Abstract
The function of GLN3, a GATA factor encoding gene, in nitrogen metabolism of C. albicans was examined. GLN3 null mutants had reduced growth rates on multiple nitrogen sources. More severe growth defects were observed in mutants lacking both GLN3 and GAT1, a second GATA factor gene. GLN3 was an activator of two genes involved in ammonium assimilation, GDH3, encoding NADP-dependent glutamate dehydrogenase, and MEP2, which encodes an ammonium permease. GAT1 contributed to MEP2 expression, but not that of GDH3. A putative general amino acid permease gene, GAP2, was also activated by both GLN3 and GAT1, but activation by GLN3 was nitrogen source dependent. GLN3 was constitutively expressed, but GAT1 expression varied with nitrogen source and was reduced 2–3 fold in gln3 mutants. Both gln3 and gat1 mutants exhibited reduced sensitivity to rapamycin, suggesting they function downstream of TOR kinase. Hyphae formation by gln3 and gat1 mutants differed in relation to nitrogen source. The gln3 mutants formed hyphae on several nitrogen sources, but not ammonium or urea, suggesting a defect in ammonium assimilation. Virulence of gln3 mutants was reduced in a murine model of disseminated disease. We conclude that GLN3 has a broad role in nitrogen metabolism, partially overlapping, but distinct from that of GAT1, and that its function is important for the ability of C. albicans to survive within the host environment.
Keywords: Candida albicans, nitrogen metabolism, GATA factors, GLN3, GAT1, virulence
1. Introduction
Candida albicans is a commensal inhabitant of the alimentary canal of warm-blooded animals, but capable of opportunistic infections in a debilitated or compromised host (Odds, 1988). As a pathogen, it can cause disease in most host tissues and organs (Odds, 1988). Essential to its survival as either commensal or pathogen is the ability to adapt to the various and dynamic nutritive environments presented by the host. For instance, within the alimentary tract nutrient availability ranges widely in association with periods of feeding and fasting and, in addition, C. albicans must compete within a large and complex resident microbial community. Invasion of internal tissues poses similar adaptive challenges, such as transitions between the amino acid rich plasma and the nutrient limited environment of immune cell phagosomes (Lorenz et al., 2004; Lorenz and Fink, 2002; Rubin-Bejerano et al., 2003).
Several regulatory systems have been implicated in the response of C. albicans to nitrogen source availability. The external amino acid sensing system detects the presence of at least seven different amino acids and induces the expression of several permeases that facilitate uptake of amino acids from the medium (Brega et al., 2004). CSY1 encodes a component of the putative sensor complex and csy1 mutants fail to activate permease expression and exhibit reduced filamentation (Brega et al., 2004). Enhanced expression of permeases is mediated by the transcription factors Stp1p and Stp2p, which are proteolytically activated in response to external amino acids (Martinez and Ljungdahl, 2005). In addition to CSY1, activation of these proteins is dependent upon the chaperone protein Csh3p (Martinez and Ljungdahl, 2004). Mutants lacking CSH3 have reduced virulence in a murine disseminated disease model suggesting that acquisition of amino acids from the host is important for pathogenesis. In accord with this idea is the observation that C. albicans strains auxotrophic for histidine, leucine, arginine, or methionine are virulent in this animal model (Kirsch and Whitney, 1991; Manning et al., 1984; Noble and Johnson, 2005).
Responses to internal amino acid pools are regulated by the general amino acid control system (Tripathi et al., 2002). Starvation for a single amino acid induces multiple amino acid biosynthetic pathways and induces hyphae formation (Pereira and Livi, 1995; Tripathi et al., 2002). Both responses require the transcription activator encoded by GCN4, a functional homolog of S. cerevisiae GCN4 (Tripathi et al., 2002).
Additional control over nitrogen metabolism is provided by GAT1, which encodes a member of the GATA factor family of transcription factors (Limjindaporn et al., 2003). Members of this transcription factor family contain a zinc-finger domain that binds the nucleotide sequence 5′-GATA-3′, for which they are named (Wilson and Arst, 1998). These factors are conserved in fungi and regulate expression of core enzymes of nitrogen metabolism as well as genes required for the utilization of secondary nitrogen sources (Coffman et al., 1997; Magasanik and Kaiser, 2002; Marzluf, 1997). When preferred nitrogen sources such as ammonium or glutamine are limiting or absent, the genes necessary for metabolism of secondary sources are activated, a response commonly called nitrogen catabolite repression (NCR) (Coffman et al., 1997; Magasanik and Kaiser, 2002; Marzluf, 1997). Nitrogen metabolism in fungi is typically regulated by multiple GATA factors, some activate transcription while others negatively regulate expression (Coffman et al., 1997; Magasanik and Kaiser, 2002; Marzluf, 1997). C. albicans gat1 null mutants have reduced capacity to metabolize certain secondary nitrogen sources and induction of permeases in response to secondary nitrogen sources is impaired. Furthermore, the mutants were avirulent in a disseminated disease model demonstrating the importance of GAT1 and nitrogen metabolism to virulence (Limjindaporn et al., 2003).
In a recent study a second C. albicans GATA factor was described, GLN3, which was found to activate expression of MEP2 (Dabas and Morschhauser, 2007). MEP2 has dual roles, acting as an ammonium permease and as a sensor of nitrogen starvation. In its latter role MEP2 provides a nitrogen starvation signal that induces differentiation from the yeast to the hyphal growth form (Biswas and Morschhauser, 2005). Here we show that GLN3 has a more global role in nitrogen metabolism. It influences metabolism of various nitrogen sources and activates expression of genes of central nitrogen metabolism as well as various permeases. Its function partially overlaps that of GAT1, yet it has separate and distinct roles. Expression of GLN3 was independent of nitrogen source and GAT1, but GAT1 requires GLN3 for full expression. Sensitivity to the drug rapamycin is decreased in gln3 and gat1 mutants suggesting they lie downstream of the nutrient sensing TOR kinase pathway. GLN3 was also required for full virulence further demonstrating that C. albicans must adapt its nitrogen metabolic capacity to constraints imposed by the host.
2. Materials and methods
2.1 Strains and culture conditions
C. albicans strains used in this study are listed in Table 1. Strains were routinely cultured in YPD medium (2% glucose, 1% yeast extract, 2% Bacto peptone) or in YNB medium (2% glucose, 0.17% Difco yeast nitrogen base without amino acids and ammonium sulfate, 0.5% ammonium sulfate). Uridine was added at a concentration of 25 μg/ml as needed.
Table 1. C. albicans.
strains used in this study
| Strain | Parental strain | Genotypea | Source |
|---|---|---|---|
| CAI-4 | (Fonzi and Irwin, 1993) | ||
| CAI-12 | CAI-4 | iro1-ura3Δ::λimm434/IRO1-URA3 | (Porta et al., 1999) |
| CTL-7 | gat1-Δ1::hisG/gat1-Δ1::hisG iro1-ura3Δ::λimm434/IRO1-URA3 | (Limjindaporn et al., 2003) | |
| CTL-5 | GAT1-pUC18-URA3-gat1Δ::hisG/gat1Δ::hisG | (Limjindaporn et al., 2003) | |
| CWL-1A | CAI-4 | gln3Δ::hisG-URA3-hisG/GLN3 | This work |
| CWL-1B | CAI-4 | gln3Δ::hisG-URA3-hisG/GLN3 | This work |
| CWL-1C | CAI-4 | gln3Δ::hisG-URA3-hisG/GLN3 | This work |
| CWL-2A | CWL-1A | gln3Δ::hisG/GLN3 | This work |
| CWL-2B | CWL-1B | gln3Δ::hisG/GLN3 | This work |
| CWL-2C | CWL-1C | gln3Δ::hisG/GLN3 | This work |
| CWL-3A | CWL-2A | gln3Δ::hisG/gln3Δ::hisG-URA3-hisG | This work |
| CWL-3B | CWL-2B | gln3Δ::hisG/gln3Δ::hisG-URA3-hisG | This work |
| CWL-3C | CWL-2C | gln3Δ::hisG/gln3Δ::hisG-URA3-hisG | This work |
| CWL-4A | CWL-3A | gln3Δ::hisG/gln3Δ::hisG | This work |
| CWL-4B | CWL-3B | gln3Δ::hisG/gln3Δ::hisG | This work |
| CWL-4C | CWL-3C | gln3Δ::hisG/gln3Δ::hisG | This work |
| CWL-5A | CWL-2A | gln3Δ::hisG/GLN3 iro1-ura3Δ::λimm434/IRO1-URA3 | This work |
| GUI-11 | CWL-4A | gln3Δ::hisG/gln3Δ::hisG iro1-ura3Δ:: λimm434/IRO1-URA3 | This work |
| GUI-21 | CWL-4B | gln3Δ::hisG/gln3Δ::hisG iro1-ura3Δ:: λimm434/IRO1-URA3 | This work |
| GUI-31 | CWL-4C | gln3Δ::hisG/gln3Δ::hisG iro1-ura3Δ:: λimm434/IRO1-URA3 | This work |
| GLR-11 | CWL-4A | GLN3-URA3-gln3Δ::hisG/gln3Δ::hisG | This work |
| GLR-21 | CWL-4B | GLN3-URA3-gln3Δ::hisG/gln3Δ::hisG | This work |
| GLR-31 | CWL-4C | GLN3-URA3-gln3Δ::hisG/gln3Δ::hisG | This work |
| GLG-1 | CWL-4A | gat1Δ::hisG-URA3-hisG/GAT1 gln3Δ::hisG/gln3Δ::hisG | This work |
| GLG-2 | CWL-4B | gat1Δ::hisG-URA3-hisG/GAT1 gln3Δ::hisG/gln3Δ::hisG | This work |
| GLG-11 | GLG-1 | gat1Δ::hisG/GAT1 gln3Δ::hisG/gln3Δ::hisG | This work |
| GLG-21 | GLG-2 | gat1Δ::hisG/GAT1 gln3Δ::hisG/gln3Δ::hisG | This work |
| GLG-111 | GLG-11 | gat1Δ::hisG/gat1Δ::hisG-URA3-hisG gln3Δ::hisG/gln3Δ::hisG | This work |
| GLG-211 | GLG-21 | gat1Δ::hisG/gat1Δ::hisG-URA3-hisG gln3Δ::hisG/gln3Δ::hisG | This work |
| GLG-1111 | GLG-111 | gat1Δ::hisG/gat1Δ::hisG gln3Δ::hisG/gln3Δ::hisG | This work |
| GLG-2111 | GLG-211 | gat1Δ::hisG/gat1Δ::hisG gln3Δ::hisG/gln3Δ::hisG | This work |
| GGU-11 | GLG-1111 |
gat1Δ::hisG/gat1Δ::hisG gln3Δ::hisG/gln3Δ::hisG iro1-ura3:: λimm434/IRO1-URA3 |
This work |
| GGU-21 | GLG-2111 |
gat1Δ::hisG/gat1Δ::hisG gln3Δ::hisG/gln3Δ::hisG iro1-ura3:: λimm434/IRO1-URA3 |
This work |
Unless indicated otherwise, the background genotype is iro1-ura3Δ::λ imm434/iro1-ura3Δ::λimm434.
To evaluate utilization of nitrogenous compounds, test strains were pre-cultured 24 h in YNB medium at 30°C. Cells were collected by centrifugation, washed with sterile water, suspended in an equal volume of nitrogen depletion medium (NDM) consisting of 100 mM glucose, 0.17% yeast nitrogen base without amino acids and ammonium sulfate (Difco), and incubated 24 h at 30°C. The depleted cells were diluted 1:1000 into 100 μl of fresh medium consisting of 100 mM glucose, 0.17% yeast nitrogen base without amino acids and ammonium sulfate (Difco), 100 mM MES buffer, pH 5.0, and containing 10 mM nitrogenous test compound, except for ammonium sulfate which was added at 5 mM. OD595 of the cultures was monitored in a Tecan GENios plate reader at 30°C for 24 to 48 h. Growth in fetal calf serum was assessed by measuring changes in cell dry weight as previously described (Limjindaporn et al., 2003).
Hyphae formation was assessed by spotting 2 μl of a suspension of nitrogen-depleted cells (5 × 108 cells/ml) onto medium consisting of 1 mM nitrogen source, 100 mM glucose, 0.17% yeast nitrogen base without amino acids and ammonium sulfate (Difco), 100 mM MOPS buffer, pH 7.0, 1.5% washed agar. Spots were imaged after 4 days incubation at 37°C. To assess growth rate differences on these media, 5 μl of serial 10-fold dilutions of the cell suspensions were spotted on the plates and incubated 48 h at 37°C.
2.2 Rapamycin sensitivity
Sensitivity to rapamycin was assessed on YPD agar medium supplemented with 30 ng/ml or 100 ng/ml of rapamycin. Stationary phase cells cultured in YPD were washed and suspended to a density of 2×108 cells per ml. Five μl of cells from serial 10-fold dilutions were spotted on the plates, which were imaged after 5 days incubation at 30°C.
2.3 Construction of GLN3 mutants
A 3 kb DNA fragment encompassing GLN3 and 500 bp of upstream and downstream sequence was amplified by PCR from genomic DNA of strain CAI-12 (Porta et al., 1999). Extend High Fidelity PCR System (Roche Diagnostic Corporation) and the primers CaGLN3-F and CaGLN3-R (Table 2), which incorporated non-template XhoI and PstI restriction sites respectively, were used. Thermal cycles were 95°C for 3 min, 20 cycles of 94°C for 1 min, 48°C for 1 min, and 68°C for 3 min, and a final extension at 68°C for 7 min. PCR products were precipitated, digested with XhoI and PstI, and the purified XhoI-PstI digested DNA was cloned into the like sites of plasmid LITMUS28i (New England Biolabs) to create plasmid pLGLN1.
Table 2.
Oligonucleotide primers used for PCR
| Target gene | Primer name | Primer sequence |
|---|---|---|
| GAT1 | GAT1-F | 5′-ATCATTTTAATACTTCGTTATCTGTG -3′ |
| GAT1-R | 5′-ATCCCAATCTTGATTATTAGCAG -3′ | |
| GLN3 | GLN3-F | 5′-AATGCACCTTCTCCTGATGG -3′ |
| GLN3-R | 5′-AATGTCAAACTTCAACCAATCC -3 | |
| GLN3 | CaGLN3-F | 5′-GGCTCGAGGGTCTTTTTTCATTTTGTGTCG -3′ |
| CaGLN3-R | 5′-GGCTGCAGCAGCTAATGAAATATCGGCAAC -3′ | |
| GLN1 | GLN1-F | 5′-ATGACTACTTCCCTTACAGA-3′ |
| GLN1-R | 5′-TTAGTTATCATCACTGCTTTC-3′ | |
| GDH3 | GDH1-F | 5′-CATGAGACAATTGGCCAGATAC-3′ |
| GDH1-R | 5′-TCAAACATGGCATCAGCAAC-3′ | |
| MEP2 | MEP2-F | 5′-TTATCTGGTTGACTGTTGTTTACTG -3′ |
| MEP2-R | 5′-TACTAGACCCATCAGCAACACC -3′ | |
| GAP2 | GAP2-F | 5′-AAAACTGGTATATTGAAGGTGCTC-3′ |
| GAP2-R | 5′-TCAACACCAAGCATTGTAGACTC-3′ | |
| HHT1 | HHT1-F | 5′-CAAACAGCAAGAAAATCTACTGGTGG-3′ |
| HHT2-R | 5′-CTTTCACCTCTTAATCTTCTAGCTAATTGC-3′ |
GLN3 was deleted by homologous recombination and marker recycling using a hisG-URA3-hisG cassette (Fonzi and Irwin, 1993). To construct a deleted allele, plasmid pLGLN1 was digested with MfeI, made blunt with Klenow fragment, and digested with SalI. This removed a 1712 bp SalI-MfeI fragment encompassing nucleotides +52 to +1763 relative to the AUG start codon, which was replaced with a 3.9 kb SalI-Ecl136II fragment from plasmid pMB-7 containing the hisG-URA3-hisG cassette (Fonzi and Irwin, 1993). The resulting plasmid was designated pLGLN2.
Plasmid pLGLN2 was digested with XhoI and PstI to release a 5.2kb fragment containing the deletion allele, which was transformed into strain CAI-4 as described by Walther and Wendland (Walther and Wendland, 2003). The CAI-4 parental isolate was verified to be disomic for chromosome#1 prior to use (Chen et al., 2004). Uri+ transformants were selected on YNB agar at 30°C. Strain CWL-1A was a representative heterozygous mutant. Spontaneous Uri−derivatives of CWL-1A were obtained by selection on 5-fluoroorotic acid (5-FOA) medium (Boeke et al., 1984). A Uri− isolate heterozygous for the deletion, CWL-2A, was transformed with the XhoI-PstI fragment as above to delete the remaining wild-type allele, generating strain CWL-3A. A Uri− derivative of CWL-3A was selected on 5-FOA and designated CWL-4A. Three independent lineages of mutants were constructed, as designated by the suffix A, B, or C, e.g. CWL-1A, CWL-1B, and CWL-1C.
Restoration of a functional GLN3 allele in the gln3 null mutants was achieved by integrative transformation with plasmid pLGLN1-URA3. Plasmid pLGLN1-URA3 was constructed by subcloning a 2.1 kb Ecl136II-XbaI fragment of URA3 from plasmid pSMS44 (Saporito-Irwin et al., 1995) into the EcoRV-XbaI sites of pLGLN1. Plasmid pLGLN1-URA3 DNA was linearized with BsmF1, which cuts 7 bp upstream of the GLN3 coding region, to target integration to the GLN3 locus. The resulting rescued strains derived from the three mutant lineages were designated GLR-11, GLR-21, and GLR-31.
Mutants lacking both GLN3 and GAT1 were produced by deletion of GAT1 from two lineages of gln3Δ mutants. GAT1 was deleted as previously described (Limjindaporn et al., 2003). The Uri− double mutants were designated GLG-1111, and GLG-2111.
The iro1-ura3Δ mutation each lineage was reverted (Garcia et al., 2001) by transformation with a 4.9 kb BglII-PstI fragment of URA3 from plasmid pLUBP. Integration events were verified by Southern blot analysis in all strains. The strains and their genotypes are listed in Table 1.(Ramon and Fonzi, 2003).
2.4 Northern blot analysis
Cultures for RNA extraction were inoculated to an OD595 of 0.07 and incubated at 30°C with vigorous aeration until the OD595 reached 0.6 to 0.7. The medium consisted of 100 mM glucose, 0.17% Difco yeast nitrogen base without amino acids and ammonium sulfate, and 5 mM ammonium sulfate or 10 mM of alternate nitrogen sources. Inoculum was prepared from cells cultured approximately 24 h at 30°C in YNB, harvested, and washed twice with sterile dH2O. After achieving the desired OD, cultures were harvested by centrifugation, immediately frozen in dry ice-ethanol, and stored at −70°C. RNA isolation and northern blotting were conducted as previously described (Ramon et al., 1999). Hybridization probes were prepared from DNA amplified by PCR using primers listed in Table 2. Hybridization bands were detected with a STORM 840 PhosphorImager, quantified using ImageJ (NIH, http://rsb.info.nih.gov/ij/), and normalized to that of HHT1.
2.5 Virulence assay
Virulence in a murine model of disseminated disease was tested as described by Sharkey et al. (Sharkey et al., 2005), except that groups of 8–10 male BALB/c mice (Harlan), 8–10 weeks old, were used. Two independent trials were performed. Survival data were compared using the generalized Wilcoxon and log-rank tests as implemented in the NCSS software package. P values < 0.05 were considered statistically significant.
3. Results
3.1 Structural conservation of GLN3 proteins
GATA-factors are a family of zinc-finger containing transcription factors that regulate diverse processes in fungi including nitrogen metabolism (Cooper, 2002; Marzluf, 1997). The C. albicans genome contains multiple ORFs encoding putative GATA-factors (Jones et al., 2004), one of which, GAT1, was previously shown to participate in nitrogen metabolism (Limjindaporn et al., 2003). A recent study demonstrated that another GATA factor of C. albicans, designated GLN3 (orf19.3912), regulated expression of ammonium permease MEP2 (Dabas and Morschhauser, 2007). The designation GLN3 reflects the fact that the most closely related S. cerevisiae protein is encoded by GLN3 (Braun et al., 2005). However, global alignment of CaGln3p and ScGln3p shows little overall sequence conservation (approximately 21% identity) and only two notable blocks of conservation, the zinc-finger domain and the carboxyl terminus (Fig. 1A). The single type IVa zinc-finger motif, C-X2-C-X17-C-X2-C, (Teakle and Gilmartin, 1998) characteristic of GATA-family transcriptional factors was approximately 75% identical between the proteins and this included the nuclear export signal, LHGTMRPLSL (Carvalho and Zheng, 2003). The ten C-terminal amino acids were conserved between the GLN3 proteins and though distinct, this sequence block was clearly related to a conserved C-terminal domain present in a broad range of fungal GATA-factors (Platt et al., 1996)(Fig. 1B). The functional significance of this region has been demonstrated for the GATA factors NIT2 of N. crassa and AreA of A. nidulan (Pan et al., 1997; Platt et al., 1996).
Fig. 1. Sequence relatedness of CaGln3p.
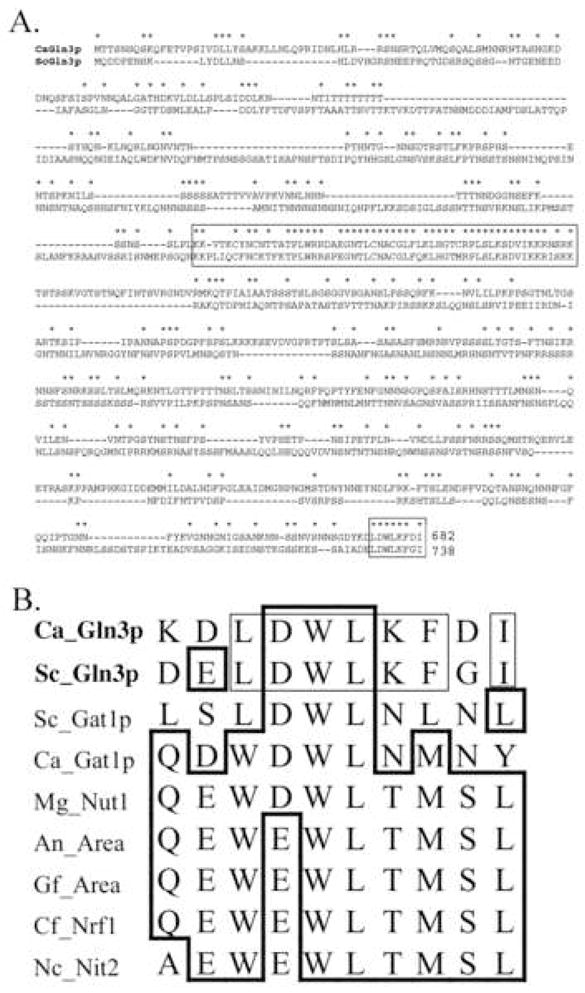
A ClustalX alignment (Jeanmougin et al., 1998) of CaGln3p and ScGln3p is shown in panel A. Identical residues are marked with an asterisk. The zinc-finger and carboxy-terminal domains are boxed. Panel B shows an alignment of the carboxy-termini of CaGln3p and GATA factors of other fungi; Sc, S. cerevisiae; Mg, Magnaporthe grisea Nut1 (Acc.# Q01168), An, A. nidulans Area (Acc.# CAA36731); Gf, Gibberella fujikuroi Area (Acc.# P78688); Cf, Cladosporium fulvum Nrf1 (Acc.# AAG48616); Nc, Neurospora crassa Nit-2 (Acc.# A34755). The thin lines enclose residues that are identical in Ca_Gln3p and Sc_Gln3p. The thick lines enclose amino acids conserved in >50% of the sequences shown.
3.2 GLN3 influences metabolism of multiple nitrogen sources
Given the limited homology, it was not clear that CaGln3p has a global role in nitrogen metabolism, as does ScGln3p (Magasanik and Kaiser, 2002). To assess whether GLN3 has such a global role, a set of deletion mutants lacking GLN3, GAT1, or both was constructed and their ability to grow on a variety of nitrogen sources was evaluated. Mutational inactivation of fungal GATA factors that regulate nitrogen metabolism generally restricts the spectrum of nitrogen sources that can be used for growth, since expression of the enzymes needed to metabolize secondary nitrogen sources is impaired (Fu and Marzluf, 1987; Limjindaporn et al., 2003; Stanbrough et al., 1995; Tanzer et al., 2003). The parental control strain, CAI-12, grew equally well with 10 mM glutamine, ammonium, arginine, or urea as nitrogen source, but progressively slower with γ-aminobutryric acid (GABA), glutamate, proline, isoleucine, or tryptophan (Table 3). In comparison, the gln3 mutant exhibited a reduction in growth rate of 25 to 40% on the preferred nitrogen sources, glutamine, ammonium, arginine, and urea, as well as the secondary source isoleucine (Table 3). The growth rate was reduced 2-fold on tryptophan, but identical to the control strain with GABA, glutamate, or proline as nitrogen sources (Table 3). These growth rate differences were observed in three independent gln3 mutants and were restored to the control rate in rescued strains, verifying their association with GLN3.
Table 3.
Effect of GATA factor mutation on growth rate with various nitrogen sources.
| Nitrogen source | Growth rate (h)a |
|||||
|---|---|---|---|---|---|---|
| CAI-12 (parental) | GUI-11 (Gln3−) | GLR-11 (Gln3+ rescued) | CTL-7 (Gat1−) | CTL-5 (Gat1+ rescued) | GGU-11 (Gln3− Gat1−) | |
| Glutamine | 1.6 ± 0.1 | 2.0 ± 0.2c | 1.8 ± 0.2 | 1.6 ± 0.2 | 1.6 ± 0.1 | 1.9 ± 0.2c |
| Ammonium | 1.6 ± 0.1 | 2.3 ± 0.4c | 1.6 ± 0.1 | 1.6 ± 0.1 | 1.6 ± 0.1 | 2.3 ± 0.2c |
| Arginine | 1.5 ± 0.1 | 2.0 ± 0.2c | 1.6 ± 0.2 | 1.7 ± 0.2 | 1.5 ± 0.2 | 2.1 ± 0.2c |
| Urea | 1.7 ± 0.1 | 2.3 ± 0.2c | 1.8 ± 0.2 | 1.7 ± 0.1 | 1.7 ± 0.2 | 47 ± 6c |
| GABA | 1.9 ± 0.1b | 2.1 ± 0.2 | 2.0 ± 0.1 | 1.9 ± 0.1 | 1.9 ± 0.1 | 7.5 ± 2.0c |
| Glutamate | 2.4 ± 0.2b | 2.3 ± 0.2 | 2.3 ± 0.2 | 2.3 ± 0.1 | 2.2 ± 0.1 | 2.4 ± 0.2 |
| Proline | 2.3 ± 0.1b | 2.5 ± 0.1 | 2.4 ± 0.1 | 2.1 ± 0.1 | 2.5 ± 0.2 | 2.5 ± 0.3 |
| Isoleucine | 4.2 ± 0.5b | 5.6 ± 1.2c | 4.2 ± 0.4 | 20 ± 3c | 4.5 ± 0.5 | 22 ± 4c |
| Tryptophan | 5.0 ± 0.6b | 10.2 ± 2.3c | 5.4 ± 0.9 | 29 ± 7 | 5.2 ± 0.9 | 33 ± 7c |
Values are mean doubling time in hours of at least four independent determinations ± standard deviation.
Growth rate significantly different from strain CAI-12 cultured with glutamine (P<0.05 in 2-tailed t-test).
Growth rate significantly different from strain CAI-12 cultured with the respective nitrogen source (P<0.05 in 2-tailed t-test).
The phenotype of the gln3 mutants was distinct from that of a gat1 mutant. Deletion of GAT1 had no measurable effect on utilization of any of the tested nitrogen sources except for isoleucine and tryptophan (Table 3). In agreement with our previous report (Limjindaporn et al., 2003), a gat1 mutation resulted in a 5 to 6-fold reduction in growth rate when isoleucine or tryptophan was provided as the sole nitrogen source.
The observation that metabolism of isoleucine and tryptophan was impaired by mutation of either gene suggested that they have partially redundant functions, as seen for Gln3p and Gat1p of S. cerevisiae (Magasanik and Kaiser, 2002). To explore the extent of overlap, a mutant lacking both genes was examined. With glutamine, ammonium, or arginine as nitrogen source, the growth rate of the double mutant was the same as that of the gln3 mutant, indicating that GLN3 and GAT1 do not overlap in controlling the metabolism of these compounds (Table 3). The growth rate of the gln3 gat1 mutant on isoleucine or tryptophan was comparable to that of the gat1 mutant, demonstrating that the gat1 mutation is epistatic to the gln3 mutation (Table 3). Interestingly, the double mutant had a more severe phenotype with either urea or GABA as nitrogen source. Although the gat1 mutant was unaffected in urea metabolism and the gln3 mutant had only a 30% reduced growth rate, the growth rate of the double mutant was reduced nearly 30-fold (Table 3). Similarly, neither single mutation influenced growth on GABA, yet the double mutant grew approximately 3 times slower. Thus, GLN3 and GAT1 have largely redundant functions in controlling metabolism of these compounds. Growth of the gln3 gat1 mutant on glutamate or proline was comparable to the parental control. These phenotypes were confirmed in an independent double mutant. Thus, GLN3 appears to have a global role in nitrogen metabolism and its role, while overlapping, is also distinct from that of GAT1.
3.3 GLN3 activates expression of core nitrogen metabolic genes and participates in NCR
The role of GLN3 in nitrogen metabolism was further explored by examining its ability to influence transcription of genes involved in nitrogen metabolism. As potential targets, several genes were chosen based on their homology with S. cerevisiae genes that are directly activated by S. cerevisiae Gln3p. ScGDH1 and ScGDH3 encode isozymes of NADP-dependent glutamate dehydrogenase, a key enzyme in ammonium assimilation, converting ammonium and α-ketoglutarate to glutamate (Magasanik and Kaiser, 2002; ter Schure et al., 2000). GDH1 encodes the major isozyme, which is ~87% identical to Gdh3p. The C. albicans genome contains a single homolog designated GDH3 (orf19.4716). Expression of this gene, like ScGDH1 (Daugherty et al., 1993; Riego et al., 2002), varied little in cells cultured on a range of nitrogen sources (data not shown). However, its expression was highly GLN3-depenent. The abundance of GDH3 mRNA was reduced about 5-fold in mutants lacking GLN3 and genetic rescue of the mutant restored normal expression (Fig. 2A). Several nitrogen sources were tested including ammonium, glutamine, and proline and GDH3 expression was GLN3-dependent in each instance (Fig. 2A and data not shown). In contrast, deletion of GAT1 was without measurable effect and a gln3 gat1 double mutant was comparable to the gln3 mutant (Fig. 2A). Thus, GLN3positively regulates GDH3 expression independent of GAT1 and irrespective of nitrogen source.
Fig. 2. Effect of nitrogen source and GATA-factor mutations on gene expression.
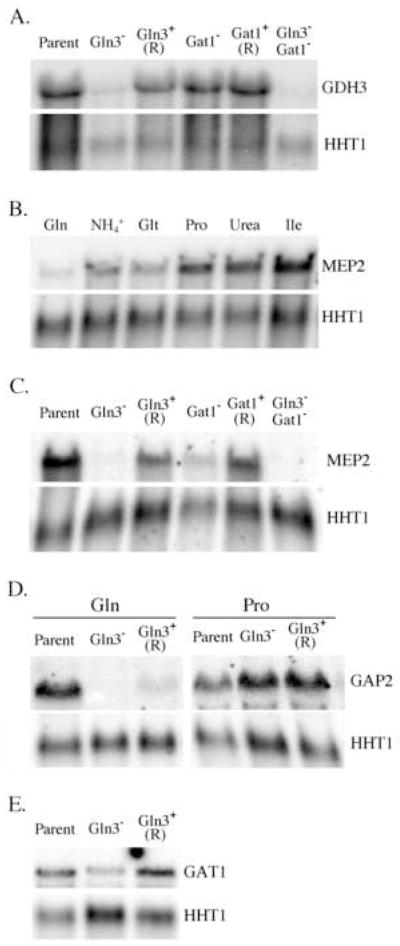
Northern blot analysis of RNA hybridized with GDH3 (A.), MEP2 (B. and C.), GAP2 (D.) or GAT1 (E.). RNA was extracted from strains CAI-12 (Parent), GUI-11 (Gln3−), the rescued gln3 mutant GLR-11 (Gln3+-R), CTL-7 (Gat1−), the rescued gat1 mutant CTL-5 (Gat1+-R), or GGU-11 (Gln3− Gat1−) cultured with 10 mM proline (A., C., D., and E.) or glutamine (D.). RNA samples used in B. were prepared from strain CAI-12 cultured with 10 mM of the indicated nitrogen source. As a control, all blots were hybridized with HHT1.
A second enzyme in ammonium assimilation, glutamine synthetase, combines ammonia and glutamate to yield glutamine and is encoded by GLN1. In S. cerevisiae GLN1 expression is 100-fold higher in glutamate versus glutamine grown cells and this activation is dependent upon GLN3 (Minehart and Magasanik, 1992). In contrast, C. albicans GLN1 varied little in relation to nitrogen source and was only about 2-fold higher in glutamate versus glutamine grown cells. Furthermore, neither gln3 nor gat1 mutations, singly or in combination, significantly influenced GLN1 expression in cells cultured with glutamine or proline (data not shown).
Neither GDH3 nor GLN1 were sensitive to nitrogen catabolite repression, a phenomenon that in S. cerevisiae relies on GLN3 and GAT1 (Coffman et al., 1997). To assess whether C. albicans GLN3 participates in nitrogen catabolite repression, expression of MEP2 and GAP2 was examined. MEP2 encodes a high affinity ammonium transporter (Biswas and Morschhauser, 2005; Marini et al., 1997) and its expression in S. cerevisiae is sensitive to nitrogen catabolite repression in a GLN3 and GAT1-dependent manner (Marini et al., 1997). Analogous to S. cerevisiae, expression of C. albicans MEP2 varied with the quality of the nitrogen source, characteristic of genes sensitive to nitrogen catabolite repression (Fig. 2B). Expression was lowest in cells cultured with glutamine, increased about 5-fold in ammonium or glutamate cultured cells, and at least 15-fold higher when the nitrogen source was proline, urea or isoleucine. Expression of MEP2 was influenced by both GLN3 and GAT1. In gln3 mutants expression was reduced >20-fold (Fig. 2C). The absence of GAT1 had a less pronounced effect and reduced MEP2 expression about 5-fold (Fig. 2C). In both instances, genetic rescue of the mutants restored MEP2 expression, though only to about half of the wild-type level, suggesting a gene dosage effect. No MEP2 mRNA was detected in the double mutant (Fig. 2C). These results were obtained with proline as the nitrogen source. Because of the low level of expression with glutamine, the effect of the mutations could not be reliably measured, although expression appeared to be reduced in both the gln3 and gat1 mutants (data not shown).
Expression of another permease, GAP2 (orf19.6993), was also examined. GAP2, previously referred to as GAP1 (Limjindaporn et al., 2003), encodes a protein similar to the general amino acid permease of S. cerevisiae. In cells cultured with glutamine, GAP2 expression was strongly GLN3-dependent, being reduced to undetectable levels in a gln3 null mutant (Fig. 2D). Genetic rescue of the mutant restored GAP2 expression, but not to control levels, suggestive of a gene dosage effect. As previously reported (Limjindaporn et al., 2003), a gat1 null mutant exhibited a 10-fold reduction in GAP2 expression (data not shown). However, a different expression pattern was observed with proline. Under these conditions loss of GLN3 had no measurable effect on GAP2 expression (Fig. 2D). However, GAT1 was still required since GAP2 expression was significantly reduced in the gat1 null mutant. Mutants lacking both GLN3 and GAT1 expressed no detectable GAP2 (data not shown). This suggests that GLN3and GAT1 both contribute to GAP2 expression, even with proline as the nitrogen source.
3.4 Expression of GLN3 and GAT1
In S. cerevisiae GLN3 is constitutively expressed and, in response to secondary nitrogen sources, activates expression of GAT1 (Magasanik and Kaiser, 2002). Thus the expression and interrelationship of C. albicans GLN3 and GAT1 was examined. Northern blot analysis of RNA from the parental control strain cultured on several nitrogen sources showed no reproducible variation in expression of GLN3 (data not shown). GAT1 expression, however, was about 3-fold less with glutamine than with any of the other nitrogen sources tested (data not shown). Comparison of GLN3 expression in the parental strain and GAT1 null mutant showed similar amounts of GLN3 mRNA in both strains (data not shown). In contrast, with proline as nitrogen source, GAT1 mRNA abundance was reduced approximately 3-fold in the GLN3 null mutant relative to the parental control or the genetically rescued Gln3+ strain (Fig 2E). Thus, GLN3 is constitutively expressed independent of nitrogen source, but GAT1 is moderately influenced by nitrogen source and dependent on GLN3 for induction.
3.5 GLN3 and GAT1 contribute to rapamycin sensitivity
In S. cerevisiae the TOR (target of rapamycin) kinase pathway controls cell growth in response to nutrient availability (Beck and Hall, 1999; Cardenas et al., 1999). Cell growth is inhibited in the presence of rapamycin, an inhibitor of TOR kinases, and this inhibition is relieved in GLN3 and GAT1 mutants, indicating that the TOR kinase pathway functions, in part, through these GATA factors (Beck and Hall, 1999; Bertram et al., 2000; Cardenas et al., 1999). To determine if a similar signaling relationship exists in C. albicans, the rapamycin sensitivity of gln3 and gat1 mutants was tested. As shown in Fig. 7, deletion of either GLN3 or GAT1 imparted resistance to rapamycin and to a similar degree. Sensitivity was restored by genetic rescue of the mutants (Fig. 7). Resistance was further enhanced in mutants lacking both genes (Fig. 7), which was even more evident at higher concentrations of rapamycin (data not shown). This suggested that the TOR kinase signaling pathway acts through GLN3 and GAT1 and that they have overlapping function in this regard.
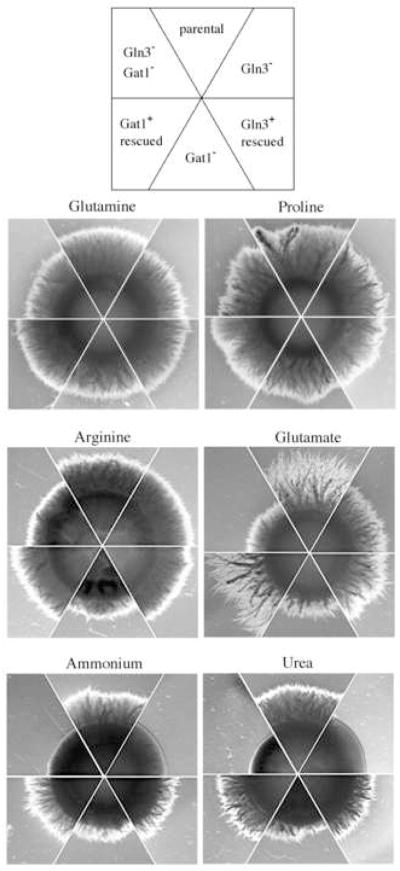
3.6 Effect of GLN3 and GAT1 on filamentation
It has long been known that the transition of C. albicans from yeast to hyphal morphology is sensitive to the quality and quantity of nitrogen source available (Dabrowa et al., 1976; Holmes and Shepherd, 1987; Hrmova and Drobnica, 1981; Land et al., 1975) and GLN3 is necessary for nitrogen starvation induced filamentation (Dabas and Morschhauser, 2007). When filamentation of gln3 and gat1 mutants was tested under nitrogen sufficient conditions, each mutant responded differently. The ability of colonies to form laterally spreading hyphae was marginally affected by gln3 or gat1 mutations when either 1 mM glutamine or proline was provided as the nitrogen source (Fig. 8). Even a double gln3 gat1 mutant filamented well, indicating that filamentation of the single mutants was not the result of the redundant function of these genes. The results also show that under these conditions nitrogen quality did not have a significant effect, since cells filamented as well with the primary nitrogen source glutamine as with the secondary source proline.
A different response was seen with arginine and glutamate. On these nitrogen sources, the gat1 mutant was notably impaired. Though abundant hyphae were produced, they were substantially shorter than the parental control or the rescued control (Fig. 8). In contrast, the gln3 mutation had little effect and the phenotype of the double mutant was similar to a gat1 mutant (Fig. 8). Filamentation of gat1 mutants was similarly impaired on media containing ammonium or urea. However, on these media gln3 mutants were grossly affected and essentially devoid of hyphae (Fig. 8). The double mutant was also non-filamentous, behaving as a gln3, rather than a gat1, mutant (Fig. 8). Except for the gln3 gat1 double mutant cultured on urea, none of the strains had substantial growth defects on these media as tested in spot plate assays (data not shown). Thus, GLN3 and GAT1 are not essential for hyphae formation per se, but influence the process depending upon the nitrogen sources available.
Interestingly, gln3 mutants were non-filamentous when the nitrogen source was either ammonium or urea. Urea is degraded to ammonium and then assimilated in like manner. This suggested that the mutants could be deficient in ammonium incorporation under hyphal inducing conditions. Increasing the concentration of ammonium from 1 mM to 25 mM, a concentration that supports growth of mutants lacking ammonium transporters (Biswas and Morschhauser, 2005; Marini et al., 1997), did not promote filamentation of gln3 mutants and was inhibitory to the parental strain (Fig. 9). In contrast, reducing the glucose concentration from 100 mM to 10 mM enhanced hyphae formation by the parental strain and allowed a low, but significant level of filamentation by the mutant (Fig. 9). When both low glucose and high ammonium concentrations were supplied, filamentation of the mutant was further enhanced, approaching control levels (Fig. 9). Analogous experiments with urea showed the same pattern of enhancement, although filamentation of the mutant was still reduced relative to the control (data not shown). As elaborated in the discussion, these results are consistent with a defect in ammonium assimilation.
3.7 Effect of gln3 mutations on virulence
The importance of nitrogen regulation in pathogenesis was demonstrated previously with the observation that mutants lacking GAT1 were avirulent in a murine model of hematogenously disseminated infection (Limjindaporn et al., 2003). Since GLN3 participates in nitrogen regulation, virulence of gln3 mutants was assessed. As a prelude to virulence testing, the growth rate of a gln3 mutant was assessed in 100% fetal calf serum at 37°C. The mean doubling time of the control strain CAI-12 was 2.8 ± 0.5 h versus 3.3 ± 0.3 h for the gln3 null mutant GUI-11. The growth yield at 24 h was also comparable.
To simulate a disseminated infection, male BALB/c mice were injected via the lateral tail vein with 1 × 106 cells of the test strains. All mice infected with the control strain rapidly succumbed to the infection and mortality was 100% by day 8. Survival of mice infected with a heterozygous gln3 mutant was significantly prolonged relative to the control strain (P < 0.005, generalized Wilcoxon test) (Fig. 10). Survival time was further increased in mice infected with the gln3 null mutant and 50% of these mice survived the duration of the experiment (Fig. 10). The rescued strain in which a functional GLN3 allele was introduced into the null mutant yielded an intermediate survival curve comparable to the heterozygous gln3 mutant. Statistically indistinguishable results were obtained in a second independent trial (data not shown) demonstrating that GLN3, like GAT1, is needed within the host environment and that a full complement of alleles is required for normal cell function.
4. Discussion
The foregoing results demonstrated that the C. albicans GATA-factor encoded by GLN3 plays a global regulatory role and is integrated into multiple aspects of nitrogen metabolism. It was required for optimal growth on a variety of nitrogen sources and was a positive regulator of several genes that contribute to nitrogen metabolism. At the core of nitrogen metabolism is the synthesis and interconversion of glutamate and glutamine, which are the principal nitrogen donors for all other biosynthetic reactions of the cell (Magasanik and Kaiser, 2002). One of the core metabolic reactions is under GLN3 control, the conversion of ammonium and α-ketoglutarate to glutamate catalyzed by NADP-dependent glutamate dehydrogenase. This enzyme is presumably encoded by GDH3 and expression of this gene was reduced 5-fold in a gln3 null mutant.
GLN3 also controlled expression of NCR sensitive genes. The NCR response promotes efficient utilization of available nitrogen sources by preventing expression of genes involved in assimilation of less preferred compounds when preferred nitrogen sources are present (Coffman et al., 1997). MEP2 exhibited a characteristic NCR-sensitive expression pattern, exhibiting low expression in the presence of glutamine and enhanced expression with secondary nitrogen sources such as proline. Expression of MEP2 in proline grown cells was GLN3, as well as GAT1, dependent. However, GLN3 and GAT1 cannot be conclusively linked to induction by secondary nitrogen sources, since expression with a preferred nitrogen source, glutamine, appeared also to be reduced. It’s possible that GLN3 and GAT1 are strictly required for expression, regardless of nitrogen source, and that an alternate factor(s) controls expression in relation to nitrogen quality. In this regard, previous studies showed that under conditions of nitrogen limitation MEP2 expression was independent of nitrogen source and GLN3 and GAT1-dependent on all nitrogen sources tested (Biswas and Morschhauser, 2005; Dabas and Morschhauser, 2007). Nitrogen sources were supplied at a concentration of 100 μM in those studies versus the 10 mM concentration used here. Thus MEP2 expression is responsive to both nitrogen quantity and quality and may be regulated differently in response to these variables.
GAP2 expression was also regulated by GLN3 and the results suggest a possible interaction between GLN3 and the external amino acid sensing pathway. Expression of GAP2 is induced by the presence of external amino acids, even when the preferred nitrogen source ammonium is abundant (Brega et al., 2004). This induction requires the amino acid sensor Csy1p and the downstream transcription factor Stp2p (Brega et al., 2004; Martinez and Ljungdahl, 2005). The presence of glutamine causes proteolytic activation of Stp2 and subsequent activation of GAP2 (Martinez and Ljungdahl, 2005). Since a gln3 mutation blocked GAP2 expression in the presence of glutamine, this indicates that Gln3p directly or indirectly interacts with the external amino acid sensing pathway. The observation that GLN3 was not required for GAP2 expression in the presence of proline, an amino acid that does not activate the external amino acid sensing system (Brega et al., 2004), is also consistent with this idea. GAT1, in contrast, activated expression independent of nitrogen source. This control arrangement has similarities to that of AGP1 permease of S. cerevisiae wherein Gln3p strongly enhances, but is not required for, induction of AGP1 by the amino acid sensing pathway (Abdel-Sater et al., 2004). However, induction does depend on the GATA-factor Uga35p/Dal81p (Abdel-Sater et al., 2004).
Another nutrient sensing pathway, the TOR kinase pathway, also relied on GLN3. The TOR pathway is a global nutrient sensing pathway and is the target of inhibition by the drug rapamycin in C. albicans and other fungi (Cruz et al., 2001). In C. albicans rapamycin binds Rbp1p, a homolog of the rapamycin target protein FKBP12, and this complex interacts with and inhibits the kinase Tor1p (Cruz et al., 2001). The sensitivity of C. albicans to rapamycin was greatly decreased by deletion of either GLN3 or GAT1. Sensitivity was further reduced in gln3 gat1 double mutants. This suggests that Gln3p and Gat1p are subject to TOR kinase control and function downstream of TOR kinase. An analogous arrangement is seen in S. cerevisiae (Beck and Hall, 1999; Bertram et al., 2000; Cardenas et al., 1999), in contrast to A. nidulans (Fitzgibbon et al., 2005).
From the foregoing it is evident that GLN3 and GAT1 both contribute to the regulation of nitrogen metabolism through partially overlapping as well as distinct roles. Expression of GLN3 mRNA, however, was not nitrogen regulated and was not altered in a gat1 null mutant. In contrast, GAT1 mRNA abundance was enhanced several fold with nitrogen sources other than glutamine and enhanced expression was dependent on GLN3. This again is similar to the situation in S. cerevisiae wherein GAT1/NIL1 is nitrogen regulated and partially controlled by GLN3 (Coffman et al., 1996; Rowen et al., 1997).
One of the phenotypic consequences of null mutations in GLN3 and GAT1 was reduced growth rates on certain nitrogen sources. Loss of either gene altered growth on isoleucine or tryptophan, but a substantial growth rate reduction on urea or GABA was observed only in a double mutant. Interestingly, even the double mutant grew well with a range of nitrogen sources, including arginine, glutamate, proline, ammonium and glutamine. This contrasts with S. cerevisiae gln3 gat1 deletion mutants, which grow poorly with nitrogen sources other than glutamine (Stanbrough et al., 1995). GLN3 and GAT1 also contributed to morphogenesis. Neither gene was essential to hyphae formation per se, rather, null mutations in these genes conferred a nitrogen source-dependent phenotype. Deletion of either or both genes caused only a small diminution of filamentation with glutamine or proline as nitrogen sources. When arginine or glutamate were provided, filamentation of the gat1 null mutant was substantially reduced, though not eliminated. Deletion of gln3 had little effect on these nitrogen sources, while the gln3 gat1 mutant was indistinguishable from mutants lacking GAT1 alone. Conversely, mutation of gln3 eliminated filamentation on ammonium or urea containing medium. The gat1 mutant was partially compromised and the double mutant mirrored the gln3 mutant. Interestingly, the mutants all grew well as yeast under these conditions, suggesting that hyphae have metabolic needs or constraints distinct form the yeast form.
These results have similarities and differences with those of Dabas and Morschhauser (Dabas and Morschhauser, 2007). They found gln3 mutants to be more compromised for filamentation on glutamine or proline than observed here (Dabas and Morschhauser, 2007) and reported that gat1 mutants filament normally on ammonium and urea (Dabas and Morschhauser, 2007), whereas we observed a noticeable reduction. These differences may be attributable to the nitrogen limiting conditions used in those studies, 100 μM versus 1 mM in the present studies, other methodological differences, or possibly the genetic background of the strains (Dabas and Morschhauser, 2007). Both studies agree, however, that loss of GLN3 profoundly diminishes the ability to filament with ammonium or urea as nitrogen source. Under nitrogen limiting conditions, this defect was traced to the reduction in MEP2 expression in the gln3 mutant (Dabas and Morschhauser, 2007). MEP2 was previously shown to provide a nitrogen starvation signal that induces hyphae formation (Biswas and Morschhauser, 2005).
In addition to the effect of reduced MEP2 expression, hyphae formation may also be influenced by the ability of the gln3 null mutant to metabolize ammonium, at least under the conditions used here. Filamentation of the gln3 mutant was abolished on both ammonium and urea, but not other nitrogen sources. Urea is degraded to ammonium then assimilated in the same manner as ammonium. An important reaction in ammonium assimilation is reductive amination of α-ketoglutarate to yield glutamate, a reaction catalyzed by NADP-dependent glutamate dehydrogenase. Expression of the presumptive gene for this enzyme, GDH3, is reduced several fold in the gln3 mutant. This reaction lies at the intersection of carbon and nitrogen metabolism and the filamentation defect of the gln3 mutant was partially alleviated by reducing the concentration of glucose and further mitigated by increasing the ammonium concentration. The effect of ammonium concentration is consistent with an impairment of the cell’s ability to assimilate ammonium. Reduction of the glucose concentration to 10 mM would be expected to relieve glucose repression (Meijer et al., 1998) and conceivably enhance mitochondrial production of α-ketoglutarate. Increasing the concentration of either or both substrates would increase the forward reaction and enhance assimilation.
Previous work demonstrated the importance of nitrogen regulation in disseminated disease (Limjindaporn et al., 2003) and this conclusion was further substantiated in these studies. In a mouse model of disseminated candidiasis, a gln3 null mutant was significantly reduced in virulence compared to the control or heterozygous mutants. Strains containing a single functional copy of GLN3 were also attenuated, but to a lesser extent, suggestive of a gene dosage effect. While the gln3 null mutant retained some measure of virulence, a gat1 mutant is avirulent (Limjindaporn et al., 2003), indicating that each factor makes distinct contributions to the ability of C. albicans to survive in the host niche. The relationship between GATA factors and virulence may be a common theme in fungal pathogens as areA null mutations attenuate virulence of Aspergillus fumigatus in pulmonary disease model (Hensel et al., 1998). While the significance of GLN3 and GAT1 to virulence is clearly demonstrated, full appreciation of their role awaits a more complete analysis of the gene targets and processes regulated by these factors.
Fig. 3. Effect of GATA-factor mutations on rapamycin sensitivity.
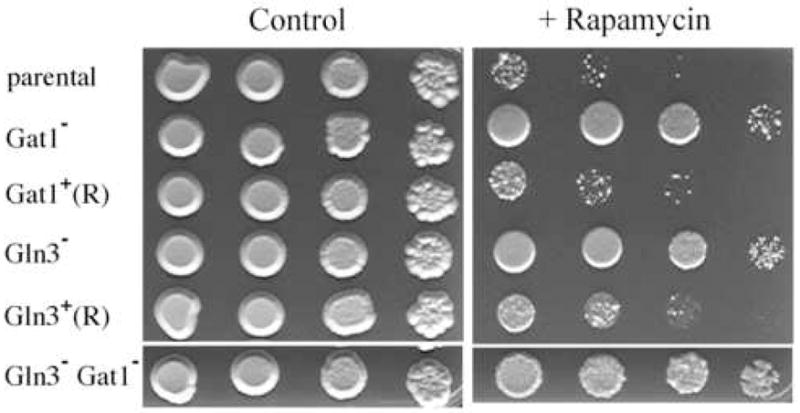
Serial dilutions of strains CAI-12 (Parent), GUI-11 (Gln3−), the rescued gln3 mutant GLR-11 (Gln3+-R), CTL-7 (Gat1−), the rescued gat1 mutant CTL-5 (Gat1+-R), or GGU-11 (Gln3− Gat1−) were spotted on YPD or YPD + 30 ng/ml rapamycin and imaged after incubation 5 days at 30°C.
Fig. 4. Filamentation phenotype of GATA-factor mutants.
Strains CAI-12 (parental), GUI-11 (Gln3−), GLR-11 (Gln3+ rescued), CTL-7 (Gat1−), CTL-5 (Gat1+ rescued), or GGU-11 (Gln3− Gat1−) were nitrogen starved and 2 μl of each, containing 1 × 106 cells, was spotted on medium containing 1 mM of the indicated nitrogen source. Colonies were imaged after incubated 4 d at 37°C. A section of the colony from each strain is shown.
Fig. 5. Effect of nutrient conditions on hyphae formation by gln3 mutant.
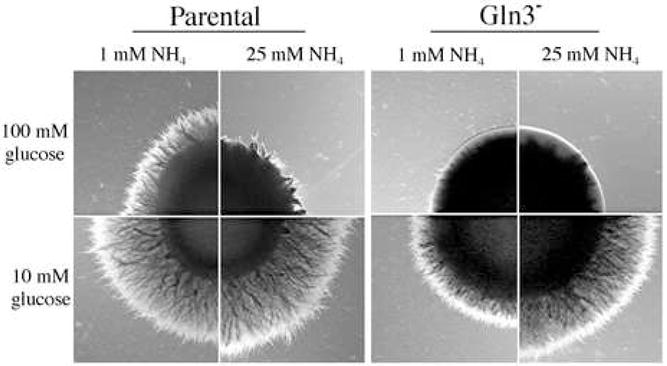
Strains CAI-12 (parental), GUI-11 (Gln3−) were nitrogen starved and 2 μl of each, containing 1 × 106 cells, was spotted on medium containing the indicated concentration of ammonium and glucose. Colonies were imaged after incubated 4 d at 37°C. A section of the colony from each strain is shown.
Fig. 6. Virulence phenotype of gln3 mutants.
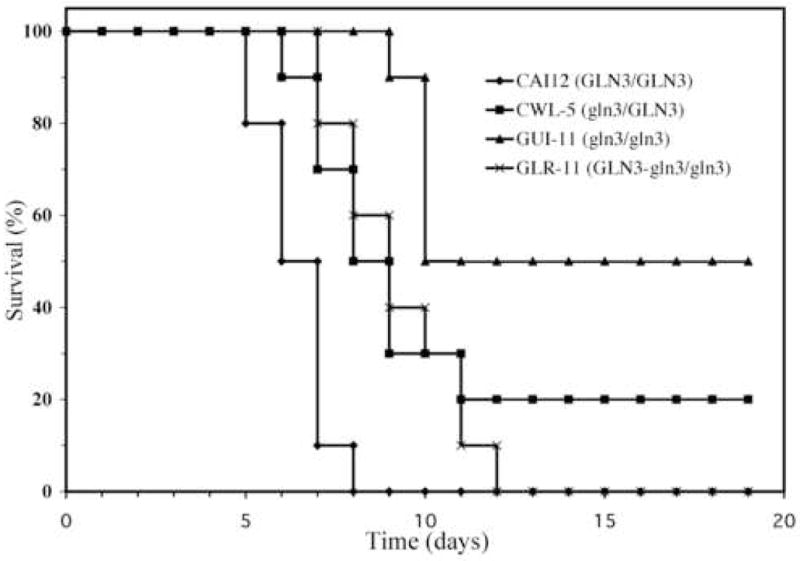
Ten BALB/c mice were infected with 1 × 106 cells of strain CAI-12 (GLN3/GLN3), a heterozygous mutant CWL-5A (gln3/GLN3), a null mutant, GUI-11 (gln3/gln3) or a rescued mutant, GLR-11 (GLN3/gln3) and monitored for 19 days post infection.
Acknowledgments
This work was supported by Public Health Service grant AI50800 from the National Institutes of Health to WAF.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abdel-Sater F, et al. The External Amino Acid Signaling Pathway Promotes Activation of Stp1 and Uga35/Dal81 Transcription Factors for Induction of the AGP1 Gene in Saccharomyces cerevisiae. Genetics. 2004;166:1727–39. doi: 10.1534/genetics.166.4.1727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beck T, Hall MN. The TOR signalling pathway controls nuclear localization of nutrient- regulated transcription factors. Nature. 1999;402:689–692. doi: 10.1038/45287. [DOI] [PubMed] [Google Scholar]
- Bertram PG, et al. Tripartite regulation of Gln3p by TOR, Ure2p, and phosphatases. J Biol Chem. 2000;275:35727–33. doi: 10.1074/jbc.M004235200. [DOI] [PubMed] [Google Scholar]
- Biswas K, Morschhauser J. The Mep2p ammonium permease controls nitrogen starvation-induced filamentous growth in Candida albicans. Mol Microbiol. 2005;56:649–69. doi: 10.1111/j.1365-2958.2005.04576.x. [DOI] [PubMed] [Google Scholar]
- Boeke JD, et al. A positive selection for mutants lacking orotidine-5′-phosphate decarboxylase activity in yeast: 5-fluoro-orotic acid resistance. Mol Gen Genet. 1984;197:345–346. doi: 10.1007/BF00330984. [DOI] [PubMed] [Google Scholar]
- Braun BR, et al. A human-curated annotation of the Candida albicans genome. PLoS Genet. 2005;1:36–57. doi: 10.1371/journal.pgen.0010001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brega E, et al. Candida albicans Csy1p is a nutrient sensor important for activation of amino acid uptake and hyphal morphogenesis. Eukaryot Cell. 2004;3:135–43. doi: 10.1128/EC.3.1.135-143.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cardenas ME, et al. The TOR signaling cascade regulates gene expression in response to nutrients. Genes Dev. 1999;13:3271–9. doi: 10.1101/gad.13.24.3271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carvalho J, Zheng XF. Domains of Gln3p interacting with karyopherins, Ure2p, and the target of rapamycin protein. J Biol Chem. 2003;278:16878–86. doi: 10.1074/jbc.M300429200. [DOI] [PubMed] [Google Scholar]
- Chen X, et al. Chromosome 1 trisomy compromises the virulence of Candida albicans. Mol Microbiol. 2004;51:551–65. doi: 10.1046/j.1365-2958.2003.03852.x. [DOI] [PubMed] [Google Scholar]
- Coffman JA, et al. Gat1p, a GATA family protein whose production is sensitive to nitrogen catabolite repression, participates in transcriptional activation of nitrogen-catabolic genes in Saccharomyces cerevisiae. Mol Cell Biol. 1996;16:847–58. doi: 10.1128/mcb.16.3.847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coffman JA, et al. Cross regulation of four GATA factors that control nitrogen catabolic gene expression in Saccharomyces cerevisiae. J Bacteriol. 1997;179:3416–3429. doi: 10.1128/jb.179.11.3416-3429.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper TG. Transmitting the signal of excess nitrogen in Saccharomyces cerevisiae from the Tor proteins to the GATA factors: connecting the dots. FEMS Microbiol Rev. 2002;26:223–38. doi: 10.1111/j.1574-6976.2002.tb00612.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cruz MC, et al. Rapamycin and less immunosuppressive analogs are toxic to Candida albicans and Cryptococcus neoformans via FKBP12-dependent inhibition of TOR. Antimicrob Agents Chemother. 2001;45:3162–70. doi: 10.1128/AAC.45.11.3162-3170.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dabas N, Morschhauser J. Control of ammonium permease expression and filamentous growth by the GATA transcription factors GLN3 and GAT1 in Candida albicans. Eukaryot Cell. 2007 doi: 10.1128/EC.00307-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dabrowa N, et al. Germination of Candida albicans induced by proline. Infect Immun. 1976;13:830–835. doi: 10.1128/iai.13.3.830-835.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daugherty JR, et al. Regulatory circuit for responses of nitrogen catabolic gene expression to the GLN3 and DAL80 proteins and nitrogen catabolite repression in Saccharomyces cerevisiae. J Bacteriol. 1993;175:64–73. doi: 10.1128/jb.175.1.64-73.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitzgibbon GJ, et al. Genetic analysis of the TOR pathway in Aspergillus nidulans. Eukaryot Cell. 2005;4:1595–8. doi: 10.1128/EC.4.9.1595-1598.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fonzi WA, Irwin MY. Isogenic strain construction and gene mapping in Candida albicans. Genetics. 1993;134:717–728. doi: 10.1093/genetics/134.3.717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu YH, Marzluf GA. Characterization of nit-2, the major nitrogen regulatory gene of Neurospora crassa. Mol Cell Biol. 1987;7:1691–6. doi: 10.1128/mcb.7.5.1691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia MG, et al. Isolation of a Candida albicans gene, tightly linked to URA3, coding for a putative transcription factor that suppresses a Saccharomyces cerevisiae aft1 mutation. Yeast. 2001;18:301–11. doi: 10.1002/1097-0061(20010315)18:4<301::AID-YEA672>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- Hensel M, et al. The role of the Aspergillus fumigatus areA gene in invasive pulmonary aspergillosis. Mol Gen Genet. 1998;258:553–557. doi: 10.1007/s004380050767. [DOI] [PubMed] [Google Scholar]
- Holmes AR, Shepherd MG. Proline-induced germ-tube formation in Candida albicans: role of proline uptake and nitrogen metabolism. J Gen Microbiol. 1987;133:3219–28. doi: 10.1099/00221287-133-11-3219. [DOI] [PubMed] [Google Scholar]
- Hrmova M, Drobnica L. Induction of mycelial type of development in Candida albicans by low glucose concentration. Mycopathologia. 1981;76:83–96. doi: 10.1007/BF00443755. [DOI] [PubMed] [Google Scholar]
- Jeanmougin F, et al. Multiple sequence alignment with Clustal X. Trends Biochem Sci. 1998;23:403–5. doi: 10.1016/s0968-0004(98)01285-7. [DOI] [PubMed] [Google Scholar]
- Jones T, et al. The diploid genome sequence of Candida albicans. Proc Natl Acad Sci U S A. 2004;101:7329–34. doi: 10.1073/pnas.0401648101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirsch DR, Whitney RR. Pathogenicity of Candida albicans auxotrophic mutants in experimental infections. Infect Immun. 1991;59:3297–3300. doi: 10.1128/iai.59.9.3297-3300.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Land GA, et al. Factors affecting filamentation in Candida albicans: changes in respiratory activity of Candida albicans during filamentation. Infect Immun. 1975;12:119–27. doi: 10.1128/iai.12.1.119-127.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Limjindaporn T, et al. Nitrogen metabolism and virulence of Candida albicans require the GATA-type transcriptional activator encoded by GAT1. Mol Microbiol. 2003;50:993–1004. doi: 10.1046/j.1365-2958.2003.03747.x. [DOI] [PubMed] [Google Scholar]
- Lorenz MC, et al. Transcriptional response of Candida albicans upon internalization by macrophages. Eukaryot Cell. 2004;3:1076–87. doi: 10.1128/EC.3.5.1076-1087.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorenz MC, Fink GR. Life and death in a macrophage: role of the glyoxylate cycle in virulence. Eukaryot Cell. 2002;1:657–62. doi: 10.1128/EC.1.5.657-662.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magasanik B, Kaiser CA. Nitrogen regulation in Saccharomyces cerevisiae. Gene. 2002;290:1–18. doi: 10.1016/s0378-1119(02)00558-9. [DOI] [PubMed] [Google Scholar]
- Manning M, et al. Comparative pathogenicity of auxotrophic mutants of Candida albicans. Can J Microbiol. 1984;30:31–35. doi: 10.1139/m84-005. [DOI] [PubMed] [Google Scholar]
- Marini AM, et al. A family of ammonium transporters in Saccharomyces cerevisiae. Mol Cell Biol. 1997;17:4282–4293. doi: 10.1128/mcb.17.8.4282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez P, Ljungdahl PO. An ER packaging chaperone determines the amino acid uptake capacity and virulence of Candida albicans. Mol Microbiol. 2004;51:371–84. doi: 10.1046/j.1365-2958.2003.03845.x. [DOI] [PubMed] [Google Scholar]
- Martinez P, Ljungdahl PO. Divergence of Stp1 and Stp2 transcription factors in Candida albicans places virulence factors required for proper nutrient acquisition under amino acid control. Mol Cell Biol. 2005;25:9435–46. doi: 10.1128/MCB.25.21.9435-9446.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marzluf GA. Genetic regulation of nitrogen metabolism in the fungi. Microbiol Molec Biol Rev. 1997;61:17–32. doi: 10.1128/mmbr.61.1.17-32.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meijer MM, et al. Glucose repression in Saccharomyces cerevisiae is related to the glucose concentration rather than the glucose flux. J Biol Chem. 1998;273:24102–7. doi: 10.1074/jbc.273.37.24102. [DOI] [PubMed] [Google Scholar]
- Minehart PL, Magasanik B. Sequence of the GLN1 gene of Saccharomyces cerevisiae: role of the upstream region in regulation of glutamine synthetase expression. J Bacteriol. 1992;174:1828–36. doi: 10.1128/jb.174.6.1828-1836.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noble SM, Johnson AD. Strains and strategies for large-scale gene deletion studies of the diploid human fungal pathogen Candida albicans. Eukaryot Cell. 2005;4:298–309. doi: 10.1128/EC.4.2.298-309.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Odds FC. A Review and Bibliography. Bailliere Tindal; London: 1988. Candida and Candidosis. [Google Scholar]
- Pan H, et al. Two distinct protein-protein interactions between the NIT2 and NMR regulatory proteins are required to establish nitrogen metabolite repression in Neurospora crassa. Mol Microbiol. 1997;26:721–9. doi: 10.1046/j.1365-2958.1997.6041979.x. [DOI] [PubMed] [Google Scholar]
- Pereira SA, Livi GP. A GCN-like response in Candida albicans. Cell Biol Int. 1995;19:65–9. doi: 10.1006/cbir.1995.1009. [DOI] [PubMed] [Google Scholar]
- Platt A, et al. Nitrogen metabolite signalling involves the C-terminus and the GATA domain of the Aspergillus transcription factor AREA and the 3′ untranslated region of its mRNA. Embo J. 1996;15:2791–801. [PMC free article] [PubMed] [Google Scholar]
- Porta A, et al. PRR1, a homolog of Aspergillus nidulans palF, controls pH-dependent gene expression and filamentation in Candida albicans. J Bacteriol. 1999;181:7516–7523. doi: 10.1128/jb.181.24.7516-7523.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramon AM, Fonzi WA. Diverged binding specificity of Rim101p, the Candida albicans ortholog of PacC. Eukaryot Cell. 2003;2:718–28. doi: 10.1128/EC.2.4.718-728.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramon AM, et al. Effect of environmental pH on morphological development of Candida albicans is mediated via the PacC-related transcription factor encoded by PRR2. J Bacteriol. 1999;181:7524–7530. doi: 10.1128/jb.181.24.7524-7530.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riego L, et al. GDH1 expression is regulated by GLN3, GCN4, and HAP4 under respiratory growth. Biochem Biophys Res Commun. 2002;293:79–85. doi: 10.1016/S0006-291X(02)00174-2. [DOI] [PubMed] [Google Scholar]
- Rowen DW, et al. Role of GATA factor Nil2p in nitrogen regulation of gene expression in Saccharomyces cerevisiae. J Bacteriol. 1997;179:3761–6. doi: 10.1128/jb.179.11.3761-3766.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubin-Bejerano I, et al. Phagocytosis by neutrophils induces an amino acid deprivation response in Saccharomyces cerevisiae and Candida albicans. Proc Natl Acad Sci U S A. 2003;100:11007–12. doi: 10.1073/pnas.1834481100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saporito-Irwin SM, et al. PHR1, a pH-regulated gene of Candida albicans, is required for morphogenesis. Mol Cell Biol. 1995;15:601–613. doi: 10.1128/mcb.15.2.601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharkey LL, et al. Flanking Direct Repeats of hisG Alter URA3 Marker Expression at the HWP1 locus of Candida albicans. Microbiology. 2005;151:1061–1071. doi: 10.1099/mic.0.27487-0. [DOI] [PubMed] [Google Scholar]
- Stanbrough M, et al. Role of the GATA factors Gln3p and Nil1p of Saccharomyces cerevisiae in the expression of nitrogen-regulated genes. Proc Natl Acad Sci U S A. 1995;92:9450–4. doi: 10.1073/pnas.92.21.9450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanzer MM, et al. Global nutritional profiling for mutant and chemical mode-of-action analysis in filamentous fungi. Funct Integr Genomics. 2003;3:160–70. doi: 10.1007/s10142-003-0089-3. [DOI] [PubMed] [Google Scholar]
- Teakle GR, Gilmartin PM. Two forms of type IV zinc-finger motif and their kingdom-specific distribution between the flora, fauna and fungi. Trends Biochem Sci. 1998;23:100–2. doi: 10.1016/s0968-0004(98)01174-8. [DOI] [PubMed] [Google Scholar]
- ter Schure EG, et al. The role of ammonia metabolism in nitrogen catabolite repression in Saccharomyces cerevisiae. FEMS Microbiol Rev. 2000;24:67–83. doi: 10.1111/j.1574-6976.2000.tb00533.x. [DOI] [PubMed] [Google Scholar]
- Tripathi G, et al. GCN4 co-ordinates morphogenetic and metabolic responses to amino acid starvation in Candida albicans. Embo J. 2002;21:5448–5456. doi: 10.1093/emboj/cdf507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walther A, Wendland J. An improved transformation protocol for the human fungal pathogen Candida albicans. Curr Genet. 2003;42:339–43. doi: 10.1007/s00294-002-0349-0. [DOI] [PubMed] [Google Scholar]
- Wilson RA, Arst HN., Jr Mutational analysis of AREA, a transcriptional activator mediating nitrogen metabolite repression in Aspergillus nidulans and a member of the “streetwise” GATA family of transcription factors. Microbiol Mol Biol Rev. 1998;62:586–96. doi: 10.1128/mmbr.62.3.586-596.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]