Abstract
The cathelin-like domain (CLD) of the antimicrobial cathelicidin family constitutes a unique protein family with structural similarity to cystatins, the cysteine protease inhibitors. CLDs are derived from the processed amino-terminal prosequence of the cathelicidin precursors with conservation across the vertebrate lineage ranging from fish to human. Initial attempt to characterize a possible inhibitory activity of protegrin-3 (PG3) CLD protein (a member of the multigene family of porcine cathelicidins) against several proteases led to an unexpected finding that PG3 CLD efficiently activated rather than inhibited human cathepsin L. Partial deletion of the L2 loop of PG3 CLD, a structurally equivalent region important in interaction of cystatins with proteases, significantly decreased its activating effect on cathepsin L. A complex model based on this functional loop was proposed to explain this unexpected effect, in which evolutionary emergence of completely opposite biological activity could be associated with structural discrepancies of the loop due to sequence variations between pig and human. Our results provide new insights into deeper understanding of the immune-related biological activity of this so-called pro-domain of the cathelicidin family.
Keywords: Innate immunity, Antimicrobial peptide, Cystatin, Cathelicidin
1. Introduction
Cathelicidins are a family of bipartite effector molecules of innate immunity identified by a substantial heterogenic carboxyl-terminal antimicrobial domain of 12–100 residues linked to an evolutionarily conserved amino-terminal cathelin-like domain (CLD) of approximately 99–114 residues. This heterogeneity is reflected by their structural diversity that includes all three major folding types of antimicrobial peptides (Tomasinsig and Zanetti, 2005; Zaiou and Gallo, 2002; Zanetti, 2005). Since the initial discovery in bovine, a large number of cathelicidins have been characterized from an array of phylogenetically distant vertebrates (Tomasinsig and Zanetti, 2005; Uzzell et al., 2003; Zaiou and Gallo, 2002; Zanetti, 2005).
The cathelicidin gene is translated as a precursor in the cytoplasmic granules of neutrophil/polymorphonuclear leukocytes (PMN) with a signal peptide removed during translation to yield the proform with two domains including the CLD and the antimicrobial domain. During the inflammatory response, the proform will further be processed to make functionally active by protease cleavage of the antimicrobial domain that will be released to sites of microbial infection. The proform isolated from the rabbit PMN in vitro exhibits high affinity to bind Escherichia coli and modulate the antibacterial actions of other leukocyte proteins on this Gram-negative bacterium (Zarember et al., 1997). Recent literature reported that some proforms can be mapped onto the cell surface of PMN. For example, a 15 kDa proform derived from a porcine cathelicidin was found to be associated with FcγRIIIaα on the cell surface (Sweeney and Kim, 2004), whereas the human proform hCAP-18/LL37, a well-characterized component of PMN-specific granules, was characterized to be translocated to the human PMN surface after the chemoattractant fMLF stimulation (Stie et al., 2007).
Although extensive studies have focused on the antimicrobial domains of the cathelicidin family due to their central roles in both innate and adaptive immunity through direct antimicrobial activity and as immune modulators and mediators of inflammation, the body of evidence for their possible immune-related defense functions of CLDs has been growing in recent years. For instance, Zaiou et al. (2003) demonstrated that human hCAP-18/LL37 CLD was able to inhibit protease activity of cathepsin L and exhibited clear toxicity against both Gram-positive and -negative bacteria. Such inhibitory activity on cathepsin L could be associated with its structural similarity to type 2 cystatins which belong to secreted natural inhibitors of family C1 (papain-like) cysteine peptidases (Dieckmann et al., 1993). Given the key role of cathepsin L in antigen presentation (Honey and Rudensky, 2003), it is possible that the regulation of its activity by CLD can establish a link between innate and adaptive immunity, which will undoubtedly provide new insights into more understanding of specific and independent functions of CLD in host defense.
Here, we report an unexpected activating effect of porcine PG3 CLD which is completely contrary to its human counterpart hCAP-18/LL37. Mutational experiments combined with a structure complex model allow us to correlate this activity to a structurally flexible loop of PG3 CLD which could be involved in a direct interaction with cathepsin L. Biological significance of the activating effect on cathepsin L has been discussed in the context of antigen presentation.
2. Materials and methods
2.1. Construction of the CLD mutant (CLD-M)
To generate the mutant of PG3 CLD with seven residues in the L2 loop deleted, we designed a pair of back-to-back primers (FP: 5′-ATCACCTGCAATGAGGTTCAAGGT-3′; RP: 5′-ATCCAGGGTGACTGTCCCCACACA-3′) to perform inverse PCR amplification of the plasmid pET-15b-ProS (Sanchez et al., 2002). Primers FP and RP, respectively correspond to the amino acid sequences of ITCNEVQG and CVGTVTLD of PG3 CLD. Phosphorylation of FP and RP was carried out by T4 polynucleotide kinase and ATP (Takara, Dalian). PCR components include: 14 µl ddH2O; 2 µl 10×Ex Taq buffer; 1 µl 10mM dNTPs; 1 µl 5 µM kinased FP; 1 µl 5 µM kinased RP; 1 µl pET-15b-ProS [0.1 ng/µl]; 0.25 µl TaKaRa Ex Taq. Subsequently, the linear PCR product was circularized by T4 DNA ligase after end polishing using pfu polymerase and transformed into E. coli DH5α. Positive clone was confirmed by DNA sequencing and the plasmid pET-15b-ProS-m was transformed into E. coli BL21 (DE3) for protein expression.
2.2. Expression and purification of recombinant proteins
We used the similar method described by Sanchez et al. (2002) with some minor modifications to express and purify both CLD-M (mutant) and CLD-W (wild type). For the detailed description of the expression and purification methods, see Supplemental material 2. Protein concentration was determined according to the biuret method (Layne, 1957).
2.3. Analytical assays
The mass spectra were acquired on a time-of-flight delayed extraction MALDI mass spectrometer (Bruker Autoflex. with a nitrogen laser (337 nm). The samples were mixed in an eppendorf tube with the same volume of the matrix solution.A solution of a-CHCA was prepared at a concentration of 15 mg/ml in 2:1 (v/v) ACN/0.1% TFA. 1 µl of the mixtures were applied to a steel plate and introduced into the mass spectrometer after drying. The spectra were obtained in the linear mode by summing 200 laser shots with an ion source voltage 1 of 19 kV, ion source 2 of 16.27 kV. The instrument was calibrated externally by cytochrome c (Bruker). Circular dichroism (CD) spectra were recorded on a JASCO J-715 spectropolarimeter (Japan) at a protein concentration of 0.3 mg/ml dissolved in water. Spectra were measured at room temperature from 190 to 250 nmusing a quartz cell of 1.0mm path length. Data were collected at 1-nm intervals with a scan rate of 200 nm/min. The CD spectra measure was performed by averaging three scans. Secondary structure content was estimated by JASCO CD standard analysis.
2.4. Protease activity assays
Protease inhibitory activities of recombinant CLDs were assayed with fluorescence-conjugated casein substrate (EnzCheck® Protease Assay Kit green fluorescence, #E-6638, Invitrogen) by measuring their inhibitory action against human liver cathepsin L (#219402, Calbiochem, CA, USA), human neutrophil elastase (#324681, Calbiochem), bovine pancreas trypsin (#T1426, Sigma–Aldrich, MO, USA). Protease assay reaction mixture includes: 100 µl of FL-conjugated casein substrate; 97 µl of buffer; 2 µl of 100 µM PG3 CLD-W or CLD-M samples (final concentration 1 µM) and 1 µl of proteases. Cathepsin L (0.1 or 0.05 mU) in the assay buffer (340 nM sodium acetate, 60 mM acetic acid, 8 mM dithiothreitol, and 4 mM EDTA, pH 5.2), elastase (2 or 0.2 mU) in 20 mM Tris (pH 7.8), or trypsin (0.5 µg or 50 ng) in 20 mM Tris (pH 7.8) were preincubated for 2 min at RT with cathelin-like protein (PG3 CLD-W or PG3 CLD-M, at designated concentration) before adding BODIPY FL casein substrate. Reaction mixtures were incubated at 37 °C for designated time periods, and protease activity was monitored as fluorescence level with SpectraMax GEMINI EM (Molecular Devices Corporation, Sunnyvale, CA). Statistical analyses were done by two-way ANOVA with Graphpad PRISM 4 (Graphpad Software Inc.).
3. Results and discussion
3.1. Characterization of recombinant CLD-W and CLD-M
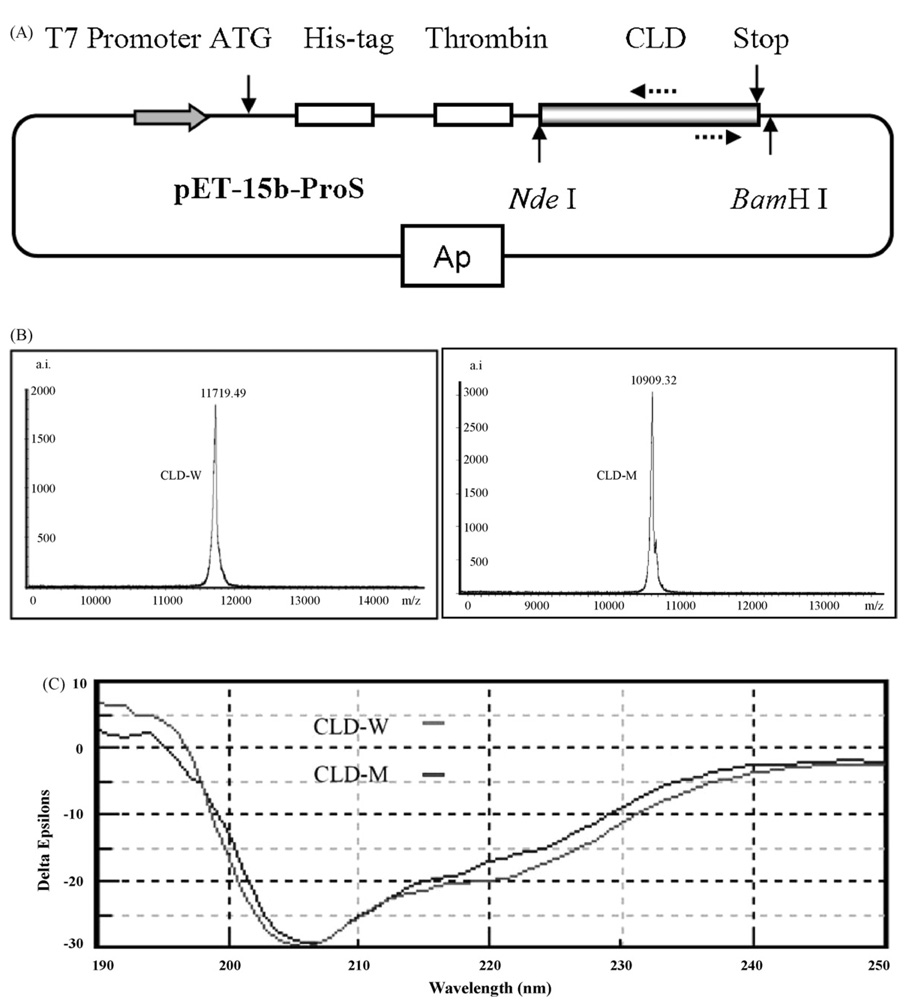
CLD-W and CLD-M were expressed in E. coli as His-tagged proteins (Fig. 1(A)) which were purified on a nickel column. After the removal of His-tag and further purified by rp-HPLC (Supplemental material 1), recombinant CLD-W and CLD-M were used for analytical assays. Molecular weights (MWs) of purified products were determined by MALDI-TOF, which, respectively gave 11719.49 and 10909.32 Da for CLD-W and CLD-M, highly consistent with their theoretical MWs of 11718.23 and 10908.31 (Fig. 1(B)). CD spectra of CLD-W and CLD-M shown in Fig. 1(C) are similar each other, characterized by a minimum at 205 nm and a shoulder in the 222–225 nm range, indicating that mutant and wild CLD proteins display globally similar three-dimensional structure and the deletion of the 7-aa segment in the L2 loop does not significantly affect its native structure.
Fig. 1.
Expression and characterization of CLD-W and CLD-M. (A) Schematic representation of CLD expression system in E. coli. Two dotted arrows label the positions of two primers for inverse PCR to generate the mutant of PG3 CLD. (B) Analytical assays of recombinant proteins by MALDI-TOF mass spectrometry, and (C) CD spectra recorded from 190 to 250 nm. Δε corresponds to the variation of molar amino acid residue absorption expressed in M−1 cm−1. Spectra were taken at a peptide concentration of 0.3 mg/ml in water.
3.2. PG3 CLD is an activator of cathepsin L
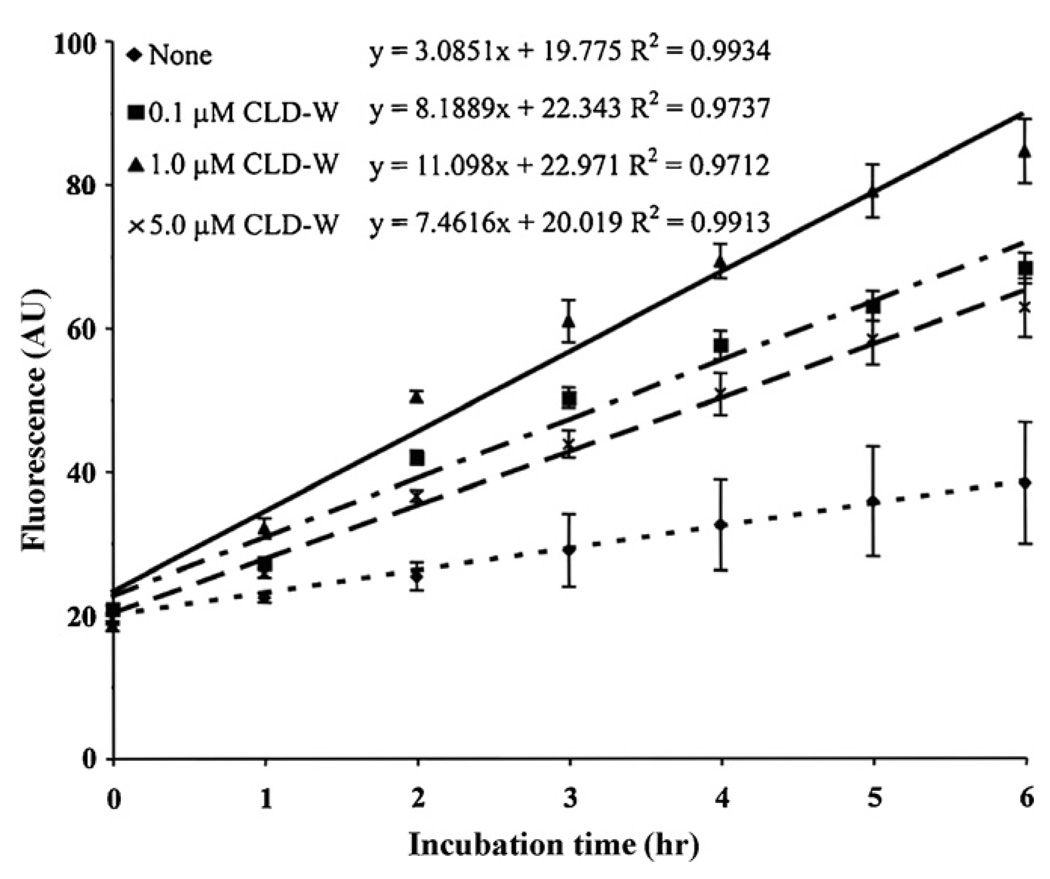
Firstly, we assayed the inhibitory activity of CLD-W on three proteases (human neutrophil elastase, human liver cathepsin L and bovine pancreas trypsin) in the presence or absence of the recombinant protein using the fluorescence-conjugated casein substrate. As expected on the basis of their structural feature, CLD-W lacked of any detectable effect on trypsin and elastase (data not shown). However, unexpectedly, we found for the first time that PG3 CLD can efficiently activate rather than inhibit the activity of human cathepsin L, an opposite effect previously observed in human hCAP-18 CLD. As presented in Fig. 2, addition of CLD-W statistically increased protease activity higher than control after 2 h (0.1 µM CLD-W: 2 h (p < 0.05), 3 h (p < 0.01), after 4 h (p < 0.001); 1 µM CLD-W: after 2 h (p < 0.001); 5 µM CLD-W: 3 h (p < 0.05), 4 h (p < 0.01), after 5 h (p < 0.001)) and the activity of cathepsin L was increased by CLDs at 0.1 and 1 µM, but a higher concentration (5 µM) obtained lower activating efficiency on protease activity by cathepsin L compared to 0.1 and 1 µM, exhibiting a non-typical concentration–dose dependency.
Fig. 2.
Activation of cathepsin L by different concentrations of PG3 CLD-W. Concentration of cathepsin L is 0.1 mU/reaction mixture (0.1 mU/200 µl). Concentrations of PG3 CLD-W are 0.1, 1 and 5 µM, respectively. The assays were performed in triplicate in one experiment. ‘None’ contains cathepsin L, but not CLDs.
Next, we evaluated the antibacterial activity of PG3 CLD against two representative bacterial strains (Gram+ M. luteus and Gram− E. coli) using inhibition zone assay and liquid-growth inhibition assay (Gao et al., 2007), which both showed no detectable antibacterial activity even in the concentration of PG3 CLD up to 64 µM (data not shown).
3.3. The loop 2 of PG3 CLD is one functional determinant
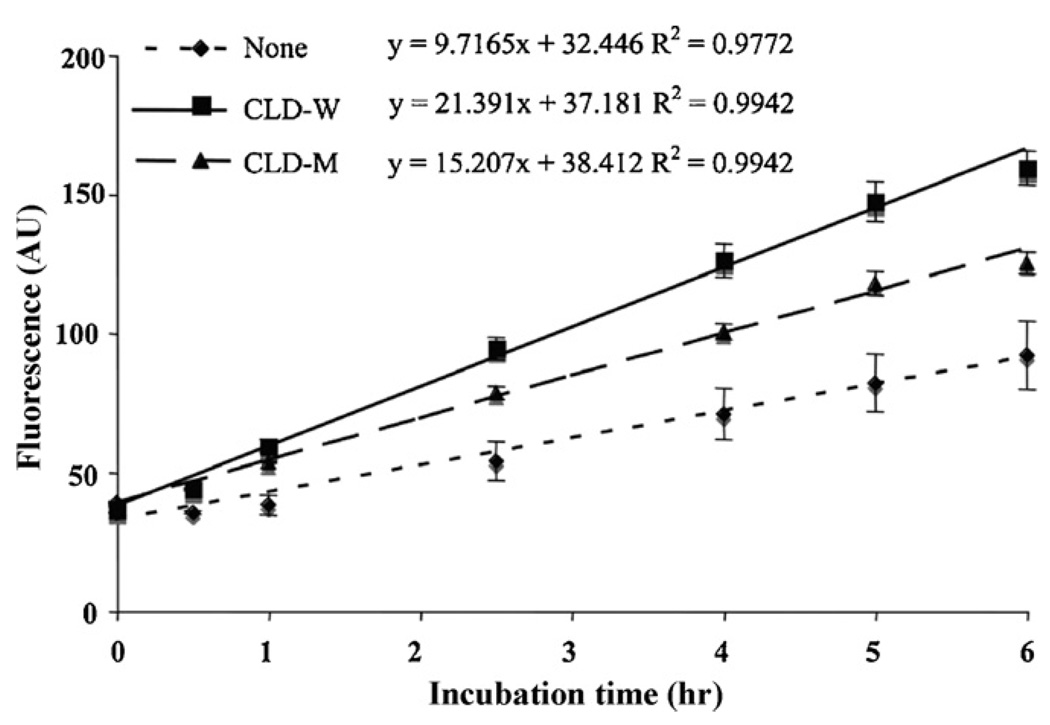
Previous studies have identified the functional motifs of cystatins involved in interacting with cathepsin L, including: (1) QxVxG motif in the first binding loop (where x represents any amino acid; (2) PW in the second binding loop; (3) a glycine at the extremity of the N-terminal region (Rzychon et al., 2004). However, these motifs are completely lacking in all characterized CLDs. Interestingly, both human and pig CLDs target cathepsin L either inhibiting or activating its activity. These observations suggest that the cystatin-fold itself other than sequence conservation is the key functional determinant. We thus hypothesized that CLDs and cystatins could adopt a similar strategy to bind cathepsin L, in which the L1 and L2 loops are presumably involved. To confirm this assumption, we designed a PG3 CLD mutant where a 7-aa segment of the L2 loop (QIKDPLD) was deleted. We assayed the activating activity of CLD-M on human liver cathepsin L (Fig. 3). There was a linear relationship (R2 = 0.9772–0.9942) between the control (cathepsin L alone) and the tests (cathepsin L + CLD-W or CLD-M) that showed that the fluorescence level (=protease activity) in the presence of 1 µM of CLD-W or CLD-M is statistically higher than control after 1 h (p < 0.001, except CLD-M at 1 h; p < 0.05. CLD-M activity was statistically lower than CLD-W after 3 h (p < 0.001, except CLD-M at 3 h; p < 0.05). This result showed that the activating effect significantly decreased after the partial deletion of the L2 loop, which provides evidence in support of the involvement of the L2 loop in the activation of cathepsin L. It is also worthy mentioning that the deletion of the 7-aa of the L2 loop alone was not completely sufficient to diminish its activating function, suggesting that other minor determinants of activation also may exist. Deletion of residues in the L1 loop will provide additional evidence for the functional site information of PG3 CLD.
Fig. 3.
Decreased activation of effect of CLD-M on cathepsin L. The concentration of cathepsin L is 0.1 mU/reaction mixture (0.1 mU/200 µl). Concentrations of CLD-W and CLD-M are 1 µM. The assays were performed in triplicate in one experiment. Same trend was confirmed at least three independent experiments in this concentration. ‘None’ indicates only the enzyme included. Data demonstrate activation of cathepsin L by the addition of recombinant CLD-W and CLD-M. Slopes, intercepts, and correlation coefficients were calculated by linear regression.
3.4. A complex model for activation of cathepsin L by PG3 CLD
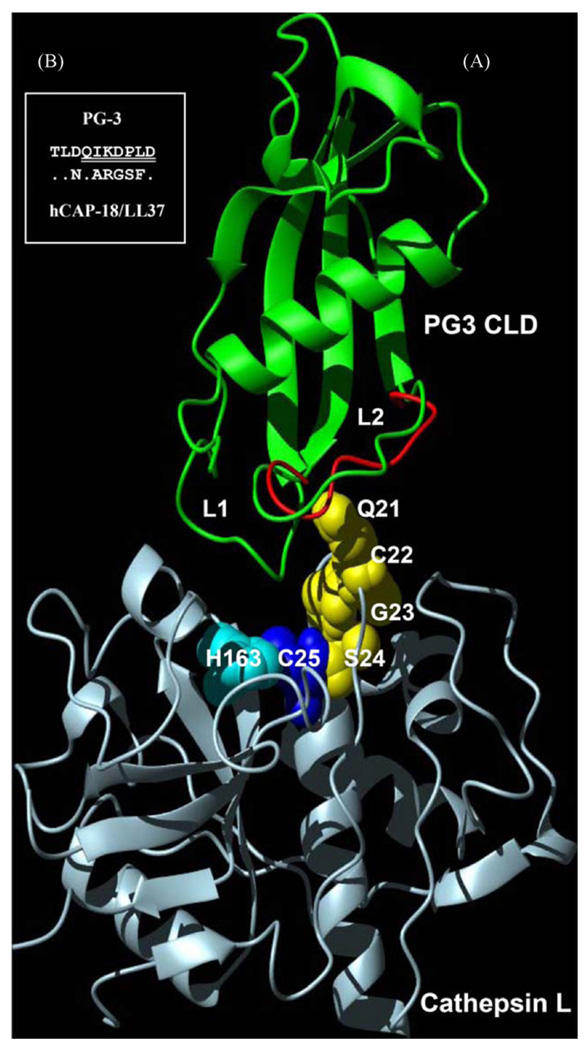
The unexpected discovery of activating effect of PG3 CLD on cathepsin L prompts us to study its possible action mode. On the basis of the possible similarity in interacting mode between cystatins and CLDs, we constructed a hypothetical complex model by substituting papain (cysteine protease) and stefin B (cystatin B) in the crystal structure of complex (pdb entry 1STF) using cathepsin L (pdb entry 1ICF) and PG3 CLD (pdb entry 1N5H) (Yang et al., 2003), respectively (Fig. 4(A)). It is known that cystatins inhibits the papain-like proteases (e.g. cathepsin L) by binding to sites on either side of the active site and the cystatin molecule itself remains intact but still prevents interactions with substrates (Rzychon et al., 2004). In the complex model it can be easily seen that PG3 CLD fits into the active cleft of the enzyme in an analogous way as cystatin B does. This suggests that CLD could interact with cathepsin L by such a mechanism. Despite a shorter amino-terminal segment diminished its contact with the enzyme, the L1 and L2 loops of PG3 CLD were close to the active site of enzyme, especially the conformationally flexible L2 loop was adjacent to the active site C25 located in amino-terminal subdomain of cathepsin L and could provide a surface to interact with the loop comprising residues 21–25 of cathepsin L.
Fig. 4.
Hypothesized complex model between PG3 CLD and cathepsin L. (A) The L2 loop of PG3 CLD with conformational flexibility due to the prolyl cis–trans isomerization is close to the active site of cathepsin L in which a putative conformational pathway mediated by Q21-S24 of cathepsin L (highlighted by yellow sphere models) could play a role in generating activating effect. H163 and C25 constitute the active centre of cathepsin L. To show flexibility of the L2 loop, two conformations were superimposed based on their coordinates (1N5H and 1N5P, respectively. Green: cis; red: trans); (B) comparison of amino acid sequences of the CLD L2 loops between hCAP-18/LL37 and PG3. Identical residues in hCAP-18/LL37 are represented by dots. Seven deleted residues in PG3 CLD are underlined twice. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of the article.)
Previous structural analysis has shown that the cis–trans alterations mediated by P119 are extended all along the L2 loop and lead to the main conformational changes. If we hypothesize that there exists a putative conformational pathway between cathepsin L and PG3 CLD, functional switch from inhibition to activation can be well explained. In this case, the cis–trans isomerization-mediated conformational variability of the L2 loop might have ability to transfer the interacting signal to the active site of cathepsin L through the loop of the enzyme (residues Q21 to C25) and induce the conformational change of the active site which could be facilitated for substrates’ binding to produce an activating effect. This hypothesis is consistent with the observation that higher concentration (e.g. 5 µM) of PG3 CLD significantly decreased its activating effect on cathepsin L given more molecules hampering substrates’ binding. On the contrary, the absence of cis–trans alterations in the L2 loop of human CLD due to sequence variations makes it impossible to undergo big conformational changes and thus human CLD displays a classical inhibitory activity. A similar conformational pathway hypothesis has also been proposed to explain the complicated binding mode between the chaperone hsp90 and its co-chaperone p23 (Zhu and Tytgat, 2004). To reach a decisive conclusion, the determination of experimental structure of PG3 CLD and cathepsin L complex will be needed. In addition, given considerable sequence variations in the CLD L2 loops between hCAP-18/LL37 and PG3 (Fig. 4(B)), it is reasonable to infer that functional switch from inhibition to activation could occur if their loops are exchanged.
3.5. Biological significance of cathepsin L activated: possible role of PG3 CLD in adaptive immunity
To answer the possible biological significance of such an activating effect, one should take into account the immune-related roles of cathepsin L regulated by PG3 CLD. As we know, cathepsin L is a key lysosomal cysteine protease of the papain family and has been identified to be involved in antigen presentation in the early events of immunological response upon infection (Honey and Rudensky, 2003). This enzyme is present in some antigen presenting cells (APCs) as procathepsin L that matures when hosts are immunized with foreign antigens such as soluble Leishmania antigen (SLA) (Onishi et al., 2004). An increase in mature cathepsin L in APCs is crucial for the proteolytic process of endocytosed antigens and for presentation of those antigens to the immune system. On the basis of these facts, it is obvious that activation of cathepsin L by PG3 CLD will prompt adaptive immune response through the antigen presentation pathway. This is further strengthened by the role of cystatins in controlling degradation of the invariant chain (Ii) by cathepsins and modulating antigen presentation (Vray et al., 2002). Dendritic cells (DCs) are potent APCs and their maturation process leads to a reduced level of cystatin C that favors the protease activity of cathepsin L. In turn, activation of cysteine proteases should more facilitate antigen presentation. As pointed out by Kopitar-Jerala (2006), the real challenge that lies in front of us is to discover proteases which interact with cystatins that are differentially up-regulated in cells of the immune system. Therefore, the experimental confirmation of cathepsin L being efficiently activated by PG3 CLDs in vivo upon infection will offer important evidence in favor of our hypothesis.
Finally, one related question remaining to be answered is how CLDs can enter into APCs to exert their regulatory effects on cathepsin L? Given some cathelicidin proforms carrying CLDs can be secreted into plasma where they circulate associated with plasma lipoproteins (Zarember et al., 2002), we assume that CLDs might firstly be released into the circulating system and finally enter APCs by a yet uncharacterized lipoprotein-mediated uptake mechanism. Alternatively, some APCs themselves could express cathelicidin genes. In this aspect, bone marrow-derived dendritic cells are possible candidates because porcine bone marrow has been found to express several cathelicidins that contain protegrins (Bellm et al., 2000).
Supplementary Material
Acknowledgments
We thank Dr. André Aumelas for the gift of pET-15b-ProS plasmid and Miss Yue Jiang for constructing the pET-15b-ProS-m vector. This work was supported by grants from the National Natural Science Foundation of China (90608009 and 30621003) and the ‘Bairen Plan’ from the Chinese Academy of Sciences to S.Z.
Appendix A. Supplementary data
Supplementary data associated with this article can be found, in the online version, at doi:10.1016/j.molimm.2008.01.007.
References
- Bellm L, Lehrer RI, Ganz T. Protegrins: new antibiotics of mammalian origin. Expert Opin. Investig. Drugs. 2000;9:1731–1742. doi: 10.1517/13543784.9.8.1731. [DOI] [PubMed] [Google Scholar]
- Dieckmann T, Mitschang L, Hofmann M, Kos J, Turk V, Auerswald EV, Jaenicke R, Oschkinat H. The structures of native phosphorylated chicken cystatin and of a recombinant unphosphorylated variant in solution. J. Mol. Biol. 1993;234:1048–1059. doi: 10.1006/jmbi.1993.1658. [DOI] [PubMed] [Google Scholar]
- Gao B, Tian C, Zhu S. Inducible antibacterial response of scorpion venom gland. Peptides. 2007;28:2299–2305. doi: 10.1016/j.peptides.2007.10.004. [DOI] [PubMed] [Google Scholar]
- Honey K, Rudensky AY. Lysosomal cysteine proteases regulate antigen presentation. Nat. Rev. Immunol. 2003;3:472–482. doi: 10.1038/nri1110. [DOI] [PubMed] [Google Scholar]
- Kopitar-Jerala N. The role of cystatins in cells of the immune system. FEBS Lett. 2006;580:6295–6301. doi: 10.1016/j.febslet.2006.10.055. [DOI] [PubMed] [Google Scholar]
- Layne E. Spectrophotometric and turbidimetric methods for measuring proteins. Methods Enzymol. 1957;10:447–455. [Google Scholar]
- Onishi K, Li Y, Ishii K, Hisaeda H, Tang L, Duan X, Dainichi T, Maekawa Y, Katunuma N, Himeno K. Cathepsin L is crucial for a Th1-type immune response during Leishmania major infection. Microbes Infect. 2004;6:468–474. doi: 10.1016/j.micinf.2004.01.008. [DOI] [PubMed] [Google Scholar]
- Rzychon M, Chmiel D, Stec-Niemczyk J. Modes of inhibition of cysteine proteases. Acta Biochim. Pol. 2004;51:861–873. [PubMed] [Google Scholar]
- Sanchez JF, Wojcik F, Yang YS, Strub MP, Strub JM, van Dorsselaer A, Martin M, Lehrer R, Ganz T, Chavanieu A, Calas B, Aumelas A. Overexpression and structural study of the cathelicidin motif of the protegrin-3 precursor. Biochemistry. 2002;41:21–30. doi: 10.1021/bi010930a. [DOI] [PubMed] [Google Scholar]
- Stie J, Jesaitis AV, Lord CI, Gripentrog JM, Taylor M, Burritt JB, Jesaitis AJ. Localization of hCAP-18 on the surface of chemoattractant-stimulated human granulocytes: analysis using two novel hCAP-18-specific monoclonal antibodies. J. Leukoc. Biol. 2007;82:161–172. doi: 10.1189/jlb.0906586. [DOI] [PubMed] [Google Scholar]
- Sweeney SE, Kim YB. Identification of a novel FcγRIIIaα-associated molecule that contains significant homology to porcine cathelin. J. Immunol. 2004;172:1203–1212. doi: 10.4049/jimmunol.172.2.1203. [DOI] [PubMed] [Google Scholar]
- Tomasinsig L, Zanetti M. The cathelicidins—structure, function and evolution. Curr. Protein Peptide Sci. 2005;6:23–34. doi: 10.2174/1389203053027520. [DOI] [PubMed] [Google Scholar]
- Uzzell T, Stolzenberg ED, Shinnar AE, Zasloff M. Hagfish intestinal antimicrobial peptides are ancient cathelicidins. Peptides. 2003;24:1655–1667. doi: 10.1016/j.peptides.2003.08.024. [DOI] [PubMed] [Google Scholar]
- Vray B, Hartmann S, Hoebeke J. Immunomodulatory properties of cystatins. Cell. Mol. Life Sci. 2002;59:1503–1510. doi: 10.1007/s00018-002-8525-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y, Sanchez JF, Strub MP, Brutscher B, Aumelas A. NMR structure of the cathelin-like domain of the protegrin-3 precursor. Biochemistry. 2003;42:4669–4680. doi: 10.1021/bi027133c. [DOI] [PubMed] [Google Scholar]
- Zaiou M, Gallo RL. Cathelicidins, essential gene-encoded mammalian antibiotics. J. Mol. Med. 2002;80:549–561. doi: 10.1007/s00109-002-0350-6. [DOI] [PubMed] [Google Scholar]
- Zaiou M, Nizet V, Gallo RL. Antimicrobial and protease inhibitory functions of the human cathelicidin (hCAP18/LL37) prosequence. J. Invest. Dermatol. 2003;120:810–816. doi: 10.1046/j.1523-1747.2003.12132.x. [DOI] [PubMed] [Google Scholar]
- Zanetti M. The role of cathelicidins in the innate host defenses of mammals. Curr. Issues Mol. Biol. 2005;7:179–196. [PubMed] [Google Scholar]
- Zarember KA, Elsbach P, Shin-Kim K, Weiss J. p15s (15-kD antimicrobial proteins) are stored in the secondary granules of Rabbit granulocytes: implications for antibacterial synergy with the bactericidal/permeability-increasing protein in inflammatory fluids. Blood. 1997;89:672–679. [PubMed] [Google Scholar]
- Zarember KA, Katz SS, Tack BF, Doukhan L, Weiss J, Elsbach P. Host defense functions of proteolytically processed and parent (unprocessed cathelicidins of rabbit granulocytes) Infect. Immun. 2002;70:569–576. doi: 10.1128/iai.70.2.569-576.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu S, Tytgat J. Evolutionary epitopes of Hsp90 and p23: implications for their interaction. FABES J. 2004;18:940–947. doi: 10.1096/fj.04-1570hyp. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.