Abstract
Most studies of the relationship between cardiorespiratory fitness (CRF) and depression have been limited to cross-sectional designs. The objective of this study was to follow individuals over time to examine whether those with higher levels of CRF have lower risk of developing depressive symptoms. Participants were 11,258 men and 3,085 women enrolled in the Aerobics Center Longitudinal Study in Dallas, TX. All participants completed a maximal treadmill exercise test at baseline (1970–1995) and a follow-up health survey in 1990 and/or 1995. Individuals with a history of a mental disorder, cardiovascular disease, or cancer were excluded. CRF was quantified by exercise test duration, and categorized into age-stratified groups as low (lowest 20%), moderate (middle 40%), or high (upper 40%). Depressive symptoms were assessed using the 20-item Center for Epidemiologic Studies Depression Scale (CES-D). Those who scored 16 or more on the CES-D were considered to have depressive symptoms. After an average of 12 years of follow-up, 282 women and 740 men reported depressive symptoms. After adjusting for age, baseline examination year, and survey response year, the odds of reporting depressive symptoms were 31% lower for men with moderate CRF (odds ratio, OR 0.69; 95% confidence interval, CI 0.56–0.85) and 51% lower for men with high CRF (OR 0.49, CI 0.39–0.60), compared to men with low CRF. Corresponding ORs for women were 0.56 (CI 0.40–0.80) and 0.46 (CI 0.32–0.65). Higher CRF is associated with lower risk of incident depressive symptoms independent of other clinical risk predictors.
Keywords: Cardiorespiratory fitness, CES-D, depressive symptoms, physical activity
1. Introduction
In any given year, depressive disorders affect about 18.8 million Americans, 9.5% of all adults (NIMH, 2006). The costs associated with depression for the year 2000 have been estimated at $83.1 billion (Greenberg et al., 2003). Depressive symptoms are associated with greater morbidity and mortality, less ability to function independently, and lower occupational performance (Penninx et al., 2001;Kouzis et al., 1995;Druss et al., 2000;Krishnan et al., 2002). There is also evidence suggesting that depression may increase the risks of developing Alzheimer’s disease and cognitive decline (Jorm, 2000;Green et al., 2003).
Many population-based studies have reported that physical activity may reduce the risk of developing depression (Farmer et al., 1988;Camacho et al., 1991;Paffenbarger et al., 1994;Kritz-Silverstein et al., 2001;Strawbridge et al., 2002;Wiles et al., 2007;Thirlaway & Benton, 1992;Galper et al., 2006;Tolmunen et al., 2006). Meta-analyses based on cross-sectional studies, longitudinal studies, and randomized clinical trials appear to confirm this association (Lawlor & Hopker, 2001;Dunn et al., 2001;Stathopoulou et al., 2006;Blumenthal et al., 1999). However, some studies have not found this effect (Lennox et al., 1990;Cooper-Patrick et al., 1997;Weyerer, 1992). The inconsistent findings of previous research may be due to: cross-sectional analyses or experimental designs with short term follow-up; selection biases associated with age, gender, or other non-representative sampling; nonstandardized measurement of depression; and the variety of ways in which physical activity has been measured, most commonly with self-reports (Hopko et al., 2008).
For the measure of depressive symptoms, we employed the validated and widely-used Center for Epidemiologic Studies Depression Scale (CES-D). Although the CES-D cannot diagnose depression, it is not diagnostic, the CES-D is a valid and reliable measure of depressive symptoms among adults in the general population (Beekman et al., 1997). We sought to overcome many of the limitations of previous research in the current study by examining the prospective relationship between cardiorespiratory fitness (CRF) and depressive symptoms. CRF is an objective and reproducible physiological measure that reflects functional influences of physical activity habits, genetics, and disease status. CRF has been found to be inversely associated with the risks of developing fatal and nonfatal chronic diseases (Bouchard et al., 2006;Blair et al., 1989;Blair et al., 1996;Sui et al., 2007). Less research has examined associations between CRF and emotional well being. To our knowledge, only three previous studies have investigated the association between symptoms of depression and CRF (Tolmunen et al., 2006;Galper et al., 2006;Thirlaway & Benton, 1992). One focused on middle-aged men, reporting that those with low CRF (measured by VO2max, maximal oxygen uptake) were more likely to have depressive symptoms (Tolmunen et al., 2006). The others included both women and men (Thirlaway & Benton, 1992;Galper et al., 2006). Thirlaway et al (Thirlaway & Benton, 1992) studied a group of relatively young subjects, the majority between ages 30 and 40, finding an inverse association between CRF and depressive symptoms. This result was found only for those who were physically inactive, however, not for those who were at least moderately active. More recently, in the Aerobics Center Longitudinal Study (ACLS), Galper et al (Galper et al., 2006) also reported that CRF was inversely associated with depressive symptoms. All of these studies were limited by their cross-sectional designs. The present study will expand the earlier report from the ACLS, by examining the longitudinal association between CRF and depressive symptoms.
2. Methods
2.1. Study population
The ACLS is a prospective epidemiological study investigating health outcomes associated with physical activity and CRF at the Cooper Clinic, Dallas, TX (Blair et al., 1989;Blair et al., 1996;Sui et al., 2007). All patients had a baseline health examination. All patients included in this study had normal resting electrocardiograms (ECGs), and were able to complete an exercise stress test to at least 85% of their age-predicted maximal heart rate (defined as 220 minus age) during 1970 – 1995. The mean (SD) percentage of age-predicted maximal heart rate achieved during exercise was 101.2 (6.0) in women, and 102.5 (7.0) in men, thus indicating maximal or near maximal exertion. Those who reported having previously been diagnosed with a mental disorder—such as depression, anxiety, thoughts of suicide, nervous breakdown, difficulty sleeping, nervous disorder, or psychiatric counseling—were excluded from the analysis. We also excluded individuals with a history of myocardial infarction, stroke, or cancer, because these diseases might be related to depression and/or fitness. The current analysis included 14,343 individuals (aged 20 to 81 years) who met the above criteria and responded to at least one mail-back health survey in 1990 and/or 1995. Most participants were Caucasian, relatively well-educated, and from middle and upper socioeconomic strata. Participants were told the purpose of the study and provided their written informed consent to participate. The study protocol was approved annually by the Cooper Institute’s institutional review board.
2.2. Baseline examination
After participants completed an overnight fast of at least 12 hours, an extensive physical examination and preventive health evaluation were performed (Blair et al., 1989;Blair et al., 1996). During the examination, participants completed a maximal exercise treadmill test, provided blood for chemistry analyses, had measures of blood pressure taken, and responded to a comprehensive questionnaire that elicited demographic characteristics, personal health history, and lifestyle habits. Height and weight were measured on a standard physician’s balance beam scale and stadiometer. Body mass index (BMI) was computed as weight (kg)/height (m)2. Resting blood pressure was measured using standard auscultation methods after a brief period of quiet sitting. Blood chemistry was analyzed for lipids and glucose using standardized automated bioassays. The presence of hypertension and diabetes was determined based on a self-reported history of physician diagnosis or measured phenotypes that met clinical thresholds for each condition. Information on smoking habits (current smoker or not), alcohol intake (drinks per week), physician diagnosed mental disorder, and stressful occupation (yes or no) was obtained from a standardized questionnaire.
CRF was defined as the total time of a symptom-limited maximal treadmill exercise test, using a modified Balke protocol (Blair et al., 1989;Balke & Ware, 1959). Total time of the test on this protocol correlates highly with measured maximal oxygen uptake in both men (r = 0.92) (Pollock et al., 1976) and women (r = 0.94) (Pollock et al., 1982). The test endpoint was volitional exhaustion or termination by the supervising physician. Maximal metabolic equivalents (METs, 1 MET = 3.5 ml O2 uptake·kg−1·min−1) were calculated from the final treadmill speed and grade.(American College of Sports Medicine, 2005) Previous ACLS reports have shown that low CRF is an independent predictor of mortality and morbidity (Blair et al., 1989;Blair et al., 1996;Sui et al., 2007). In previous ACLS reports, we have defined low, moderate, and high CRF exposures as the lowest 20% and the middle and upper 40%, respectively, of the age and sex-specific distribution of treadmill duration in the overall ACLS population (Sui et al., 2007). Abnormal exercise ECG responses were broadly defined as rhythm and conduction disturbances and ischemic ST-T wave abnormalities, as described in detail elsewhere (Gibbons et al., 2000). We have found 90% agreement between the ECG interpretation recorded in our database and that of a group of three physicians who read a random sample of 357 patient records and were blinded to the recorded interpretation (Gibbons et al., 2000).
2.3. Assessment of outcome
The presence of depressive symptoms was assessed using the 20-item version of the CES-D, a measurement of depressive mood experienced during the past week. This 20-item self-report scale is designed to measure depressive symptoms in the general population (Radloff, 1977). Although the CES-D is not a diagnostic instrument, it is a valid, reliable, and widely-used tool for screening and detecting depressive symptoms in the general population (Beekman et al., 1997;Cheung et al., 2007). The range of possible scores is 0 to 60, with higher scores indicating a higher degree of depressive symptoms. A CES-D score of 16 or higher is commonly interpreted as indicating the presence of depressive symptoms. This cutpoint has been used extensively in studies of the general population (Radloff, 1977), in studies of older subjects (Beekman et al., 1997;Heikkinen & Kauppinen, 2004), and also in studies that separately examined women and men (Ried & Planas, 2002). We used this cutpoint to define the presence of depressive symptoms. All participants completed the CES-D as part of a mail survey in 1990 and/or 1995. There were 10,190 and 9,674 participants who responded to the 1990 and 1995 surveys, respectively; 5,521 responded to both surveys. The aggregate survey response rate across these two survey periods was about 65%. Nonresponse bias is a concern in epidemiological surveillance. This issue has been investigated in the ACLS (Macera et al., 1990). Baseline health histories and clinical measures were similar between responders and nonresponders, and between early and late responders (Macera et al., 1990). We also investigated whether differential mortality might have affected response rates, using the National Death Index to identify decedents. We found that mortality rates were similar for responders and nonresponders (unpublished data). In participants who replied to both surveys, the more recent depressive symptom score was used for analysis.
2.4. Statistical analysis
All analyses were sex-specific. Descriptive statistics were calculated for both women and men. We used logistic regression to evaluate the association between CRF and depressive symptoms. Multivariate analyses included 9 covariables: age (years), baseline examination year, current smoker (yes/no), alcohol intake (≥5 drinks/wk, or less), abnormal exercise ECG responses (present or not), BMI, hypertension (present or not), and diabetes (present or not). To account for differences in survey response patterns among study participants, and for the possibility that external events may have differentially affected responses to the CES-D during the two survey periods, we created a dummy variable that indicated whether the outcome measurement was from 1990 or 1995. Incidence rates were calculated by dividing the number of individuals reporting elevated levels of depressive symptoms by the population at risk, both for the total sample and within categories of fitness. Tests of linear trend across CRF categories were conducted by entering the three-category fitness variable into the regression models as an ordinal term. To examine potential modifying effects of selective variables on the fitness-depressive symptoms association, we stratified data in the following groups: age (<65 vs. ≥65 years), BMI (18.5–24.9 vs. 25.0–29.9 vs. ≥30.0), current smoker, alcohol consumption, hypertension, and diabetes. All p values are 2-sided with an alpha level of 0.05. All analyses were performed using SAS, version 9.1 (SAS Institute, Inc., Cary, North Carolina).
3. Results
The mean baseline age of the study sample was 44.9 years (SD, 9.7); 22% were female. Baseline treadmill time ranged from 1.0 to 38.3 minutes, with a mean of 17.4 (SD, 5.4). Among the participants, the prevalence of low, moderate, and high CRF was 12.7% (1,816), 37.1% (5,317), and 50.3% (7,210), respectively. Time to follow-up averaged 12 years (range 1 to 25 years), with 174,554 total person-years of exposure. Among women, 9.1% reported depressive symptoms at follow-up (CES-D scores above 16). The corresponding rate for men was 6.6%. Table 1 presents characteristics of the women (n=3,085) and men (n=11,258) in the study.
Table 1.
Baseline Characteristics of Men and Women, Aerobics Center Longitudinal Study, 1970–1995.
| Characteristic | Men (n=11,258) | Women (n=3,085) |
|---|---|---|
| Age (years) | 45.0±9.5 | 44.6±10.3 |
| Maximal METs | 12.0±2.6 | 9.6±2.2 |
| Treadmill time (minutes) | 18.5±5.1 | 13.5±4.7 |
| Low fitness (%) | 12.9 | 11.8 |
| Moderate fitness (%) | 37.2 | 36.7 |
| High fitness (%) | 49.9 | 51.6 |
| Body Mass Index (kg/m2) | 25.7±3.3 | 22.5±3.3 |
| Lipids (mmol/L) | ||
| Total cholesterol | 5.5±1.0 | 5.3±1.2 |
| HDL-C | 1.2±0.3 | 1.6±0.5 |
| Triglycerides | 1.4±1.0 | 1.0±1.1 |
| Fasting blood glucose (mmol/L) | 5.6±0.9 | 5.2±0.7 |
| Blood pressure (mmHg) | ||
| Systolic | 122±14 | 113±14 |
| Diastolic | 81±9 | 75±9 |
| Stressful occupation* (%) | 36.8 | 17.9 |
| Current smoker (%) | 13.4 | 8.8 |
| Alcohol intake (≥5 drinks/week) † (%) | 41.7 | 25.1 |
| Hypertension‡ (%) | 24.1 | 12.3 |
| Diabetes§ (%) | 4.1 | 1.9 |
| Abnormal exercise ECG (%) | 5.5 | 4.7 |
Data shown as Means ± SD unless specified otherwise.
METs= maximal metabolic equivalents achieved during the treadmill test; HDL- C= high density lipoprotein cholesterol; ECG=electrocardiogram.
Defined as yes or no based on a standardized questionnaire
One unit of alcohol is defined as 12 ounces (3.41 dL) of beer, 5 ounces (1.421 dL) of wine, or 1.5 ounces (0.4262 dL) of hard liquor.
Hypertension is defined as systolic blood pressure ≥140 mmHg, diastolic blood pressure ≥90 mmHg, or a history of physician diagnosis.
Diabetes mellitus is defined as a fasting plasma glucose concentration ≥7.0 mmol/L (126 mg/dL), a history of physician diagnosis, or insulin use.
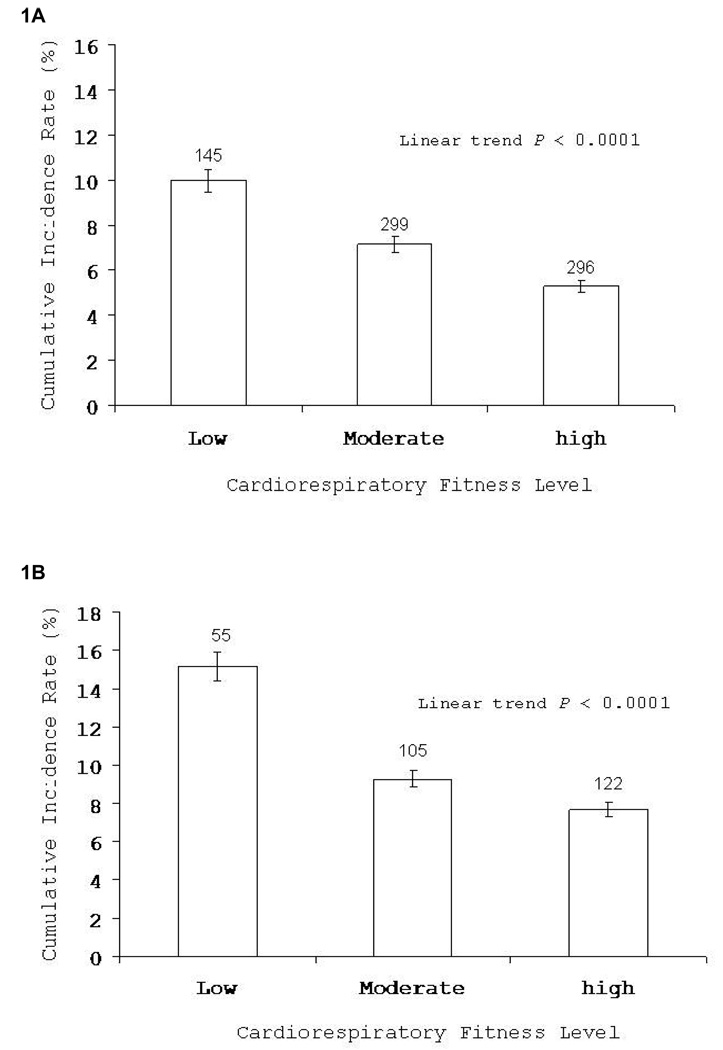
Fig. 1A (representing men) and Fig. 1B (representing women) present unadjusted incidence rates of elevated depressive symptoms across low, moderate, and high categories of CRF. There was an inverse gradient for the incidence of depressive symptoms across incremental levels of CRF (ptrend < 0.0001, for both men and women).
Fig. 1.
Incidence of depressive symptoms by cardiorespiratory fitness categories among men (1A) and women (1B). Bars indicate 95% confidence intervals. The number of cases is shown above the bars.
Table 2 shows odds ratios (ORs) for depressive symptoms, and their associated 95% confidence intervals (CIs), adjusted for age, baseline examination year, and the survey response year. Each additional minute of treadmill test duration at baseline was associated with 5% lower odds of reporting depressive symptoms at follow-up, for both men and women (OR 0.95; CI 0.94–0.97 for men, 0.92–0.98 for women). The analogous adjusted OR for depressive symptoms associated with each additional MET of maximal energy expenditure was 0.91 (CI 0.88–0.94) in men, and 0.89 (CI 0.83–0.95) in women. The remaining data rows of Table 2 show comparable ORs and CIs associated with other risk factors in the analysis, all adjusted for the same set of potential confounders. For example, obese individuals had higher depressive symptoms risk in comparison with those of normal weight in both men (OR 1.55, CI 1.22–1.96) and women (OR 1.93, CI 1.11–3.35). Those with stressful occupations had higher depressive symptoms risk than others. Current smokers had higher depressive symptoms risk than non-current smokers (Table 2).
Table 2.
Odds ratios (ORs)* for depressive symptoms, by study risk factors.
| Men |
Women |
|
|---|---|---|
| Variable | OR (95% CI) | OR (95% CI) |
| Treadmill test duration (per 1-minute increment) | 0.95 (0.94–0.97) | 0.95 (0.92–0.98) |
| Treadmill test maximal METs (per 1-MET increment) | 0.91 (0.88–0.94) | 0.89 (0.83–0.95) |
| Body mass index, kg/m2 | ||
| 18.5–24.9 | 1.00 (Referent) | 1.00 (Referent) |
| 25.0–29.9 | 1.14 (0.97–1.34) | 1.30 (0.91–1.85) |
| ≥30.0 | 1.55 (1.22–1.96) | 1.93 (1.11–3.35) |
| P-linear trend | 0.0007 | 0.01 |
| Stressful occupation | 1.56 (1.34–1.82) | 1.31 (0.97–1.76) |
| Current smoker | 1.42 (1.16–1.72) | 1.23 (0.82–1.84) |
| Alcohol consumption (≥5drinks/week) | 1.01 (0.87–1.18) | 1.00 (0.75–1.33) |
| Hypertension | 1.06 (0.89–1.27) | 1.11 (0.76–1.62) |
| Diabetes | 1.00 (0.68–1.47) | 1.80 (0.84–3.85) |
| Abnormal exercise ECG responses | 1.04 (0.73–1.46) | 1.14 (0.63–2.08) |
OR, odds ratio; CI, confidence interval. OR, odds ratio; CI, confidence interval; ECG, electrocardiogram; METs, metabolic equivalents.
Adjusted for age, baseline examination year, and survey response year.
Table 3 shows, separately for men and women, the number of study participants in each of the 3 CRF categories, the number of cases where respondents reported depressive symptoms at follow-up (events), and the ORs and CIs from 2 sets of logistic regression models focused on the 3 CRF categories. Model 1 adjusted for age, baseline examination year, and the year in which the participant completed the follow-up survey. In the results from Model 1, low CRF was a notable predictor of depressive symptoms for both women and men. Compared to women with low CRF, the odds of reporting depressive symptoms were 44% lower for women with moderate CRF (OR 0.56, CI 0.40–0.80), and 54% lower for women with high CRF (OR 0.46, CI 0.32–0.65, ptrend < 0.0001). Compared to men with low CRF, the odds of reporting depressive symptoms were 31% lower for men with moderate CRF (OR 0.69, CI 0.56–0.85) and 51% lower for men with high CRF (OR 0.49, CI 0.39–0.60, ptrend < 0.0001).
Table 3.
Risks for depressive symptoms, by level of cardiorespiratory fitness.
| Model 1* |
Model 2† |
|||||
|---|---|---|---|---|---|---|
| N | Events | OR | 95% CI | OR | 95% CI | |
| Men | ||||||
| Cardiorespiratory fitness | ||||||
| Low | 1,453 | 145 | 1.00 | Referent | 1.00 | Referent |
| Moderate | 4,186 | 299 | 0.69 | 0.56–0.85 | 0.73 | 0.58–0.91 |
| High | 5,619 | 296 | 0.49 | 0.39–0.60 | 0.53 | 0.42–0.68 |
| P-linear trend | <0.0001 | <0.0001 | ||||
| Women | ||||||
| Cardiorespiratory fitness | ||||||
| Low | 363 | 55 | 1.00 | Referent | 1.00 | Referent |
| Moderate | 1,131 | 105 | 0.56 | 0.40–0.80 | 0.60 | 0.42–0.87 |
| High | 1,591 | 122 | 0.46 | 0.32–0.65 | 0.51 | 0.34–0.75 |
| P-linear trend | <0.0001 | 0.002 | ||||
OR=odds ratio; CI=confidence interval; ECG=electrocardiogram.
Adjusted for age, baseline examination year and survey response year.
Adjusted for the above plus stressful occupation (yes or no), current smoking (yes or no), alcohol consumption (≥ 5 drinks/week or not), body mass index, hypertension, diabetes (present or not for each), and abnormal exercise ECG responses (present or not).
Model 2 of Table 3 further adjusts for stressful occupation, current smoking, alcohol consumption, body mass index, hypertension, diabetes, and abnormal exercise ECG responses. The results shown in Model 2 suggest only modest attenuation of the association between CRF and depressive symptoms with the additional controls for this larger set of potential confounders.
The associations between baseline CRF and the risks of reporting depressive symptoms at follow-up within categories of other possible risk factors are presented in Table 4. In men, after adjusting for age, baseline examination year, survey response year, and each of the other variables in the table, each additional MET of maximal exercise was, on average, associated with 4% to 14% (p < 0.05) lower odds of reporting depressive symptoms in each risk factor group, adverse or not. The consistency in the direction and magnitude of association between CRF and depressive symptoms suggested that there was little effect modification across risk factor categories. In women, the pattern of association between CRF and depressive symptoms was less consistent across risk factors, although in all instances the point estimates for the ORs were below 1.0. The somewhat larger variation among the point estimates for women, and the marginal statistical significance in many instances, may be attributable to a small number of events in many categories, and limited statistical power. Given the smaller number of women in the sample, it is also possible that the sample size for women may not have been sufficient to provide stable estimates.
Table 4.
Odds ratios for depressive symptoms per 1-MET increment in maximal exercise test, by study risk factors.*
| Men |
Women |
|||||||
|---|---|---|---|---|---|---|---|---|
| Risk Factor | N | Events | OR | 95% CI | N | Events | OR | 95% CI |
| Age, years† | ||||||||
| <55 | 9,380 | 621 | 0.88 | 0.80–0.97 | 2,523 | 238 | 0.95 | 0.88–1.02 |
| ≥55 | 1,878 | 119 | 0.96 | 0.92–1.00 | 562 | 44 | 0.86 | 0.69–1.07 |
| Body mass index, kg/m2† | ||||||||
| 18.5–24.9 | 5,268 | 317 | 0.94 | 0.89–0.99 | 2,592 | 226 | 0.91 | 0.84–0.99 |
| 25.0–29.9 | 4,889 | 326 | 0.91 | 0.85–0.97 | 385 | 40 | 0.93 | 0.73–1.18 |
| ≥30 | 1,101 | 97 | 0.86 | 0.75–0.99 | 108 | 16 | 0.61 | 0.35–1.04 |
| Stressful occupation† | ||||||||
| No | 7,113 | 390 | 0.94 | 0.89–0.99 | 2,534 | 220 | 0.94 | 0.87–1.02 |
| Yes | 4,145 | 350 | 0.91 | 0.86–0.96 | 551 | 62 | 0.78 | 0.65–0.93 |
| Current smoker† | ||||||||
| No | 9,746 | 609 | 0.94 | 0.90–0.98 | 2,814 | 252 | 0.91 | 0.84–0.99 |
| Yes | 1,512 | 131 | 0.87 | 0.78–0.96 | 271 | 30 | 0.85 | 0.63–1.05 |
| Alcohol consumption† | ||||||||
| <5 drinks/week | 6,560 | 427 | 0.94 | 0.89–0.99 | 2,312 | 210 | 0.92 | 0.84–1.00 |
| ≥5 drinks/week | 4,698 | 313 | 0.90 | 0.97–0.995 | 773 | 72 | 0.87 | 0.74–1.01 |
| Hypertension† | ||||||||
| No | 8,541 | 560 | 0.93 | 0.89–0.97 | 2,707 | 247 | 0.93 | 0.86–1.00 |
| Yes | 2,717 | 180 | 0.91 | 0.83–0.99 | 378 | 35 | 0.73 | 0.87–0.96 |
| Diabetes† | ||||||||
| No | 10,795 | 711 | 0.93 | 0.89–0.97 | 3,028 | 274 | 0.91 | 0.84–0.98 |
| Yes | 463 | 29 | 0.87 | 0.70–1.09 | 57 | 8 | 0.92 | 0.50–1.68 |
OR, odds ratio; CI, confidence interval
The point and interval estimates represent the relative odds of reporting depressive symptoms at follow-up that are associated, on average, with each 1-MET increment in the baseline treadmill exercise test.
Adjusted for baseline examination year, survey response year, and each of the other risk factors in the table.
4. Discussion
4.1. Principal findings
In longitudinal analyses, we found a sharply graded inverse dose-response relationship between CRF at baseline and depressive symptoms at follow-up, in both men and women. Participants with low CRF were at significantly greater risk of developing depressive symptoms, even after adjustment for age, baseline examination year, follow-up survey response year, smoking status, alcohol consumption, body mass index, hypertension, diabetes, and abnormal exercise ECG responses. The inverse association between CRF and depressive symptoms was generally consistent within strata of other potential risk factors.
The findings that obese men and women had significantly higher risk of depressive symptoms are consistent with other studies. Recently, Petry et al studied 41,654 participants and found that obesity was associated with significantly increased odds of major depression compared with normal weight individuals (Petry et al., 2008). It is becoming more evident that there may be a relationship between BMI and depressive symptoms. Future studies will be warranted to provide more evidence in this area.
4.2. Possible mechanisms of action
The precise physiological mechanisms associating physical activity with depression are not well established. Some possible explanations are proposed. Neuroscientific evidence suggests that exercise can increase the release of brain neurotransmitters such as monoamines and endorphins (Morgan, 1985;Thoren et al., 1990) or brain-derived neurotrophic factor (Zheng et al., 2006;Russo-Neustadt et al., 2000), which inhibit cell death and provide antidepressant effects. Other plausible mechanisms focus on psychosocial factors such as self-esteem (Stewart et al., 1994), social interaction (Peluso & Guerra de Andrade, 2005), and a sense of ownership (Stephens, 1988), which could result from improved fitness and may affect mental health and mood
Comparison with other published studies
The prospective results of the current study are consistent with previous follow-up studies that have suggested an inverse association between physical activity and depressive disorder not only in younger and middle-aged persons (Farmer et al., 1988;Camacho et al., 1991;Paffenbarger et al., 1994), but also in older men and women (Strawbridge et al., 2002;Kritz-Silverstein et al., 2001). Only a limited number of previous studies have objectively measured CRF (Thirlaway & Benton, 1992;Galper et al., 2006;Tolmunen et al., 2006). These studies have inconsistent findings, and are all limited by cross-sectional designs. Thirlaway and Benton did not find a relationship between CRF and depression in active individuals (Thirlaway & Benton, 1992), while the most recent two studies did report lower rates of depressive symptoms in persons with higher levels of CRF (Galper et al., 2006;Tolmunen et al., 2006). In the ACLS, Galper et al (Galper et al., 2006) found that both women and men had lower mean CES-D scores across higher fitness levels after adjustment for age, BMI, and years of participation in physical activity. In a group of middle-aged men from the Kuopio Ischemic Heart Disease Risk Factor study, Tolmunen and colleagues (Tolmunen et al., 2006) observed that men with low oxygen uptake (less than 28.1 ml/kg/min) had a 3.4fold higher risk of being depressed, compared with those with a VO2max exceeding 36.2 ml/kg/min. Furthermore, they reported a correlation between the severity of the depression scores and levels of fitness (Tolmunen et al., 2006). To our knowledge, the present study is the first to explore the association between CRF and depressive symptoms in longitudinal analysis. Future follow-up studies are needed to confirm our findings.
4.3. Strengths and limitations
The main strength of the current study is its use of maximal exercise testing to quantify CRF. CRF is considerably less prone to misclassification than other measures of physical activity. It may better reflect the adverse health consequences of a sedentary lifestyle than self-reported physical activity (Haskell et al., 1992). Thus, our approach improves on previous population-based studies, which have generally relied on self-reported measures of physical activity (Wiles et al., 2007;Paffenbarger et al., 1994;Farmer et al., 1988;Camacho et al., 1991). Second, we used measured risk factors and validated instruments to determine depressive symptoms. Third, the large number of person-years of follow-up enabled us to investigate dose-response effects in both men and women, and within categories of other exposures. Fourth, we accounted for variable patterns of survey responses in our analyses, an approach not usually used in cohort studies such as ours (Wiles et al., 2007). Finally, we excluded individuals with cancer, cardiovascular disease, or a self-report of having had a physician diagnosis of any mental disorder at baseline, reducing the likelihood of residual confounding. It is possible that participants with low CRF at baseline had high CES-D scores at baseline, which will underestimate the true association. The sociodemographic homogeneity of our study participants should enhance the internal validity of our findings, by reducing the likelihood of notable confounding by factors such as education, income, occupational and residential characteristics, prestige, and lifestyle. The self-referred origin and homogeneity of our cohort may be seen as a limitation. However, the ACLS study group is similar in many respects to other cohorts that have provided important information on disease prevention (Paffenbarger et al., 1994;Lee & Paffenbarger, 1998).
Nonetheless, generalizations to other adult populations should be made with caution. CES-D scores were not available at baseline. For that reason, we excluded those who reported having been clinically diagnosed with mental health disorders or chronic diseases, providing a degree of control for initial level of mental health. It should be recognized that relying on self-reports of previous diagnoses may introduce measurement error into the analysis. However, we have found in previous analyses that the sensitivity and specificity of self-report of other health problems in this cohort are quite high. For example, the percentage of agreement between self-reported events and participants’ medical records was 88%, 100%, 89% for myocardial infarction, revascularization, and stroke, respectively (Sui et al., 2007). We previously also verified the accuracy of self-reported, physician-diagnosed hypertension in this cohort and observed 98% sensitivity and 99% specificity (Blair et al., 1984). We did not use clinical diagnostic criteria to diagnose depression. Some of the study participants who were classified as having depressive symptoms might not have qualified for the diagnosis of clinical depression. It is also possible that the CES-D cut-point of 16 may be too low, in which case our outcome variable would mis-classify some individuals, as false positives. There is no widely accepted “optimal” cut-off score for the CES-D (Cheung et al., 2007).
Studies of the validity of the CES-D for identifying symptoms associated with major depressive disorder in adults have suggested a range of optimal scores for screening, generally ranging from 16 to 25 (Parikh et al., 1988;Lyness et al., 1997;Haringsma et al., 2004;Wada et al., 2007). A recent detailed study of the performance of the CES-D for screening for major depression in cancer patients identified the optimal cut-off score of 17, which was associated with 100% sensitivity, 79% specificity, and 92% positive predictive value (Hopko et al., 2008); thus, this score missed no cases of clinical depression, and correctly identified 79% of those without depression, while only 8% of those screening positive would not be clinically diagnosed with major depression. Nonetheless, we stress that case-finding tools such as the CES-D were not developed to make clinical diagnoses. Although results from the CES-D are commonly used for outcome measurement in epidemiological studies, this tool was developed primarily to identify individuals with clinically significant symptoms that require more intensive evaluation. We therefore advise caution when interpreting the results of this study. To examine the sensitivity of the cut-point used in this analysis, we conducted additional analyses using cut-points of 20 and 24 to define depressive symptoms. In these sensitivity analyses, the association between CRF and the depressive symptoms outcome was not materially changed (data not shown). The findings of our study also may be affected by unmeasured factors related to fitness and/or depressive symptoms, although it seems unlikely that such factors would explain all of the observed association between CRF and depressive symptoms. We do not have sufficient information on medication use, treatments, menopausal status, pregnancy status, or dietary habits to include these factors in our analysis. Such information should be included in future studies, to expand on the findings reported here.
4.4. Implications of the current study
We computed population attributable risk (Rothman & Greenland, 1998) values to estimate the burden of depressive symptoms attributable to low fitness and other risk predictors. If all individuals with low fitness in our population sample became fit, the incidence of depressive symptoms cases might have been xx% and xx% lower in men and women, respectively (data not shown). Currently, there is not enough data to determine how much of the depressive symptoms burden may be due to low fitness. These findings from the ACLS cohort suggest that assessing and possibly increasing population fitness levels should be given consideration for primary prevention and for lowering the burden of depressive symptoms through lifestyle changes such as increasing physical activity. CRF can be enhanced through participation in moderate and vigorous physical activities, such as brisk walking, bicycling, and jogging for 30 minutes or more on most days of the week (Pate et al., 1995). Our recent randomized trial showed a significant improvement in VO2max in overweight or obese postmenopausal women with as little as 72 minutes of moderate intensity physical activity/week (Church et al., 2007). The clear dose-response relationship between fitness and depressive symptoms found in the current study should encourage individuals to lead a physically active lifestyle. Doing so may reduce not only cardiovascular disease, the leading cause of death in the United States, but also the risk of depression. These findings are also helpful for health care professionals. They suggest the usefulness of advising sedentary patients about the benefits of physical activity for mental well-being.
4.5. Conclusions
In a large sample of men and women, this prospective study showed that CRF is inversely associated with the risk of developing elevated depressive symptoms. This result is consistent with previous longitudinal studies of self-reported physical activity and depressive symptoms. Further studies are needed to expand our findings on functional capacity assessment using exercise testing to clinically defined depression in the general population.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference List
- American College of Sports Medicine. ACSM's Guidelines For Exercise Testing And Prescription. Philadelphia: Lippincott Williams and Wilkins; 2005. [Google Scholar]
- Balke B, Ware RW. An experimental study of physical fitness in Air Force personnel. US Armed Forces Medical Journal. 1959;10:675–688. [PubMed] [Google Scholar]
- Beekman AT, Deeg DJ, Van Limbeek J, Braam AW, De Vries MZ, van Tilburg W. Criterion validity of the Center for Epidemiologic Studies Depression scale (CES-D): results from a community-based sample of older subjects in The Netherlands. Psychology Medicine. 1997;27:231–235. doi: 10.1017/s0033291796003510. [DOI] [PubMed] [Google Scholar]
- Blair SN, Goodyear NN, Gibbons LW, Cooper KH. Physical fitness and incidence of hypertension in healthy normotensive men and women. Journal of the American Medical Association. 1984;252:487–490. [PubMed] [Google Scholar]
- Blair SN, Kampert JB, Kohl HW, Barlow CE, Macera CA, Paffenbarger RSJ, Gibbons LW. Influences of cardiorespiratory fitness and other precursors on cardiovascular disease and allcause mortality in men and women. Journal of the American Medical Association. 1996;276:205–210. [PubMed] [Google Scholar]
- Blair SN, Kohl HW, III, Paffenbarger RS, Jr, Clark DG, Cooper KH, Gibbons LW. Physical fitness and all-cause mortality: a prospective study of healthy men and women. Journal of the American Medical Association. 1989;262:2395–2401. doi: 10.1001/jama.262.17.2395. [DOI] [PubMed] [Google Scholar]
- Blumenthal JA, Babyak MA, Moore KA, Craighead WE, Herman S, Khatri P, Waugh R, Napolitano MA, Forman LM, Appelbaum M, Doraiswamy PM, Krishnan KR. Effects of exercise training on older patients with major depression. Arch Intern Med. 1999;159:2349–2356. doi: 10.1001/archinte.159.19.2349. [DOI] [PubMed] [Google Scholar]
- Bouchard C, Blair SN, Haskell WL. Physical activity and health. Human kinetics, Inc.; 2006. [Google Scholar]
- Camacho TC, Roberts RE, Lazarus NB, Kaplan GA, Cohen RD. Physical activity and depression: evidence from the Alameda County Study. Am J Epidemiol. 1991;134:220–231. doi: 10.1093/oxfordjournals.aje.a116074. [DOI] [PubMed] [Google Scholar]
- Cheung YB, Liu KY, Yip PS. Performance of the CES-D and its short forms in screening suicidality and hopelessness in the community. Suicide Life Threat.Behav. 2007;37:79–88. doi: 10.1521/suli.2007.37.1.79. [DOI] [PubMed] [Google Scholar]
- Church TS, Earnest CP, Skinner JS, Blair SN. Effects of different doses of physical activity on cardiorespiratory fitness among sedentary, overweight or obese postmenopausal women with elevated blood pressure: a randomized controlled trial. Journal of the American Medical Association. 2007;297:2081–2091. doi: 10.1001/jama.297.19.2081. [DOI] [PubMed] [Google Scholar]
- Cooper-Patrick L, Ford DE, Mead LA, Chang PP, Klag MJ. Exercise and depression in midlife: A prospective study. Am J Public Health. 1997;87:670–673. doi: 10.2105/ajph.87.4.670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Druss BG, Rosenheck RA, Sledge WH. Health and disability costs of depressive illness in a major U.S. corporation. Am J Psychiatry. 2000;157:1274–1278. doi: 10.1176/appi.ajp.157.8.1274. [DOI] [PubMed] [Google Scholar]
- Dunn AL, Trivedi MH, O'Neal HA. Physical activity dose-response effects on outcomes of depression and anxiety. Medicine and Science in Sports and Exercise. 2001;33:S587–S597. doi: 10.1097/00005768-200106001-00027. [DOI] [PubMed] [Google Scholar]
- Farmer ME, Locke BZ, Moscicki EK, Dannenberg AL, Larson DB, Radloff LS. Physical activity and depressive symptoms: the NHANES I Epidemiologic Follow-up Study. Am J Epidemiol. 1988;128:1340–1351. doi: 10.1093/oxfordjournals.aje.a115087. [DOI] [PubMed] [Google Scholar]
- Galper DI, Trivedi MH, Barlow CE, Dunn AL, Kampert JB. Inverse Association between Physical Inactivity and Mental Health in Men and Women. Medicine and Science in Sports and Exercise. 2006;38:173–178. doi: 10.1249/01.mss.0000180883.32116.28. [DOI] [PubMed] [Google Scholar]
- Gibbons LW, Mitchell TL, Wei M, Blair SN, Cooper KH. Maximal exercise test as a predictor of risk for mortality from coronary heart disease in asymptomatic men. American Journal of Cardiology. 2000;86:53–58. doi: 10.1016/s0002-9149(00)00827-4. [DOI] [PubMed] [Google Scholar]
- Green RC, Cupples LA, Kurz A, Auerbach S, Go R, Sadovnick D, Duara R, Kukull WA, Chui H, Edeki T, Griffith PA, Friedland RP, Bachman D, Farrer L. Depression as a risk factor for Alzheimer disease: the MIRAGE Study. Arch Neurol. 2003;60:753–759. doi: 10.1001/archneur.60.5.753. [DOI] [PubMed] [Google Scholar]
- Greenberg PE, Kessler RC, Birnbaum HG, Leong SA, Lowe SW, Berglund PA, Corey-Lisle PK. The economic burden of depression in the United States: how did it change between 1990 and 2000? J Clin Psychiatry. 2003;64:1465–1475. doi: 10.4088/jcp.v64n1211. [DOI] [PubMed] [Google Scholar]
- Haringsma R, Engels GI, Beekman AT, Spinhoven P. The criterion validity of the Center for Epidemiological Studies Depression Scale (CES-D) in a sample of self-referred elders with depressive symptomatology. Int J Geriatr Psychiatry. 2004;19:558–563. doi: 10.1002/gps.1130. [DOI] [PubMed] [Google Scholar]
- Haskell WL, Leon AS, Caspersen CJ, Froelicher VF, Hagberg JM, Harlan W, Holloszy JO, Regensteiner JG, Thompson PD, Washburn RA. Cardiovascular benefits and assessment of physical activity and physical fitness in adults. Med Sci.Sports Exerc. 1992;24:S201–S220. [PubMed] [Google Scholar]
- Heikkinen RL, Kauppinen M. Depressive symptoms in late life: a 10-year follow-up. Arch Gerontol Geriatr. 2004;38:239–250. doi: 10.1016/j.archger.2003.10.004. [DOI] [PubMed] [Google Scholar]
- Hopko DR, Bell JL, Armento ME, Robertson SM, Hunt MK, Wolf NJ, Mullane C. The phenomenology and screening of clinical depression in cancer patients. J Psychosoc.Oncol. 2008;26:31–51. doi: 10.1300/j077v26n01_03. [DOI] [PubMed] [Google Scholar]
- Jorm AF. Is depression a risk factor for dementia or cognitive decline? A review. Gerontology. 2000;46:219–227. doi: 10.1159/000022163. [DOI] [PubMed] [Google Scholar]
- Kouzis A, Eaton WW, Leaf PJ. Psychopathology and mortality in the general population. Soc Psychiatry Psychiatr.Epidemiol. 1995;30:165–170. doi: 10.1007/BF00790655. [DOI] [PubMed] [Google Scholar]
- Krishnan KR, Delong M, Kraemer H, Carney R, Spiegel D, Gordon C, McDonald W, Dew M, Alexopoulos G, Buckwalter K, Cohen PD, Evans D, Kaufmann PG, Olin J, Otey E, Wainscott C. Comorbidity of depression with other medical diseases in the elderly. Biol Psychiatry. 2002;52:559–588. doi: 10.1016/s0006-3223(02)01472-5. [DOI] [PubMed] [Google Scholar]
- Kritz-Silverstein D, Barrett-Connor E, Corbeau C. Cross-sectional and prospective study of exercise and depressed mood in the elderly: the Rancho Bernardo study. American Journal of Epidemiology. 2001;153:596–603. doi: 10.1093/aje/153.6.596. [DOI] [PubMed] [Google Scholar]
- Lawlor DA, Hopker SW. The effectiveness of exercise as an intervention in the management of depression: systematic review and meta-regression analysis of randomised controlled trials. BMJ. 2001;322:763–767. doi: 10.1136/bmj.322.7289.763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee I-M, Paffenbarger RSJ. Physical activity and stroke incidence: The Harvard Alumni Health Study. Stroke. 1998;29:2049–2054. doi: 10.1161/01.str.29.10.2049. [DOI] [PubMed] [Google Scholar]
- Lennox SS, Bedell JR, Stone AA. The effect of exercise on normal mood. Journal of Psychosomatic Research. 1990;34:629–636. doi: 10.1016/0022-3999(90)90106-e. [DOI] [PubMed] [Google Scholar]
- Lyness JM, Noel TK, Cox C, King DA, Conwell Y, Caine ED. Screening for depression in elderly primary care patients. A comparison of the Center for Epidemiologic Studies-Depression Scale and the Geriatric Depression Scale. Arch Intern Med. 1997;157:449–454. [PubMed] [Google Scholar]
- Macera CA, Jackson KL, Davis DR, Kronenfeld JJ, Blair SN. Patterns of non-response to a mail survey. Journal of Clinical Epidemiology. 1990;43:1427–1430. doi: 10.1016/0895-4356(90)90112-3. [DOI] [PubMed] [Google Scholar]
- Morgan WP. Affective beneficence of vigorous physical activity. Medicine and Science in Sports and Exercise. 1985;17:94–100. [PubMed] [Google Scholar]
- NIMH. 2006. “The numbers count: mental illness in America,”. Science on our minds fact sheet series. In: [Google Scholar]
- Paffenbarger RSJ, Lee IM, Leung R. Physical activity and personal characteristics associated with depression and suicide in American college men. Acta Psychiatrica Scandinavica Supplement. 1994;377:16–22. doi: 10.1111/j.1600-0447.1994.tb05796.x. [DOI] [PubMed] [Google Scholar]
- Parikh RM, Eden DT, Price TR, Robinson RG. The sensitivity and specificity of the Center for Epidemiologic Studies Depression Scale in screening for post-stroke depression. Int J Psychiatry Med. 1988;18:169–181. doi: 10.2190/bh75-euya-4fm1-j7qa. [DOI] [PubMed] [Google Scholar]
- Pate RR, Pratt M, Blair SN, Haskell WL, Macera CA, Bouchard C, Buchner D, Ettinger W, Heath GW, King AC, Kriska A, Leon AS, Marcus BH, Morris J, Paffenbarger RS, Jr, Patrick K, Pollock ML, Rippe JM, Sallis J, Wilmore JH. Physical activity and public health: a recommendation from the Centers for Disease Control and Prevention and the American College of Sports Medicine. Journal of the American Medical Association. 1995;273:402–407. doi: 10.1001/jama.273.5.402. [DOI] [PubMed] [Google Scholar]
- Peluso MA, Guerra de Andrade LH. Physical activity and mental health: the association between exercise and mood. Clinics. 2005;60:61–70. doi: 10.1590/s1807-59322005000100012. [DOI] [PubMed] [Google Scholar]
- Penninx BW, Beekman AT, Honig A, Deeg DJ, Schoevers RA, van Eijk JT, van TW. Depression and cardiac mortality: results from a community-based longitudinal study. Arch Gen.Psychiatry. 2001;58:221–227. doi: 10.1001/archpsyc.58.3.221. [DOI] [PubMed] [Google Scholar]
- Petry NM, Barry D, Pietrzak RH, Wagner JA. Overweight and obesity are associated with psychiatric disorders: results from the National Epidemiologic Survey on Alcohol and Related Conditions. Psychosom Med. 2008;70:288–297. doi: 10.1097/PSY.0b013e3181651651. [DOI] [PubMed] [Google Scholar]
- Pollock ML, Bohannon RL, Cooper KH, Ayres JJ, Ward A, White SR, Linnerud AC. A comparative analysis of four protocols for maximal treadmill stress testing. American Heart Journal. 1976;92:39–46. doi: 10.1016/s0002-8703(76)80401-2. [DOI] [PubMed] [Google Scholar]
- Pollock ML, Foster C, Schmidt D, Hellman C, Linnerud AC, Ward A. Comparative analysis of physiologic responses to three different maximal graded exercise test protocols in healthy women. American Heart Journal. 1982;103:363–373. doi: 10.1016/0002-8703(82)90275-7. [DOI] [PubMed] [Google Scholar]
- Radloff LS. The CES-D Scale: a self-report depression scale for research in the general population. Applied Psychological Measurement. 1977;1:385–401. [Google Scholar]
- Ried LD, Planas LG. Aging, health, and depressive symptoms: are women and men different? J Womens Health (Larchmt.) 2002;11:813–824. doi: 10.1089/15409990260430963. [DOI] [PubMed] [Google Scholar]
- Rothman KJ, Greenland S. Modern epidemiology. Philadelphia, PA: Lippincott-Raven Publishers; 1998. [Google Scholar]
- Russo-Neustadt AA, Beard RC, Huang YM, Cotman CW. Physical activity and antidepressant treatment potentiate the expression of specific brain-derived neurotrophic factor transcripts in the rat hippocampus. Neuroscience. 2000;101:305–312. doi: 10.1016/s0306-4522(00)00349-3. [DOI] [PubMed] [Google Scholar]
- Stathopoulou G, Powers MB, Berry AC, Smits AJ, Otto MW. Exercise interventions for mental health: A quantitative and qualitative review. Clin Psychol Sci Prac. 2006;13:179–193. [Google Scholar]
- Stephens T. Physical activity and mental health in the United States and Canada: evidence from four population surveys. Preventive Medicine. 1988;17:35–47. doi: 10.1016/0091-7435(88)90070-9. [DOI] [PubMed] [Google Scholar]
- Stewart AL, Hays RD, Wells KB, Rogers WH, Spritzer KL, Greenfield S. Long-term functioning and well-being outcomes associated with physical activity and exercise in patients with chronic conditions in the Medical Outcomes Study. Journal of Clinical Epidemiology. 1994;47:719–730. doi: 10.1016/0895-4356(94)90169-4. [DOI] [PubMed] [Google Scholar]
- Strawbridge WJ, Deleger S, Roberts RE, Kaplan GA. Physical activity reduces the risk of subsequent depression for older adults. Am J Epidemiol. 2002;156:328–334. doi: 10.1093/aje/kwf047. [DOI] [PubMed] [Google Scholar]
- Sui X, LaMonte MJ, Blair SN. Cardiorespiratory fitness as a predictor of nonfatal cardiovascular events in asymptomatic women and men. American Journal of Epidemiology. 2007;165:1413–1423. doi: 10.1093/aje/kwm031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thirlaway K, Benton D. Participation in physical activity and cardiovascular fitness have different effects on mental health and mood. Journal of Psychosomatic Research. 1992;36:657–665. doi: 10.1016/0022-3999(92)90055-7. [DOI] [PubMed] [Google Scholar]
- Thoren P, Floras JS, Hoffmann P, Seals DR. Endorphins and exercise: physiological mechanisms and clinical implications. Medicine and Science in Sports and Exercise. 1990;22:417–428. [PubMed] [Google Scholar]
- Tolmunen T, Laukkanen JA, Hintikka J, Kurl S, Viinamaki H, Salonen R, Kauhanen J, Kaplan GA, Salonen JT. Low maximal oxygen uptake is associated with elevated depressive symptoms in middle-aged men. Eur J Epidemiol. 2006;21:701–706. doi: 10.1007/s10654-006-9038-5. [DOI] [PubMed] [Google Scholar]
- Wada K, Tanaka K, Theriault G, Satoh T, Mimura M, Miyaoka H, Aizawa Y. Validity of the Center for Epidemiologic Studies Depression Scale as a screening instrument of major depressive disorder among Japanese workers. Am J Ind.Med. 2007;50:8–12. doi: 10.1002/ajim.20403. [DOI] [PubMed] [Google Scholar]
- Weyerer S. Physical inactivity and depression in the community. Evidence from the Upper Bavarian Field Study. Int J Sports Med. 1992;13:492–496. doi: 10.1055/s-2007-1021304. [DOI] [PubMed] [Google Scholar]
- Wiles NJ, Haase AM, Gallacher J, Lawlor DA, Lewis G. Physical activity and common mental disorder: results from the caerphilly study. Am J Epidemiol. 2007;165:946–954. doi: 10.1093/aje/kwk070. [DOI] [PubMed] [Google Scholar]
- Zheng H, Liu Y, Li W, Yang B, Chen D, Wang X, Jiang Z, Wang H, Wang Z, Cornelisson G, Halberg F. Beneficial effects of exercise and its molecular mechanisms on depression in rats. Behav Brain Res. 2006;168:47–55. doi: 10.1016/j.bbr.2005.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]