Abstract
The central nervous system (CNS) of adult mammals regenerates poorly; in vivo, neurogenesis occurs only in two restricted areas, the hippocampal subgranular zone (SGZ) and the subventricular zone (SVZ). Neurogenic potential depends on both the intrinsic properties of neural progenitors and the environment, or niche, in which progenitor cells reside. Isolation of multipotent progenitor cells from broad CNS regions suggests that the neurogenic potential of the adult CNS is dictated by local environmental cues. Here, we report that astrocytes in the neurogenic brain regions, the SGZ and SVZ, of adult mice release molecular signals, such as sonic hedgehog (Shh), that stimulate adult neural progenitors to reenter the cell cycle and generate new neurons in vitro and in vivo. Transplantation of SGZ astrocytes or application of Shh caused de novo neurogenesis from the non-neurogenic neocortex of adult mice. These findings identify a molecular target that can activate the dormant neurogenic potential from nonconventional neurogenic regions of the adult CNS and suggest a novel mechanism of neural replacement therapy for treating neurodegenerative disease and injury without transplanting exogenous cells.
Keywords: Adult stem cell, Neural stem cell, Astrocytes, Stem cell-microenvironment interactions
INTRODUCTION
Neural progenitors and their niches are spatially ephemeral and temporally transient in development. In the central nervous system (CNS) of adult mammals, constitutive neurogenesis is retained in only two regions—the subgranular zone (SGZ) and subventricular zone (SVZ), which give rise to neurons of the hippocampal dentate gyrus and olfactory bulb [1–3]. In contrast, most adult CNS areas outside the SGZ and SVZ do not exhibit constitutive neurogenesis in vivo under normal conditions. Glial-lineage progenitors, which can give rise to neurons in vitro, have been found in areas outside the classic neurogenic regions, including the optic nerve, striatum, hypothalamus, and subcortical white matter [4–7]. The fact that limited neurogenesis can be induced either in vitro or in vivo by injury-induced signals [8] or by specific cues [4, 7, 9, 10] suggests that the neurogenic potential of a certain CNS region may be determined by local environmental cues.
Compelling evidence indicates that neural progenitors in neurogenic areas—the SVZ and SGZ—are in intimate contact with endothelial cells and astrocytes [11, 12], which helps to generate an instructive niche that promotes neurogenesis [13]. Most interestingly, astrocytes isolated from different CNS regions exhibit regional heterogeneity with regard to their ability to promote progenitor cell proliferation and differentiation [14]. SVZ and SGZ astrocytes are capable of supporting neurogenesis by participating in the creation of an instructive niche, whereas astrocytes isolated from the spinal cord fail to do so [11]. These observations support a notion that the neurogenic potential of the adult CNS is controlled by environmental cues associated with local astrocytes.
Astrocytes have classically been considered support cells in the brain and key mediators of many brain processes, including neural development, function, and plasticity [15]. An emerging view now points to the heterogeneity and developmental specification of subpopulations of astrocytes throughout the brain, allowing them to function uniquely as sensors and regulators of the CNS microenvironment. However, the molecular signals associated with astrocytes that regulate the ability of neural stem cells to self-renew and differentiate are largely unknown. One of the signals that are involved in proliferation in adult neurogenic regions is thought to be Sonic hedgehog (Shh) [16–19]. The cellular source of Shh in the SVZ and SGZ remains unclear. During development, Shh acts as both a morphogen and a mitogen, playing crucial roles in ventral patterning of the neuraxis and neural precursor cell proliferation [20]. Mice deficient in shh signaling have malformation of neural stem cell niches in the postnatal brain [21]. Uncovering the molecular signals underlying neural stem cell and niche interactions will provide invaluable insight into the potential use of neural stem cells for neuroregeneration and repair in disease or damage.
In the present study, we showed that neural progenitors similar to those found in the SVZ and SGZ are widely distributed in the entire CNS of adult mice. The neurogenic potential of certain CNS regions is dictated by local environmental cues or astrocyte production of growth-stimulating signals, in particular Shh. These findings suggest the exciting possibility of a novel mechanism for neural repair and cell replacement therapy in the adult CNS by activating an endogenous source of neural progenitors.
MATERIALS AND METHODS
Animals
Green fluorescent protein transgenic (GFPtg) mice driven under the control of a chicken β-actin promoter and cytomegalovirus enhancer were courtesy of Dr. Okabe, Osaka University (Osaka, Japan) [22]. Glial fibrillary acidic protein (GFAP)-green fluorescent protein (GFP) mice driven under the control of a human GFAP promoter were purchased from the Jackson Laboratory (Bar Harbor, ME, http://www.jax.org) [23]. GFAP-TK mice (line 7.1) were generated under the control of mouse GFAP promoter [24]. Mice older than 2 months are referred to as adult. All experimental procedures and use and care of animals followed a protocol approved by the Animal Care and Use Committee at the Schepens Eye Research Institute, Harvard Medical School.
Preparation of Astrocyte Cultures
Postnatal day 0 (P0) cortical or adult SGZ astrocytes were prepared as described [25]. In brief, corresponding brain regions were dissected and trypsinized. Dissociated cells were cultured in Dulbecco’s modified Eagle’s medium (DMEM)/Ham’s F-12 medium (F12) (1:1) with 10% fetal bovine serum (FBS) for 7 days. Astrocytes were then purified by subjecting cultured cells to 24 hours of continuous shaking at 110 rpm, and detached cells were removed. More than 95% of cells that remained attached to culture plates were found to be positive for GFAP. For coculturing assays, purified astrocytes were transferred to six-well transwell culture inserts at 1 × 104 cells per well at the same time that dissociated cortical, cerebellar, or spinal cord cells were plated.
Dissociated CNS Cell-Astrocyte Cocultures
The gray matter of cortex, cerebellum, or spinal cord of adult wild-type (WT), GFPtg, or GFAP-GFP mice was dissected and dissociated with a 15-minute treatment of 0.05% papain in Eagle’s buffered salt solution at 37°C. Enzymatic digestion was stopped by addition of 1% ovomucoid. Cells were washed twice with serumfree DMEM/F12, triturated, and passed through a 40-µm cell strainer (BD Biosciences, San Jose, CA, http://www.bdbiosciences.com) to remove cell aggregates and debris. Cells were then plated in six-well culture plates at clonal density (1 × 105 cells per well) in the same well with transwell inserts containing niche astrocytes. For control conditions, NIH3T3 fibroblasts, age-matched cortical cells, or astrocytes of the adult cortex were plated in the transwell inserts at the same density. Cells were cultured in DMEM/F12 and Neurobasal medium (mixed 1:1 [Invitrogen, Carlsbad, CA, http://www.beta.invitrogen.com]) supplemented with N2, B27, epidermal growth factor (20 ng/ml), and basic fibroblast growth factor (10 ng/ml). After an 8-day incubation, neurospheres larger than 50 cells were counted. For cell expansion and self-renewal studies, individual neurospheres were collected, dissociated, and plated at a low density. Counts of secondary, tertiary, and subsequent spheres were always performed at 8 days after plating. To search for molecules affecting CNS neurogenesis, candidate proteins were added to cortical cell-astrocyte cocultures, and after an 8-day incubation, neurospheres were counted.
Immunohistochemistry
Immunodetection was performed using standard protocols. For 5′-bromo-2′-deoxyuridine (BrdU) labeling, sections were also treated with HCl (2.0 M) for 2 hours at 37°C. Primary antibodies used included rat anti-BrdU (1:100; Novus Biologicals, Inc., Littleton, CO, http://www.novusbio.com), mouse monoclonal antibodies specific for β-III-tubulin (Tuj1; 1:200; BD Biosciences), doublecortin (Dcx), HuD (1:200; Chemicon, Temecula, CA, http://www.chemicon.com), NeuN, microtubule-associated protein 2 (MAP2), γ-aminobutyric acid (GABA), acetylcholine transporter, glutamate decarboxylase-67 (1:200; all from Chemicon), RIP (1:200; Chemicon) O4 and GFAP (1:2,000; Sigma-Aldrich, St. Louis, http://www.sigmaaldrich.com), and rabbit anti-S100 (1:300; Lab Vision, Fremont, CA, http://www.labvision.com). Secondary antibodies conjugated with Cy2, Cy3, or Cy5 (Jackson Immunoresearch Laboratories, West Grove, PA, http://www.jacksonimmuno.com) were used.
Cell Differentiation Assay
For differentiating neurosphere-derived cells in vitro, neurospheres were dissociated with trypsin, plated in Matrigel (BD Biosciences)-coated four-well culture chamber slides (Nalge Nunc International, Rochester, NY, http://www.nalgenunc.com), and incubated in DMEM/F12 supplemented with N2, B27, retinoid acid (1 µM), and 2% FBS. Eighty percent of the exhausted medium was replaced every other day. Cell differentiation was analyzed, after a 7-day incubation, by immunocytochemistry. Most data presented were generated using cells derived from secondary and tertiary spheres. For studying cell differentiation in vivo, sphere-derived cells were injected into the cortex of P0 mouse pups. Mice were sacrificed at 4 weeks after transplantation and transcardially perfused with phosphate-buffered saline (PBS) followed by 4% paraformaldehyde. Frozen brain sections were subjected to immunohistochemistry.
In Vivo Neurogenesis Experiments
Two microliters of cell suspension containing 1 × 104 niche astrocytes isolated from P0 GFPtg mice or 2 µl of PBS containing 100 µg/ml Shh were injected slowly through a glass micropipette into the cortexes of adult mice. BrdU (50 mg/kg) was administered through daily intraperitoneal injection for seven consecutive days. Mice were sacrificed 14 days after treatment and transcardially perfused with PBS followed by 4% paraformaldehyde.
RESULTS
Neural Progenitor Cells in the Non-Neurogenic Cerebral Cortex of Adult Mice
To confirm that neural progenitor cells distribute widely in nonconventional neurogenic regions of the adult CNS, cells dissociated from the non-neurogenic cerebral cortex (the posterior region to exclude the SVZ) were cocultured with niche astrocytes isolated from neurogenic areas. Because the white matter of the adult brain harbors a pool of NG2-positive oligodendrocyte progenitor cells, which in culture can give rise to neurospheres [6], we dissected only gray matter of the cerebral cortex and placed these cells in cocultures. Astrocytes of P0 mouse brains and the SGZ of adult mice are considered niche astrocytes, which present an environment permissive for neural progenitor cell proliferation.
Dissociated gray matter cortical cells were plated at a clonal density on the base of six-well culture plates, whereas upper transwells were seeded with niche astrocytes isolated from the adult SGZ or P0 mouse brain (Fig. 1A). In control experiments, niche astrocytes were replaced with either NIH3T3 fibroblasts, age-matched cortical cells of adult mice, or no cells. Cells in the upper and lower compartments were separated by a membrane with 0.4-µm-diameter pores. After 8 days, cortical cell cultures provided with either adult SGZ or P0 brain astrocytes developed large neurospheres that were positive for the neural progenitor marker nestin (Fig. 1B). In contrast, control cultures that were seeded with either NIH3T3 fibroblasts, adult cortical cells, or adult spinal cord astrocytes or without cells in the upper compartment generated few neurospheres or colonies (Fig. 1B). The number of neurospheres in cultures plated in the presence of SGZ or P0 brain astrocytes was 10–20-fold higher than the number in control groups (Fig. 1C), indicating that neural progenitor-like cells exist in the adult cortex.
Figure 1.
Development of neurospheres in adult cortical cell cultures. (A): Schematic illustration of adult cortical cells that were cocultured with feeder cells. (B): Niche astrocytes induced neurosphere formation from adult cortical cells. Large floating neurospheres developed after 8 days of incubation in cortical cell cultures prepared from adult mice that were plated in the same well with A-SGZ or AST-P0 but not in those that were cocultured with NIH3T3. Neurospheres were stained positive for antinestin. Scale bar = 40 µm. (C): Numbers of neurospheres developed in dissociated adult cortical cell cultures plated in the presence or absence of various feeder cells after an 8-day incubation. Neurospheres larger than 50 cells were counted (*, p < .001; Student’s t test and analysis of variance). (D): A green fluorescent protein+ neurosphere developed in astrocyte-cortical cell cocultures, in which cortical cells were derived from a GFPtg mouse. Scale bar = 25 µm. (E): Number of neurospheres developed in cortical cell cultures in the presence of control medium (control) or N-CM or A-CM culture. Abbreviations: A-CM, P0 subgranular zone astrocyte; A-Cx, adult cortical cells; A-SC, adult spinal cord astrocytes; A-SGZ, adult subgranular zone; AST-P0, P0 brain astrocytes; N-CM, medium conditioned by NIH3T3; NIH3T3, NIH3T3 fibroblasts.
To further demonstrate the existence of neural progenitors in the adult cortex and exclude the possibility that neurospheres were formed by cells in the upper transwell (niche astrocytes) that had migrated through the membrane pores to the basal compartment, we isolated either cortical cells or astrocytes from tg mice carrying the GFP transgene. In this mouse line, GFP transgene is driven by a chicken β-actin promoter and cytomeg-alovirus enhancer (GFPtg), and all cells isolated from these mice are GFP-positive (+) [22]. In the following experiments, P0 SGZ astrocytes were used as niche cells, as they were easier to obtain and resulted in a sphere-inducing effect similar to that of adult SGZ astrocytes. Notably, when cortical cells isolated from GFPtg mice were cocultured with astrocytes of P0 GFP-negative (−) wild-type mice, all neurospheres were GFP+ (Fig. 1D). In contrast, when GFP− cortical cells were cocultured with astrocytes of P0 GFPtg mice, all of the spheres were GFP− (not shown). Moreover, in the absence of niche astrocytes, medium conditioned by P0 brain astrocytes stimulated neurosphere formation in cortical cell cultures in comparison with the control culture or cultures treated with medium conditioned by NIH3T3 cells (Fig. 1E). These results demonstrate that sphere-forming cells are derived from the cerebral cortex, but not niche astrocytes, of adult mice.
Sphere-Forming Cells Are Widely Distributed in Non-Neurogenic CNS Regions of Adult Mice
We then further confirmed the general presence of neural progenitors in other non-neurogenic regions of the adult CNS. Cells harvested from the gray matter of the adult cerebellum and spinal cord were cocultured with P0 astrocytes. After 8–14 days, both cerebellum and spinal cord cell cultures plated with P0 astrocytes developed large neurospheres, whereas few neurospheres were seen in cultures in the absence of niche astrocytes or plated with control NIH3T3 fibroblasts (Fig. 2A). These results show that sphere-forming cells can be derived from many areas of heretofore non-neurogenic CNS regions.
Figure 2.
Wide distribution of neural progenitors in the adult central nervous system. (A): Number of neurospheres developed in cultures derived from gray-matter cells of the cerebellum and spinal cord of adult mice that were cultured in the absence (control) or presence of P0 Astro for 8 days. Results represent the mean ± SD (n = 9; * p < .01; Student’s t test). (B): Sphere-forming cells undergo active cell division. Sphere-derived cells were dissociated and cultured in the presence of BrdU for 24 hours and were labeled by anti-BrdU (red) and nuclei (DAPI; blue) staining. Scale bar = 40 µm. Abbreviations: Astro, astrocytes; BrdU, 5′-bromo-2′-deoxyuridine; DAPI, 4′,6-diamidino-2-phenylindole dihydrochloride.
To investigate the progenitor cell potential of sphere-forming cells, we examined their capacity for self-renewal and multipotency. Secondary or tertiary neurospheres derived from cortical cell-niche astrocyte cocultures were dissociated and cultured in the presence of BrdU. After 24 hours, 91% ± 4% of the sphere-forming nuclei incorporated BrdU (Fig. 2B), suggesting that these cells actively underwent mitosis. In another experiment, secondary and tertiary neurospheres were dissociated and plated as single-cell suspensions. After 8 days in culture, an average of 2.1 ± 0.5 secondary spheres developed. Cells dissociated from secondary spheres then gave rise to 1.9 ± 0.9 tertiary spheres. Spheres could still be generated after at least 10 passages without diminution of sphere-forming ability (not shown), indicating their undiminished capacity for self-renewal.
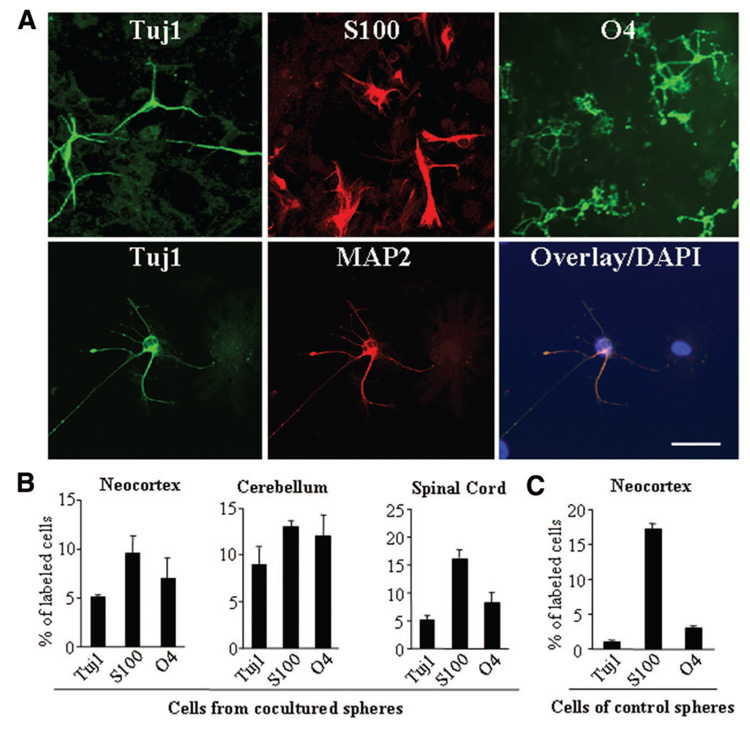
Next, we tested the potential of sphere-forming cells to give rise to different cell types in vitro. Once removed from suspension and plated onto Matrigel-coated coverslips, cells dissociated from secondary or tertiary neurospheres readily attached and began to differentiate. After 8 days, the sphere-derived cells displayed morphologies of neurons, astrocytes, and oligoden-drocytes (Fig. 3A). By quantifying cells immunolabeled for neuronal (Tuj1), astrocyte (S100), and oligodendrocyte (O4) markers, we demonstrated that sphere-derived cells isolated from either the cortex, cerebellum, and spinal cord exhibited similar ability to differentiate into various CNS cells (Fig. 3B). Moreover, in the absence of niche astrocytes, cells dissociated from neurospheres developed in cortical cell-astrocyte cocultures displayed a significantly higher potency to differentiate into neurons (Tuj1+) (p < .001) than those dissociated from control cultures (without feeder cells) (Fig. 3B, 3C), whereas the majority of cells isolated from neurospheres of control cortical cell cultures differentiated into astroglia (S100), compared with those from spheres that had been cocultured with niche astrocytes (Fig. 3B, 3C). These data suggest that niche astrocytes release factors that may selectively expand the population of progenitor cells that are capable of making neurons or directly enhance the neurogenic potential of these cells.
Figure 3.
Neural progenitor cells display multipotency in vitro. (A): Sphere-derived cells differentiated into neurons, astrocytes, and oligodendrocytes in culture. Neurospheres isolated from cortical cell cultures were dissociated, plated on Matrigel-coated chamber slides, and cultured in N2 medium containing retinoic acid and 2% fetal bovine serum. After a 7-day incubation, cultures were fixed and stained with antibodies specific for Tuj1, MAP-2, S100, and O4. Overlay image shows a Tuj1+ (green) neuron coexpressing the mature neuronal marker MAP2 (red) and DAPI (blue). Scale bar = 40 µm. (B, C): Quantification of cell differentiation in dissociated neurosphere cell cultures, of which the neurospheres were isolated either from the neocortex-, cerebellum-, and spinal cord-niche astrocyte cocultures (B) or from cortical cell cultures in the absence of niche astrocytes (C). Data represent percentage of cells immunostained for neuronal (Tuj1-positive), astrocyte (S100), and oligodendrocyte (O4) markers over total number of cells in culture ± SD. Note that in the absence of niche astrocytes, neurosphere cells derived from the neocortex exhibited lower neurogenic potential but preferentially differentiated into astroglia compared with those cells derived from niche astrocyte cocultures. Abbreviations: DAPI, 4′,6-diamidino-2-phenylindole dihydrochloride; MAP2, microtubule-associated protein 2; Tuj1, β-III-tubulin.
The multipotency of sphere-derived cells was also examined in vivo. Neurospheres prepared from the cerebral cortex of adult GFPtg mice were dissociated and transplanted into the P0 mouse hippocampus, which presents a rich environment for neural progenitor cell differentiation. After 4 weeks, many engrafted GFP+ cells differentiated and expressed neural and glial cell markers. We detected cells positive for neuronal markers Tuj1 (not shown), MAP2, HuD, and MAP2 (supplemental online Fig. 1) and glial markers O4 (supplemental online Fig. 1) and GFAP (not shown). These results show that sphere-forming cells that are derived from the adult cortex retain neurogenic capacity and the multilineage competence of neural progenitors in vitro and in vivo.
Neural Progenitor Cells Are GFAP-Expressing Cells
To determine the cellular identity of the cells from which sphere-forming cells originated in vivo, we isolated RNA from neurospheres derived from gray-matter cells of the adult cortex and examined the expression of different cell markers with reverse transcriptase-polymerase chain reaction (RT-PCR). Similar to neural progenitors in the SVZ and SGZ, cortical neurosphere-derived cells expressed the neural progenitor markers GFAP and nestin but were negative for mature astroglial marker S100 (Fig. 4A). Staining of the brain sections of adult mice with primary antibodies against GFAP and nestin revealed a subpopulation of GFAP+ cells that were nestin+ (Fig. 4A). By examining BrdU incorporation, we noted that these GFAP+/ nestin+ cells stayed quiescent and failed to incorporate BrdU under the normal conditions in vivo, consistent with the notion that the adult cortical environment is nonpermissive for neural progenitor cell proliferation.
Figure 4.
Sphere-forming cells are originated from GFAP-expressing cells. (A): Sphere-forming cells are GFAP+ and nestin+. Results of reverse transcription-polymerase chain reaction show that NS were GFAP+ and nestin+, whereas primary Cx expressed GFAP and S100. (B): Fluorescence confocal images of single optical slices were taken from adult cortical sections double-labeled by primary antibodies specific for GFAP and nestin, identifying a subpopulation of GFAP+ (red)/nestin+ (green) cells. Arrows point to cells positively labeled by both anti-GFAP and anti-nestin. Scale bars = 30 µm. (C): A GFP+ neurosphere formed in cocultures using GFP+ Cx sorted out from GFAP-GFP mice. Note that because GFP expression is driven under the GFAP promoter, only GFAP+ cells from these mice were GFP+. Scale bar = 20 µm. (D): Number of neurospheres developed from unsorted (Cx) and sorted GFP+ (GFAP+) and GFP− (GFAP−) cortical cell cultures in the absence ((−) astro) or presence ((+) astro) of P0 astro. Results represent the mean ± SD (n = 6; *, p < .001; Student’s t test and analysis of variance). Abbreviations: Astro, astrocytes; Cx, cortical cells; GFAP, glial fibrillary acidic protein; GFP, green fluorescent protein; NS, neurosphere cells.
To determine whether cortical neural progenitor cells, like those in the SGZ, originated from GFAP-expressing cells, we took advantage of another transgenic mouse line in which a GFP transgene is driven by the GFAP promoter (GFAP-GFP) [23]. GFP expression in the adult GFAP-GFP mouse brain is confined to GFAP-expressing cells [23] (supplemental online Fig. 2A). When cortical cells of GFAP-GFP mice were cocultured with P0 brain astrocytes, neurospheres were GFP+ (Fig. 4C), indicating that sphere-forming cells were GFAP+ cells. Next, GFP+ and GFP− cells were sorted out from the cerebral cortex of adult GFAP-GFP mice with fluorescence-activated cell sorting (FACS), and the result was corroborated using fluorescence microscopy (supplemental online Fig. 2B). Both GFP+ and GFP− cell populations were cocultured with niche (P0) astrocytes isolated from the SGZ of adult WT mice (GFP−). As predicted, in the absence of niche astrocytes, GFP+ or GFP− cells developed few neurospheres (Fig. 4D). Addition of niche astrocytes, however, induced a 10-fold increase in neurosphere formation in the GFP+ cell compartment compared with control cultures that did not contain P0 astrocytes, whereas the GFP− cell compartment developed few neurospheres under similar culture conditions (Fig. 4D). The frequency of neurosphere formation in GFP+ cell/niche astrocyte cocultures was significantly higher than that in unsorted cortical cell/niche astrocyte cocultures (Fig. 4D), suggesting that the GFP+ (GFAP-expressing) cell population is rich in neural progenitors.
To further support the notion that GFAP-expressing cells are the primary source of neural progenitors in the adult CNS, we used a transgenic mouse line that carries the targeted expression of herpes simplex virus thymidine kinase (TK) driven by GFAP promoter (GFAP-TK) [24]. Application of the antiviral agent ganciclovir (GCV) to cells derived from this mouse line results in specific ablation of dividing GFAP+ cells in vitro and in vivo [26]. Immunohistochemistry confirmed that TK was expressed in GFAP+ cells in the adult cortex of transgenic mice (Fig. 5A). In coculture preparations, gray-matter cortical cells derived from adult GFAP-TK mice were plated with P0 astrocytes of wild-type mice. Administration of GCV selectively eliminated GFAP-expressing cells from the cortical cell compartment but had no effect on niche astrocytes derived from wild-type mice (not shown). In the absence of GCV, the neurospheres developed normally in cortical cell-astrocyte cocultures; however, application of GCV completely abolished sphere formation under similar culture conditions (Fig. 5B). Application of GCV to cortical cells isolated from wild-type mice had no effect on neurosphere formation (Fig. 5B). Similar results were obtained using dissociated gray-matter cells derived from the cerebellum or spinal cord of adult GFAP-TK mice (not shown). These data demonstrate that GFAP-expressing cells, similar to those seen in the SVZ and SGZ, are a primary source of neural progenitors in the adult CNS.
Figure 5.
Depletion of GFAP-expressing cells abolishes neurosphere formation in cortical-astrocyte cocultures. (A): TK+ cells in GFAP-TK mice are GFAP+. Confocal photomicrographs of single optical slices taken from the cortex of adult GFAP-TK mice were double-labeled by anti-TK (green) and anti-GFAP (red). Scale bars = 40 µm. (B): Number of neurospheres counted from cortical-astrocyte cocultures, in which cortical cells were derived from GFAP-TK or WT mice, in the absence or presence of GCV. Note that GCV does not affect the number of neurospheres in WT cultures. Results represent the mean ± SD (n = 6; *, p < .01; Student’s t test). (C): Confocal photomicrographs of NG2 immunolabeling showing that the GFAP+ neurosphere was NG2−. GFAP+ neurospheres were derived from dissociated neocortical cell cultures, and NG2+ neurospheres were collected from cultured NG2+ cells sorted out from the neocortex by fluorescence-activated cell sorting using anti-A2B5. Scale bars = 40 µm. (D): Results of reverse transcription-polymerase chain reaction reveal low levels of NG2 and A2B5 mRNAs in GFAP+ neurosphere cells compared with NG2+ neurospheres. (E, F): Quantification of neural and glial differentiation in cultures derived from secondary or tertiary GFAP+ and NG2+ spheres isolated from the neocortex or WT (E) or from GFAP-TK (F) mice in the absence (E) or presence (F) of GCV. Note the reduction of astrocyte, but not neuron or oligodendrocyte, differentiation in NG2+ cell cultures derived from GFAP-TK mice after GCV treatment (F), indicating the selective effect of GCV on GFAP+ cells. Data represent percentage of cells immunostained for Tuj1, S100, and O4 over total number of cells in culture ± SD. Abbreviations: GCV, ganciclovir; GFAP, glial fibrillary acidic protein; immuno+, immunopositive; TK, thymidine kinase; Tuj1, β-III-tubulin; WT, wild-type.
As the gray matter and white matter of the CNS are abundant in NG2+ oligodendrocyte precursor cells that can give rise to neurons and glial cells in culture [6], we asked whether GFAP+/nestin+ neural progenitors differ from NG2+ progenitors. Using immunohistochemistry (Fig. 5C) and RT-PCR (Fig. 5D), we showed that GFAP+/nestin+ progenitors expressed a much lower level of NG2 and A2B5 than NG2+ cells, suggesting they represent different populations of progenitor cells. We then compared the differentiation of GFAP+/nestin+ cells with that of the NG2+ cells. NG2+ progenitors were sorted out with FACS using anti-A2B5 antibody and cultured in the absence of feeder cells. After 8 days, neurospheres were collected; cells were dissociated and plated onto Matrigel-coated coverslips. Quantification of cells expressing the neural marker (Tuj1), the astrocyte marker (S100), and the oligodendrocyte marker (O4) indicated that the NG2+ progenitors preferentially differentiate into oligodendrocytes in culture (Fig. 5E). In contrast, GFAP+/nestin+ progenitors exhibited a significantly higher potential to generate astroglia (Fig. 5E). Presence or absence of GCV did not affect neuronal and oligodendrocyte differentiation by NG2+ progenitors that were isolated from GFAP-TK mice (Fig. 5F). The data are consistent with the notions that NG2+ cells function as oligodendrocyte precursors and that GFAP+/nestin+ and NG2+ cells represent different populations of progenitors in the adult CNS.
Induction of Adult Neurogenesis from Non-Neurogenic CNS Regions In Vivo by Niche Astrocyte Implants
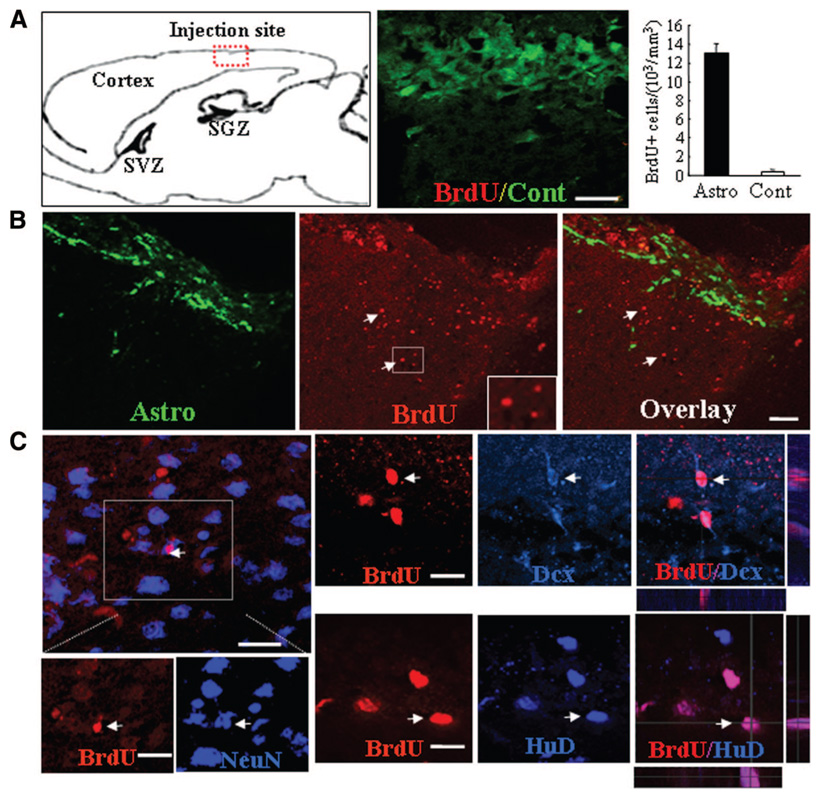
The facts that neural progenitors with similar cellular properties exist in both neurogenic and non-neurogenic CNS areas and that they can be stimulated to re-enter the cell cycle by niche astrocytes suggest that these astrocytes release growth-stimulating signals for neural progenitors. To further investigate whether neural progenitors in non-neurogenic CNS regions can be induced to re-enter the cell-cycle by niche astrocytes in vivo, we grafted astrocytes derived from P0 GFP mice into the nonneurogenic cortex of adult mice (Fig. 6A). Proliferating neural progenitors were detected using BrdU pulse-labeling combined with immunolabeling of either neural progenitor or mature neuron markers. Two weeks after astrocyte transplantation, numerous BrdU+ cells were detected in the host cortex, around engrafted GFP+ astrocytes (Fig. 6B). In contrast, in shamoperated controls, few BrdU+ cells were noted (Fig. 6A). Approximately 13,150 ± 102 BrdU+ cells per mm3 were counted in cortical sections that received niche astrocyte implants compared with 491 ± 18 BrdU+ cells per mm3 in control injected brain sections, or an increase in BrdU+ cells of more than 30-fold was induced by astrocyte implants compared with control implants. Many of the BrdU+ cells were seen to colocalize with early (Dcx and HuD) and late (NeuN) neuronal markers [8] (Fig. 6C), strongly suggesting induction of neurogenesis by niche astrocyte transplantation. These results indicate that endogenous neural precursors of non-neurogenic CNS regions can be induced to proliferate and generate new neurons in vivo.
Figure 6.
Transplantation of niche Astro induces progenitor cell proliferation and neurogenesis in the adult neocortex. (A): Schematic illustration of a sagittal section of a mouse brain and a photomicrograph of an overlay image of anti-BrdU (red) and green fluorescent protein (GFP) immunofluorescence in a cortical section of a mouse that received a control injection (cont). Very few BrdU+ cells were observed in the control mouse brain. Quantification of BrdU+ cells in brain sections taken from mice treated with Cont or niche Astro. (B): Numerous BrdU+ cells were detected in cortical areas surrounding GFP+ Astro transplants (green). Two weeks after receiving niche Astro transplants, mouse brain sections were collected and immunolabeled for BrdU (red). Arrowheads indicate BrdU+ nuclei. Note that BrdU+ cells appeared adjacent to the Astro implant. Scale bar = 40 µm. (C): Confocal photomicrographs of single optic slices taken from the vicinity of Astro-injected cortical areas showing newly born, BrdU+ cells (red) colocalized with immunolabeling of neuronal markers NeuN (green), Dcx (blue), and HuD (blue). Scale bars = 40 µm (A, B) and 20 µm (C). Abbreviations: Astro, astrocytes; BrdU, 5′-bromo-2′-deoxyuridine; Cont, control injection; Dcx, doublecortin; SGZ, subgranular zone; SVZ, subventricular zone.
Shh As a Molecular Component of the Neurogenic Niche
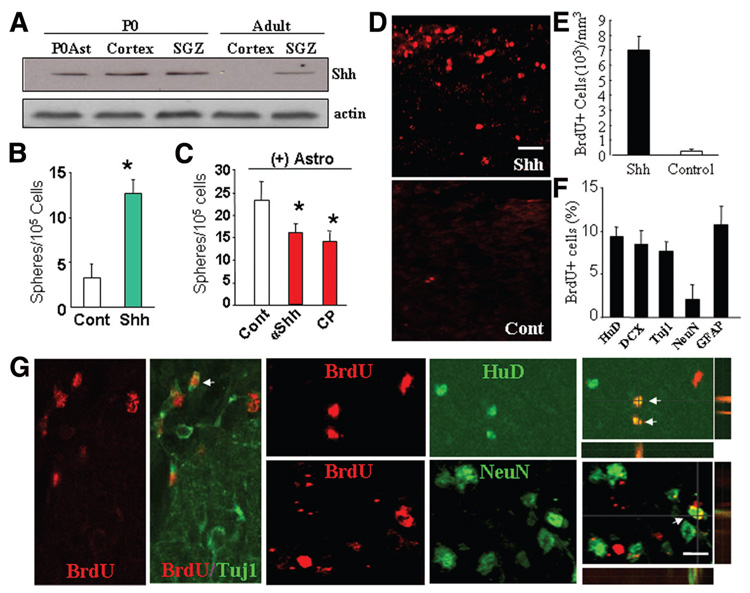
To identify the neurogenic signal produced by niche astrocytes, we screened for molecules that are reported to regulate neurogenesis using RT-PCR and Western blotting. We anticipated that these factors would be differentially expressed in the neurogenic and non-neurogenic CNS regions or astrocytes. Mature astrocytes were isolated from GFAP-GFP mice by FACS. We compared the expression of Shh, bone morphogen proteins [27], wnts and wnt inhibitors [28–31], and other molecules. The ability of these candidates to induce neurosphere formation from non-neurogenic CNS regions was analyzed using cortical cell cultures. We found that niche astrocytes isolated from P0 and adult SGZ expressed high levels of the neurogenic factor Shh, whose expression was virtually absent from astrocytes of the cerebral cortex in adult mice (Fig. 7A). Moreover, in the absence of niche astrocytes, addition of Shh alone is sufficient to stimulate the proliferation of neural progenitors and sphere formation from adult cortical cell cultures (Fig. 7B). In contrast, Shh antagonist attenuated the sphere-forming effect by niche astrocytes in cortical-astrocyte cocultures, confirming that Shh is a growth signal produced by niche astrocytes to stimulate neural progenitor proliferation (Fig. 7C).
Figure 7.
Shh as a component of niche astrocyte-produced neurogenic niche. (A): Shh expression by niche astrocytes or neurogenic brain regions of adult mice. Representative Western blot analyses of triplicate experiments showing Shh expression in P0Ast, the cerebral cortex and SGZ of P0 and adult mice, but not in the non-neurogenic cortex of adult mice. (B): Shh is a positive regulator for neural progenitor cell proliferation in culture. Quantification of neurosphere formation in dissociated cortical cell cultures in the absence (Cont) or presence of Shh (50 ng/ml). (C): αShh (1 µg/ml) and its inhibitor CP (5 µM) diminish neurosphere formation in cortical-astrocyte ((+)Astro) cocultures. Results represent the mean value ± SD (n = 5; *, p < .01; Student’s t test). (D): Administration of Shh induced cell proliferation in the adult mouse neocortex in vivo. A solution containing soluble Shh or phosphate-buffered saline (PBS) (as Cont) was administered to the neocortex of adult mice. Two weeks postinjection, numerous BrdU+ cells (red) were observed in the cortical area surrounding the Shh injection site (Shh) but not the PBS injection site (Cont). (E, F): Quantification of BrdU+ cells (E) and BrdU+ cells that were double-labeled for another cell marker (HuD, DCX, Tuj1, NeuN, or GFAP) (F) in brain sections taken from mice received PBS (white bar) or Shh (50 ng/ml) (black bar) injection. (G): Shh-induced BrdU+ cells differentiate into neurons in vivo. Confocal photomicrographs of single optical slices taken form the cortex of adult of mice received Shh injection showing subsets of BrdU+ precursors are positive for neuronal marker HuD, NeuN, and βIII-tubulin (Tuj1). Scale bars = 50 µm (D) and 30 µm (E). Abbreviations: BrdU, 5′-bromo-2′-deoxyuridine; Cont, control; CP, cyclopamine; DCX, doublecortin; GFAP, glial fibrillary acidic protein; P0Ast, P0 brain astrocytes; SGZ, subgranular zone; Shh, sonic hedgehog; αShh, sonic hedgehog neutralizing antibody; Tuj1, β-III-tubulin.
To determine whether application of Shh alone is sufficient to stimulate adult neurogenesis from non-neurogenic brain regions in vivo, we injected Shh to the cerebral cortex of adult mice. Under normal conditions, as expected, few BrdU+ cells were detected in the cortex of adult mice. However, 24 hours after Shh injection, many BrdU+ cells appeared in the neocortex of adult mice, primarily colocalizing with GFAP immunolabeling (supplemental online Fig. 3A). Counts of BrdU+ cells consistently revealed that more than 80% of BrdU+ cells induced by either Shh injection or niche astrocyte transplantation were GFAP+, whereas less than 10% of BrdU+ cells were NG2+ (supplemental online Fig. 3A). Two weeks after a single Shh administration, there was a significant increase in the number of BrdU+ cells in the cortex of the adult mice compared with PBS-injected controls (Fig. 7D, 7E). Double immunolabeling of anti-BrdU with primary antibodies against the developing and mature neuron and glial markers revealed many BrdU+/ HuD+, Brdu+/Tuj1+, BrdU+/NeuN+, and BrdU+/GABA+ neurons around the Shh injection site (Fig. 7G; supplemental online Fig. 3C). To further determine the ability of Shh to regulate neurogenesis, we studied neural progenitor cell differentiation in the presence and absence of Shh in culture. Cells dissociated from secondary cortical neurospheres were plated onto Matrigel-coated coverslips. After 7 days of incubation, we quantified cells immunolabeled for Tuj1, S100, and O4. We found that sphere-derived cells cultured in the presence of Shh behaved like those cultured in the presence of niche astrocytes, showing a significant increase in neural differentiation compared with untreated cultures (supplemental online Fig. 3D). These data demonstrate that Shh is a key component of the instructive niche produced by SGZ or niche astrocytes, which can induce the neurogenic behavior from the adult CNS.
DISCUSSION
The results of this study demonstrate that multipotent neural progenitor cells are widely distributed in the entire CNS of adult mice, including the cortex, cerebellum, and spinal cord. Like those in the SVZ and SGZ, these progenitor cells are GFAP-expressing cells [26, 32] that have the capacity to generate neurons, oligodendrocytes, and astrocytes both in vitro and in vivo. The neurogenic potential of certain CNS regions is dictated by signals produced by the resident astrocytes, such as Shh, or by neurogenic permissiveness of the local environment. The fact that neural progenitors with cellular properties similar to those in the SGZ and SVZ distribute widely in the adult CNS argues strongly that the critical differences between neurogenic and non-neurogenic regions reflect not distinctions in intrinsic properties of their progenitor cells but differences in their microenvironment. These data therefore provide the first strong evidence of the exciting possibility of directly activating the dormant neurogenic or regenerative potential in nonconventional neurogenic regions of the adult CNS in vivo.
Our data further suggest that these neural progenitor cells represent a different population from NG2+ cells. They are negative for the NG2+ cell markers, NG2 and A2B5 and display higher neuro- and astrogliogenic potential compared with NG2+ progenitors. Under normal conditions, NG2+ cells preferentially differentiate into oligodendrocytes and are thus called oligodendrocyte precursors. Emerging evidence suggests that NG2+ progenitors can also give rise to astrocytes and neurons in vitro and in vivo [33, 34] and that the astroglio- and neurogenic potentials of NG2+ cells also depend on their site of origin. For instance, in the SVZ of the adult brain, the neurogenic niche produces factors that inhibit Olig2 signaling and thus oligodendrogenesis of neural progenitors. Bone morphogenetic protein and Smads signaling have been shown to play a key role in controlling the fate of NG2+ cells [34–37]. Likely intrinsic determinants, such as the expression of transcription factor Pax6, may also participate in the regulation of neuronal or glial cell fate of neural progenitors [38].
Astrocytes, as a cellular component of the stem cell microenvironment, are reported to display regional heterogeneity in their ability to regulate neurogenesis [11]. SGZ astrocytes have been shown to provide signals that can increase the proliferation of hippocampal neural stem cells and instruct these cells to adopt a neuronal fate; these signals have been proposed to contribute, in part, to the active neurogenesis in the adult SGZ [11]. Astrocytes isolated from CNS regions outside the SGZ and SVZ, in contrast, are inhibitory for neural progenitor proliferation, as shown in cortical-astrocyte cocultures. In the present study, we show that neural progenitors with similar properties are widely distributed in the adult CNS. In agreement with the previous report [11], our results also indicate that niche astrocytes isolated from the adult SGZ produce factors that increase progenitor cell proliferation and promote neuronal differentiation. Niche astrocytes isolated from the adult SVZ and neonatal mouse brains showed effects similar to those of SGZ astrocytes (not shown) in promoting cell proliferation. Interestingly, progenitor cells isolated from neurospheres derived from cocultures with niche astrocytes exhibited increased neurogenic potential compared with those taken from the control cultures (without feeder cells). The data suggest that factors produced by niche astrocytes may initiate a neurogenic program in progenitor cells or selectively promote the proliferation of a subpopulation of progenitors that are capable of making neurons. Supplying niche astrocytes in vivo is sufficient to activate the dormant capacity of neural progenitors and induce neurogenesis from the larger CNS regions in the adult. These data indicate that astrocytes, acting as key regulatory switches, dictate the neurogenic behavior of both neurogenic and non-neurogenic CNS regions in the adult. The findings suggest that a neurogenic program and cell replacement therapy may be activated in any given CNS regions by providing the injury site with niche astrocytes or neurogenic signals.
Our results further elucidate that Shh is a key component of the instructive signal produced by niche astrocytes that promotes neural progenitor cell proliferation and neurogenesis in the adult. Shh has been implicated in controlling the fate of neural stem cells throughout development and adult neurogenesis in the SGZ and SVZ [17, 19, 39]. Our studies show that non-neurogenic astrocytes derived from the cerebral cortex of adult mice fail to produce Shh or support neural progenitor proliferation. Likely, they also present inhibitory molecules for neural progenitor cell growth and differentiation. Niche astrocytes, in contrast, are known to release Shh and other signaling molecules, such as wnts, which may interact with each other to dictate the neurogenic behavior in the adult CNS [28]. In addition, a recent finding indicates that other molecules, such as interleukins 1β and 6, might also be involved in the process of adult neurogenesis [40]. An emerging view points to overlapping pathways of growth factors, metalloproteases, neurotransmitters, and hormones to regulate different aspects of neurogenesis within the neurogenic niches [41]. For example, neuroblast migration is precisely regulated by cooperation among several repellants, attractants, and guidance molecules that are located within specific CNS regions. Further elucidation of crucial molecular regulators and integration of their signaling cascades should lead to more rational and effective approaches to harnessing the exciting phenomenon of adult CNS neurogenesis.
CONCLUSION
Together, our study indicates that neural progenitors distinct from NG2+ cells but with cellular properties similar to those in the neurogenic brain regions are widely distributed. These findings suggest that it may be possible to manipulate endogenous neural progenitors in diverse CNS areas to undergo neurogenesis. These results thus provide a new therapeutic avenue toward future neuronal replacement therapy for neurodegenerative diseases and other CNS injury.
Supplementary Material
See www.StemCells.com for supplemental material available online.
ACKNOWLEDGMENTS
We thank Dr. Micheal Sofroniew for GFAP-TK mice and critical comments, William C. Summers for HSV-TK antibody, and Drs. Jeff Macklis and Alice Adlier for critical discussion. This work was supported by grants from the Sybil B. Harrington Scholar Award from the Research to Prevent Blindness Foundation, the American Health Foundation (G2007-058), the Department of Defense (W81XWH-04-2-0008), the Lion’s Foundation, Harvard Stem Cell Institute, and NIH/National Eye Institute (R01-EY017641 [to D.F.C.] and P30 EY0 03790).
Footnotes
Disclosure of Potential Conflicts of Interest
The authors indicate no potential conflicts of interest.
REFERENCES
- 1.Alvarez-Buylla A, Garcia-Verdugo JM. Neurogenesis in adult subventricular zone. J Neurosci. 2002;22:629–634. doi: 10.1523/JNEUROSCI.22-03-00629.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lie DC, Song H, Colamarino SA, et al. Neurogenesis in the adult brain: New strategies for central nervous system diseases. Annu Rev Pharmacol Toxicol. 2004;44:399–421. doi: 10.1146/annurev.pharmtox.44.101802.121631. [DOI] [PubMed] [Google Scholar]
- 3.Kornack DR, Rakic P. Cell proliferation without neurogenesis in adult primate neocortex. Science. 2001;294:2127–2130. doi: 10.1126/science.1065467. [DOI] [PubMed] [Google Scholar]
- 4.Kokoeva MV, Yin H, Flier JS. Neurogenesis in the hypothalamus of adult mice: Potential role in energy balance. Science. 2005;310:679–683. doi: 10.1126/science.1115360. [DOI] [PubMed] [Google Scholar]
- 5.Lie DC, Dziewczapolski G, Willhoite AR, et al. The adult substantia nigra contains progenitor cells with neurogenic potential. J Neurosci. 2002;22:6639–6649. doi: 10.1523/JNEUROSCI.22-15-06639.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nunes MC, Roy NS, Keyoung HM, et al. Identification and isolation of multipotential neural progenitor cells from the subcortical white matter of the adult human brain. Nat Med. 2003;9:439–447. doi: 10.1038/nm837. [DOI] [PubMed] [Google Scholar]
- 7.Markakis EA, Palmer TD, Randolph-Moore L, et al. Novel neuronal phenotypes from neural progenitor cells. J Neurosci. 2004;24:2886–2897. doi: 10.1523/JNEUROSCI.4161-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Magavi SS, Leavitt BR, Macklis JD. Induction of neurogenesis in the neocortex of adult mice. Nature. 2000;405:951–955. doi: 10.1038/35016083. [DOI] [PubMed] [Google Scholar]
- 9.Pencea V, Bingaman KD, Wiegand SJ, et al. Infusion of brain-derived neurotrophic factor into the lateral ventricle of the adult rat leads to new neurons in the parenchyma of the striatum, septum, thalamus, and hypothalamus. J Neurosci. 2001;21:6706–6717. doi: 10.1523/JNEUROSCI.21-17-06706.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Palmer TD, Markakis EA, Willhoite AR, et al. Fibroblast growth factor-2 activates a latent neurogenic program in neural stem cells from diverse regions of the adult CNS. J Neurosci. 1999;19:8487–8497. doi: 10.1523/JNEUROSCI.19-19-08487.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Song H, Stevens CF, Gage FH. Astroglia induce neurogenesis from adult neural stem cells. Nature. 2002;417:39–44. doi: 10.1038/417039a. [DOI] [PubMed] [Google Scholar]
- 12.Shen Q, Goderie SK, Jin L, et al. Endothelial cells stimulate self-renewal and expand neurogenesis of neural stem cells. Science. 2004;304:1338–1340. doi: 10.1126/science.1095505. [DOI] [PubMed] [Google Scholar]
- 13.Ma DK, Ming GL, Song H. Glial influences on neural stem cell development: Cellular niches for adult neurogenesis. Curr Opin Neurobiol. 2005;15:514–520. doi: 10.1016/j.conb.2005.08.003. [DOI] [PubMed] [Google Scholar]
- 14.Horner PJ, Palmer TD. New roles for astrocytes: The nightlife of an ‘astrocyte’. La Vida loca! Trends Neurosci. 2003;26:597–603. doi: 10.1016/j.tins.2003.09.010. [DOI] [PubMed] [Google Scholar]
- 15.Pixley SK. CNS glial cells support in vitro survival, division, and differentiation of dissociated olfactory neuronal progenitor cells. Neuron. 1992;8:1191–1204. doi: 10.1016/0896-6273(92)90139-5. [DOI] [PubMed] [Google Scholar]
- 16.Riquelme PA, Drapeau E, Doetsch F. Brain micro-ecologies: Neural stem cell niches in the adult mammalian brain. Philos Trans R Soc Lond B Biol Sci. 2008;363:123–137. doi: 10.1098/rstb.2006.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ahn S, Joyner AL. In vivo analysis of quiescent adult neural stem cells responding to Sonic hedgehog. Nature. 2005;437:894–897. doi: 10.1038/nature03994. [DOI] [PubMed] [Google Scholar]
- 18.Palma V, Lim DA, Dahmane N, et al. Sonic hedgehog controls stem cell behavior in the postnatal and adult brain. Development. 2005;132:335–344. doi: 10.1242/dev.01567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lai K, Kaspar BK, Gage FH, et al. Sonic hedgehog regulates adult neural progenitor proliferation in vitro and in vivo. Nat Neurosci. 2003;6:21–27. doi: 10.1038/nn983. [DOI] [PubMed] [Google Scholar]
- 20.Ruiz i Altaba A, Palma V, Dahmane N. Hedgehog-Gli signalling and the growth of the brain. Nat Rev Neurosci. 2002;3:24–33. doi: 10.1038/nrn704. [DOI] [PubMed] [Google Scholar]
- 21.Machold R, Hayashi S, Rutlin M, et al. Sonic hedgehog is required for progenitor cell maintenance in telencephalic stem cell niches. Neuron. 2003;39:937–950. doi: 10.1016/s0896-6273(03)00561-0. [DOI] [PubMed] [Google Scholar]
- 22.Okabe M, Ikawa M, Kominami K, et al. ‘Green mice’ as a source of ubiquitous green cells. FEBS Lett. 1997;407:313–319. doi: 10.1016/s0014-5793(97)00313-x. [DOI] [PubMed] [Google Scholar]
- 23.Zhuo L, Sun B, Zhang CL, et al. Live astrocytes visualized by green fluorescent protein in transgenic mice. Dev Biol. 1997;187:36–42. doi: 10.1006/dbio.1997.8601. [DOI] [PubMed] [Google Scholar]
- 24.Bush TG, Savidge TC, Freeman TC, et al. Fulminant jejuno-ileitis following ablation of enteric glia in adult transgenic mice. Cell. 1998;93:189–201. doi: 10.1016/s0092-8674(00)81571-8. [DOI] [PubMed] [Google Scholar]
- 25.Cole R. Preparation of astrocyte, oligodendrocyte, and microglia cultures from primary rat cerebal cultures. In: Fedoroff S, Richardson A, editors. Protocols for Neural Cell Culture. Totowa, NJ: Humana Press; 2001. pp. 117–127. [Google Scholar]
- 26.Garcia AD, Doan NB, Imura T, et al. GFAP-expressing progenitors are the principal source of constitutive neurogenesis in adult mouse forebrain. Nat Neurosci 2004. 2004;7:1233–1241. doi: 10.1038/nn1340. [DOI] [PubMed] [Google Scholar]
- 27.Ying QL, Nichols J, Chambers I, et al. BMP induction of Id proteins suppresses differentiation and sustains embryonic stem cell self-renewal in collaboration with STAT3. Cell. 2003;115:281–292. doi: 10.1016/s0092-8674(03)00847-x. [DOI] [PubMed] [Google Scholar]
- 28.Lie DC, Colamarino SA, Song HJ, et al. Wnt signalling regulates adult hippocampal neurogenesis. Nature. 2005;437:1370–1375. doi: 10.1038/nature04108. [DOI] [PubMed] [Google Scholar]
- 29.Aubert J, Dunstan H, Chambers I, et al. Functional gene screening in embryonic stem cells implicates Wnt antagonism in neural differentiation. Nat Biotechnol. 2002;20:1240–1245. doi: 10.1038/nbt763. [DOI] [PubMed] [Google Scholar]
- 30.Lee HY, Kleber M, Hari L, et al. Instructive role of Wnt/beta-catenin in sensory fate specification in neural crest stem cells. Science. 2004;303:1020–1023. doi: 10.1126/science.1091611. [DOI] [PubMed] [Google Scholar]
- 31.Hirabayashi Y, Itoh Y, Tabata H, et al. The Wnt/beta-catenin pathway directs neuronal differentiation of cortical neural precursor cells. Development. 2004;131:2791–2801. doi: 10.1242/dev.01165. [DOI] [PubMed] [Google Scholar]
- 32.Doetsch F. The glial identity of neural stem cells. Nat Neurosci. 2003;6:1127–1134. doi: 10.1038/nn1144. [DOI] [PubMed] [Google Scholar]
- 33.Zhu X, Bergles DE, Nishiyama A. NG2 cells generate both oligoden-drocytes and gray matter astrocytes. Development. 2008;135:145–157. doi: 10.1242/dev.004895. [DOI] [PubMed] [Google Scholar]
- 34.Gross RE, Mehler MF, Mabie PC, et al. Bone morphogenetic proteins promote astroglial lineage commitment by mammalian subventricular zone progenitor cells. Neuron. 1996;17:595–606. doi: 10.1016/s0896-6273(00)80193-2. [DOI] [PubMed] [Google Scholar]
- 35.Mabie PC, Mehler MF, Marmur R, et al. Bone morphogenetic proteins induce astroglial differentiation of oligodendroglial-astroglial progenitor cells. J Neurosci. 1997;17:4112–4120. doi: 10.1523/JNEUROSCI.17-11-04112.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Grinspan JB, Edell E, Carpio DF, et al. Stage-specific effects of bone morphogenetic proteins on the oligodendrocyte lineage. J Neurobiol. 2000;43:1–17. [PubMed] [Google Scholar]
- 37.Samanta S, Burke GM, McGuire K, et al. BMPR1a signaling determines numbers of oligodendrocytes and calbindin-expressing interneurons in the cortex. J Neurosci. 2007;27:7397–7407. doi: 10.1523/JNEUROSCI.1434-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kohwi M, Osumi N, Rubenstein JLR, et al. Pax6 is required for making specific subpopulations of granule and periglomerular neurons in the olfactory bulb. J Neurosci. 2005;25:6997–7003. doi: 10.1523/JNEUROSCI.1435-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Machold R, Hayashi S, Rutlin M, et al. Sonic hedgehog is required for progenitor cell maintenance in telencephalic stem cell niches. Neuron. 2003;39:937–950. doi: 10.1016/s0896-6273(03)00561-0. [DOI] [PubMed] [Google Scholar]
- 40.Barkho BZ, Song H, Aimone JB, et al. Identification of astrocyte-expressed factors that modulate neural stem/progenitor cell differentiation. Stem Cells Dev. 2006;15:407–421. doi: 10.1089/scd.2006.15.407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hagg T. Molecular regulation of adult CNS neurogenesis: An integrated view. Trends Neurosci. 2005;28:589–595. doi: 10.1016/j.tins.2005.08.009. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
See www.StemCells.com for supplemental material available online.