Abstract
Background:
Despite the high incidence of acute stroke, only a minority of patients are enrolled in acute stroke treatment trials. We aimed to identify factors associated with participation in clinical trials of novel therapeutic agents for acute stroke.
Methods:
Prospective survey of patients with acute stroke <72 hours from onset. A structured interview was administered to the patient or primary decision-maker. If offered participation in an actual acute treatment trial, questions focused on decisions about that trial; otherwise a similar mock trial was proposed. The primary outcome was whether the subject agreed to participate in the proposed trial.
Results:
A total of 200 subjects (47% patients, 53% proxies) completed the survey: mean age 63 ± 14 years, 47% women, 44% white, 50% black. A real acute trial was offered to 22%; others were offered a mock trial. Overall, 57% (95% confidence interval: 50%–64%) of respondents stated they would participate in the proposed acute treatment trial. There were no differences with respect to age, sex, race, educational level, self-assessed stroke severity or stroke type, vascular risk factors, or comorbidities. Misconceptions about key research concepts were found in 50% but did not impact participation. Participation was associated with the perceived risk of the proposed trial intervention (p < 0.001), prior general attitudes about research (p < 0.001), and influences attributed to family, religion, and other personal beliefs (p < 0.001). Patients were more likely to participate than proxy decision-makers (p = 0.04).
Conclusions:
Demographic factors, clinical factors, and prior knowledge about research have little impact on the decision to participate in acute stroke clinical trials. Preexisting negative attitudes and external influences about research strongly inhibit participation. Patients are more inclined to participate than their proxy decision-makers.
GLOSSARY
- CI
= confidence interval;
- OR
= odds ratio.
Acute neurologic emergencies such as stroke, traumatic brain injury, status epilepticus, and others must be treated within the first few hours after onset and sometimes within the first few minutes of contact with the health care system. Few proven treatments are available, and the brief therapeutic window to initiate existing therapies poses an enormous challenge.1–5 In many centers, patients with acute neurologic emergencies are offered participation in clinical trials of novel acute therapies. Despite the substantial probability of a poor outcome and the lack of alternative proven therapy, many eligible patients (or their surrogate decision makers) choose not to participate in these trials or are unable to come to a decision during the allotted time. Consequently, trial recruitment is typically quite slow.6,7 Further, the generalizability of a trial may be called into question if only a small fraction of eligible patients actually participate in the trial.8,9
There has been relatively little research related to recruitment of patients into acute, time-sensitive clinical trials. Most existing studies addressed trials in which patients had ample time to consider their options, such as studies of disease prevention or treatment of chronic illness. None have investigated the specific issues related to acute stroke trials. When faced with the very urgent decision about participation in an acute treatment trial, patients or their surrogate decision-makers have little time to consider the many factors involved in such a decision. Moreover, patients with acute neurologic illness are often partially or completely unable to take part in the process due to their impairment.10 It is unclear which factors are most important in making decisions in this context. Gaining an understanding of how patients choose to participate or not and their attitudes toward acute stroke treatment trials may enhance patient recruitment procedures and the informed consent process with the overall goals of reaching optimal participants and improving enrollment.9
METHODS
A survey instrument was developed to gather information including demographic features, level of education, prior general medical knowledge, prior knowledge about stroke, prior research-related experience, and issues related to the stroke itself, including severity, impact on quality of life, and comorbidities. The survey also assessed general attitudes toward clinical trials; external influences on research participation; concerns about study safety and efficacy; and others. The survey instrument was developed into a structured interview and all interviewers were trained in its administration. After assessment of face validity by stroke physicians and nurses, the survey instrument was piloted on several stroke survivors and modified accordingly to improve clarity. The final version is provided in appendix e-1 on the Neurology® Web site at www.neurology.org.
During a 2-year period (2006–2008), all patients admitted to a single urban university hospital with acute ischemic stroke or intracerebral hemorrhage were considered for participation in the survey if the structured interview could be initiated <72 hours of the onset of symptoms, preferably <24 hours of admission. The person who would have made the decision about the patient’s participation in a trial was asked to be the respondent: either the patient if he or she would have been able to consent on his or her own behalf at the time of admission or an appropriate proxy.
For subjects offered the opportunity to participate in an actual acute stroke trial upon admission, the interview focused on that initial decision. For all others, a real trial was not an option because of timing or other eligibility criteria. In those cases, a mock trial (based on prior real trials) was proposed which attempted to replicate the decision-making process as if a real trial had been offered, though subjects were explicitly informed that the mock trials were hypothetical and they were not going to receive any experimental treatment. The real and mock trials were all designed as two-arm double-blinded randomized clinical trials of experimental drug vs placebo with outcomes determined at 90 days. There were two mock trials, each accompanied by mock informed consent forms (appendices e-2 and e-3). The mock trial for ischemic stroke offered an IV antiplatelet agent posing a risk of cerebral and systemic bleeding, and the mock trial for intracerebral hemorrhage offered a procoagulant drug carrying a potential risk of major thromboembolic events. When this study was initiated, the available real trials had similar risk-benefit profiles to these mock trials. However, a subsequent real trial was launched with a relatively low-risk intervention. Consequently, the risks of the proposed real and mock trials were graded by the investigators as low (minor potential adverse effects, no known major adverse effects), medium (known minor adverse effects, known but extremely rare major adverse effects), or high (known risk of life-threatening adverse effects, such as major hemorrhage or thromboembolism) to adjust for this factor. In general, subjects were allotted <30 minutes to make a decision, though occasionally additional time was provided if requested by the respondent and if possible for a real trial.
The primary outcome was whether or not the respondent agreed to participation in the proposed trial. Univariate analysis was performed using t test for continuous variables and χ2 or Fisher exact test for categorical variables, as appropriate. Linear regression was used for tests for trend. Stepwise multivariable logistic regression was performed to explore factors independently associated with participation, selecting candidates from univariate analysis at the p < 0.15 level due to the relatively limited power for multivariable analysis. A series of hierarchical models were created, first incorporating factors related to the subject, then incorporating potential external and modifiable factors. Multivariable models were adjusted for trial risk. Statistical analyses were performed using Stata 10 (Stata Corp, College Station, TX).
We estimated that about half of the subjects would agree to participate in the proposed acute stroke trial. We therefore planned a sample size of 200 subjects in order to provide 80% power (at alpha = 0.05) to detect a 20% absolute difference in the likelihood of trial participation between subgroups representing about half of the overall population. A ≥20% difference between subgroups was deemed by the investigators to be large enough to potentially merit changes in consent procedures or policies.
This study was reviewed and approved by the local Institutional Review Board. All subjects verbally consented to participation in the survey.
RESULTS
During the study period, 297 subjects were screened to take part in this study. Of these, 97 were not included: 36 had no suitable respondent available, 9 stated they were too busy with testing or treatment, 9 were too ill (i.e., moribund, on hospice, family too distraught), 19 stated only that they were not interested, and 24 declined without stating a reason.
The survey was completed by 200 subjects: 94 (47%) were the stroke patients themselves and 106 (53%) were proxy decision-makers (37 spouses, 51 children, 7 siblings, 11 others). A proxy decision-maker was more likely to be the respondent when the stroke was severe (p < 0.001).
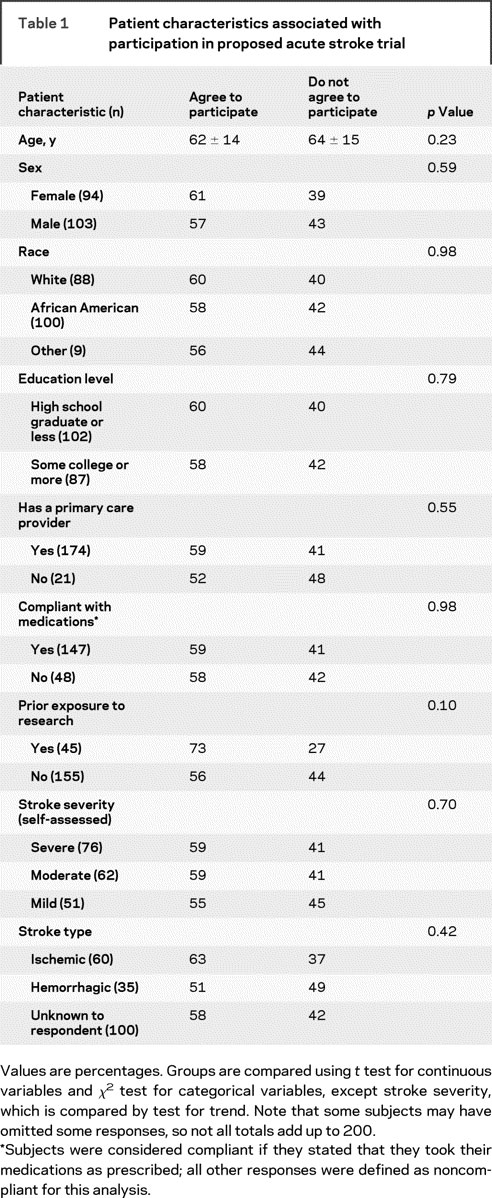
Overall, 57% (95% confidence interval: 50%–64%) of respondents stated that they would participate in an acute treatment trial. Table 1 compares the characteristics of participants vs nonparticipants. There were no differences with respect to demographic factors or access to health care. There were also no differences based on prior medical history, including diabetes mellitus, hypertension, hyperlipidemia, prior stroke, coronary artery disease, peripheral vascular disease, or smoking (p values 0.40–0.97, data not shown). There was a trend toward prior personal or family research experience being associated with a greater likelihood of trial participation. There were no differences based on self-assessed stroke severity or the patient’s understanding of his or her stroke type.
Table 1 Patient characteristics associated with participation in proposed acute stroke trial
The minority (19%) of subjects offered a low/medium risk trial were much more likely to participate than the majority (81%) offered a high risk trial (92% vs 50%; p < 0.001). A real trial was offered to 44 (22%) and the remainder were offered a mock trial. There were no differences between those offered real vs mock trials with respect to demographic characteristics, stroke severity, or type (p values 0.22–0.73, data not shown). After adjustment for trial risk, there was no difference in the likelihood of participating in the real and mock trials (p = 0.19). For those offered a real trial, there was no difference in response between patients and proxies (83% vs 89%, p = 0.60). For those offered a mock trial, patients favored participation more than their proxies (59% vs 40%, p = 0.040). Further, patients differed from proxies more with high risk (60% vs 41%, p = 0.017) than with low risk trial participation (93% vs 92%, p = 1.0). The time allotted to make a decision was <30 minutes for 85% of respondents. The amount of time was deemed sufficient by 56%, insufficient by 38%, and no opinion in the remainder. Subjects offered a real trial were more likely to report sufficient time for their decisions than those offered a mock trial (76% vs 54%, p = 0.011). Subjects tended to participate in more if they felt that they had enough time (64% vs 53%, p = 0.12).
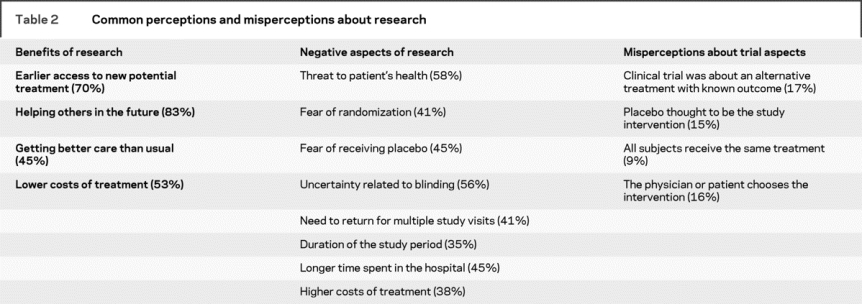
Nearly half of respondents (45%) had heard of negative consequences related to clinical trials but this had no impact on their decision to participate (58% vs 59%, p = 0.88). A total of 63% reported hearing of benefits related to trials and those respondents were more likely to participate (65% vs 35%, p = 0.024). These groups overlapped, as 33% of respondents were aware of both. Potential perceived benefits and negative aspects of participation are summarized in table 2. Advertisements or announcements about trial participation had been noticed by 75% of subjects, but these respondents were not more inclined to participate (56% vs 67%, p = 0.20).
Table 2 Common perceptions and misperceptions about research
General attitude about clinical trials was rated as very positive by 5%, positive by 45%, neutral by 44%, negative by 5%, and very negative by 2% of subjects. Attitude was not associated with any demographic characteristic, but was strongly associated with participation in the proposed stroke trial (p for trend <0.001). Subjects who were very positive or positive were much more likely to participate than those with stated neutral or negative attitudes (76% vs 42%, p < 0.001).
Misconceptions about key trial aspects were prevalent, even after reading the trial informed consent form and making a decision about participation, as summarized in table 2. In total, 48% of respondents made at least one of these specific errors. Neither age, sex, nor educational level was associated with misconceptions, but white subjects had fewer misconceptions than nonwhite subjects (34% vs 59%, p = 0.001 unadjusted; p = 0.003 adjusted for age, sex, and educational level). Respondents with prior research exposure were less likely to have a misconception than those who had no prior experience (33% vs 51%; p = 0.037). Having a misconception about research did not impact the decision to participate in the proposed stroke trial (57% vs 59%, p = 0.78).
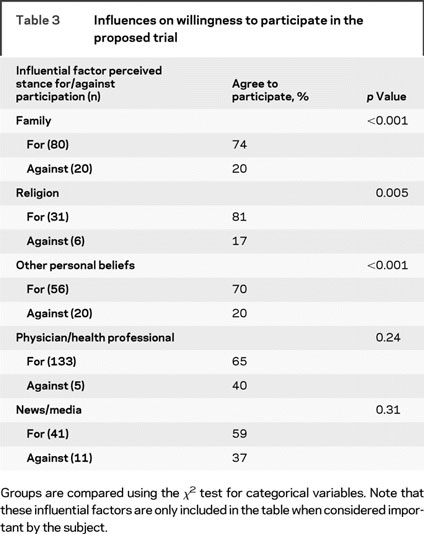
Influential factors in the decisions about participation in the proposed trial included family, religion, other unspecified personal beliefs, health professionals, and news/media. The impact of these factors on the decision is summarized in table 3, only for those who cited them as important. Overall, 20% of respondents cited at least one of these to be both important and against trial participation, and this group was much less likely to agree to participate (20% vs 68%, p < 0.001).
Table 3 Influences on willingness to participate in the proposed trial
Subjects offered a real trial commonly discussed the choice with others, usually with family (74%) or with a treating physician or nurse not part of the investigational team (58%). Only a minority (10%) of subjects offered a mock trial included others in their decision-making process. Discussion with others did not influence participation (p = 0.29). Overall, a decision was made solely by 72% of respondents without discussion with others. However, of this latter group, 57% would have liked to confer with family if given the opportunity, 51% with the treating physician or nurse, and 4% with clergy.
In a multivariable model incorporating subject characteristics and adjusted for trial risk, response by the patient rather than a proxy-decision maker increased the likelihood of participation (odds ratio [OR] 2.10, 95% confidence interval [CI] 1.04–4.3; p = 0.040). In a model then incorporating prior attitudes and perceived influences, preexisting negative attitudes or external influences dramatically reduced participation (OR 0.18, 95% CI 0.09–0.36; p < 0.001) and attenuated the patient vs proxy effect (OR 1.85, 95% CI 0.95–3.6; p = 0.069). No modifiable factor related to the consent process, such as discussing the trial with others or giving sufficient time to make the decision, was significantly associated with the decision when including the above factors in the model. The model characteristics include a Pearson goodness-of-fit test p = 0.87 and a C-statistic of 0.80.
DISCUSSION
Clinical trials involving patients with acute neurologic injury pose numerous challenges that are familiar to investigators, most notably narrow therapeutic time windows, cognitively impaired subjects, complex eligibility criteria, and frequent comorbidities. Patients and their decision-makers likely have an entirely different perspective, with a substantial amount of uncertainty about the treatment, fear about their future, and too little time to process all of the information needed for their decisions, but these issues have not been well characterized. In this study of 200 ischemic stroke and ICH patients and their proxy decision-makers, we found that more than half would be willing to participate in an acute treatment trial. Our study population was diverse and reflective of the greater Philadelphia population and encompassed a wide range of stroke severity. There appeared to be no impact of demographic characteristics or access to health care on trial participation. Previous studies of chronic illness and prevention trials are inconsistent but suggest a possible influence of age, sex, race/ethnicity, and educational level.11–20 In our study, we were unable to identify any such factors associated with trial consent. Further, we expected that subjects with more severe strokes would be more inclined to participate in the face of a more dire prognosis, but could not find any relationship between participation and stroke severity. However, not surprisingly, participation was inversely related to the risk of the proposed trial intervention.
In our study, patients themselves were much more inclined to participate than their proxies, even in the face of greater potential risk from the trial intervention. Prior studies in less urgent circumstances have shown that patients and family members varied substantially in such decisions.21 Patients are likely more worried about the impact of the stroke itself on their well-being and are willing to take risks to improve it, while proxies may be more concerned about choosing to expose the patient to an unproven intervention.21–22 Since surrogate consent in acute stroke research may account for the majority of participants,23 it may be reasonable to inform families that patients tend to favor participation in the face of uncertainty. On the other hand, prior research has suggested that surrogates may overenroll compared to patients themselves in the setting of other acute illness.24 Ultimately, the goal of surrogate consent is beneficence on behalf of the patient.
Many subjects had some awareness about research at the time of the survey and many had preexisting perspectives and attitudes. Prior positive experiences and attitudes had a modestly favorably impact upon participation and may have been attributable to pro-research media exposures. In prevention and chronic disease trials, frequently cited motivators for participation are altruism and perceived personal benefit.11,25 From the few studies that discuss why patients choose not to participate, negative external influences from family or physician and personal fears have been frequently noted as reasons for refusal.11,26 Those who choose to participate or not tend to be attitudinally different in other aspects of their medical treatment, and also differ with regard to medical knowledge and education.27 We similarly found that prior negative attitudes markedly impeded trial enrollment, but did not correlate with educational level or any other demographic feature. These negative attitudes may be amenable to public advocacy and education campaigns.
Frankly erroneous ideas about key research concepts were common. Although these did not impact participation, this finding nevertheless suggests that the informed consent process could be unclear or overwhelming in the setting of an acute stroke despite the best intentions of study personnel and Institutional Review Boards. Others have shown that potential participants in non-acute trials are often satisfied with the consent process despite numerous inaccurate perceptions about research.18,28–29 Improvements in the consent process that address these misconceptions and inadequacies are needed.
Subjects frequently felt that they had insufficient time to make their decision, and this modestly hindered participation. Urgency is essential in acute stroke research, but it seems important that potential study subjects not feel too hurried in their decisions. In a study involving pediatric perioperative research, parents similarly were less likely to consent if they perceived insufficient time for their decisions.30 Further, many subjects in our study wished they had more time and more opportunity to discuss the choice with others, and prior research has suggested that support for research by doctors and family may improve enrollment.18 Given the short time window for many acute stroke trials, this will be challenging, but providing a limited amount of additional time and an effort to bring families and prior trusted care providers into the decision-making process, in person or by telephone if necessary, has the potential to augment participation.
This study has a number of limitations. The study population came from a single academic center and may not be generalizable to other regions or hospital types. However, the study population was relatively large and diverse with respect to major demographic characteristics. About one-third of screened subjects did not take part in our study. This was usually due to competing interests such as tests and treatments or grief related to the stroke. We cannot be certain that subjects abjectly against all research, even a survey, were fully represented. We do know that many subjects were willing and able to express their negative attitudes and decline participation in the proposed trials. Finally, the majority of subjects were offered a mock trial rather than a real trial, and the hypothetical situation may not directly translate into reality. Prior research has suggested that willingness to participate in a hypothetical trial is a valid predictor of actual participation,31 and that assessing potential enrollment preferences in hypothetical situations rather than in dire emergencies might better reflect patients’ genuine interests.9 It is nonetheless reassuring that subjects offered real vs mock trials made similar decisions after accounting for the risks of the proposed trials.
AUTHOR CONTRIBUTIONS
Statistical analysis was performed by Scott E. Kasner, MD, MSCE.
ACKNOWLEDGMENT
The authors thank Joshua Glick, Julianne Bishop, Richard Guyer, and Colleen Pellegrini for performing subject interviews for this study and MaryElizabeth DeSanto for administrative and regulatory assistance.
DISCLOSURE
This study was supported in part by an unrestricted grant from Merck. Neither Merck nor its representatives had any role in the design or conduct of the study; analysis or interpretation of the results; or preparation, review, or approval of the manuscript. Scott E. Kasner has received grant support from the National Institutes of Health, the American Heart Association, Photothera, NovoNordisk, Sanofi-Aventis, NMT Medical, Boehringer-Ingelheim, Merck (for this project), and WL Gore; has served on endpoint adjudication committees for Wyeth and Novartis; and has received honoraria for consulting with AstraZeneca, WL Gore, and Daiichi-Sankyo. Jean M. Luciano has received honoraria for speaking from Genentech. Brett L. Cucchiara has received honoraria for speaking from Boehringer-Ingelheim; has served as a consultant to Novo-Nordisk, Daiichi-Sankyo, and Wyeth; and has received grant support from the National Institutes of Health, the American Heart Association, and Vernalis. Steven R. Messé has received grant support from the American Heart Association and the National Institutes of Health. Lauren H. Sansing has received grant support from the National Institutes of Health. Jill M. Baren has received grant support from the National Institutes of Health and consulting honoraria from NovoNordisk.
Supplementary Material
Address correspondence and reprint requests to Dr. Scott Eric Kasner, University of Pennsylvania Medical Center, 3 West Gates Building, 3400 Spruce Street, Philadelphia, PA 19104 kasner@mail.med.upenn.edu
Supplemental data at www.neurology.org
Disclosure: Author disclosures are provided at the end of the article.
Received December 3, 2008. Accepted in final form February 12, 2009.
REFERENCES
- 1.The National Institute of Neurological Disorders and Stroke rt-PA Stroke Study Group. Tissue plasminogen activator for acute ischemic stroke. N Engl J Med 1995;333:1581–1587. [DOI] [PubMed] [Google Scholar]
- 2.Furlan A, Higashida R, Wechsler L, et al. Intra-arterial prourokinase for acute ischemic stroke: The PROACT II study: a randomized controlled trial. JAMA 1999;282:2003–2011. [DOI] [PubMed] [Google Scholar]
- 3.Smith WS, Sung G, Starkman S, et al. Safety and efficacy of mechanical embolectomy in acute ischemic stroke: results of the MERCI Trial. Stroke 2005;36:1432–1438. [DOI] [PubMed] [Google Scholar]
- 4.Kalviainen R. Status epilepticus treatment guidelines. Epilepsia 2007;48 suppl 8:99–102. [DOI] [PubMed] [Google Scholar]
- 5.Katzan IL, Hammer MD, Hixson ED, Furlan AJ, Abou-Chebl A, Nadzam DM. Utilization of intravenous tissue plasminogen activator for acute ischemic stroke. Arch Neurol 2004;61:346–350. [DOI] [PubMed] [Google Scholar]
- 6.Alexandrov AV. Slow recruitment in clinical trials: failure is not an option! Int J Stroke 2006;1:160. [DOI] [PubMed] [Google Scholar]
- 7.Elkins JS, Khatabi T, Fung L, Rootenberg J, Johnston SC. Recruiting subjects for acute stroke trials: a meta-analysis. Stroke 2006;37:123–128. [DOI] [PubMed] [Google Scholar]
- 8.Kramer MS, Shapiro SH. Scientific challenges in the application of randomized trials. JAMA 1984;252:2739–2745. [PubMed] [Google Scholar]
- 9.Halpern SD. Prospective preference assessment: a method to enhance the ethics and efficiency of randomized controlled trials. Control Clin Trials 2002;23:274–288. [DOI] [PubMed] [Google Scholar]
- 10.Dani KA, McCormick MT, Muir KW. Brain lesion volume and capacity for consent in stroke trials: potential regulatory barriers to the use of surrogate markers. Stroke 2008;39:2336–2340. [DOI] [PubMed] [Google Scholar]
- 11.Corbie-Smith G, Viscoli CM, Kernan WN, Brass LM, Sarrel P, Horwitz RI. Influence of race, clinical, and other socio-demographic features on trial participation. J Clin Epidemiol 2003;56:304–309. [DOI] [PubMed] [Google Scholar]
- 12.Carey MP, Senn TE, Vanable PA, Coury-Doniger P, Urban MA. Do STD clinic patients who consent to sexual health research differ from those who decline? Findings from a randomized controlled trial with implications for the generalization of research results. Sex Transm Dis 2008;35:73–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Peterson ED, Lytle BL, Biswas MS, Coombs L. Willingness to participate in cardiac trials. Am J Geriatr Cardiol 2004;13:11–15. [DOI] [PubMed] [Google Scholar]
- 14.Patel A, Wilke HJ 2nd, Mingay D, Ellis JE. Patient attitudes toward granting consent to participate in perioperative randomized clinical trials. J Clin Anesth 2004;16:426–434. [DOI] [PubMed] [Google Scholar]
- 15.Baren JM, Anicetti JP, Ledesma S, Biros MH, Mahabee-Gittens M, Lewis RJ. An approach to community consultation prior to initiating an emergency research study incorporating a waiver of informed consent. Acad Emerg Med 1999;6:1210–1215. [DOI] [PubMed] [Google Scholar]
- 16.McClure KB, DeIorio NM, Gunnels MD, Ochsner MJ, Biros MH, Schmidt TA. Attitudes of emergency department patients and visitors regarding emergency exception from informed consent in resuscitation research, community consultation, and public notification. Acad Emerg Med 2003;10:352–359. [DOI] [PubMed] [Google Scholar]
- 17.Ding EL, Powe NR, Manson JE, Sherber NS, Braunstein JB. Sex differences in perceived risks, distrust, and willingness to participate in clinical trials: a randomized study of cardiovascular prevention trials. Arch Intern Med 2007;167:905–912. [DOI] [PubMed] [Google Scholar]
- 18.Sen Biswas M, Newby LK, Bastian LA, Peterson ED, Sugarman J. Who refuses enrollment in cardiac clinical trials? Clin Trials 2007;4:258–263. [DOI] [PubMed] [Google Scholar]
- 19.Halpern SD, Karlawish JH, Casarett D, Berlin JA, Townsend RR, Asch DA. Hypertensive patients’ willingness to participate in placebo-controlled trials: implications for recruitment efficiency. Am Heart J 2003;146:985–992. [DOI] [PubMed] [Google Scholar]
- 20.Glickman SW, Anstrom KJ, Lin L, et al. Challenges in enrollment of minority, pediatric, and geriatric patients in emergency and acute care clinical research. Ann Emerg Med 2008;51:775–780. [DOI] [PubMed] [Google Scholar]
- 21.Ciroldi M, Cariou A, Adrie C, et al. Ability of family members to predict patient’s consent to critical care research. Intens Care Med 2007;33:807–813. [DOI] [PubMed] [Google Scholar]
- 22.Blixen CE, Agich GJ. Stroke patients’ preferences and values about emergency research. J Med Ethics 2005;31:608–611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Flaherty ML, Karlawish J, Khoury JC, Kleindorfer D, Woo D, Broderick JP. How important is surrogate consent for stroke research? Neurology 2008;71:1566–1571. [DOI] [PubMed] [Google Scholar]
- 24.Coppolino M, Ackerson L. Do surrogate decision makers provide accurate consent for intensive care research? Chest 2001;119:603–612. [DOI] [PubMed] [Google Scholar]
- 25.Cassileth BR, Lusk EJ, Miller DS, Hurwitz S. Attitudes toward clinical trials among patients and the public. JAMA 1982;248:968–970. [PubMed] [Google Scholar]
- 26.Madsen S, Holm S, Riis P. Ethical aspects of clinical trials: the attitudes of the public and out-patients. J Intern Med 1999;245:571–579. [DOI] [PubMed] [Google Scholar]
- 27.Llewellyn-Thomas HA, McGreal MJ, Thiel EC, Fine S, Erlichman C. Patients’ willingness to enter clinical trials: measuring the association with perceived benefit and preference for decision participation. Soc Sci Med 1991;32:35–42. [DOI] [PubMed] [Google Scholar]
- 28.Lavori PW, Wilt TJ, Sugarman J. Quality assurance questionnaire for professionals fails to improve the quality of informed consent. Clin Trials 2007;4:638–649. [DOI] [PubMed] [Google Scholar]
- 29.Sugarman J, Lavori PW, Boeger M, et al. Evaluating the quality of informed consent. Clin Trials 2005;2:34–41. [DOI] [PubMed] [Google Scholar]
- 30.Tait AR, Voepel-Lewis T, Malviya S. Factors that influence parents’ assessments of the risks and benefits of research involving their children. Pediatrics 2004;113:727–732. [DOI] [PubMed] [Google Scholar]
- 31.Halpern SD, Metzger DS, Berlin JA, Ubel PA. Who will enroll? Predicting participation in a phase II AIDS vaccine trial J Acquir Immune Defic Syndr 2001;27:281–288. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


