Abstract
Some spontaneous preterm deliveries (PTD) are caused by occult infections of the fetal membranes (histologic chorioamnionitis [HCA]). High levels of infection-related markers, including some cytokines, sampled from maternal circulation in mid-pregnancy have been linked to PTD, but whether these specifically identify HCA has not been established. We have tested associations between thirteen Th1, Th2 and Th17 cytokines and PTD with and without HCA in a prospective cohort study. The study sample included 926 Pregnancy Outcomes and Community Health Study subcohort women; women with medically indicated PTD or incomplete data excluded. A panel of cytokines was assessed using a multiplex assay in maternal plasma collected at 15–27 weeks of gestation. Severe HCA was scored by a placental pathologist blinded to clinical variables. Multivariable polytomous logistic regression was used to estimate adjusted odds ratios (OR) per 1 standard deviation (SD) increase in cytokine levels using a 5 level outcome variable: PTD <35 weeks with HCA, PTD <35 weeks without HCA, PTD 35–36 weeks with HCA, PTD 35–36 weeks without HCA, and term (referent). Interleukin (IL)-1β, IL-2, IL-12, interferon-γ, IL-4, IL-6 and transforming growth factor-β were all significantly associated with PTD <35 weeks with HCA, with ORs of 1.6 – 2.3 per SD increase. None of these were associated with PTD <35 weeks without HCA or PTD 35–36 weeks with HCA. Although the tissues of origin of circulating cytokines are unclear, the observed elevations across many cytokines among women who later delivered <35 weeks with HCA may represent a robust immune response to infection within gestational tissues. These results suggest that women with HCA could be identified using relatively non-invasive means.
Keywords: pregnancy, cytokines, histologic chorioamnionitis, premature birth, inflammation
1. Introduction
Preterm delivery (PTD), defined as delivery prior to 37 completed weeks, increases risks of neonatal morbidity and mortality (Saigal and Doyle, 2008). It is now well accepted that intrauterine infection causes a significant proportion of spontaneous PTD, particularly earlier deliveries. Bacteria ascend from the lower genital tract before or during pregnancy, infect the membranes and initiate an inflammatory response culminating in preterm labor (PTL) or preterm premature rupture of membranes (PROM) (Romero et al., 2006). These typically asymptomatic infections are identified via sampling of amniotic fluid, fetal cord blood or delivered placental tissue that shows evidence of histologic chorioamnionitis (HCA) (Holzman et al., 2007). Other inflammatory pathways, perhaps involving maternal metabolic syndrome and vascular disease, may impact placentation and/or perturb later stages in the course of pregnancy to result in PTD (Catov et al., 2007).
Cytokines are a diverse group of soluble proteins that mediate inflammation and many other processes (Balkiwill, 2000). These proteins exhibit pleiotropy and functional redundancy, up- and down-regulating one another to result in complex networks (Balkiwill, 2000) involved in establishment and maintenance of pregnancy (Kharfi et al., 2003) and complications such as miscarriage (Jasper et al., 2007), preeclampsia (Saito et al., 2007) and spontaneous PTD (Romero et al., 2006). A long-standing paradigm classifies cytokines based on their T-cell lineage as Th1 or Th2. The balance between these two types has been considered a measure of the immune milieu: cell-mediated if Th1 cytokines predominate, humoral if Th2 cytokines predominate (Aris et al., 2008). Normal pregnancy has been characterized as a Th2-dominant state (Wilczynski, 2005), although the timecourse of the expected shift to Th2 dominance in peripheral blood is not clear (Aris et al., 2008; Okun and Coussons-Read, 2007; Stewart et al., 2007). Many researchers have conceived of both recurrent spontaneous abortion (Chaouat et al., 2004) and preeclampsia (Saito and Sakai, 2003) as resulting from Th1 dominance.
A third T-cell lineage, named ‘Th17’ after its main product, interleukin (IL)-17, has been recently characterized (Veldhoen and Stockinger, 2006; Weaver et al., 2006). IL-17 is a potent pro-inflammatory cytokine that promotes expansion and recruitment of neutrophils and supports mucosal barriers (Cua and Kastelein, 2006). Paradoxically, Th17 differentiation depends on the classically regulatory or ‘anti-inflammatory’ cytokine transforming growth factor (TGF)-β in the presence of the Th2 cytokine IL-6 (Kimura et al., 2007, Veldhoen and Stockinger, 2006; Weaver et al., 2006). IL-17 up-regulates production of Th1 cytokines (IL-1β, tumor necrosis factor (TNF)-α), certain chemokines and growth factors (Korn et al., 2007). At the local level, interferon (IFN)-γ, the main Th1 product, and IL-4, the main Th2 product, both interfere with Th17 differentiation, while TGF-β blocks differentiation of Th1 and Th2 cells (Weaver et al., 2006). Th1, Th2 and Th17 may all participate in pathogen defense, with Th17 upregulated in early stages, and Th1 and Th2 both proliferating in later stages (Cua and Kastelein, 2006).
Most studies that have evaluated cytokines as markers of subclinical intrauterine infection have not focused on T-cell lineage, and have instead applied the ‘pro-inflammatory’ label to include various Th-1 (TNF-α, IL-1β) and Th-2 (IL-6) cytokines, chemokines (IL-8) and growth factors (G-CSF) (Arntzen et al., 1998; Dollner et al., 2002; Greig et al., 1993, 1997; Murtha et al., 2007; Wenstrom et al., 1998). These studies have primarily sampled during PTL or from the fetal compartment and, while such studies have contributed to understanding of mechanism, they have not enabled identification of at-risk women in a general obstetric population. Predictive biomarkers from the maternal circulation would be valuable because blood is already routinely drawn several times during prenatal care, but studies evaluating these are sparse and less consistent. High levels of IgM (Holzman et al., 1999), ferritin (Goldenberg et al., 1996a; Tamura et al., 1996; Xiao et al., 2002), C-reactive protein (Catov et al., 2007; Lohsoonthorn et al., 2007; Pitiphat et al., 2005) and G-CSF (Goldenberg et al., 2000), measured in mid-pregnancy serum or plasma, have been linked to increased risk of spontaneous PTD. A high-risk cohort study found that many serum cytokines were associated with recurrent PTD <35 weeks (Vogel et al., 2007), but another study concluded that five cytokines sampled from mid-pregnancy maternal serum in low-risk women offered little useful predictive value (Curry et al., 2007).
Generally, maternal mid-pregnancy biomarkers have been more strongly associated with earlier PTD, but specificity to intrauterine infection has not been established. We are not aware of any prospective studies linking mid-pregnancy circulating cytokines directly to placental findings of HCA. The objective of this study was to assess a panel of cytokines measured in maternal plasma collected in mid-pregnancy in relation to PTD and HCA, according to a Th1, Th2 and Th17 lineage framework.
2. Materials and Methods
2.1 Cohort and subcohort sampling
The Pregnancy Outcomes and Community Health (POUCH) Study enrolled 3019 pregnant women at 15–27 weeks’ gestation from 52 clinics in five Michigan communities from 1998 to 2004. Eligibility criteria included age ≥15, English-speaking, no preexisting diabetes, maternal serum alpha-fetoprotein (MSAFP) screening at 15–22 weeks’ gestation, and singleton pregnancy with no identified congenital anomalies. From the pool of potential participants, the study invited all women with high MSAFP (≥2 multiples of the median, 7% of cohort) and a sample of women with normal MSAFP. High MSAFP was of particular interest because of this biomarker’s consistent association with PTD (Holzman et al., 2001). The POUCH cohort has been shown previously to be largely representative of the 5 communities in terms of demographics and reproductive histories (Holzman et al., 2006). Human subjects approval was obtained, and all participants provided written informed consent.
2.2 Study protocol
Information on demographics, pregnancy history and smoking were elicited during an interview conducted at enrollment. Pregnancy outcome was determined through chart review. Gestational age was calculated using the last menstrual period and modified if it disagreed by >2 weeks with ultrasound conducted prior to 25 weeks. Medically indicated PTD was defined as delivery initiated by induction or Cesarean section without prior spontaneous labor or rupture of membranes. At enrollment, women provided non-fasting venous blood samples. These were collected into EDTA or clot tubes using cooled syringes, spun in cooled centrifuges and immediately frozen.
2.3 Subcohort exposure assessment
A subcohort of the POUCH cohort was selected for detailed assessment of biological specimens using a sampling scheme designed to optimize available resources and maximize statistical power for comparing at-risk subgroups. The subcohort included all women with elevated MSAFP, all PTD and a stratified sample of women delivering at term with normal MSAFP, with over-sampling of African-American women in this latter category. Sampling weights are used to analyze subcohort data in order to generalize results to the cohort and to account for oversampling of high MSAFP into the cohort. For subcohort women, aliquots of EDTA plasma were shipped on dry ice to the analytical laboratory (Statens Serum Institut, Copenhagen, Denmark) for analysis of an in-house panel of inflammatory markers consisting of the following: Il-1β, IL-2, IL-4, IL-5, IL-6, IL-8, IL-10, IL-12, IL-17, IL-18, soluble IL-6 receptor-α, IFN-γ, TNF-α, TNF-β, monocyte chemoattractant protein-1, TGF-β, macrophage inflammatory proteins 1α and 1β, matrix metalloproteinase-9, triggering receptor expressed on myeloid cells (TREM)-1, brain-derived neurotrophic factor, granulocyte macrophage colony stimulating factor (GM-CSF), neurotrophins 3 and 4, soluble TNF receptor I, macrophage migration inhibitory factor and regulated on activation, normal T-cell expressed and secreted (RANTES). This panel was developed for the study of inflammatory reactions in newborns (Skogstrand et al., 2005), and has been used to measure circulating cytokine levels in pregnant women (Vogel et al., 2007). The 27-plexed assay was performed in duplicates with 38 sample pairs (mean used for analysis) per plate using xMAP technology and the Luminex platform as previously described (Skogstrand et al., 2005). All assay plates included samples from both term and PTD. This multiplex assay has been thoroughly characterized previously (coefficients of variation: intraassay, 6.7–13%, interassay 7.3–25%, depending on analyte) (Skogstrand et al., 2005). Rather than truncating the sample to exclude women with values too low for precise quantification, cytokine values below the lowest point in the working ranges were assigned half that value (Haas and Scheff, 1990). In order to analyze all cytokines using a common scale and reduce the impact of interassay variability, cytokine values were standardized based on values in women with term pregnancies within each plate [standardized value = (log-transformed cytokine value – plate-specific median log-transformed cytokine value)/plate-specific standard deviation of log-transformed cytokine value] (Dibley et al., 1987). The resulting standardized values were approximately normally distributed.
For the present analysis, we selected a group of cytokines that could be classified as Th1, Th2 or Th17 to study in relation to HCA and PTD. The Th1-related cytokines selected were IL-1β, IL-2, IL-12, IL-18, IFN-γ, TNF–α and TNF–β. The Th2-related cytokines selected were IL-4, IL-6 and IL-10. To represent Th17, we selected IL-17, TGF-β and GM-CSF, a hematopoietic growth factor up-regulated by IL-17 (Korn et al., 2007; Weaver et al., 2006). This is a conceptual but probably over-simplified classification scheme given the complex cross-talk among cytokines and the ongoing basic science research in this area; other groupings could be constructed from the analytes considered here. In particular, IL-1β, TNF-α and IL-6 are all involved in Th17 responses.
2.4 Placenta protocol
Details of the placenta histopathology protocol have been published previously (Holzman et al., 2007). Briefly, nine tissue sections, representing the placental disc, cord and extraplacental membranes, were examined microscopically by a placental pathologist who was blinded to gestational age at delivery and all clinical data. The microscopic evaluation used a detailed descriptive rather than diagnostic approach, which allowed a number of potential definitions to be tested. For the present analysis, HCA was defined as severe (polymorphonuclear leukocyte inflammatory pattern in chorionic plate and/or extraplacental membrane chorion and amnion, plus karyorrhexis and or necrotizing inflammation), mild (at least one high-powered field with greater than 10 polymorphonuclear leukocytes) or none. This definition of severe HCA was found previously to identify women at markedly increased risk of PTD, while mild HCA was not associated with increased PTD risk (Holzman et al., 2007).
2.5 Statistical analyses
Pearson correlations coefficients were calculated for each pair of 13 standardized cytokines. Associations between continuous standardized cytokine levels and PTD <37 weeks were assessed using logistic regression, and odds ratios (OR) and 95% confidence intervals were calculated per 1 standard deviation (SD) increase in standardized cytokine levels (Whitcomb et al., 2007).
PTD and HCA were cross-classified as 35–36 weeks with no/mild HCA, 35–36 weeks with severe HCA, <35 weeks with no/mild HCA and <35 weeks with severe HCA. Associations between standardized maternal cytokine values and these PTD/HCA groups vs. term delivery were assessed through unadjusted and adjusted polytomous logistic regression models. To avoid inflating type I error by evaluating 13×4=52 ORs in the polytomous analyses, we decided a priori to interpret ORs only for those analytes having overall Wald p-values <0.05, indicating that cytokine levels varied significantly among the 5 outcome variable levels. Variables considered as potential confounders included race/ethnicity (African-American vs. whites/others), maternal age, body mass index, gestational week at blood draw, smoking during pregnancy (yes/no), years of sample storage and community. Variables were included if they changed any unadjusted OR estimate by ≥10%. A sensitivity analysis was performed to assess the impact of excluding cytokine values below the working range. The final models were also rerun after separating mild HCA from no HCA. Frequency and OR estimates were weighted to account for oversampling of African-Americans, women with high MSAFP, and PTD using survey procedures in SAS 9.1.3 (Statistical Analysis Software, Cary, NC).
3. Results
3.1 Study sample
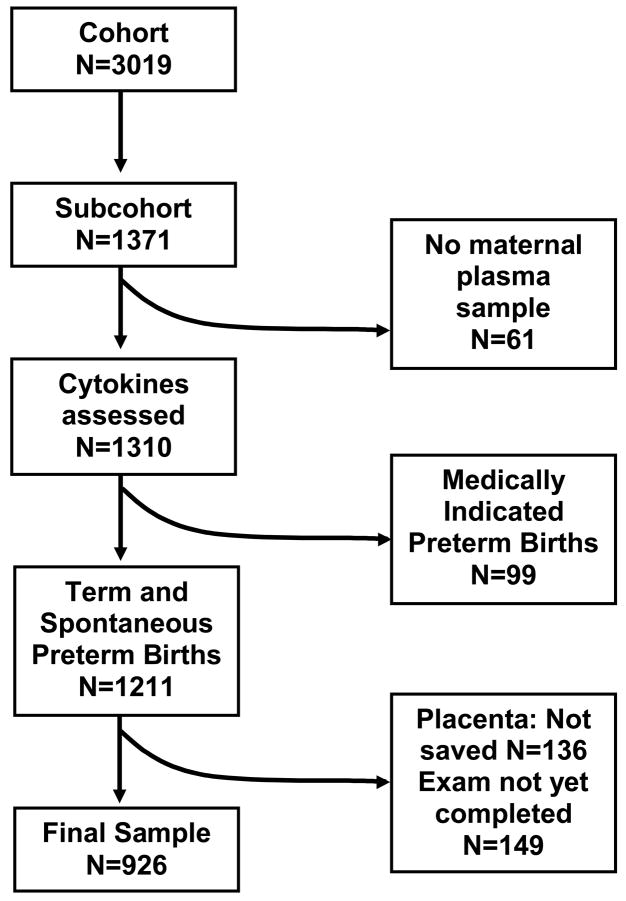
Cytokine data and placental assessments were available for 926 subcohort women (Figure 1). Sample characteristics are presented with raw and weighted percentages to illustrate how weighted analyses account for oversampling (Table 1). For example, 16.6% of subcohort women had high MSAFP, corresponding to 3.4% after weighting (i.e. the estimated population prevalence of high MSAFP).
Figure 1.
Derivation of the study sample
Table 1.
Maternal characteristics of 926 POUCH subcohort women with plasma cytokine profiles and placental histopathological examination completeda
| No. | Subcohort sample % | Weighted %b | |
|---|---|---|---|
| Total | 926 | 100.0 | 100.0 |
| Race | |||
| White | 492 | 53.1 | 65.8 |
| African-American | 363 | 39.2 | 24.4 |
| Asian, Hispanic, Native American and other ethnicities | 71 | 7.7 | 9.8 |
| Age | |||
| < 20 | 152 | 16.4 | 14.3 |
| 20 – 29 | 517 | 55.8 | 56.3 |
| ≥ 30 | 257 | 27.8 | 29.4 |
| Body Mass Index(Kg/m2) | |||
| < 18.5 | 40 | 4.3 | 3.7 |
| 18.5 –< 25 | 422 | 45.6 | 46.5 |
| 25 –< 30 | 216 | 23.3 | 24.6 |
| ≥ 30 | 248 | 26.8 | 25.2 |
| Gestational age at sampling (completed weeks) | |||
| 15–19 | 135 | 14.6 | 13.5 |
| 20–21 | 263 | 28.4 | 28.9 |
| 22–23 | 263 | 28.4 | 29.4 |
| 24–27 | 265 | 28.6 | 28.2 |
| Smoking during pregnancy | |||
| Did not smoke during pregnancy | 678 | 73.2 | 73.8 |
| Smoked during pregnancy, quit before enrollment | 79 | 8.5 | 8.9 |
| Smoked <1/2 pack/day at enrollment | 113 | 12.2 | 10.9 |
| MSAFP screening | |||
| Unexplained high (≥2 MoM) | 154 | 16.6 | 3.4 |
| Normal (< 2 MoM) | 772 | 83.4 | 96.6 |
| Placental abruption diagnosis | 21 | 2.3 | 1.7 |
| Preterm premature rupture of membranes (PPROM)d | 60 | 6.5 | 2.8 |
| Prolonged PPROM (≥24 hours)d | 24 | 2.6 | 1.1 |
| Preterm delivery and HCAa (Median GA [IQR])c | |||
| Term (394 [385 – 403]) | 767 | 82.8 | 92.2 |
| 35–36 weeks, no/mild HCA (362 [356 – 364]) | 99 | 10.7 | 4.8 |
| 35–36 weeks, severe HCA (363 [360 – 366]) | 15 | 1.6 | 0.7 |
| <35 weeks, no/mild HCA (336 [316 – 343]) | 31 | 3.4 | 1.5 |
Removed medically indicated PTD
Weighted to reflect distribution in cohort, with the exception of MSAFP which reflects % in population, since MSAFP was oversampled into the cohort as well as the subcohort.
GA, gestational age (weeksdays). IQR, interquartile range.
Plasma for cytokine assessment was sampled prior to membrane rupture.
3.2 Correlation coefficients
Pearson correlations for each pair of cytokines selected for this study and percent below working range are presented in Table 2. Many of the cytokines were highly correlated, and these correlations were not necessarily stronger within classification groups than between them. For example, the Th1 cytokine IL-1β was correlated >0.5 with four other Th1 cytokines (IL-2, IL-12, IFN-γ and TNF-α), two of the Th2 cytokines (IL-4 and IL-6) and two Th17 cytokines (TGF-b and GM-CSF). Excluding imputed values did not greatly affect correlation coefficients or alter their statistical significance (not shown).
Table 2.
Pearson correlation coefficients between standardized values of selected Th1, Th2 and Th17-related cytokines (N=926).
| Th1 |
Th2 |
Th17 |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| IL-1b | IL-2 | IL-12 | IL-18 | IFN-γ | TNF-a | TNF–β | IL-4 | IL-6 | IL-10 | IL-17 | TGF-b | GM-CSF | |
| % below working range | 18 | 14 | 11 | <1 | 17 | 16 | 9 | 18 | 7 | 17 | 6 | 24 | 16 |
| Th1 | |||||||||||||
| IL-1b | 1.00 | ||||||||||||
| IL-2 | 0.55 | 1.00 | |||||||||||
| IL-12 | 0.50 | 0.45 | 1.00 | ||||||||||
| IL-18 | 0.12 | 0.24 | 0.21 | 1.00 | |||||||||
| IFN-γ | 0.56 | 0.61 | 0.44 | 0.21 | 1.00 | ||||||||
| TNF-a | 0.52 | 0.64 | 0.46 | 0.26 | 0.59 | 1.00 | |||||||
| TNF–β | 0.39 | 0.49 | 0.44 | 0.14 | 0.32 | 0.37 | 1.00 | ||||||
| Th2 | |||||||||||||
| IL-4 | 0.52 | 0.63 | 0.47 | 0.23 | 0.57 | 0.53 | 0.45 | 1.00 | |||||
| IL-6 | 0.53 | 0.47 | 0.59 | 0.23 | 0.50 | 0.50 | 0.41 | 0.49 | 1.00 | ||||
| IL-10 | 0.37 | 0.31 | 0.37 | 0.11 | 0.35 | 0.29 | 0.21 | 0.28 | 0.41 | 1.00 | |||
| Th17 | |||||||||||||
| IL-17 | 0.43 | 0.52 | 0.45 | 0.13 | 0.36 | 0.37 | 0.79 | 0.47 | 0.42 | 0.30 | 1.00 | ||
| TGF-b | 0.52 | 0.49 | 0.43 | 0.10 | 0.50 | 0.54 | 0.31 | 0.49 | 0.47 | 0.36 | 0.34 | 1.00 | |
| GM-CSF | 0.54 | 0.58 | 0.45 | 0.16 | 0.56 | 0.52 | 0.43 | 0.50 | 0.48 | 0.36 | 0.42 | 0.51 | 1.00 |
Note: all correlations are statistically significant (p<.0001).
3.3 Logistic regression
Of the thirteen cytokines evaluated, seven had modest but statistically significant relations to risk of PTD <37 weeks, with ORs indicating 1.2 to 1.4-fold increased risk per SD increase in log cytokine values (Table 3). These represented all 3 classification groups. ORs were not changed by adjustment for potential confounders.
Table 3.
Associations between mid-pregnancy plasma cytokines and PTD (N=926). Odds ratios are for 1-SD increase in log cytokine value.
| PTD <37 Weeks | |
|---|---|
| Cytokine | OR (95% CI)a |
| Th1 | |
| IL-1bb | 1.4 (1.1, 1.7) |
| IL-2 | 1.3 (1.0, 1.6) |
| IL-12 | 1.3 (1.1, 1.5) |
| IL-18 | 1.0 (0.8, 1.3) |
| IFN-γ | 1.2 (1.0, 1.5) |
| TNF-ab | 1.2 (1.0, 1.5) |
| TNF-β | 1.3 (1.0, 1.6) |
| Th2 | |
| IL-4 | 1.4 (1.2, 1.6) |
| IL-6b | 1.1 (0.9, 1.4) |
| IL-10 | 0.9 (0.8, 1.1) |
| Th17 | |
| IL-17 | 1.3 (1.0, 1.6) |
| TGF-β | 1.2 (1.0, 1.5) |
| GM-CSF | 1.1 (0.9, 1.4) |
Weighted to account for sampling scheme.
Cytokines that also participate in Th17 response.
NOTE: boldface signifies p<0.05 (some lower confidence limits are 1.0 due to rounding). Adjustments for potential confounders did not alter any odds ratios.
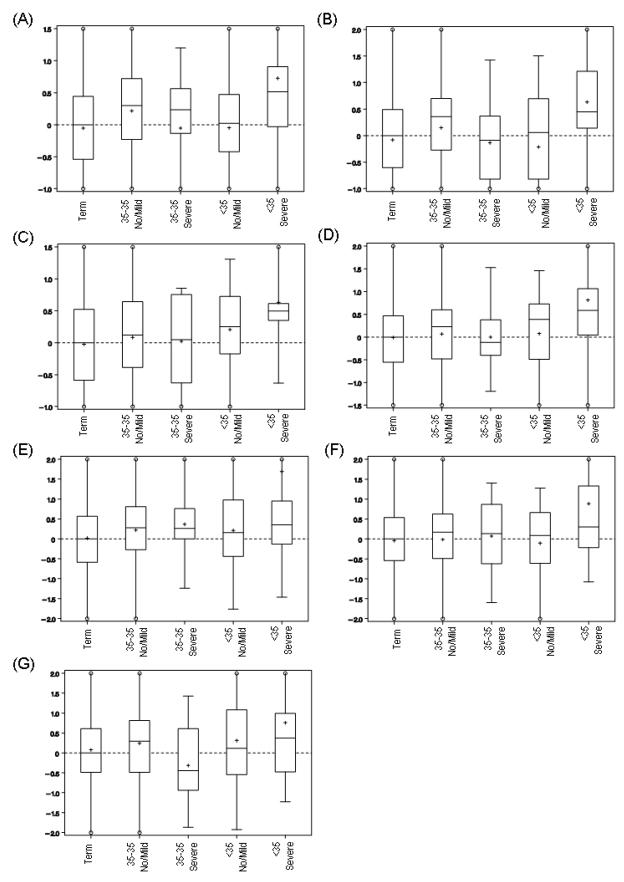
In the multivariable polytomous regression analysis, 7 cytokines had significant associations (Wald p<0.05) after adjusting for gestational age at sampling, race and community (Table 4). All 7 of these cytokines carried elevated risk of PTD <35 weeks/HCA, with ORs ranging from 1.6 to 2.3 per 1-SD increase in log cytokine values. None of these cytokines demonstrated a strong link to PTD <35 weeks without HCA or PTD 35–36 weeks with HCA. However, 3 of these 7 were associated also with modestly elevated odds of PTD 35–36 weeks without HCA. Interestingly, two cytokines that were significantly associated with PTD <37 weeks overall were not associated with HCA (TNF–β and IL-17), but two cytokines that were not significantly associated with all PTD <37 weeks overall were strongly associated with PTD <35 weeks with severe HCA (IFN-γ and IL-6). The significant OR for TNF-β and PTD 35–36 weeks without HCA should be interpreted cautiously in light of the many statistical tests performed, because the Global Wald test does not indicate statistical significance overall. The distributions of the standardized values of the 7 cytokines associated with PTD <35 weeks with HCA are plotted in Figure 2.
Table 4.
Associations between mid-pregnancy plasma cytokines and PTD and HCA (N=926). Adjusteda Odds ratios are for 1-SD increase in log cytokine value
| Cytokine | Global Wald | 35–36 Weeks | 35–36 Weeks | <35 Weeks | <35 Weeks |
|---|---|---|---|---|---|
| p-value | No/Mild HCA OR (95% CI) a | Severe HCA OR (95% CI) a | No/Mild HCA OR (95% CI) a | Severe HCA OR (95% CI) a | |
| Th1 | |||||
| IL-1bb | 0.0028 | 1.4 (1.1, 1.8) | 1.0 (0.5, 2.0) | 1.1 (0.7, 1.7) | 2.3 (1.3, 3.9) |
| IL-2 | 0.0197 | 1.4 (1.1, 1.7) | 1.0 (0.6, 1.5) | 0.9 (0.6, 1.4) | 2.0 (1.2, 3.3) |
| IL-12 | 0.0092 | 1.2 (0.9, 1.6) | 1.0 (0.7, 1.6) | 1.4 (1.0, 2.0) | 2.3 (1.3, 4.0) |
| IL-18 | 0.3996 | 0.9 (0.7, 1.1) | 0.8 (0.5, 1.4) | 1.4 (0.9, 2.4) | 1.4 (0.7, 2.7) |
| IFN-γ | 0.0434 | 1.1 (0.8, 1.5) | 1.1 (0.7, 1.6) | 1.1 (0.7, 1.7) | 1.8 (1.2, 2.5) |
| TNF-ab | 0.3050 | 1.2 (0.9, 1.6) | 1.0 (0.5, 1.9) | 1.2 (0.7, 1.9) | 1.6 (0.9, 3.2) |
| TNF-β | 0.1066 | 1.4 (1.1, 1.8) | 0.8 (0.4, 1.5) | 1.2 (0.8, 1.6) | 1.4 (0.8, 2.4) |
| Th2 | |||||
| IL-4 | 0.0032 | 1.3 (1.0, 1.6) | 1.3 (1.0, 1.8) | 1.2 (0.9, 1.8) | 1.6 (1.2, 1.9) |
| IL-6b | 0.0372 | 1.1 (0.8, 1.4) | 1.1 (0.6, 2.0) | 0.9 (1.6, 1.5) | 2.1 (1.3, 3.3) |
| IL-10 | 0.8915 | 0.9 (0.7, 1.2) | 1.0 (0.6, 1.5) | 0.8 (0.6, 1.2) | 1.0 (0.5, 2.1) |
| Th17 | |||||
| IL-17 | 0.2307 | 1.3 (1.0, 1.7) | 1.1 (0.5, 2.3) | 1.3 (0.9, 1.8) | 1.4 (0.8, 1.8) |
| TGF-β | 0.0359 | 1.2 (0.9, 1.5) | 0.7 (0.4, 1.3) | 1.4 (0.9, 2.0) | 1.7 (1.1, 2.5) |
| GM-CSF | 0.1501 | 1.1 (0.8, 1.4) | 0.7 (0.4, 1.3) | 1.5 (1.0, 2.3) | 1.5 (0.9, 2.3) |
Adjusted for race, gestational age at sampling, and community of enrollment, and weighted to account for sampling scheme.
Cytokines that also participate in Th17 response.
NOTE: boldface signifies p<0.05 for specific level of outcome variable (some lower confidence limits are 1.0 due to rounding). Significant pairwise comparisons based on odds ratios should be interpreted with caution in the absence of a significant Global Wald test p-value.
Figure 2.
Boxplots of standardized cytokine values for 7 cytokines by delivery week and HCA categories: (A) IL-1β, (B) IL-2, (C) IL-12, (D) IFN-γ, (E) IL-4, (F) IL-6, (G) TGF-β. Y-axis scale is standard deviation of log-transformed cytokine value. Solid horizontal lines denote median and interquartile range; + denotes mean. Extreme values (> 75th percentile + 2x interquartile range or < 25th percentile - 2x interquartile range) not shown (clipping indicated by open circle).
In the sensitivity analysis, after excluding subjects with cytokine values below the working range, adjusted ORs for PTD <35 weeks with HCA for the 7 cytokines significant in Table 4 were: IL-1β OR=1.9 (1.1, 3.1), IL-2: OR=1.3 (0.8, 2.1), IL-12: OR=2.1 (1.3,3.3), IFN-γ: OR=1.8 (1.4, 2.3), IL-4: OR=1.4 (1.2, 1.7), IL-6: OR=2.0 (1.3, 3.3), TGF-β: OR=1.6 (1.0, 2.5). Most ORs were attenuated, perhaps owing to differential loss of subjects with low values in the control group; however, only IL-2 lost the interpretation of being a prospective marker for HCA. Odds of delivering <35 weeks with no HCA or with mild HCA were not different by cytokine levels.
4. Discussion
Higher levels of several cytokines, representing Th1, Th2 and Th17 lineages, were associated with increased odds of PTD <35 weeks accompanied by severe HCA. These cytokines were not associated with other PTD <35 weeks, nor were they associated with later HCA-related PTD. This is the first report of an association between a biomarker from maternal circulation collected prior to onset of labor or PROM and placental findings of intrauterine infection.
Studies evaluating biochemical markers for spontaneous PTD in women prior to PTL or PROM require large prospective cohorts given the relative rarity of early spontaneous PTD. High levels of various proteins sampled from maternal circulation in mid-pregnancy have been associated with spontaneous PTD, but not with specific evidence of infection. Elevated C-reactive protein collected in early to mid-pregnancy in serum or plasma has been positively associated with PTD in several studies (Catov et al., 2007; Lohsoonthorn et al., 2007; Pitiphat et al., 2005). High plasma G-CSF (Goldenberg et al., 2000) and serum ferritin (Tamura et al., 1996; Xiao et al., 2002) were associated with increased risk of spontaneous PTD. A small study in a very high risk cohort consisting of women with prior PTD <30 weeks used a panel of cytokines similar to the one employed here, and found that twelve of eighteen (including IL-1β, IL-2, IL-5, IL-6, IL-8, IL-12, IL-18, TNF-α, soluble TNF receptor I, TGF-β, TREM-1 and GM-CSF) were associated with recurrent PTD (Vogel et al., 2007). Recently, a study reported on five serum cytokines (IL-2, IL-6, TNF-α, IFN-γ and GM-CSF) collected prior to 22 weeks, but found little evidence that these markers were strong predictors of spontaneous PTD, although there were partial positive associations for IFN-γ and IL-6 (Curry et al., 2007).
Limited evidence has linked markers in cervical or vaginal fluid among non-laboring women to infection. Low levels of IL-1β, IL-6 or IL-8 in vaginal fluid at 8–20 weeks’ gestation were associated with increased risk of clinical (i.e. symptomatic) chorioamnionitis (Simhan et al., 2003). High cervicovaginal fetal fibronectin at 23–24 weeks was associated with PTD, and among a subset of women who delivered <32 weeks and had clinical placental pathology exams available, fetal fibronectin was associated with HCA (Goldenberg et al., 1996b).
Many of the cytokines considered in this analysis were highly correlated, and these correlations were not limited to other cytokines within the same T-cell lineage. In particular, the 7 cytokines found to be associated with HCA and PTD <35 weeks were all highly correlated with one another and may mark the same pathway. Although cytokines of different T-cell lineages are theoretically up-regulated at different times in an infectious process, the precise time course of events in the immune response cannot be disentangled in this study, since each woman was only sampled at one time point. Although the tissues of origin of the circulating cytokines are unclear, the observed elevations across many cytokines among women who later delivered <35 weeks with HCA may represent a robust immune response to infection within gestational tissues.
It is possible that, by the time an occult infection has become sufficiently established to be detected in a maternal compartment, the process leading to parturition is well advanced. The lack of association between PTD and HCA near term (i.e. 35–36 weeks) suggests that, for these women, infection has not yet occurred or advanced to a point of detection in the maternal circulation at 15–27 weeks. These findings are consistent with published results on other prospective biomarkers from maternal circulation which find much stronger results for earlier PTD (Goldenberg et al., 1996a, 2000; Lohsoonthorn et al., 2007; Xiao et al., 2002). Our finding of a modest association between several cytokines and spontaneous PTD occurring at 35–36 weeks without HCA could represent a separate pathway to PTD, perhaps related to inflammation in the maternal vascular system.
A major strength of this study is the ability to link upstream biomarker data collected prior to clinical manifestations of pregnancy complications to specific placental findings, which increases the specificity of the findings between prospectively collected biologic material and intrauterine infection. The placenta samples were assessed without knowledge of delivery timing or other clinical variables using a detailed descriptive, rather than diagnostic instrument, and the HCA definition was previously linked to PTD. Because the POUCH Study sampled from several communities and included women from urban, suburban and rural populations, external validity should be greater than studies from single clinical sites or high-risk practices. Oversampling of women with high MSAFP into the subcohort should not distort the analyses, since the sampling probability was taken into account using weighted analyses.
Although the cohort from which this sample was drawn was relatively large (N=3019), the number of women with PTD <35 completed weeks is relatively small, and further stratification on HCA status led to small numbers in some outcome categories. This resulted in sufficient power to detect effect sizes of 1.6 or greater in the <35 week with HCA group, but the power was not sufficient for effects of smaller magnitude. The sample size of PTD/HCA was not sufficient to test for effect modification by timing of sample collection within the 13-week enrollment window or to consider PTD <32 weeks. While the multiplex assay has some attendant error, misclassification should be non-differential because assay plates included PTD and term samples; thus, measurement error would be expected to bias results toward the null. We attempted to minimize error associated with interassay variability by standardizing cytokine values. Thirteen analytes were considered in this analysis, thus an increased level of type I error could produce spurious results by chance. In fact, 7 of 13 cytokines tested followed a similar pattern of elevation prior to PTD <35 weeks with severe HCA, and hence we find it unlikely that this pattern is attributable to chance alone.
Before predictive values can be developed, several studies need to replicate prospective association findings, linking a relevant group of cytokines to specific evidence of an infection PTD pathway. However, the results of this study give hope that women with HCA could be screened prior to labor onset using non-invasive means, and that interventions could be targeted to these high-risk women with the goal of preventing PTD.
Acknowledgments
Sources of financial support include the National Institute of Child Health and Human Development grant number R01 HD034543, National Institute of Nursing Research (Renewal NIH POUCH) grant number R01 HD34543, March of Dimes Foundation (Perinatal Epidemiological Research Initiative Program) Grants 20-FY98-0697 through 20-FY04-37, Thrasher Research Foundation grant 02816-7 and Centers for Disease Control and Prevention grant U01 DP000143-01. The funding sources had no role in the collection, analysis or interpretation of the data.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aris A, Lambert F, Bessette P, Moutquin JM. Maternal circulating interferon-gamma and interleukin-6 as biomarkers of Th1/Th2 immune status throughout pregnancy. J Obstet Gynaecol Res. 2008;34:7–11. doi: 10.1111/j.1447-0756.2007.00676.x. [DOI] [PubMed] [Google Scholar]
- Arntzen KJ, Kjollesdal AM, Halgunset J, Vatten L, Austgulen R. TNF, IL-1, IL-6, IL-8 and soluble TNF receptors in relation to chorioamnionitis and premature labor. J Perinat Med. 1998;26:17–26. doi: 10.1515/jpme.1998.26.1.17. [DOI] [PubMed] [Google Scholar]
- Balkiwill F. The Cytokine Network. Oxford: Oxford University Press; 2000. [Google Scholar]
- Catov JM, Bodnar LM, Ness RB, Barron SJ, Roberts JM. Inflammation and dyslipidemia related to risk of spontaneous preterm birth. Am J Epidemiol. 2007;166:1312–1319. doi: 10.1093/aje/kwm273. [DOI] [PubMed] [Google Scholar]
- Chaouat G, Ledee-Bataille N, Dubanchet S, Zourbas S, Sandra O, Martal J. TH1/TH2 paradigm in pregnancy: paradigm lost? Cytokines in pregnancy/early abortion: reexamining the TH1/TH2 paradigm. Int Arch Allergy Immunol. 2004;134:93–119. doi: 10.1159/000074300. [DOI] [PubMed] [Google Scholar]
- Cua DJ, Kastelein RA. TGF-beta, a ‘double agent’ in the immune pathology war. Nat Immunol. 2006;7:557–559. doi: 10.1038/ni0606-557. [DOI] [PubMed] [Google Scholar]
- Curry AE, Vogel I, Drews C, Schendel D, Skogstrand K, Flanders WD, Hougaard D, Olsen J, Thorsen P. Mid-pregnancy maternal plasma levels of interleukin 2, 6, and 12, tumor necrosis factor-alpha, interferon-gamma, and granulocyte-macrophage colony-stimulating factor and spontaneous preterm delivery. Acta Obstet Gynecol Scand. 2007;86:1103–1110. doi: 10.1080/00016340701515423. [DOI] [PubMed] [Google Scholar]
- Dibley MJ, Goldsby JB, Staehling NW, Trowbridge FL. Development of normalized curves for the international growth reference: historical and technical considerations. Am J Clin Nutr. 1987;46:736–748. doi: 10.1093/ajcn/46.5.736. [DOI] [PubMed] [Google Scholar]
- Dollner H, Vatten L, Halgunset J, Rahimipoor S, Austgulen R. Histologic chorioamnionitis and umbilical serum levels of pro-inflammatory cytokines and cytokine inhibitors. Brit J Obstet Gynaecol. 2002;109:534–539. [PubMed] [Google Scholar]
- Goldenberg RL, Andrews WW, Mercer BM, Moawad AH, Meis PJ, Iams JD, Das A, Caritis SN, Roberts JM, Miodovnik M, Menard K, Thurnau G, Dombrowski MP, Mcnellis D. The preterm prediction study: granulocyte colony-stimulating factor and spontaneous preterm birth. National Institute of Child Health and Human Development Maternal-Fetal Medicine Units Network. Am J Obstet Gynecol. 2000;182:625–630. doi: 10.1067/mob.2000.104210. [DOI] [PubMed] [Google Scholar]
- Goldenberg RL, Tamura T, Dubard M, Johnston KE, Copper RL, Neggers Y. Plasma ferritin and pregnancy outcome. Am J Obstet Gynecol. 1996a;175:1356–1359. doi: 10.1016/s0002-9378(96)70054-6. [DOI] [PubMed] [Google Scholar]
- Goldenberg RL, Thom E, Moawad AH, Johnson F, Roberts J, Caritis SN. The preterm prediction study: fetal fibronectin, bacterial vaginosis, and peripartum infection. NICHD Maternal Fetal Medicine Units Network. Obstet Gynecol. 1996b;87:656–660. doi: 10.1016/0029-7844(96)00034-8. [DOI] [PubMed] [Google Scholar]
- Greig PC, Ernest JM, Teot L, Erikson M, Talley R. Amniotic fluid interleukin-6 levels correlate with histologic chorioamnionitis and amniotic fluid cultures in patients in premature labor with intact membranes. Am J Obstet Gynecol. 1993;169:1035–1044. doi: 10.1016/0002-9378(93)90050-s. [DOI] [PubMed] [Google Scholar]
- Greig PC, Murtha AP, Jimmerson CJ, Herbert WN, Roitman-Johnson B, Allen J. Maternal serum interleukin-6 during pregnancy and during term and preterm labor. Obstet Gynecol. 1997;90:465–469. doi: 10.1016/s0029-7844(97)00294-9. [DOI] [PubMed] [Google Scholar]
- Haas C, Scheff P. Estimation of Averages in Truncated Samples. Environ Sci Technol. 1990;24:912–919. [Google Scholar]
- Holzman C, Bullen B, Fisher R, Paneth N, Reuss L. Pregnancy outcomes and community health: the POUCH study of preterm delivery. Paediatr Perinat Epidemiol. 2001;15(Suppl 2):136–158. doi: 10.1046/j.1365-3016.2001.00014.x. [DOI] [PubMed] [Google Scholar]
- Holzman C, Eyster J, Tiedje LB, Roman LA, Seagull E, Rahbar MH. A life course perspective on depressive symptoms in mid-pregnancy. Matern Child Health J. 2006;10:127–138. doi: 10.1007/s10995-005-0044-0. [DOI] [PubMed] [Google Scholar]
- Holzman C, Jetton J, Fisher R, Senagore P, Mohan M, Paneth N. Association of maternal IgM concentrations above the median at 15–19 weeks of gestation and early preterm delivery. Lancet. 1999;354:1095–1096. doi: 10.1016/S0140-6736(99)03781-2. [DOI] [PubMed] [Google Scholar]
- Holzman C, Lin X, Senagore P, Chung H. Histologic chorioamnionitis and preterm delivery. Am J Epidemiol. 2007;166:786–794. doi: 10.1093/aje/kwm168. [DOI] [PubMed] [Google Scholar]
- Jasper MJ, Tremellen KP, Robertson SA. Reduced expression of IL-6 and IL-1alpha mRNAs in secretory phase endometrium of women with recurrent miscarriage. J Reprod Immunol. 2007;73:74–84. doi: 10.1016/j.jri.2006.06.003. [DOI] [PubMed] [Google Scholar]
- Kharfi A, Giguere Y, Sapin V, Masse J, Dastugue B, Forest JC. Trophoblastic remodeling in normal and preeclamptic pregnancies: implication of cytokines. Clin Biochem. 2003;36:323–331. doi: 10.1016/s0009-9120(03)00060-2. [DOI] [PubMed] [Google Scholar]
- Kimura A, Naka T, Kishimoto T. IL-6-dependent and -independent pathways in the development of interleukin 17-producing T helper cells. Proc Natl Acad Sci USA. 2007;104:12099–12104. doi: 10.1073/pnas.0705268104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korn T, Oukka M, Kuchroo V, Bettelli E. Th17 cells: Effector T cells with inflammatory properties. Semin Immunol. 2007;19:362–371. doi: 10.1016/j.smim.2007.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lohsoonthorn V, Qiu C, Williams MA. Maternal serum C-reactive protein concentrations in early pregnancy and subsequent risk of preterm delivery. Clin Biochem. 2007;40:330–335. doi: 10.1016/j.clinbiochem.2006.11.01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murtha AP, Sinclair T, Hauser ER, Swamy GK, Herbert WN, Heine RP. Maternal serum cytokines in preterm premature rupture of membranes. Obstet Gynecol. 2007;109:121–127. doi: 10.1097/01.AOG.0000250474.35369.12. [DOI] [PubMed] [Google Scholar]
- Okun ML, Coussons-Read ME. Sleep disruption during pregnancy: how does it influence serum cytokines? J Reprod Immunol. 2007;73:158–165. doi: 10.1016/j.jri.2006.06.006. [DOI] [PubMed] [Google Scholar]
- Pitiphat W, Gillman MW, Joshipura KJ, Williams PL, Douglass CW, Rich-Edwards JW. Plasma C-reactive protein in early pregnancy and preterm delivery. Am J Epidemiol. 2005;162:1108–1113. doi: 10.1093/aje/kwi323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romero R, Espinoza J, Goncalves LF, Kusanovic JP, Friel LA, Nien JK. Inflammation in preterm and term labour and delivery. Semin Fetal Neonatal Med. 2006;11:317–326. doi: 10.1016/j.siny.2006.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saigal S, Doyle LW. An overview of mortality and sequelae of preterm birth from infancy to adulthood. Lancet. 2008;371:261–269. doi: 10.1016/S0140-6736(08)60136-1. [DOI] [PubMed] [Google Scholar]
- Saito S, Sakai M. Th1/Th2 balance in preeclampsia. J Reprod Immunol. 2003;59:161–173. doi: 10.1016/s0165-0378(03)00045-7. [DOI] [PubMed] [Google Scholar]
- Saito S, Shiozaki A, Nakashima A, Sakai M, Sasaki Y. The role of the immune system in preeclampsia. Mol Aspects Med. 2007;28:192–209. doi: 10.1016/j.mam.2007.02.006. [DOI] [PubMed] [Google Scholar]
- Simhan HN, Caritis SN, Krohn MA, Martinez De Tejada B, Landers DV, Hillier SL. Decreased cervical proinflammatory cytokines permit subsequent upper genital tract infection during pregnancy. Am J Obstet Gynecol. 2003;189:560–567. doi: 10.1067/s0002-9378(03)00518-0. [DOI] [PubMed] [Google Scholar]
- Skogstrand K, Thorsen P, Norgaard-Pedersen B, Schendel DE, Sorensen LC, Hougaard DM. Simultaneous measurement of 25 inflammatory markers and neurotrophins in neonatal dried blood spots by immunoassay with xMAP technology. Clin Chem. 2005;51:1854–1866. doi: 10.1373/clinchem.2005.052241. [DOI] [PubMed] [Google Scholar]
- Stewart FM, Freeman DJ, Ramsay JE, Greer IA, Caslake M, Ferrell WR. Longitudinal assessment of maternal endothelial function and markers of inflammation and placental function throughout pregnancy in lean and obese mothers. J Clin Endocrinol Metab. 2007;92:969–975. doi: 10.1210/jc.2006-2083. [DOI] [PubMed] [Google Scholar]
- Tamura T, Goldenberg RL, Johnston KE, Cliver SP, Hickey CA. Serum ferritin: a predictor of early spontaneous preterm delivery. Obstet Gynecol. 1996;87:360–365. doi: 10.1016/0029-7844(95)00437-8. [DOI] [PubMed] [Google Scholar]
- Veldhoen M, Stockinger B. TGFbeta1, a ‘Jack of all trades’: the link with pro-inflammatory IL-17-producing T cells. Trends Immunol. 2006;27:358–361. doi: 10.1016/j.it.2006.06.001. [DOI] [PubMed] [Google Scholar]
- Vogel I, Goepfert AR, Thorsen P, Skogstrand K, Hougaard DM, Curry AH, Cliver S, Andrews WW. Early second-trimester inflammatory markers and short cervical length and the risk of recurrent preterm birth. J Reprod Immunol. 2007;75:133–140. doi: 10.1016/j.jri.2007.02.008. [DOI] [PubMed] [Google Scholar]
- Weaver CT, Harrington LE, Mangan PR, Gavrieli M, Murphy KM. Th17: an effector CD4 T cell lineage with regulatory T cell ties. Immunity. 2006;24:677–688. doi: 10.1016/j.immuni.2006.06.002. [DOI] [PubMed] [Google Scholar]
- Wenstrom KD, Andrews WW, Hauth JC, Goldenberg RL, Dubard MB, Cliver SP. Elevated second-trimester amniotic fluid interleukin-6 levels predict preterm delivery. Am J Obstet Gynecol. 1998;178:546–550. doi: 10.1016/s0002-9378(98)70436-3. [DOI] [PubMed] [Google Scholar]
- Whitcomb BW, Schisterman EF, Klebanoff MA, Baumgarten M, Rhoton-Vlasak A, Luo X, Chegini N. Circulating chemokine levels and miscarriage. Am J Epidemiol. 2007;166:323–331. doi: 10.1093/aje/kwm084. [DOI] [PubMed] [Google Scholar]
- Wilczynski JR. Th1/Th2 cytokines balance--yin and yang of reproductive immunology. Eur J Obstet Gynecol Reprod Biol. 2005;122:136–143. doi: 10.1016/j.ejogrb.2005.03.008. [DOI] [PubMed] [Google Scholar]
- Xiao R, Sorensen TK, Frederick IO, El-Bastawissi A, King IB, Leisenring WM, Williams MA. Maternal second-trimester serum ferritin concentrations and subsequent risk of preterm delivery. Paediatr Perinat Epidemiol. 2002;16:297–304. doi: 10.1046/j.1365-3016.2002.00448.x. [DOI] [PubMed] [Google Scholar]