Abstract
Pain perception begins with the activation of primary sensory nociceptors. Over the past decade, flourishing research has revealed that members of the Transient Receptor Potential (TRP) ion channel family are fundamental molecules that detect noxious stimuli and transduce a diverse range of physical and chemical energy into action potentials in somatosensory nociceptors. Here we highlight the roles of TRP vanilloid 1 (TRPV1), TRP melastatin 8 (TRPM8) and TRP ankyrin 1 (TRPA1) in the activation of nociceptors by heat and cold environmental stimuli, mechanical force, and by chemicals including exogenous plant and environmental compounds as well as endogenous inflammatory molecules. The contribution of these channels to pain and somatosensation is discussed at levels ranging from whole animal behavior to molecular modulation by intracellular signaling proteins. An emerging theme is that TRP channels are not simple ion channel transducers of one or two stimuli, but instead serve multidimensional roles in signaling sensory stimuli that are exceptionally diverse in modality and in their environmental milieu.
Keywords: TRPA1, TRPV1, TRPM8, Cold, Mechanotransduction, Nociceptor, Sensory neuron, Cutaneous, Inflammation, Prostaglandin, NGF, Artemin, Bradykinin, Kinase, Scaffold protein
1. Introduction
The Transient Receptor Potential (TRP) superfamily of ion channels comprises proteins with six transmembrane domains and cytoplasmic N- and C-termini. TRP proteins assemble as homo- or heterotetramers to form cation-permeable ion channels (Voets et al., 2005). Currently, 28 TRP channels have been discovered in mammals and based on their sequence homology, are classified into six subfamilies: TRPC, TRPV, TRPM, TRPA, TRPP and TRPML (Montell, 2005). This review highlights recent discoveries describing the roles of TRPV1, TRPM8 and TRPA1 in transduction and sensitization in primary afferent somatosensory neurons. The first TRP channel discovered in mammalian sensory neurons was Transient Receptor Potential Vanilloid 1 (TRPV1). TRPV1 is the obligate receptor for capsaicin, the spicy ingredient in hot chili peppers, and also a key receptor for noxious physical heat (>42 °C; Caterina et al., 1997). TRPV1 is found on many small-medium-diameter nociceptive sensory neurons which are likely to have C fiber axons (Tominaga et al., 1998). The identification of TRPV1 was the major catalyst that launched the fields of somatosensory and pain transduction research to the molecular level, and discovery of additional TRP family members rapidly followed.
TRP melastatin 8 (TRPM8) was subsequently discovered in 2002 and found to be strongly activated by the cool-mimetic chemical menthol and by physical cooling (McKemy et al., 2002; Peier et al., 2002). TRPM8 is located in small- and medium-diameter sensory neurons within the trigeminal and dorsal root ganglia. Recently, three independent studies of TRPM8-null mice have firmly established that TRPM8 is a major cold and cooling transduction channel in mammalian sensory neurons (McKemy et al., 2002; Peier et al., 2002; Bautista et al., 2007; Dhaka et al., 2007; Colburn et al., 2007).
Transient Receptor Potential Ankyrin 1 (TRPA1) is the only member of the ankyrin subfamily found in mammals. Originally called ANKTM1, TRPA1 was identified by a homology search for ankyrin domains and six transmembrane domains and is 20% homologous at the amino acid level to TRPV1 (Jaquemar et al., 1999; Story et al., 2003). Its structure is distinct from other TRP channels as it is the only member with an extended (14–17) ankryin repeat domain in the N-terminus (Clapham, 2003). TRPA1 channels are expressed in a subpopulation of unmyelinated nociceptors that also express the capsaicin receptor TRPV1, suggesting an important role in nociception. Consistent with this posit, TRPA1 is activated by a diverse assortment of pungent or irritating reactive chemical compounds including those found in mustard oil (allyl isothiocyanate), cinnamon oil (cinnamaldehyde), gas exhaust (acrolein), raw garlic and onions (allicin) and formalin (formaldehyde); all of these elicit a painful burning or prickling sensation (Story et al., 2003; Bandell et al., 2004; Jordt et al., 2004; Macpherson et al., 2005; Bautista et al., 2006; McNamara et al., 2007). Moreover, TRPA1 has been put forth as a putative transducer of natural physical stimuli including both cold and mechanical force (Story et al., 2003, Corey et al., 2004). Thus, a common theme has emerged whereby TRPA1 is found in nociceptors and the modality(s) of nociception to which it mechanistically contributes appear to be quite diverse. Like TRPV1 (Caterina et al., 2000), TRPA1 may be a molecular “switchboard” integrator for a range of diverse noxious stimuli.
In this article, we focus on these three principal sensory transduction channels and their roles in processes that span from whole animal pain behavior down to their molecular modulation by signaling proteins. On an anatomical level, we review recent work illuminating the central and peripheral projections of TRPM8-expressing neurons. At the primary afferent function level, we review the evidence supporting a role for TRPA1 in both cold transduction and mechanotransduction incutaneous sensory neurons. On a cellular level, we discuss recent discoveries of both endogenous inflammatory mediators and exogenous irritating chemicals that activate TRPA1. Finally, on a membrane signaling level, we detail the capacity of growth factors to modulate TRPA1 and TRPV1 channel activity, and discuss the roles of scaffolding proteins that assist kinases and phosphatases in diversely modulating these ion channels.
2. Diversity in the neural circuitry of cold sensing revealed by genetic axonal labeling of TRPM8 neurons
Cold temperatures activate a small cohort of sensory neurons, yet generate a variety of distinct sensations that range from pleasantly cool to painfully aching, prickling, and burning. Cold responses are mediated through both C and Aδ fibers, each of which likely provides one or more of these distinct cold sensations. Remarkably the majority of cold responses in vivo are dependent upon the cold and menthol receptor TRPM8. In vitro, TRPM8 currents are evoked as temperatures drop below ~26 °C, and over a range of both innocuous cool and noxious cold temperatures (McKemy et al., 2002; Peier et al., 2002). In vivo, TRPM8-null mice are deficient in detecting innocuous cool temperatures and exhibit a partially defective phenotype in responding to noxious cold (Bautista et al., 2007; Colburn et al., 2007; Dhaka et al., 2007). Cold-evoked responses in both Aδ and C fibers are greatly reduced, if not absent in these animals (see section 5). Moreover, activation of TRPM8 is required for the analgesia provided by cooling and cooling compounds (Proudfoot et al., 2006; Dhaka et al., 2007) and, paradoxically, is essential in injury-evoked hypersensitivity to cold (Colburn et al., 2007; Dhaka et al., 2007). Thus, TRPM8 plays a key role in the perception of cold temperatures and raises the question: how can a single receptor contribute to multiple, and in some cases antagonistic (pain versus analgesia), aspects of sensory signaling? To begin to address this question, transgenic mice expressing a genetically encoded axonal tracer (Green Fluorescent Protein; GFP) that fluorescently labels TRPM8 neurons and axons in vivo were generated, and their neurochemical phenotype and central and peripheral projections examined (Takashima et al., 2007).
2.1. TRPM8 is expressed by somata of nociceptors and non-nociceptors
Consistent with the broad range of functional roles of the channel, TRPM8 neurons express markers of nociceptors as well as non-nociceptors, and have axonal properties indicative of both Aδ and C fibers. TRPM8 expression co-labels with the neurofilaments NF200 (Fig. 1a) and peripherin (Fig. 1b) demonstrating that these neurons have both Aδ and C fibers, respectively (Lawson and Waddell, 1991) and provide consistency with the large deficits in cold-evoked discharges observed in both fiber types in TRPM8-null mice (see section 5 and Bautista et al., 2007). Interestingly, a large proportion of TRPM8 neurons do not label with either intermediate filament marker (~40% in TG and 60% in DRG), an observation that suggests that these cells represent a population of small-diameter afferent neurons that can only be categorized by TRPM8 expression. Functional recordings from cultured sensory neurons show that approximately one-half of menthol-sensitive neurons are also capsaicin-sensitive, suggesting co-expression of both TRPM8 and TRPV1 in a neuronal subset (McKemy et al., 2002; Viana et al., 2002; Hjerling-Leffler et al., 2007). Thus, co-expression of TRPM8 and TRPV1 immunoreactivity in somatosensory neurons was examined and, consistent with functional data, ~one-fourth of TRPM8 neurons also express TRPV1 (Fig. 1c). Additionally, a significant overlap with the neuropeptide CGRP was observed (Fig. 1d). Thus, as predicted from behavioral and functional data, a fraction of TRPM8 neurons can be considered nociceptors neurochemically, providing a cellular rationale for the role of this channel in detecting noxious as well as innocuous thermal stimuli. Whether these presumptive nociceptors (TRPM8/TRPV1-expressing neurons) account for all cold nociceptors remains to be determined.
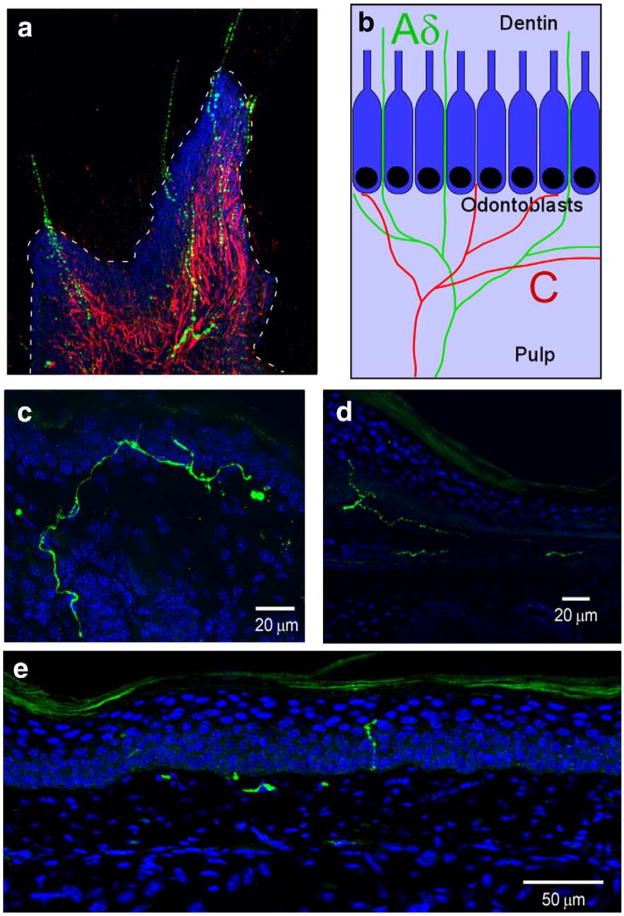
Fig. 1.
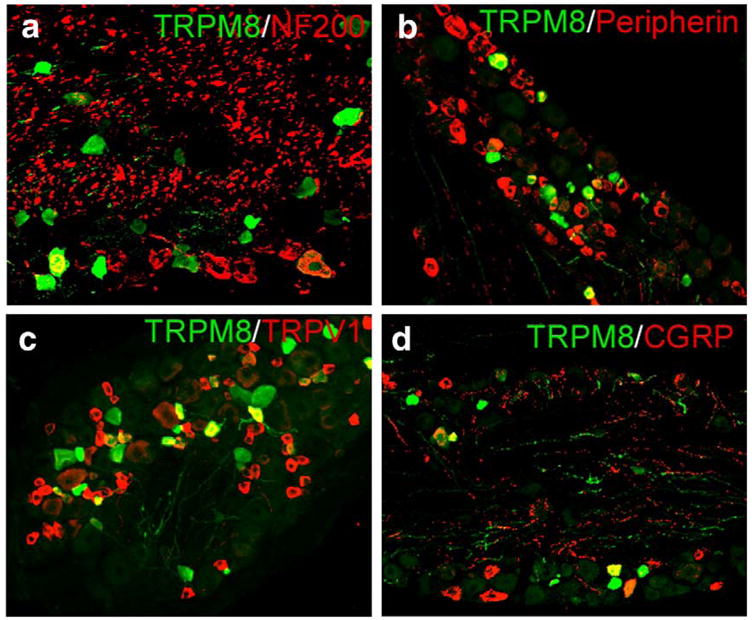
TRPM8 co-localizes with a diverse array of afferent markers in somatosensory neurons. TRPM8 neurons in both TG and DRG were immunoreactive for the intermediate filaments NF200 (a; TG: 25.5±2.6%, n=329; DRG: 13.9±3.7%, n=129) and peripherin (b; TG: 28.0±3.8%, n=239; DRG: 24.8±2.8%, n=272), as well as TRPV1 (c; TG: 38.8±2.2%, n=719; DRG: 23.7±4.6%, n=221) and CGRP (d; TG: 32.1±3.1%, n=511; DRG: 19.9±2.4%, n=288; see Takashima et al., 2007).
2.2. TRPM8 is expressed by nociceptors and thermoreceptors innervating the tooth
These data show that TRPM8 neurons are heterogeneous neurochemically and that TRPM8 is expressed in both C and Aδ fibers which are presumptive nociceptors. These findings suggest that TRPM8 is involved in both nociceptive and non-nociceptive sensory signaling. However, these results are based on somal expression and may not be indicative of the properties of the terminals of these neurons in the periphery. Psychophysical and functional data have shown cool fibers and noxious cold fibers to have distinct receptive zones in many peripheral tissues, such as skin and teeth (Darian-Smith et al., 1973; Jyvasjarvi and Kniffki, 1987; Davis, 1998). Thus, various peripheral projections of TRPM8 fibers were examined to determine if they innervate tissues in a manner that suggests both innocuous and noxious cold signaling, with the hypothesis that expression would be found in morphologically distinct subtypes of neurons or neural circuits. The best studied fibers for localization of receptive zones of distinct cold fiber-types are found in the tooth dentin and pulp (Byers and Narhi, 2002). In human and animal studies, the first and second pains evoked by cold stimulation of the tooth are attributed to cold-sensitive Aδ and C fibers, respectively (Jyvasjarvi and Kniffki, 1987). More critically, cold-sensitive Aδ and C fibers have distinct receptive fields, with superficial endings of Aδ fibers located to the dentin and C fibers deeper in the pulp (Fig. 2b) (Jyvasjarvi and Kniffki, 1987; Byers and Narhi, 2002). Since GFP was observed in both C and Aδ fibers in TG neurons, it was hypothesized that TRPM8 is expressed in both intradentinal and pulpal fibers in the tooth and thus mediates both first and second pain sensations. Consistent with this hypothesis, TRPM8 fibers in mouse molars cross the odontoblast layer that demarcates the pulp–dentin border and extend into the dentinal tubules (Fig. 2a), indicating that these are Aδ-fibers (Jyvasjarvi and Kniffki, 1987; Byers and Narhi, 2002). In addition to dentinal localization of TRPM8 fibers, TRPM8-expressing axons were found in the pulp as a fraction that co-label with peripherin, which is known to be restricted to the pulp (Byers and Narhi, 2002). Thus, TRPM8 neurons comprise both Aδ and C fibers in the tooth, and these results, taken with both functional recordings in animals and psychophysical data in humans, suggest that TRPM8 can serve dual roles in cold signaling based upon expression in functionally- and anatomically distinct fiber-types.
Fig. 2.
Peripheral terminals of TRPM8 axons are found in functionally distinct regions in the tooth and skin. (a) In a longitudinal section of a de-calcified molar, TRPM8 fibers (green) cross the odontoblast layer located at the pulp–dentin border (dashed line) and extend into the dentin, while peripherin+ axons (red) are restricted to the pulp. Cell nuclei were stained with DAPI (blue). (b) Schematic diagram of mouse molars indicating the termination zones of cold-sensitive C (red) and Aδ fibers (green) localized in the pulp and dentin, respectively. Cell rich odontoblast layer that separates the pulp and dentin is shown in blue (blue box). (c–e) In mouse skin, TRPM8 axons (green) terminate in a heterogeneous manner throughout the skin dermis and epidermis. Cell nuclei were stained with DAPI (blue).
2.3. TRPM8 is expressed by diverse cutaneous afferents that mediate cooling and noxious cold
Next, it was determined if this diversity in receptive zones innervated by TRPM8 fibers is restricted to the tooth, or also occurs in other peripheral tissues such as skin. For cold-sensitive nerves, psychophysical and functional data suggest that innocuous cool fibers are located superficial to noxious cold fibers in the skin (Davis, 1998; Harrison and Davis, 1999). From these data, along with findings that TRPM8 neurons are both peptidergic and non-peptidergic, it was determined if the peripheral terminals of GFP-positive axons are restricted to a single epidermal domain, or terminate throughout the epidermis in a manner indicative of disparate functional fiber-types. The localization of TRPM8 axon terminals in glabrous skin was examined, where the epidermis is separated into 4 distinct layers demarcated by keratinocyte organization. TRPM8-expressing axon terminals were found near the epidermal/dermal boundary as well as in the stratum granulosum (SG), the most superficial live cell layer of the epidermis (Figs. 2c–e). Thus, these results from both skin and teeth indicate that TRPM8 axons terminate in several receptive zones in which cold fibers are known to mediate temporally-and perceptually distinct cooling and cold pain sensations.
2.4. TRPM8 is a molecular fulcrum that conveys both innocuous cooling and painful cold
In conclusion, the preponderance of evidence coming from human, animal, and cellular studies suggests that the neural substrates for cold signaling are diverse. However, recent analyses of TRPM8-null mice suggest that this lone ion channel is the prime molecular detector of cold, and initiates the majority of these responses (Bautista et al., 2007; Colburn et al., 2007; Dhaka et al., 2007). Here evidence shows that TRPM8 is expressed in several neuronal subtypes and that, while it endows these cells with cold sensitivity, other mechanisms likely help determine the resulting perceptual outcome of their activation. These results further demonstrate that the peripheral neural circuitry of cold sensing is cellularly and anatomically complex, yet suggest that cold fibers, due to the diverse neuronal context of TRPM8 expression, use a single molecular sensor to convey a wide range of cold sensations. Whether different TRPM8-expressing afferents are endowed with distinct functional properties, or are in segregated neural circuits, needs to be addressed by functional recordings in intact preparations and circuit tracings in vivo. Such analyses of these and other afferent subtypes will shed light on the neural correlates of somatosensory signaling and establish the basis for genetic manipulation of neural function, the mapping of somatosensory neural networks, and the elucidation of intricacies of sensory coding in the somatosensory system.
3. Role of TRPA1 in acute cold and mechanical transduction in cutaneous nociceptors
3.1. TRPM8 is a major receptor for cooling and noxious cold
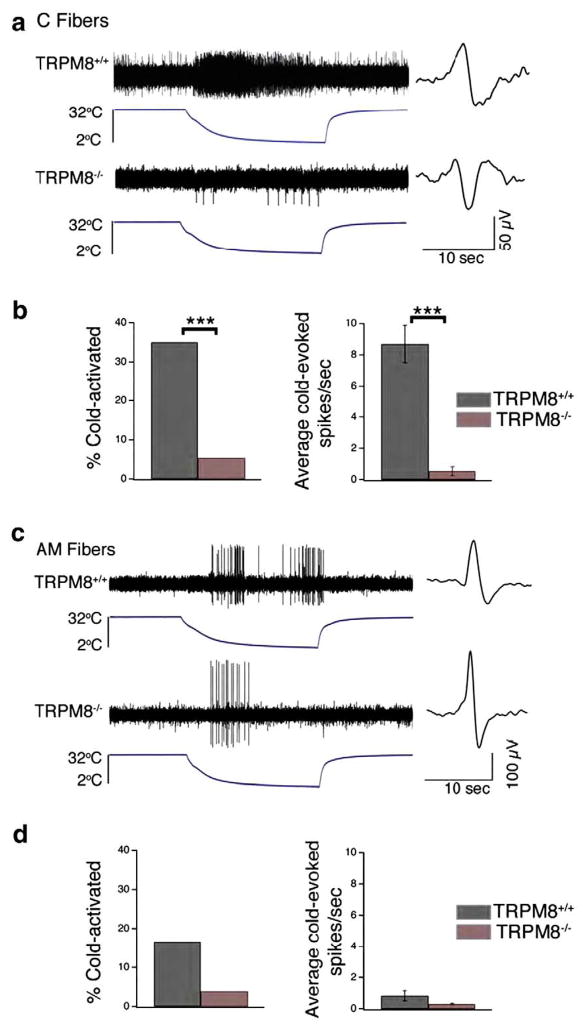
As detailed earlier in section 2.0, the majority of cold responses in mammals are mediated by the cold and menthol receptor TRPM8. Three independent laboratories generated TRPM8-null mice and through an array of phenotypic analyses from molecular to behavioral levels, demonstrate collectively that TRPM8 is a key receptor for both cooling and noxious cold (Bautista et al., 2007; Colburn et al., 2007; Dhaka et al., 2007). In one of these reports (Bautista et al., 2007), our laboratory utilized an ex vivo skin-saphenous nerve preparation to characterize the physiological responses of cutaneous C and Aδ fibers to cold stimuli (32 to 2 °C in 20 s) applied to the receptive terminals in situ (Fig. 3a). The proportion of C fibers that responded to cold was reduced from 35% in wild type preparations to only 5.4% in TRPM8-null preparations (Fig. 3b, left). Furthermore, the residual cold-sensitive C fibers fired only one-tenth the number of action potentials in response to cold (Fig. 3b, right). In addition, Aδ fibers were also reduced in cold responsiveness in the TRPM8-null mice (Figs. 3c, d). Behaviorally, these TRPM8-null mice showed little discrimination of floor plate temperatures in the range of 25–15 °C, but they nonetheless showed clear avoidance of temperatures colder than 15 °C (Bautista et al., 2007). These data indicate that TRPM8 is largely responsible for mediating sensory responses to innocuous cooling, and partially underlies responsiveness to noxious cold. However, the finding that TRPM8-null mice possess a small but finite number of cold-sensitive neurons and retain an ability to discriminate and avoid noxious cold temperatures suggests that there are other mechanisms that transduce responsiveness to cold, particularly in the noxious range. A plausible candidate is TRPA1.
Fig. 3.
TRPM8-deficient cutaneous primary afferent fibers show a significant loss of cold sensitivity. (a) Example of a typical response of a wild type (top) and TRPM8-deficient (bottom) cutaneous C fiber to a cold ramp (32 to 2 °C, over 20 s). The action potential waveform is shown to right of trace. (b) Percentage of C fibers responding to cold ramp in wild type (black; n=60) versus TRPM8−/− (red; n=56) mice (left). Average cold-evoked action potential firing rate in C fibers of wild type (n=21) versus TRPM8−/− (n=3) mice (right; Mean±S.E.M.). (c) Example of a typical response of wild type (top) and TRPM8-deficient (bottom) cutaneous AM fiber to a cold ramp (32 to 2 °C over 20 s). The action potential waveform is shown to right of trace. (d) Percentage of AM fibers responding to cold ramp in wild-type (black; n=24) versus TRPM8−/− (red; n=26) mice (left). Average cold-evoked action potential firing rate in AM fibers of wild type (n=4; Mean±S.E.M.) versus TRPM8−/− (n=1) mice (right). ***p<0.001, Students t-test or Fisher’s exact test (See Bautista et al., 2007).
3.2. Is TRPA1 a noxious acute cold receptor?
Whether or not TRPA1 is a noxious cold sensor, via either direct or indirect mechanism, is uncertain. On one hand, some research groups have shown that when TRPA1 is expressed in heterologous systems, human embryonic kidney (HEK) cells or Chinese hamster ovary (CHO) cells, it is activated by cold temperatures, ~17 °C and below that are in the noxious range (Story et al., 2003, Bandell et al., 2004; Sawada et al., 2007). Alternatively, other laboratories have found that exogenously-expressed TRPA1 is not activated by noxious cold (Bautista et al., 2006, Jordt et al., 2004, Nagata et al., 2005). A plausible answer to this discrepancy has come from two recent studies which showed that TRPA1 can be directly activated by intracellular Ca2+ via an EF-hand domain in its N-terminal domain (Doerner et al., 2007; Zurborg et al., 2007), leading one group to conclude that the cold-sensitivity of TRPA1 occurs indirectly through cold-induced release of intracellular Ca2+ (Zurborg et al., 2007). Definitive evidence of the role of TRPA1 in cold somatosensation could be provided by analysis of mice lacking the TRPA1 gene. Two groups generated TRPA1-deficient mice, but came to different conclusions based on behavioral tests. One laboratory found significant deficits in assays for acute noxious cold sensation, including withdrawal responses from ice-cold plate and acetone-evoked cooling (Kwan et al., 2006), whereas the other group found no significant cooling deficits in TRPA1-null mice compared to wild type littermates in cellular or behavioral assays (Bautista et al., 2006). Some explanations for the discrepancy in behavioral results may be differences in the test methods used or differences in gender of animals used. Beyond its disputed role in acute cold transduction, TRPA1 has also been proposed to contribute to cold hyperalgesia or allodynia after injury. In animal models, TRPA1 mRNA is reported to be upregulated after both tissue inflammation and peripheral nerve injury, and antisense knock down of TRPA1 alleviates cold hyperalgesia (Obata et al., 2005; Katsura et al., 2006).
3.3. Is TRPA1 involved in sensory mechanotransduction?
Adding further diversity to its potential physiological roles, TRPA1 has also been proposed to be involved in mechanotransduction. Evidence has been put forth from a range of evolutionary levels of species from fly to mammals. Drosophila larvae deficient in a TRPA homologue called painless have decreased behavioral responses to intense mechanical stimuli (Tracey et al., 2003). C. elegans with mutations in the Trpa1 gene fail to show head withdrawal or to cease feeding following nose touch (Kindt et al., 2007). Mice with a deletion of the pore domain of TRPA1 exhibit decreased behavioral responses to intense mechanical force in the noxious range (Kwan et al., 2006), although behavioral deficits to mechanical stimuli were not observed in a similar TRPA1 mutant mouse (Bautista et al., 2006). A small molecule inhibitor of TRPA1 reverses mechanical hyperalgesia induced by inflammation in mice (Petrus et al., 2007). No cellular studies have provided clear evidence that TRPA1 is directly gated by mechanical force, although a recent study shows that heterologously-expressed TRPA1 is activated by hypertonic saline, suggesting that TRPA1 is sensitive to osmotic stimuli (Zhang et al., 2008). However, it should be noted that the nature of osmotic stimuli and how it activates channels in a cell membrane may differ substantially from that of punctuate mechanical force applied to a localized region of the neuronal membrane.
3.4. Factors to consider when investigating sensory transduction molecules
To understand the physiological role of TRPA1 in acute cold or mechanical transduction, or in sensitization to cold or mechanical stimuli after tissue injury, it is important to carefully consider the models and techniques used because a wide range of factors could influence the results and conclusions. Some of the important variables to consider are as follows: 1) the fact that the cellular milieu surrounding the TRPA1 channel differs substantially when it is expressed in heterologous cells versus native dorsal or trigeminal ganglia, 2) the physiological relevance of the region of the sensory neuron should be considered because sensory terminals in vivo or in situ may contain different levels of ion channel expression than the soma membrane. 3) the tissue setting because TRPA1 expressed by neurons in injured or inflamed tissue may have different physiological properties than that expressed in naïve, uninjured skin; 4) the manipulation of tissue used in experiments since isolated somata from dorsal or trigeminal ganglia may be “injured” via the axotomy induced during ganglia removal or dissociation, and neurons may be altered by the effects of the culture milieu and growth factors; 5) the fact that different components that are integrated into the response of a single cell, neural pathway or whole animal behavior.
The ex vivo skin nerve preparation provides a way to measure the response properties of single identified cutaneous fibers to natural cold and mechanical stimuli. Advantages of this approach are that terminals of sensory neurons remain intact in their natural environment in the skin and that quantitative physiological stimuli can be applied to the receptive terminal. Our goals are to determine the role of TRPA1 in the response properties of C and Aδ fibers in non-injured skin to acute cold and mechanical stimuli by using TRPA1-deficient mice. A further goal is to determine the role of TRPA1 in the sensitization of nociceptors to cold or mechanical force following persistent nerve or tissue injury.
4. TRPA1 activation by endogenous mediator prostaglandin D2 metabolite, 15d-PGJ2
The TRPA1 channel is best characterized as a “chemoTRP” activated by a diverse myriad of noxious environmental chemicals (see section 1). Besides exogenous compounds, TRPA1 is also activated and/or modulated by chemicals originating from within the body, including arachadonic acid, bradykinin and products of oxidative stress (Andersson et al., 2008; Bandell et al., 2004). Our search for endogenous chemical activators utilized a bioactive lipid library screen and identified a cyclopentane prostaglandin D2 metabolite, 15-deoxy-Δ12,14-prostaglandin J2 (15d-PGJ2), as a TRPA1 agonist (Cruz-Orengo et al., 2008). Unlike other TRPA1 agonists, 15d-PGJ2 has not been previously shown to evoke nociceptive responses; rather it mediates anti-inflammatory processes. However, we find that 15d-PGJ2 evokes acute nociceptive behaviors in rodents when injected into the skin and does so via a TRPA1-dependent mechanism.
4.1. Synthesis of 15d-PGJ2 and roles in vivo
The prostaglandins (PGs) are a class of biomolecules derived from arachidonic acid that are involved in a variety of signaling processes. The synthesis of all prostaglandins begins with the enzymatic cyclooxygenation of arachidonic acid to produce prostaglandin H2 (PGH2). PGH2 is then converted into other PGs by the activity of several prostaglandin synthases, which are differentially-expressed depending on cell type and on response to external stimuli. The arachadonic acid cascade generally promotes an inflammatory response. For example, PGE2 and PGI2 are produced during inflammation, resulting in vasodilation, edema and rubor. They also contribute to the direct sensitization of nociceptive neurons of the dorsal root ganglia (DRG), resulting in hyperalgesia and allodynia (Nakae et al., 2005; Stucky et al., 1996). For example, one mechanism whereby PGE2 sensitizes nociceptive neurons to thermal stimuli occurs via the vanilloid Transient Receptor Potential channel, TRPV1. Downstream of binding to its G protein-coupled receptor (GPCR), PGE2 sensitizes TRPV1 via specific PKA-dependent phosphorylation (Hu et al., 2002).
Prostaglandin D2 (PGD2) is the most abundant prostanoid in mammalian brain tissue. Although PGD2 has been implicated primarily in the regulation of sleep, evidence also indicates that it contributes to the processing of pain signals in the central nervous system (Urade and Hayaishi, 2000). Like other prostaglandins, PGD2 acts via specific GPCRs, designated DP1 and DP2 (D-type prostanoid receptors), which are expressed in the membranes of target cells. Two subsequent non-enzymatic dehydrations of PGD2 result in the production of Δ12-Prostaglandin-J2 (Δ12-PG J2) and 15d-PGJ2.
Recently, 15d-PGJ2 has been characterized as a potent anti-inflammatory agent rather than a molecule that induces acute or long-term inflammatory pain. In fact, the resolution of the inflammatory state appears to correlate with increasing levels of 15d-PGJ2 within tissue fluids (Gilroy et al., 1999). Like other cyclopentane prostaglandins, 15d-PGJ2 does not function via a specific membrane-bound GPCR. Rather, the anti-inflammatory effects of 15d-PGJ2 are mediated by activation of the transcription factor peroxisome proliferator-activated receptor gamma (PPARγ; Forman et al., 1996, Soares et al., 2005; Straus et al., 2000). The 15d-PGJ2 is suspected to release cytoplasmic PPARγ from repressor proteins, enabling it to translocate to the nucleus and initiate the transcription of genes containing the PPAR-response element. For example, 15dPGJ2 represses the transcription of a number of pro-inflammatory factors including inducible nitric oxide synthase, cyclooxygenase-2 and tumor necrosis factor-α (Jiang et al. 1998; Ricote et al., 1998). 15d-PGJ2 also acts independent of PPARγ to alter the activity of inflammatory molecules. It is thought to directly alkylate nucleophilic cysteine residues of NF-κB, thereby inhibiting DNA binding by NF-κB (Kliewer et al., 1995).
4.2. 15d-PGJ2 directly activates TRPA1 expressed in heterologous cells
Results of our studies reveal a previously uncharacterized role of 15d-PGJ2 in peripheral nociception. We show that 15d-PGJ2, like all other known TRPA1 ligands, induces pain. We further demonstrate a causal link between 15d-PGJ2-induced nociception and TRPA1 activation at the cellular and behavioral levels. Similar to allyl isothiocyanate (AITC) and cinnamaldehyde, 15d-PGJ2 is characterized by an electrophilic α,β-unsaturated carbonyl group that is capable of undergoing a Michael addition with nucleophilic groups on cysteine residues. Two groups have shown that TRPA1 is activated by the covalent binding of electrophiles to cysteines, providing a likely mechanism whereby 15d-PGJ2 activates this channel (Hinman et al., 2006; Macpherson et al., 2007a).
The results of our initial compound library screen were borne out by follow-up electrophysiological recordings showing that 15d-PGJ2 directly activates TRPA1. In contrast to 15d-PGJ2, the J series PGD2 metabolites PGJ2 and 12d-PGJ2, which also contain reactive electrophilic carbons, failed to activate TRPA1 in our studies. A recent study by Taylor-Clark et al. (2008) which examined the activation of human TRPA1 using heterologous expression in HEK cells, found that 12d-PGJ2 was able to activate this channel. Discrepancies between this study and our study could be due to concentrations tested (5 times greater concentrations were utilized by Taylor-Clark and colleagues), expression systems used or to subtle species differences in channel structure. The in vivo behavioral studies described here extend those of Taylor-Clark and colleagues by demonstrating that 15d-PGJ2 activation of TRPA1 is physiologically relevant. During the preparation of this manuscript, Andersson and co-workers published an article on the activation of TRPA1 by mediators of oxidative stress (including 15d-PGJ2) which confirms our findings presented here (Andersson et al., 2008; Cruz-Orengo et al., 2008).
4.3. 15d-PGJ2 directly activates TRPA1 in native dorsal root ganglia neurons
Consistent with a physiological role of 15d-PGJ2 in the activation of TRPA1, we identified a population of DRG neurons that are sensitive to 15d-PGJ2, AITC and capsaicin. TRPA1-expressing neurons occur as a subset of TRPV1-expressing neurons in the DRG; whereas TRPM8 is expressed in a population distinct from TRPA1 (Story et al., 2003). These results support our electrophysiological studies showing that 15d-PGJ2 does not activate TRPV1 or TRPM8. In further support of specific activation of TRPA1, a negligible number of 15d-PGJ2/AITC responsive neurons were detected in cultures derived from TRPA1 knockout mice compared to wildtype (~2% vs. 90% respectively).
4.4. 15d-PGJ2 causes acute behavioral nociception via TRPA1
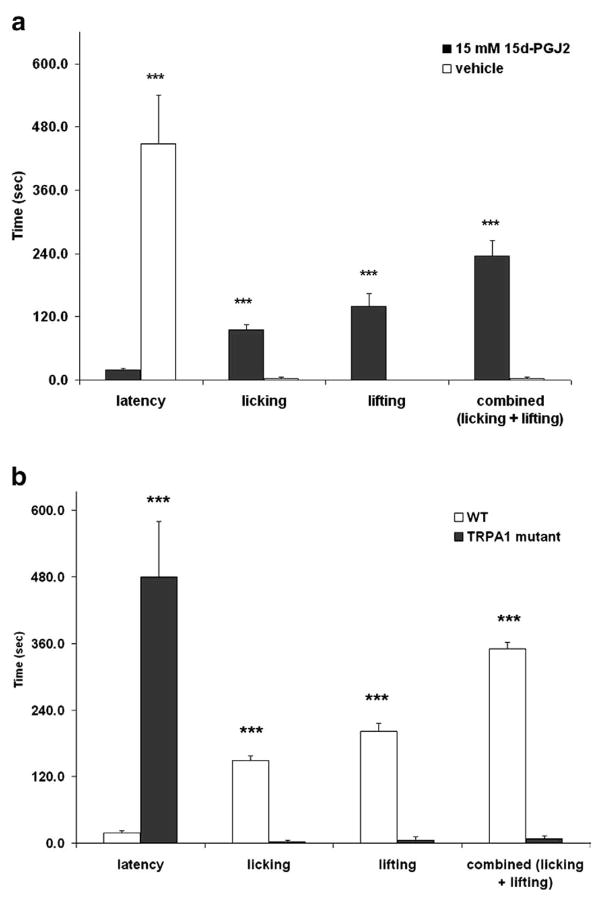
Specific agonists of TRPA1 cause acute nociceptive behaviors in vivo when injected into hind paw skin (Bandell et al., 2004; Bautista et al., 2006). Therefore, we investigated the behavioral responses to intraplantar injection of 15d-PGJ2 in mice. Both C57BL/6J and TRPA1 wildtype mice responded robustly by licking of the injected paw as well as lifting of the paw from the surface of the testing apparatus (Figs. 4a, b). These behaviors were dramatically abolished in TRPA1 knockout mice, suggesting that the acute peripheral nociceptive responses induced by 15d-PGJ2 are mediated by TRPA1.
Fig. 4.
Intraplantar injection of 15d-PGJ2 causes acute nociceptive responses via TRPA1. (a) 10 μL of vehicle (10% DMSO in saline) or 15 mM 15d-PGJ2 was injected into the hind paw of C57BL/6J mice (n =5 per group) and nociceptive behaviors (licking and lifting of the paw) for 10 min. 15d-PGJ2 caused significant nociceptive responses compared to vehicle. (b) 15d-PGJ2-induced nociceptive behaviors are absent in TRPA1 knockout mice (n=5 per group; ***p<0.001).
Previous studies have demonstrated a definitive role of TRPA1 in transmitting acute and inflammatory pain (Bandell et al., 2004; Bautista et al., 2006; Hinman et al., 2006; Macpherson et al., 2005; McNamara et al., 2007; Kwan et al., 2006). Here we show that 15d-PGJ2, a molecule with no previously-identified membrane receptor, specifically activates TRPA1, an ion channel expressed in the cell membrane of nociceptive neurons. Whereas previous studies have implicated roles for 15d-PGJ2 in anti-inflammatory pathways, we demonstrate a novel role for this molecule in acute pain. We demonstrate that similar to other TRPA1 agonists, 15d-PGJ2 induces robust, acute nociceptive behaviors in vivo. Our data also support that 15d-PGJ2-induced peripheral nociception in vivo occurs via TRPA1 signaling. Collectively, our findings elaborate a novel function of 15d-PGJ2 in peripheral nociception and identify TRPA1 as its principal receptor in pain-sensing DRG neurons.
4.5. Open questions
Does 15d-PGJ2 have therapeutic potential? Is TRPA1 involved at all in the pathways whereby 15d-PGJ2 participates in the resolution of inflammation? What are the effects of 15d-PGJ2 on peripheral inflammatory pain? Two studies have examined the effects of peripheral 15d-PGJ2 administration on nociception in the context of inflammation using the carrageenan and formalin models and found it to be anti-nociceptive (Napimoga et al., 2008a, b). Further studies using TRPA1 knockout animals will resolve whether this channel is involved in these anti-nociceptive effects of 15d-PGJ2. A possible mechanism might involve 15d-PGJ2-induced direct desensitization of the TRPA1 channel in mechanically-sensitive sensory neurons.
5. Mechanisms of TRPA1 activation and modulation in inflammatory pain
Defining the extent of the contribution of TRPA1 to the etiology of spontaneous pain and thermal and mechanical hyperalgesia in persistent inflammation is important for analgesic discovery. As mentioned earlier, TRPA1 signals the presence of a plethora of noxious stimuli in our environment and endogenous molecules released in inflamed tissues. TRPA1 is activated by a large set of chemically diverse compounds that have in common their reactivity with amino acid residues in the N terminal cytoplasmic domain (Macpherson et al., 2007a; Hinman et al., 2006). One widely used inflammatory pain model utilizes formalin, a small reactive aldehyde (formaldehyde; Fig. 5a, inset) which can act as a fixative by irreversibly crosslinking proteins. It is generally believed that the pain induced by formalin injection is attributable to general non-specific effects of tissue damage and inflammation. Here we investigated whether TRPA1 activation was required for the spontaneous nociceptive behavior evoked by subcutaneous injection of formalin. We further investigated the role of TRPA1 in mechanical and thermal hyperalgesia after inflammation produced by Complete Freund’s Adjuvant (CFA) using pharmacological and genetic approaches employing a recently identified antagonist AP18 and TRPA1 null mice.
Fig. 5.
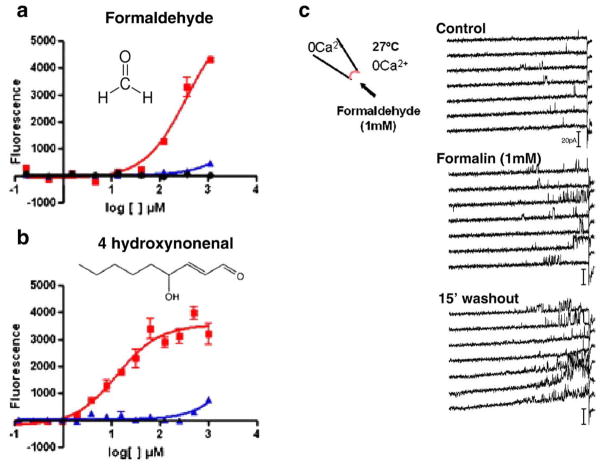
Formaldehyde and the endogenous inflammatory mediator 4-hydroxynonenal activate recombinant TRPA1. a,b) Increased intracellular calcium was observed at concentrations below 1 mM formaldehyde (a) and 4-hydroxynonenal (b) in CHO cells expressing TRPA1 (red) but not naïve CHO cells (black). CHO cells expressing TRPV1 (blue) were not responsive at low concentrations of these electrophiles. (c) Formaldehyde (1 mM, ~0.003%) increased mTRPA1 single channel activity by 1 min (middle panel) and activity remained high after 15 min of washout. Channel activity was monitored via the inside-out configuration using voltage ramps from −50 to +120 mV, rate of 2.8 mV/s every 5 s.
5.1. Formalin directly and selectively activates TRPA1
Because of its reactive properties, it was hypothesized that formaldehyde would activate TRPA1. Activation of TRPA1 by formaldehyde was initially tested in heterologous cells transfected with TRPA1 by using the high throughput fluorescent imaging plate reader (FLIPR) and electrophysiology (Figs. 5a, c). Formaldehyde increased intracellular calcium in a concentration-dependent manner (Fig. 5a; red: TRPA1-expressing CHO cells, black: naïve CHO). The estimated EC50 was ~350 μM, a concentration likely achieved after intraplantar injection of 0.2% (66 mM) formalin into the rodent hind paw. Subsequently, it was demonstrated that TRPA1 could be activated essentially irreversibly by formaldehyde in cell-detached patches of membrane (inside-out configuration) from HEK cells transiently transfected with mouse TRPA1 (Fig. 5c). Importantly, ongoing channel activity was reversibly inhibited by a high concentration of menthol (Macpherson et al., 2007b), as was similarly demonstrated for TRPA1 activation by mustard oil (Macpherson et al., 2007a). The effects of formaldehyde were specific for TRPA1 as a similar increase in fluorescence was not observed in cells transfected with TRPV1 (Figs. 5a b, blue symbols). The endogenous inflammatory mediator 4-hydroxynonenal which is known to be released during oxidative stress (Uchida, 2003) also activated TRPA1 (Fig. 5b). Similar findings were reported by two other groups (McNamara et al., 2007 and Trevisani et al., 2007). Further evidence for specificity comes from the observation that formaldehyde increases TRPA1 activity (as measured by Fura-2 ratiometric calcium imaging) in DRG neurons isolated from wild type but not TRPA1−/− mice (Fig. 6a).
Fig. 6.
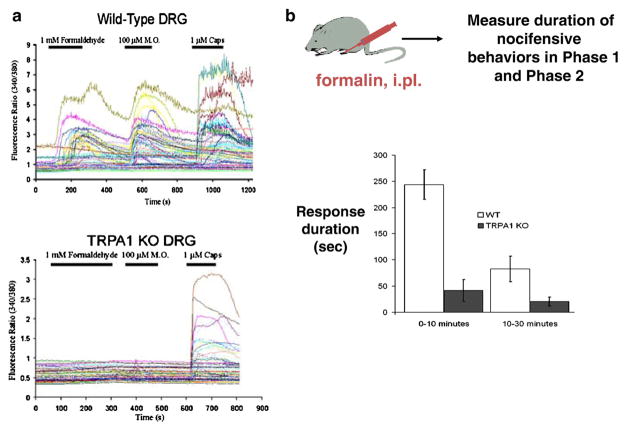
Formalin elicits inflammatory pain behaviors through TRPA1. (a) Ratiometric Fura-2 calcium imaging illustrates that formaldehyde (1 mM) evokes increase in Fura-2 ratios in DRG neurons from wild type mice, specifically in neurons also activated by mustard oil and capsaicin (top) but formaldehyde has no effect on neurons from TRPA1-null mice (bottom). (b) Formalin (10 μl of 0.2%) was injected intraplantar (i.pl.) into one hind paw of a mouse. The duration of formalin-induced nocifensive behaviors during the first phase (0–10 min) and second phase (10–30 min) after injection was scored for wild type and TRPA1−/− littermates. TRPA1-deficient mice showed a severe deficit in nociceptive responses during both phases of formalin-induced pain behavior. Responses represent the time mice spent licking, flicking, or lifting the injected hind paw (n>10 mice).
5.2. Behavioral responses to formalin depend on TRPA1
We next sought to determine whether formalin elicits inflammatory pain behaviors via a TRPA1-dependent mechanism by using a genetic approach employing TRPA1-null mice. The early phase (0–10 min) of the behavioral response to formalin is thought to be predominantly caused by peripherally-driven C-fiber activation, while the late phase (10–30 min) depends on a combination of peripheral tissue factors and functional changes in the dorsal horn of the spinal cord initiated by the C-fiber barrage during the first phase (Tjolsen et al., 1992). Both the early and late phases of formalin-induced nocifensive behaviors were significantly reduced in TRPA1−/− mice relative to their wild type littermates (Fig. 6b; Macpherson et al., 2007b). Thus, in vivo, at least at moderate concentrations, the nociceptive effects of formaldehyde are strongly reduced in TRPA1-deficient mice. These observations in genetically-modified mice were subsequently confirmed with a pharmacological approach employing TRPA1 specific antagonists (McNamara et al., 2007).
5.3. TRPA1 contributes to inflammatory mechanical hyperalgesia
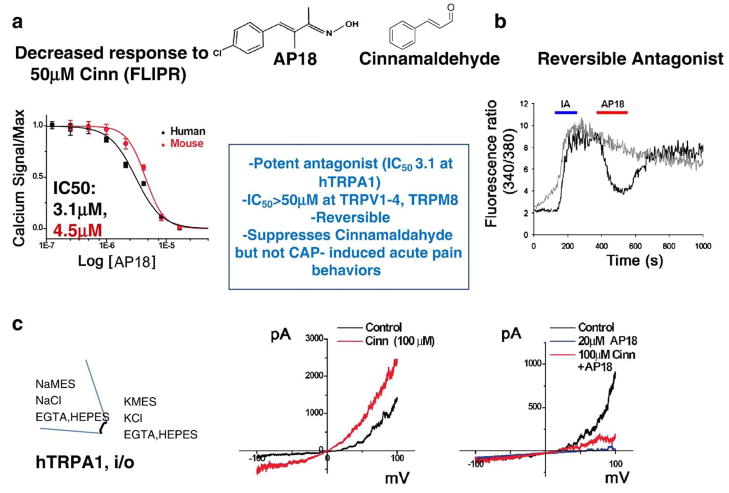
To evaluate the role of TRPA1 activity in animal models of pain, we sought to find a selective pharmacological inhibitor of TRPA1. Using an agonist–antagonist formatted FLIPR assay to screen a library of ~44,000 chemicals, we identified AP18. Compound AP18 effectively blocked the response to cinnamaldehyde, had no inherent agonist activity (Figs. 7a–c), and exhibited high selectivity for TRPA1 as other TRP channel family members were relatively insensitive to AP18 blockade (Fig. 3, inset box).
Fig. 7.
AP18 is a potent, selective and reversible antagonist for TRPA1. (a, b) AP18 inhibits increases in intracellular calcium evoked by cinnamaldehyde (Cinn; a; FLIPR technique) and iodoacetamide (IA; b; Fura-2 ratiometric calcium imaging) in CHO cells expressing TRPA1. (c) AP18 (20 μM) strongly inhibited the Cinn-activated TRPA1 currents in inside-out macropatches derived from Xenopus oocytes. The selectivity profile of AP18 is described in the box inset.
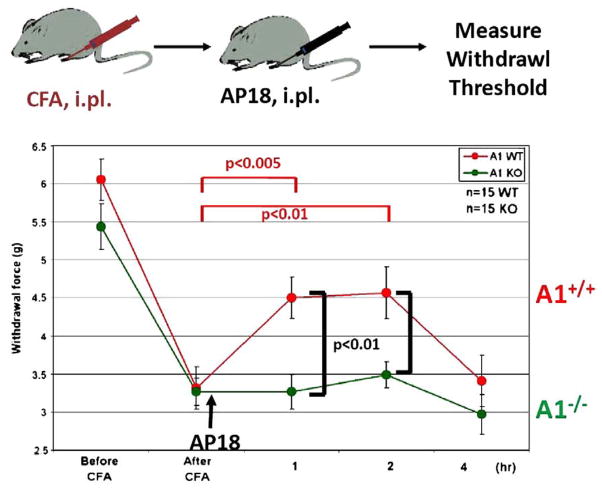
Next, AP18 was used to explore the relative contribution of TRPA1 to the hyperalgesia and allodynia associated with persistent inflammation. Cutaneous inflammation was induced via injection of the hind paw with Complete Freund’s Adjuvant (CFA) in both wild type and TRPA1 null mice. By 24 h later, both genotypes had developed marked mechanical hyperalgesia (Fig. 8). Subsequent intraplantar injection of AP18 significantly reversed the mechanical hyperalgesia in wild type but not TRPA1-null animals. AP18 did not reverse CFA-induced heat hyperalgesia, or block acute responses to intense mechanical force or noxious heat in normal non-inflamed mice (data not shown; Petrus et al., 2007). The similarity in mechanical threshold in pre-CFA injected paws from wild type and null animals determined using the automated von Frey device (Dynamic Plantar Aesthesiometer) contrasts with the results from a previous study (Kwan et al., 2006); however, the difference between studies was attributable to instrumentation since we also observed that non-injured TRPA1-deficient mice exhibited a significantly increased acute mechanical threshold to intense force when the method of Kwan was employed. Interestingly, the data presented in Fig. 8 indicate that TRPA1-deficient mice develop and sustain normal mechanical hyperalgesia following CFA injection, suggesting that compensatory mechanisms in the developmental knockout likely mask the requirement for TRPA1 in the maintenance of mechanical sensitization.
Fig. 8.
AP18 partially reverses inflammation-induced mechanical hyperalgesia. CFA injection induces marked mechanical hyperalgesia in both wild type (red) and TRPA1−/− mice (green). AP18 was injected (i.pl.) after the 24 h measurement time point. AP18 significantly reversed the mechanical hyperalgesia in wild type mice but not TRPA1 null littermates (n=12, females). Mice were injected with 10 μg CFA in 10 μL; AP18 (1 mM in 10 μL) was injected in a PBS based vehicle containing 0.5% Tween80 and 1% DMSO. Statistical significance was determined using a two-tailed Student’s t-test.
5.4. TRPA1 contributes to inflammatory cold hyperalgesia
Direct activation of TRPA1 by noxious cold temperatures has been heatedly debated by a number of different investigators, and it is likely that second messengers such as intracellular calcium contribute to the activation of TRPA1 in response to cold stimuli (Zurborg et al., 2007). Using a pharmacological approach we sought to determine whether TRPA1 contributed to CFA induced cold hyperalgesia. Conflicting results have been reported on the role of TRPA1 in sensing noxious cold temperatures in TRPA1-deficient mice. One group observed a partial deficit in response to cold in two different behavioral assays (Kwan et al., 2006) while the other group found no cold deficits (Bautista et al., 2006). The differences observed may depend on the choice of gender used (the cold deficits were observed only in females; Kwan et al., 2006), the different methods used for assaying cold-evoked behavioral responses, or the difficulty of observing responses to noxious cold stimuli in mice, particularly of different genetic backgrounds. However, a robust cold-induced hyperalgesia has been reported in rats (Obata et al., 2005; Allchorne et al., 2005). Placing a rat on a 5 °C cold plate induces nocifensive behaviors such as licking/flicking the paw and non-coordinated paw lifting. TRPA1 antisense but not control oligodeoxynucleotides reverse the cold hyperalgesia after CFA-induced inflammation, indicating a role for TRPA1 in cold hyperalgesia (Obata et al., 2005). Thus, we investigated whether AP18 could reverse CFA-induced cold hyperalgesia in Sprague–Dawley rats. In comparison to basal nocifensive responses before CFA injection, CFA-induced inflammation elicited a hyperalgesic response to noxious cold as evident by a marked increase in the number of cold-evoked responses (Fig. 9). Treatment with AP18, but not vehicle, substantially reversed the number of nociceptive responses observed 24 h after CFA injection (Fig. 9). These data provide pharmacological evidence for a major contribution of TRPA1 to CFA-induced cold hyperalgesia in rats.
Fig. 9.
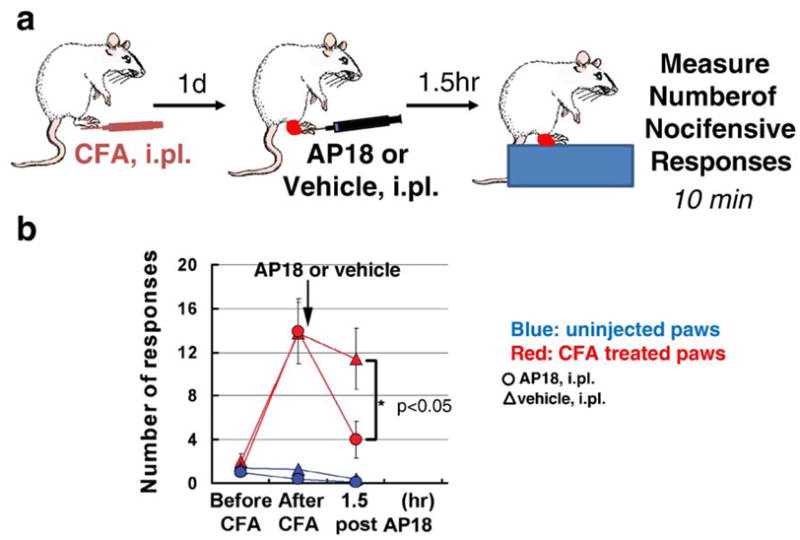
AP18 partially reverses cold hyperalgesia in rats with CFA-induced inflammation. Sprague Dawley rats (n=8) were injected with 50 μg CFA in 100 μL (1:1 emulsion of mineral oil and saline). AP18 (1 mM in 10 μL) was injected in a PBS based vehicle containing 0.5% Tween80 and 1% DMSO. Statistical significance was determined using a two-tailed Student’s t-test.
Thus, using genetic and pharmacological approaches, the composite of these data suggest that TRPA1 plays a major role in the inflammatory pain induced by formalin injection as well as in the mechanical and cold hyperalgesia that is associated with peripheral inflammation. The mechanism by which TRPA1 contributes to inflammatory pain is not known. Whether native mammalian TRPA1 can be directly gated by noxious mechanical forces and cold temperatures, and the extent that upstream messengers or accessory proteins participate in TRPA1-mediated pain behaviors are currently unanswered questions.
6. Growth factor modulation of TRP channels
6.1. Proposed role for TRPA1 in inflammatory pain
Previous studies have demonstrated that TRPV1 is critical to a variety of chronic pain states: cutaneous inflammatory pain (Caterina et al., 2000), bone cancer pain (Ghilardi et al., 2005), incisional pain (Pogatzki-Zahn et al., 2005), bladder cystitis pain (Charrua et al., 2007), and colonic hypersensitivity (Jones et al., 2007). Interestingly, TRPA1−/− mice lack responsiveness to bradykinin, a major effector of TRPV1 potentiation and inflammatory pain, and recent studies using TRPA1-specific inhibitors also suggest a role for TRPA1 in inflammatory hyperalgesia (Kwan et al., 2006; McNamara et al., 2007; Petrus et al., 2007). However, little is known about the function and modulation of TRPA1 in chronic pain.
6.2. Growth factor modulation of TRPA1 and TRPV1
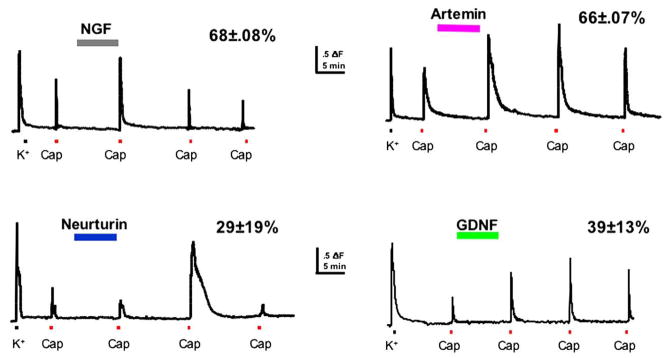
Modulation of TRPA1 by bradykinin-, growth factor- and TRPV1-gated pathways has important implications for possible roles for this channel in acute and chronic pain conditions. Growth factors such as NGF, GDNF, neurturin and artemin are dynamically regulated in peripheral tissues in response to injury. In particular, expression of NGF and artemin is dramatically elevated in inflamed tissue during the period of hyperalgesia (Malin et al., 2006; Fig. 10). Previous studies have demonstrated that the elevation of NGF is critical to the development of inflammatory and incisional thermal hyperalgesia (Banik et al., 2005; Wu et al., 2007), and acute application of NGF to sensory neurons blocks tachyphylaxis of the capsaicin response (Shu and Mendell, 1999; Zhu et al., 2004; Fig. 11) by reducing desensitization of TRPV1. We recently reported that acute application of artemin, neurturin and GDNF also potentiates TRPV1 function in sensory neurons in vitro (Fig. 11), and that this mechanism likely contributes to inflammatory pain in vivo. Artemin and NGF potentiate TRPV1 in most (>60%) capsaicin-responsive sensory neurons. Interestingly, the effects of artemin and NGF are synergistic: potentiation of TRPV1 responses by combined NGF and artemin application is 4-fold greater than that seen with either growth factor alone (Malin et al., 2006). Similar studies examining modulation of TRPA1 by growth factors have not been done and are critical to understanding the role of TRPA1 in inflammation.
Fig. 10.
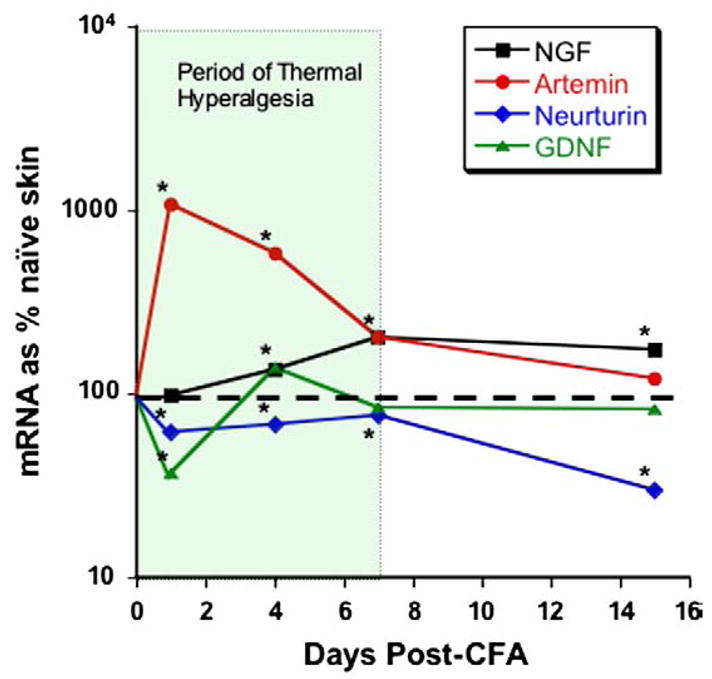
Growth factors are upregulated during inflammation. PCR was used to measure changes in growth factor levels following a single injection of CFA into hindpaw glabrous skin. Artemin mRNA levels increased 10-fold within 24 h.
Fig. 11.
Growth factors inhibit TRPV1 tachyphylaxis. Fura-2 calcium imaging of primary afferent responses to capsaicin (Cap) before and after 10 min exposure to growth factor. In the absence of growth factor, repeated capsaicin responses normally exhibit tachyphylaxis. All four growth factors block tachyphylaxis and produce potentiation (neurturin effect is delayed). Percentage (%) indicates the capsaicin-responsive cells potentiated by each growth factor.
We have also shown that injections of GDNF family members (artemin, neurturin and GDNF) or NGF into the hindpaw cause acute thermal hyperalgesia lasting >4 h (Fig. 12). Interestingly, combined injection of NGF and artemin, the growth factors elevated during inflammation in vivo, results in significant thermal hyperalgesia lasting >6 days (Fig. 12). Taken together, these data suggest that growth factor modulation of TRP channel function is profound and critical to inflammatory thermal hyperalgesia.
Fig. 12.
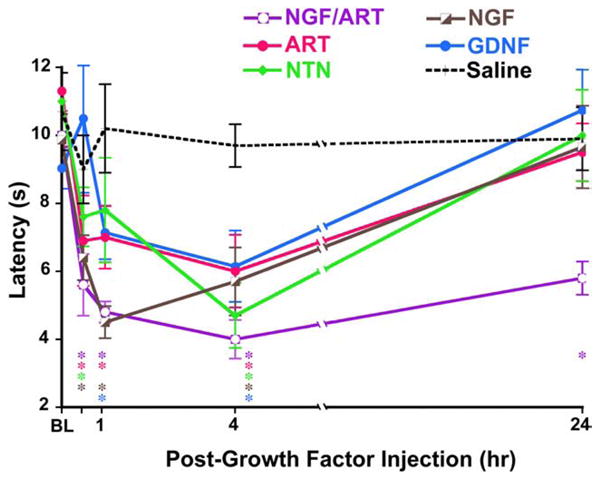
Growth factors induce hyperalgesia in vivo. Latency to thermal nociceptive response measured with a Hargreaves apparatus before and after injection of 0.2 μg/20 μl growth factor (or saline control) into the hindpaw of wildtype mice. In wildtype animals, all growth factors tested caused significant thermal hyperalgesia during the first 4 h. Co-injection of NGF/ART resulted in persistent thermal hyperalgesia lasting 6d. *p<0.05.
6.3. TRPA1 and TRPV1 may functionally interact
TRPV1 and TRPA1 are expressed inlargely overlapping subsets of nociceptors (Bautista et al., 2005; Kobayashi et al., 2005), and both channels are implicated in inflammatory thermal hyperalgesia. Surprisingly, TRPV1−/− mice show reduced response to mustard oil, a TRPA1 ligand (Caterina et al., 2000). Thisresult suggests that TRPA1 function is impaired in the absence of TRPV1. Furthermore, behavioral and electrophysiological responses to the pro-inflammatory peptide bradykinin, previously demonstrated to require TRPV1, are impaired in TRPA1−/− mice (Ferreira et al., 2004; Bautista et al., 2006; Kwan et al., 2006). These results suggest that TRPA1 may be critical to TRPV1 function. A recent report describes cross-desensitization of TRPA1 and TRPV1 in vitro, further supporting the hypothesis that TRPA1 and TRPV1 are functionally linked in sensory neurons in vivo (Akopian et al., 2007). The mechanism for this interaction is unknown, but may involve regulation of one channel by calcium entry through the other (Doerner et al., 2007; Zurborg et al., 2007), direct channel–channel interaction within the plasma membrane and/or co-assembly of TRPV1 and TRPA1 subunits into heteromultimeric channels. In addition, TRP channel activation results in activation of second messenger signaling cascades that may modify both TRPV1 and TRPA1. These findings suggest a previously-unsuspected mechanism for the regulation of noxious sensation, and beg the question of whether TRPA1, like TRPV1, is potentiated by endogenous inflammatory mediators.
6.4. Sensitization of TRPA1 and TRPV1 by second messengers
In sensory neurons, repeated TRPA1 activation by 100 μM mustard oil results in profound desensitization of the TRPA1 channel (Fig. 13). Similar desensitization has been described for TRPV1 and variously attributed to 3 distinct pathways: 1) activation of calcineurin, which causes dephosphorylation of TRPV1 (Docherty et al., 1996; Mohapatra and Nau, 2005), 2) activation of PLC and subsequent PIP2 hydrolysis (Chuang et al., 2001; Liu et al., 2005), and 3) activation of calcium-dependent PKC isoforms and subsequent channel phosphorylation (Akopian et al., 2007). PIP2 has been shown to reduce TRPV1 activation and drive TRPV1 desensitization; NGF- and bradykinin-induced potentiation of TRPV1 (Shu and Mendell, 1999) may result from blockade of this TRPV1 densensitization pathway by hydrolysis of PIP2. However, the role of PIP2 in TRP channel desensitization is controversial and an area of active study. Recently, inhibition of TRPA1 by PIP2 has been reported (Kim et al., 2008), and scavenging of PIP2 has been demonstrated to reduce desensitization of TRPA1 (Dai et al., 2007; Karashima et al., 2009). Sensitization of TRPA1 by the GPCR Protease Activated Receptor 2 (PAR2) has been demonstrated, and appears to occur via a PLC/PIP2 mechanism in sensory neurons (Dai et al., 2007). In addition, activation of the ion channel TRPV1 has been shown to desensitize TRPA1 via a PIP2-dependent mechanism (Akopian et al., 2007). These data suggest that growth factors and other inflammatory mediators that activate PLC and reduce membrane PIP2 will sensitize TRPA1, however this has not yet been tested directly.
Fig. 13.
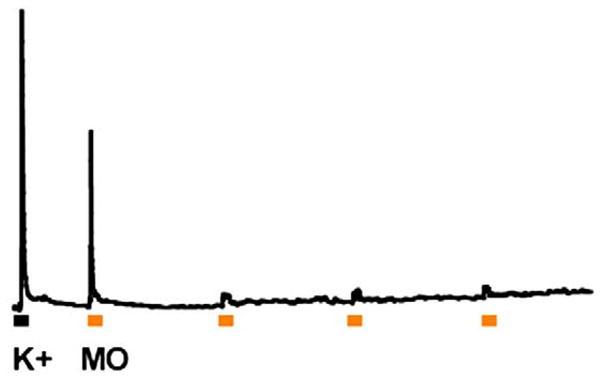
TRPA1 shows prominent tachyphylaxis. Fura-2 calcium imaging was used to record responses to repeated application of 100 μM mustard oil (yellow squares) at 10 min intervals. Cells were first tested with a depolarizing stimulus (50 mM K+) to evaluate cell health and then tested 4 times with mustard oil.
Recently, bradykinin-induced sensitization of TRPA1 has been demonstrated in vivo and appears to involve both PLC and PKA pathways in sensory neurons (Wang et al., 2008). Furthermore, these effects are additive, suggesting that TRPA1 may integrate signals from multiple activators of PLC and PKA during sensory signaling (Wang et al., 2008). Because of the high structural and functional homology between TRPV1 and TRPA1, it seems likely that growth factors and inflammatory mediators potentiate TRPA1 function through inhibition/blockade of TRPA1 desensitization. In addition to modulatory action of bradykinin on TRPA1 channels through PLC and PKA, TRPA1 channels are also opened by bradykinin. This effect requires the bradykinin receptor, and is, therefore, likely to occur through second messenger pathways downstream of bradykinin receptors, as opposed to direct channel gating of TRPA1 by bradykinin (Bandell et al., 2004; Bautista et al., 2006; Kwan et al., 2006). These data suggest that other PLC and PKA activators may gate or modulate TRPA1 channels in sensory neurons (Fig. 14)
Fig. 14.
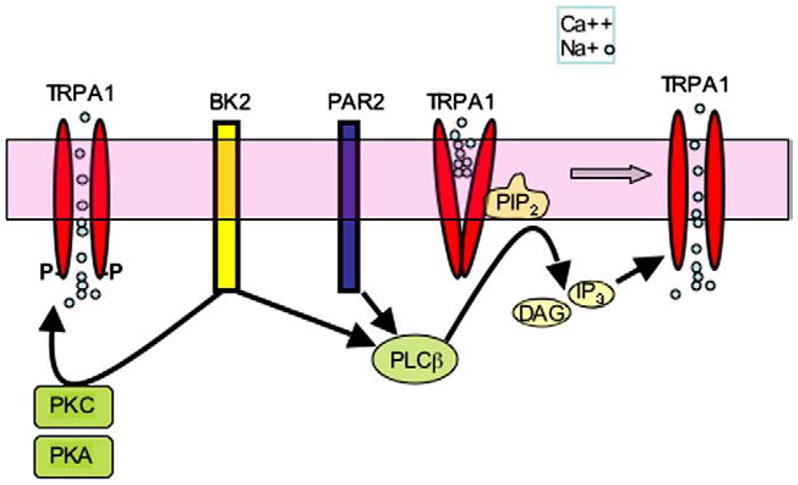
TRPA1 activity is modulated in sensory neurons. TRPA1 activation is sensitized by intracellular Ca++ and kinases PKC and PKA. TRPA1 is inhibited by PIP2, and polyphosphates such as IP3 are required for TRPA1 function. Bradykinin, acting through the bradykinin receptor BK2, activates PLC, PKC and PKA to sensitize TRPA1; proteases trypsin and tryptase, acting through PAR2, sensitize TRPA1 through PLC. The effects of these pathways are largely additive.
6.5. TRPA1 may integrate multiple stimuli in sensory neurons
Interestingly, a soluble intracellular polyphosphate co-factor is required for activation of TRPA1 by irritant chemicals (Kim and Cavanaugh, 2007). Although this polyphosphate is required for activation of TRPA1 by pungent compounds, it is not required for cannabinoid activation of TRPA1 (Cavanaugh et al., 2008). These data suggest that ion channel or GPCR activity that affects intracellular IP3 concentrations will modulate the activation state and effective agonists of TRPA1. TRPA1 is also activated by elevation of intracellular calcium (Doerner et al., 2007) This effect is not dependent on polyphosphates or another soluble cysolic factor and is, therefore, functionally distinct from activation by pungent compounds or cannabinoids (Cavanaugh et al., 2008). It seems likely that pathways which result in elevation of intracellular calcium will enhance TRPA1 function; this calcium-driven modulation may allow TRPA1 to function as a coincidence detector of multiple stimuli in sensory neurons.
Although less-studied than TRPV1, initial studies suggest that TRPA1 channel activity is modulated by PKA, PKC, PIP2 and IP3 in sensory neurons. TRPA1 is sensitized by both bradykinin and PAR2, suggesting a role for this channel in inflammatory pain. Furthermore, a recent study has shown immunohistochemical and functional upregulation of TRPA1 following the SNL model of nerve injury and increased behavioral responses to TRPA1 ligands in vivo (Ji et al., 2008). These data suggest that TRPA1 expression and ion channel modulation play an important role in both inflammatory and nerve injury-induced chronic pain.
7. Sensitization of TRPV1 by kinases is modulated by scaffold proteins
Signaling mechanisms throughout the body are tightly coordinated and regulated by dynamic processes that control virtually every physiological system. The targeting, association, and orientation of signaling molecules in a transduction pathway are considered the points of highest control along the route of the original signal. Proteins including Membrane-Associated Guanylate Kinase homologs (MAGUK proteins; Willott et al., 1993; Woods and Bryant, 1991) and Ran Binding Protein in the Microtubule-Organizing Center (RanBPM; Yokoyama et al., 1995) serve as scaffolding proteins that organize multi-protein complexes. Scaffolding proteins serve to mediate and propagate signaling events, thereby reducing temporal, structural, and functional obstacles that would hinder the efficient coupling and association of pathway partners. Indeed, post-translational events that regulate the sensitivity of plasma membrane receptor/channels have been demonstrated to depend upon the organizational nature of scaffolding proteins. Recent biochemical studies of TRP family channels have identified the plasma membrane-localized isoform of A-Kinase Anchoring Protein (AKAP) scaffolding proteins as a critical link for inflammatory mediator-induced sensitization of TRPV1. Here we discuss the modulatory effects of AKAP scaffolding proteins on TRPV1 activity and sensitization.
7.1. TRPV1 channel activity is tightly regulated by post-translational modification
Like other members of the TRP superfamily, native TRPV1 likely consists of four subunits, with each containing 6 transmembrane domains (Clapham, 2003). TRPV1 is expressed within a major class of nociceptive neurons (Helliwell et al., 1998) and appears to be an important receptor for detecting noxious stimuli such as acidic pH (Tominaga et al., 1998), heat (>43 °C) (Caterina et al., 1997), and chemicals including capsaicin (Caterina et al., 1997) and anandamide (Zygmunt et al., 1999). Analysis of TRPV1-deficient mice confirmed the specificity and activation kinetics of various receptor-specific stimuli (Caterina et al., 2000). Due to the multimodal sensory stimuli that can activate the receptor, TRPV1 is a particularly useful model for studying the biochemical regulation of receptor-activated sensitization of peripheral nociceptors.
Early work that characterized release of neuropeptides from rat spinal cord following intrathecal infusion of capsaicin suggested that activation of the TRPV1 receptor/channel was regulated, at least in part, by post-translational modification (Jhamandas et al., 1984). The activation state of TRPV1 has been consistently shown to depend on the degree of post-translational modification, primarily phosphorylation (Lee et al., 2005). For example, tachyphylaxis, the pharmacological desensitization that follows repeated application of capsaicin, highlights the key role of phosphorylation and dephosphorylation in regulation of TRPV1 channel activity. Specifically, dephosphorylation of TRPV1 results in decreased channel activity. A key study by Docherty and colleagues showed that the neuronal phosphatase calcineurin plays a major role in dephosphorylating TRPV1, and is fundamentally responsible for the use-dependent decreases in channel activity in the presence of extracellular Ca2+ (Docherty et al., 1996; Wu et al., 2005). Conversely, phosphorylation of TRPV1 increases its activity and at least four unique serine/threonine kinases are known to phosphorylate and sensitize TRPV1, including Protein Kinase A (PKA), Protein Kinase C (PKC), Ca2+/calmodulin-dependent kinase II (CaMK II), and cyclin-dependent kinase 5 (Cdk5) (Ahern and Premkumar, 2002; Crandall et al., 2002; Jung et al., 2004; Numazaki et al., 2002; Pareek et al., 2007; Vellani et al., 2001). For example, PKA phosphorylates TRPV1 at amino acid sites Ser116, Thr144, Thr370, Ser502, Ser774, and Ser820 (Mohapatra and Nau, 2003; Rathee et al., 2002), and PKA activity is positively correlated with increased channel activity (Bhave et al., 2002; Hu et al., 2002; Mohapatra and Nau, 2003; Rathee et al., 2002). Surprisingly however, TRPV1 lacks a known binding domain for PKA association, suggesting that PKA must act via a scaffolding protein accomplice in order to modulate TRPV1 activity.
7.2. A-Kinase Anchoring Proteins serve as scaffolding partners for kinases
A-Kinase Anchoring Proteins (AKAPs) were initially characterized as mediators of PKA signaling events and phosphorylation cascades (Bregman et al., 1989; Carr et al., 1992). Since their initial designation, over 20 AKAP isoforms have been identified and characterized in species ranging from C. elegans to Homo sapiens for their specific roles and subcellular localization (for review see Colledge and Scott, 1999). Among the AKAP isoforms that have been characterized, AKAP79/150 is predominantly localized to the plasma membrane and postsynaptic densities of neurons within the CNS (Dell’Acqua et al., 1998). In humans, AKAP79 is homologous with AKAP150 in rodents, and for convenience, we will refer to this protein as AKAP150. At postsynaptic densities, AKAP150 is found to bind to the RII subunits (RIIα and RIIβ) of PKA with nanomolar affinity (Carr et al., 1992). Importantly, AKAP150 is also present in rodent DRG neurons where it is essential for TRPV1 modulation (Schnizler et al., 2008).
Further work has identified various other proteins that can associate with AKAP proteins in several tissues. For instance, AKAP150 has binding affinity with protein phosphatase 2B (PP2B, calcineurin) in addition to PKA (Coghlan et al., 1995). Interestingly, AKAP150 could potentially regulate both the phosphorylation and dephosphorylation of various proximal substrates, effectively controlling phosphorylation-dependent activities of substrate proteins (Faux and Scott, 1996), and thereby channel activity. Following this line of reasoning, several labs hypothesized that AKAP79/150 could regulate PKA-dependent sensitization of TRPV1 in peripheral neurons, thereby controlling the activity of the receptor and the sensation of pain. These observations support the suggestion that AKAP may play a role in the dynamic regulation of TRPV1 via both decreases and increases in activity by anchoring two different proteins that differentially modulate TRPV1 channel function.
7.3. AKAP150 and TRPV1 physically and functionally interact
The initial suggestion of a functional AKAP/TRPV1 association was made in 2002, when Rathee and colleagues reported that forskolin-potentiated heat-induced currents in cultured DRG neurons could be blocked by inhibiting AKAP/PKA association (Rathee et al., 2002). Indeed, the Ca2+-mediated potentiation of heat-currents in dissociated DRG neurons was inhibited when PKA coupling to AKAP was disrupted. (Distler et al., 2003). Despite this, Rathee and colleagues were unable to demonstrate any physical interaction between AKAP and TRPV1 (Rathee et al., 2002). Nevertheless, a physical interaction between AKAP150 and TRPV1 was subsequently demonstrated in co-immunoprecipitation assays using both sensory neurons and Chinese Hamster Ovary (CHO) cells that express AKAP150 and TRPV1. Interestingly, MAGUK proteins that have been shown to bridge AKAP/effector protein interactions including PSD95 and SAP97 (Colledge et al., 2000), failed to co-immunoprecipitate with AKAP150 or TRPV1 in trigeminal sensory neurons. These results indicate that AKAP150 physically associates with TRPV1, either directly, or via the assistance of an unknown bridging protein that may be selectively expressed in sensory neurons.
Given the number of TRP channels that exhibit heat sensitivity, a more specific link between TRPV1 and AKAP was established with the selective TRPV1 agonist capsaicin. When 8-Br-cAMP was used to stimulate PKA phosphorylation, the 8-Br-cAMP-induced sensitization of capsaicin-evoked Ca2+ transients in CHO cells required co-expression of TRPV1 and full-length AKAP150 proteins. In contrast, co-expression of TRPV1 with an AKAP150 mutant missing the PKA-binding domain (AKAP150ΔPKA) prevented 8-Br-cAMP from sensitizing the capsaicin-evoked Ca2+ influx. Furthermore, the 8-Br-cAMP-stimulated phosphorylation of TRPV1 and sensitization of capsaicin-evoked current in dissociated trigeminal neurons was significantly impaired in neurons transfected with AKAP150-specific siRNA compared to mock- or scrambled siRNA-transfected neurons, despite the fact that similar levels of PKA kinase activities were found in all groups of transfected neurons. These findings demonstrated that AKP150 functionally regulates the CAP-mediated activation and PKA-mediated sensitization of TRPV1.
Further evidence for a function link between AKAP150 and PKA-induced modulation of TRPV1 comes from studies employing the peptide St-HT31, which inhibits PKA binding to AKAP150 and therefore the proper positioning of this kinase (Vijayaraghavan et al., 1997). In dissociated trigeminal neurons, pre-treatment with the St-HT31 blocked 8-Br-cAMP-stimulated TRPV1 phosphorylation. Importantly, the peripheral injection of St-HT31 significantly attenuates PGE2-stimulated thermal hyperalgesia (Jeske et al., 2008), confirming that both PKA and TRPV1 underlie this behavioral change.
7.4. AKAP serves as a linchpin for inflammatory mediators
As illustrated in Fig. 15, AKAP may serve as a point of convergence for an array of inflammatory mediators including prostaglandin E2 (PGE2) receptors (Hu et al., 2002), β-adrenergic type 1–3 receptors (β AR1–3; Faisy et al., 2004), serotonin type 7 receptors (5-HT7; Ohta et al., 2006), and histamine type 2 receptors (H2), although only the sensitizing effects of H1 receptor activation by histamine on TRPV1 activity have been reported (Kim et al., 2004; Koda et al., 1996).
Fig. 15.
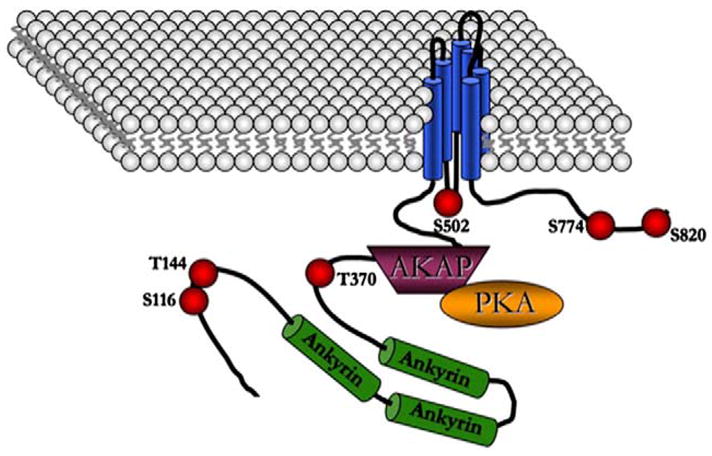
Illustration of hypothetical AKAP150 association with TRPV1 at the plasma membrane. PKA phosphorylation sites, as listed and referenced in the text, are identified by red spheres.
Biochemical characterizations of receptor-mediated signaling systems often fail to account for supporting proteins that play a significant role in modulating post-translational changes in the receptor complex. The AKAP150 scaffolding protein serves such a purpose as it allows for the close spatial association of PKA, and other potential signaling enzymes, with TRPV1 at the level of the plasma membrane in primary afferent neurons. Indeed, such an association supports an efficient pathway for inflammatory sensitization, thereby identifying the scaffolding protein as a potential therapeutic target for inflammatory hyperalgesia over the signaling enzymes themselves. The identification and subsequent characterization of peptide sequences responsible for TRPV1/AKAP150 association would provide a unique target for the generation of peripheral inflammatory analgesics, and could even be used to dissect the associations of other potential signaling enzymes that post-translationally modify TRPV1.
8. Closing remarks
Knowledge about the function of TRPV1, TRPM8 and TRPA1 has rapidly developed since their discoveries within the past eleven years. A theme that emerges from this review is that TRPV1, TRPM8 and TRPA1 are not simple channel mediators of heat, cold and pungency, respectively, but instead serve multidimensional roles in signaling multimodal sensory stimuli. TRPM8 is required for cold detection, and contributes to cold pain, cold hypersensitivity after injury, and cooling analgesia. TRPV1 is a molecular fulcrum for activation and sensitization of primary afferent neurons to heat, acid, and numerous endogenous inflammatory mediators and is a plays an essential role in the peripheral hyperalgesia that accompanies tissue injury. Growing evidence indicates that TRPA1 acts as a similar molecular integrator, via its activation, sensitization and facilitation of multiple signaling pathways, including sensory transduction, nociception, inflammation and oxidative stress.
Whereas significant strides for roles of these channels are being made at the cellular level in nociceptors, other levels of the nervous system that need to be charted include their roles in synaptic plasticity in the spinal cord, brainstem and descending systems. We anticipated that by the next Spring Pain meeting, the information about functional roles of TRPV1, TRPM8 and TRPA1 will have multiplied exponentially.
Acknowledgments
The authors wish to thank Yoshio Takashima and the members of the McKemy lab for their support and insights related to this work. Furthermore, the authors wish to thank Joshua Glazer and Diana Bautista for their contributions. Thanks to Ardem Patapoutian, Bialong Xiao, Lindsey Macpherson, Matt Petrus, Andrea Peier, Michael Bandell, Sun Wook Hwang, Truc Huynh, Nicholas Olney and Tim Jegla. This research was supported by grants NS40538 (C.L.S), NS054069 (D.D.M), NS061884 (N.A.J), NS31826 and NS050758 (to B.M. Davis for S.A.M) and NIDDK 063922 (S.A.M).
References
- Ahern GP, Premkumar LS. Voltage-dependent priming of rat vanilloid receptor: effects of agonist and protein kinase C activation. J Physiol. 2002;545:441–451. doi: 10.1113/jphysiol.2002.029561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akopian AN, Ruparel NB, Jeske NA, Hargreaves KM. Transient receptor potential TRPA1 channel desensitization in sensory neurons is agonist dependent and regulated by TRPV1-directed internalization. J Physiol. 2007;583:175–193. doi: 10.1113/jphysiol.2007.133231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allchorne AJ, Broom DC, Woolf CJ. Detection of cold pain, cold allodynia and cold hyperalgesia in freely behaving rats. Mol Pain. 2005;1:36–41. doi: 10.1186/1744-8069-1-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersson DA, Gentry C, Moss S, Bevan S. Transient receptor potential A1 is a sensory receptor for multiple products of oxidative stress. J Neurosci. 2008;28:2485–2494. doi: 10.1523/JNEUROSCI.5369-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bandell M, Story GM, Hwang SW, Viswanath V, Eid SR, Petrus MJ, Earley TJ, Patapoutian A. Noxious cold ion channel TRPA1 is activated by pungent compounds and bradykinin. Neuron. 2004;41:849–857. doi: 10.1016/s0896-6273(04)00150-3. [DOI] [PubMed] [Google Scholar]
- Banik RK, Subieta AR, Wu C, Brennan TJ. Increased nerve growth factor after rat plantar incision contributes to guarding behavior and heat hyperalgesia. Pain. 2005;117:68–76. doi: 10.1016/j.pain.2005.05.017. [DOI] [PubMed] [Google Scholar]
- Bautista DM, Movahed P, Hinman A, Axelsson HE, Sterner O, Hogestatt ED, Julius D, Jordt SE, Zygmunt PM. Pungent products from garlic activate the sensory ion channel TRPA1. Proc Natl Acad Sci U S A. 2005;102:12248–12252. doi: 10.1073/pnas.0505356102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bautista DM, Jordt SE, Nikai T, Tsuruda PR, Read AJ, Poblete J, Yamoah EN, Basbaum AI, Julius D. TRPA1 mediates the inflammatory actions of environmental irritants and proalgesic agents. Cell. 2006;124:1269–1282. doi: 10.1016/j.cell.2006.02.023. [DOI] [PubMed] [Google Scholar]
- Bautista DM, Siemens J, Glazer JM, Tsuruda PR, Basbaum AI, Stucky CL, Jordt SE, Julius D. The menthol receptor TRPM8 is the principal detector of environmental cold. Nature. 2007;448:204–208. doi: 10.1038/nature05910. [DOI] [PubMed] [Google Scholar]
- Bhave G, Zhu W, Wang H, Brasier DJ, Oxford GS, Gereau RWT. cAMP-dependent protein kinase regulates desensitization of the capsaicin receptor (VR1) by direct phosphorylation. Neuron. 2002;35:721–731. doi: 10.1016/s0896-6273(02)00802-4. [DOI] [PubMed] [Google Scholar]
- Bregman DB, Bhattacharyya N, Rubin CS. High affinity binding protein for the regulatory subunit of cAMP-dependent protein kinase II-B. Cloning, characterization, and expression of cDNAs for rat brain P150. J Biol Chem. 1989;264:4648–4656. [PubMed] [Google Scholar]
- Byers MR, Narhi MVO. Nerve supply of the pulpodentin complex and responses to injury. In: Hargreaves KM, Goodis HE, editors. Dental Pulp. Quintessence Publishing Co., Inc; Carol Stream, IL: 2002. pp. 151–179. [Google Scholar]
- Carr DW, Stofko-Hahn RE, Fraser ID, Cone RD, Scott JD. Localization of the cAMP-dependent protein kinase to the postsynaptic densities by A-kinase anchoring proteins. Characterization of AKAP 79. J Biol Chem. 1992;267:16816–16823. [PubMed] [Google Scholar]
- Caterina MJ, Schumacher MA, Tominaga M, Rosen TA, Levine JD, Julius D. The capsaicin receptor: a heat-activated ion channel in the pain pathway. Nature. 1997;389:816–824. doi: 10.1038/39807. [DOI] [PubMed] [Google Scholar]
- Caterina MJ, Leffler A, Malmberg AB, Martin WJ, Trafton J, Petersen-Zeitz KR, Koltzenburg M, Basbaum AI, Julius D. Impaired nociception and pain sensation in mice lacking the capsaicin receptor. Science. 2000;288:306–313. doi: 10.1126/science.288.5464.306. [DOI] [PubMed] [Google Scholar]
- Cavanaugh EJ, Simkin D, Kim D. Activation of transient receptor potential A1 channels by mustard oil, tetrahydrocannabinol and Ca(2+) reveals different functional channel states. Neuroscience. 2008;154:1467–1476. doi: 10.1016/j.neuroscience.2008.04.048. [DOI] [PubMed] [Google Scholar]
- Charrua A, Cruz CD, Cruz F, Avelino A. Transient receptor potential vanilloid subfamily 1 is essential for the generation of noxious bladder input and bladder overactivity in cystitis. J Urol. 2007;177:1537–1541. doi: 10.1016/j.juro.2006.11.046. [DOI] [PubMed] [Google Scholar]
- Chuang HH, Prescott ED, Kong H, Shields S, Jordt SE, Basbaum AI, Chao MV, Julius D. Bradykinin and nerve growth factor release the capsaicin receptor from PtdIns (4,5)P2-mediated inhibition. Nature. 2001;411:957–962. doi: 10.1038/35082088. [DOI] [PubMed] [Google Scholar]
- Clapham DE. TRP channels as cellular sensors. Nature. 2003;426:517–524. doi: 10.1038/nature02196. [DOI] [PubMed] [Google Scholar]
- Coghlan VM, Perrino BA, Howard M, Langeberg LK, Hicks JB, Gallatin WM, Scott JD. Association of protein kinase A and protein phosphatase 2B with a common anchoring protein. Science. 1995;267:108–111. doi: 10.1126/science.7528941. [DOI] [PubMed] [Google Scholar]
- Colburn RW, Lubin ML, Stone DJ, Jr, Wang Y, Lawrence D, D’Andrea MR, Brandt MR, Liu Y, Flores CM, Qin N. Attenuated cold sensitivity in TRPM8 null mice. Neuron. 2007;54:379–386. doi: 10.1016/j.neuron.2007.04.017. [DOI] [PubMed] [Google Scholar]
- Colledge M, Scott JD. AKAPs: from structure to function. Trends Cell Biol. 1999;9:216–221. doi: 10.1016/s0962-8924(99)01558-5. [DOI] [PubMed] [Google Scholar]
- Colledge M, Dean RA, Scott GK, Langeberg LK, Huganir RL, Scott JD. Targeting of PKA to glutamate receptors through a MAGUK–AKAP complex. Neuron. 2000;27:107–119. doi: 10.1016/s0896-6273(00)00013-1. [DOI] [PubMed] [Google Scholar]
- Corey DP, Garcia-Anoveros J, Holt JR, Kwan KY, Lin SY, Vollrath MA, Amalfitano A, Cheung EL, Derfler BH, Duggan A, Geleoc GS, Gray PA, Hoffman MP, Rehm HL, Tamasauskas D, Zhang DS. TRPA1 is a candidate for the mechanosensitive transduction channel of vertebrate hair cells. Nature. 2004;432:723–730. doi: 10.1038/nature03066. [DOI] [PubMed] [Google Scholar]
- Crandall M, Kwash J, Yu W, White G. Activation of protein kinase C sensitizes human VR1 to capsaicin and to moderate decreases in pH at physiological temperatures in Xenopus oocytes. Pain. 2002;98:109–117. doi: 10.1016/s0304-3959(02)00034-9. [DOI] [PubMed] [Google Scholar]
- Cruz-Orengo L, Dhaka A, Young TJ, Montana MC, Cavanaugh EJ, Kim D, Story GM. Cutaneous nociception evoked by 15-delta PGJ2 via activation of ion channel TRPA1. Mol Pain. 2008;4:30. doi: 10.1186/1744-8069-4-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dai Y, Wang S, Tominaga M, Yamamoto S, Fukuoka T, Higashi T, Kobayashi K, Obata K, Yamanaka H, Noguchi K. Sensitization of TRPA1 by PAR2 contributes to the sensation of inflammatory pain. J Clin Invest. 2007;117:1979–1987. doi: 10.1172/JCI30951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darian-Smith I, Johnson KO, Dykes R. “Cold” fiber population innervating palmar and digital skin of the monkey: responses to cooling pulses. J Neurophysiol. 1973;36:325–346. doi: 10.1152/jn.1973.36.2.325. [DOI] [PubMed] [Google Scholar]
- Davis KD. Cold-induced pain and prickle in the glabrous and hairy skin. Pain. 1998;75:47–57. doi: 10.1016/S0304-3959(97)00203-0. [DOI] [PubMed] [Google Scholar]
- Dell’Acqua ML, Faux MC, Thorburn J, Thorburn A, Scott JD. Membrane-targeting sequences on AKAP79 bind phosphatidylinositol-4, 5-bisphosphate. EMBO J. 1998;17:2246–2260. doi: 10.1093/emboj/17.8.2246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhaka A, Murray AN, Mathur J, Earley TJ, Petrus MJ, Patapoutian A. TRPM8 is required for cold sensation in mice. Neuron. 2007;54:371–378. doi: 10.1016/j.neuron.2007.02.024. [DOI] [PubMed] [Google Scholar]
- Distler C, Rathee PK, Lips KS, Obreja O, Neuhuber W, Kress M. Fast Ca2+-induced potentiation of heat-activated ionic currents requires cAMP/PKA signaling and functional AKAP anchoring. J Neurophysiol. 2003;89:2499–2505. doi: 10.1152/jn.00713.2002. [DOI] [PubMed] [Google Scholar]
- Docherty RJ, Yeats JC, Bevan S, Boddeke HW. Inhibition of calcineurin inhibits the desensitization of capsaicin-evoked currents in cultured dorsal root ganglion neurones from adult rats. Pflugers Arch. 1996;431:828–837. doi: 10.1007/s004240050074. [DOI] [PubMed] [Google Scholar]
- Doerner JF, Gisselmann G, Hatt H, Wetzel CH. Transient receptor potential channel A1 is directly gated by calcium ions. J Biol Chem. 2007;282:13180–13189. doi: 10.1074/jbc.M607849200. [DOI] [PubMed] [Google Scholar]
- Faisy C, Naline E, Rouget C, Risse PA, Guerot E, Fagon JY, Chinet T, Roche N, Advenier C. Nociceptin inhibits vanilloid TRPV-1-mediated neurosensitization induced by fenoterol in human isolated bronchi. Naunyn-Schmiedeberg’s Arch Pharmacol. 2004;370:167–175. doi: 10.1007/s00210-004-0974-x. [DOI] [PubMed] [Google Scholar]
- Faux MC, Scott JD. Molecular glue: kinase anchoring and scaffold proteins. Cell. 1996;85:9–12. doi: 10.1016/s0092-8674(00)81075-2. [DOI] [PubMed] [Google Scholar]
- Ferreira J, da Silva GL, Calixto JB. Contribution of vanilloid receptors to the overt nociception induced by B2 kinin receptor activation in mice. Br J Pharmacol. 2004;141:787–794. doi: 10.1038/sj.bjp.0705546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forman BM, Chen J, Evans RM. The peroxisome proliferator-activated receptors: ligands and activators. Ann N Y Acad Sci. 1996;804:266–275. doi: 10.1111/j.1749-6632.1996.tb18621.x. [DOI] [PubMed] [Google Scholar]
- Ghilardi JR, Rohrich H, Lindsay TH, Sevcik MA, Schwei MJ, Kubota K, Halvorson KG, Poblete J, Chaplan SR, Dubin AE, Carruthers NI, Swanson D, Kuskowski M, Flores CM, Julius D, Mantyh PW. Selective blockade of the capsaicin receptor TRPV1 attenuates bone cancer pain. J Neurosci. 2005;25:3126–3131. doi: 10.1523/JNEUROSCI.3815-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilroy DW, Colville-Nash PR, Willis D, Chivers J, Paul-Clark MJ, Willoughby DA. Inducible cyclooxygenase may have anti-inflammatory properties. Nat Med. 1999;5:698–701. doi: 10.1038/9550. [DOI] [PubMed] [Google Scholar]
- Harrison JL, Davis KD. Cold-evoked pain varies with skin type and cooling rate: a psychophysical study in humans. Pain. 1999;83:123–135. doi: 10.1016/s0304-3959(99)00099-8. [DOI] [PubMed] [Google Scholar]
- Helliwell RJ, McLatchie LM, Clarke M, Winter J, Bevan S, McIntyre P. Capsaicin sensitivity is associated with the expression of the vanilloid (capsaicin) receptor (VR1) mRNA in adult rat sensory ganglia. Neurosci Lett. 1998;250:177–180. doi: 10.1016/s0304-3940(98)00475-3. [DOI] [PubMed] [Google Scholar]
- Hinman A, Chuang HH, Bautista DM, Julius D. TRP channel activation by reversible covalent modification. Proc Natl Acad Sci U S A. 2006;103:19564–19568. doi: 10.1073/pnas.0609598103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hjerling-Leffler J, Alqatari M, Ernfors P, Koltzenburg M. Emergence of functional sensory subtypes as defined by transient receptor potential channel expression. J Neurosci. 2007;27:2435–2443. doi: 10.1523/JNEUROSCI.5614-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu HJ, Bhave G, Gereau RW. Prostaglandin and protein kinase A-dependent modulation of vanilloid receptor function by metabotropic glutamate receptor 5: potential mechanism for thermal hyperalgesia. J Neurosci. 2002;22:7444–7452. doi: 10.1523/JNEUROSCI.22-17-07444.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaquemar D, Schenker T, Trueb B. An ankyrin-like protein with transmembrane domains is specifically lost after oncogenic transformation of human fibroblasts. J Biol Chem. 1999;274:7325–7333. doi: 10.1074/jbc.274.11.7325. [DOI] [PubMed] [Google Scholar]
- Jeske NA, Diogenes A, Ruparel NB, Fehrenbacher JC, Henry M, Akopian AN, Hargreaves KM. A-kinase anchoring protein mediates TRPV1 thermal hyperalgesia through PKA phosphorylation of TRPV1. Pain. 2008;138:604–616. doi: 10.1016/j.pain.2008.02.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jhamandas K, Yaksh TL, Harty G, Szolcsanyi J, Go VL. Action of intrathecal capsaicin and its structural analogues on the content and release of spinal substance P: selectivity of action and relationship to analgesia. Brain Res. 1984;306:215–225. doi: 10.1016/0006-8993(84)90371-8. [DOI] [PubMed] [Google Scholar]
- Ji G, Zhou S, Carlton SM. Intact Adelta-fibers up-regulate transient receptor potential A1 and contribute to cold hypersensitivity in neuropathic rats. Neuroscience. 2008;154:1054–1066. doi: 10.1016/j.neuroscience.2008.04.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang C, Ting AT, Seed B. PPAR-gamma agonists inhibit production of monocyte inflammatory cytokines. Nature. 1998;391:82–86. doi: 10.1038/34184. [DOI] [PubMed] [Google Scholar]
- Jones RC, 3rd, Otsuka E, Wagstrom E, Jensen CS, Price MP, Gebhart GF. Short-term sensitization of colon mechanoreceptors is associated with long-term hypersensitivity to colon distention in the mouse. Gastroenterology. 2007;133:184–194. doi: 10.1053/j.gastro.2007.04.042. [DOI] [PubMed] [Google Scholar]
- Jordt SE, Bautista DM, Chuang HH, McKemy DD, Zygmut PM, Högestätt ED, Meng ID, Julius D. Mustard oils and cannabinoids excite sensory nerve fibres through the TRP channel ANKTM1. Nature. 2004;427:260–265. doi: 10.1038/nature02282. [DOI] [PubMed] [Google Scholar]
- Jung J, Shin JS, Lee SY, Hwang SW, Koo J, Cho H, Oh U. Phosphorylation of vanilloid receptor 1 by Ca2+/calmodulin-dependent kinase II regulates its vanilloid binding. J Biol Chem. 2004;279:7048–7054. doi: 10.1074/jbc.M311448200. [DOI] [PubMed] [Google Scholar]
- Jyvasjarvi E, Kniffki KD. Cold stimulation of teeth: a comparison between the responses of cat intradental A delta and C fibres and human sensation. J Physiol. 1987;391:193–207. doi: 10.1113/jphysiol.1987.sp016733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karashima Y, Talavera K, Everaerts W, Janssens A, Kwan KY, Vennekens R, Nilius B, Voets T. TRPA1 acts as a cold sensor in vitro and in vivo. Proc Natl Acad Sci USA. 2009;106:1273–1280. doi: 10.1073/pnas.0808487106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katsura H, Obata K, Mizushima T, Yamanaka H, Kobayashi K, Dai Y, Fukuoka T, Tokunaga A, Sakagami M, Noguchi K. Antisense knock down of TRPA1, but not TRPM8, alleviates cold hyperalgesia after spinal nerve ligation in rats. Exp Neurol. 2006;200:112–123. doi: 10.1016/j.expneurol.2006.01.031. [DOI] [PubMed] [Google Scholar]
- Kim D, Cavanaugh EJ. Requirement of a soluble intracellular factor for activation of transient receptor potential A1 by pungent chemicals: role of inorganic polyphosphates. J Neurosci. 2007;27:6500–6509. doi: 10.1523/JNEUROSCI.0623-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim BM, Lee SH, Shim WS, Oh U. Histamine-induced Ca(2+) influx via the PLA(2)/lipoxygenase/TRPV1 pathway in rat sensory neurons. Neurosci Lett. 2004;361:159–162. doi: 10.1016/j.neulet.2004.01.019. [DOI] [PubMed] [Google Scholar]
- Kim D, Cavanaugh E, Simkin D. Inhibition of transient receptor potential A1 by phosphatidylinositol-4,5-bisphosphate. Am J Physiol Cell Physiol. 2008;295:C92–C99. doi: 10.1152/ajpcell.00023.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kindt KS, Viswanath V, Macpherson L, Quast K, Hu H, Patapoutian A, Schafer WR. Caenorhabditis elegans TRPA-1 functions in mechanosensation. Nat Neurosci. 2007;10:568–577. doi: 10.1038/nn1886. [DOI] [PubMed] [Google Scholar]
- Kliewer SA, Lenhard JM, Willson TM, Patel I, Morris DC, Lehmann JM. A prostaglandin J2 metabolite binds peroxisome proliferator-activated receptor gamma and promotes adipocyte differentiation. Cell. 1995;83:813–819. doi: 10.1016/0092-8674(95)90194-9. [DOI] [PubMed] [Google Scholar]
- Kobayashi K, Fukuoka T, Obata K, Yamanaka H, Dai Y, Tokunaga A, Noguchi K. Distinct expression of TRPM8, TRPA1, and TRPV1 mRNAs in rat primary afferent neurons with adelta/c-fibers and colocalization with trk receptors. J Comp Neurol. 2005;493:596–606. doi: 10.1002/cne.20794. [DOI] [PubMed] [Google Scholar]
- Koda H, Minagawa M, Si-Hong L, Mizumura K, Kumazawa T. H1-receptor-mediated excitation and facilitation of the heat response by histamine in canine visceral polymodal receptors studied in vitro. J Neurophysiol. 1996;76:1396–1404. doi: 10.1152/jn.1996.76.3.1396. [DOI] [PubMed] [Google Scholar]
- Kwan KY, Allchorne AJ, Vollrath MA, Christensen AP, Zhang DS, Woolf CJ, Corey DP. TRPA1 contributes to cold, mechanical, and chemical nociception but is not essential for hair-cell transduction. Neuron. 2006;50:277–289. doi: 10.1016/j.neuron.2006.03.042. [DOI] [PubMed] [Google Scholar]
- Lawson SN, Waddell PJ. Soma neurofilament immunoreactivity is related to cell size and fibre conduction velocity in rat primary sensory neurons. J Physiol. 1991;435:41–63. doi: 10.1113/jphysiol.1991.sp018497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee SY, Lee JH, Kang KK, Hwang SY, Choi KD, Oh U. Sensitization of vanilloid receptor involves an increase in the phosphorylated form of the channel. Arch Pharm Res. 2005;28:405–412. doi: 10.1007/BF02977669. [DOI] [PubMed] [Google Scholar]
- Liu B, Zhang C, Qin F. Functional recovery from desensitization of vanilloid receptor TRPV1 requires resynthesis of phosphatidylinositol 4,5-bisphosphate. J Neurosci. 2005;25:4835–4843. doi: 10.1523/JNEUROSCI.1296-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macpherson LJ, Geierstanger BH, Viswanath V, Bandell M, Eid SR, Hwang S, Patapoutian A. The pungency of garlic: activaiton of TRPA1 and TRPV1 in response to allicin. Curr Biol. 2005;15:929–934. doi: 10.1016/j.cub.2005.04.018. [DOI] [PubMed] [Google Scholar]
- Macpherson LJ, Dubin AE, Evans MJ, Marr F, Schultz PG, Cravatt BF, Patapoutian A. Noxious compounds activate TRPA1 ion channels through covalent modification of cysteines. Nature. 2007a;445:541–545. doi: 10.1038/nature05544. [DOI] [PubMed] [Google Scholar]
- Macpherson LJ, Xiao B, Kwan KY, Petrus MJ, Dubin AE, Hwang SW, Cravatt B, Corey DP, Patapoutian A. An ion channel essential for sensing chemical damage. J Neurosci. 2007b;27:11412–11415. doi: 10.1523/JNEUROSCI.3600-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malin SA, Molliver DC, Koerber HR, Cornuet P, Frye R, Albers KM, Davis BM. Glial cell line-derived neurotrophic factor family members sensitize nociceptors in vitro and produce thermal hyperalgesia in vivo. J Neurosci. 2006;26:8588–8599. doi: 10.1523/JNEUROSCI.1726-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKemy DD, Neuhausser WM, Julius D. Identification of a cold receptor reveals a general role for TRP channels in thermosensation. Nature. 2002;416:52–58. doi: 10.1038/nature719. [DOI] [PubMed] [Google Scholar]
- McNamara CR, Mandel-Brehm J, Bautista DM, Siemens J, Deranian KL, Zhao M, Hayward NJ, Chong JA, Julius D, Moran MM, Fanger CM. TRPA1 mediates formalin-induced pain. Proc Natl Acad Sci U S A. 2007;104:13525–13530. doi: 10.1073/pnas.0705924104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohapatra DP, Nau C. Desensitization of capsaicin-activated currents in the vanilloid receptor TRPV1 is decreased by the cyclic AMP-dependent protein kinase pathway. J Biol Chem. 2003;278:50080–50090. doi: 10.1074/jbc.M306619200. [DOI] [PubMed] [Google Scholar]
- Mohapatra DP, Nau C. Regulation of Ca2+-dependent desensitization in the vanilloid receptor TRPV1 by calcineurin and cAMP-dependent protein kinase. J Biol Chem. 2005;280:13424–13432. doi: 10.1074/jbc.M410917200. [DOI] [PubMed] [Google Scholar]
- Montell C. The TRP superfamily of cation channels. Sci STKE. 2005;272:re3. doi: 10.1126/stke.2722005re3. [DOI] [PubMed] [Google Scholar]
- Nagata K, Duggan A, Kumar G, García-Añoveros J. Nociceptor and hair cell transducer properties of TRPA1, a channel for pain and hearing. J Neurosci. 2005;25:4052–4061. doi: 10.1523/JNEUROSCI.0013-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakae K, Saito K, Iino T, Yamamoto N, Wakabayashi M, Yoshikawa S, Matsushima S, Miyashita H, Sugimoto H, Kiba A, Gupta J. A prostacyclin receptor antagonist inhibits the sensitized release of substance P from rat sensory neurons. J Pharmacol Exp Ther. 2005;315:1136–1142. doi: 10.1124/jpet.105.091967. [DOI] [PubMed] [Google Scholar]
- Napimoga MH, Souza GR, Cunha TM, Ferrari LF, Clemente-Napimoga JT, Parada CA, Verri WA, Jr, Cunha FQ, Ferreira SH. 15d-prostaglandin J2 inhibits inflammatory hypernociception: involvement of peripheral opioid receptor. J Pharmacol Exp Ther. 2008a;324:313–321. doi: 10.1124/jpet.107.126045. [DOI] [PubMed] [Google Scholar]
- Napimoga MH, Vieira SM, Dal-Secco D, Freitas A, Souto FO, Mestriner FL, Alves-Filho JC, Grespan R, Kawai T, Ferreira SH, Cunha FQ. Peroxisome proliferator-activated receptor-gamma ligand, 15-deoxy-Delta12,14-prostaglandin J2, reduces neutrophil migration via a nitric oxide pathway. J Immunol. 2008b;180:609–617. doi: 10.4049/jimmunol.180.1.609. [DOI] [PubMed] [Google Scholar]
- Numazaki M, Tominaga T, Toyooka H, Tominaga M. Direct phosphorylation of capsaicin receptor VR1 by protein kinase Cepsilon and identification of two target serine residues. J Biol Chem. 2002;277:13375–13378. doi: 10.1074/jbc.C200104200. [DOI] [PubMed] [Google Scholar]
- Obata K, Katsura H, Mizushima T, Yamanaka H, Kobayashi K, Dai Y, Fukuoka T, Tokunaga A, Tominaga M, Noguchi K. TRPA1 induced in sensory neurons contributes to cold hyperalgesia after inflammation and nerve injury. J Clin Invest. 2005;115:2393–2401. doi: 10.1172/JCI25437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohta T, Ikemi Y, Murakami M, Imagawa T, Otsuguro K, Ito S. Potentiation of transient receptor potential V1 functions by the activation of metabotropic 5-HT receptors in rat primary sensory neurons. J Physiol. 2006;576:809–822. doi: 10.1113/jphysiol.2006.112250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pareek TK, Keller J, Kesavapany S, Agarwal N, Kuner R, Pant HC, Iadarola MJ, Brady RO, Kulkarni AB. Cyclin-dependent kinase 5 modulates nociceptive signaling through direct phosphorylation of transient receptor potential vanilloid 1. Proc Natl Acad Sci U S A. 2007;104:660–665. doi: 10.1073/pnas.0609916104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peier AM, Moqrich A, Hergarden AC, Reeve AJ, Andersson DA, Story GM, Earley TJ, Dragoni I, McIntyre P, Bevan S, Patapoutian A. A TRP channel that senses cold stimuli and menthol. Cell. 2002;108:705–715. doi: 10.1016/s0092-8674(02)00652-9. [DOI] [PubMed] [Google Scholar]
- Petrus M, Peier AM, Bandell M, Hwang SW, Huynh T, Olney N, Jegla T, Patapoutian A. A role of TRPA1 in mechanical hyperalgesia is revealed by pharmacological inhibition. Mol Pain. 2007;3:40. doi: 10.1186/1744-8069-3-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pogatzki-Zahn EM, Shimizu I, Caterina M, Raja SN. Heat hyperalgesia after incision requires TRPV1 and is distinct from pure inflammatory pain. Pain. 2005;115:296–307. doi: 10.1016/j.pain.2005.03.010. [DOI] [PubMed] [Google Scholar]
- Proudfoot CJ, Garry EM, Cottrell DF, Rosie R, Anderson H, Robertson DC, Fleetwood-Walker SM, Mitchell R. Analgesia mediated by the TRPM8 cold receptor in chronic neuropathic pain. Curr Biol. 2006;16:1591–1605. doi: 10.1016/j.cub.2006.07.061. [DOI] [PubMed] [Google Scholar]
- Rathee PK, Distler C, Obreja O, Neuhuber W, Wang GK, Wang SY, Nau C, Kress M. PKA/AKAP/VR-1 module: a common link of Gs-mediated signaling to thermal hyperalgesia. J Neurosci. 2002;22:4740–4745. doi: 10.1523/JNEUROSCI.22-11-04740.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ricote M, Li AC, Willson TM, Kelly CJ, Glass CK. The peroxisome proliferator-activated receptor-gamma is a negative regulator of macrophage activation. Nature. 1998;391:79–82. doi: 10.1038/34178. [DOI] [PubMed] [Google Scholar]
- Sawada Y, Hosokawa H, Hori A, Matsumura K, Kobayashi S. Cold sensitivity of recombinant TRPA1 channels. Brain Res. 2007;1160:39–46. doi: 10.1016/j.brainres.2007.05.047. [DOI] [PubMed] [Google Scholar]
- Schnizler K, Shutov LP, Van Kanegan MJ, Merrill MA, Nichols B, McKnight GS, Strack S, Hell JW, Usachev YM. Protein kinase A anchoring via AKAP150 is essential for TRPV1 modulation by forskolin and prostaglandin E2 in mouse sensory neurons. J Neurosci. 2002;28 (19):4904–4917. doi: 10.1523/JNEUROSCI.0233-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shu X, Mendell LM. Nerve growth factor acutely sensitizes the response of adult rat sensory neurons to capsaicin. Neurosci Lett. 1999;274:159–162. doi: 10.1016/s0304-3940(99)00701-6. [DOI] [PubMed] [Google Scholar]
- Soares AF, Nosjean O, Cozzone D, D’Orazio D, Becchi M, Guichardant M, Ferry G, Boutin JA, Lagarde M, Géloën A. Covalent binding of 15-deoxy-delta12,14-prostaglandin J2 to PPARgamma. Biochem Biophys Res Commun. 2005;337:521–525. doi: 10.1016/j.bbrc.2005.09.085. [DOI] [PubMed] [Google Scholar]
- Story GM, Peier AM, Reeve AJ, Eid SR, Mosbacher J, Hricik TR, Earley TJ, Hergarden AC, Andersson DA, Hwang SW, McIntyre P, Jegla T, Bevan S, Patapoutian A. ANKTM1, a TRP-like channel expressed in nociceptive neurons, is activated by cold temperatures. Cell. 2003;112:819–829. doi: 10.1016/s0092-8674(03)00158-2. [DOI] [PubMed] [Google Scholar]
- Straus DS, Pascual G, Li M, Welch JS, Ricote M, Hsiang CH, Sengchanthalangsy LL, Ghosh G, Glass CK. 15-deoxy-delta 12,14-prostaglandin J2 inhibits multiple steps in the NF-kappa B signaling pathway. Proc Natl Acad Sci U S A. 2000;97:4844–4849. doi: 10.1073/pnas.97.9.4844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stucky CL, Thayer SA, Seybold VS. Prostaglandin E2 increases the proportion of neonatal rat dorsal root ganglion neurons that respond to bradykinin. Neuroscience. 1996;74:1111–1123. doi: 10.1016/0306-4522(96)00264-3. [DOI] [PubMed] [Google Scholar]
- Takashima Y, Daniels RL, Knowlton W, Teng J, Liman ER, McKemy DD. Diversity in the neural circuitry of cold sensing revealed by genetic axonal labeling of transient receptor potential melastatin 8 neurons. J Neurosci. 2007;27:14147–14157. doi: 10.1523/JNEUROSCI.4578-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor-Clark TE, Undem BJ, Macglashan DW, Jr, Ghatta S, Carr MJ, McAlexander MA. Prostaglandin-induced activation of nociceptive neurons via direct interaction with transient receptor potential A1 (TRPA1) Mol Pharmacol. 2008;73:274–281. doi: 10.1124/mol.107.040832. [DOI] [PubMed] [Google Scholar]
- Tjolsen A, Berge OG, Hunskaar S, Rosland JH, Hole K. The formalin test: an evaluation of the method. Pain. 1992;51:5–17. doi: 10.1016/0304-3959(92)90003-T. [DOI] [PubMed] [Google Scholar]
- Tominaga M, Caterina MJ, Malmberg AB, Rosen TA, Gilbert H, Skinner K, Raumann BE, Basbaum AI, Julius D. The cloned capsaicin receptor integrates multiple pain-producing stimuli. Neuron. 1998;21:531–543. doi: 10.1016/s0896-6273(00)80564-4. [DOI] [PubMed] [Google Scholar]
- Tracey WD, Jr, Wilson RI, Laurent G, Benzer S. painless, a Drosophila gene essential for nociception. Cell. 2003;113:261–273. doi: 10.1016/s0092-8674(03)00272-1. [DOI] [PubMed] [Google Scholar]
- Trevisani M, Siemens J, Materazzi S, Bautista DM, Nassini R, Campi B, Imamachi N, Andre E, Patacchini R, Cottrell GS, Gatti R, Basbaum AI, Bunnett NW, Julius D, Geppetti P. 4-Hydroxynonenal, an endogenous aldehyde, causes pain and neurogenic inflammation through activation of the irritant receptor TRPA1. Proc Natl Acad Sci U S A. 2007;104:13519–13524. doi: 10.1073/pnas.0705923104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uchida K. 4-Hydroxy-2-nonenal: a product and mediator of oxidative stress. Prog Lipid Res. 2003;42:318–343. doi: 10.1016/s0163-7827(03)00014-6. [DOI] [PubMed] [Google Scholar]
- Urade Y, Hayaishi O. Prostaglandin D synthase: structure and function. Vitam Horm. 2000;58:89–120. doi: 10.1016/s0083-6729(00)58022-4. [DOI] [PubMed] [Google Scholar]
- Vellani V, Mapplebeck S, Moriondo A, Davis JB, McNaughton PA. Protein kinase C activation potentiates gating of the vanilloid receptor VR1 by capsaicin, protons, heat and anandamide. J Physiol. 2001;534:813–825. doi: 10.1111/j.1469-7793.2001.00813.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viana F, de la Peña E, Belmonte C. Specificity of cold thermotransduction is determined by differential ionic channel expression. Nat Neurosci. 2002;5:254–260. doi: 10.1038/nn809. [DOI] [PubMed] [Google Scholar]
- Vijayaraghavan S, Goueli SA, Davey MP, Carr DW. Protein kinase A-anchoring inhibitor peptides arrest mammalian sperm motility. J Biol Chem. 1997;272:4747–4752. doi: 10.1074/jbc.272.8.4747. [DOI] [PubMed] [Google Scholar]
- Voets T, Talavera K, Owsianik G, Nilius B. Sensing with TRP channels. Nat Chem Biol. 2005;1:85–92. doi: 10.1038/nchembio0705-85. [DOI] [PubMed] [Google Scholar]
- Wang S, Dai Y, Fukuoka T, Yamanaka H, Kobayashi K, Obata K, Cui X, Tominaga M, Noguchi K. Phospholipase C and protein kinase A mediate bradykinin sensitization of TRPA1: a molecular mechanism of inflammatory pain. Brain. 2008;131:1241–1251. doi: 10.1093/brain/awn060. [DOI] [PubMed] [Google Scholar]
- Willott E, Balda MS, Fanning AS, Jameson B, Van Itallie C, Anderson JM. The tight junction protein ZO-1 is homologous to the Drosophila discs-large tumor suppressor protein of septate junctions. Proc Natl Acad Sci U S A. 1993;90:7834–7838. doi: 10.1073/pnas.90.16.7834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woods DF, Bryant PJ. The discs-large tumor suppressor gene of Drosophila encodes a guanylate kinase homolog localized at septate junctions. Cell. 1991;66:451–464. doi: 10.1016/0092-8674(81)90009-x. [DOI] [PubMed] [Google Scholar]
- Wu ZZ, Chen SR, Pan HL. Transient receptor potential vanilloid type 1 activation down-regulates voltage-gated calcium channels through calcium-dependent calcineurin in sensory neurons. J Biol Chem. 2005;280:18142–18151. doi: 10.1074/jbc.M501229200. [DOI] [PubMed] [Google Scholar]
- Wu C, Boustany L, Liang H, Brennan TJ. Nerve growth factor expression after plantar incision in the rat. Anesthesiology. 2007;107:128–135. doi: 10.1097/01.anes.0000267512.08619.bd. [DOI] [PubMed] [Google Scholar]
- Yokoyama N, Hayashi N, Seki T, Pante N, Ohba T, Nishii K, Kuma K, Hayashida T, Miyata T, Aebi U, et al. A giant nucleopore protein that binds Ran/TC4. Nature. 1995;376:184–188. doi: 10.1038/376184a0. [DOI] [PubMed] [Google Scholar]
- Zhang XF, Chen J, Faltynek CR, Moreland RB, Neelands TR. Transient receptor potential A1 mediates an osmotically activated ion channel. Eur J Neurosci. 2008;27:606–611. doi: 10.1111/j.1460-9568.2008.06030.x. [DOI] [PubMed] [Google Scholar]
- Zhu W, Galoyan SM, Petruska JC, Oxford GS, Mendell LM. A developmental switch in acute sensitization of small dorsal root ganglion (DRG) neurons to capsaicin or noxious heating by NGF. J Neurophysiol. 2004;92:3148–3152. doi: 10.1152/jn.00356.2004. [DOI] [PubMed] [Google Scholar]
- Zurborg S, Yurgionas B, Jira JA, Caspani O, Heppenstall PA. Direct activation of the ion channel TRPA1 by Ca2+ Nat Neurosci. 2007;10:277–279. doi: 10.1038/nn1843. [DOI] [PubMed] [Google Scholar]
- Zygmunt PM, Petersson J, Andersson DA, Chuang H, Sorgard M, Di Marzo V, Julius D, Hogestatt ED. Va nilloid receptors on sensory nerves mediate the vasodilator action of anandamide. Nature. 1999;400:452–457. doi: 10.1038/22761. [DOI] [PubMed] [Google Scholar]