Bengston L, Rosen GJ, Simerly RB, Forger NG. Overexpression of bcl–2 reduces sex differences in neuron number in the brain and spinal cord. J Neurosci. 2003;23:2357–2362. doi: 10.1523/JNEUROSCI.23-06-02357.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Arai Y, Sekine Y, Murakami S. Estrogen and apoptosis in the developing sexually dimorphic preoptic area in female rats. Neurosci Res. 1996;75:573–585. doi: 10.1016/0168-0102(96)01070-x. [DOI] [PubMed] [Google Scholar]
- 46.Simerly RB, Swanson LW, Handa RJ, Gorski RA. Influence of perinatal androgen on the sexually dimorphic distribution of tyrosine hydroxylase-immunoreactive cells and fibers in the anteroventral periventricular nucleus of the rat. Neuroendocrinol. 1985;40:501–510. doi: 10.1159/000124122. [DOI] [PubMed] [Google Scholar]
- 47.Simerly RB, Zee MC, Pendleton JW, Lubahn DB, Korach KS. Estrogen receptor-dependent sexual differentiation of dopaminergic neurons in the preoptic region of the mouse. Proc Natl Acad Sci U S A. 1997;94:14077–14082. doi: 10.1073/pnas.94.25.14077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Yu LY, Saarma M, Arumae U. Death receptors and caspases but not mitochondria are activated in the GDNF- or BDNF-deprived dopaminergic neurons. J Neurosci. 2008;28:7467–7475. doi: 10.1523/JNEUROSCI.1877-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Breedlove SM, Arnold AP. Hormone accumulation in a sexually dimorphic motor nucleus of the rat spinal cord. Science. 1980;210:564–566. doi: 10.1126/science.7423210. [DOI] [PubMed] [Google Scholar]
- 50.Wee BE, Clemens LG. Characteristics of the spinal nucleus of the bulbocavernosus are influenced by genotype in the house mouse. Brain Res. 1987;424:305–310. doi: 10.1016/0006-8993(87)91475-2. [DOI] [PubMed] [Google Scholar]
- 51.Breedlove SM, Arnold AP. Hormonal control of a developing neuromuscular system. II. Sensitive periods for the androgen-induced masculinization of the rat spinal nucleus of the bulbocavernosus. J Neurosci. 1983;3:424–432. doi: 10.1523/JNEUROSCI.03-02-00424.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Breedlove SM, Arnold AP. Sexually dimorphic motor nucleus in the rat lumbar spinal cord: response to adult hormone manipulation, absence in androgen-insensitive rats. Brain Res. 1981;225:297–307. doi: 10.1016/0006-8993(81)90837-4. [DOI] [PubMed] [Google Scholar]
- 53.Sengelaub DR, Forger NG. The spinal nucleus of the bulbocavernosus: firsts in androgen-dependent neural sex differences. Horm Behav. 2008;53:596–612. doi: 10.1016/j.yhbeh.2007.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Jacob DA, Bengston CL, Forger NG. Effects of Bax gene deletion on muscle and motoneuron degeneration in a sexually dimorphic neuromuscular system. J Neurosci. 2005;25:5638–5644. doi: 10.1523/JNEUROSCI.1200-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Tobin C, Joubert Y. Testosterone-induced development of the rat levator ani muscle. Dev Biol. 1991;146:131–138. doi: 10.1016/0012-1606(91)90453-a. [DOI] [PubMed] [Google Scholar]
- 56.Lindsten T, Ross AJ, King A, Zong WX, Rathmell JC, Shiels HA, Ulrich E, Waymire KG, Mahar P, Frauwirth K, Chen Y, Wei M, Eng VM, Adelman DM, Simon MC, Ma A, Golden JA, Evan G, Korsmeyer SJ, MacGregor GR, Thompson CB. The combined functions of proapoptotic Bcl-2 family members bak and bax are essential for normal development of multiple tissues. Mol Cell. 2000;6:1389–1399. doi: 10.1016/s1097-2765(00)00136-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Jacob DA, Ray T, Bengston CL, Lindsten T, Wu J, Thompson CB, Forger NG. The role of cell death in sexually dimorphic muscle development: Male-specific muscles are retained in female bax/bak knockout mice. Dev Neurobiol. 2008;68:1303–1314. doi: 10.1002/dneu.20658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Pike CJ. Estrogen modulates neuronal Bcl-xL expression and beta-amyloid-induced apoptosis: relevance to Alzheimer’s disease. J Neurochem. 1999;72:1552–1563. doi: 10.1046/j.1471-4159.1999.721552.x. [DOI] [PubMed] [Google Scholar]
- 59.Lin CC, Tsai YL, Huang MT, Lu YP, Ho CT, Tseng SF, Teng SC. Inhibition of estradiol-induced mammary proliferation by dibenzoylmethane through the E2-ER-ERE-dependent pathway. Carcinogenesis. 2006;27:131–136. doi: 10.1093/carcin/bgi199. [DOI] [PubMed] [Google Scholar]
- 60.Tsukahara S, Kakeyama M, Toyofuku Y. Sex differences in the level of Bcl-2 family proteins and caspase-3 activation in the sexually dimorphic nuclei of the preoptic area in postnatal rats. J Neurobiol. 2006;66:1411–1419. doi: 10.1002/neu.20276. [DOI] [PubMed] [Google Scholar]
- 61.Tsukahara S, Hojo R, Kuroda Y, Fujimaki H. Estrogen modulates Bcl-2 family protein expression in the sexually dimorphic nucleus of the preoptic area of postnatal rats. Neurosci Lett. 2008;432:58–63. doi: 10.1016/j.neulet.2007.12.006. [DOI] [PubMed] [Google Scholar]
- 62.Jenuwein T, Allis CD. Translating the histone code. Science. 2001;293:1074–1080. doi: 10.1126/science.1063127. [DOI] [PubMed] [Google Scholar]
- 63.Cosgrove MS, Wolberger C. How does the histone code work? Biochem Cell Biol. 2005;83:468–476. doi: 10.1139/o05-137. [DOI] [PubMed] [Google Scholar]
- 64.Kishimoto M, Fujiki R, Takezawa S, Sasaki Y, Nakamura T, Yamaoka K, Kitagawa H, Kato S. Nuclear receptor mediated gene regulation through chromatin remodeling and histone modifications. Endocr J. 2006;53:157–172. doi: 10.1507/endocrj.53.157. [DOI] [PubMed] [Google Scholar]
- 65.Chen J, Kinyamu HK, Archer TK. Changes in attitude, changes in latitude: nuclear receptors remodeling chromatin to regulate transcription. Mol Endocrinol. 2006;20:1–13. doi: 10.1210/me.2005-0192. [DOI] [PubMed] [Google Scholar]
- 66.Auger AP, Tetel MJ, McCarthy MM. Steroid receptor coactivator-1 (SRC-1) mediates the development of sex-specific brain morphology and behavior. Proc Natl Acad Sci U S A. 2000;97:7551–7555. doi: 10.1073/pnas.97.13.7551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Auger AP, Tetel MJ, McCarthy MM. Steroid receptor coactivator-1 (SRC-1) mediates the development of sex-specific brain morphology and behavior. Proc Natl Acad Sci U S A. 2000;97:7551–7555. doi: 10.1073/pnas.97.13.7551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Murray EK, Hien A, De Vries GJ, Forger NG. Epigenetic control of sexual differentiation of the bed nucleus of the stria terminalis in mice. Society for Neuroscience Meeting Planner. 2008 doi: 10.1210/en.2009-0458. Abstract 278.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Glaser KB, Staver MJ, Waring JF, Stender J, Ulrich RG, Davidsen SK. Gene expression profiling of multiple histone deacetylase (HDAC) inhibitors: defining a common gene set produced by HDAC inhibition in T24 and MDA carcinoma cell lines. Mol Cancer Ther. 2003;2:151–163. [PubMed] [Google Scholar]
- 70.Chuang DM. The antiapoptotic actions of mood stabilizers: molecular mechanisms and therapeutic potentials. Ann N Y Acad Sci. 2005;1053:195–204. doi: 10.1196/annals.1344.018. [DOI] [PubMed] [Google Scholar]
- 71.Sinn DI, Kim SJ, Chu K, Jung KH, Lee ST, Song EC, Kim JM, Park DK, Kun Lee S, Kim M, Roh JK. Valproic acid-mediated neuroprotection in intracerebral hemorrhage via histone deacetylase inhibition and transcriptional activation. Neurobiol Dis. 2007;26:464–472. doi: 10.1016/j.nbd.2007.02.006. [DOI] [PubMed] [Google Scholar]
- 72.Putcha GV, Johnson EM., Jr Men are but worms: neuronal cell death in C elegans and vertebrates. Cell Death Differ. 2004;11:38–48. doi: 10.1038/sj.cdd.4401352. [DOI] [PubMed] [Google Scholar]
- 73.Buss RR, Sun W, Oppenheim RW. Adaptive roles of programmed cell death during nervous system development. Annu Rev Neurosci. 2006;29:1–35. doi: 10.1146/annurev.neuro.29.051605.112800. [DOI] [PubMed] [Google Scholar]
- 74.Knudson CM, Tung KS, Tourtellotte WG, Brown GA, Korsmeyer SJ. Bax-deficient mice with lymphoid hyperplasia and male germ cell death. Science. 1995;270:96–9. doi: 10.1126/science.270.5233.96. [DOI] [PubMed] [Google Scholar]
- 75.Porciatti V, Pizzorusso T, Maffei L. Vision in mice with neuronal redundancy due to inhibition of developmental cell death. Vis Neurosci. 1999;16:721–6. doi: 10.1017/s0952523899164113. [DOI] [PubMed] [Google Scholar]
- 76.Rondi-Reig L, Lohof A, Dubreuil YL, Delhaye-Bouchaud N, Martinou JC, Caston J, Mariani J. Hu-Bcl-2 transgenic mice with supernumerary neurons exhibit timing impairment in a complex motor task. Eur J Neurosci. 1999;11:2285–2290. doi: 10.1046/j.1460-9568.1999.00643.x. [DOI] [PubMed] [Google Scholar]
- 77.Rondi-Reig L, Lemaigre-Dubreuil Y, Montecot C, Muller D, Martinou JC, Caston J, Mariani J. Transgenic mice with neuronal overexpression of bcl-2 gene present navigation disabilities in a water task. Neuroscience. 2001;104:207–215. doi: 10.1016/s0306-4522(01)00050-1. [DOI] [PubMed] [Google Scholar]
- 78.Buss RR, Gould TW, Ma J, Vinsant S, Prevette D, Winseck A, Toops KA, Hammarback JA, Smith TL, Oppenheim RW. Neuromuscular development in the absence of programmed cell death: phenotypic alteration of motoneurons and muscle. J Neurosci. 2006;26:13413–13427. doi: 10.1523/JNEUROSCI.3528-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Jyotika J, McCutcheon J, Laroche J, Blaustein JD, Forger NG. Deletion of the Bax gene disrupts sexual behavior and modestly impairs motor function in mice. Dev Neurobiol. 2007;67:1511–9. doi: 10.1002/dneu.20525. [DOI] [PubMed] [Google Scholar]
- 80.Bakker J, Van Ophemert J, Slob AK. Sexual differentiation of odor and partner preference in the rat. Physiol Behav. 1996;60:489–494. doi: 10.1016/s0031-9384(96)80023-0. [DOI] [PubMed] [Google Scholar]
- 81.Holmes MM, Niel L, Monks DA, Forger NG. Bax gene deletion disrupts sex differences in social behavior. Society for Behavioral Neuroendocrinology 12thAnnual Meeting Program. 2008:118. [Google Scholar]
- 82.Segovia S, Guillamon A. Sexual dimorphism in the vomeronasal pathway and sex differences in reproductive behaviors. Brain Res Rev. 1993;18:51–74. doi: 10.1016/0165-0173(93)90007-m. [DOI] [PubMed] [Google Scholar]
- 83.Halem HA, Cherry JA, Baum MJ. Vomeronasal neuroepithelium and forebrain Fos responses to male pheromones in male and female mice. J Neurobiol. 1999;39:249–263. [PubMed] [Google Scholar]
- 84.Kang N, Janes A, Baum MJ, Cherry JA. Sex difference in Fos induced by male urine in medial amygdala-projecting accessory olfactory bulb mitral cells of mice. Neurosci Lett. 2006;398:59–62. doi: 10.1016/j.neulet.2005.12.062. [DOI] [PubMed] [Google Scholar]
- 85.Bodo C, Rissman EF. Androgen receptor is essential for sexual differentiation of responses to olfactory cues in mice. Eur J Neurosci. 2007;25:2182–90. doi: 10.1111/j.1460-9568.2007.05484.x. [DOI] [PubMed] [Google Scholar]