Abstract
Low vitamin K status is associated with low BMD and increased fracture risk. Additionally, a specific menaquinone, menatetrenone (MK4), may reduce fracture risk. However, whether vitamin K plays a role in the skeletal health of North American women remains unclear. Moreover, various K vitamers (e.g., phylloquinone and MK4) may have differing skeletal effects. The objective of this study was to evaluate the impact of phylloquinone or MK4 treatment on markers of skeletal turnover and BMD in nonosteoporotic, postmenopausal, North American women. In this double-blind, placebo-controlled study, 381 postmenopausal women received phylloquinone (1 mg daily), MK4 (45 mg daily), or placebo for 12 mo. All participants received daily calcium and vitamin D3 supplementation. Serum bone-specific alkaline phosphatase (BSALP) and n-telopeptide of type 1 collagen (NTX) were measured at baseline and 1, 3, 6, and 12 mo. Lumbar spine and proximal femur BMD and proximal femur geometry were measured by DXA at baseline and 6 and 12 mo. At baseline, the three treatment groups did not differ in demographics or study endpoints. Compliance with calcium, phylloquinone, and MK4 treatment was 93%, 93%, and 87%, respectively. Phylloquinone and MK4 treatment reduced serum undercarboxylated osteocalcin but did not alter BSALP or NTX. No effect of phylloquinone or MK4 on lumbar spine or proximal femur BMD or proximal femur geometric parameters was observed. This study does not support a role for vitamin K supplementation in osteoporosis prevention among healthy, postmenopausal, North American women receiving calcium and vitamin D supplementation.
Key words: vitamin K, osteoporosis, undercarboxylated osteocalcin, phylloquinone, menatetrenone
INTRODUCTION
Amultitude of observations suggest that vitamin K and the K-dependent proteins may have importance in skeletal health. This was initially suggested by the observation that anticoagulation with warfarin, a compound that produces functional vitamin K deficiency, during pregnancy produced skeletal abnormalities.(1) Because vitamin K is essential for γ-carboxylation of specific glutamic acid residues in a limited number of proteins, it is plausible that inadequate vitamin K status adversely impacts skeletal health. Consistent with this, four vitamin K–dependent proteins (osteocalcin, matrix Gla protein, protein S, and Gas 6) have been identified as components of bone matrix.(2) Additionally, vitamin K inadequacy, if defined as less than maximal γ-carboxylation of osteocalcin, is common.(3–5)
A number of findings have related vitamin K status with bone health. For example, vitamin K treatment may modestly reduce bone resorption,(6) low dietary vitamin K intake is associated with low BMD(7) and increased hip fracture risk,(8,9) and elevations of undercarboxylated osteocalcin (indicating suboptimal vitamin K status) are associated with increased hip fracture risk.(10) Based on the above, it is plausible that unappreciated vitamin K inadequacy commonly contributes to the development of osteoporosis and fracture.(11) In fact, a recent meta-analysis concluded that supplementation with vitamin K (MK4 in most of the studies) reduces bone loss and incident fractures.(12) In contrast, other studies do not identify an effect of vitamin K intake on BMD or fracture risk in women,(13) and long-term vitamin K antagonism with warfarin in nonhuman primates shows no effect on bone turnover or bone mass.(14) Finally, prospective human clinical trials of vitamin K supplementation are discordant with two studies of vitamin K1, calcium, and vitamin D reporting beneficial effects on BMD at some, but not all, sites measured,(15,16) whereas a recent work found no effect.(17) As such, the role of vitamin K in bone health remains unclear.
Two forms of vitamin K occur in nature.(18) The major dietary form is 2-metyl-3-phytyl-1, 4-napthoquinone or phylloquinone (vitamin K1), which is found in vegetables. Additionally, a series of 2-methyl-3-multiprenyl-1,4 naphthoquinones or menaquinones (vitamin K2) are synthesized in bacteria. The majority of menaquinones contain a long side-chain (6–11 prenyl groups) and are prevalent in the lower bowel, but one specific menaquinone, menatetrenone (menaquinone-4 [MK-4]), contains only four prenyl units and can be synthesized in animals from phylloquinone.(19) It is generally accepted that all K vitamers can function as required cofactors for the vitamin K–dependent γ-glutamyl carboxylase enzyme that is responsible for the conversion of specific glutamyl (Glu) to γ-carboxyglutamyl (Gla) residues in a limited number of proteins.(1) Whereas the K1 data are conflicting as noted above, studies of MK4 are more consistent and report preservation of bone mass(20–22) and reduction of osteoporotic fractures in estrogen-deficient Asian women.(23–25) Finally, it has been proposed that MK4 may favorably alter bone quality(26); consistent with this, changes in proximal femur geometry were observed in a 3-yr study of MK4.(27)
Given the above, it is possible that vitamin K supplementation may affect skeletal status in postmenopausal North American women. Furthermore, it is plausible that phylloquinone and MK4 could have differing skeletal effects. Although prior studies have evaluated the effect of phylloquinone(15–17) or MK4(27) on skeletal status, there has been no prior prospective randomized study directly comparing the effect of phylloquinone with MK4 on bone turnover or density in postmenopausal North American women. Results from a prospective study of vitamin K treatment with phylloquinone or MK4 involving 381 postmenopausal women are reported here.
MATERIALS AND METHODS
Subjects and study design
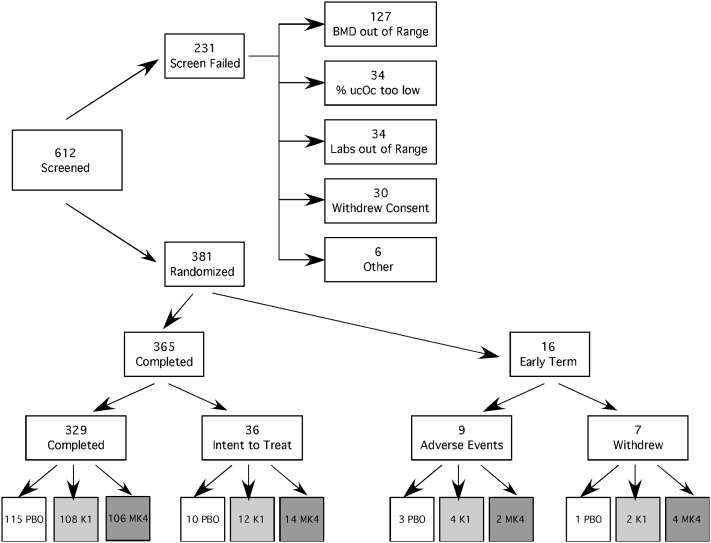
Ambulatory community-dwelling postmenopausal women were recruited for this study. Inclusion criteria required they be ≥5 yr postmenopausal with a lumbar spine and proximal femur T-score above −2.0 or above −1.5 if a National Osteoporosis Foundation (NOF)-defined risk factor was present; as such, they did not meet existing criteria for receipt of pharmacologic osteoporosis therapy. Additionally, to enhance detection of potential vitamin K effects, inclusion criteria required evidence of vitamin K inadequacy, defined as less than maximal osteocalcin carboxylation. The value selected for this criterion was an undercarboxylated osteocalcin (ucOC) ≥4.0%. It should be noted that the value defining “maximal gamma-carboxylation” is that obtained in our laboratory using a hydroxyapatite binding assay and is impacted by the assay methodology used to measure undercarboxylated osteocalcin.(28) Potential volunteers were excluded for use of vitamin K antagonist medications (e.g., warfarin) or skeletally active agents (e.g., bisphosphonates, glucocorticoids, estrogen, calcitonin). Study participants could not have a history of liver or kidney disease or have clinically significant laboratory abnormalities. It was also required that participants have a self-reported low dietary vitamin K intake during the study and abstain from compounds that interfere with fat absorption (e.g., Olestra). Study participant screening and recruitment is shown in Fig. 1.
FIG. 1.
Study participant recruitment.
All participants received open-label calcium with vitamin D3 (315 mg/200 IU; Citracal + D, Mission Pharmacal, San Antonio, TX, USA) twice daily for a two-mo lead-in phase and throughout the study. After this lead in phase, participants were randomly assigned to one of three treatment groups in a double-dummy, double-blind fashion as follows:
Phylloquinone: 1 mg phylloquinone (Roche Vitamins, Parsippany, NJ, USA) daily plus placebo matching vitamin MK4 three times daily. Roche Vitamins supplied the phylloquinone and matching placebo used in this study.
MK4: 15 mg menatetrenone (Eisai, Tokyo, Japan) three times daily plus placebo matching phylloquinone once daily. Eisai supplied the MK4 and matching placebo used in this study.
Placebo phylloquinone daily and placebo MK4 three times daily.
Fasting serum specimens were obtained at time of randomization and subsequently at months 1, 3, 6, and 12 between 8:00 and 11:00 a.m. Blood specimens were allowed to clot for 30 min and were centrifuged, and serum aliquots were quick-frozen using liquid nitrogen. Serum aliquots were stored at −80°C until thawed for analyses. Lumbar spine (L1–L4) and proximal femur BMD measurements were performed using DXA technology at baseline and 6 and 12 mo. Additionally, calcaneal ultrasound was performed on a random subset of study participants (n = 208; 71 placebo, 67 phylloquinone, 70 MK4) at baseline and 12 mo.
Subjects were interviewed about adverse events at every study visit. Severe adverse or life-threatening events, including hospitalization, were immediately reported to the study safety officer. Additionally, all events were compiled and reported to the safety officer every 6 mo. Study compliance was assessed by pill count at all study visits. This study was reviewed and approved by the University of Wisconsin Health Sciences Human Subjects Committee. All participants provided written informed consent.
Biochemical measurements
Chemistry panels were performed at a regional laboratory (Meriter Medical Laboratories, Madison, WI, USA) in a routine clinical manner using a Roche Integra autoanalyzer. Commercially available kits were used to measure bone-specific alkaline phosphatase (BSALP) by immunoassay (Metra BAP; Quidel, San Diego, CA, USA) and n-telopeptide (NTX) by competitive-inhibition ELISA (Osteomark, Seattle, WA, USA). Osteocalcin (Oc) was determined by immunoradiometric assay (Osteo-riact; CIS bio international, Gif-Sur-Yvette Cedex, France), and undercarboxylated osteocalcin (ucOc) percentage was determined by hydroxyapatite binding as previously reported.(29) Intra- and interassay percentage CVs for these analytes in our laboratory are as follows: osteocalcin, 3.3%/7.7%; BSALP, 7.5%/5.1%; NTX, 4.5%/7.9%. To minimize variability, serum from all time points for each individual was run on the same assay kit.
Bone densitometry, proximal femur geometry, and quantitative ultrasound measurements
DXA scans of the lumbar spine and proximal femur were acquired and analyzed on the same DPX-IQ densitometer (GE Healthcare Lunar, Madison, WI, USA) using DXA and software version 4.6b. BMD change over time was assessed using the lumbar spine (L1–L4) and total proximal femur measurement. Densitometer stability was evaluated by performance of phantom scans on the dates of all data acquisition. No densitometer drift or shift occurred during the course of this study. Proximal femur scans geometric parameter data were obtained by one technologist (N.V.A.) performing analyses for femur neck and total femur BMD, BMC, area, geometric measurements, and femur strength calculations using the GE Healthcare Lunar proprietary femur strength index using software version 4.7. The GE Healthcare Lunar software automatically determines hip axis length (HAL), femur neck cross-sectional area (CSA), cross-sectional moment of inertia (CSMI), and a proprietary femur strength index (FSI). Briefly, CSMI is a measure of how mass is distributed around a central axis with small increases in diameter increasing CSMI. FSI is the compressive yield strength of bone divided by the compressive stress from a fall on the greater trochanter. Compressive stress includes height and weight to calculate the fall force. CSA and CSMI are included in the FSI calculation, which is available in Yoshikawa et. al.(30) Using this methodology, FSI is an independent predictor of hip fracture risk.(31) For this study, femur axis length was defined as the distance from the most medial aspect of the femur head to the most lateral aspect of the trochanter.
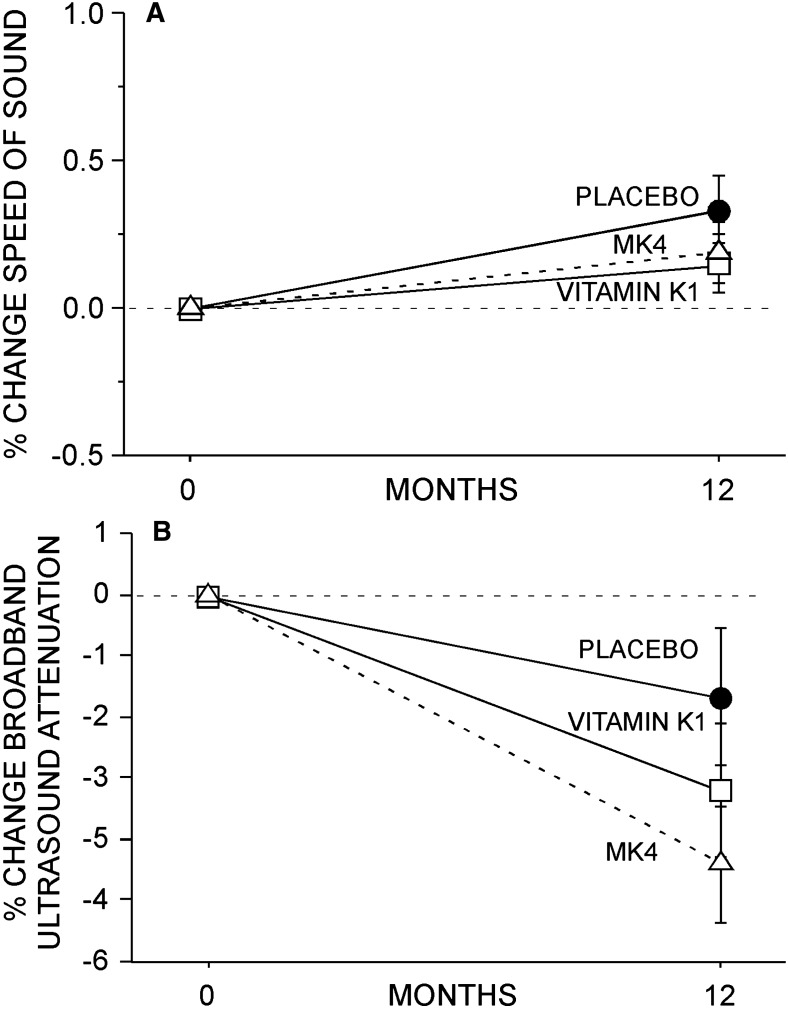
A subset of subjects also had calcaneal ultrasound measurements performed with an Achilles Insight ultrasonometer (GE Healthcare Lunar, Madison, WI, USA). Calcaneal broadband ultrasound attenuation (BUA) and speed of sound (SOS) were measured at baseline and after 12 mo of study.
Statistical analysis
Treatment group baseline demographics were compared at time of randomization using a three-level one-way ANOVA on the log scale to induce normality. An intent to treat (ITT) analysis was performed for standardized outcome variables. “Standardized” indicates that all variables were divided by their values at baseline. These ratios were averaged on the logarithmic scale, yielding geometric means. The ITT analyses were performed using Splus statistical software.(32) These results are presented in Table 2. Additionally, for robustness, a per-protocol analysis of the 365 study completers was performed. The results of this analysis (presented in the figures) show no effect of MK4 or vitamin K1 on study outcome measures and are thus consistent with the ITT analysis.
Table 2.
Relative Effects of Vitamin K1 and MK4 by Intent to Treat Analysis
| Outcome | Treatment group | Average percent effect vs. placebo | 95% CI | p |
| Weight | K1 | −0.87% | (−1.88%, 0.15%) | 0.20 |
| MK4 | −0.70% | (−1.70%, 0.31%) | ||
| BSALP | K1 | 3.55% | (−1.64, 9.02%) | 0.36 |
| MK4 | 0.49% | (−4.47%, 5.71%) | ||
| NTX | K1 | −0.24% | (−7.05%, 7.07%) | 1.00 |
| MK4 | −0.24% | (−6.96%, 6.97%) | ||
| ucOc | K1 | −61.1% | (−65.5%, −56.1%) | <0.0001 |
| MK4 | −60.7% | (−65.1%, −55.8%) | ||
| Osteocalcin | K1 | −8.38% | (−13.15%, −3.35%) | 0.005 |
| MK4 | −5.65% | (−10.50%, −0.54%) | ||
| L-spine BMD | K1 | −0.66% | (−1.32%, −0.01%) | 0.09 |
| MK4 | −0.60% | (−1.24%, 0.05%) | ||
| Total femur BMD | K1 | −0.06% | (−0.55%, 0.43%) | 0.81 |
| MK4 | 0.10% | (−0.38%, 0.59%) | ||
| Ultrasound BUA | K1 | −1.59% | (−4.44%, 1.35%) | 0.18 |
| MK4 | −2.72% | (−5.51%, 0.15%) | ||
| Ultrasound SOS | K1 | −0.18% | (−0.47%, 0.12%) | 0.45 |
| MK4 | −0.15% | (−0.44%, 0.15%) |
The results were averaged over 6- and 12-mo visits (weight, lumbar spine, and total femur BMD) or averaged over 1-, 3-, 6-, and 12-mo visits (BSALP, NTX, % ucOc, and total osteocalcin), or for the 12-mo visit only (ultrasound parameters). All outcomes were standardized by dividing by values at baseline (month 0).
RESULTS
Subject demographics
A total of 612 postmenopausal women were screened to obtain the 381 who were enrolled. Over one half of the screen failures were caused by BMD values being too low (Fig. 1). At randomization, there were no differences (p > 0.1) in demographic characteristics, serum chemistries, or percent ucOc observed between treatment groups (Table 1). Body weight did not change over the course of the study (data not shown).
Table 1.
Study Participant Baseline Demographic Characteristics
| Group | Age (yr) | Weight (lb) | Alb (g/dl) | Creat (mg/dl) | AST (U/liter) | μcOc (%) | BSALP (μg/liter) | NTX (nM BCE) | Oc (ng/ml) | LSBMD (g/cm2) | TFBMD (g/cm2) |
| Placebo (n = 129) | 62.4 (0.6) | 168.8 (3.1) | 4.19 (0.03) | 0.8 (0.01) | 24.1 (0.5) | 13.0 (0.6) | 27.8 (0.9) | 18.2 (0.6) | 19.6 0.6 | 1.189 (0.014) | 1.004 (0.010) |
| Phylloquinone (n = 126) | 62.7 (0.7) | 165.6 (3.0) | 4.15 (0.03) | 0.8 (0.01) | 24.0 (0.6) | 11.6 (0.5) | 27.3 (0.9) | 18.7 (0.8) | 21.8 0.8 | 1.180 (0.014) | 1.009 (0.012) |
| MK4 (n = 126) | 62.4 (0.7) | 167.4 (3.2) | 4.16 (0.03) | 0.8 (0.01) | 23.5 (0.4) | 12.0 (0.5) | 28.3 (1.0) | 19.2 (0.7) | 20.5 0.7 | 1.197 (0.015) | 1.006 (0.012) |
Data as mean (SE).
Albumin, creatinine, and AST determinations obtained at screening 2 mo before randomization.
BMI, body mass index; Alb, serum albumin; Creat, serum creatinine; AST, serum aspartate amino transferase; ucOC, undercarboxylated osteocalcin; BSALP, bone-specific alkaline phosphatase; NTX, serum n-telopeptide of type 1 collagen; Oc, osteocalcin; LSBMD, L1–L4 BMD; TFBMD, total proximal femur BMD.
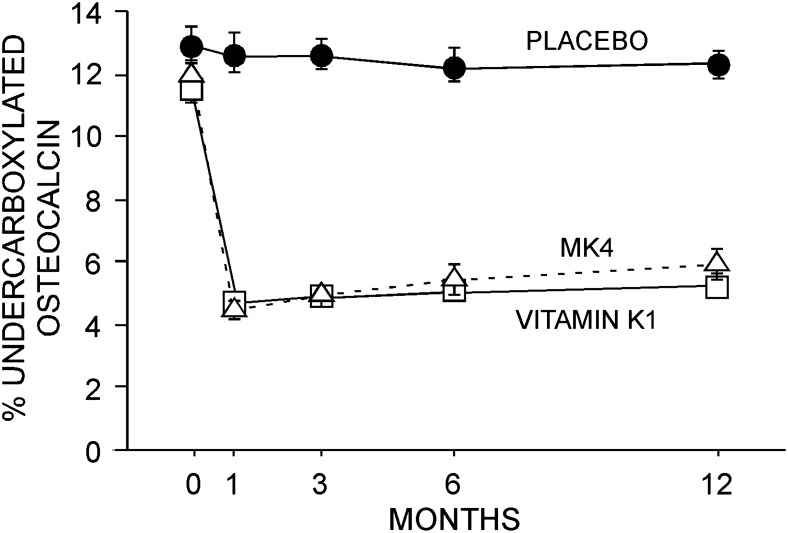
Vitamin K1 compliance was 93.2% in those receiving the active preparation and 92.1% for those receiving the corresponding placebo. Similarly, MK4 compliance was 87.1% for those receiving the active preparation and 89.6% for those receiving the corresponding placebo. Finally, calcium compliance was 92.9%, 92.8%, and 93.0% for those receiving placebo, vitamin K1, and MK4, respectively. An expected effect of vitamin K was observed in the prompt and sustained reduction in %ucOc in the phylloquinone and MK4 groups (Fig. 2).
FIG. 2.
Undercarboxylated osteocalcin. Treatment with either phylloquinone or MK4 rapidly reduced (p < 0.001) circulating percent undercarboxylated osteocalcin. No difference between phylloquinone and MK4 treatment was observed. Data (mean ± SE) presented for study completers.
Outcome measures
Geometric means of relative changes in outcomes along with 95% CIs by ITT analysis are presented in Table 2. Each result is compared with the effect of placebo by dividing it by the corresponding mean for the placebo group. For example, the change in lumbar spine BMD attributed to vitamin K1 is arrived at by dividing the geometric mean relative change in lumbar spine BMD for those who received vitamin K1 by the mean change in lumbar spine BMD for volunteers in the placebo group resulting in 0.9934, or a nonsignificant −0.66% difference (Table 2).
Bone turnover markers
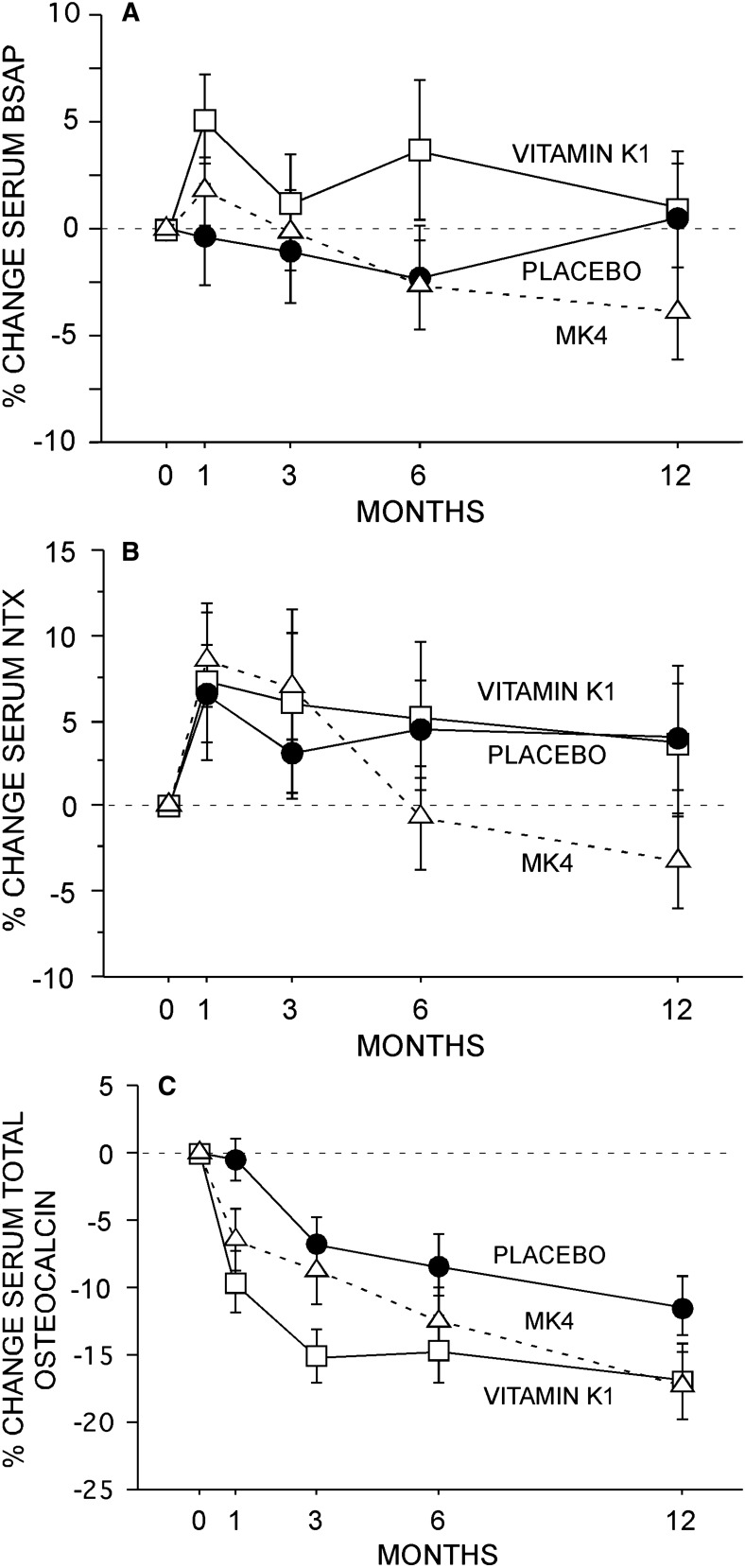
No between-group difference in serum BSALP or serum NTX was observed in this study (Figs. 3A and 3B). Serum total osteocalcin declined by a mean of 11.4% at 1 yr in the placebo group, with a further reduction of ∼5% observed with phylloquinone and MK4 (Fig. 3C).
FIG. 3.
Bone turnover. No effect of phylloquinone or MK4 was observed on serum BSALP (A) or serum NTX (B). Serum osteocalcin declined in all groups (C). Specifically, total osteocalcin declined (p < 0.001) over the 1-yr study duration by 11.4% in the placebo group. In comparison with placebo, slightly greater declines (p < 0.05) were observed for the two treatment groups. Data (mean ± SE) presented for study completers.
BMD, femur geometry, and ultrasound parameters
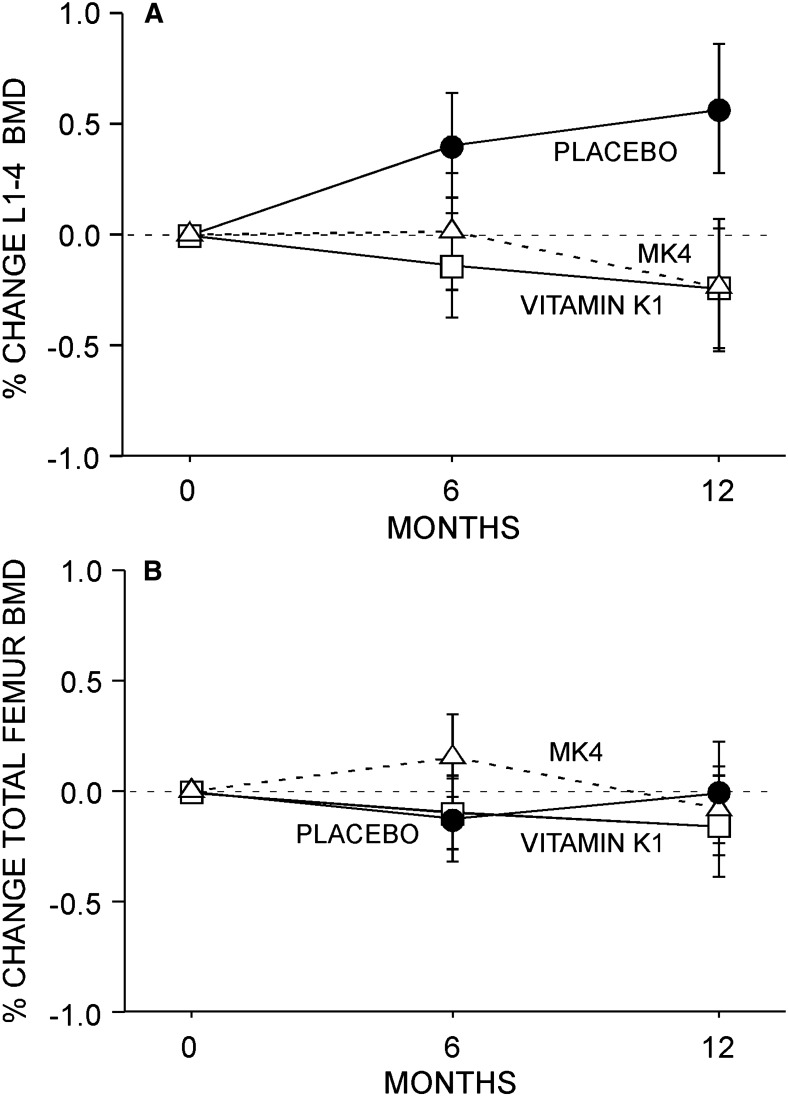
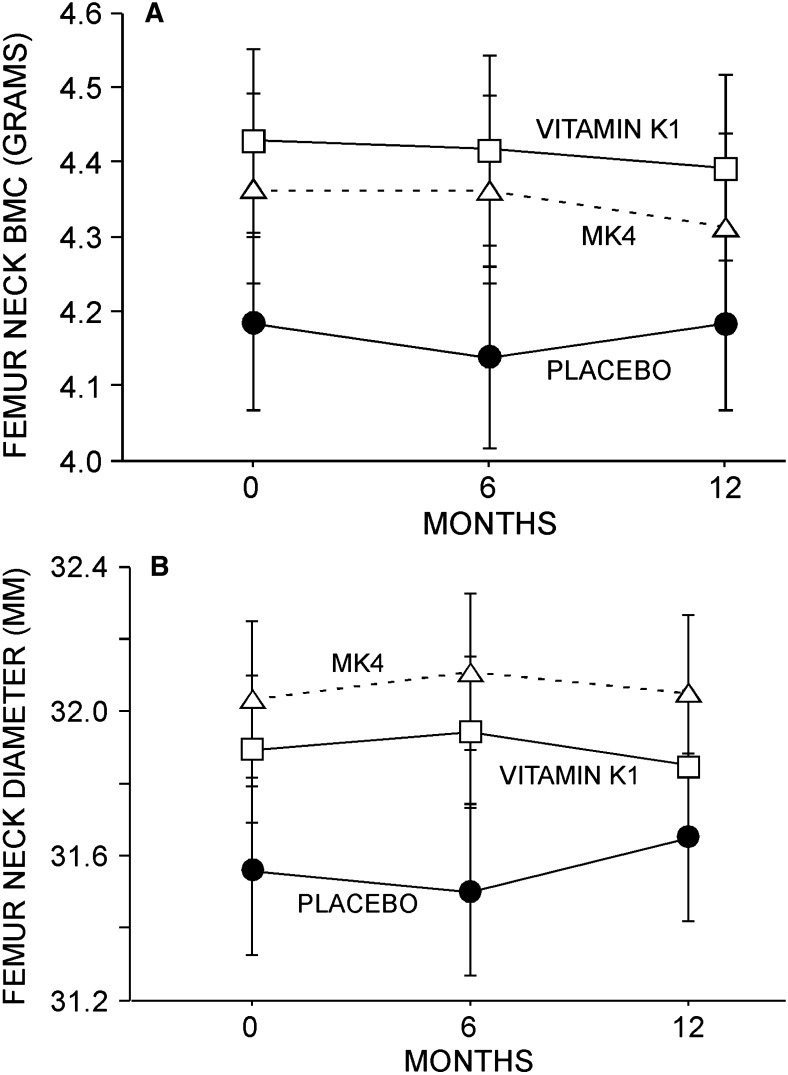
No between-group difference in L1–L4 spine or total femur BMD was observed in this study (Figs. 4A and 4B). Similarly, no between-group difference in SOS or BUA was observed (Figs. 5A and 5B). Finally, no effect of K1 or MK4 was seen on femur neck BMC or diameter (Figs. 6A and 6B) or on femur neck BMD, area, CSA, CSMI, femur neck length, or calculated FSI (data not shown).
FIG. 4.
BMD. No effect of either phylloquinone or MK4 was observed at the L1–L4 spine (A) or left total proximal femur (B). Data (mean ± SE) presented for study completers.
FIG. 5.
Calcaneal ultrasound parameters. No effect of either phylloquinone or MK4 was observed on SOS (A) or BUA (B). Data (mean ± SE) reported for 208 study completers; 71, 67, and 70 in the placebo, phylloquinone, and MK4 groups, respectively.
FIG. 6.
Femur geometric parameters. No effect of either phylloquinone or MK4 was observed on femur neck BMC (A) or diameter (B). Although not shown here, no effect on femur neck CSA, CSMI, or FSI was observed. Data (mean ± SE) presented for study completers.
Adverse events
All study preparations were well tolerated. Serious adverse events occurred in 29 participants and did not differ between groups (Table 3). Nonserious adverse events were more common and also equally distributed among the three treatment groups (data not shown). No specific side effects or adverse events were related to either phylloquinone or MK4.
Table 3.
Reported SAEs by Group
| SAE | Phylloquinone (n = 123) | MK4 (n = 129) | Placebo (n = 123) |
| Malignancy | 3 (2 basal cell, 1 breast) | 5 (2 basal cell, 1 breast 1 lymphoma, 1 uterus | 4 (2 basal cell, 1 colon, 1 lung) |
| Cardiac | 3 (chest pain, atrial tachycardia) | 0 | 2 (angina, MI) |
| GI | 1 (GI bleed) | 2 (appendix, gastric ulcer) | 2 (GERD, pancreatitis) |
| Respiratory | 1 (pneumonia) | 0 | 0 |
| Neurologic | 1 (vertigo) | 0 | 1 (syncope) |
| Other hospitalizations | 1 (motor vehicle accident) | 1 (back lesion) | 1 hysterectomy, 1 tympanoplasty, 1 colon lesion |
SAE, serious adverse effect; GI, gastrointestinal; GERD, gastroesophageal reflux disease.
DISCUSSION
This is the first prospective study directly evaluating the skeletal effects of phylloquinone (vitamin K1) and menaquinone (MK4). No effect of either phylloquinone or MK4 was observed on serum markers of bone turnover, BMD of the lumbar spine and hip, femur geometric properties as measured by DXA, or calcaneal ultrasound parameters. As such, this study does not support a role for vitamin K supplementation in osteoporosis prevention among healthy, postmenopausal, North American women receiving calcium and vitamin D supplementation.
The daily doses of vitamin K used in this study are substantially higher than currently recommended. Specifically, the U.S. current vitamin K adequate intake (AI) for women is 90 μg. Thus, the doses used in this study are ∼11 times higher for phylloquinone and 500 times higher for MK4. These doses were chosen because this MK4 dose is reported to be effective in reducing bone loss or fracture risk in published studies(21,23) and the phylloquinone dose is that which maximally reduces undercarboxylated osteocalcin.(4) Because the classical role of vitamin K is to produce γ-carboxylation of a limited number of proteins, including osteocalcin, the prompt and sustained reduction of undercarboxylated osteocalcin with these high vitamin K doses is expected. Despite this, no skeletal effects were observed.
A potential role of vitamin K in skeletal health has been suggested by epidemiologic studies in which low vitamin K status(33) has been linked to lower BMD(34) and increased fracture risk.(9,10,35,36) Moreover, small studies have reported that MK4 treatment reduces fracture risk.(23,37) In fact, a recent meta-analysis involving ∼700 vitamin K–treated patients found MK4 to have a strong effect in reducing incident fractures in Japan.(12) However, it is important to recognize that one of the studies contributing the greatest weight to effect of vitamin K on hip and nonvertebral fractures(38) studied women with Alzheimer's disease who were severely vitamin D deficient (mean serum 25-hydroxyvitamin D of ∼9 ng/ml with optimal often defined as >30 ng/ml(39)). In this study, while the untreated group received no medications, supplements, or placebo, the treated group received MK4, 1000 IU of vitamin D, and 600 mg of calcium daily. Because the mean vitamin D intake of these subjects was ∼80 IU, the 1000 IU administered to the treatment group resulted in an ∼150% increase in serum 25-hydroxyvitamin D over the 2 yr of study.(38) Thus, the observed reduction in fracture risk noted in the study of Sato et al.,(38) which strongly influenced the meta-analysis,(12) could easily be the result of established efficacy of vitamin D in reducing fracture risk,(40) rather than being attributable to a vitamin K effect. The authors of this meta-analysis(12) did caution that routine vitamin K supplementation is not justified until randomized trials confirm a reduction in fracture risk. Such caution seems justified, because some preclinical studies found no effect of vitamin K deficiency on skeletal health,(14,41) and others found no association between vitamin K dietary intake and BMD or fracture risk over 10 yr of follow-up(13) or with calcaneal quantitative ultrasound parameters.(42)
Because osteoporosis is recognized as low BMD combined with altered bone quality(43) (e.g., altered bone microarchitecture, mineralization, collagen status),(44) it has been suggested that vitamin K may have a beneficial effect on bone quality,(26,45) thereby leading to a reduction in fracture risk with minimal or no effect on BMD or markers of bone turnover. Although such effects remain plausible, and some preclinical work finds vitamin K to impact bone strength,(46) whereas other studies do not,(41,47) it is important to recognize, as emphasized by Tamura et al.,(48) that the aforementioned meta-analysis(12) did not include a pharmacoepidemiological study involving >3000 Japanese participants that was released by Eisai and is available online.(49) In this large study, vertebral fracture reduction efficacy was not noted in the entire study cohort but was observed in a small subpopulation with at least five vertebral fractures. As Tamura et al. suggest,(48) it seems probable the meta-analysis results would have been different if this report had been included in the analysis. Thus, the reasoning that vitamin K reduces fractures and, given the absence of changes in bone turnover or density, that such effects must occur by changes in bone quality, seems open to debate.
Randomized trials such as reported here seem necessary to resolve the potential role of vitamin K in bone health. In this regard, a recently reported 3-yr prospective study of a supplement containing 600 mg elemental calcium, 400 IU vitamin D, and either 500 μg phylloquinone or placebo daily involving men and women found no effect of vitamin K on lumbar spine, femur neck, or total body BMD or on markers of bone turnover.(17) Similarly, a recent randomized trial found 200 μg of phylloquinone daily to have no influence on bone mass despite improvement in osteocalcin carboxylation.(16) In this study, an effect on radius, but not on proximal femur, bone mass was observed only when vitamin K was combined with 1000 mg of calcium and 400 IU of vitamin D.(16) An additional prospective study found no effect on markers of bone turnover or lumbar spine BMD with 3 yr of phylloquinone (1000 μg daily) when combined with calcium zinc, magnesium, and vitamin D.(15) However, in this same report,(15) modest reduction in femur neck bone loss was observed after 3 yr of vitamin K treatment when co-administered with minerals and vitamin D. Finally, a 3-yr prospective study of 325 postmenopausal women receiving 45 μg of MK4 daily or placebo found no effect on femur BMD but a beneficial effect of MK4 on femur neck BMC and bone width.(27) Because bone size profoundly impacts strength, such geometric changes would be expected to reduce fracture risk. In this study, no effect of either phylloquinone or MK4 on multiple measures of femur geometry was observed. Although the mechanism by which vitamin K may alter femur geometry is unclear, it remains possible that longer-duration studies will detect changes in femur geometry.
Taken together, the existing data, in combination with the absence of skeletal effects observed in this prospective study, makes it likely that the relationship of undercarboxylated osteocalcin with low BMD and increased fracture risk is not indicative of adverse skeletal consequences related to vitamin K inadequacy. Rather, it seems probable that elevated ucOC is simply a marker for generally inadequate nutrition; thus, the adverse skeletal consequences previously reported could reflect deficiencies of other (possibly multiple) nutrients or general protein/calorie undernutrition and frailty which lead to an increase in fracture risk.
Limitations of this study include the relatively short (1 yr) study duration and the inclusion of only healthy women. Thus, the exclusion of osteoporotic women and, importantly, the short duration of this study prohibited use of fracture reduction as a study endpoint. Consistent with this approach, this study was not powered for, and did not evaluate, fractures as an endpoint and spinal radiographs were not performed. However, the absence of change in bone turnover markers or BMD makes it unlikely that vitamin K supplementation will be of benefit in osteoporosis prevention among North American women who are receiving calcium and vitamin D supplementation. As noted above, changes in bone quality may be invoked to explain fracture reduction; however, the absence of vitamin K effects on ultrasound parameters and femur geometry do not support such an explanation. Finally, it is unclear if the slight serum osteocalcin decline in the phylloquinone and MK4 groups (∼5%) is of consequence. Although statistically significant, this minimal change (which is not congruent with two other markers of bone turnover, i.e., BSALP and NTX) seems unlikely to have physiologic skeletal significance.
In summary, 1 yr of phylloquinone or MK4 supplementation in healthy postmenopausal, North American women receiving calcium and vitamin D supplementation does not alter serum markers of bone turnover, BMD, proximal femur geometry, or calcaneal ultrasound parameters. This study does not support the use of vitamin K supplementation in osteoporosis prevention among postmenopausal North American women receiving calcium and vitamin D supplementation.
ACKNOWLEDGMENTS
The authors thank Eisai for supplying the MK4, Roche Vitamins for supplying the phylloquinone, and Mission Pharmacal for supplying the Citracal used in this study. This work was supported by RO1 DK58363 and Eisai.
Footnotes
The authors state that they have no conflicts of interest.
REFERENCES
- 1.Suttie JW. Warfarin and vitamin K. Clin Cardiol. 1990;13:16–18. [PubMed] [Google Scholar]
- 2.Price PA. Gla-containing proteins of bone. Connect Tissue Res. 1989;21:51–60. doi: 10.3109/03008208909049995. [DOI] [PubMed] [Google Scholar]
- 3.Knapen MH, Jie KS, Hamulyak K, Vermeer C. Vitamin K-induced changes in markers for osteoblast activity and urinary calcium loss. Calcif Tissue Int. 1993;53:81–85. doi: 10.1007/BF01321883. [DOI] [PubMed] [Google Scholar]
- 4.Binkley NC, Krueger DC, Foley AL, Engelke JA, Suttie JW. Vitamin K supplementation reduces serum under gamma-carboxylated osteocalcin concentration in healthy adults. Am J Clin Nutr. 2000;72:1523–1528. doi: 10.1093/ajcn/72.6.1523. [DOI] [PubMed] [Google Scholar]
- 5.Sokoll LJ, Booth SL, O'Brien ME, Davidson KW, Tsaioun KI, Sadowski JA. Changes in serum osteocalcin, plasma phylloquinone, and urinary gamma-carboxyglutamic acid in response to altered intakes of dietary phylloquinone in human subjects. Am J Clin Nutr. 1997;65:779–784. doi: 10.1093/ajcn/65.3.779. [DOI] [PubMed] [Google Scholar]
- 6.Martini LA, Booth SL, Saltzman E, do Rosario Dias E, de Oliveria Latorre M, Wood RJ. Dietary phylloquinone depletion and repletion in postmenopausal women: Effects on bone and mineral metabolism. Osteoporos Int. 2006;17:929–935. doi: 10.1007/s00198-006-0086-1. [DOI] [PubMed] [Google Scholar]
- 7.Booth SL, Broe KE, Gagnon DR, Tucker KL, Hannan MT, McLean RR, Dawson-Hughes B, Wilson PW, Cupples LA, Kiel DP. Vitamin K intake and bone mineral density in women and men. Am J Clin Nutr. 2003;77:512–516. doi: 10.1093/ajcn/77.2.512. [DOI] [PubMed] [Google Scholar]
- 8.Feskanich D, Weber P, Willett WC, Rockett H, Booth SL, Colditz GA. Vitamin K intake and hip fractures in women: A prospective study. Am J Clin Nutr. 1999;69:74–79. doi: 10.1093/ajcn/69.1.74. [DOI] [PubMed] [Google Scholar]
- 9.Booth SL, Tucker KL, Chen H, Hannan MT, Gagnon DR, Cupples LA, Wilson PWF, Ordovas J, Schaeffer EJ, Dawson-Hughes B, Kiel DP. Dietary vitamin K intakes are associated with hip fracture but not with bone mineral density in elderly men and women. Am J Clin Nutr. 2000;71:1201–1208. doi: 10.1093/ajcn/71.5.1201. [DOI] [PubMed] [Google Scholar]
- 10.Szulc P, Chapuy MC, Meunier PJ, Delmas PD. Serum undercarboxylated osteocalcin is a marker of the risk of hip fracture in elderly women. J Clin Invest. 1993;91:1769–1774. doi: 10.1172/JCI116387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Price PA. Vitamin K nutrition and postmenopausal osteoporosis. J Clin Invest. 1993;91:1268. doi: 10.1172/JCI116324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cockayne S, Adamson J, Lanham-New S, Shearer MJ, Gilbody S, Torgerson DJ. Vitamin K and the prevention of fractures: Systematic review and meta-analysis of randomized controlled trials. Arch Intern Med. 2006;166:1256–1261. doi: 10.1001/archinte.166.12.1256. [DOI] [PubMed] [Google Scholar]
- 13.Rejnmark L, Vestergaard P, Charles P, Hermann AP, Brot C, Eiken P, Mosekilde L. No effect of vitamin K1 intake on bone mineral density and fracture risk in perimenopausal women. Osteoporos Int. 2006;17:1122–1132. doi: 10.1007/s00198-005-0044-3. [DOI] [PubMed] [Google Scholar]
- 14.Binkley N, Krueger D, Engelke JA, Suttie JW. Long-term warfarin treatment does not alter skeletal status in male rhesus monkeys. J Bone Miner Res. 2007;22:695–700. doi: 10.1359/jbmr.070208. [DOI] [PubMed] [Google Scholar]
- 15.Braam LAJLM, Knapen MHJ, Geusens P, Brouns F, Hamulyak K, Gerichhausen MJW, Vermeer C. Vitamin K1 supplementation retards bone loss in postmenopausal women between 50 and 60 years of age. Calcif Tissue Int. 2003;73:21–26. doi: 10.1007/s00223-002-2084-4. [DOI] [PubMed] [Google Scholar]
- 16.Bolton-Smith C, McMurdo ETM, Paterson CR, Mole PA, Harvey JM, Fenton ST, Prynne CJ, Mishra GD, Shearer MJ. Two-year randomized controlled trial of vitamin K1 (phylloquinone) and vitamin D3 plus calcium on the bone health of older women. J Bone Miner Res. 2007;22:509–519. doi: 10.1359/jbmr.070116. [DOI] [PubMed] [Google Scholar]
- 17.Booth SL, Dallal G, Shea MK, Gundberg C, Peterson JW, Dawson-Hughes B. Effect of vitamin K supplementation on bone loss in elderly men and women. J Clin Endocrinol Metab. 2008;93:1217–1223. doi: 10.1210/jc.2007-2490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Suttie JW. The importance of menaquinones in human nutrition. Annu Rev Nutr. 1995;15:399–417. doi: 10.1146/annurev.nu.15.070195.002151. [DOI] [PubMed] [Google Scholar]
- 19.Davidson RT, Foley AL, Engelke JA, Suttie JW. Conversion of dietary phylloquinone to tissue menaquinone-4 in rats is not dependent on gut bacteria. J Nutr. 1998;128:220–223. doi: 10.1093/jn/128.2.220. [DOI] [PubMed] [Google Scholar]
- 20.Iwamoto I, Kosha S, Noguchi S, Murakami M, Fujino T, Tsutomu D, Yukihiro N. A longitudinal study of the effect of vitamin K2 on bone mineral density in postmenopausal women a comparative study with vitamin D3 and estrogen-progestin therapy. Maturitas. 1999;31:161–164. doi: 10.1016/s0378-5122(98)00114-5. [DOI] [PubMed] [Google Scholar]
- 21.Iwamoto J, Takeda T, Ichimura S. Effect of menatetrenone on bone mineral density and incidence of vertebral fractures in postmenopausal women with osteoporosis: A comparison of the effect with etidronate. J Orthop Sci. 2001;6:487–492. doi: 10.1007/s007760100002. [DOI] [PubMed] [Google Scholar]
- 22.Sato Y, Honda Y, Kuno H, Oizumi K. Menatetrenone ameliorates osteopenia in disuse-affected limbs of vitamin D- and K-deficient stroke patients. Bone. 1998;23:291–296. doi: 10.1016/s8756-3282(98)00108-2. [DOI] [PubMed] [Google Scholar]
- 23.Shiraki M, Shiraki Y, Aoki C, Miura M. Vitamin K2 (menatetrenone) effectively prevents fractures and sustains lumbar bone mineral density in osteoporosis. J Bone Miner Res. 2000;15:515–521. doi: 10.1359/jbmr.2000.15.3.515. [DOI] [PubMed] [Google Scholar]
- 24.Orimo H, Shiraki M, Fujita T, Onomura T, Inoue T, Kushida K. Clinical evaluation of menatetrenone in the treatment of involutional osteoporosis-a double-blind multicenter comparative study with 1alpha hydroxyvitamin D. J Bone Miner Res. 1992;7(S1):S122. [Google Scholar]
- 25.Orimo H, Shiraki M, Tomita A, Morii H, Fujita T, Ohata M. Effects of menatetrenone on the bone and calcium metabolism in osteoporosis: A double-blind placebo-controlled study. J Bone Miner Metab. 1998;16:106–112. [Google Scholar]
- 26.Sugiyama T. Possible involvements of vitamin K in bone quality. Arch Intern Med. 2007;167:93. doi: 10.1001/archinte.167.1.93. [DOI] [PubMed] [Google Scholar]
- 27.Knapen MHJ, Schurgers LJ, Vermeer C. Vitamin K2 supplementation improves hip bone geometry and bone strength indices in postmenopausal women. Osteoporos Int. 2007;18:963–972. doi: 10.1007/s00198-007-0337-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gundberg C, Nieman SD, Abrams S, Rosen H. Vitamin K status and bone health: An analysis of methods for determination of undercarboxylated osteocalcin. J Clin Endocrinol Metab. 1998;83:3258–3266. doi: 10.1210/jcem.83.9.5126. [DOI] [PubMed] [Google Scholar]
- 29.Binkley NC, Krueger DC, Kawahara TN, Engelke JA, Chappell RJ, Suttie JW. A high phylloquinone intake is required to achieve maximal osteocalcin gamma-carboxylation. Am J Clin Nutr. 2002;76:1055–1060. doi: 10.1093/ajcn/76.5.1055. [DOI] [PubMed] [Google Scholar]
- 30.Yoshikawa T, Turner CH, Peacock M, Slemenda CW, Weaver CM, Teegarden D, Markwardt P, Burr DB. Geometric structure of the femoral neck measured using dual-energy x-ray absorptiometry. J Bone Miner Res. 1994;10:1053–1064. doi: 10.1002/jbmr.5650090713. [DOI] [PubMed] [Google Scholar]
- 31.Faulkner KG, Wacker WK, Barden HS, Simonelli C, Burke PK, Ragi S, Del Rio L. Femur strength index predicts hip fracture independent of bone density and hip axis length. Osteoporos Int. 2006;17:593–599. doi: 10.1007/s00198-005-0019-4. [DOI] [PubMed] [Google Scholar]
- 32.Venables WN, Ripley BD. 4th ed. NY, USA: Springer, New York; 2002. Modern Applied Statistics with S, [Google Scholar]
- 33.Gundberg CM, Nieman SD, Abrams S, Rosen H. Vitamin K status and bone health: An analysis of methods for determination of undercarboxylated osteocalcin. J Clin Endocrinol Metab. 1998;83:3258–3266. doi: 10.1210/jcem.83.9.5126. [DOI] [PubMed] [Google Scholar]
- 34.Szulc P, Arlot M, Chapuy MC, Duboeuf F, Meunier PJ, Delmas PD. Serum undercarboxylated osteocalcin correlates with hip bone mineral density in elderly women. J Bone Miner Res. 1994;9:1591–1595. doi: 10.1002/jbmr.5650091012. [DOI] [PubMed] [Google Scholar]
- 35.Vergnaud P, Garnero P, Meunier PJ, Breart G, Kamihagi K, Delmas PD. Undercarboxylated osteocalcin measured with a specific immunoassay predicts hip fracture in elderly women: The EPIDOS study. J Clin Endocrinol Metab. 1997;82:719–724. doi: 10.1210/jcem.82.3.3805. [DOI] [PubMed] [Google Scholar]
- 36.Szulc P, Chapuy MC, Meunier PJ, Delmas PD. Serum undercarboxylated osteocalcin is a marker of the risk of hip fracture: A three year follow-up study. Bone. 1996;18:487–488. doi: 10.1016/8756-3282(96)00037-3. [DOI] [PubMed] [Google Scholar]
- 37.Ishida Y, Kawai S. Comparative efficacy of hormone replacement therapy, etidronate, calcitonin, alfacalcidol and vitamin K in postmenopausal women with osteoporosis: The Yamaguchi osteoporosis prevention study. Am J Med. 2004;117:549–555. doi: 10.1016/j.amjmed.2004.05.019. [DOI] [PubMed] [Google Scholar]
- 38.Sato Y, Kanoko T, Satoh K, Iwamoto J. Menatetrenone and vitamin D2 with calcium supplements prevent nonvertebral fracture in elderly women with Alzheimer's disease. Bone. 2005;36:61–68. doi: 10.1016/j.bone.2004.09.018. [DOI] [PubMed] [Google Scholar]
- 39.Dawson-Hughes B, Heaney RP, Holick MF, Lips P, Meunier PJ, Vieth R. Estimates of optimal vitamin D status. Osteoporos Int. 2005;16:713–716. doi: 10.1007/s00198-005-1867-7. [DOI] [PubMed] [Google Scholar]
- 40.Bischoff-Ferrari HA, Willett WC, Wong JB, Giovannucci E, Dietrich T, Dawson-Hughes B. Fracture prevention with vitamin D supplementation: A meta-analysis of randomized controlled trials. JAMA. 2005;293:2257–2264. doi: 10.1001/jama.293.18.2257. [DOI] [PubMed] [Google Scholar]
- 41.Haffa A, Krueger D, Bruner J, Engelke J, Gundberg C, Akhter M, Binkley N. Diet or warfarin induced vitamin K insufficiency elevates circulating undercarboxylated osteocalcin without altering skeletal status in growing female rats. J Bone Miner Res. 2000;15:872–878. doi: 10.1359/jbmr.2000.15.5.872. [DOI] [PubMed] [Google Scholar]
- 42.McLean RR, Booth SL, Kiel DP, Broe KE, Gagnon DR, Tucker KL, Cupples LA, Hannan MT. Association of dietary and biochemical measures of vitamin K with quantitative ultrasound of the heel in men and women. Osteoporos Int. 2006;17:600–607. doi: 10.1007/s00198-005-0022-9. [DOI] [PubMed] [Google Scholar]
- 43.Anonymous Osteoporosis prevention, diagnosis and therapy NIH Consensus Development Panel on Osteoporosis Prevention, Diagnosis and Therapy. JAMA. 2001;285:785–795. doi: 10.1001/jama.285.6.785. [DOI] [PubMed] [Google Scholar]
- 44.Seeman E, Delmas P. Bone quality-The material and structural basis of bone strength and fragility. N Engl J Med. 2006;354:2250–2261. doi: 10.1056/NEJMra053077. [DOI] [PubMed] [Google Scholar]
- 45.Iwamoto J, Takeda T, Sato Y. Menatetrenone (vitamin K2) and bone quality in the treatment of postmenopausal osteoporosis. Nutr Rev. 2006;64:509–517. doi: 10.1111/j.1753-4887.2006.tb00184.x. [DOI] [PubMed] [Google Scholar]
- 46.Akiyama Y, Hara K, Ohkawa I, Tajima T. Effects of menatetrenone on bone loss induced by ovariectomy in rats. Jpn J Pharmacol. 1993;62:145–153. doi: 10.1254/jjp.62.145. [DOI] [PubMed] [Google Scholar]
- 47.Binkley N, Krueger D, Engelke JA, Crenshaw T, Suttie JW. Vitamin K supplementation does not affect ovariectomy-induced bone loss in rats. Bone. 2002;30:897–900. doi: 10.1016/s8756-3282(02)00734-2. [DOI] [PubMed] [Google Scholar]
- 48.Tamura T, Morgan SL, Takimoto H. Vitamin K and the prevention of fractures. Arch Intern Med. 2007;167:94. doi: 10.1001/archinte.167.1.94-a. [DOI] [PubMed] [Google Scholar]
- 49.Anonymous. Eisai announces the intermediate analysis of anti-osteoporosis treatment post-marketing research to investigate the benefits of menatetrenone as part of the ministry of health, labour and welfare's pharmacoepidemiological drug preview program. Available online at www.eisai.co.jp/enews/enews200506.html. Accessed September 2, 2008.