Abstract
Mitochondria are central to energy metabolism as the source of much of the cell’s ATP, as well as being a hub for cellular Ca2+ signaling. Mitochondrial Ca2+ is a positive effector of ATP synthesis, yet Ca2+ overload can lead to mitochondrial dysfunction and cell death. Moreover, Ca2+ uptake by mitochondria is involved in shaping cellular Ca2+ dynamics by regulating the concentrations of Ca2+ within microdomains between mitochondria and sarco/endoplasmic reticulum and plasma membrane Ca2+ transporters. Reactive oxygen species (ROS) generated as a consequence of ATP production in the mitochondria are important for cellular signaling, yet contribute to oxidative stress and cellular damage. ROS regulate the activity of redox sensitive enzymes and ion channels within the cell, including Ca2+ channels. For both Ca2+ and ROS, a delicate balance exists between the beneficial and detrimental effects on mitochondria. In this review we bring together current data on mitochondrial Ca2+ uptake, ROS generation, and redox modulation of Ca2+ transport proteins. We present a model for crosstalk between Ca2+ and ROS signaling pathways within mitochondrial microdomains.
Keywords: Calcium, Mitochondria, Reactive Oxygen Species, Crosstalk, Sarcoplasmic Reticulum, Redox, Microdomain, Mitochondrial Permeability Transition, Permeability Transition Pore, Apoptosis, Ryanodine Receptor, Review
2. INTRODUCTION
In the past decade, mitochondria have become a focal point of modern biomedical research due in part to the central role the organelle plays in numerous pathologies including, but not limited to cardiovascular disorders such as atherosclerosis, ischemic heart disease, ischemia-reperfusion injury, and cardiac failure, neurodegenerative disorders related to mitochondrially derived oxidative stress such Huntington’s disease, Parkinson’s disease, Alzheimer’s disease, as well as playing a role in the aging process and diabetes (1–7).
The main function of mitochondria is ATP production, which occurs during mitochondrial oxidative phosphorylation (ox-phos). During ox-phos, electrons from reduced substrates are transferred to O2 through a chain of respiratory electron transporters including the complex I, III, and IV H+ pumps, which in turn generate a proton gradient across the mitochondrial inner membrane. The electrochemical energy of this gradient is then used by the ATP synthesis (complex V) which couples H+ reuptake with ADP phosphorylation in the matrix to generate ATP. Electrons however, may leak from reduced sites in the respiratory chain and react with oxygen to form reactive oxygen species (ROS) which play an important role in cell signaling, but are better known for creating oxidative stress (8).
In several cell types, mitochondria also serve as a very efficient Ca2+ buffer, taking up substantial amounts of cytosolic Ca2+ at the expense of mitochondrial membrane potential (ΔΨm). The pathways of Ca2+ entry into mitochondrial matrix are known as the mitochondrial calcium uniporter (MCU) (9), the “rapid mode” mechanism (10), and the mitochondrial ryanodine receptor (11). The main role of mitochondrial Ca2+ is the stimulation of the ox-phos enzymes (12). In addition to ox-phos, mitochondria are central players in cellular Ca2+ signaling by shaping and buffering cellular Ca2+ signals (13–15).
As a consequence of Ca2+ uptake, mitochondria can suffer Ca2+ overload, triggering the opening of the permeability transition pore (PTP) which is associated with apoptosis via the mitochondrial pathway or necrosis due to mitochondrial damage (16). The PTP has been shown to be promoted by thiol oxidation and inhibited by antioxidants, lending support for a role of ROS in pore opening (17). Furthermore, it has been demonstrated that mitochondrial Ca2+ uptake can lead to free radical production (18, 19). From a thermodynamic point of view, however, it has been noted that Ca2+ uptake occurring at the expense of membrane potential should result in a decrease in ROS production (20). The mechanism for Ca2+-induced ROS generation is not understood and is a topic of great debate in the field.
A delicate balance exists between moderate ROS production to modulate physiological signaling and overproduction of ROS that ultimately leads to oxidative stress. ROS detoxification pathways exist to minimize oxidative damage, but insults that lead to excessive ROS production precipitate changes in cellular redox homeostasis, including changes in Ca2+ handling and further alterations of ROS production. It is clear that a complex interdependence exists between mitochondrial energy production, Ca2+ uptake, ROS generation, ROS detoxification, and redox signaling. In this review, we will discuss each of these points independently, and then examine how crosstalk between mitochondrial Ca2+ handling and ROS generation play a role in normal cell function, and how deviations from normal signaling lead to mitochondrial dysfunction, and ultimately, cell death.
3. MITOCHONDRIAL Ca2+ DYNAMICS
The driving force for Ca2+ uptake by mitochondria is provided by an electrochemical membrane potential (ΔΨm) across the inner mitochondrial membrane (IMM). The ΔΨm is established by outward proton pumping from the matrix via the respiratory electron transport chain (ETC) H+ pumps. The outer mitochondrial membrane (OMM) is made permeable to Ca2+ via the voltage dependant anion channel (VDAC) which regulates the movement of Ca2+ to the intermembrane space (IMS) (21). The relatively impermeable IMM contains multiple mechanisms responsible for both Ca2+ influx and efflux (for extensive review of mitochondrial Ca2+ transporters, see (22, 23)).
3.1. Mitochondrial Ca2+ mobilization: uptake and efflux
Uptake via the mitochondrial calcium uniporter (MCU) is considered the primary route of Ca2+ influx in the mitochondria (see figure 1 for a schematic of mitochondrial Ca2+ uptake and efflux mechanisms). Recent patch clamp studies have determined that the MCU is a highly selective Ca2+ channel with a Kd < 2nM (24). The MCU is blocked by ruthenium red (RuR) and a colorless ruthenium red derivative, Ru360 (25, 26). The MCU has activation and a transport site for Ca2+, thus exhibiting second order kinetics (27–29). Despite the wealth of pharmacologic and electrophysiological data describing the MCU, the molecular identity of the channel is still unknown. Attempts to identify the MCU have led to the isolation of a number of soluble Ca2+ binding glycoproteins from ox and rat liver and beef heart mitochondria (30–32). Depletion of a 34–40 kDa glycoprotein from the mitochondria IM space reduced Ca2+ transport that was partially restored upon re-addition of the glycoprotein faction. Subsequently, antibodies raised against purified glycoproteins inhibited MCU Ca2+ transport (33, 34). A recent study has suggested that mitochondrial uncoupling proteins (UCP2 and UCP3) may comprise or play a role in MCU Ca2+ transport as well (35).
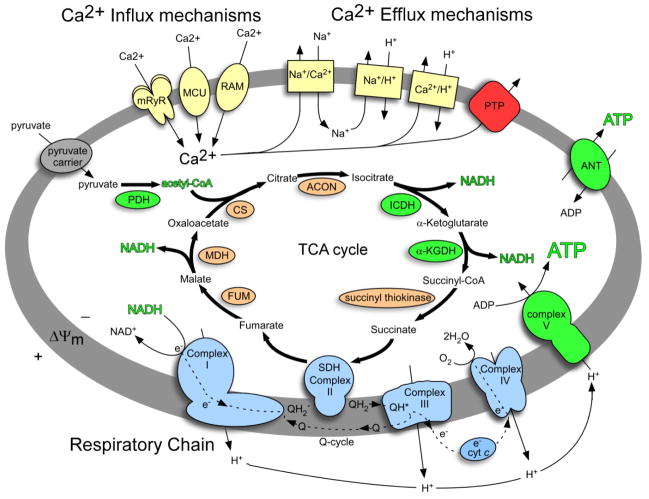
Figure 1.
Mitochondrial Ca2+ dynamics and stimulation of the TCA cycle and oxidative phosphorylation. Metabolite and ion transport are represented by thin arrows passing through membrane carriers, metabolic pathways by thick arrows, respiratory chain electron transport by broken arrows, and TCA cycle enzymes by shaded ovals. Mitochondrial Ca2+ influx and efflux mechanisms are displayed in yellow. Enzymes and complexes stimulated by Ca2+ are displayed in green, and the respiratory chain is displayed in blue. The mitochondrial outer membrane has been omitted for clarity. MCU, mitochondrial calcium uniporter; mRrR, mitochondrial ryanodine receptor; RAM, rapid mode; PTP, permeability transition pore; ANT, adenine nucleotide transporter; PDH, pyruvate dehydrogenase; CS, citrate synthase; ACON, aconitase; ICDH, isocitrate dehydrogenase; α-KGDH, α-ketogluterate dehydrogenase; SDH, succinate dehydrogenase; FUM, fumarase; MDH, malate dehydrogenase; ΔΨm, membrane potential.
A second mechanism of Ca2+ uptake in mitochondria is the rapid mode (RaM) described by Sparanga et. al. (10). RaM facilitates very rapid Ca2+ influx at the onset of a [Ca2+]c pulse, and is subsequently inhibited by binding of Ca2+ from the pulse at an external binding site. This mechanism may provide mitochondria with a burst of Ca2+ to facilitate an immediate activation of Ca2+ dependent processes without a possible delay from slower MCU mediated influx. In accord with the frequency of Ca2+ pulses in different tissues, the RaM in liver and heart mitochondria differ in their regulation. In heart cells in which excitation-contraction generated Ca2+ pulses are rapid, at least one minute is required to reset the RaM prior to subsequent reactivation, whereas the liver RaM resets in a fraction of a second after the local [Ca2+] drops to 100 nM (36). As with the MCU, the molecular identity of RaM is unknown.
The identification of a ryanodine receptor (RyR) in the IMM of cardiac mitochondria represents a third mechanism of mitochondrial Ca2+ import (mRyR) (11, 37, 38). The mRyR identified in rat heart mitochondria was characterized using subtype specific antisera as RyR1 (skeletal muscle subtype) (38). Via ryanodine titration, it was determined that mRyR is activated at low concentrations of Ca2+ with a peak open channel probability at 10–30 μM Ca2+, similar to skeletal muscle RyR1 (11). Purified mRyR channels were found to have characteristics similar to those of the skeletal muscle SR-RyR1 at the single channel level (37). High affinity Ca2+ transport by the mRyR suggest a mechanism to sequester Ca2+ on a beat-to-beat basis in cardiac cells. Accordingly, mitochondrial Ca2+ uptake occurs on a beat for beat basis in cardiomyocytes (39–42). Thus, cardiac mitochondria can match excitation-contraction derived Ca2+ signals to increased ATP production in order to meet the high energy demand of the heart, a mechanism termed excitation-metabolism coupling (11).
Mitochondria have a finite capacity for Ca2+. In the presence of inorganic phosphate, mitochondrial calcium phosphate precipitates form, facilitating total accumulation of Ca2+ approaching 1M while maintaining low [Ca2+]m (43, 44). This extraordinary capacity allows mitochondria to form a significant cellular Ca2+ buffer, more so than the ER and SR. To this end, mitochondrial Ca2+ overload can occur, leading to failure and eventual cell death without an effective Ca2+ export mechanism. Two mechanisms exist for the controlled export of Ca2+ from mitochondria, a Na+ dependent and a Na+ independent exchanger (figure 1). Mitochondrial Ca2+ efflux has been comprehensively reviewed (45, 46). Briefly, the predominant Ca2+ efflux mechanism in excitable cells such as heart, brain, and skeletal muscle is the Na+ dependant Na+/Ca2+ exchanger (Na+/Ca2+). Na+/Ca2+ is an electrogenic antiporter with a stoichiometry of transport of 3:1 (Na+: Ca2+) (47, 48). The Na+ imported into the mitochondria in exchange for Ca2+ is subsequently exchanged for H+ via the Na+/H+ antiporter, thus Ca2+ efflux comes at the cost of ΔΨm. The alternative mechanism, a 2H+/Ca2+ exchanger (2H+/Ca2+) is predominantly expressed in liver, kidney, lung, smooth muscle and is an electroneutral transporter (49–51). Both Na+/Ca2+ and 2H+/Ca2+ have been found in mitochondria from all tissues examined indicating Ca2+ efflux is not mediated by a single mechanism in any given cell type. In energized mitochondria, a steady-state Ca2+ “set point” exists that is established by matched Ca2+ influx and efflux (52). The permeability transition pore (PTP, discussed in detail below 3.3. Ca2+ overload: The permeability transition and apoptosis) has also been suggested to act as a Ca2+ release mechanism by transiently opening and releasing Ca2+ to the cytoplasm (53, 54).
3.2. Ca2+ regulation of mitochondrial metabolism
ATP production via oxidative phosphorylation is the primary function of mitochondria, and Ca2+ is the hallmark stimulatory signal for activation of numerous mitochondrial enzymes (55–57). Physiological increases in [Ca2+]m lead to the allosteric activation of TCA cycle enzymes including isocitrate dehydrogenase and α-ketogluterate dehydrogenase, as well as pyruvate dehydrogenase (57). The net effect of TCA cycle activation is a boost in reduced ox-phos substrate synthesis (NADH and FADH), enhanced respiratory chain activity and a subsequent increase in H+ pumping. In experiments that monitored the redox state of NADH/NAD+ and FADH2/FAD, it was shown that following Ca2+ uptake, ΔΨm dropped and pyridine nucleotide/flavoprotein pools became transiently oxidized as ox-phos was stimulated to restore ΔΨm (58, 59). Ca2+ pulses also stimulate the adenine nucleotide transporter (60) and Complex V (mitochondrial F0F1 ATP synthase) (61), harnessing the H+ gradient to up-regulate ATP production. As well as stimulating ox-phos, Ca2+ also stimulates α-glycerolphosphate dehydrogenase (62), a component of the glycerol phosphate shuttle that supplies NAD+ for glycolysis.
3.3. Ca2+ overload: the permeability transition pore and apoptosis
There are two sides to the effects of Ca2+ on mitochondrial function. On the beneficial side, Ca2+ is a positive effector of ox-phos. However, when overloaded, Ca2+ becomes a pathological signal leading to opening of the PTP and the subsequent initiation of apoptosis (63). The PTP is composed of a number of mitochondrial proteins in both the IMM and OMM that associate to form a large conductance channel that, when opened, dissipates ΔΨm and allows matrix solutes < 1.5 kDa and Ca2+ to be released from the mitochondria (64–66). The primary factors that are thought to compose the PTP are the OMM VDAC, the IMM ANT, and the soluble matrix protein cyclophilin D (67). However, there appears to be a great deal of variability, as the PTP has been shown to function in the absence ANT1 and ANT2 in double-knockout mice (68) as well as in the absence of cyclophilin D (69). ANT and VDAC form contact sites between the IMM and OMM that often associates with and are modulated by additional proteins (70) including hexokinase (65), the mitochondrial benzodiazepine receptor (71), Bax (72), and creatine kinase (67). PTP opening can be inhibited by cyclosporine A, which binds to matrix cyclophilin D, preventing its association with ANT. Ca2+ overload is the primary stimulator of the opening of the PTP, but other stimuli are involved in sensitizing the PTP such as oxidative stress (eg. ROS, oxidized GSH and pyridine nucleotide pools) (45, 73, 74), ADP/ATP depletion (with a concurrent rise in matrix Pi) (75, 76). Likewise, ADP, a reduced GSH and pyridine nucleotide pool, and acidic pH (77) are all inhibitors of PTP opening. A model proposed by He and Lemasters suggests that clusters of proteins that have been modified by oxidative damage can form unregulated pores in the mitochondrial inner membrane (78). These protein cluster pores are in turn blocked by mitochondrial chaperones and cyclophilin D. This model provides a possible explanation for the variability of PTP components by proposing that the blocked pores are regulated by the sensitizers and inhibitors described above.
Opening of the PTP leads to mitochondrial depolarization, loss of matrix solutes including GSH, pyridine nucleotides, ADP/ATP, and leads to the release of cytochrome c from the intermembrane space (comprehensively reviewed in (53, 63). Cytochrome c release is required for caspase activation that initiates the apoptotic program (79). In isolated mitochondria, Ca2+ induced opening of the PTP results in mitochondrial swelling and release of IMS contents as the OMM ruptures and mitoplasts expand (80). Although the PTP is a leading pathway leading to cytochrome c release (81), PTP is not necessary for apoptosis as there are reports of non-PTP cytochrome c release (82, 83). The actual mechanism (s) for in vivo cytochrome c release are not known.
PTP opening is not always an all-or-nothing event. Transient opening, or flicker, of the PTP has been observed in many cell types (84, 85), as well as in isolated mitochondria (54). The frequency of transient PTP opening is primarily determined by free matrix [Ca2+] (76, 86). Physiological PTP flicker has been suggested to be a mechanism for the release of Ca2+ from overloaded mitochondria (53, 54, 87, 88). In this manner, PTP flicker serves as a physiological safety valve to prevent Ca2+ overload, mitochondrial failure, and cell death.
4. Ca2+ AND MITOCHONDRIAL REACTIVE OXYGEN SPECIES
Mitochondrial oxidative stress is caused by an imbalance between ROS generation and ROS detoxification. There are physiological benefits to controlled ROS generation such as cell signaling (8), but unregulated, ROS can lead to oxidative stress, cellular damage, and eventually cell death. In the following section, mitochondrial sources of ROS and the defense mechanisms that maintain a physiological oxidative homeostasis will be discussed, as well as the role Ca2+ plays in ROS generation.
4.1. Reactive oxygen species
Reactive oxygen species are typically defined as molecules or ions formed by the incomplete one-electron reduction of oxygen. Categorically, ROS include free radicals such as superoxide, hydroxyl radical, and singlet oxygen, as well as non-radical species such as hydrogen peroxide. Oxygen free radicals are highly reactive and have the capacity to damage cellular components such as proteins, lipids, and nucleic acids. Mitochondria are considered a major source of ROS in the cell. In liver, the total rate of H2O2 production has been calculated to be on the order of 90 nmol/min/g tissue wet weight, mostly of which could be attributed to mitochondrial generation (89, 90). The respiratory chain complexes I and III are the primary mitochondrial sources of univalent reduction of O2 into superoxide (O2·−) (91–94). O2·− is very unstable and quickly reacts with nearby molecules, naturally dismutes into hydrogen peroxide (H2O2) and O2, or is enzymatically dismuted by the superoxide dismutase (SOD) enzyme family. In contrast to O2·−, H2O2 is membrane permeable. H2O2 can in turn react with metal ions to form the highly reactive hydroxyl radical (OH·) via the Fenton reaction.
4.2. ROS defense mechanisms
Cellular oxidative stress is the result in an imbalance between ROS generation and ROS detoxification. Cells employ a number of mechanisms to scavenge and detoxify ROS to maintain a permissive redox environment. Figure 2 details the mechanisms by which mitochondrially generated ROS are neutralized. The glutathione redox couple (GSH/GSSG) is the primary cellular redox buffer and is maintained at a very high ratio of reduced to oxidized glutathione (>30:1, GSH:GSSG) (95, 96). GSH is a cysteine containing tripeptide that can directly scavenge ROS or act as a cofactor for glutathione peroxidase which oxidizes glutathione to reduce H2O2. GSH is subsequently reduced by glutathione reductase which uses NADPH as a substrate. In the mitochondria, NADPH is generated at the expense of NADH by NADH transhydrogenase. The NADH/NAD+ redox couple also plays a role in redox buffering, but is maintained primarily in the oxidized (NAD+) form (97). Thioredoxin is a disulfide containing protein that can directly scavenge H2O2 as part of the thioredoxin reductase and thioredoxin peroxidase system (98, 99).
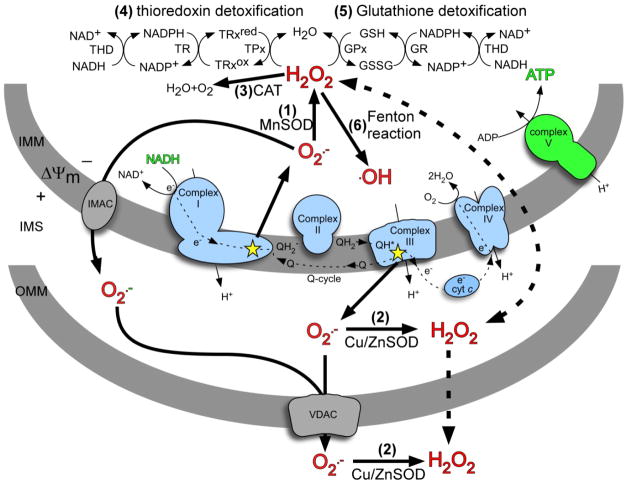
Figure 2.
Mitochondrial ROS production and defense. Superoxide (O2·−) generated by the respiratory chain is mostly released to the matrix at complex I and the IMS at complex III (indicated by stars). O2·− can naturally dismute to hydrogen peroxide (H2O2) or is enzymatically dismuted by matrix MnSOD (1) or Cu/ZnSOD (2) in the IMS or cytosol. H2O2 is detoxified in the matrix by catalase (3), the thioredoxin/thioredoxin peroxidase system (4), or the glutathione/glutathione peroxidase system (5). Alternately, H2O2 can react with metal ions to generate the highly reactive hydroxyl radical (·OH) via Fenton chemistry (6). O2·− is not membrane permeable but can pass through ion channels (solid lines), whereas H2O2 can pass freely through membranes (dashed lines). IMM, inner mitochondrial membrane; IMS, intermembrane space; OMM, outer mitochondrial membrane; O2·−, superoxide; H2O2, hydrogen peroxide; MnSOD, manganese superoxide dismutase; Cu/ZnSOD, copper/zinc superoxide dismutase; CAT, catalase; THD, NADH transhydrogenase; TR, thioredoxin reductase; TPx, thioredoxin peroxidase; TRxred, reduced thioredoxin; TRxox, oxidized thioredoxin; GSH, glutathione; GSSG, glutathione disulfide; IMAC, inner membrane ion channel; VDAC, voltage dependant anion channel; ΔΨm, membrane potential.
ROS detoxification enzymes including superoxide dismutase and catalase also play a direct role in ROS defense. Superoxide dismutase (SOD) catalyzes the dismutation of O2·− into H2O2 and O2. In mitochondria, SOD uses a manganese cofactor (MnSOD) while the predominantly cytosolic SOD uses copper and zinc (Cu/ZnSOD). ROS released into the mitochondrial matrix is therefore neutralized by MnSOD (100). The fate of O2·− in the IMS is short lived, as the superoxide anion within IMS may be quickly inactivated by Cu/ZnSOD which has been described in the IMS (101), or by cytochrome c which can be directly reduced by O2−· and subsequently pass electrons to complex IV (102). H2O2 is detoxified by the enzyme catalase. It has been reported that Ca2+ can regulate cellular antioxidant defense systems by stimulating catalase and GSH reductase, interacting with calmodulin (CaM) which then interacts with enzymes involved in ROS homeostasis, or via release GSH early in Ca2+ induced PTP opening (12, 103). Ultimately, changes in redox homeostasis, especially at the level of the GSH/GSSG pool, can lead to oxidative damage, especially at the level of protein thiol oxidation by virtue of the high protein content in the IMM, leading to respiratory chain inhibition and further ROS generation (104).
4.3. ROS production at complex I
Complex I of the respiratory chain plays a major role in ROS production. Three mechanisms have been proposed to explain the mechanisms of ROS formation at this level, two of which are associated with forward electron flow through the respiratory chain complexes. Initial electron transport from NAD-linked substrates to complex I produce little O2·−. Reduced sites within complex I including FMN (92), iron sulfur clusters (105) and semiquinones (106) have been suggested to be potential redox sites responsible for O2·− production. Under conditions in which inhibitors that poison complex I function are absent, and in the presence of NAD+ linked substrates (pyruvate, glutamate and malate), O2·− production is stimulated by high mitochondrial membrane potential (ΔΨm) generated by respiratory chain electrons transport (107). When mitochondria are energized via succinate oxidation (a complex II linked substrate), the rate of ROS production by complex I increase significantly. Under such conditions, O2·− production by complex I is caused by a reverse net electron flow from succinate to NAD+. Reverse flow is enhanced by high ΔΨm′ which underlies the rate of ROS production (108, 109). Mitochondrial Ca2+ uptake causes mild uncoupling of ΔΨm, hence, it should decrease ROS production as has been shown in other studies, investigating the role of mild uncoupling on mitochondrial ROS generation (20). Mitochondria from Drosophila melanogaster, exhibit 70% decrease of ROS generation from complex I upon drop of mitochondrial membrane potential of about 10 mV (110). This result is in agreement with the observation that proton leak mediated by uncoupling proteins (UCPs) has been shown to decrease ROS production (111). Moreover, it has been demonstrated the proton leak can be cytoprotective in several model of ischemic injury (112).
4.3.1. Role of Ca2+ on complex I derived ROS
There is an increasing body of evidence suggesting that Ca2+ in mitochondria can augment the oxidative stress. Increases of ROS production in isolated mitochondria have been reported upon activation of the PTP, despite the requisite mitochondrial uncoupling (19). It has been suggested that PTP opening (triggered by Ca2+) induces a specific conformational change of complex I, which results in an increase of H2O2 production when electrons are provided to complex I, and it may also inhibit the electron pathway inside complex I (113). This hypothesis finds its confirmation in studies in which an increase of ROS production upon inhibition of complex I has been demonstrated (91, 92, 114). The direct inhibitory effect of Ca2+ on complex I activity was observed in the presence of nitric oxide (NO); high levels of NO and calcium together, but not separately, caused irreversible inhibition of respiration supported by complex I substrates (glutamate and malate), but not succinate (the complex II substrate) (115). Supporting these observations, there is evidence that Ca2+ stimulates activity of nitric oxide synthase to generate NO·, leading to inhibition of complex IV (116). A study on the role of peroxynitrite–induced permeability transition in isolated mitochondria revealed that Ca2+ exposes novel mitochondrial targets for nitration by ONOO−, consistent with protein conformational changes (117). Because mitochondrial complex I has a very complex composition and is the least well understood component of mitochondrial electron transport chain, it remains unknown if Ca2+ indeed leads to conformational changes. It has been reported that Ca2+ can alter the spectrum of cytochromes a/a3 in isolated complex IV (118). Therefore, it is plausible that Ca2+ may lead to changes in complex I conformation.
4.4. ROS production at complex III
Another significant source of ROS in mitochondria is the ubisemiquinone radical intermediate (QH·) formed during Q cycle at the Q0 site of complex III (119). The rate of ROS production is accelerated by inhibition of sites within complex III distal to Q0 (the site of QH· formation) and further down the respiratory chain (i.e. at level of complex IV) (120). Two parameters that regulate ROS generation derived at complex III have been proposed (12). The first is dependent on the concentration of QH· In the Q0 site which is increased when the distal respiratory chain is inhibited. The second involves the frequency of QH· occurrence which is increased when the respiratory chain turns over more quickly. Stimulation by Ca2+ of the TCA cycle and ox-phos enhance ROS production, at the level of respiratory chain complexes, by increasing the respiration rate and increasing the concentration of reduced substrates (121). Indeed, it has been shown that ROS generation correlates with metabolic rate (122, 123).
4.4.1. Role of Ca2+ on complex III derived ROS
It is not known whether Ca2+ can directly influence ROS production at complex III, but it is likely that Ca2+ can indirectly induce ROS production. Ca2+ can stimulate nitric oxide synthase to produce NO· (116) which has been shown to inhibit complex IV. Hence, augmented radical generation at Q0 forms a link between Ca2+ and ROS production at complex III. Furthermore, upon activation of PTP by Ca2+, inhibition of complex III may occur due to dislocation and loss of cytochrome c. This can result from efflux of cytochrome c through an open pore or by Ca2+ competing with cytochrome c for cardiolipin binding sites, which subsequently disrupts electron transport and results in increased ROS generation (124). Finally, as was indicated earlier, stimulation by Ca2+ of the TCA cycle and ox-phos enhance ROS production, at the level of respiratory chain complexes, by increasing the respiration rate and increasing the concentration of reduced substrates (121).
The study on topology of O2·− production from different sites in the mitochondrial electron transport chain has revealed that ROS produced at complex I are released on the matrix side of the inner membrane, whereas production centered at Q0 of complex III liberates ROS to the IMS (119). Because O2·− cannot cross membrane, there is evidence suggesting the role of voltage-dependent anion channel (VDAC) in the outer mitochondrial membrane in liberating anionic O2·− from the IMS into the cytosol where it can act as a signaling molecule (125). Furthermore, Ca2+ ions stimulate activation of VDAC (126). Alternatively, O2·− may be protonated to form the perhydroxyl radical which is membrane permeable (127).
4.5. Other sources of mitochondrial ROS
The respiratory chain complexes are not the only sources of mitochondrial ROS generation. Non-complex I matrix dehydrogenases are involved in ROS production in the absence of mitochondrial respiratory chain inhibitors. Reduced flavins and flavoproteins have been demonstrated to generate O2·− (128). Purified lactate dehydrogenase (129) and glyceraldehyde-3-phosphate dehydrogenase (130) where shown to catalyze NADH-dependent O2·− production. Flavin-dependent O2·− production in the absence of an electron acceptor has been demonstrated for isolated mitochondrial succinate dehydrogenase (131). Among the NAD+-linked dehydrogenases that generated ROS, the α-ketoglutarate dehydrogenase complex (KGDHC) plays a specific role in Ca2+-induced mitochondrial ROS generation. Mammalian KGDHC is composed of several copies of three enzymes: α-ketoglutarate dehydrogenase (E1; EC 1.2.4.2), dihydrolipoamide succinyltransferase (E2; EC 2.3.1.61) and dihydrolipoamide dehydrogenase (E3 or Dld; EC 1.8.1.4). Dld is also a part of other multienzyme complexes such as the pyruvate dehydrogenase complex (PDHC), ketoacid dehydrogenase complex and glycyne cleavage system (132). The KGDHC is a mitochondrial enzyme tightly bound to the matrix side of the inner mitochondrial membrane and forms part of the TCA cycle enzyme super-complex (133). The fact that KGDHC is responsible for the majority of ROS production (among other non respiratory chain dehydrogenases) under maximal ADP induced respiration (state 3) or uncoupling (134), makes this enzyme an attractive focus of investigation. This is nicely illustrated by the recent discovery in yeast that dihydrolipoyl-dehydrogenases are a major source of ROS (135). Moreover, it has been demonstrated that Ca2+ activates ROS generation by isolated KGDHC (136), as well as in other well known [Ca2+]m regulated citric TCA cycle enzymes: isocitrate dehydrogenase and alpha-ketoglutarate dehydrogenase, as well as pyruvate dehydrogenase (121). It seems very plausible that increased ROS production induced by Ca2+ may be also a result of increased activity of KGDHC. Conditions promoting KGDHC-mediated ROS production may be mostly related to the increases in the intra-mitochondrial NADH/NAD+ ratio (136). On the other hand, KGDHC is inhibited by H2O2 (137), and because providing NADH is the rate-limiting step of TCA cycle, KGDHC inhibition would result in decreased complex I function (138). It has also been demonstrated that H2O2 can impair function of other enzymes in the mitochondrial matrix such as succinate dehydrogenase (SDH) and aconitase (137). In contrast to KGDHC and SDH, aconitase activity is impaired due to direct oxidation by H2O2. Because aconitase contains iron-sulfur cluster ( (4Fe-4S)2+), the interaction with H2O2 leads to oxidation of the cluster to inactive (3Fe-4S)1+ aconitase, which is accompanied with the release of iron III from (4Fe-4S)2+ and H2O2. This sequence of events leads to formation of hydroxyl radical (·OH) by the Fenton reaction, which can exacerbate mitochondrial oxidative stress (139).
4.6. Does Ca2+ induce or decrease ROS production?
Ca2+-induced ROS generation, at the level of respiratory chain complexes is dependent on respiratory rate (i.e. state 4 vs. state 3), the source of mitochondria, and the presence of inhibitors and uncouplers. Addition of Ca2+ to mitochondria isolated from heart, in the presence of antimycin A (complex III inhibitor) results in a significant increase in ROS generation (140). On the other hand, it has been shown that addition of Ca2+ to brain mitochondria in the presence of antimycin A did not induce ROS formation at complex III, but Ca2+ stimulated formation of ROS at the level of complex I in the presence of rotenone (141). It has been also shown, that an increase in Ca2+ uptake was correlated with O2·− production by mitochondria from guinea pig and rat neonatal ventricular myocytes (142). A possibility exists that under physiological conditions, respiratory chain complexes involved in ROS production might be partially inhibited, hence the observed Ca2+-induced ROS production (12).
A major unanswered question in the field of mitochondrial ROS production is whether mild uncoupling, or controlled H+ leak through the IMM, stimulates ROS production or is a mechanism to minimize oxidative stress (143–145). There are multiple pathways for H+ leak including a basal membrane leak (146), ANT mediated leak (147), and uncoupling protein (UCP) mediated leak (143). UCPs are hypothesized to protect the cell from excessive oxidative stress by reducing a high ΔΨm and minimizing ROS production (144). This hypothesis is based on the idea that proton leak is sufficient to reduce ΔΨm such that the protonmotive force is lowered to a point that increases respiration (and a subsequent reduction of O2·− production generated by a highly reduced electron transport chain), but does not ameliorate ATP production by complex IV, the predominant H+ channel in the IMM. Support for this hypothesis is found in data demonstrating that UCP2 and UCP3 mediated proton conductance is induced by O2·− and oxidized fatty acids (94, 148, 149), but are otherwise inactive as uncouplers. A model presented by Brand and Esteves (model #2 in (144)) suggests that high electron flux resulting from fatty acid oxidation increases ΔΨm and O2·− production in mitochondria. Subsequent oxidation of n-6-polyunsaturates fatty acids by ROS leads to production of reactive alkenes that in turn activate proton conductance in UCPs, thus forming a feedback loop to control ROS production. Along these lines, Ca2+ influx, which consumes ΔΨm, should result in a partial uncoupling that would reduce reverse electron flow from Complex II and decrease ROS generation.
A fundamentally different model has been proposed in which uncoupling leads to increased ROS generation by virtue of increased in electron flux (to restore and maintain ΔΨm), thus increasing the probability of electron slippage and an increased rate of O2·− production at complex III (140). However, one caveat to this observation is that the complex III inhibitor antimycin A was used, thus blocking oxidative phosphorylation. In this model, Ca2+ can alter ROS production by simply increasing metabolic rate via TCA cycle stimulation, subsequently increasing electron flux through the electron transport chain when ox-phos is partially inhibited. In fact, increased metabolic rate has been shown to increase ROS production (150). Moreover, Ca2+ can activate NOS and generate ·NO which has been shown to inhibit complex IV, which in turn can lead to ROS production at the Qo site of complex III (151). In support of a Ca2+ induced ROS generation model is data demonstrating that a mildly uncoupling drop of less than 10 mV in ΔΨm induced by low concentrations of the protonophore FCCP in cerebellar granule neurons led to an increase in oxidative stress and ultimate loss of Complex V activity and ATP starvation (152). Experiments on UCP (n) mediated uncoupling are made even more difficult by the fact that UCP2 and UCP3 are found at a tiny relative abundance of 1% or less than that of the well characterized UCP1 in brown adipose tissue mitochondria (153). Thus, Ca2+ may both stimulate ox-phos electron flux, as well as leading to partial inhibition of the electron transport chain, leading to an increased probability of electron slippage to O2.
An additional possible avenue of Ca2+ and ROS crosstalk involves influx of K+ through the mitochondrial KATP channel (mitoKATP) which has been suggested induce an increase in mitochondrial ROS generation. Andrukhiv et al. recently demonstrated that mitoKATP activation (or low concentrations of the K+ ionophore valinomycin) increase mitochondrial ROS generation in isolated rat cardiac mitochondria despite an increase in respiratory electron flux (154). Similar results were seen upon K+ influx via opening of the mitochondrial Ca2+ sensitive K+ channel (mtBKCa2+) (155). Garlid’s group showed that mitochondrial K+ influx leads to matrix alkalinization as H+ ions pumped out via the respiratory chain are replaced by K+ ions (156). Moreover, it was determined that matrix alkalinization leads to inhibition of electron transport at complex I (154) supporting data that matrix alkalinization leads to increased mitochondrial ROS production (157, 158). Therefore, while slight uncoupling due to electrophoretic K+ influx leads lead to a decrease in ΔΨm, a secondary effect of complex I inhibition is matrix alkalinization which stimulates ROS production. Furthermore, PKCε, which is a protein kinase present in cardiac mitochondria (159) is an activator of mitoKATP (160). PKCε is activated by PKG (160), which is in turn stimulated by Ca2+ via the NO/cGMP/PKG signaling pathway, linking Ca2+ to mitoKATP mediated ROS production. However, as discussed above, these results are conflict with studies that demonstrate that uncoupling lead to a decrease in ROS generation (161, 162). In fact, a number of studies from Kowaltowski’s group have demonstrated that mitoKATP opening reduces ROS generation (163–165). Ferranti et al showed that the mitoKATP opener diazoxide (DZX) leads to a decrease in H2O2 released from isolated rat heart, brain and liver mitochondria using the ROS indicator Amplex Red (165). These conflicting data have been suggested to result from direct interactions between DZX and commonly used ROS probes such as dichlorofluorescein, or by changes in indicator fluorescence by pH changes induced by ΔΨm dependant probe uptake (164, 166). Despite these conflicting data, mitochondrial K+ flux appears to affect mitochondrial ROS generation.
5. ROS AND Ca2+ SIGNALING
Supported by an expansive library of literature describing the pathological effects of ROS and oxidative damage, a developing field of study has initiated investigations on ROS as a physiological mediator of normal cellular function (167). As described above, mitochondria are crucial to proper cellular Ca2+ signaling and energy production, and as a consequence, are a significant source of cellular ROS. Just as Ca2+ plays a role in the production of ROS, cellular redox state can significantly modulate Ca2+ signaling (for review see, (103, 168–172)). The reciprocal interaction between Ca2+ modulated ROS production and ROS modulated Ca2+ signaling underlies the concept of ROS and Ca2+ crosstalk. This section will discuss how redox state and ROS modulate Ca2+ ion channels and pumps and the intricate crosstalk between ROS and Ca2+ signaling.
5.1. Redox modulation of Ca2+ transporters
Many cellular processes are modulated by the redox state of proteins. Changes in structure caused by oxidizing neighboring cysteine residues (resulting in disulfide bridge formation) can change the activity of enzymes and ion transporters. In the context of Ca2+ signaling, redox state and ROS can stimulate as well as inhibit Ca2+ channels/pumps/exchangers, generally increasing Ca2+ channel activity and inhibiting Ca2+ pumps. Regulation is found intracellularly at cellular store Ca2+ channels and at PM channels. In the following section, we will discuss known instances of redox regulation of Ca2+ ion channels, pumps, and exchangers, and when observed, discuss how ROS can directly modulate those Ca2+ transporting proteins.
5.1.1. Ryanodine receptors (RyR) are stimulated by oxidation
Following action potential-induced depolarization of the plasma membrane, Ca2+ enters the cell via L-type Ca2+ channels, evoking Ca2+-induced Ca2+ release (CICR) from SR Ca2+ stores (173). Ryanodine receptors are the primary Ca2+ release channel involved in SR Ca2+ release to evoke muscle contraction in skeletal (RyR1 isoform) and cardiac (RyR2 isoform) muscle cells. RyR are large (>500 kDa) proteins that form tetramers in the SR and ER membranes (174). Despite having up to 89 cystine residues per monomer, only a small subset has been shown to be redox active and play a role in channel function (175–177). Experimentally, generation of RyR protein disulfides led to reversible activation of channel activity (13, 178–181). Moreover, shifts in the ratios of cellular redox buffers such as the GSH/GSSG redox pair (178, 182–184) and the NADH/NAD+ redox pair (185–187) modulate RyR channel activity. In addition to changes in RyR channel modulation by changes in whole cell redox homeostasis, ROS has been shown to directly modulate RyR (188). O2·− (187, 189) and H2O2/hydroxyl radical (190–192) directly oxidize redox-sensing thiols on the RyR leading to channel stimulation, and effect that is reversible by agents that reduce thiol groups. Moreover, ROS induction of NOS can oxidize NO to nitrosium (NO+) and subsequently react with free RyR thiols to form S-nitrosothiol which stimulates RyR channel activity (175, 193).
5.1.2. IP3R are stimulated by oxidation
Inositol 1,4,5-triphosphate receptors (IP3R) channels are the primary Ca2+ release channel in the endoplasmic reticulum (ER) in non-excitable cells and constitute a minor proportion of SR Ca2+ release channels in cardiac cells (194). ER Ca2+ release via IP3R is initiated by binding of the signaling molecule inositol 1,4,5-triphosphate (IP3). In non-excitable cells such as HeLa and the cerebellum, redox modification of sulfhydrals is thought to be responsible for a thimerosal dependant sensitization to IP3 (195). GSSG has also been shown to stimulate IP3R channel activity by increasing the binding affinity of IP3 to hepatocyte IP3R (196, 197). ROS has been shown to directly stimulate IP3R mediated Ca2+ release from the ER. ROS generated by tert-butyl hydroperoxide treatment of hepatocytes resulted in DTT reversible stimulation of IP3R Ca2+ release (198). H2O2 treatment resulted in Ca2+ release from endothelial cells (199). Xanthine/Xanthine oxidase generated O2·− was shown to stimulate IP3R channel activity in smooth muscle cells (200).
5.1.3. SERCA and PMCA are inhibited by oxidation
Muscle relaxation following Ca2+ release from SR/ER Ca2+ stores is facilitated by Ca2+ re-uptake by the sarco/endoplasmic reticulum Ca2+ ATPase (SERCA). SERCA activity is sensitive to the redox state, but unlike RyR and IP3R, SERCA is inhibited by oxidation and ROS (201). There are three SERCA isoforms expressed differentially in different tissue types that have up to 25 cysteine residues with one or two essential catalytic cysteines (202). Thiol oxidizing compounds inhibit SERCA Ca2+ pumping activity while reducing agents including DTT and GSH stimulate SERCA (203). ROS have a striking effect on SERCA on two levels. Oxygen radicals have been shown to depress SERCA in cardiac cells (189, 204–206) and nearly inhibit SERCA in smooth muscle cells (207, 208). SERCA is dependent on ATP hydrolysis to energize Ca2+ pumping. H2O2 and O2−· have also been shown to directly inhibit ATP binding to SERCA, uncoupling ATP hydrolysis from Ca2+ pumping (188, 189, 209, 210).
The plasma membrane Ca2+ ATPase (PMCA) is a much slower pump than SERCA. Although Na+/Ca2+ exchange is the dominated efflux mechanism, PMCA works alongside with Na+/Ca2+ exchange for maintenance of low intracellular Ca2+ during the relaxation phase of muscle contraction (211). As with the SERCA, ROS have been shown to modify PMCA sulfhydrals, depressing activity as well as inhibiting ATP hydrolysis, thus reducing Ca2+ pumping in the heart (206, 212), pancreas (213), and brain (214).
5.1.4. Plasma membrane Na+/Ca2+ exchanger is modulated by oxidation
The Plasma membrane Na+/Ca2+ exchange (NCX) is a high capacity, low affinity Ca2+ pump that utilizes the transmembrane Na+ gradient to exchange extracellular Na+ for cellular Ca2+ in a ratio of 3:1. There is evidence suggesting ROS both stimulate and decrease NCX activity. In cardiac cells, ROS in the form of both H2O2 (215) and O2·− (216) was shown to stimulate NCX activity. In another study, H2O2 stimulated NCX while the strong oxidant HOCL led to NCX inhibition. H2O2 was not found to augment NCX activity when expressed in CHO-K1 cells, but peroxynitrite and peroxyl radical generation led to a decrease in NCX activity (217, 218). During oxidative stress, either stimulation or inhibition of NCX could lead to Ca2+ disregulation and have pathological effects. It is known that extended membrane depolarization or high intracellular Na+ can lead to NCX operating in reverse, ultimately acting as a Ca2+ importer
6. CROSSTALK BETWEEN Ca2+ AND ROS
As illustrated above, there is clearly an intricate level of crosstalk between Ca2+ signaling in the cell and the redox environment, which in turn is modulated to a large extent by both physiological and pathological generation of ROS. In mitochondria, Ca2+ signals are central to metabolic regulation of the TCA cycle and ox-phos of which ROS generation is an important by-product. Most ROS are buffered and detoxified very quickly by mitochondrial and cellular antioxidant defenses (SOD, catalase, GSH for example), so the extent of ROS modulation of Ca2+ signaling is most likely dependent on spatiotemporal positioning within microenvironments of transiently higher oxidative potential. There is considerable data supporting Ca2+ microdomains (219–221), yet relatively little discussion of redox microdomains. The following section will describe the features of mitochondrial Ca2+ microdomains, followed by discussion of crosstalk with ROS microdomains and localized regions of differential redox potential.
6.1. Mitochondrial Ca2+ microdomains
There are many types of subcellular Ca2+ release events that occur as a result of different species and numbers of Ca2+ channels opening (222). The physiological basis of Ca2+ microdomains depends on a localized concentration of Ca2+ around the mouth of an open Ca2+ release channel that is significantly above that of the steady state [Ca2+]c. for instance, it has been estimated that [Ca2+]c can reach 100 μM within 20 nm of open plasma membrane Ca2+ channels (223). Moreover, measurements of [Ca2+]c formed by localized ER Ca2+ release (sparks) has been estimated to be between 20 and 100 μM (224–227). These loci of [Ca2+] orders of magnitude higher than resting [Ca2+]c have profound effects on mitochondrial Ca2+ uptake. Recent data characterizing the Ca2+ affinity of the MCU calculated the half-saturation of current to be 19 +/− 2 mM Ca2+ (24), which is much higher than previous estimates of between 1 and 189 μM (53), indicating the MCU is most effective at high [Ca2+]c. In contrast, resting cytosolic [Ca2+]c has been estimated to be < 100–300 nM (228, 229). Therefore, mitochondrial Ca2+ uptake by the MCU would be most effective when [Ca2+]c reach tens to hundreds of μM, as occurs in the direct vicinity of SR/ER Ca2+ release channels upon activation.
For mitochondrial Ca2+ microdomains to serve a physiological role, there must be a close apposition between ER/SR Ca2+ release channels and mitochondrial Ca2+ uptake channels to take advantage of the high localized [Ca2+]. This has been shown to be true immunologically as well as via direct visualization. In HeLa cells, a large fraction of mitochondria lie within 100 nm of the ER as determined by co-localization of fluorescence from GFPs localized to the ER lumina and mitochondrial matrix (230). IP3R are enriched in regions apposed to mitochondria as determined via increased IP3R immunoreactivity near mitochondria in astrocytes and oligodendrocytes (231, 232) and by direct electron microscopy in Purkinje neurons (233, 234). Via electron microscopy, it was demonstrated that the distance between SR RyR and the nearest mitochondrial surface is very short (between 37 and 270 nm) in cardiac ventricular myocytes (235). In fact, peptide linkages have been observed in that may help to position mitochondria near calcium release channels, leading to a distance between ER and mitochondria of between 5–30 nm at the junctions (236). Privileged Ca2+ exchange between the ER/SR and mitochondria may be further enhanced if mitochondrial Ca2+ uptake channels are enriched in the vicinity of corresponding ER/SR Ca2+ release channels. However, until the molecular identity of the MCU can be established, this facet of microdomain Ca2+ transfer remains unclear.
6.2. Mitochondrial ROS microdomains
In part because the technology to spatially and temporally visualize ROS currently lacks the resolution found with modern Ca2+ visualization tools, the concept of redox or ROS microdomains has not received much attention. Nonetheless, some of the fundamental properties of Ca2+ microdomains can be extended to ROS signaling. As described above, mitochondria are the primary source of cellular ROS. By virtue of cellular architecture, mitochondria define a localized source of ROS, similar to the spatially defined Ca2+ release mechanisms that define Ca2+ microdomains. Moreover, the action of ROS buffering and defense mechanisms ensure the lifetime of ROS is fairly short within the cell, facilitating temporal fluctuations by eliminating ROS pulses. Redox microdomains differ from Ca2+ microdomains however, in that changes in the redox environment are generally formed by a steady state change in ROS production or decrease in ROS defense rather than a transient release of bulk ROS. Thus, a redox microdomain may be spatially defined, but persist as a concentration gradient rather than as a pulse. Additionally, ROS microdomains result from the combined redox effects of different species with differing diffusion abilities including membrane permeable H2O2, membrane impermeable O2·−, as well as changes in the ratios of redox couples such as GSH/GSSG and the local availability of defensive enzymes. To summarize, ROS microdomains are generally formed by diffusion from spatially defined sources rather than by release from a mitochondrial store of ROS. However, the recent discovery of an ROS activated inner mitochondrial anion channel (IMAC) (237) provides the possibility of transient ROS pulses, similar to the well known phenomena of Ca2+ sparks (238).
In cardiac mitochondria loaded with TMRM (a ΔΨm sensitive fluorescent probe), a local loss ΔΨm was detected upon application of a laser flash resulting in the production of O2·− and ·OH by photoexcitation (239). Laser de-energized mitochondria transiently produced ROS in two distinct phases; an initial slow rise termed “trigger ROS” caused by accumulation of photoexcitation-related ROS, followed by a “ROS burst” that accrued simultaneously with transient mitochondrial membrane depolarization. This phenomenon, called “ROS-induced-ROS release” (RIRR), was initially postulated to be dependent on the PTP for propagation of ROS in mitochondria (239). Further studies have shown that the inner mitochondrial anion channel (IMAC) plays a crucial role in propagation of ROS in RIRR. The IMAC channel is itself activated by a ROS dependent reduction in GSH:GSSG ratio, indicative of a change in mitochondrial protein thiol status, and opens prior to PTP opening (237, 240, 241). In a model presented by Aon et. al. (237), recycling of oxidized glutathione to restore a high GSH:GSSH ratio is carried out by glutathione reductase, consuming NADPH in the process. NADPH is subsequently regenerated by NADH transhydrogenase which has been suggested to involve partial uncoupling via UCP2 (242). Thus, in the face of enhanced ROS production, NADH is shunted to restore the GSH:GSSG ratio in order to maintain redox homeostasis at the expense of ox-phos reducing power (NADH supply) and a diminution of ΔΨm. The extent to which Ca2+ regulation in this process is currently unknown.
6.3. Mitochondrial Ca2+ and ROS crosstalk
As a central player in cellular Ca2+ signaling, energy metabolism, and apoptosis, it is essential that mitochondria have the capacity to micromanage Ca2+ handling. As such, it not surprising that ROS produced as a by-product of ox-phos is utilized as a signaling molecule to fine tune Ca2+ transport, both at the mitochondrial level, as well as the ER/SR. The sheer number of diseases and disorders that are caused by Ca2+ deregulation and/or oxidative stress emphasize the importance of tight regulation in these pathways.
Figure 3 illustrates how a Ca2+ signal initiated during cardiomyocyte excitation-contraction can lead to changes in both Ca2+ handling, as well as ROS generation. Briefly, an excitation-contraction derived Ca2+ transient generated by SR-RyR channel opening results in mitochondrial Ca2+ uptake. Increased mitochondrial Ca2+ stimulates metabolic pathways leading to an increase in reduced substrates and electron transport which can evoke subsequent increases in ROS generation by the mechanisms detailed above. ROS can then stimulate further Ca2+ release by oxidizing SR-RyR cysteine residues. For example, it has been shown that mitochondrially derived ROS can elicit cerebral artery dilation by activating RyR Ca2+ sparks (243). Moreover, there is some evidence that mitochondrial Ca2+ transport is under redox control as well (our results, unpublished and (244)), although the mechanisms are not known. In this model, a mitochondrial Ca2+ signal is utilized to match energy production via ox-phos to the energy demands of muscle contraction. A slight increase in ROS may sensitize the Ca2+ signaling pathways during times of rapid signaling or high energy demand. Furthermore, this crosstalk is made possible by virtue of microdomain architecture. Based on similarities to neuronal structures, microdomain Ca2+ signaling has been described as being “quasi-synaptic” between SR and mitochondria (225). The comparison was conceptually extended by Camello’s group to include ROS as a “neuro-modulator” (170). Another instance in which ROS may affect Ca2+ signaling is illustrated by Ca2+ oscillations in HeLa cells. Ca2+ oscillation were shown to be invoked upon release of mitochondrial Ca2+ via Na+/Ca2+ exchange near ER-IP3R, invoking secondary Ca2+ induced release from the ER through IP3R (245). Oscillations caused by IP3R sensitization by low levels of mitochondrially generated ROS in Ca2+ treated cells were subsequently abolished by antioxidants.
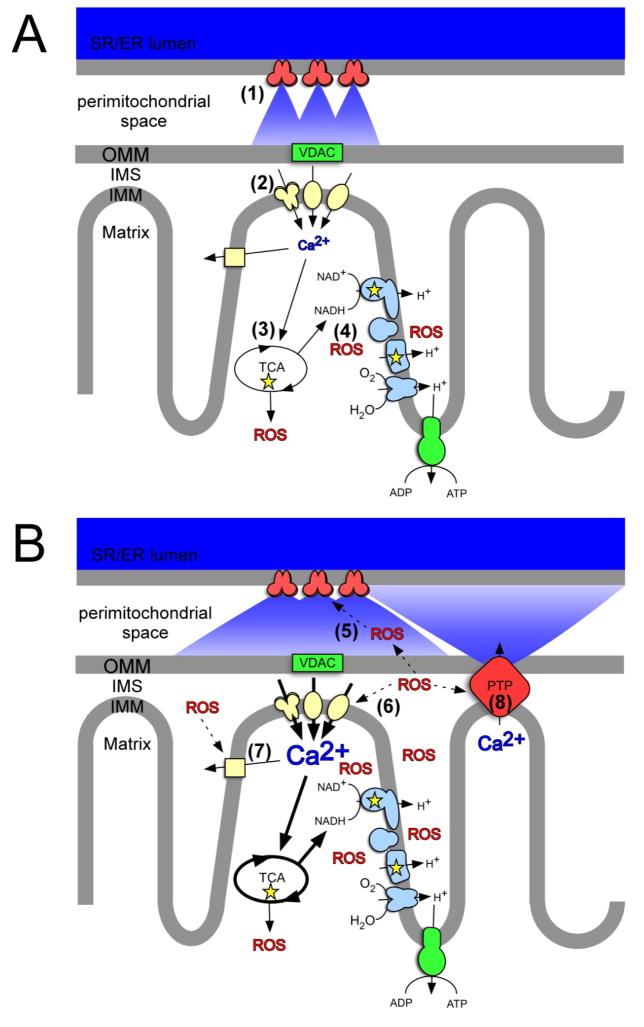
Figure 3.
Scheme for Ca2+/ROS crosstalk in cardiac mitochondrial microdomains. A: Ca2+ stimulation of ox-phos and ROS production. Pulses of Ca2+ resulting from excitation-contraction derived SR RyR Ca2+ release ((in red, (1)) leads to mitochondrial Ca2+ uptake via MCU, RaM, and mRyR (in yellow, (2)). Increased [Ca2+]m stimulates TCA cycle enzymes (3) which generates NADH that feeds into the respiratory electron transport chain (in blue), in turn increasing APT synthesis and ROS production (4). B: ROS modulation of Ca2+ channel activity. Diffusion of ox-phos derived ROS eads to a shift in redox homeostasis resulting in a locally oxidizing environment. Redox and ROS modulation of Ca2+ channels increases Ca2+ release from SR RyR channels (5), increased mitochondrial Ca2+ uptake by mitochondrial Ca2+ channels (6), and changes in Na+/Ca2+ exchange (7). Continued Ca2+ uptake can lead to Ca2+ overload and further Ca2+ induced ROS generation which can ultimately lead to PTP opening (8) and mitochondrial dysfunction. IMM, inner mitochondrial membrane; IMS, intermembrane space; OMM, outer mitochondrial membrane; SR/ER, sarcoplasmic reticulum/endoplasmic reticulum; VDAC, voltage dependant anion channel; PTP, permeability transition pore.
The positive stimulation of mitochondrial Ca2+ signals by ROS and increased ROS generation resulting from increased [Ca2+]m can lead to a positive feedback loop. While physiological increases in [Ca2+]m are beneficial for metabolism, overload is detrimental to mitochondrial function and can become pathological. Likewise, ROS as a signal transduction molecule is physiologically relevant, but excess ROS leads to oxidative stress and mitochondrial dysfunction. Following short periods of excitation and enhanced Ca2+ signaling, mitochondrial antioxidant defenses should restore ROS to physiological levels and mitochondrial Ca2+ efflux channels should expel excess Ca2+. However, during prolonged stimulation or in the face of oxidative stress, there must be mechanisms to break the feedback cycle to prevent Ca2+ overload. As mentioned above, there is evidence that transient PTP flicker can act as a “pressure relief valve”, expelling excess Ca2+. One problem with this hypothesis is that a full opening of PTP would lead to the release of GSH, diminishing the antioxidant capacity of the mitochondria. Therefore, the existence of a “sub-conductance” state of PTP must be proposed. Additionally, there is evidence that ROS activation of c-Jun N-terminal kinase can lead to an up-regulation of glycogen synthase. Increased glycogen synthase activity effectively shunts glucose metabolism away from pyruvate production. Lower pyruvate levels subsequently translate into a reduction of mitochondrial substrate and a reduction in metabolism, reducing ROS production (246). A third possibility is that mitochondrial Ca2+ efflux mechanisms such as Na+/Ca2+ are also modulated by ROS, thereby resulting in an increase of total Ca2+ flux, both in and out of the mitochondria.
7. PERSPECTIVES
Despite great advances in mitochondrial Ca2+ dynamics and ROS metabolism and signaling, there are clearly more questions than answers in this field. From what was once believed to be a dangerous waste product of respiration, ROSs have only recently come to the forefront as important signaling molecules. Moreover, the idea that ROS may play a required role in modulating Ca2+ signaling has been a startling discovery. It is becoming increasingly important to address the mechanisms by which mitochondrial Ca2+ regulate mitochondrial ROS generation. There is considerable evidence that ROS and the redox environment modulate cellular Ca2+ channels. However, our knowledge of how ROS and the redox environment modulate mitochondrial Ca2+ transport is lacking. Perhaps one of the biggest hurdles in this field is that no mitochondrial inner membrane ion channel has ever been cloned. Furthermore, specific ROS probes targeted to the mitochondria of living cells are still underdeveloped. Clearly, the field of mitochondrial microdomains and the crosstalk between Ca2+ and ROS represents a new frontier in biomedical research. Once we understand the dynamic interplay between these two crucial signaling pathways we will be poised to develop therapies for the large number of insidious diseases involving mitochondrial dysfunction.
Acknowledgments
We thank the members of the Mitochondrial Research & Innovations Group (MRIG) at the University of Rochester Medical Center for insightful discussions and helpful comments on this manuscript. This work was supported by NIH grants HL-33333 and ES-07026, NYS Spinal Cord Injury research programs CO17688 and C020941, and URMC Department of Pediatrics research funds.
Abbreviations
- ACON
aconitase
- ADP
adenosine diphosphate
- α-KGDH
α-ketogluterate dehydrogenase
- ANT
adenine nucleotide transporter
- ATP
adenosine triphosphate
- [Ca2+]c
cytoplasmic calcium concentration
- [Ca2+]m
mitochondrial calcium concentration
- CAT
catalase
- CICR
calcium-induced calcium release
- CS
citrate synthase
- Cu/ZnSOD
copper/zinc superoxide dismutase
- ER
endoplasmic reticulum
- ETC
electron transport chain
- FADH
reduced flavin adenine dinucleotide
- FUM
fumarase
- GSH
glutathione
- GSSG
glutathione disulfide
- H2O2
hydrogen peroxide
- ICDH
isocitrate dehydrogenase
- IMAC
inner membrane ion channel
- IMM
inner mitochondrial membrane
- IMS
intermembrane space
- IP3R
inositol-1,4,5-triphosphate receptor
- MCU
mitochondrial calcium uniporter
- MDH
malate dehydrogenase
- MnSOD
manganese superoxide dismutase
- MPT
mitochondrial permeability transition
- mRrR
mitochondrial ryanodine receptor
- NADH
reduced nicotinamide adenine dinucleotide
- NADPH
reduced nicotinamide adenine dinucleotide phosphate
- O2·−
superoxide
- OMM
outer mitochondrial membrane
- ox-phos
oxidative phosphorylation
- PDH
pyruvate dehydrogenase
- PTP
permeability transition pore
- RAM
rapid mode
- RIRR
ROS-induced ROS release
- ROS
reactive oxygen species
- RuR
ruthenium red
- SDH
succinate dehydrogenase
- SR
sarcoplasmic reticulum
- TCA
tricarboxylic acid cycle
- THD
NADH transhydrogenase
- TMRM
tetramethylrhodamine methyl ester
- TPx
thioredoxin peroxidase
- TR
thioredoxin reductase
- TRxox
oxidized thioredoxin
- TRxred
reduced thioredoxin
- VDAC
voltage dependant anion channel
- ΔΨm
membrane potential
Footnotes
Publisher's Disclaimer: This is an, un-copyedited, author manuscript that has been accepted for publication in the Frontiers in Bioscience". Cite this article as appearing in the Journal of Frontiers in Bioscience. Full citation can be found by searching the Frontiers in Bioscience (http://bioscience.org/search/authors/htm/search.htm) following publication and at PubMed (http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?CMD=search&DB=pubmed) following indexing. This article may not be duplicated or reproduced, other than for personal use or within the rule of "Fair Use of Copyrighted Materials" (section 107, Title 17, U.S. Code) without permission of the copyright holder, the Frontiers in Bioscience. From the time of acceptance following peer review, the full final copy edited article of this manuscript will be made available at http://www.bioscience.org/. The Frontiers in Bioscience disclaims any responsibility or liability for errors or omissions in this version of the un-copyedited manuscript or in any version derived from it by the National Institutes of Health or other parties.
References
- 1.DiMauro S. Mitochondrial diseases. Biochim Biophys Acta. 2004;1658(1–2):80–8. doi: 10.1016/j.bbabio.2004.03.014. [DOI] [PubMed] [Google Scholar]
- 2.Zeviani M, Di Donato S. Mitochondrial disorders. Brain. 2004;127(Pt 10):2153–72. doi: 10.1093/brain/awh259. [DOI] [PubMed] [Google Scholar]
- 3.Mancuso M, Coppede F, Migliore L, Siciliano G, Murri L. Mitochondrial dysfunction, oxidative stress and neurodegeneration. J Alzheimers Dis. 2006;10(1):59–73. doi: 10.3233/jad-2006-10110. [DOI] [PubMed] [Google Scholar]
- 4.Brand MD, Buckingham JA, Esteves TC, Green K, Lambert AJ, Miwa S, Murphy MP, Pakay JL, Talbot DA, Echtay KS. Mitochondrial superoxide and aging: uncoupling-protein activity and superoxide production. Biochem Soc Symp. 2004;(71):203–13. doi: 10.1042/bss0710203. [DOI] [PubMed] [Google Scholar]
- 5.Fiskum G, Starkov A, Polster BM, Chinopoulos C. Mitochondrial mechanisms of neural cell death and neuroprotective interventions in Parkinson’s disease. Ann N Y Acad Sci. 2003;991:111–9. doi: 10.1111/j.1749-6632.2003.tb07469.x. [DOI] [PubMed] [Google Scholar]
- 6.Lowell BB, Shulman GI. Mitochondrial dysfunction and type 2 diabetes. Science. 2005;307(5708):384–7. doi: 10.1126/science.1104343. [DOI] [PubMed] [Google Scholar]
- 7.Rego AC, Oliveira CR. Mitochondrial dysfunction and reactive oxygen species in excitotoxicity and apoptosis: implications for the pathogenesis of neurodegenerative diseases. Neurochem Res. 2003;28(10):1563–74. doi: 10.1023/a:1025682611389. [DOI] [PubMed] [Google Scholar]
- 8.Brookes PS, Levonen AL, Shiva S, Sarti P, Darley-Usmar VM. Mitochondria: regulators of signal transduction by reactive oxygen and nitrogen species. Free Radic Biol Med. 2002;33(6):755–64. doi: 10.1016/s0891-5849(02)00901-2. [DOI] [PubMed] [Google Scholar]
- 9.Nicholls DG. Mitochondria and calcium signaling. Cell Calcium. 2005;38(3–4):311–7. doi: 10.1016/j.ceca.2005.06.011. [DOI] [PubMed] [Google Scholar]
- 10.Sparagna GC, Gunter KK, Sheu SS, Gunter TE. Mitochondrial calcium uptake from physiological-type pulses of calcium. A description of the rapid uptake mode. J Biol Chem. 1995;270(46):27510–5. doi: 10.1074/jbc.270.46.27510. [DOI] [PubMed] [Google Scholar]
- 11.Beutner G, Sharma VK, Giovannucci DR, Yule DI, Sheu SS. Identification of a ryanodine receptor in rat heart mitochondria. J Biol Chem. 2001;276(24):21482–8. doi: 10.1074/jbc.M101486200. [DOI] [PubMed] [Google Scholar]
- 12.Brookes PS, Yoon Y, Robotham JL, Anders MW, Sheu SS. Calcium, ATP, and ROS: a mitochondrial love-hate triangle. Am J Physiol Cell Physiol. 2004;287(4):C817–33. doi: 10.1152/ajpcell.00139.2004. [DOI] [PubMed] [Google Scholar]
- 13.Eager KR, Roden LD, Dulhunty AF. Actions of sulfhydryl reagents on single ryanodine receptor Ca (2+)-release channels from sheep myocardium. Am J Physiol. 1997;272(6 Pt 1):C1908–18. doi: 10.1152/ajpcell.1997.272.6.C1908. [DOI] [PubMed] [Google Scholar]
- 14.Herrington J, Park YB, Babcock DF, Hille B. Dominant role of mitochondria in clearance of large Ca2+ loads from rat adrenal chromaffin cells. Neuron. 1996;16(1):219–28. doi: 10.1016/s0896-6273(00)80038-0. [DOI] [PubMed] [Google Scholar]
- 15.Boitier E, Rea R, Duchen MR. Mitochondria exert a negative feedback on the propagation of intracellular Ca2+ waves in rat cortical astrocytes. J Cell Biol. 1999;145(4):795–808. doi: 10.1083/jcb.145.4.795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zoratti M, Szabo I. The mitochondrial permeability transition. Biochim Biophys Acta. 1995;1241(2):139–76. doi: 10.1016/0304-4157(95)00003-a. [DOI] [PubMed] [Google Scholar]
- 17.Kowaltowski AJ, Castilho RF, Vercesi AE. Mitochondrial permeability transition and oxidative stress. FEBS Lett. 2001;495(1–2):12–5. doi: 10.1016/s0014-5793(01)02316-x. [DOI] [PubMed] [Google Scholar]
- 18.Kowaltowski AJ, Naia-da-Silva ES, Castilho RF, Vercesi AE. Ca2+-stimulated mitochondrial reactive oxygen species generation and permeability transition are inhibited by dibucaine or Mg2+ Arch Biochem Biophys. 1998;359(1):77–81. doi: 10.1006/abbi.1998.0870. [DOI] [PubMed] [Google Scholar]
- 19.Maciel EN, Vercesi AE, Castilho RF. Oxidative stress in Ca(2+)-induced membrane permeability transition in brain mitochondria. J Neurochem. 2001;79(6):1237–45. doi: 10.1046/j.1471-4159.2001.00670.x. [DOI] [PubMed] [Google Scholar]
- 20.Starkov AA, Chinopoulos C, Fiskum G. Mitochondrial calcium and oxidative stress as mediators of ischemic brain injury. Cell Calcium. 2004;36(3–4):257–64. doi: 10.1016/j.ceca.2004.02.012. [DOI] [PubMed] [Google Scholar]
- 21.Gincel D, Zaid H, Shoshan-Barmatz V. Calcium binding and translocation by the voltage-dependent anion channel: a possible regulatory mechanism in mitochondrial function. Biochem J. 2001;358(Pt 1):147–55. doi: 10.1042/0264-6021:3580147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.O’Rourke B. Mitochondrial ion channels. Annu Rev Physiol. 2007;69:19–49. doi: 10.1146/annurev.physiol.69.031905.163804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gunter TE, Buntinas L, Sparagna G, Eliseev R, Gunter K. Mitochondrial calcium transport: mechanisms and functions. Cell Calcium. 2000;28(5–6):285–96. doi: 10.1054/ceca.2000.0168. [DOI] [PubMed] [Google Scholar]
- 24.Kirichok Y, Krapivinsky G, Clapham DE. The mitochondrial calcium uniporter is a highly selective ion channel. Nature. 2004;427(6972):360–4. doi: 10.1038/nature02246. [DOI] [PubMed] [Google Scholar]
- 25.Moore CL. Specific inhibition of mitochondrial Ca++ transport by ruthenium red. Biochem Biophys Res Commun. 1971;42(2):298–305. doi: 10.1016/0006-291x(71)90102-1. [DOI] [PubMed] [Google Scholar]
- 26.Reed KC, Bygrave FL. A low molecular weight ruthenium complex inhibitory to mitochondrial Ca2+ transport. FEBS Lett. 1974;46(1):109–14. doi: 10.1016/0014-5793(74)80346-7. [DOI] [PubMed] [Google Scholar]
- 27.Bygrave FL, Reed KC, Spencer T. Cooperative interactions in energy-dependent accumulation of Ca2+ by isolated rat liver mitochondria. Nat New Biol. 1971;230(11):89. doi: 10.1038/newbio230089a0. [DOI] [PubMed] [Google Scholar]
- 28.Scarpa A, Cecchetto A, Azzone GF. The mechanism of anion translocation and pH equilibration in erythrocytes. Biochim Biophys Acta. 1970;219(1):179–88. doi: 10.1016/0005-2736(70)90073-8. [DOI] [PubMed] [Google Scholar]
- 29.Vinogradov A, Scarpa A. The initial velocities of calcium uptake by rat liver mitochondria. J Biol Chem. 1973;248(15):5527–31. [PubMed] [Google Scholar]
- 30.Sottocasa G, Sandri G, Panfili E, De Bernard B, Gazzotti P, Vasington FD, Carafoli E. Isolation of a soluble Ca 2+ binding glycoprotein from ox liver mitochondria. Biochem Biophys Res Commun. 1972;47(4):808–13. doi: 10.1016/0006-291x(72)90564-5. [DOI] [PubMed] [Google Scholar]
- 31.Sottocasa GL, Sandri G, Panfili E, De Bernard B. A glycoprotein located in the intermembrane space of rat liver mitochondria. FEBS Lett. 1971;17(1):100–105. doi: 10.1016/0014-5793(71)80574-4. [DOI] [PubMed] [Google Scholar]
- 32.Mironova GD, Sirota TV, Pronevich LA, Trofimenko NV, Mironov GP, Grigorjev PA, Kondrashova MN. Isolation and properties of Ca2+-transporting glycoprotein and peptide from beef heart mitochondria. J Bioenerg Biomembr. 1982;14(4):213–25. doi: 10.1007/BF00751016. [DOI] [PubMed] [Google Scholar]
- 33.Panfili E, Sandri G, Sottocasa GL, Lunazzi G, Liut G, Graziosi G. Specific inhibition of mitochondrial Ca2+ transport by antibodies directed to the Ca2+-binding glycoprotein. Nature. 1976;264(5582):185–6. doi: 10.1038/264185a0. [DOI] [PubMed] [Google Scholar]
- 34.Saris NE, Sirota TV, Virtanen I, Niva K, Penttila T, Dolgachova LP, Mironova GD. Inhibition of the mitochondrial calcium uniporter by antibodies against a 40-kDa glycoproteinT. J Bioenerg Biomembr. 1993;25(3):307–12. doi: 10.1007/BF00762591. [DOI] [PubMed] [Google Scholar]
- 35.Trenker M, Malli R, Fertschai I, Levak-Frank S, Graier WF. Uncoupling proteins 2 and 3 are fundamental for mitochondrial Ca2+ uniport. Nat Cell Biol. 2007;9(4):445–52. doi: 10.1038/ncb1556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Buntinas L, Gunter KK, Sparagna GC, Gunter TE. The rapid mode of calcium uptake into heart mitochondria (RaM): comparison to RaM in liver mitochondria. Biochim Biophys Acta. 2001;1504(2–3):248–61. doi: 10.1016/s0005-2728(00)00254-1. [DOI] [PubMed] [Google Scholar]
- 37.Altschafl BA, Beutner G, Sharma VK, Sheu SS, Valdivia HH. The mitochondrial ryanodine receptor in rat heart: A pharmaco-kinetic profile. Biochim Biophys Acta. 2007;1768(7):1784–95. doi: 10.1016/j.bbamem.2007.04.011. [DOI] [PubMed] [Google Scholar]
- 38.Beutner G, Sharma VK, Lin L, Ryu SY, Dirksen RT, Sheu SS. Type 1 ryanodine receptor in cardiac mitochondria: transducer of excitation-metabolism coupling. Biochim Biophys Acta. 2005;1717(1):1–10. doi: 10.1016/j.bbamem.2005.09.016. [DOI] [PubMed] [Google Scholar]
- 39.Maack C, Cortassa S, Aon MA, Ganesan AN, Liu T, O’Rourke B. Elevated cytosolic Na+ decreases mitochondrial Ca2+ uptake during excitation-contraction coupling and impairs energetic adaptation in cardiac myocytes. Circ Res. 2006;99(2):172–82. doi: 10.1161/01.RES.0000232546.92777.05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chacon E, Ohata H, Harper IS, Trollinger DR, Herman B, Lemasters JJ. Mitochondrial free calcium transients during excitation-contraction coupling in rabbit cardiac myocytes. FEBS Lett. 1996;382(1–2):31–6. doi: 10.1016/0014-5793(96)00138-x. [DOI] [PubMed] [Google Scholar]
- 41.Isenberg G, Han S, Schiefer A, Wendt-Gallitelli MF. Changes in mitochondrial calcium concentration during the cardiac contraction cycle. Cardiovasc Res. 1993;27(10):1800–9. doi: 10.1093/cvr/27.10.1800. [DOI] [PubMed] [Google Scholar]
- 42.Robert V, Gurlini P, Tosello V, Nagai T, Miyawaki A, Di Lisa F, Pozzan T. Beat-to-beat oscillations of mitochondrial [Ca2+] in cardiac cells. EMBO J. 2001;20(17):4998–5007. doi: 10.1093/emboj/20.17.4998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chalmers S, Nicholls DG. The relationship between free and total calcium concentrations in the matrix of liver and brain mitochondria. J Biol Chem. 2003;278(21):19062–70. doi: 10.1074/jbc.M212661200. [DOI] [PubMed] [Google Scholar]
- 44.Gunter TE, Gunter KK, Sheu SS, Gavin CE. Mitochondrial calcium transport: physiological and pathological relevance. Am J Physiol. 1994;267(2 Pt 1):C313–39. doi: 10.1152/ajpcell.1994.267.2.C313. [DOI] [PubMed] [Google Scholar]
- 45.Bernardi P. Mitochondrial transport of cations: channels, exchangers, and permeability transition. Physiol Rev. 1999;79(4):1127–55. doi: 10.1152/physrev.1999.79.4.1127. [DOI] [PubMed] [Google Scholar]
- 46.Pfeiffer DR, Gunter TE, Eliseev R, Broekemeier KM, Gunter KK. Release of Ca2+ from mitochondria via the saturable mechanisms and the permeability transition. IUBMB Life. 2001;52(3–5):205–12. doi: 10.1080/15216540152846019. [DOI] [PubMed] [Google Scholar]
- 47.Baysal K, Jung DW, Gunter KK, Gunter TE, Brierley GP. Na (+)-dependent Ca2+ efflux mechanism of heart mitochondria is not a passive Ca2+/2Na+ exchanger. Am J Physiol. 1994;266(3 Pt 1):C800–8. doi: 10.1152/ajpcell.1994.266.3.C800. [DOI] [PubMed] [Google Scholar]
- 48.Jung DW, Baysal K, Brierley GP. The sodium-calcium antiport of heart mitochondria is not electroneutral. J Biol Chem. 1995;270(2):672–8. doi: 10.1074/jbc.270.2.672. [DOI] [PubMed] [Google Scholar]
- 49.Brand MD. The stoichiometry of the exchange catalysed by the mitochondrial calcium/sodium antiporter. Biochem J. 1985;229(1):161–6. doi: 10.1042/bj2290161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Fiskum G, Reynafarje B, Lehninger AL. The electric charge stoichiometry of respiration-dependent Ca2+ uptake by mitochondria. J Biol Chem. 1979;254(14):6288–95. [PubMed] [Google Scholar]
- 51.Gunter TE, Chace JH, Puskin JS, Gunter KK. Mechanism of sodium independent calcium efflux from rat liver mitochondria. Biochemistry. 1983;22(26):6341–51. doi: 10.1021/bi00295a046. [DOI] [PubMed] [Google Scholar]
- 52.Nicholls DG, Crompton M. Mitochondrial calcium transport. FEBS Lett. 1980;111(2):261–8. doi: 10.1016/0014-5793(80)80806-4. [DOI] [PubMed] [Google Scholar]
- 53.Gunter TE, Pfeiffer DR. Mechanisms by which mitochondria transport calcium. Am J Physiol. 1990;258(5 Pt 1):C755–86. doi: 10.1152/ajpcell.1990.258.5.C755. [DOI] [PubMed] [Google Scholar]
- 54.Huser J, Blatter LA. Fluctuations in mitochondrial membrane potential caused by repetitive gating of the permeability transition pore. Biochem J. 1999;343(Pt 2):311–7. [PMC free article] [PubMed] [Google Scholar]
- 55.Balaban RS. Cardiac energy metabolism homeostasis: role of cytosolic calcium. J Mol Cell Cardiol. 2002;34(10):1259–71. doi: 10.1006/jmcc.2002.2082. [DOI] [PubMed] [Google Scholar]
- 56.Hansford RG, Zorov D. Role of mitochondrial calcium transport in the control of substrate oxidation. Mol Cell Biochem. 1998;184(1–2):359–69. [PubMed] [Google Scholar]
- 57.McCormack JG, Denton RM. Mitochondrial Ca2+ transport and the role of intramitochondrial Ca2+ in the regulation of energy metabolism. Dev Neurosci. 1993;15(3–5):165–73. doi: 10.1159/000111332. [DOI] [PubMed] [Google Scholar]
- 58.Duchen MR. Ca (2+)-dependent changes in the mitochondrial energetics in single dissociated mouse sensory neurons. Biochem J. 1992;283( Pt 1):41–50. doi: 10.1042/bj2830041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Pralong WF, Hunyady L, Varnai P, Wollheim CB, Spat A. Pyridine nucleotide redox state parallels production of aldosterone in potassium-stimulated adrenal glomerulosa cells. Proc Natl Acad Sci U S A. 1992;89(1):132–6. doi: 10.1073/pnas.89.1.132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Mildaziene V, Baniene R, Nauciene Z, Bakker BM, Brown GC, Westerhoff HV, Kholodenko BN. Calcium indirectly increases the control exerted by the adenine nucleotide translocator over 2-oxoglutarate oxidation in rat heart mitochondria. Arch Biochem Biophys. 1995;324(1):130–4. doi: 10.1006/abbi.1995.9918. [DOI] [PubMed] [Google Scholar]
- 61.Das AM, Harris DA. Control of mitochondrial ATP synthase in heart cells: inactive to active transitions caused by beating or positive inotropic agents. Cardiovasc Res. 1990;24(5):411–7. doi: 10.1093/cvr/24.5.411. [DOI] [PubMed] [Google Scholar]
- 62.Wernette ME, Ochs RS, Lardy HA. Ca2+ stimulation of rat liver mitochondrial glycerophosphate dehydrogenase. J Biol Chem. 1981;256(24):12767–71. [PubMed] [Google Scholar]
- 63.Crompton M. The mitochondrial permeability transition pore and its role in cell death. Biochem J. 1999;341( Pt 2):233–49. [PMC free article] [PubMed] [Google Scholar]
- 64.Crompton M, Ellinger H, Costi A. Inhibition by cyclosporin A of a Ca2+-dependent pore in heart mitochondria activated by inorganic phosphate and oxidative stress. Biochem J. 1988;255(1):357–60. [PMC free article] [PubMed] [Google Scholar]
- 65.Beutner G, Ruck A, Riede B, Welte W, Brdiczka D. Complexes between kinases, mitochondrial porin and adenylate translocator in rat brain resemble the permeability transition pore. FEBS Lett. 1996;396(2–3):189–95. doi: 10.1016/0014-5793(96)01092-7. [DOI] [PubMed] [Google Scholar]
- 66.Halestrap AP, Davidson AM. Inhibition of Ca2 (+)-induced large-amplitude swelling of liver and heart mitochondria by cyclosporin is probably caused by the inhibitor binding to mitochondrial-matrix peptidyl-prolyl cis-trans isomerase and preventing it interacting with the adenine nucleotide translocase. Biochem J. 1990;268(1):153–60. doi: 10.1042/bj2680153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Beutner G, Ruck A, Riede B, Brdiczka D. Complexes between porin, hexokinase, mitochondrial creatine kinase and adenylate translocator display properties of the permeability transition pore. Implication for regulation of permeability transition by the kinases. Biochim Biophys Acta. 1998;1368(1):7–18. doi: 10.1016/s0005-2736(97)00175-2. [DOI] [PubMed] [Google Scholar]
- 68.Kokoszka JE, Waymire KG, Levy SE, Sligh JE, Cai J, Jones DP, MacGregor GR, Wallace DC. The ADP/ATP translocator is not essential for the mitochondrial permeability transition pore. Nature. 2004;427(6973):461–5. doi: 10.1038/nature02229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Basso E, Fante L, Fowlkes J, Petronilli V, Forte MA, Bernardi P. Properties of the permeability transition pore in mitochondria devoid of Cyclophilin D. J Biol Chem. 2005;280(19):18558–61. doi: 10.1074/jbc.C500089200. [DOI] [PubMed] [Google Scholar]
- 70.Brdiczka DG, Zorov DB, Sheu SS. Mitochondrial contact sites: their role in energy metabolism and apoptosis. Biochim Biophys Acta. 2006;1762(2):148–63. doi: 10.1016/j.bbadis.2005.09.007. [DOI] [PubMed] [Google Scholar]
- 71.McEnery MW, Snowman AM, Trifiletti RR, Snyder SH. Isolation of the mitochondrial benzodiazepine receptor: association with the voltage-dependent anion channel and the adenine nucleotide carrier. Proc Natl Acad Sci U S A. 1992;89(8):3170–4. doi: 10.1073/pnas.89.8.3170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Capano M, Crompton M. Biphasic translocation of Bax to mitochondria. Biochem J. 2002;367(Pt 1):169–78. doi: 10.1042/BJ20020805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Nieminen AL, Byrne AM, Herman B, Lemasters JJ. Mitochondrial permeability transition in hepatocytes induced by t-BuOOH: NAD (P)H and reactive oxygen species. Am J Physiol. 1997;272(4 Pt 1):C1286–94. doi: 10.1152/ajpcell.1997.272.4.C1286. [DOI] [PubMed] [Google Scholar]
- 74.Vercesi AE, Kowaltowski AJ, Grijalba MT, Meinicke AR, Castilho RF. The role of reactive oxygen species in mitochondrial permeability transition. Biosci Rep. 1997;17(1):43–52. doi: 10.1023/a:1027335217774. [DOI] [PubMed] [Google Scholar]
- 75.Crompton M, Costi A. Kinetic evidence for a heart mitochondrial pore activated by Ca2+, inorganic phosphate and oxidative stress. A potential mechanism for mitochondrial dysfunction during cellular Ca2+ overload. Eur J Biochem. 1988;178(2):489–501. doi: 10.1111/j.1432-1033.1988.tb14475.x. [DOI] [PubMed] [Google Scholar]
- 76.Al-Nasser I, Crompton M. The reversible Ca2+-induced permeabilization of rat liver mitochondria. Biochem J. 1986;239(1):19–29. doi: 10.1042/bj2390019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Nicolli A, Petronilli V, Bernardi P. Modulation of the mitochondrial cyclosporin A-sensitive permeability transition pore by matrix pH. Evidence that the pore open-closed probability is regulated by reversible histidine protonation. Biochemistry. 1993;32(16):4461–5. doi: 10.1021/bi00067a039. [DOI] [PubMed] [Google Scholar]
- 78.He L, Lemasters JJ. Regulated and unregulated mitochondrial permeability transition pores: a new paradigm of pore structure and function? FEBS Lett. 2002;512(1–3):1–7. doi: 10.1016/s0014-5793(01)03314-2. [DOI] [PubMed] [Google Scholar]
- 79.Liu X, Kim CN, Yang J, Jemmerson R, Wang X. Induction of apoptotic program in cell-free extracts: requirement for dATP and cytochrome c. Cell. 1996;86(1):147–57. doi: 10.1016/s0092-8674(00)80085-9. [DOI] [PubMed] [Google Scholar]
- 80.Ott M, Robertson JD, Gogvadze V, Zhivotovsky B, Orrenius S. Cytochrome c release from mitochondria proceeds by a two-step process. Proc Natl Acad Sci U S A. 2002;99(3):1259–63. doi: 10.1073/pnas.241655498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Kroemer G, Zamzami N, Susin SA. Mitochondrial control of apoptosis. Immunol Today. 1997;18(1):44–51. doi: 10.1016/s0167-5699(97)80014-x. [DOI] [PubMed] [Google Scholar]
- 82.Madesh M, Hajnoczky G. VDAC-dependent permeabilization of the outer mitochondrial membrane by superoxide induces rapid and massive cytochrome c release. J Cell Biol. 2001;155(6):1003–15. doi: 10.1083/jcb.200105057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Kluck RM, Bossy-Wetzel E, Green DR, Newmeyer DD. The release of cytochrome c from mitochondria: a primary site for Bcl-2 regulation of apoptosis. Science. 1997;275(5303):1132–6. doi: 10.1126/science.275.5303.1132. [DOI] [PubMed] [Google Scholar]
- 84.O’Reilly CM, Fogarty KE, Drummond RM, Tuft RA, Walsh JV., Jr Quantitative analysis of spontaneous mitochondrial depolarizations. Biophys J. 2003;85(5):3350–7. doi: 10.1016/S0006-3495(03)74754-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Vergun O, Votyakova TV, Reynolds IJ. Spontaneous changes in mitochondrial membrane potential in single isolated brain mitochondria. Biophys J. 2003;85(5):3358–66. doi: 10.1016/S0006-3495(03)74755-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Al-Nasser I, Crompton M. The entrapment of the Ca2+ indicator arsenazo III in the matrix space of rat liver mitochondria by permeabilization and resealing. Na+-dependent and -independent effluxes of Ca2+ in arsenazo III-loaded mitochondria. Biochem J. 1986;239(1):31–40. doi: 10.1042/bj2390031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Bernardi P, Broekemeier KM, Pfeiffer DR. Recent progress on regulation of the mitochondrial permeability transition pore; a cyclosporin-sensitive pore in the inner mitochondrial membrane. J Bioenerg Biomembr. 1994;26(5):509–17. doi: 10.1007/BF00762735. [DOI] [PubMed] [Google Scholar]
- 88.Vergun O, Reynolds IJ. Fluctuations in mitochondrial membrane potential in single isolated brain mitochondria: modulation by adenine nucleotides and Ca2+ Biophys J. 2004;87(5):3585–93. doi: 10.1529/biophysj.104.042671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Boveris A, Oshino N, Chance B. The cellular production of hydrogen peroxide. Biochem J. 1972;128(3):617–30. doi: 10.1042/bj1280617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Grivennikova VG, Vinogradov AD. Generation of superoxide by the mitochondrial Complex I. Biochim Biophys Acta. 2006;1757(5–6):553–61. doi: 10.1016/j.bbabio.2006.03.013. [DOI] [PubMed] [Google Scholar]
- 91.Kudin AP, Debska-Vielhaber G, Kunz WS. Characterization of superoxide production sites in isolated rat brain and skeletal muscle mitochondria. Biomed Pharmacother. 2005;59(4):163–8. doi: 10.1016/j.biopha.2005.03.012. [DOI] [PubMed] [Google Scholar]
- 92.Kudin AP, Bimpong-Buta NY, Vielhaber S, Elger CE, Kunz WS. Characterization of superoxide-producing sites in isolated brain mitochondria. J Biol Chem. 2004;279(6):4127–35. doi: 10.1074/jbc.M310341200. [DOI] [PubMed] [Google Scholar]
- 93.Kussmaul L, Hirst J. The mechanism of superoxide production by NADH:ubiquinone oxidoreductase (complex I) from bovine heart mitochondria. Proc Natl Acad Sci U S A. 2006;103(20):7607–12. doi: 10.1073/pnas.0510977103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Brand MD, Affourtit C, Esteves TC, Green K, Lambert AJ, Miwa S, Pakay JL, Parker N. Mitochondrial superoxide: production, biological effects, and activation of uncoupling proteins. Free Radic Biol Med. 2004;37(6):755–67. doi: 10.1016/j.freeradbiomed.2004.05.034. [DOI] [PubMed] [Google Scholar]
- 95.Gilbert HF. Molecular and cellular aspects of thiol-disulfide exchange. Adv Enzymol Relat Areas Mol Biol. 1990;63:69–172. doi: 10.1002/9780470123096.ch2. [DOI] [PubMed] [Google Scholar]
- 96.Schafer FQ, Buettner GR. Redox environment of the cell as viewed through the redox state of the glutathione disulfide/glutathione couple. Free Radic Biol Med. 2001;30(11):1191–212. doi: 10.1016/s0891-5849(01)00480-4. [DOI] [PubMed] [Google Scholar]
- 97.Lin SJ, Guarente L. Nicotinamide adenine dinucleotide, a metabolic regulator of transcription, longevity and disease. Curr Opin Cell Biol. 2003;15(2):241–6. doi: 10.1016/s0955-0674(03)00006-1. [DOI] [PubMed] [Google Scholar]
- 98.Yamawaki H, Haendeler J, Berk BC. Thioredoxin: a key regulator of cardiovascular homeostasis. Circ Res. 2003;93(11):1029–33. doi: 10.1161/01.RES.0000102869.39150.23. [DOI] [PubMed] [Google Scholar]
- 99.Zhang H, Go YM, Jones DP. Mitochondrial thioredoxin-2/peroxiredoxin-3 system functions in parallel with mitochondrial GSH system in protection against oxidative stress. Arch Biochem Biophys. 2007 doi: 10.1016/j.abb.2007.05.001. [DOI] [PubMed] [Google Scholar]
- 100.Melov S, Coskun P, Patel M, Tuinstra R, Cottrell B, Jun AS, Zastawny TH, Dizdaroglu M, Goodman SI, Huang TT, Miziorko H, Epstein CJ, Wallace DC. Mitochondrial disease in superoxide dismutase 2 mutant mice. Proc Natl Acad Sci U S A. 1999;96(3):846–51. doi: 10.1073/pnas.96.3.846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Okado-Matsumoto A, Fridovich I. Subcellular distribution of superoxide dismutases (SOD) in rat liver: Cu, Zn-SOD in mitochondria. J Biol Chem. 2001;276(42):38388–93. doi: 10.1074/jbc.M105395200. [DOI] [PubMed] [Google Scholar]
- 102.Mailer K. Superoxide radical as electron donor for oxidative phosphorylation of ADP. Biochem Biophys Res Commun. 1990;170(1):59–64. doi: 10.1016/0006-291x(90)91240-s. [DOI] [PubMed] [Google Scholar]
- 103.Yan Y, Wei CL, Zhang WR, Cheng HP, Liu J. Cross-talk between calcium and reactive oxygen species signaling. Acta Pharmacol Sin. 2006;27(7):821–6. doi: 10.1111/j.1745-7254.2006.00390.x. [DOI] [PubMed] [Google Scholar]
- 104.Schild L, Reinheckel T, Wiswedel I, Augustin W. Short-term impairment of energy production in isolated rat liver mitochondria by hypoxia/reoxygenation: involvement of oxidative protein modification. Biochem J. 1997;328( Pt 1):205–10. doi: 10.1042/bj3280205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Genova ML, Ventura B, Giuliano G, Bovina C, Formiggini G, Parenti Castelli G, Lenaz G. The site of production of superoxide radical in mitochondrial Complex I is not a bound ubisemiquinone but presumably iron-sulfur cluster N2. FEBS Lett. 2001;505(3):364–8. doi: 10.1016/s0014-5793(01)02850-2. [DOI] [PubMed] [Google Scholar]
- 106.Lambert AJ, Brand MD. Inhibitors of the quinone-binding site allow rapid superoxide production from mitochondrial NADH:ubiquinone oxidoreductase (complex I) J Biol Chem. 2004;279(38):39414–20. doi: 10.1074/jbc.M406576200. [DOI] [PubMed] [Google Scholar]
- 107.Starkov AA, Fiskum G. Regulation of brain mitochondrial H2O2 production by membrane potential and NAD (P)H redox state. J Neurochem. 2003;86(5):1101–7. doi: 10.1046/j.1471-4159.2003.01908.x. [DOI] [PubMed] [Google Scholar]
- 108.Andreyev AY, Kushnareva YE, Starkov AA. Mitochondrial metabolism of reactive oxygen species. Biochemistry (Mosc) 2005;70(2):200–14. doi: 10.1007/s10541-005-0102-7. [DOI] [PubMed] [Google Scholar]
- 109.Turrens JF. Mitochondrial formation of reactive oxygen species. J Physiol. 2003;552(Pt 2):335–44. doi: 10.1113/jphysiol.2003.049478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Miwa S, St-Pierre J, Partridge L, Brand MD. Superoxide and hydrogen peroxide production by Drosophila mitochondria. Free Radic Biol Med. 2003;35(8):938–48. doi: 10.1016/s0891-5849(03)00464-7. [DOI] [PubMed] [Google Scholar]
- 111.Negre-Salvayre A, Hirtz C, Carrera G, Cazenave R, Troly M, Salvayre R, Penicaud L, Casteilla L. A role for uncoupling protein-2 as a regulator of mitochondrial hydrogen peroxide generation. FASEB J. 1997;11(10):809–15. [PubMed] [Google Scholar]
- 112.Nadtochiy SM, Tompkins AJ, Brookes PS. Different mechanisms of mitochondrial proton leak in ischaemia/reperfusion injury and preconditioning: implications for pathology and cardioprotection. Biochem J. 2006;395(3):611–8. doi: 10.1042/BJ20051927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Batandier C, Leverve X, Fontaine E. Opening of the mitochondrial permeability transition pore induces reactive oxygen species production at the level of the respiratory chain complex I. J Biol Chem. 2004;279(17):17197–204. doi: 10.1074/jbc.M310329200. [DOI] [PubMed] [Google Scholar]
- 114.Barrientos A, Moraes CT. Titrating the effects of mitochondrial complex I impairment in the cell physiology. J Biol Chem. 1999;274(23):16188–97. doi: 10.1074/jbc.274.23.16188. [DOI] [PubMed] [Google Scholar]
- 115.Jekabsone A, Ivanoviene L, Brown GC, Borutaite V. Nitric oxide and calcium together inactivate mitochondrial complex I and induce cytochrome c release. J Mol Cell Cardiol. 2003;35(7):803–9. doi: 10.1016/s0022-2828(03)00137-8. [DOI] [PubMed] [Google Scholar]
- 116.Alderton WK, Cooper CE, Knowles RG. Nitric oxide synthases: structure, function and inhibition. Biochem J. 2001;357(Pt 3):593–615. doi: 10.1042/0264-6021:3570593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Brookes PS, Darley-Usmar VM. Role of calcium and superoxide dismutase in sensitizing mitochondria to peroxynitrite-induced permeability transition. Am J Physiol Heart Circ Physiol. 2004;286(1):H39–46. doi: 10.1152/ajpheart.00742.2003. [DOI] [PubMed] [Google Scholar]
- 118.Wikstrom M, Saari H. A spectral shift in cytochrome a induced by calcium ions. Biochim Biophys Acta. 1975;408(2):170–9. doi: 10.1016/0005-2728(75)90009-2. [DOI] [PubMed] [Google Scholar]
- 119.St-Pierre J, Buckingham JA, Roebuck SJ, Brand MD. Topology of superoxide production from different sites in the mitochondrial electron transport chain. J Biol Chem. 2002;277(47):44784–90. doi: 10.1074/jbc.M207217200. [DOI] [PubMed] [Google Scholar]
- 120.Brookes P, Darley-Usmar VM. Hypothesis: the mitochondrial NO (*) signaling pathway, and the transduction of nitrosative to oxidative cell signals: an alternative function for cytochrome C oxidase. Free Radic Biol Med. 2002;32(4):370–4. doi: 10.1016/s0891-5849(01)00805-x. [DOI] [PubMed] [Google Scholar]
- 121.Wan B, LaNoue KF, Cheung JY, Scaduto RC., Jr Regulation of citric acid cycle by calcium. J Biol Chem. 1989;264(23):13430–9. [PubMed] [Google Scholar]
- 122.Sohal RS, Allen RG. Relationship between metabolic rate, free radicals, differentiation and aging: a unified theory. Basic Life Sci. 1985;35:75–104. doi: 10.1007/978-1-4899-2218-2_4. [DOI] [PubMed] [Google Scholar]
- 123.Merry BJ. Molecular mechanisms linking calorie restriction and longevity. Int J Biochem Cell Biol. 2002;34(11):1340–54. doi: 10.1016/s1357-2725(02)00038-9. [DOI] [PubMed] [Google Scholar]
- 124.Grijalba MT, Vercesi AE, Schreier S. Ca2+-induced increased lipid packing and domain formation in submitochondrial particles. A possible early step in the mechanism of Ca2+-stimulated generation of reactive oxygen species by the respiratory chain. Biochemistry. 1999;38(40):13279–87. doi: 10.1021/bi9828674. [DOI] [PubMed] [Google Scholar]
- 125.Han D, Antunes F, Canali R, Rettori D, Cadenas E. Voltage-dependent anion channels control the release of the superoxide anion from mitochondria to cytosol. J Biol Chem. 2003;278(8):5557–63. doi: 10.1074/jbc.M210269200. [DOI] [PubMed] [Google Scholar]
- 126.Bathori G, Csordas G, Garcia-Perez C, Davies E, Hajnoczky G. Ca2+-dependent control of the permeability properties of the mitochondrial outer membrane and voltage-dependent anion-selective channel (VDAC) J Biol Chem. 2006;281(25):17347–58. doi: 10.1074/jbc.M600906200. [DOI] [PubMed] [Google Scholar]
- 127.Liu SS. Cooperation of a “reactive oxygen cycle” with the Q cycle and the proton cycle in the respiratory chain--superoxide generating and cycling mechanisms in mitochondria. J Bioenerg Biomembr. 1999;31(4):367–76. doi: 10.1023/a:1018650103259. [DOI] [PubMed] [Google Scholar]
- 128.Massey V. Activation of molecular oxygen by flavins and flavoproteins. J Biol Chem. 1994;269(36):22459–62. [PubMed] [Google Scholar]
- 129.Chan PC, Bielski BH. Enzyme-catalyzed free radical reactions with nicotinamide adenine nucleotides. II. Lactate dehydrogenase-catalyzed oxidation of reduced nicotinamide adenine dinucleotide by superoxide radicals generated by xanthine oxidase. J Biol Chem. 1974;249(4):1317–9. [PubMed] [Google Scholar]
- 130.Chan PC, Bielski BH. Glyceraldehyde-3-phosphate dehydrogenase-catalyzed chain oxidation of reduced nicotinamide adenine dinucleotide by perhydroxyl radicals. J Biol Chem. 1980;255(3):874–6. [PubMed] [Google Scholar]
- 131.Zhang L, Yu L, Yu CA. Generation of superoxide anion by succinate-cytochrome c reductase from bovine heart mitochondria. J Biol Chem. 1998;273(51):33972–6. doi: 10.1074/jbc.273.51.33972. [DOI] [PubMed] [Google Scholar]
- 132.Chinopoulos C, Adam-Vizi V. Calcium, mitochondria and oxidative stress in neuronal pathology. Novel aspects of an enduring theme. FEBS J. 2006;273(3):433–50. doi: 10.1111/j.1742-4658.2005.05103.x. [DOI] [PubMed] [Google Scholar]
- 133.Maas E, Bisswanger H. Localization of the alpha-oxoacid dehydrogenase multienzyme complexes within the mitochondrion. FEBS Lett. 1990;277(1–2):189–90. doi: 10.1016/0014-5793(90)80840-f. [DOI] [PubMed] [Google Scholar]
- 134.Starkov AA, Fiskum G, Chinopoulos C, Lorenzo BJ, Browne SE, Patel MS, Beal MF. Mitochondrial alpha-ketoglutarate dehydrogenase complex generates reactive oxygen species. J Neurosci. 2004;24(36):7779–88. doi: 10.1523/JNEUROSCI.1899-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Tahara EB, Barros MH, Oliveira GA, Netto LE, Kowaltowski AJ. Dihydrolipoyl dehydrogenase as a source of reactive oxygen species inhibited by caloric restriction and involved in Saccharomyces cerevisiae aging. FASEB J. 2007;21(1):274–83. doi: 10.1096/fj.06-6686com. [DOI] [PubMed] [Google Scholar]
- 136.Tretter L, Adam-Vizi V. Generation of reactive oxygen species in the reaction catalyzed by alpha-ketoglutarate dehydrogenase. J Neurosci. 2004;24(36):7771–8. doi: 10.1523/JNEUROSCI.1842-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Nulton-Persson AC, Szweda LI. Modulation of mitochondrial function by hydrogen peroxide. J Biol Chem. 2001;276(26):23357–61. doi: 10.1074/jbc.M100320200. [DOI] [PubMed] [Google Scholar]
- 138.Tretter L, Adam-Vizi V. Inhibition of Krebs cycle enzymes by hydrogen peroxide: A key role of (alpha)-ketoglutarate dehydrogenase in limiting NADH production under oxidative stress. J Neurosci. 2000;20(24):8972–9. doi: 10.1523/JNEUROSCI.20-24-08972.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Vasquez-Vivar J, Kalyanaraman B, Kennedy MC. Mitochondrial aconitase is a source of hydroxyl radical. An electron spin resonance investigation. J Biol Chem. 2000;275(19):14064–9. doi: 10.1074/jbc.275.19.14064. [DOI] [PubMed] [Google Scholar]
- 140.Cadenas E, Boveris A. Enhancement of hydrogen peroxide formation by protophores and ionophores in antimycin-supplemented mitochondria. Biochem J. 1980;188(1):31–7. doi: 10.1042/bj1880031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Sousa SC, Maciel EN, Vercesi AE, Castilho RF. Ca2+-induced oxidative stress in brain mitochondria treated with the respiratory chain inhibitor rotenone. FEBS Lett. 2003;543(1–3):179–83. doi: 10.1016/s0014-5793(03)00421-6. [DOI] [PubMed] [Google Scholar]
- 142.Viola HM, Arthur PG, Hool LC. Transient exposure to hydrogen peroxide causes an increase in mitochondria-derived superoxide as a result of sustained alteration in L-type Ca2+ channel function in the absence of apoptosis in ventricular myocytes. Circ Res. 2007;100(7):1036–44. doi: 10.1161/01.RES.0000263010.19273.48. [DOI] [PubMed] [Google Scholar]
- 143.Brookes PS. Mitochondrial H (+) leak and ROS generation: an odd couple. Free Radic Biol Med. 2005;38(1):12–23. doi: 10.1016/j.freeradbiomed.2004.10.016. [DOI] [PubMed] [Google Scholar]
- 144.Brand MD, Esteves TC. Physiological functions of the mitochondrial uncoupling proteins UCP2 and UCP3. Cell Metab. 2005;2(2):85–93. doi: 10.1016/j.cmet.2005.06.002. [DOI] [PubMed] [Google Scholar]
- 145.Cannon B, Shabalina IG, Kramarova TV, Petrovic N, Nedergaard J. Uncoupling proteins: a role in protection against reactive oxygen species--or not? Biochim Biophys Acta. 2006;1757(5–6):449–58. doi: 10.1016/j.bbabio.2006.05.016. [DOI] [PubMed] [Google Scholar]
- 146.Nicholls DG. The non-Ohmic proton leak--25 years on. Biosci Rep. 1997;17(3):251–7. doi: 10.1023/a:1027376426860. [DOI] [PubMed] [Google Scholar]
- 147.Cadenas S, Buckingham JA, St-Pierre J, Dickinson K, Jones RB, Brand MD. AMP decreases the efficiency of skeletal-muscle mitochondria. Biochem J. 2000;351(Pt 2):307–11. [PMC free article] [PubMed] [Google Scholar]
- 148.Echtay KS, Esteves TC, Pakay JL, Jekabsons MB, Lambert AJ, Portero-Otin M, Pamplona R, Vidal-Puig AJ, Wang S, Roebuck SJ, Brand MD. A signalling role for 4-hydroxy-2-nonenal in regulation of mitochondrial uncoupling. EMBO J. 2003;22(16):4103–10. doi: 10.1093/emboj/cdg412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Echtay KS, Roussel D, St-Pierre J, Jekabsons MB, Cadenas S, Stuart JA, Harper JA, Roebuck SJ, Morrison A, Pickering S, Clapham JC, Brand MD. Superoxide activates mitochondrial uncoupling proteins. Nature. 2002;415(6867):96–9. doi: 10.1038/415096a. [DOI] [PubMed] [Google Scholar]
- 150.Perez-Campo R, Lopez-Torres M, Cadenas S, Rojas C, Barja G. The rate of free radical production as a determinant of the rate of aging: evidence from the comparative approach. J Comp Physiol (B) 1998;168(3):149–58. doi: 10.1007/s003600050131. [DOI] [PubMed] [Google Scholar]
- 151.Cleeter MW, Cooper JM, Darley-Usmar VM, Moncada S, Schapira AH. Reversible inhibition of cytochrome c oxidase, the terminal enzyme of the mitochondrial respiratory chain, by nitric oxide. Implications for neurodegenerative diseases. FEBS Lett. 1994;345(1):50–4. doi: 10.1016/0014-5793(94)00424-2. [DOI] [PubMed] [Google Scholar]
- 152.Johnson-Cadwell LI, Jekabsons MB, Wang A, Polster BM, Nicholls DG. ‘Mild Uncoupling’ does not decrease mitochondrial superoxide levels in cultured cerebellar granule neurons but decreases spare respiratory capacity and increases toxicity to glutamate and oxidative stress. J Neurochem. 2007;101(6):1619–31. doi: 10.1111/j.1471-4159.2007.04516.x. [DOI] [PubMed] [Google Scholar]
- 153.Harper JA, Stuart JA, Jekabsons MB, Roussel D, Brindle KM, Dickinson K, Jones RB, Brand MD. Artifactual uncoupling by uncoupling protein 3 in yeast mitochondria at the concentrations found in mouse and rat skeletal-muscle mitochondria. Biochem J. 2002;361(Pt 1):49–56. doi: 10.1042/0264-6021:3610049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Andrukhiv A, Costa AD, West IC, Garlid KD. Opening mitoKATP increases superoxide generation from complex I of the electron transport chain. Am J Physiol Heart Circ Physiol. 2006;291(5):H2067–74. doi: 10.1152/ajpheart.00272.2006. [DOI] [PubMed] [Google Scholar]
- 155.Heinen A, Camara AK, Aldakkak M, Rhodes SS, Riess ML, Stowe DF. Mitochondrial Ca2+-induced K+ influx increases respiration and enhances ROS production while maintaining membrane potential. Am J Physiol Cell Physiol. 2007;292(1):C148–56. doi: 10.1152/ajpcell.00215.2006. [DOI] [PubMed] [Google Scholar]
- 156.Costa AD, Quinlan CL, Andrukhiv A, West IC, Jaburek M, Garlid KD. The direct physiological effects of mitoK (ATP) opening on heart mitochondria. Am J Physiol Heart Circ Physiol. 2006;290(1):H406–15. doi: 10.1152/ajpheart.00794.2005. [DOI] [PubMed] [Google Scholar]
- 157.Galkin A, Brandt U. Superoxide radical formation by pure complex I (NADH:ubiquinone oxidoreductase) from Yarrowia lipolytica. J Biol Chem. 2005;280(34):30129–35. doi: 10.1074/jbc.M504709200. [DOI] [PubMed] [Google Scholar]
- 158.Krishnamoorthy G, Hinkle PC. Studies on the electron transfer pathway, topography of iron-sulfur centers, and site of coupling in NADH-Q oxidoreductase. J Biol Chem. 1988;263(33):17566–75. [PubMed] [Google Scholar]
- 159.Baines CP, Zhang J, Wang GW, Zheng YT, Xiu JX, Cardwell EM, Bolli R, Ping P. Mitochondrial PKCepsilon and MAPK form signaling modules in the murine heart: enhanced mitochondrial PKCepsilon-MAPK interactions and differential MAPK activation in PKCepsilon-induced cardioprotection. Circ Res. 2002;90(4):390–7. doi: 10.1161/01.res.0000012702.90501.8d. [DOI] [PubMed] [Google Scholar]
- 160.Costa AD, Garlid KD, West IC, Lincoln TM, Downey JM, Cohen MV, Critz SD. Protein kinase G transmits the cardioprotective signal from cytosol to mitochondria. Circ Res. 2005;97(4):329–36. doi: 10.1161/01.RES.0000178451.08719.5b. [DOI] [PubMed] [Google Scholar]
- 161.Lambert AJ, Brand MD. Superoxide production by NADH:ubiquinone oxidoreductase (complex I) depends on the pH gradient across the mitochondrial inner membrane. Biochem J. 2004;382(Pt 2):511–7. doi: 10.1042/BJ20040485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Vinogradov AD, Grivennikova VG. Generation of superoxide-radical by the NADH:ubiquinone oxidoreductase of heart mitochondria. Biochemistry (Mosc) 2005;70(2):120–7. doi: 10.1007/s10541-005-0090-7. [DOI] [PubMed] [Google Scholar]
- 163.Facundo HT, Carreira RS, de Paula JG, Santos CC, Ferranti R, Laurindo FR, Kowaltowski AJ. Ischemic preconditioning requires increases in reactive oxygen release independent of mitochondrial K+ channel activity. Free Radic Biol Med. 2006;40(3):469–79. doi: 10.1016/j.freeradbiomed.2005.08.041. [DOI] [PubMed] [Google Scholar]
- 164.Facundo HT, de Paula JG, Kowaltowski AJ. Mitochondrial ATP-sensitive K+ channels are redox-sensitive pathways that control reactive oxygen species production. Free Radic Biol Med. 2007;42(7):1039–48. doi: 10.1016/j.freeradbiomed.2007.01.001. [DOI] [PubMed] [Google Scholar]
- 165.Ferranti R, da Silva MM, Kowaltowski AJ. Mitochondrial ATP-sensitive K+ channel opening decreases reactive oxygen species generation. FEBS Lett. 2003;536(1–3):51–5. doi: 10.1016/s0014-5793(03)00007-3. [DOI] [PubMed] [Google Scholar]
- 166.Drose S, Brandt U, Hanley PJ. K+-independent actions of diazoxide question the role of inner membrane KATP channels in mitochondrial cytoprotective signaling. J Biol Chem. 2006;281(33):23733–9. doi: 10.1074/jbc.M602570200. [DOI] [PubMed] [Google Scholar]
- 167.Droge W. Free radicals in the physiological control of cell function. Physiol Rev. 2002;82(1):47–95. doi: 10.1152/physrev.00018.2001. [DOI] [PubMed] [Google Scholar]
- 168.Hool LC, Corry B. Redox control of calcium channels: from mechanisms to therapeutic opportunities. Antioxid Redox Signal. 2007;9(4):409–35. doi: 10.1089/ars.2006.1446. [DOI] [PubMed] [Google Scholar]
- 169.Zima AV, Blatter LA. Redox regulation of cardiac calcium channels and transporters. Cardiovasc Res. 2006;71(2):310–21. doi: 10.1016/j.cardiores.2006.02.019. [DOI] [PubMed] [Google Scholar]
- 170.Camello-Almaraz C, Gomez-Pinilla PJ, Pozo MJ, Camello PJ. Mitochondrial reactive oxygen species and Ca2+ signaling. Am J Physiol Cell Physiol. 2006;291(5):C1082–8. doi: 10.1152/ajpcell.00217.2006. [DOI] [PubMed] [Google Scholar]
- 171.Davidson SM, Duchen MR. Calcium microdomains and oxidative stress. Cell Calcium. 2006;40(5–6):561–74. doi: 10.1016/j.ceca.2006.08.017. [DOI] [PubMed] [Google Scholar]
- 172.Vercesi AE, Kowaltowski AJ, Oliveira HC, Castilho RF. Mitochondrial Ca2+ transport, permeability transition and oxidative stress in cell death: implications in cardiotoxicity, neurodegeneration and dyslipidemias. Front Biosci. 2006;11:2554–64. doi: 10.2741/1990. [DOI] [PubMed] [Google Scholar]
- 173.Fabiato A. Calcium-induced release of calcium from the cardiac sarcoplasmic reticulum. Am J Physiol. 1983;245(1):C1–14. doi: 10.1152/ajpcell.1983.245.1.C1. [DOI] [PubMed] [Google Scholar]
- 174.Fill M, Copello JA. Ryanodine receptor calcium release channels. Physiol Rev. 2002;82(4):893–922. doi: 10.1152/physrev.00013.2002. [DOI] [PubMed] [Google Scholar]
- 175.Xu L, Eu JP, Meissner G, Stamler JS. Activation of the cardiac calcium release channel (ryanodine receptor) by poly-S-nitrosylation. Science. 1998;279(5348):234–7. doi: 10.1126/science.279.5348.234. [DOI] [PubMed] [Google Scholar]
- 176.Abramson JJ, Salama G. Critical sulfhydryls regulate calcium release from sarcoplasmic reticulum. J Bioenerg Biomembr. 1989;21(2):283–94. doi: 10.1007/BF00812073. [DOI] [PubMed] [Google Scholar]
- 177.Liu G, Abramson JJ, Zable AC, Pessah IN. Direct evidence for the existence and functional role of hyperreactive sulfhydryls on the ryanodine receptor-triadin complex selectively labeled by the coumarin maleimide 7-diethylamino-3- (4′-maleimidylphenyl)-4-methylcoumarin. Mol Pharmacol. 1994;45(2):189–200. [PubMed] [Google Scholar]
- 178.Zable AC, Favero TG, Abramson JJ. Glutathione modulates ryanodine receptor from skeletal muscle sarcoplasmic reticulum. Evidence for redox regulation of the Ca2+ release mechanism. J Biol Chem. 1997;272(11):7069–77. doi: 10.1074/jbc.272.11.7069. [DOI] [PubMed] [Google Scholar]
- 179.Haarmann CS, Fink RH, Dulhunty AF. Oxidation and reduction of pig skeletal muscle ryanodine receptors. Biophys J. 1999;77(6):3010–22. doi: 10.1016/S0006-3495(99)77132-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Xia R, Stangler T, Abramson JJ. Skeletal muscle ryanodine receptor is a redox sensor with a well defined redox potential that is sensitive to channel modulators. J Biol Chem. 2000;275(47):36556–61. doi: 10.1074/jbc.M007613200. [DOI] [PubMed] [Google Scholar]
- 181.Sun J, Xu L, Eu JP, Stamler JS, Meissner G. Classes of thiols that influence the activity of the skeletal muscle calcium release channel. J Biol Chem. 2001;276(19):15625–30. doi: 10.1074/jbc.M100083200. [DOI] [PubMed] [Google Scholar]
- 182.Marengo JJ, Hidalgo C, Bull R. Sulfhydryl oxidation modifies the calcium dependence of ryanodine-sensitive calcium channels of excitable cells. Biophys J. 1998;74(3):1263–77. doi: 10.1016/S0006-3495(98)77840-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Feng W, Liu G, Allen PD, Pessah IN. Transmembrane redox sensor of ryanodine receptor complex. J Biol Chem. 2000;275(46):35902–7. doi: 10.1074/jbc.C000523200. [DOI] [PubMed] [Google Scholar]
- 184.Balshaw DM, Xu L, Yamaguchi N, Pasek DA, Meissner G. Calmodulin binding and inhibition of cardiac muscle calcium release channel (ryanodine receptor) J Biol Chem. 2001;276(23):20144–53. doi: 10.1074/jbc.M010771200. [DOI] [PubMed] [Google Scholar]
- 185.Cherednichenko G, Zima AV, Feng W, Schaefer S, Blatter LA, Pessah IN. NADH oxidase activity of rat cardiac sarcoplasmic reticulum regulates calcium-induced calcium release. Circ Res. 2004;94(4):478–86. doi: 10.1161/01.RES.0000115554.65513.7C. [DOI] [PubMed] [Google Scholar]
- 186.Zima AV, Copello JA, Blatter LA. Differential modulation of cardiac and skeletal muscle ryanodine receptors by NADH. FEBS Lett. 2003;547(1–3):32–6. doi: 10.1016/s0014-5793(03)00664-1. [DOI] [PubMed] [Google Scholar]
- 187.Zima AV, Copello JA, Blatter LA. Effects of cytosolic NADH/NAD (+) levels on sarcoplasmic reticulum Ca (2+) release in permeabilized rat ventricular myocytes. J Physiol. 2004;555(Pt 3):727–41. doi: 10.1113/jphysiol.2003.055848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Kourie JI. Interaction of reactive oxygen species with ion transport mechanisms. Am J Physiol. 1998;275(1 Pt 1):C1–24. doi: 10.1152/ajpcell.1998.275.1.C1. [DOI] [PubMed] [Google Scholar]
- 189.Rowe GT, Manson NH, Caplan M, Hess ML. Hydrogen peroxide and hydroxyl radical mediation of activated leukocyte depression of cardiac sarcoplasmic reticulum. Participation of the cyclooxygenase pathway. Circ Res. 1983;53(5):584–91. doi: 10.1161/01.res.53.5.584. [DOI] [PubMed] [Google Scholar]
- 190.Boraso A, Williams AJ. Modification of the gating of the cardiac sarcoplasmic reticulum Ca (2+)-release channel by H2O2 and dithiothreitol. Am J Physiol. 1994;267(3 Pt 2):H1010–6. doi: 10.1152/ajpheart.1994.267.3.H1010. [DOI] [PubMed] [Google Scholar]
- 191.Oba T, Koshita M, Yamaguchi M. H2O2 modulates twitch tension and increases Po of Ca2+ release channel in frog skeletal muscle. Am J Physiol. 1996;271(3 Pt 1):C810–8. doi: 10.1152/ajpcell.1996.271.3.C810. [DOI] [PubMed] [Google Scholar]
- 192.Anzai K, Ogawa K, Kuniyasu A, Ozawa T, Yamamoto H, Nakayama H. Effects of hydroxyl radical and sulfhydryl reagents on the open probability of the purified cardiac ryanodine receptor channel incorporated into planar lipid bilayers. Biochem Biophys Res Commun. 1998;249(3):938–42. doi: 10.1006/bbrc.1998.9244. [DOI] [PubMed] [Google Scholar]
- 193.Stoyanovsky D, Murphy T, Anno PR, Kim YM, Salama G. Nitric oxide activates skeletal and cardiac ryanodine receptors. Cell Calcium. 1997;21(1):19–29. doi: 10.1016/s0143-4160(97)90093-2. [DOI] [PubMed] [Google Scholar]
- 194.Berridge MJ. Inositol trisphosphate and calcium signalling. Nature. 1993;361(6410):315–25. doi: 10.1038/361315a0. [DOI] [PubMed] [Google Scholar]
- 195.Bootman MD, Taylor CW, Berridge MJ. The thiol reagent, thimerosal, evokes Ca2+ spikes in HeLa cells by sensitizing the inositol 1,4,5-trisphosphate receptor. J Biol Chem. 1992;267(35):25113–9. [PubMed] [Google Scholar]
- 196.Renard-Rooney DC, Joseph SK, Seitz MB, Thomas AP. Effect of oxidized glutathione and temperature on inositol 1,4,5-trisphosphate binding in permeabilized hepatocytes. Biochem J. 1995;310( Pt 1):185–92. doi: 10.1042/bj3100185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Missiaen L, Taylor CW, Berridge MJ. Spontaneous calcium release from inositol trisphosphate-sensitive calcium stores. Nature. 1991;352(6332):241–4. doi: 10.1038/352241a0. [DOI] [PubMed] [Google Scholar]
- 198.Rooney TA, Renard DC, Sass EJ, Thomas AP. Oscillatory cytosolic calcium waves independent of stimulated inositol 1,4,5-trisphosphate formation in hepatocytes. J Biol Chem. 1991;266(19):12272–82. [PubMed] [Google Scholar]
- 199.Wesson DE, Elliott SJ. The H2O2-generating enzyme, xanthine oxidase, decreases luminal Ca2+ content of the IP3-sensitive Ca2+ store in vascular endothelial cells. Microcirculation. 1995;2(2):195–203. doi: 10.3109/10739689509146767. [DOI] [PubMed] [Google Scholar]
- 200.Suzuki YJ, Ford GD. Superoxide stimulates IP3-induced Ca2+ release from vascular smooth muscle sarcoplasmic reticulum. Am J Physiol. 1992;262(1 Pt 2):H114–6. doi: 10.1152/ajpheart.1992.262.1.H114. [DOI] [PubMed] [Google Scholar]
- 201.Kaplan P, Babusikova E, Lehotsky J, Dobrota D. Free radical-induced protein modification and inhibition of Ca2+-ATPase of cardiac sarcoplasmic reticulum. Mol Cell Biochem. 2003;248(1–2):41–7. doi: 10.1023/a:1024145212616. [DOI] [PubMed] [Google Scholar]
- 202.Murphy AJ. Sulfhydryl group modification of sarcoplasmic reticulum membranes. Biochemistry. 1976;15(20):4492–6. doi: 10.1021/bi00665a025. [DOI] [PubMed] [Google Scholar]
- 203.Scherer NM, Deamer DW. Oxidative stress impairs the function of sarcoplasmic reticulum by oxidation of sulfhydryl groups in the Ca2+-ATPase. Arch Biochem Biophys. 1986;246(2):589–601. doi: 10.1016/0003-9861(86)90314-0. [DOI] [PubMed] [Google Scholar]
- 204.Morris TE, Sulakhe PV. Sarcoplasmic reticulum Ca (2+)-pump dysfunction in rat cardiomyocytes briefly exposed to hydroxyl radicals. Free Radic Biol Med. 1997;22(1–2):37–47. doi: 10.1016/s0891-5849(96)00238-9. [DOI] [PubMed] [Google Scholar]
- 205.Kukreja RC, Okabe E, Schrier GM, Hess ML. Oxygen radical-mediated lipid peroxidation and inhibition of Ca2+-ATPase activity of cardiac sarcoplasmic reticulum. Arch Biochem Biophys. 1988;261(2):447–57. doi: 10.1016/0003-9861(88)90361-x. [DOI] [PubMed] [Google Scholar]
- 206.Kaneko M, Beamish RE, Dhalla NS. Depression of heart sarcolemmal Ca2+-pump activity by oxygen free radicals. Am J Physiol. 1989;256(2 Pt 2):H368–74. doi: 10.1152/ajpheart.1989.256.2.H368. [DOI] [PubMed] [Google Scholar]
- 207.Ermak G, Davies KJ. Calcium and oxidative stress: from cell signaling to cell death. Mol Immunol. 2002;38(10):713–21. doi: 10.1016/s0161-5890(01)00108-0. [DOI] [PubMed] [Google Scholar]
- 208.Grover AK, Samson SE, Misquitta CM. Sarco (endo)plasmic reticulum Ca2+ pump isoform SERCA3 is more resistant than SERCA2b to peroxide. Am J Physiol. 1997;273(2 Pt 1):C420–5. doi: 10.1152/ajpcell.1997.273.2.C420. [DOI] [PubMed] [Google Scholar]
- 209.Xu KY, Zweier JL, Becker LC. Oxygen-free radicals directly attack the ATP binding site of the cardiac Na+, K (+)-ATPase. Ann N Y Acad Sci. 1997;834:680–3. doi: 10.1111/j.1749-6632.1997.tb52349.x. [DOI] [PubMed] [Google Scholar]
- 210.Xu KY, Zweier JL, Becker LC. Hydroxyl radical inhibits sarcoplasmic reticulum Ca (2+)-ATPase function by direct attack on the ATP binding site. Circ Res. 1997;80(1):76–81. doi: 10.1161/01.res.80.1.76. [DOI] [PubMed] [Google Scholar]
- 211.Carafoli E. The calcium pumping ATPase of the plasma membrane. Annu Rev Physiol. 1991;53:531–47. doi: 10.1146/annurev.ph.53.030191.002531. [DOI] [PubMed] [Google Scholar]
- 212.Kaneko M, Elimban V, Dhalla NS. Mechanism for depression of heart sarcolemmal Ca2+ pump by oxygen free radicals. Am J Physiol. 1989;257(3 Pt 2):H804–11. doi: 10.1152/ajpheart.1989.257.3.H804. [DOI] [PubMed] [Google Scholar]
- 213.Lajas AI, Sierra V, Camello PJ, Salido GM, Pariente JA. Vanadate inhibits the calcium extrusion in rat pancreatic acinar cells. Cell Signal. 2001;13(6):451–6. doi: 10.1016/s0898-6568(01)00161-9. [DOI] [PubMed] [Google Scholar]
- 214.Zaidi A, Michaelis ML. Effects of reactive oxygen species on brain synaptic plasma membrane Ca (2+)-ATPase. Free Radic Biol Med. 1999;27(7–8):810–21. doi: 10.1016/s0891-5849(99)00128-8. [DOI] [PubMed] [Google Scholar]
- 215.Goldhaber JI. Free radicals enhance Na+/Ca2+ exchange in ventricular myocytes. Am J Physiol. 1996;271(3 Pt 2):H823–33. doi: 10.1152/ajpheart.1996.271.3.H823. [DOI] [PubMed] [Google Scholar]
- 216.Reeves JP, Bailey CA, Hale CC. Redox modification of sodium-calcium exchange activity in cardiac sarcolemmal vesicles. J Biol Chem. 1986;261(11):4948–55. [PubMed] [Google Scholar]
- 217.Huschenbett J, Zaidi A, Michaelis ML. Sensitivity of the synaptic membrane Na+/Ca2+ exchanger and the expressed NCX1 isoform to reactive oxygen species. Biochim Biophys Acta. 1998;1374(1–2):34–46. doi: 10.1016/s0005-2736(98)00121-7. [DOI] [PubMed] [Google Scholar]
- 218.Dixon IM, Kaneko M, Hata T, Panagia V, Dhalla NS. Alterations in cardiac membrane Ca2+ transport during oxidative stress. Mol Cell Biochem. 1990;99(2):125–33. doi: 10.1007/BF00230342. [DOI] [PubMed] [Google Scholar]
- 219.Rizzuto R, Pozzan T. Microdomains of intracellular Ca2+: molecular determinants and functional consequences. Physiol Rev. 2006;86(1):369–408. doi: 10.1152/physrev.00004.2005. [DOI] [PubMed] [Google Scholar]
- 220.Rizzuto R, Duchen MR, Pozzan T. Flirting in little space: the ER/mitochondria Ca2+ liaison. Sci STKE. 2004;2004(215):re1 . doi: 10.1126/stke.2152004re1. [DOI] [PubMed] [Google Scholar]
- 221.Hajnoczky G, Csordas G, Madesh M, Pacher P. The machinery of local Ca2+ signalling between sarco-endoplasmic reticulum and mitochondria. J Physiol. 2000;529(Pt 1):69–81. doi: 10.1111/j.1469-7793.2000.00069.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Niggli E, Shirokova N. A guide to sparkology: The taxonomy of elementary cellular Ca (2+) signaling events. Cell Calcium. 2007 doi: 10.1016/j.ceca.2007.02.010. [DOI] [PubMed] [Google Scholar]
- 223.Naraghi M, Neher E. Linearized buffered Ca2+ diffusion in microdomains and its implications for calculation of [Ca2+] at the mouth of a calcium channel. J Neurosci. 1997;17(18):6961–73. doi: 10.1523/JNEUROSCI.17-18-06961.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Neher E. Vesicle pools and Ca2+ microdomains: new tools for understanding their roles in neurotransmitter release. Neuron. 1998;20(3):389–99. doi: 10.1016/s0896-6273(00)80983-6. [DOI] [PubMed] [Google Scholar]
- 225.Csordas G, Thomas AP, Hajnoczky G. Quasi-synaptic calcium signal transmission between endoplasmic reticulum and mitochondria. EMBO J. 1999;18(1):96–108. doi: 10.1093/emboj/18.1.96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Augustine GJ, Neher E. Calcium requirements for secretion in bovine chromaffin cells. J Physiol. 1992;450:247–71. doi: 10.1113/jphysiol.1992.sp019126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Montero M, Alonso MT, Carnicero E, Cuchillo-Ibanez I, Albillos A, Garcia AG, Garcia-Sancho J, Alvarez J. Chromaffin-cell stimulation triggers fast millimolar mitochondrial Ca2+ transients that modulate secretion. Nat Cell Biol. 2000;2(2):57–61. doi: 10.1038/35000001. [DOI] [PubMed] [Google Scholar]
- 228.Cobbold PH, Rink TJ. Fluorescence and bioluminescence measurement of cytoplasmic free calcium. Biochem J. 1987;248(2):313–28. doi: 10.1042/bj2480313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Fiskum G. Intracellular levels and distribution of Ca2+ in digitonin-permeabilized cells. Cell Calcium. 1985;6(1–2):25–37. doi: 10.1016/0143-4160(85)90032-6. [DOI] [PubMed] [Google Scholar]
- 230.Rizzuto R, Pinton P, Carrington W, Fay FS, Fogarty KE, Lifshitz LM, Tuft RA, Pozzan T. Close contacts with the endoplasmic reticulum as determinants of mitochondrial Ca2+ responses. Science. 1998;280(5370):1763–6. doi: 10.1126/science.280.5370.1763. [DOI] [PubMed] [Google Scholar]
- 231.Simpson PB, Mehotra S, Lange GD, Russell JT. High density distribution of endoplasmic reticulum proteins and mitochondria at specialized Ca2+ release sites in oligodendrocyte processes. J Biol Chem. 1997;272(36):22654–61. doi: 10.1074/jbc.272.36.22654. [DOI] [PubMed] [Google Scholar]
- 232.Simpson PB, Mehotra S, Langley D, Sheppard CA, Russell JT. Specialized distributions of mitochondria and endoplasmic reticulum proteins define Ca2+ wave amplification sites in cultured astrocytes. J Neurosci Res. 1998;52(6):672–83. doi: 10.1002/(SICI)1097-4547(19980615)52:6<672::AID-JNR6>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- 233.Otsu H, Yamamoto A, Maeda N, Mikoshiba K, Tashiro Y. Immunogold localization of inositol 1, 4, 5-trisphosphate (InsP3) receptor in mouse cerebellar Purkinje cells using three monoclonal antibodies. Cell Struct Funct. 1990;15(3):163–73. doi: 10.1247/csf.15.163. [DOI] [PubMed] [Google Scholar]
- 234.Satoh T, Ross CA, Villa A, Supattapone S, Pozzan T, Snyder SH, Meldolesi J. The inositol 1,4,5,-trisphosphate receptor in cerebellar Purkinje cells: quantitative immunogold labeling reveals concentration in an ER subcompartment. J Cell Biol. 1990;111(2):615–24. doi: 10.1083/jcb.111.2.615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Sharma VK, Ramesh V, Franzini-Armstrong C, Sheu SS. Transport of Ca2+ from sarcoplasmic reticulum to mitochondria in rat ventricular myocytes. J Bioenerg Biomembr. 2000;32(1):97–104. doi: 10.1023/a:1005520714221. [DOI] [PubMed] [Google Scholar]
- 236.Csordas G, Renken C, Varnai P, Walter L, Weaver D, Buttle KF, Balla T, Mannella CA, Hajnoczky G. Structural and functional features and significance of the physical linkage between ER and mitochondria. J Cell Biol. 2006;174(7):915–21. doi: 10.1083/jcb.200604016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Aon MA, Cortassa S, Maack C, O’Rourke B. Sequential opening of mitochondrial ion channels as a function of glutathione redox thiol status. J Biol Chem. 2007 doi: 10.1074/jbc.M702841200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Cheng H, Lederer WJ, Cannell MB. Calcium sparks: elementary events underlying excitation-contraction coupling in heart muscle. Science. 1993;262(5134):740–4. doi: 10.1126/science.8235594. [DOI] [PubMed] [Google Scholar]
- 239.Zorov DB, Filburn CR, Klotz LO, Zweier JL, Sollott SJ. Reactive oxygen species (ROS)-induced ROS release: a new phenomenon accompanying induction of the mitochondrial permeability transition in cardiac myocytes. J Exp Med. 2000;192(7):1001–14. doi: 10.1084/jem.192.7.1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Aon MA, Cortassa S, Marban E, O’Rourke B. Synchronized whole cell oscillations in mitochondrial metabolism triggered by a local release of reactive oxygen species in cardiac myocytes. J Biol Chem. 2003;278(45):44735–44. doi: 10.1074/jbc.M302673200. [DOI] [PubMed] [Google Scholar]
- 241.Akar FG, Aon MA, Tomaselli GF, O’Rourke B. The mitochondrial origin of postischemic arrhythmias. J Clin Invest. 2005;115(12):3527–35. doi: 10.1172/JCI25371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Rydstrom J. Mitochondrial transhydrogenase--a key enzyme in insulin secretion and, potentially, diabetes. Trends Biochem Sci. 2006;31(7):355–8. doi: 10.1016/j.tibs.2006.05.003. [DOI] [PubMed] [Google Scholar]
- 243.Xi Q, Cheranov SY, Jaggar JH. Mitochondria-derived reactive oxygen species dilate cerebral arteries by activating Ca2+ sparks. Circ Res. 2005;97(4):354–62. doi: 10.1161/01.RES.0000177669.29525.78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Arieli Y, Gursahani H, Eaton MM, Hernandez LA, Schaefer S. Gender modulation of Ca (2+) uptake in cardiac mitochondria. J Mol Cell Cardiol. 2004;37(2):507–13. doi: 10.1016/j.yjmcc.2004.04.023. [DOI] [PubMed] [Google Scholar]
- 245.Ishii K, Hirose K, Iino M. Ca2+ shuttling between endoplasmic reticulum and mitochondria underlying Ca2+ oscillations. EMBO Rep. 2006;7(4):390–6. doi: 10.1038/sj.embor.7400620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Nemoto S, Takeda K, Yu ZX, Ferrans VJ, Finkel T. Role for mitochondrial oxidants as regulators of cellular metabolism. Mol Cell Biol. 2000;20(19):7311–8. doi: 10.1128/mcb.20.19.7311-7318.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]