Abstract
The tolerance of normal brain tissues limits the radiation dose than can be delivered safely during cranial radiotherapy, and one of the potential complications that can arise involves cognitive impairment. Extensive laboratory data have appeared recently showing that hippocampal neurogenesis is significantly impacted by irradiation and that such changes are associated with altered cognitive function and involves, in part, changes in the microenvironment (oxidative stress, inflammation). While there is considerable uncertainty about exactly how these changes evolve, new in vitro and in vivo approaches have provided a means by which new mechanistic insights can be gained relevant to the topic. Together, the data from cell culture and animal-based studies provide complementary information relevant to a potentially serious complication of cranial radiotherapy, and should enhance our understanding of the tolerance of normal brain after cranial irradiation.
Introduction
In the US, a large number of patients with primary or metastatic tumors in the brain will require large volume or whole brain irradiation, and in at least some of these patients, the likelihood of developing adverse reactions in terms of cognitive decline is quite high (1, 2). Currently there are no successful long-term treatments or preventive strategies for radiation-induced cognitive impairments, and only a few possibilities have been suggested (3, 4). A better understanding of how cognitive injury develops after irradiation is critical for the development of approaches to manage this potentially serious complication and for our understanding of radiation tolerance of the brain.
Radiation injury can involve multiple regions and cell/tissue types, and a large number of physical and biologic factors influence the expression and extent of damage (5). While overt tissue injury generally occurs only after relatively high doses (>60 Gy, fractionated) (5, 6), less severe morphologic changes can occur after relatively lower doses, resulting in variable degrees of cognitive impairment, particularly in children (7-9). Such impairment has a diverse character, but often includes hippocampus-dependent functions involving learning, memory and spatial information processing. Similar findings have been reported under laboratory conditions, confirming the importance of hippocampal-related effects in the evolution of radiation-induced cognitive injury (10-14). It is particularly noteworthy that the hippocampus is an active site of neurogenesis, having multipotent stem/precursors that produce cells that migrate away and produce neurons or glia (15).
The cellular and molecular mechanisms underlying radiation-induced cognitive impairments are still not known, but may involve alterations in hippocampal neurogenesis (10-14, 16). In fact, recent data from human patients show that neurogenic cells are significantly reduced after treatment of malignant brain tumors with irradiation (17). The association between reduced neurogenesis after irradiation and a variety of cognitive impairments, suggests a mechanistic link between these factors. However, other elements may also be involved, including loss of mature neurons in the hippocampal dentate gyrus (DG) (10, 18), alterations in NMDA subunits (19), and genetic risk factors (20). Furthermore, cognitive injury due to irradiation almost certainly will involve changes associated with neuronal function, either through direct cell damage or damage mediated through factors from the irradiated microenvironment.
Laboratory studies clearly suggest that alterations in neurogenesis, and presumably cognitive impairment, involve at least in part, inflammation (12, 21-24) and/or factors related to redox homeostasis (21,25). Understanding how neural stem/precursor cells respond to irradiation and, in particular, how microenvironmental factors impact that response, should provide critical information relevant to the development of strategies and approaches to ameliorate or treat radiation-induced injuries that are associated with behavioral performance. These important issues are being addressed using both in vitro and in vivo approaches; some of the most pertinent ideas germane to this topic, as well as some new concepts, are summarized below.
In vitro approaches for studying the radiation response of multipotent neural precursor cells (MNPs)
Studies of radiation-induced effects on neurogenesis as they relate to cognitive function are necessarily difficult because they rely on complicated in vivo models. However, it is possible to address specific mechanisms associated with radiation effects on neural stem/precursor cells by using in vitro approaches. Primary multipotent neural precursor cells (MNPs) derived either from the dentate subgranular zone (SGZ) in the hippocampus or from the subventricular zone (SVZ) lining the lateral ventricles of the brain (15), have been isolated from various rodent and human sources and successfully grown in culture (26-31). Cells isolated from either of these regions can be grown as multicellular spheroids or neurospheres (Fig. 1), and while the formation of these structures is believed to depend on the presence of self-renewing neural stem cells (32-34), it has been difficult to demonstrate a 1:1 relationship between sphere formation and the presence of bona fide neural stem cell. Discriminating the difference between stem vs. precursor cells may be important under certain circumstances, and can impact the types of conclusions drawn from a given study (32-34). MNPs can also be grown as monolayer cultures, and this approach can provide certain benefits over neurospheres, such as an easier detection and quantification of differentiated phenotypes (35). While other primary and transformed cell lines have also been used to characterize the response of the 3 primary neural phenotypes in the brain, neuronal, astrocytic and oligodendrocytic, this section will focus strictly on in vitro approaches for studying the radiation response of MNPs.
Fig. 1.
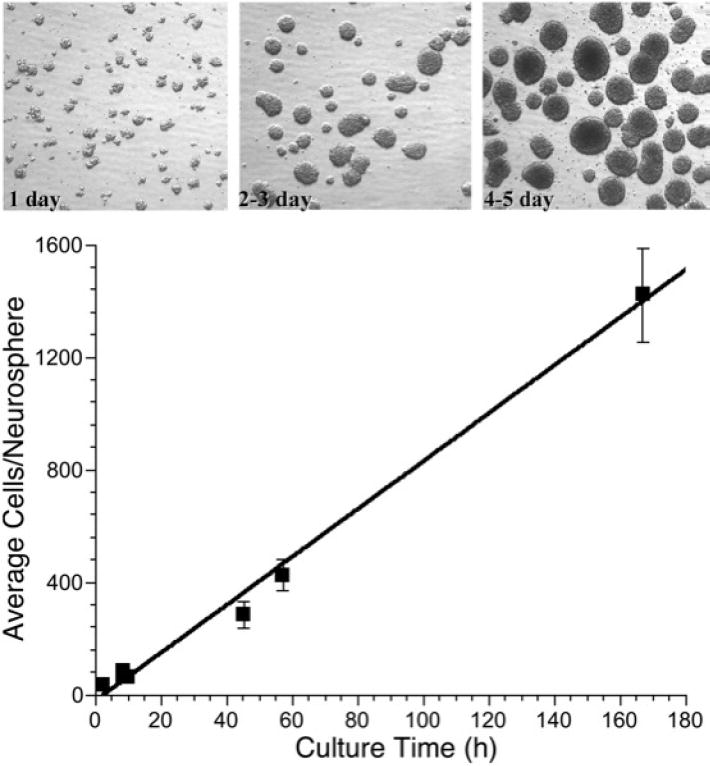
Photomicrographs (60X) of neural precursor cell neurospheres (top) as a function of time in culture, showing progressively larger size. Quantitative assessment of the numbers of cells/neurosphere as a function of time in culture shows a linear response over 7-8 days (bottom).
The growth and maintenance of MNPs is significantly influenced by specific culturing conditions which can vary greatly between laboratories. Typical conditions for maintaining these cells in an undifferentiated state requires serum free culturing in the presence of FGF and EGF (35, 58), although additional factors may also be required, depending on tissue source and experimental requirements. Hippocampal derived monolayer cultures of MNPs typically double every day, and are maintained on substrates that impede their tendency to differentiate, e.g. laminin (29, 35). Neurosphere cultures derived from the SVZ and maintained in suspension, typically have similar doubling times, but show greater variation in this parameter due to different culture strategies (i.e. shaking, spinner flask etc.). When neuropheres are dissociated, single MNPs reaggregate rapidly, forming loosely defined spheres of ~40 cells within an hour (Fig. 1). Under standard growth conditions in a humidified incubator, and with mild agitation (using shaker platforms), these spheres grow and increase in size linearly over the course of 5 days, ultimately averaging ~ 1400 cells (Fig. 1). Over this timeframe, typical labeling indexes, as measured by BrdU pulse labeling, vary from 20 to 35% (data not shown). This rapid expansion of neurospheres is important because many critical radiobiological parameters including radiosensitivity, redox-dependent signaling and differentiation may well depend on sphere size, cell heterogeneity and growth fraction. Similar considerations apply for monolayer cultures of MNPs, and we have shown that subtle changes in culture density have a marked impact on changes in oxidative stress, mitochondrial function, proliferation, and radiosensitivity (35). While these in vitro models are likely to be very valuable for addressing the mechanisms of the radiation-induced stress responses, they must be well characterized and used carefully, inasmuch as relatively simple manipulations (culture density, neurosphere size) can have a profound impact on many measurements, particularly those that are sensitive to physiologic changes such as redox state (i.e. metabolism, oxidative stress, hypoxia).
The characterization and use of MNP cultures is complicated by the tendency of these cells to differentiate. Differentiation can be induced by a variety of methods including anchorage to various substrates, presence of serum, retinoic acid and/or other morphogenic factors, and representative yields of glial and neural cell types can be obtained in as little as two days, with the proportion of differentiated cell types increasing with time (35). After differentiation, monolayers of hippocampal MNPs typically yield 5-10% astrocytes, 1-2% oligodendrocytes, 5-10% immature neurons, and 1-3% mature neurons, with the remainder of the population being immunoreactive for the undifferentiated cell marker nestin (35). These values vary somewhat, depending on the specifics of the culture and differentiation conditions. Sectioned neurospheres can provide a geographical snapshot of the cellular architecture and proliferative characteristics of the sphere, and provide a more relevant intercellular context, e.g. cell-cell contact. For instance, after pulse labeling with BrdU, there is a high fraction of BrdU-positive cells (green) near the periphery of the sphere (Fig. 2A), with less frequent staining in the center. Additionally, the pattern of differentiation is likely to be dependent on the spatial location of cells within the mass of the neurosphere. When whole spheres are induced to undergo differentiation, they show evidence of highly developed processes and intercellular connectivity (Fig. 2B). Finally, when spheres are dissociated and subjected to a variety of differentiation regimes, they exhibit similar yields of differentiated cell types as their monolayer counterparts (Fig. 2C, D). The ability to maintain cultures of MNPs and subject them to differentiation provides a powerful tool with which to analyze how radiation impacts cell fate under a variety of experimental manipulations.
Fig. 2.
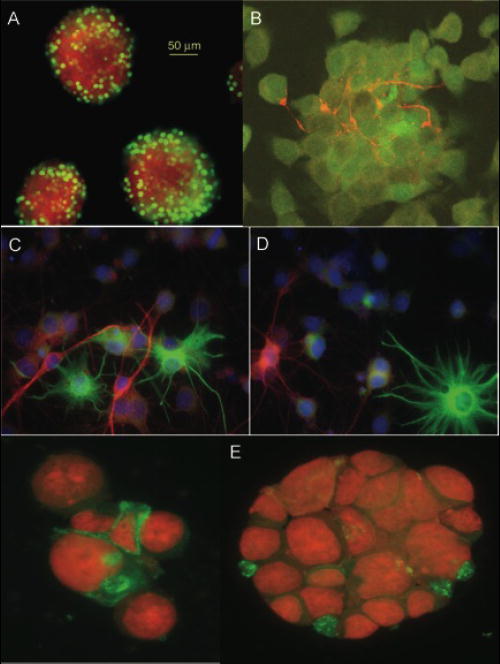
Confocal images of multipotent neural precursor cell neurospheres. A) neurospheres were pulse labeled (2h) with BrdU, embedded in agarose, sectioned, and stained for BrdU. Positively stained, BrdU+ cells (green) were seen primarily at the periphery of the neurosphere; counterstaining was done with the nuclear dye 7-AAD. B) Neurospheres were differentiated for 5 days with serum, fixed in para-formaldehyde and processed for neuronal (β-tubulin, red) and astrocytic (GFAP, green) markers. C and D) Dissociated neurospheres were plated on matrigel and 2% serum for 2 days and stained with the astrocytic marker GFAP (green) or the immature neuronal markers doublecortin (red, C) or β-tubulin (red, D). E) Primary neurospheres were stained for CD133 (green); counterstaining was done with the nuclear dye 7-AAD.
The use of cultured MNPs has proven invaluable for addressing a key factor in the radiation response of these cells: oxidative stress. We have shown that MNPs irradiated over a wide range of biologically relevant doses (i.e. where cell survival > 50%) show increased oxidative stress over acute (hours – days) and chronic (weeks –months) times post-irradiation (35, 36-40). Such measurements depend on the use of fluorogenic dyes that can enter cells and become oxidized, yielding a fluorescent analog that can be quantified using fluorescent activated cell sorting (FACS). There are many caveats associated with the use of such dyes, because few (if any) show absolute specificity for a certain reactive oxygen (ROS) or reactive nitrogen (RNS) species (41). However, when these probes are coupled with the use of their fluorescent, but oxidation-insensitive analogs to control for intercellular variability in dye uptake and retention, they provide useful measures for determining changes in the overall status of the intracellular redox environment (41). We have used these and related approaches to demonstrate that after a variety of radiation types and irradiation conditions that there are significant increases in radiation-induced oxidative stress in MNPs (36, 39, 40). Increases in free radical oxidants can be visualized in living MNPs 1-day after 0, 2 or 5 Gy of irradiation, using the carboxy-methyl diacetate analog of reduced fluorescein (CM-H2DCFDA) (Fig. 3, upper three panels) or Mitosox (Fig. 3, lower three panels). A higher magnification of cells treated with Mitosox (Fig. 3, far right), which has a relative specificity for superoxide, shows fluorescent mitochondria (red), the chief source of intracellular superoxide in these cells. Cells stained with these types of dyes can be analyzed using a variety of techniques to assess how a given intervention might modulate oxidative stress. When irradiated samples are analyzed in parallel to sham irradiated controls set to unity, one can quantify relative increases (or decreases) in MNPs (Fig. 4) (36, 39, 40).
Fig. 3.
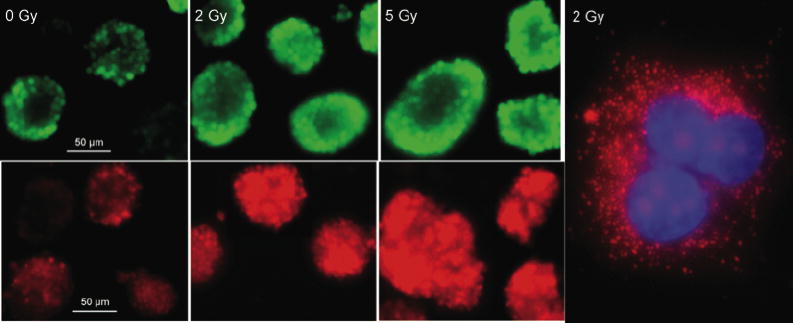
Imaging oxidative stress in living multipotent neural precursor cells. Neural precursor cells received either 0, 2 or 5 Gy and were incubated 1 day later with the carboxy-methyl diacetate analog of reduced fluorescein (CM-H2DCFDA) (upper panels), or Mitosox (lower panels). Cell were rapidly imaged at 60X to prevent photobleaching. Irradiation induced increased staining with both dyes, when compared to cells that were unirradiated. Neural precursor cells were irradiated with 1 Gy and 1 day later, superoxide output from mitochondria the was detected using Mitosox staining (red) and confocal microscopy (right panel). Nuclear counterstaining was done with DAPI.
Fig. 4.
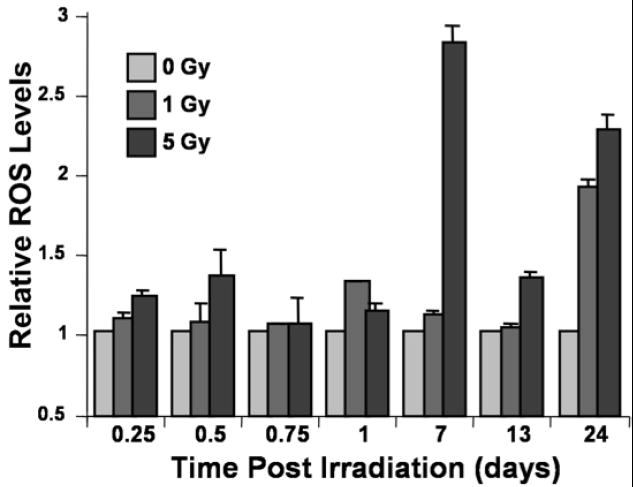
Radiation-induced oxidative stress in neural precursor cells. The data show the temporal response of ROS/RNS production in cells treated with an oxidation sensitive fluorogenic dye precursor (CM-H2DCFDA). Acute and chronic increases in ROS/RNS levels are evident in x-irradiated cells, when data are normalized to un-irradiated controls arbitrarily set to unity.
We have also used cultured MNPs to investigate their sensitivity to agents known to deplete mitochondrial DNA (mtDNA). Extended culture time in the presence of low levels of ethidium bromide (EtBr) (~ 50 ng/ml) has been shown to gradually deplete mtDNA (42). This eventually leads to a population of metabolically compromised cells containing mitochondria devoid of mtDNA. We have used this technique to generate mtDNA deficient cells in the past, but have yet been unsuccessful in generating MNPs devoid of mtDNA. Our past work has demonstrated the marked sensitivity of these cells to changes in redox state (35, 40), and our attempts at generating mtDNA deficient MNPs highlights their sensitivity to redox manipulations. Where other cell lines (e.g., GM10115, HeLa, XP30RO) can tolerate months (~5 months) of culture under EtBr (50 ng/ml) MNPs die from severe metabolic acidosis within 4 days after culturing in as little as 5 ng/ml of EtBr (data not shown). This underscores the sensitivity MNPs to interventions targeting mitochondrial metabolism, and suggest that these in vitro culture models may be useful for dissecting pathways that converge on mitochondrial metabolism and radiation sensitivity.
While the study of cultured MNPs will provide useful information important to understanding a critical aspect of normal tissue response in the brain, their presence and response to irradiation may also be germane to the topic of brain tumors. The recent emergence of the cancer stem cell hypothesis has created a tremendous amount of research activity aimed at identifying putative cancer stem cells from a variety of neoplasms (43-45). The hypothesis is based on the idea that many, if not all, cancers have a self renewing stem cell that when serially transplanted into recipient hosts, will recapitulate all the aberrant phenotypes of the original cancer. The idea that MNPs might represent a latent reservoir of brain tumor stem cells has received a great deal of attention (46-48), and was triggered by seminal reports showing that CD133 positive cells were highly tumorigenic (compared to their CD133 negative counterparts) when orthotopically transplanted into the brains of mice (48). While this marker may or may not be the critical brain tumor stem cell marker, we have found that neurospheres derived from normal brain do express CD133 in a small fraction of cells (1-3%, Fig. 2E). We also have evidence that dose fractionation increases the fraction of CD133 positive MNPs (data not shown), a finding that, in principal, is consistent with a report analyzing the radioresponse of CD133 positive tumor cells derived from high grade glioblastoma (49). Recent work (50) has also identified another important marker shared between normal MNPs and brain tumor stem cells, maternal embryonic leucine zipper kinase (MELK), which is a member of the snf1/AMPK serine-threonine kinase family. Past work (51) has shown this kinase to be a critical regulator of proliferation in MNPs of the SVZ but not the SGZ, an interesting finding that highlights critical differences between different populations of MNPs in the CNS (52, 53). These findings strengthen the link between MNPs and brain tumor stem cells.
In summary, in vitro cultures of MNPs provide a useful tool for the analysis of critical mechanistic parameters associated with radiation response of the brain. The susceptibility and sensitivity of these cells to radiation-induced oxidative stress and related redox changes is supported by numerous observations from our lab. Interestingly, a recent report detailing the role of SirT1 as a master metabolic switch capable dictating the redox-dependent fate of MNPs in vivo and in vitro (54) adds further evidence in support for the importance of redox state to the overall functionality of these critical CNS cells. Such data provide compelling insight into the biology of these cells and how they respond to irradiation. The clinically applicability of information derived from such models is also starting to be addressed, given that more mouse genetic models are becoming available for confirming the in vitro findings. While our results have demonstrated a marked coincidence between in vitro and in vivo data, further work is needed to firmly establish the translational utility of culture-based models for understanding radiation-induced sequelae in the CNS.
In vivo approaches to study neural precursor cell response after irradiation
A number of experimental reports have clearly shown that neural stem/precursor cells in dentate SGZ are extremely radiosensitive, undergoing apoptosis shortly after treatment with relatively low doses (12, 21, 22). While this provides useful information regarding the intrinsic sensitivity of neural precursor cells and their progeny, it does not directly address the longer-term issue of the fate of the surviving precursor cells. To assess this issue, different approaches are required. Neurogenesis describes a series of developmental steps that progress from the division of a stem/precursor cell to the development of a mature cell (55). The most commonly used method to determine the survival and phenotypic fate of newly born cells is to give repeated administration of the thymidine analog 5-bromo-2’deoxyuridine (BrdU), and 3-4 weeks later quantify the fraction of newly born BrdU-positive cells that co-express mature cell markers. Using this approach, it has clearly been shown that irradiation significantly impacts new neuron production in the DG (10, 12, 16, 18, 22, 23, 25, 56); effects on newly born astrocytes and oligodendrocytes are not as obvious (12, 22, 23). To date, our studies are the only ones showing that neurogenesis is reduced in a dose dependent fashion (22, 56) (Fig. 5). Furthermore, such changes are persistent, at least for the first 2-4 months after irradiation (12). Taken together, these data, along with the correlative studies associating altered neurogenesis with behavioral performance after irradiation (10-13, 16, 18) suggest that neurogenesis may play a contributory if not causal role in the effects of irradiation on cognitive function.
Fig. 5.
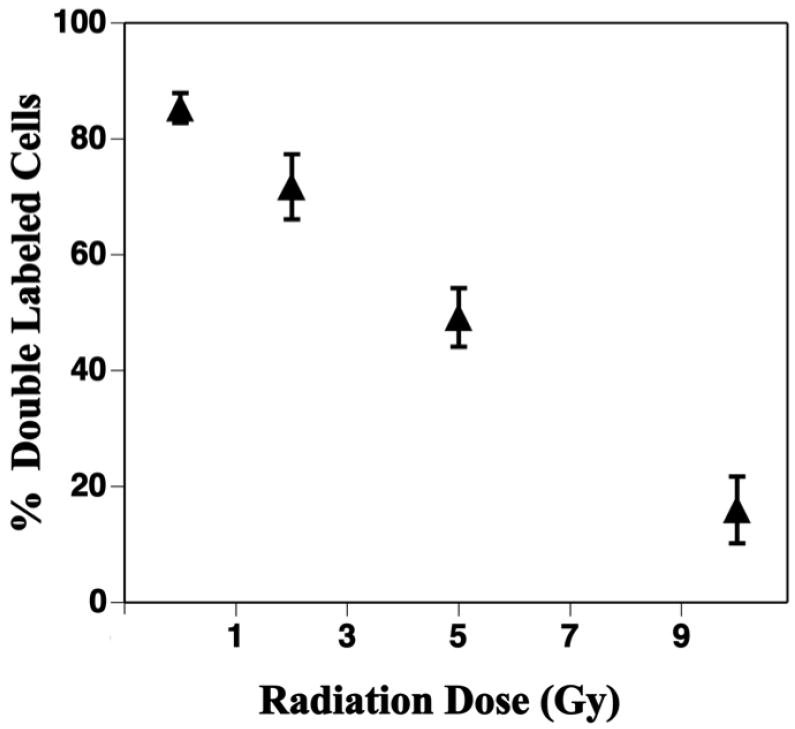
Neurogenesis in the dentate subgranular zone is reduced 2 months after local brain irradiation. Newly born neurons were co-labeled with antibodies against BrdU and the neuron specific antigen NeuN and the fractions of double labeled cells were quantified with confocal microscopy. Approximately 85% of newly born cells differentiated into neurons in sham-irradiated controls and there was significant (p < 0.001) dose-related decrease after irradiation. Each datum point represents 4 mice and error bars are SEM. Adapted from Mizumatsu et al (22) with permission.
Considerable data exist showing that radiation effects on neurogenesis involve alterations in the microenvironment, such as oxidative stress and inflammation (12, 21-25). Regarding the former, the redox environment is of particular importance in the CNS, where there is a relatively high rate of oxygen consumption and metabolic turnover (57), and relatively low levels of endogenous antioxidants which render the CNS inherently susceptible to oxidative injury (58). Altered redox state is critical in regulating the response of the CNS after a variety of insults including ionizing irradiation (57, 59), and may involve increased production of reactive oxygen species (ROS), which can contribute to the spread and ultimate expression of tissue injury (5). Indications of persistent oxidative stress after irradiation have been demonstrated in the brains of mice (21, 35) and rats (60), and along with the in vitro data described above, suggest that ROS may constitute a critical environmental cue to control precursor cell survival and differentiation (35).
There are several pathways that mitigate the physiological and pathological effects of ROS in mammalian cells. One of these involves the antioxidant enzyme superoxide dismutase (SOD), which exists as 3 genetically and geographically distinct isoenzymes (61). The SODs convert superoxide anions to hydrogen peroxide, which is then enzymatically removed by catalase and glutathione peroxidase. Although the physiological roles of the SOD isoforms in mammalian cells are not completely understood, the extracellular isoform (EC-SOD, SOD3) is associated with certain cognitive functions (62, 63), and its removal interfers with signaling cascades critical for learning (63). It is interesting that in EC-SOD knock out (KO) mice, the baseline level of neurogenesis is significantly lower than wild type (WT) mice (Fig. 6A); whether or not this finding is responsible for the cognitive deficits observed in these mice (35) is not yet known.
Fig. 6.
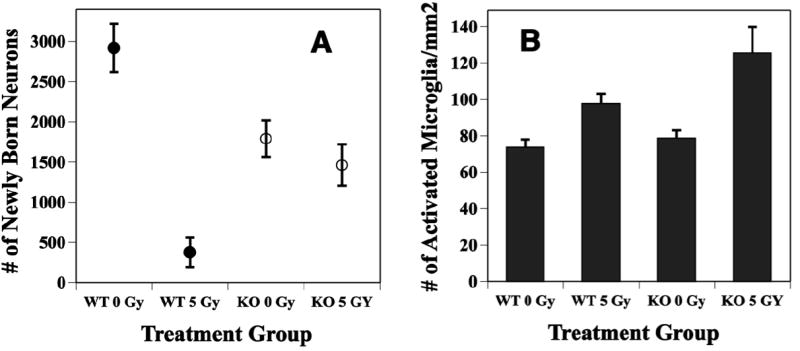
Radiation effects on neurogenesis are reduced in animals deficient in EC-SOD. Two month-old WT and EC-SOD KO mice received whole brain irradiation with 5 Gy, and the fractions of newly born neurons and activated microglia were quantified either 2 months later using immunohistochemistry and confocal microscopy. A: In un-irradiated KO mice, the baseline level of neurogenesis was significantly (p < 0.01) reduced relative to WT. Irradiation caused a significant (p < 0.01) reduction in the production of newly born neurons in WT but only a minor (p = ns) reduction in KO mice. Newly born neurons were detected using antibodies against BrdU and the neuron specific marker NeuN. Each datum point represents a mean value of 4 mice; error bars are SEM. B: Irradiation increased total numbers of activated microglia in and around the dentate gyrus of both WT and EC-SOD KO mice, with the largest effect in KO mice. Activated microglia were detected using an antibody against CD-68. Each bar represents a mean value of 4 mice; error bars are SEM. Both panels were adapted from Rola et al (12) with permission.
When EC-SOD KO mice are irradiated with a modest dose of x-rays (5 Gy), the changes in numbers of proliferating cells or immature neurons in the DG seen 48 hr after irradiation are not different from those seen in wild type (WT) mice (25). This suggests that the absence of EC-SOD and the increased oxidative stress status of KO animals, does not influence mechanisms responsible for acute cell death after irradiation. However, in terms of neurogenesis, while there is a very substantial reduction in newly generated neurons in irradiated WT mice, the same dose of irradiation has virtually no effect in KO mice (Fig. 6A). The precise mechanism for this effect is not clear, but it apparently does not involve simple compensatory changes in the expression or activities of other anti-oxidant enzymes (25). Rather it leads to a ‘protective’ type of effect not unlike the preconditioning (64), adaptive (65) or inducible-like radioprotective responses (66) observed by others. One possible explanation for this finding may involve inflammation, as defined by changes in the numbers of intrinsic inflammatory cells of the brain, the microglia. After irradiation of EC-SOD KO mice, the total number of activated microglia in and around the DG is higher that seen in WT (Fig. 6B) (25). These data are surprising taking into account a number of recent studies suggesting that increased neuroinflammation is generally linked with an inhibition of hippocampal neurogenesis (12, 21-25) (Fig. 7). Given recent data showing that microglial phenotype critically influences the ability of those cells to support or impair renewal processes (67), it may be that in a microenvironment characterized by persistent oxidative stress (e.g. EC-SOD KO), the subsequent activation of microglia may in fact have a beneficial effect, at least in terms of neurogenesis. This would suggest that microglial response to a given stimulus (e.g. irradiation) may be context dependent; this idea has been recently reviewed (68). While many questions remain, these types of data highlight the complexities associated with understanding stem/precursor cell radiation response in vivo.
Fig. 7.
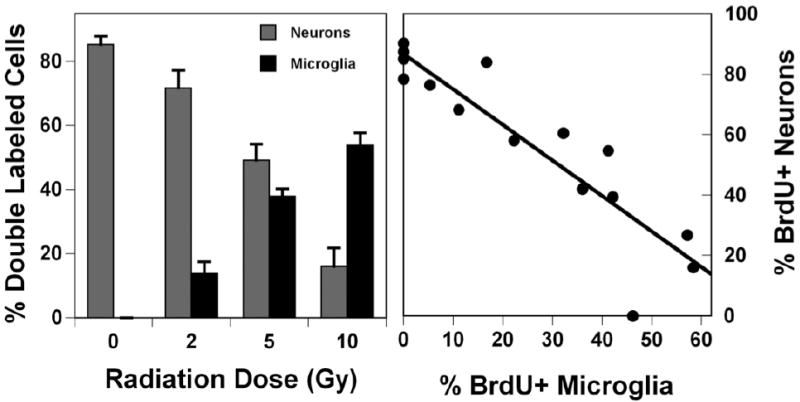
Radiation-induced changes in neurogenesis are associated with indications of neuroinflammation. Two month-old C57BL6J mice were irradiated with whole brain doses ranging from 2 – 10 Gy and 2 months later, dual labeling immunohistochemistry and confocal microscopy were used to quantify the fractions of newly born (BrdU+) cells that were co-labeled with NeuN (neurons; left panel, light bars) or CD68 (activated microglia; left panel, dark bars). The significant decrease in numbers of newly born neurons was associated with a significant increase in the numbers of newly born activated microglia. When considered in terms of individual animals, when neurogenesis (percentage of BrdU+ neurons) was plotted against the percentage of BrdU+ activated microglia, there was a strong correlation (r = 0.83) suggesting a relationship between neuroinflammation and reduced neurogenesis. In the left panel each bar represents a mean of 4 mice; error bars are SEM. In the right panel each point represents a single animals. Reprinted from Fike et al (21) with permission.
While there certainly seems to be a relationship between altered neurogenesis and the development of cognitive dysfunctions after irradiation, it is reasonable to assume that other factors can also play a role (10, 18-20). One issue that has not had much emphasis to date, is the idea of radiation-induced alterations in neuronal activity, particularly as it is coupled to macromolecular synthesis (gene expression) associated with learning and memory. Gene expression induced during learning produces proteins that alter the composition of hippocampal neuronal networks, and provide a mechanism for translating synaptic plasticity into changes in synaptic strength (memory). Information is starting to appear regarding gene expression changes in brain cells after exposure to ionizing irradiation but most of the available experimental data relate to very early changes (<1 – 24 hr) (69, 70), and there is only one consideration of an immediate early gene (IEG), c-fos, specifically known to be linked to learning and memory (69). Among the IEGs, Arc (activity-regulated cytoskeleton-associated protein) has a distinct role in modulating hippocampal synaptic plasticity (71). Arc is expressed following stimuli that induce long term potentiation (75) as well as after exploration of an environment and repeated learning tasks (73), and Arc protein is essential for consolidation of synaptic plasticity and memory (74, 75). The temporal and spatial characteristics of Arc expression corroborate neuronal activity profiles obtained using well accepted electrophysiological recordings. Perhaps most importantly, Arc is the only known IEG whose mRNA moves rapidly to the dendrites closest to active synapses where it is locally translated (76). Therefore Arc expression has been utilized extensively to map neuronal networks that underlie information processing and plasticity (76-78). Taken together, this information provides a mechanistic link between Arc and hippocampal-dependent functions, and provides a strong rationale for using Arc expression to assess specific neuronal activities associated with cognitive impairments induced by cranial irradiation.
When animals are placed in a novel learning environment, Arc mRNA is induced in neuronal nuclei within 2-5 minutes, is translocated into the cytoplasm a few minutes later (Fig. 8A, B), and within 30 minutes is translated into protein. In contrast, in caged control animals that do not explore a novel environment, very little Arc mRNA or protein are expressed. If animals are allowed to explore a novel environment twice for 5 min, with 25 min between sessions, it is possible to detect intranuclear foci of mRNA from the second exploration and cytoplasmic Arc mRNA from the first exploration (Fig. 8C). This provides information relevant to the stability of neuronal networks inasmuch as under normal conditions approximately 90% of neurons are activated in both exploration sessions. Any deviation from that value would suggest alterations in neuronal circuitry. In a preliminary study, we irradiated mice with 0 Gy or 10 Gy, a dose that induces cognitive deficits (10, 11). Two months later, we used exploration of a novel environment to induce Arc. Following exploration, the number of neurons expressing Arc in the hippocampus was significantly higher than that seen in caged control mice that did not explore the novel environment. Irradiation reduced the fractions of neurons expressing behaviorally-induced Arc mRNA and Arc protein in the DG (Fig. 9), without any radiation-induced cell loss. Importantly, this difference occurred only when animals were engaged in the exploration of a novel environment; no differences were seen in caged control animals. While the activation value (fraction of neurons expressing Arc) for the DG was < 10%, this area is the major subfield of the hippocampus and contains more neurons (~ 240,000 in C57BL6 mice) than the other regions (CA1, CA3) combined (79). The maintenance of a small fraction of Arc activity during a behavioral experience is critical for proper hippocampal function, and is consistent with electrophysiological recordings showing sparse activity in the DG during behavior, the so-called sparse coding theory (80). Given the information on relative cell numbers in the DG (79), the modest changes in Arc mRNA and Arc protein we saw in our study represent a relatively large number of cells in the DG and could be extremely important for proper hippocampal function. While this study involved a relatively small number of mice, it suggests that this type of approach, i.e. addressing the molecular distribution of Arc at the level of mRNA and protein, could provide a novel way to consider radiation-induced brain injury. Of particular interest, as it relates to the neurogenesis, it has recently been shown that the proportion of newly born neurons that express Arc in response to a learning experience (Fig. 8) is nearly 2 fold higher than what is seen in mature granule cell neurons (81), and those cells are preferentially recruited into circuits supporting spatial memory (82). This provides a potential mechanistic link between Arc expression, neurogenesis and behavior, and may offer new insight into how irradiation may impact cognitive function.
Fig. 8.
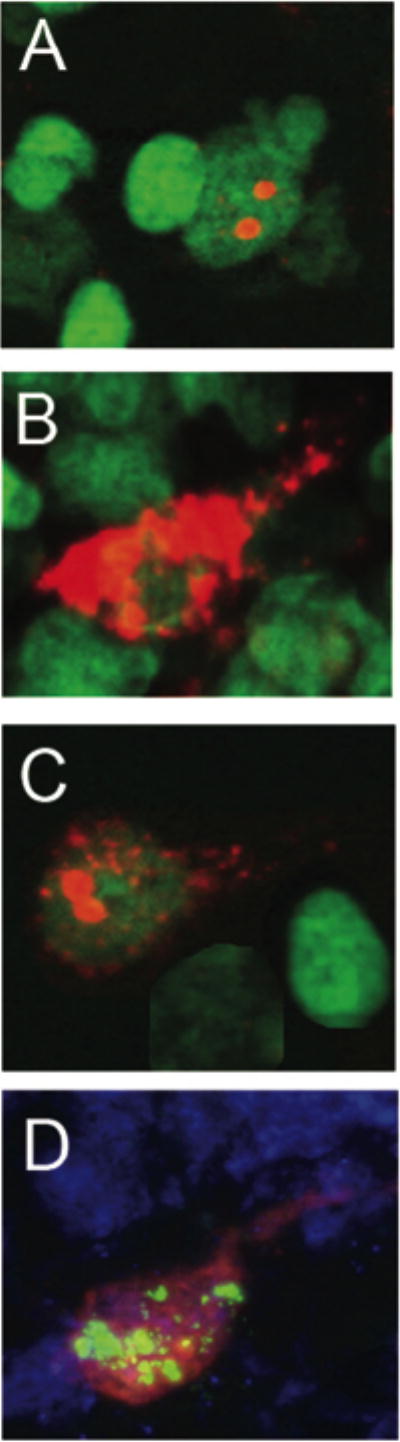
Qualitative characterization of Arc expression in the granule cell layer neurons of the dentate gyrus. Arc was induced by 2 five minute behavioral explorations of a novel environment that were separated by 25 minutes. Intranuclear foci of Arc mRNA were induced by the second exploration, ~ 5 minutes before tissue collection, and were detected using fluorescent in situ hybridization (A). Cytoplasmic Arc mRNA (B) and Arc protein (D) could be detected in neurons activated by the first exploration. Both nuclear foci and cytoplasmic Arc mRNA were seen in ~ 90% of cells immunoreactive for Arc (C). In animals given BrdU to label newly born neurons, labeling with antibodies against BrdU (green) and Arc protein (red) showed dual labeling, indicating that newly born neurons were functionally integrated into the dentate gyrus (D). Digoxine labeled Arc antisense probe was detected with Cy3 (red, A-D), and immunofluorescence staining detected Arc protein (D). Cell nuclei were counterstained green or blue and the magnification for all the images was 63X.
Fig. 9.
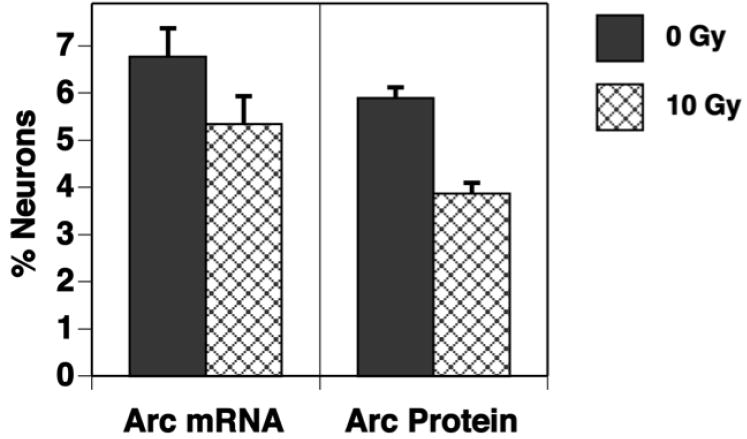
The fractions of neurons in the dentate gyrus expressing Arc mRNA and Arc protein are reduced by irradiation. Mice received a single dose of 0 Gy or 10 Gy and 2 months later were allowed to explore a novel environment twice for 5 minutes with 25 minutes between sessions. Tissues were collected 5 minutes after the second exploration and Arc mRNA and Arc protein were detected using fluorescence in situ hybridization and immunohistochemistry, respectively. While there is a trend toward reduction in the fraction of neurons expressing Arc mRNA, the difference is significant with respect to the fraction of neurons expressing Arc protein. Each bar represents a mean of 3 animals; error bars are SEM.
Conclusions
While extensive data have appeared over the past few years showing that neurogenesis/cognitive function are significantly affected by ionizing irradiation (summarized in table 1), there still is considerable uncertainty about the exactly how those changes evolve. In vitro techniques for the study of neural stem/precursor cells provide a unique way to address mechanistic elements associated with radiation response. With respect to in vivo strategies, genetic models (e.g. EC-SOD KO mice) or novel approaches (e.g. neuronal activity via Arc) provide new tools with which the complexities of whole animal models can be dissected to better understand how cellular radiation injury evolves into deficits affecting behavioral performance. Together, the combination of in vitro and in vivo models provide novel quantitative methods to address the tolerance of normal brain after exposure to ionizing irradiation.
Table 1.
Factors That Influence Neural Stem/Precursor Cell Survival and Function
| Measured Responses | In vitro | In vivo |
|---|---|---|
| Cell growth and differentiation | Multipotent cells can be obtained and grown from neurogenic areas in brain. | Neurogenesis results in new neurons/glia in the hippocampal dentate gyrus. |
| Proliferation and differentiation | Neural precursor cell growth is dependent upon cell density and cell-cell contact. | Changes in neurogenesis are associated with changes in cognitive function. |
| Radiation effects have an early impact on cell viability | Irradiation induces acute (hrs-days) oxidative stress and apoptosis. | Neural precursor cells extremely sensitive, undergoing apoptosis after clinically relevant doses within 48 hr. |
| Reactive oxygen species, radiation dose, and new neurons | Radiation-induced oxidative stress in multipotent neural precursor cells is dose dependent. | Radiation reduces neurogenesis in a dose dependent fashion. |
| Radiation effects have a chronic impact on cell viability and differentiation | Irradiation induces persistent (weeks-months) oxidative stress. | Radiation-induced changes in neurogenesis are persistent |
| Microenvironmental factors can impact the response of neural precursor cells | Protracted irradiation enriches cultures for radioresistant CD133+ neural precursor cells | Alterations in the microenvironment (inflammation, oxidative stress) are associated with altered neurogenesis and cognitive function after irradiation. |
| Neuronal activity | Neuronal activity in the dentate gyrus is adversely affected by irradiation |
Acknowledgments
Supported in part by: NIH grant NS 46051(JRF), NASA grants NNJ04HC90G (JRF), NNJ05HE33G (JRF), NNA06CB37G (CLL), ACS grant RSG-00-036-04-CNE (CLL), AND DOE Grant DE-FG02-07ER64349 (CLL)
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Meyers CA, Brown PD. Role and relevance of neurocognitive assessment in clinical trials of patients with CNS tumors. J Clin Oncol. 2006;24(8):1305–9. doi: 10.1200/JCO.2005.04.6086. [DOI] [PubMed] [Google Scholar]
- 2.Butler JM, Rapp SR, Shaw EG. Managing the cognitive effects of brain tumor radiation therapy. Curr Treat Options Oncol. 2006;7(6):517–23. doi: 10.1007/s11864-006-0026-5. [DOI] [PubMed] [Google Scholar]
- 3.Yazlovitskaya EM, Edwards E, Thotala D, Fu A, Osusky KL, Whetsell WO, Jr, Boone B, Shinohara ET, Hallahan DE. Lithium treatment prevents neurocognitive deficit resulting from cranial irradiation. Cancer Res. 2006;66(23):11179–86. doi: 10.1158/0008-5472.CAN-06-2740. [DOI] [PubMed] [Google Scholar]
- 4.Zhao W, Payne V, Tommasi E, Diz DI, Hsu FC, Robbins ME. Administration of the peroxisomal proliferator-activated receptor gamma agonist pioglitazone during fractionated brain irradiation prevents radiation-induced cognitive impairment. Int J Radiat Oncol Biol Phys. 2007;67(1):6–9. doi: 10.1016/j.ijrobp.2006.09.036. [DOI] [PubMed] [Google Scholar]
- 5.Tofilon PJ, Fike JR. The radioresponse of the central nervous system: A dynamic process. Radiat Res. 2000;153:357–370. doi: 10.1667/0033-7587(2000)153[0357:trotcn]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 6.Sheline GE, Wara WM, Smith V. Therapeutic irradiation and brain injury. Int J Radiat Oncol Biol Phys. 1980;6:1215–1228. doi: 10.1016/0360-3016(80)90175-3. [DOI] [PubMed] [Google Scholar]
- 7.Abayomi OK. Pathogenesis of irradiation-induced cognitive dysfunction. Acta Oncol. 1996;35(6):659–663. doi: 10.3109/02841869609083995. [DOI] [PubMed] [Google Scholar]
- 8.Roman DD, Sperduto PW. Neuropsychological effects of cranial radiation: current knowledge and future directions. Int J Radiat Oncol Biol Phys. 1995;31(4):983–998. doi: 10.1016/0360-3016(94)00550-8. [DOI] [PubMed] [Google Scholar]
- 9.Surma-aho O, Niemela M, Vilkki J, Kouri M, Brander A, Salonen O, Paetau A, Kallio M, Pyykkonen J, Jaaskelainen J. Adverse long-term effects of brain radiotherapy in adult low-grade glioma patients. Neurology. 2001;56(10):1285–90. doi: 10.1212/wnl.56.10.1285. [DOI] [PubMed] [Google Scholar]
- 10.Raber J, Fan Y, Matsumori Y, Liu Z, Weinstein PR, Fike JR, Liu J. Irradiation attenuates neurogenesis and exacerbates ischemia-induced deficits. Ann Neurol. 2004;55(3):381–9. doi: 10.1002/ana.10853. [DOI] [PubMed] [Google Scholar]
- 11.Raber J, Rola R, LeFevour A, Morhardt DR, Curley J, Mizumatsu S, VandenBerg SR, Fike JR. Radiation-Induced cognitive impairments are associated with changes in indicators of hippocampal neurogenesis. Radiat Res. 2004;162:39–47. doi: 10.1667/rr3206. [DOI] [PubMed] [Google Scholar]
- 12.Rola R, Raber J, Rizk A, Otsuka S, VandenBerg SR, Morhardt DR, Fike JR. Radiation-induced impairment of hippocampal neurogenesis is associated with cognitive deficits in young mice. Exp Neurol. 2004;188:316–330. doi: 10.1016/j.expneurol.2004.05.005. [DOI] [PubMed] [Google Scholar]
- 13.Madsen TM, Kristjansen PE, Bolwig TG, Wortwein G. Arrested neuronal proliferation and impaired hippocampal function following fractionated brain irradiation in the adult rat. Neuroscience. 2003;119(3):635–42. doi: 10.1016/s0306-4522(03)00199-4. [DOI] [PubMed] [Google Scholar]
- 14.Winocur G, Wojtowicz JM, Sekeres M, Snyder JS, Wang S. Inhibition of neurogenesis interferes with hippocampus-dependent memory function. Hippocampus. 2006;16(3):296–304. doi: 10.1002/hipo.20163. [DOI] [PubMed] [Google Scholar]
- 15.Gage FH. Mammalian neural stem cells. Science. 2000;287(5457):1433–8. doi: 10.1126/science.287.5457.1433. [DOI] [PubMed] [Google Scholar]
- 16.Santarelli L, Saxe M, Gross C, Surget A, Battaglia F, Dulawa S, Weisstaub N, Lee J, Duman R, Arancio O, et al. Requirement of hippocampal neurogenesis for the behavioral effects of antidepressants. Science. 2003;301(5634):805–9. doi: 10.1126/science.1083328. [DOI] [PubMed] [Google Scholar]
- 17.Monje ML, Vogel H, Masek M, Ligon KL, Fisher PG, Palmer TD. Impaired human hippocampal neurogenesis after treatment for central nervous system malignancies. Ann Neurol. 2007;62(5):515–20. doi: 10.1002/ana.21214. [DOI] [PubMed] [Google Scholar]
- 18.Fan Y, Liu Z, Weinstein PR, Fike JR, Liu J. Environmental enrichment enhances neurogenesis and improves functional outcome after cranial irradiation. Eur J Neurosci. 2007;25(1):38–46. doi: 10.1111/j.1460-9568.2006.05269.x. [DOI] [PubMed] [Google Scholar]
- 19.Shi L, Adams MM, Long A, Carter CC, Bennett C, Sonntag WE, Nicolle MM, Robbins M, D’Agostino R, Brunso-Bechtold JK. Spatial Learning and Memory Deficits after Whole-Brain Irradiation are Associated with Changes in NMDA Receptor Subunits in the Hippocampus. Radiat Res. 2006;166(6):892–899. doi: 10.1667/RR0588.1. [DOI] [PubMed] [Google Scholar]
- 20.Villasana L, Acevedo S, Poage C, Raber J. Sex- and APOE isoform-dependent effects of radiation on cognitive function. Radiat Res. 2006;166(6):883–91. doi: 10.1667/RR0642.1. [DOI] [PubMed] [Google Scholar]
- 21.Fike JR, Rola R, Limoli CL. Radiation response of neural precursor cells. Neurosurg Clin N Am. 2007;18(1):115–27. doi: 10.1016/j.nec.2006.10.010. [DOI] [PubMed] [Google Scholar]
- 22.Mizumatsu S, Monje ML, Morhardt DR, Rola R, Palmer TD, Fike JR. Extreme sensitivity of adult neurogenesis to low doses of x-irradiation. Can Res. 2003;63:4021–4027. [PubMed] [Google Scholar]
- 23.Monje ML, Mizumatsu S, Fike JR, Palmer TD. Irradiation induces neural precursor-cell dysfunction. Nat Med. 2002;8(9):955–962. doi: 10.1038/nm749. [DOI] [PubMed] [Google Scholar]
- 24.Monje ML, Toda H, Palmer TD. Inflammatory blockade restores adult hippocampal neurogenesis. Science. 2003;302(5651):1760–5. doi: 10.1126/science.1088417. [DOI] [PubMed] [Google Scholar]
- 25.Rola R, Zou Z, Huang T-T, Fishman K, Baure J, Rosi S, Milliken H, Limoli CL, Fike JR. Lack of EC-SOD in the microenvironment impacts radiation-induced changes in neurogenesis. Free Rad Biol & Med. 2007;42:1133–1145. doi: 10.1016/j.freeradbiomed.2007.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chojnacki A, Shimazaki T, Gregg C, Weinmaster G, Weiss S. Glycoprotein 130 signaling regulates Notch1 expression and activation in the self-renewal of mammalian forebrain neural stem cells. J Neurosci. 2003;23(5):1730–41. doi: 10.1523/JNEUROSCI.23-05-01730.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gage FH, Kempermann G, Palmer TD, Peterson DA, Ray J. Multipotent progenitor cells in the adult dentate gyrus. J Neurobiol. 1998;36(2):249–266. doi: 10.1002/(sici)1097-4695(199808)36:2<249::aid-neu11>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- 28.Lu FG, Wong CS. Time-dependent neurosphere-forming ability of adult rat spinal cord after irradiation. Radiat Res. 2007;168(4):453–61. doi: 10.1667/RR0591.1. [DOI] [PubMed] [Google Scholar]
- 29.Palmer TD, Ray J, Gage FH. FGF-2-responsive neuronal progenitors reside in proliferative and quiescent regions of the adult rodent brain. Mol Cell Neurosci. 1995;6(5):474–86. doi: 10.1006/mcne.1995.1035. [DOI] [PubMed] [Google Scholar]
- 30.Palmer TD, Schwartz PH, Taupin P, Kaspar B, Stein SA, Gage FH. Cell culture. Progenitor cells from human brain after death. Nature. 2001;411(6833):42–3. doi: 10.1038/35075141. [DOI] [PubMed] [Google Scholar]
- 31.Tarasenko YI, Yu Y, Jordan PM, Bottenstein J, Wu P. Effect of growth factors on proliferation and phenotypic differentiation of human fetal neural stem cells. J Neurosci Res. 2004;78(5):625–36. doi: 10.1002/jnr.20316. [DOI] [PubMed] [Google Scholar]
- 32.Louis SA, Rietze RL, Deleyrolle L, Wagey RE, Thomas TE, Eaves AC, Reynolds BA. Enumeration of neural stem and progenitor cells in the neural colony-forming cell assay. Stem Cells. 2008;26(4):988–96. doi: 10.1634/stemcells.2007-0867. [DOI] [PubMed] [Google Scholar]
- 33.Lu F, Wong CS. A clonogenic survival assay of neural stem cells in rat spinal cord after exposure to ionizing radiation. Radiat Res. 2005;163(1):63–71. doi: 10.1667/rr3285. [DOI] [PubMed] [Google Scholar]
- 34.Reynolds BA, Rietze RL. Neural stem cells and neurospheres--re-evaluating the relationship. Nat Methods. 2005;2(5):333–6. doi: 10.1038/nmeth758. [DOI] [PubMed] [Google Scholar]
- 35.Limoli CL, Rola R, Giedzinski E, Mantha S, Huang T-T, Fike JR. Cell density dependent regulation of neural precursor cell function. PNAS. 2004;101:16052–16057. doi: 10.1073/pnas.0407065101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Giedzinski E, Rola R, Fike JR, Limoli CL. Efficient production of reactive oxygen species in neural precursor cells after exposure to 250 MeV protons. Radiat Res. 2005;164(4):540–4. doi: 10.1667/rr3369.1. [DOI] [PubMed] [Google Scholar]
- 37.Limoli CL, Giedzinski E, Baure J, Doctrow SR, Rola R, Fike JR. Using superoxide dismutase/catalase mimetics to manipulate the redox environment of neural precursor cells. Radiat Prot Dosimetry. 2006;122(14):228–36. doi: 10.1093/rpd/ncl458. [DOI] [PubMed] [Google Scholar]
- 38.Limoli CL, Giedzinski E, Baure J, Rola R, Fike JR. Altered growth and radiosensitivity in neural precursor cells subjected to oxidative stress. Int J Radiat Biol. 2006;82(9):640–7. doi: 10.1080/09553000600887816. [DOI] [PubMed] [Google Scholar]
- 39.Limoli CL, Giedzinski E, Baure J, Rola R, Fike JR. Redox changes induced in hippocampal precursor cells by heavy ion irradiation. Radiat Environ Biophys. 2007;46(2):167–72. doi: 10.1007/s00411-006-0077-9. [DOI] [PubMed] [Google Scholar]
- 40.Limoli CL, Giedzinski E, Rola R, Otsuka S, Palmer TD, Fike JR. Radiation response of neural precursor cells: linking cellular sensitivity to cell cycle checkpoints, apoptosis and oxidative stress. Radiation Research. 2004;161:17–27. doi: 10.1667/rr3112. [DOI] [PubMed] [Google Scholar]
- 41.Mikkelsen RB, Wardman P. Biological chemistry of reactive oxygen and nitrogen and radiation-induced signal transduction mechanisms. Oncogene. 2003;22(37):5734–54. doi: 10.1038/sj.onc.1206663. [DOI] [PubMed] [Google Scholar]
- 42.King MP, Attardi G. Isolation of human cell lines lacking mitochondrial DNA. Methods Enzymol. 1996;264:304–13. doi: 10.1016/s0076-6879(96)64029-4. [DOI] [PubMed] [Google Scholar]
- 43.Clarke MF, Dick JE, Dirks PB, Eaves CJ, Jamieson CH, Jones DL, Visvader J, Weissman IL, Wahl GM. Cancer stem cells--perspectives on current status and future directions: AACR Workshop on cancer stem cells. Cancer Res. 2006;66(19):9339–44. doi: 10.1158/0008-5472.CAN-06-3126. [DOI] [PubMed] [Google Scholar]
- 44.Dalerba P, Cho RW, Clarke MF. Cancer stem cells: models and concepts. Annu Rev Med. 2007;58:267–84. doi: 10.1146/annurev.med.58.062105.204854. [DOI] [PubMed] [Google Scholar]
- 45.Hill RP, Perris R. “Destemming” cancer stem cells. J Natl Cancer Inst. 2007;99(19):1435–40. doi: 10.1093/jnci/djm136. [DOI] [PubMed] [Google Scholar]
- 46.Nakano I, Kornblum HI. Brain tumor stem cells. Pediatr Res. 2006;59(4 Pt 2):54R–8R. doi: 10.1203/01.pdr.0000203568.63482.f9. [DOI] [PubMed] [Google Scholar]
- 47.Sanai N, Alvarez-Buylla A, Berger MS. Neural stem cells and the origin of gliomas. N Engl J Med. 2005;353(8):811–22. doi: 10.1056/NEJMra043666. [DOI] [PubMed] [Google Scholar]
- 48.Singh S, Dirks PB. Brain tumor stem cells: identification and concepts. Neurosurg Clin N Am. 2007;18(1):31–38. doi: 10.1016/j.nec.2006.10.014. [DOI] [PubMed] [Google Scholar]
- 49.Bao S, Wu Q, McLendon RE, Hao Y, Shi Q, Hjelmeland AB, Dewhirst MW, Bigner DD, Rich JN. Glioma stem cells promote radioresistance by preferential activation of the DNA damage response. Nature. 2006;444(7120):756–60. doi: 10.1038/nature05236. [DOI] [PubMed] [Google Scholar]
- 50.Nakano I, Masterman-Smith M, Saigusa K, Paucar AA, Horvath S, Shoemaker L, Watanabe M, Negro A, Bajpai R, Howes A, et al. Maternal embryonic leucine zipper kinase is a key regulator of the proliferation of malignant brain tumors, including brain tumor stem cells. J Neurosci Res. 2008;86(1):48–60. doi: 10.1002/jnr.21471. [DOI] [PubMed] [Google Scholar]
- 51.Nakano I, Paucar AA, Bajpai R, Dougherty JD, Zewail A, Kelly TK, Kim KJ, Ou J, Groszer M, Imura T, et al. Maternal embryonic leucine zipper kinase (MELK) regulates multipotent neural progenitor proliferation. J Cell Biol. 2005;170(3):413–27. doi: 10.1083/jcb.200412115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Becq H, Jorquera I, Ben-Ari Y, Weiss S, Represa A. Differential properties of dentate gyrus and CA1 neural precursors. J Neurobiol. 2005;62(2):243–61. doi: 10.1002/neu.20089. [DOI] [PubMed] [Google Scholar]
- 53.Seaberg RM, van der Kooy D. Adult rodent neurogenic regions: the ventricular subependyma contains neural stem cells, but the dentate gyrus contains restricted progenitors. J Neurosci. 2002;22(5):1784–93. doi: 10.1523/JNEUROSCI.22-05-01784.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Prozorovski T, Schulze-Topphoff U, Glumm R, Baumgart J, Schroter F, Ninnemann O, Siegert E, Bendix I, Brustle O, Nitsch R, et al. Sirt1 contributes critically to the redox-dependent fate of neural progenitors. Nat Cell Biol. 2008;10(4):385–94. doi: 10.1038/ncb1700. [DOI] [PubMed] [Google Scholar]
- 55.Kempermann G, Gast D, Kronenberg G, Yamaguchi M, Gage FH. Early determination and long-term persistence of adult-generated new neurons in the hippocampus of mice. Development. 2003;130(2):391–9. doi: 10.1242/dev.00203. [DOI] [PubMed] [Google Scholar]
- 56.Rola R, Fishman K, Baure J, Rosi S, Lamborn KR, Obenaus A, Nelson GA, Fike JR. Hippocampal neurogenesis and neuroinflammation after cranial irradiation with (56)fe particles. Radiat Res. 2008;169(6):626–32. doi: 10.1667/RR1263.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Lewen A, Matz P, Chan PH. Free radical pathways in CNS injury. J Neurotrauma. 2000;17(10):871–90. doi: 10.1089/neu.2000.17.871. [DOI] [PubMed] [Google Scholar]
- 58.Smith KJ, Kapoor R, Felts PA. Demyelination: the role of reactive oxygen and nitrogen species. Brain Pathol. 1999;9(1):69–92. doi: 10.1111/j.1750-3639.1999.tb00212.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Schultheiss TE, Stephens LC. Permanent radiation myelopathy. Brit J Radiol. 1992;65:737–753. doi: 10.1259/0007-1285-65-777-737. [DOI] [PubMed] [Google Scholar]
- 60.Lonergan PE, Martin DS, Horrobin DF, Lynch MA. Neuroprotective effect of eicosapentaenoic acid in hippocampus of rats exposed to gamma-irradiation. J Biol Chem. 2002;277(23):20804–11. doi: 10.1074/jbc.M202387200. [DOI] [PubMed] [Google Scholar]
- 61.Huang TT, Carlson EJ, Raineri I, Gillespie AM, Kozy H, Epstein CJ. The use of transgenic and mutant mice to study oxygen free radical metabolism. Ann N Y Acad Sci. 1999;893:95–112. doi: 10.1111/j.1749-6632.1999.tb07820.x. [DOI] [PubMed] [Google Scholar]
- 62.Levin ED, Brady TC, Hochrein EC, Oury TD, Jonsson LM, Marklund SL, Crapo JD. Molecular manipulations of extracellular superoxide dismutase: functional importance for learning. Behav Genet. 1998;28(5):381–90. doi: 10.1023/a:1021673703129. [DOI] [PubMed] [Google Scholar]
- 63.Thiels E, Urban NN, Gonzalez-Burgos GR, Kanterewicz BI, Barrionuevo G, Chu CT, Oury TD, Klann E. Impairment of long-term potentiation and associative memory in mice that overexpress extracellular superoxide dismutase. J Neurosci. 2000;20(20):7631–9. doi: 10.1523/JNEUROSCI.20-20-07631.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Gori T, Forconi S. The role of reactive free radicals in ischemic preconditioning--clinical and evolutionary implications. Clin Hemorheol Microcirc. 2005;33(1):19–28. [PubMed] [Google Scholar]
- 65.Yu BP, Chung HY. Adaptive mechanisms to oxidative stress during aging. Mech Ageing Dev. 2006;127(5):436–43. doi: 10.1016/j.mad.2006.01.023. [DOI] [PubMed] [Google Scholar]
- 66.Qutob SS, Multani AS, Pathak S, McNamee JP, Bellier PV, Liu QY, Ng CE. Fractionated X-radiation treatment can elicit an inducible-like radioprotective response that is not dependent on the intrinsic cellular X-radiation resistance/sensitivity. Radiat Res. 2006;166(4):590–9. doi: 10.1667/RR0514.1. [DOI] [PubMed] [Google Scholar]
- 67.Butovsky O, Ziv Y, Schwartz A, Landa G, Talpalar AE, Pluchino S, Martino G, Schwartz M. Microglia activated by IL-4 or IFN-gamma differentially induce neurogenesis and oligodendrogenesis from adult stem/progenitor cells. Mol Cell Neurosci. 2006;31(1):149–60. doi: 10.1016/j.mcn.2005.10.006. [DOI] [PubMed] [Google Scholar]
- 68.Schwartz M, Butovsky O, Bruck W, Hanisch UK. Microglial phenotype: is the commitment reversible? Trends Neurosci. 2006;29(2):68–74. doi: 10.1016/j.tins.2005.12.005. [DOI] [PubMed] [Google Scholar]
- 69.Achanta P, Thompson KJ, Fuss M, Martinez JL., Jr Gene expression changes in the rodent hippocampus following whole brain irradiation. Neurosci Lett. 2007;418(2):143–8. doi: 10.1016/j.neulet.2007.03.029. [DOI] [PubMed] [Google Scholar]
- 70.Mahmoud-Ahmed AS, Atkinson S, Wong CS. Early gene expression profile in mouse brain after exposure to ionizing radiation. Radiat Res. 2006;165(2):142–54. doi: 10.1667/rr3485.1. [DOI] [PubMed] [Google Scholar]
- 71.McIntyre CK, Miyashita T, Setlow B, Marjon KD, Steward O, Guzowski JF, McGaugh JL. Memory-influencing intra-basolateral amygdala drug infusions modulate expression of Arc protein in the hippocampus. Proc Natl Acad Sci U S A. 2005;102(30):10718–23. doi: 10.1073/pnas.0504436102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Lyford GL, Yamagata K, Kaufmann WE, Barnes CA, Sanders LK, Copeland NG, Gilbert DJ, Jenkins NA, Lanahan AA, Worley PF. Arc, a growth factor and activity-regulated gene, encodes a novel cytoskeleton-associated protein that is enriched in neuronal dendrites. Neuron. 1995;14(2):433–45. doi: 10.1016/0896-6273(95)90299-6. [DOI] [PubMed] [Google Scholar]
- 73.Guzowski JF, Setlow B, Wagner EK, McGaugh JL. Experience-dependent gene expression in the rat hippocampus after spatial learning: a comparison of the immediate-early genes Arc, c-fos, and zif268. J Neurosci. 2001;21(14):5089–98. doi: 10.1523/JNEUROSCI.21-14-05089.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Plath N, Ohana O, Dammermann B, Errington ML, Schmitz D, Gross C, Mao X, Engelsberg A, Mahlke C, Welzl H, et al. Arc/Arg3.1 is essential for the consolidation of synaptic plasticity and memories. Neuron. 2006;52(3):437–44. doi: 10.1016/j.neuron.2006.08.024. [DOI] [PubMed] [Google Scholar]
- 75.Guzowski JF, Lyford GL, Stevenson GD, Houston FP, McGaugh JL, Worley PF, Barnes CA. Inhibition of activity-dependent arc protein expression in the rat hippocampus impairs the maintenance of long-term potentiation and the consolidation of long-term memory. J Neurosci. 2000;20(11):3993–4001. doi: 10.1523/JNEUROSCI.20-11-03993.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Guzowski JF, McNaughton BL, Barnes CA, Worley PF. Environment-specific expression of the immediate-early gene Arc in hippocampal neuronal ensembles. Nat Neurosci. 1999;2(12):1120–4. doi: 10.1038/16046. [DOI] [PubMed] [Google Scholar]
- 77.Ramirez-Amaya V, Vazdarjanova A, Mikhael D, Rosi S, Worley PF, Barnes CA. Spatial exploration-induced Arc mRNA and protein expression evidence for selective, network-specific reactivation. J Neurosci. 2005;25(7):1761–8. doi: 10.1523/JNEUROSCI.4342-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Rosi S, Ramirez-Amaya V, Vazdarjanova A, Worley PF, Barnes CA, Wenk GL. Neuroinflammation alters the hippocampal pattern of behaviorally induced Arc expression. J Neurosci. 2005;25(3):723–31. doi: 10.1523/JNEUROSCI.4469-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Kempermann G, Kuhn HG, Gage FH. Genetic influence on neurogenesis in the dentate gyrus of adult mice. Proc Natl Acad Sci U S A. 1997;94(19):10409–14. doi: 10.1073/pnas.94.19.10409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Jung MW, McNaughton BL. Spatial selectivity of unit activity in the hippocampal granular layer. Hippocampus. 1993;3(2):165–82. doi: 10.1002/hipo.450030209. [DOI] [PubMed] [Google Scholar]
- 81.Ramirez-Amaya V, Marrone DF, Gage FH, Worley PF, Barnes CA. Integration of new neurons into functional neural networks. J Neurosci. 2006;26(47):12237–41. doi: 10.1523/JNEUROSCI.2195-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Kee N, Teixeira CM, Wang AH, Frankland PW. Preferential incorporation of adult-generated granule cells into spatial memory networks in the dentate gyrus. Nat Neurosci. 2007 doi: 10.1038/nn1847. [DOI] [PubMed] [Google Scholar]


