Abstract
Therapeutic vaccination of lymphoma patients with tumor-specific immunoglobulin (idiotype, Id) coupled to the carrier protein keyhole limpet hemocyanin (Id-KLH) is undergoing clinical investigation, and methods to improve the immunogenicity of these and other protein tumor antigen vaccines are being sought. Id proteins can be produced via tumor-myeloma hybridomas or recombinant methods in mammalian, bacteria, or insect cells. We now demonstrate that terminal mannose residues, characteristic of recombinant proteins produced in insect cells, yield Id proteins with significantly enhanced immunostimulatory properties compared to Id proteins derived from mammalian cells. Recombinant baculovirus-infected insect cell-derived Id showed higher binding to and activation of human dendritic cells mediated by mannose receptors. In vivo, insect cell-derived Id elicited higher levels of tumor-specific CD8+ cytotoxic T lymphocyte (CTL) and improved eradication of pre-established murine lymphoma. Insect cell and mammalian Id generated similar levels of tumor-specific antibodies, showing no impairment in antibody responses to native tumor antigen despite the glycoslylation differences in the immunogen. Combining insect cell production and maleimide-based KLH conjugation offered the highest levels of anti-tumor immunity. Our data comparing sources of recombinant Id protein tumor antigens used in therapeutic cancer vaccines demonstrate that insect cell-derived antigens can offer several immunologic advantages over proteins derived from mammalian sources.
Keywords: Baculovirus, Tumor antigen, Vaccination
1. Introduction
The tumor-specific immunoglobulin (idiotype, or Id) clonally expressed by B cell lymphomas can serve as a target for therapeutic vaccination against B cell malignancies [1]. Immunization with this and other full-length protein tumor antigens has the goal of eliciting tumor-specific T cell and/or antibody responses capable of inhibiting tumor growth [2]. Tumor-specific Id proteins are isolated from individual B cell lymphomas by either fusion with a myeloma cell line to generate a tumor-myeloma hybridoma secreting tumor-specific Ig, or by molecular cloning of the tumor's heavy- and light-chain variable region Ig genes for production of recombinant Id in a variety of expression systems. Each of these methods yields a patient-specific tumor antigen for immunization. Vaccination with tumor-derived Id may elicit a polyclonal antibody response as well as CD8+ and CD4+ T cells recognizing Id-derived peptides presented on class I and class II MHC proteins at the tumor cell surface [1]. To improve immunogenicity, Id is chemically conjugated to the highly immunogenic foreign carrier protein keyhole limpet hemocyanin (KLH) [3]. Phase I/II trials of Id-KLH vaccination in lymphoma patients demonstrated a correlation of induced anti-Id immune responses with improved progression-free and overall survival [4], [5], clearance of circulating lymphoma cells [6], and durable tumor regressions [7], [8], [9], prompting the initiation of several phase III clinical trials of Id-KLH vaccination [10].
To achieve optimal immunity, protein tumor antigens should be delivered in a highly immunogenic form, preferably one allowing efficient uptake and processing by antigen presenting cells, including dendritic cells (DCs) [11]. Such antigen processing is facilitated by targeting of antigen to “scavenger” receptors such as the mannose receptor (MR), which binds to pathogen-associated carbohydrate structures rich in terminal mannose residues [12], [13]. MR-mediated uptake of glycoprotein antigens by DCs has been shown to enhance antigen presentation to CD8+ and CD4+ T cells in both murine [14], [15], [16] and human [17], [18], [19], [20] systems. Indeed, MRs expressed by DCs appears to be critical for the uptake and “cross-presentation” of soluble glycoproteins to CD8+ T cells [14], [15].
Baculovirus expression in insect cells represents a robust method for producing recombinant glycoproteins [21], [22]. Production of recombinant protein in insect cells leads to altered glycosylation patterns, including a relative abundance of terminal mannose residues [21], [22], thereby offering improved binding to MRs present on antigen presenting cells. Baculovirus-produced proteins are currently under study as vaccines against a number of viral pathogens, including human papilloma virus [23], influenza [24], HIV [25], hepatitis E [26], and the SARS/coronavirus [27] among others [28], as well as a variety of therapeutic human cancer vaccines [29], [30], [31], [32], [33]. The efficiency by which large quantities of recombinant proteins can be generated using a baculovirus–insect cell system led to the adoption of this approach for production of individual lymphoma-specific Id proteins for human clinical trials [34]. However, whether recombinant tumor-specific Id proteins secreted by insect cells possessed more desirable immunologic properties than those from mammalian sources has remained unknown.
We thus sought to directly compare the immunologic characteristics of mammalian cell (hybridoma)-derived Id to recombinant Id produced in baculovirus-infected insect cells. We found that the terminal mannose residue content in the insect cell-derived protein endowed it with markedly improved binding to human DCs via MRs, and was accompanied by upregulation of several DC activation markers. In vivo, immunization with insect cell-derived Id induced higher levels of cytotoxic T lymphocyte (CTL) activity against the A20 murine B cell lymphoma compared to hybridoma-derived Id, with an associated improvement in the frequency of tumor eradication. Importantly, the altered glycosylation pattern on the insect cell-derived Id did not impair its ability to induce Id-specific antibodies reactive against the native protein. An approach combining the use of recombinant insect cell-derived Id and conjugation to KLH using a recently described maleimide sulfhydryl-based method [35] produced the highest level of tumor eradication and tumor-specific antibodies. These data support the use of insect cell-derived tumor antigens and maleimide conjugation in clinical trials of tumor antigen-carrier protein conjugate vaccines.
2. Materials and methods
2.1. Antibodies and cell lines
IgG purified from human serum was obtained from Sigma–Aldrich (St. Louis, MO). The BALB/c B cell lymphoma line A20 was obtained from the American Type Culture Collection (ATCC, Manassas, VA) and cultured as previously described [35].
2.2. Production of A20 and human IgG Id proteins
Murine A20 Id protein (IgG2a, κ) was affinity-purified (protein A) from culture media of a tumor-myeloma cell hybridoma (Hyb Id, clone 3D6.3) derived by fusion of A20 lymphoma cells with SP2/0 myeloma cells, as previously described [35]. Recombinant baculovirus-derived A20 Id (BV Id, IgG2a, κ) was produced by cloning A20 lymphoma-derived variable heavy- and light-chain Ig cDNA sequences into a dual expression baculovirus plasmid vector as follows. The approximately 8.4 kb pTRABacA20 insect cell expression plasmid was constructed to contain two cassettes for expression of immunoglobulin proteins containing murine kappa light chains and IgG2a heavy chains. Expression from the first cassette is controlled by the baculovirus polyhedrin promoter and polyadenylation site, and directs synthesis of a protein containing the A20 kappa variable region fused to the murine kappa light chain constant region present in the pTRABac backbone vector. The human alkaline phosphatase secretory sequence coding region is incorporated into the backbone vector upstream of the A20 kappa variable region cloning site to insure appropriate processing of the mature full length kappa light chain. Expression from the second cassette, transcribed from the opposite strand to that for light chain expression, is controlled by the baculovirus p10 promoter and the bovine growth hormone polyadenylation site, and directs synthesis of an IgG2a protein containing the A20 heavy chain variable region fused to the murine γ-2a heavy chain constant region also present in the pTRABac backbone vector. The honeybee melittin secretory sequence coding region is incorporated into the backbone vector upstream of the A20 heavy chain variable region cloning site to insure appropriate processing of the mature full length IgG2a heavy chain. Approximately 2 kb to each side of the coding regions promote homologous recombination with the baculovirus genome. This transfer vector was co-transfected with baculovirus genomic DNA (BaculoGold, BD Biosciences, San Jose, CA) into Sf9 insect cells (Invitrogen) using standard lipid based transfection methods (Insect GeneJuice, EMD Chemicals Inc., Gibbstown, NJ) to obtain a high-titer baculovirus stock. This stock was then used to infect High-Five™ insect cells (Trichoplusia ni, BTI-Tn-5B1-4, Invitrogen, Carlsbad, CA) for recombinant Ig production [21], [22]. Both insect cell lines were maintained in animal free ESF-AF insect cell media (Expression Systems, Woodland, CA). Three days post-infection, full-length IgG2a A20 Ig was purified from clarified culture supernatant by protein A affinity chromatography followed by ion exchange and size exclusion chromatography. Nucleotide sequences of the 3D6.3 tumor hybridoma and the recombinant heavy- and light-chain variable region Igs were run on an ABI 3730xl (Applied Biosystems, Foster City, CA) instrument and analyzed using Sequencher software (GeneCodes Inc., Ann Arbor, MI). While the variable region (Id) sequences were identical, a modification was made in the light-chain constant region (position 6 amino acid was changed from Thr to Ser) to facilitate the cloning of the A20 kappa chain into the baculovirus vector. A tumor-specific human recombinant IgG1 cloned from a follicular lymphoma was also produced in insect cells as above.
2.3. Carbohydrate analysis of insect cell and hybridoma-derived tumor Id proteins
To analyze the carbohydrate composition of the purified A20 Id proteins, 300 μg of purified Id was incubated for 16–24 h at 37 °C with 20 μl of 2500 units/ml of PNGase F (New England Biolabs, Ipswich, MA) in PNGase F digestion buffer (20 mM sodium phosphate, pH 7.5, containing 50 mM EDTA and 0.02% sodium azide). After digestion, 2 μl of 100 μg/ml maltopentose (DP5) as internal standard was added and the deglycosylated antibody samples were heated for 2 min at 100 °C to precipitate the protein. The samples were centrifuged at 10,000 × g for 10 min and the supernatant containing the oligosaccharides was dried in a centrifugal vacuum evaporator. The oligosaccharide pellet was dissolved in 10 μl of the fluorescence dye APTS (Invitrogen) that had been reconstituted in 15% acetic acid and 5 μl of 1 M sodium cyanoborohydride in tetrahydrofuran. The labeling solution was incubated at 55 °C for 2 h, and then diluted 20-fold with water. Samples were then analyzed by capillary electrophoresis on a Beckman Coulter Proteome Lab PA800 CE instrument (Beckman Coulter, Fullerton, CA). Data were collected and processed using the Gold software supplied by the manufacturer. Commercially available oligosaccharide standards were analyzed in a similar fashion and the migration times compared to the oligosaccharide peaks observed from the A20 Ig samples.
2.4. Conjugation of Id proteins to KLH
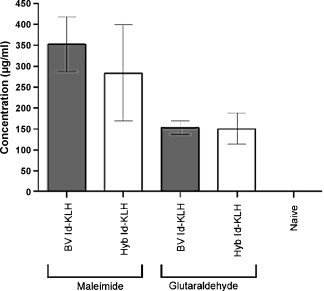
KLH was obtained form Pierce (Pierce, Rockford, IL). Glutaraldehyde conjugations were performed at room temperature using 1:1 (w:w) mixtures of Id:KLH in 0.1% glutaraldehyde (Sigma–Aldrich) as previously described [36]. Maleimide conjugations were performed as previously described [35]. Briefly, Id proteins were partially reduced in 0.1 mM DTT (Sigma–Aldrich), dialyzed into PBS containing 0.1 M EDTA to prevent re-oxidation of sulfhydryl groups, then conjugated to maleimide-activated KLH (Pierce) at a 1:1 ratio of Id to KLH (w:w, 1 mg/ml each) for 2 h at room temperature, followed by dialysis against PBS at 4 °C.
2.5. Preparation of immature human dendritic cells
Plastic-adherent peripheral blood mononuclear cells were cultured in RPMI media (Invitrogen) with 2% human AB serum (Valley Biomedical Inc., Winchester, VA), 10 ng/ml recombinant human IL-4 (R&D Systems, Minneapolis, MN), and 50 ng/ml of GM-CSF (Berlex, Montville, New Jersey), with a 50% cytokine media change every other day. After 6 days, non- and loosely-adherent cells were harvested.
2.6. Dendritic cell mannose receptor uptake and blocking
KLH, human IgG-KLH and BV-IgG-KLH conjugates were labeled with FITC using the FluoReporter® FITC protein labeling kit (Invitrogen). The FITC/protein ratio was approximately 5.5 for all conjugates. Immature monocyte-derived human DCs were incubated with 10 μg/ml of FITC-conjugated human IgG, KLH, or BV-derived human IgG antibody in the presence of 5% normal human AB serum for 20 min at 4 °C. Cells were then washed twice in staining buffer and analyzed by flow cytometry. Cells cultured without FITC-conjugated protein served as a negative control. Murine A20 Id proteins (BV- or Hyb-derived) were labeled with the Alexa Fluor® 647 protein labeling kit (Invitrogen). BV-Alexa labeled Id, Hyb-Alexa labeled Id, or FITC-Dextran (Sigma–Aldrich) were then incubated at 50 μg/ml with human DCs for 2 h at 37 °C in RPMI with 10% FCS mixed 1:1 with 1X PBS [37]. The cells were then washed 3× in ice-cold FACS buffer and fixed in 4% paraformaldehyde for flow cytometric analysis using a BD FACSCalibur flow cytometer (BD Biosciences) and FCS Express analysis software (De Novo Software, Los Angeles, CA). Aliquots of cells were also pretreated with various blocking agents for 20 min at 37 °C before the addition of Id antigen to evaluate the effects on binding to MRs on the cell surface. Blocking agents included 3 mg/ml mannan (Sigma–Aldrich), 10 μg/ml of anti-MR antibody (clone 19.2, BD Biosciences), control IgG (2B8, mouse IgG1, κ, Biogen-IDEC, San Diego, CA), or 5 μg/ml cytochalasin D (Sigma–Aldrich) [37].
2.7. Activation of dendritic cells with Id-KLH preparations
Immature human DCs were incubated for 48 h in RPMI media plus 5% human AB serum with 0, 50, or 100 μg/ml of BV- or Hyb-derived Id conjugated to KLH via glutaraldehyde. For some samples, anti-MR antibody at 50 μg/ml was added at the start of culture. Cells were harvested and stained with FITC-labeled anti-human CCR7, PE-labeled anti-human CD80, TC-labeled anti-human CD45, and APC-labeled anti-human CD14 monoclonal antibodies (BD Biosciences), and analyzed by flow cytometry as above.
2.8. In vivo immunization studies
BALB/c mice were bred and housed in the Radiation Oncology Barrier Facility, a defined pathogen colony at UCLA (Los Angeles, CA), and experiments conducted according to UCLA animal care guidelines. Groups of BALB/c mice (6–10 weeks of age) were inoculated subcutaneously (s.c.) with 1 × 105 A20 cells on day 0. Beginning on day 4, mice were given either two or three weekly vaccinations of 50 μg BV- or Hyb-derived Id-KLH, conjugated using either glutaraldehyde or maleimide chemistries [35]. Each vaccination included 4 consecutive days of 55 ng GM-CSF (BD Biosciences), given in the same location. Mice were killed when tumors reached 1.4 cm in diameter, per institutional protocol. For T cell depletion studies, animals were injected intraperitoneally (i.p.) with anti-CD8 (53.6.72) or anti-CD4 (GK1.5) antibodies (BioXCell, West Lebanon, NH), or control rat IgG (Sigma–Aldrich, St Louis, MO) on days −6, −5, −4, 0, and weekly thereafter, with depletions confirmed on day −1 by flow cytometry.
2.9. Measurement of cytotoxic T lymphocyte activity
Mice were given two bi-weekly vaccinations of BV- or Hyb-derived glutaraldehyde Id-KLH plus GM-CSF as above. Fourteen days later, post-vaccine spleen and lymph nodes were harvested and pooled from individual mice and processed into single cell suspensions. Cells were cultured for 5 days with mitomycin C (25 μg/ml, Sigma–Aldrich) treated A20 tumor cells. At the end of culture, viable cells were purified via centrifugation in Histopaque (Sigma–Aldrich). Live cells were counted and phenotyped to enumerate total CD3+CD8+ T cells by flow cytometry. 1 × 105 recovered T cells were co-incubated with 1 × 104 D275-labeled (Invitrogen) A20 cells in 1 ml of 5% FCS in RPMI media for 3 h at 37 °C. Following incubation, 20 μl of PI (Sigma–Aldrich) was added to each sample and cells were assessed immediately by flow cytometry. Percent lysis was calculated as the % of PI+D275+ A20 target cells/total D275+ A20 targets.
2.10. Quantitation of anti-idiotype antibodies by ELISA
To evaluate the antibody responses generated by BV- or Hyb-derived Id-KLH vaccines, groups of four BALB/c mice received three weekly vaccinations as above. Mice were bled 10 days after the third vaccination, and anti-Id antibodies measured as previously described [35]. F(ab)2 A20 hybridoma Id was coated onto Nunc Immunosorp (Nalge Nunc, Rochester, NY) plates at 5 μg/ml in carbonate buffer overnight at 4 °C. Plates were blocked with 5% non-fat dry milk, washed, then sera was serially diluted along with an A20 anti-Id standard 1G6 (mouse IgG1, kind gift from Dr. Ronald Levy, Stanford, CA) for 1 h at room temperature. Plates were then washed, incubated with a goat anti-mouse IgG-HRP secondary detector antibody (γ specific, Southern Biotech, Birmingham, AL), washed again, and then developed with ABTS (Sigma–Aldrich).
2.11. Statistical analysis
Survival differences among groups of mice were assessed using the Kaplan–Meier method with the log-rank test using Prism software (Graph-Pad Software, San Diego, CA). p values were considered statistically significant at p less than 0.05. CD80 and CCR7 expression, CTL, and antibody titer data were compared using the paired, two-tailed Student's t-test, and differences were considered statistically significant at p less than 0.05.
3. Results
3.1. A20 lymphoma-specific Id proteins from insect cell and hybridoma sources have distinct glycosylation patterns
The cDNA sequences of the A20 lymphoma heavy- and light-chain Ig variable (idiotype) regions were cloned into a baculovirus vector expressing a murine IgG2a, κ backbone matching that of the native Id, and produced in High-Five™ insect cells. The variable region cDNAs from the BV- and Hyb-derived Ids were sequenced and confirmed to be identical (data not shown). Comparative carbohydrate analysis of the BV- and Hyb-derived A20 Id proteins by capillary electrophoresis demonstrated a typical N-linked glycosylation pattern for the mammalian-derived Ig [38], and characteristic oligomannosidic N-glycans with five to nine mannose residues [21] in the insect cell-derived protein (Fig. 1 ). This pattern was representative of human lymphoma-derived BV Id proteins produced for Id-KLH vaccine clinical trials (data not shown).
Fig. 1.
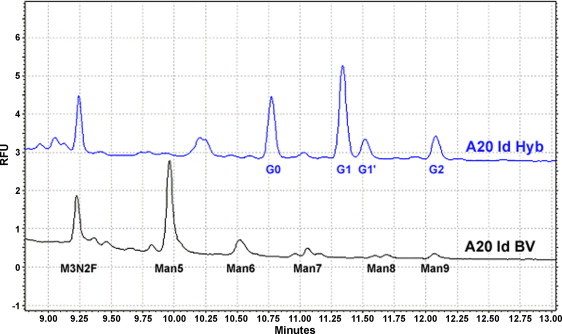
Comparative carbohydrate analysis of hybridoma-derived (Hyb) and insect cell-derived (BV) A20 Id proteins. Oligosaccharides were cleaved from purified proteins using PNGase F, stained with APTS, and analyzed by capillary electrophoresis alongside oligosaccharide standards. The elution profile is depicted as relative fluorescence units (RFU) over time. Hyb-derived Id contains oligosaccharides with terminal galactose (G) residues typical of Igs. Only the insect cell-derived Id has a high content of terminal mannose (Man) residues.
3.2. Mannose receptor mediates enhanced binding of BV-derived Id to human DCs
Given the high content of terminal mannose residues in BV-derived Id, we hypothesized that the binding and uptake of BV Id by DCs may differ from that of native, tumor-derived Id. FITC-labeled human IgG and BV-derived human IgG were conjugated to KLH via glutaraldehyde and incubated with immature human DCs. As depicted in Fig. 2A, both KLH alone and human IgG-KLH showed a modest and roughly equivalent degree of binding to the DCs. In contrast, the BV-derived human IgG-KLH conjugate demonstrated substantially higher (>10-fold increased mean fluorescence intensity, MFI) staining of human DCs. Similarly, after a 2-h incubation with DCs to allow binding and uptake of proteins, fluorescent Alexa 647-labeled Hyb A20 Id and free Alexa 647 each showed moderate uptake by human DCs, while BV-derived A20 Id displayed strikingly higher uptake (>30-fold increased MFI) (Fig. 2B). Since the amino acid sequences of these two A20 Id proteins are essentially identical, but differ substantially in their glycan content, we sought to determine the role of MRs in the affinity of these proteins for DCs. Human DCs were incubated with fluorescent-labeled BV Id, Hyb Id, or dextran in the presence of various endocytosis inhibitors (Fig. 2C). The high-level binding and uptake of BV Id and dextran (a synthetic ligand for MR) by DCs was partially blocked in the presence of the mannose-containing polysaccharide mannan, and by a blocking antibody against MR, but not affected by control antibody. In contrast, these inhibitors did not affect the binding and uptake of Hyb Id. Cytochalasin D, an inhibitor of microfilament-dependent endocytosis, reduced DC uptake of BV Id, Hyb Id, and dextran, as expected. These results demonstrate a role for DC MRs in the increased uptake of the BV-derived antigen compared to its mammalian cell-derived counterpart. Interestingly, despite the fact that KLH contains mannose residues that can bind to mannose receptors [39], the glycan content of BV-derived Id permits even higher binding to DC mannose receptors.
Fig. 2.
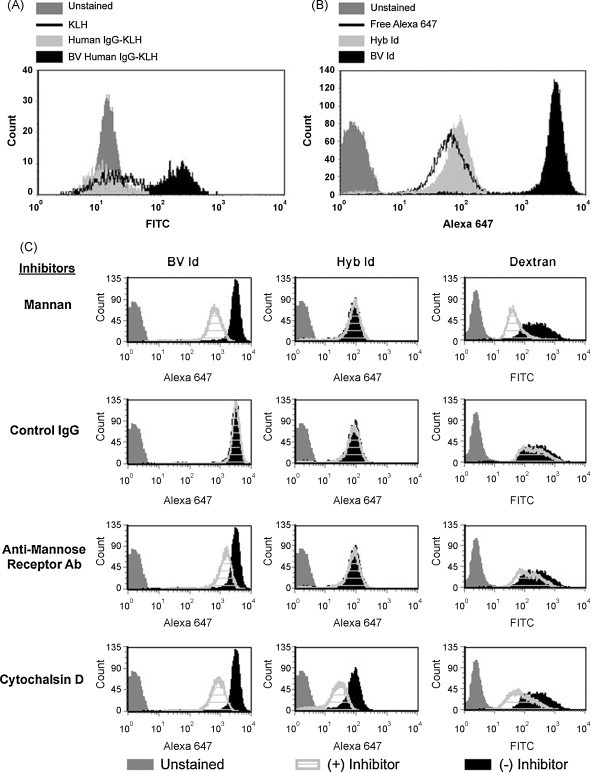
Human DC mannose receptors allow for increased binding of insect cell-derived Igs. (A) Human DCs were incubated at 4 °C with FITC-labeled KLH, human IgG-KLH, or recombinant BV-derived human IgG-KLH. IgGs were conjugated to KLH via glutaraldehyde and evaluated for surface binding. (B) Murine A20 Id produced in both BV or Hyb systems were labeled with Alexa 647 and incubated for 2 h at 37 °C with human DCs to determine surface binding of the Igs. (C) BV A20 Id-Alexa 647, Hyb A20 Id-Alexa 647, or FITC-dextran were incubated with human DCs in the presence of various inhibitors including mannan, control IgG antibody, anti-MR blocking antibody, or cytochalasin D, and bound antigen quantitated by flow cytometry.
3.3. Insect-derived Id upregulates activation markers on human DCs
Ligation of C-type lectin-like scavenger receptors such as the MR on DCs can contribute to their activation [40]. The surface expression of two representative DC activation markers were evaluated after incubation with A20 BV or Hyb glutaraldehyde Id-KLH conjugates. Immature human DCs from five donors were evaluated for surface expression of CD80 (Fig. 3A) or CCR7 (Fig. 3B) after 48 h of co-culture. DCs incubated with BV Id-KLH conjugates showed significantly higher levels of CD80 and CCR7 expression compared to DCs incubated with Hyb Id-KLH. DCs exposed to unconjugated KLH showed surface marker levels roughly equivalent to those seen with media alone controls (data not shown). The increases in CD80 expression by DCs cultured with Hyb Id-KLH (vs. media alone) may be accounted for by in part by the mannose content of KLH [39]. The addition of blocking anti-MR antibody to the co-cultures reduced the higher levels of surface marker expression seen with BV Id, in many cases, near to the levels seen with Hyb Id proteins (Fig. 3C). Moreover, marker expression in the presence of Hyb Id proteins was not greatly affected by the anti-MR antibody. Thus, the enhanced binding and uptake of BV Id by DCs is associated with the induction of a more mature immunophenotype, and appears to be mediated in part by interaction with MRs.
Fig. 3.
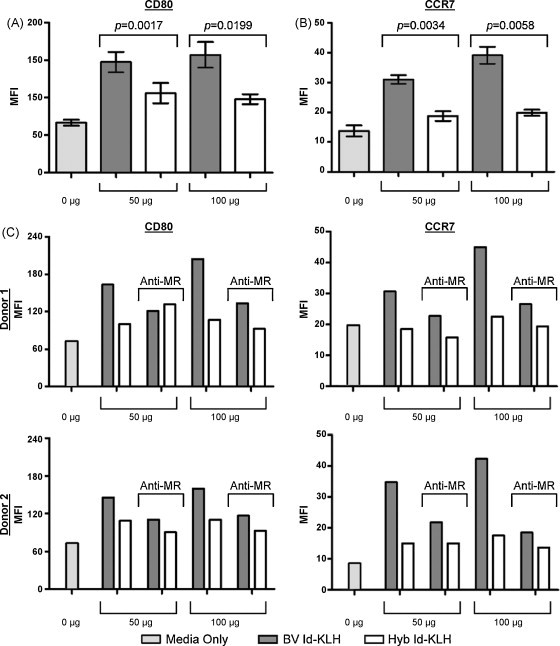
BV Id proteins stimulate expression of human DC activation markers over that seen with Hyb Id. Surface expression of (A) CD80 and (B) CCR7 in the presence of BV- or Hyb-derived glutaraldehyde Id-KLH conjugates. (C) CD80 and CCR7 surface expression after pre-treatment with an anti-MR blocking antibody. Error bars in A & B represent standard deviation of n = 5 replicates for 0 and 50 μg samples and n = 4 for 100 μg samples.
3.4. In vivo comparison of baculovirus vs. hybridoma Id-KLH conjugates
Our set of BV- and Hyb-derived A20 Id proteins permitted us to assess the impact of insect cell Id production on lymphoma-specific immunity in vivo. Mice bearing 4-day early established A20 s.c. tumors were vaccinated with BV or Hyb Id conjugated to KLH via either glutaraldehyde or maleimide chemistries. While the standard for most Id vaccine clinical trials has been Hyb Id conjugated to KLH via glutaraldehyde [1], [3], we have recently described an alternative sulfhydryl-based conjugation method with improved in vivo efficacy in multiple murine B cell lymphoma models [35]. The pooled results of three individual experiments are shown in Fig. 4 . The standard Hyb glutaraldehyde Id-KLH elicited the lowest level of tumor eradication (33%, Fig. 4A). The addition of BV Id to glutaraldehyde Id-KLH gave slightly higher survival (47%). Changing conjugation chemistries to maleimide resulted in still higher survival for both BV- and Hyb-derived Id proteins. Notably, the level of tumor eradication achieved by BV maleimide Id-KLH (64%) was significantly higher than that reached with the standard Hyb glutaraldehyde Id-KLH vaccine (33%, p value 0.0025). Considering only the source of Id protein, in all three individual experiments, BV Id protein conjugates showed a modest survival trend in favor of BV Id over Hyb Id. In the pooled analysis, BV Id resulted in a greater proportion of survivors vs. Hyb Id (53% vs. 41%, p value 0.112; Fig. 4B). Considering only the method of conjugation, maleimide conjugates resulted in significantly improved survival compared to glutaraldehyde conjugates (58% vs. 40%, p value 0.0054; Fig. 4C). These results demonstrate that the use of BV-derived Id proteins and maleimide conjugation may both contribute to the significantly improved survival seen in comparison to a traditional Hyb Id-KLH vaccine.
Fig. 4.
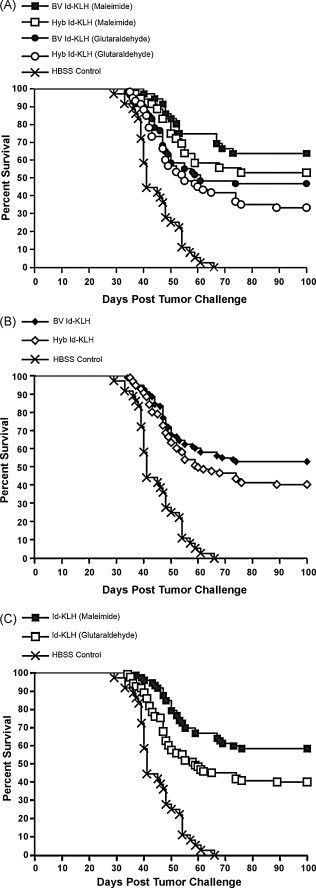
Both BV Id production and maleimide conjugation result in improvements over traditional Hyb Id production and glutaraldehyde conjugation. (A) Four days after s.c. inoculation with A20 tumor cells, groups of mice received either two or three weekly s.c. vaccinations with BV-derived (n = 96) or Hyb-derived (n = 96) Id conjugated to KLH via either maleimide (n = 72) or glutaraldehyde (n = 120), plus locally administered GM-CSF as an adjuvant. Data from three individual experiments having identical endpoint criteria were pooled. Tumor eradication was greatest using BV Id and maleimide conjugation. (B) BV and Hyb Id-KLH groups were pooled, showing a greater proportion of survivors with insect cell-derived Id proteins. (C) Id-KLH maleimide and glutaraldehyde groups were pooled, showing superiority of maleimide-conjugated Id proteins compared to glutaraldehyde conjugates.
3.5. Improved CD8+ CTL responses in BV Id vaccinated mice
We have recently demonstrated that the eradication of established A20 lymphoma by Id-KLH vaccination is dependent upon CD8+ T cells [35]. As MR-mediated uptake of soluble antigen to DCs has been shown to provide efficient CTL induction [14], [15], [16], [19], we compared the ability of BV and Hyb Id proteins to induce tumor-specific CTL activity after glutaraldehyde Id-KLH vaccination. As shown in Fig. 5A, CTL activity from mice vaccinated with the BV Id-KLH was significantly stronger than that obtained with the mammalian cell-derived Id-KLH. To confirm that CD8+ T cells were the in vivo effectors in BV glutaraldehyde Id-KLH-vaccinated mice, mice were depleted of either CD4+ or CD8+ T cells prior to tumor inoculation and subsequent immunization (Fig. 5B). Vaccination with BV glutaraldehyde Id-KLH induced significant tumor eradication compared to control-treated mice (HBSS vs. vaccinated and control antibody or CD4+ T cell-depleted, p values 0.0002 and 0.0066, respectively). Mice receiving control or CD4+ T cell-depleting antibodies showed similar levels of survival (Fig. 5B; p value 0.79), indicating no apparent role for CD4+ T cell effectors in vaccine-induced tumor protection. In contrast, mice depleted of CD8+ T cells all succumbed to tumor, just as those treated with HBSS control. Thus, enhanced CD8+ CTL induction appears to be responsible for the improved efficacy of BV Id-KLH vaccines (Fig. 4A and B).
Fig. 5.
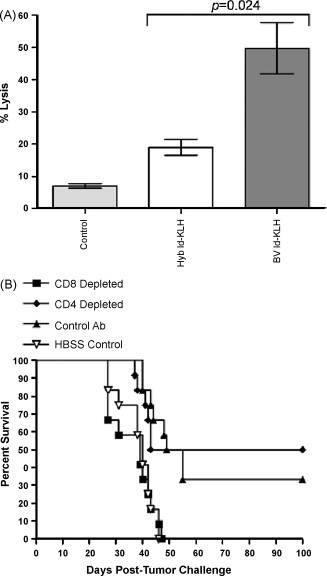
CD8+ T cells in mice vaccinated with insect cell-derived Id are required for tumor eradication and demonstrate enhanced effector function. (A) BV glutaraldehyde Id-KLH elicits greater CTL activity than Hyb glutaraldehyde Id-KLH. Mice were given two bi-weekly vaccinations, after which CTLs from the spleen and lymph nodes were tested for their ability to lyse A20 lymphoma cells at an E:T ratio of 10:1. (B) Dependence of vaccine efficacy on CD8+ T cells. BALB/c mice were pre-depleted of CD4+ or CD8+ T cells, inoculated s.c. with A20 tumor and 4 days later given three weekly vaccinations of BV glutaraldehyde Id-KLH plus GM-CSF, and followed for survival.
3.6. Humoral anti-Id responses in BV and Hyb Id vaccinated mice
Since anti-Id antibodies are potentially important effectors against some B cell lymphomas after Id vaccination [7], [35], [36], [41], [42], [43], and N-linked glycosylation is common in the variable regions of follicular lymphoma Igs [44], we assessed whether the altered glycan structures of BV Id would impair the induction of antibodies recognizing the native Id structure. Mice were vaccinated with BV or Hyb Id conjugated to KLH via glutaraldehyde or maleimide, and serum levels of antibodies binding to native (Hyb) Id were determined by ELISA. The use of BV Id neither enhanced nor inhibited the humoral response to native tumor Id (Fig. 6 ). However, anti-Id titers after immunization with maleimide conjugates of both BV and Hyb Id proteins were substantially higher than those seen with their corresponding glutaraldehyde conjugates. Notably, the highest antibody levels were seen in mice vaccinated with maleimide-conjugated BV Id-KLH.
Fig. 6.
Vaccination with BV Id-KLH maleimide conjugate results in higher tumor antigen-specific antibody titers than glutaraldehyde conjugates. Groups of four mice were vaccinated with BV or Hyb Id conjugated to KLH via either maleimide or glutaraldehyde weekly for 3 weeks together with GM-CSF. Mice were bled 10 days later and anti-Id antibody titers determined by ELISA. Error bars represent standard deviations among serum values from individual mice.
4. Discussion
Based on the established role of mannose receptors in promoting T cell immunity to protein antigens [14], [15], [16], [17], [18], [19], [20], and the mannose-rich glycans of insect cell produced glycoproteins [21], [28], baculovirus-produced antigens may offer advantages as immunogens in vaccines against both infectious agents and tumors. Indeed, several baculovirus-produced vaccines have recently demonstrated efficacy against viral infections in humans [23], [26]. To meet the challenge of producing large numbers of Id tumor antigen vaccines from individual lymphoma patients, a baculovirus production strategy was adopted [35]. While it was theorized that production of lymphoma-specific Id proteins in insect cells might yield tumor antigens with increased immunogenicity, we wished to test this hypothesis in a controlled setting. We thus performed a side-by-side comparison of Id protein antigen vaccines produced in either insect or mammalian cells for their interactions with human DCs, and their in vivo potency against a murine B cell lymphoma. To our knowledge, this study represents the first direct immunologic comparison of insect cell-derived vs. mammalian cell-derived recombinant tumor antigens. Despite numerous recent studies of BV-produced protein vaccines against a variety of infectious agents and human cancers [23], [24], [25], [26], [27], [28], [29], [30], [31], [32], [33], the degree to which recombinant tumor antigens secreted by insect cells differ in their immunologic properties from those of mammalian sources has remained incompletely characterized.
A typical pattern of N-linked oligomannosidic glycans was found in lymphoma Id proteins produced in insect cells (Fig. 1), endowing them with increased binding and uptake by human DCs (Fig. 2). Based on inhibition studies, MRs appear to play a substantial role in the increased affinity of BV Id proteins for DCs, although other C-type lectin-like and other scavenger receptors may also be involved [13]. We also confirmed that mannose-rich antigen preparations can, in addition to improving antigen uptake by DCs (Fig. 2), contribute to DC activation (Fig. 3) [40]. This property adds to the attractiveness of BV vaccines over mammalian sources of recombinant tumor antigens.
Presicce and colleagues recently reported that KLH can induce activation and maturation of human DCs in part via interactions with mannose receptors [39]. In light of this, why might conjugation of KLH to another mannose-containing protein (insect cell-derived Id) offer enhanced DC binding, uptake, and activation over that seen with mammalian Id-KLH conjugates? This may be explained by the topology of Id-KLH conjugates. Id proteins (150 kDa) are conjugated 1:1 (w:w) to the surface of the much larger KLH protein, which exists as a multimeric complex of 8000 kDa [45], yielding a KLH covered with many (approximately 50) Id molecules. Thus, mannose-containing glycans on the insect cell-derived Id could be much more accessible for interaction with DC mannose receptors than glycans on the KLH carrier protein at the core of the conjugate, making the Id protein mannose content dominant in the mannose receptor-Id-KLH interaction. The higher mannose content of a protein antigen coupled to KLH would be important in preserving mannose receptor interactions as a means of increasing immunogenicity.
In vivo, we found a reproducible trend towards improved tumor eradication using BV-produced Id compared to that produced by a tumor-myeloma hybridoma (Fig. 4; 53% vs. 41%), though this trend did not reach statistical significance. Interestingly, the optimal tumor eradication in our study was obtained by combining two relatively novel refinements in Id-KLH vaccines: the use of insect cell-derived antigen, and conjugation to KLH via maleimide chemistry [35]. Therefore, it would be worthwhile to test Id-KLH vaccines employing both MR targeting and maleimide conjugation in clinical trials against human B cell malignancies. In addition to providing increased uptake and activation of DCs, BV Id also elicited superior CTL activity against lymphoma cells (Fig. 5A). Thus, for therapeutic tumor antigen vaccines where the induction of CD8+ CTL is the goal, insect cell production of recombinant protein could prove advantageous.
Given that anti-Id antibodies can be important anti-lymphoma effectors after Id vaccination [7], [36], [41], [42], [43], it was important to exclude the possibility that the altered glycan structures of Id produced in insect cells might favor the induction of antibodies against the foreign glycans while decreasing production of those recognizing the native Id structure presented on tumor cells. Importantly, no such impairment of tumor antigen-specific antibodies was observed; BV and Hyb Id proteins elicited similar titers of antibodies recognizing native Id (Fig. 6). Thus, at least in this single case, impairment of the relevant tumor-specific humoral response by vaccination with insect cell-derived Id does not represent a concern.
The production of lymphoma-specific Id proteins using BV and insect cells remains an attractive approach for efficient generation of vaccine products. Quantities of Id sufficient for vaccination can be obtained from near 100% of human follicular lymphomas using this technology, usually within a period of only 8–12 weeks. In a phase II clinical trial in non-Hodgkin's lymphoma, several objective tumor regressions were observed in patients immunized with recombinant insect cell-derived Id-KLH [8]. Nonetheless, in a phase III clinical trial, this same vaccine was recently reported to have failed to improve progression-free survival in follicular non-Hodgkin's lymphoma patients immunized after cytoreduction with anti-CD20 monoclonal antibody therapy [46]. A second phase III vaccine trial in follicular non-Hodgkin's lymphoma using recombinant mammalian cell-derived Id also recently failed to improve progression-free survival after cytoreductive chemotherapy [47]. Only a third phase III trial using hybridoma-derived Id protein for vaccination of follicular lymphoma patients in complete remission after chemotherapy has reported positive results in improving tumor-free survival [48]. The reasons for the failure of the former two trials are unclear, but may relate to the higher tumor burdens in these patients at the time of vaccination. The patients treated on the trial using insect cell-derived Id had the highest tumor burdens of all three trials, so this vaccine may not necessarily be any less potent than the others tested in phase III trials. Our current laboratory results, comparing a single lymphoma-specific Id produced in insect cells to a hybridoma-derived Id, show modest but reproducible immunologic advantages to the insect cell-derived antigen. Furthermore, our data clearly demonstrate that vaccines utilizing both a mannose-rich tumor antigen with a novel maleimide-based carrier protein conjugation method offers significantly improved in vivo anti-tumor efficacy. Thus, combining these and other new vaccine technologies, may lead to improved clinical outcomes with therapeutic cancer vaccines.
Acknowledgements
We would like to thank Reiko Yamada and Kristopher Steward (UCLA) for helpful comments and critical evaluation of the manuscript.
Footnotes
Supported by NIH/NCI grant P50CA096888 (SPORE in lymphoma). JMT is a Damon Runyon Clinical Investigator supported in part by the Damon Runyon Cancer Research Foundation (CI-26-05).
References
- 1.Timmerman J.M. Therapeutic idiotype vaccines for non-Hodgkin's lymphoma. Adv Pharmacol (San Diego, Calif) 2004;51:271–293. doi: 10.1016/S1054-3589(04)51012-8. [DOI] [PubMed] [Google Scholar]
- 2.Rosenberg S.A. Progress in human tumour immunology and immunotherapy. Nature. 2001;411(May(6835)):380–384. doi: 10.1038/35077246. [DOI] [PubMed] [Google Scholar]
- 3.Timmerman J.M., Levy R.L. The history of the development of vaccines for lymphoma. Clin Lymph. 2000;1:129–139. doi: 10.3816/clm.2000.n.011. [DOI] [PubMed] [Google Scholar]
- 4.Kwak L.W., Campbell M.J., Czerwinski D.K., Hart S., Miller R.A., Levy R. Induction of immune responses in patients with B-cell lymphoma against the surface-immunoglobulin idiotype expressed by their tumors. N Engl J Med. 1992;327(17):1209–1215. doi: 10.1056/NEJM199210223271705. [DOI] [PubMed] [Google Scholar]
- 5.Hsu F.J., Caspar C.B., Czerwinski D., Kwak L.W., Liles T.M., Syrengelas A. Tumor-specific idiotype vaccines in the treatment of patients with B-cell lymphoma—long-term results of a clinical trial. Blood. 1997;89(9):3129–3135. [PubMed] [Google Scholar]
- 6.Bendandi M., Gocke C.D., Kobrin C.B., Benko F.A., Sternas L.A., Pennington R. Complete molecular remissions induced by patient-specific vaccination plus granulocyte-monocyte colony-stimulating factor against lymphoma [see comments] Nat Med. 1999;5(10):1171–1177. doi: 10.1038/13928. [DOI] [PubMed] [Google Scholar]
- 7.Timmerman J.M., Czerwinski D.K., Davis T.A., Hsu F.J., Benike C., Hao Z.M. Idiotype-pulsed dendritic cell vaccination for B-cell lymphoma: clinical and immune responses in 35 patients. Blood. 2002;99(5):1517–1526. doi: 10.1182/blood.v99.5.1517. [DOI] [PubMed] [Google Scholar]
- 8.Redfern C.H., Guthrie T.H., Bessudo A., Densmore J.J., Holman P.R., Janakiraman N. Phase II trial of idiotype vaccination in previously treated patients with indolent non-Hodgkin's lymphoma resulting in durable clinical responses. J Clin Oncol. 2006;24(July(19)):3107–3112. doi: 10.1200/JCO.2005.04.4289. [DOI] [PubMed] [Google Scholar]
- 9.Koc O., Redfern C., Wiernik P.H., Rosenfelt F., Winter J., Guthrie T.H. Id/KLH vaccine (FavId TM) following treatment with rituximab: an analysis of response rate immprovement (RRI) and time-to-progression (TTP) in follicular lymphoma (FL) Blood. 2004;104:170a. [abstract #587] [Google Scholar]
- 10.Hurvitz S.A., Timmerman J.M. Current status of therapeutic vaccines for non-Hodgkin's lymphoma. Curr Opin Oncol. 2005;17(September(5)):432–440. doi: 10.1097/01.cco.0000174040.52427.83. [DOI] [PubMed] [Google Scholar]
- 11.Steinman R.M., Banchereau J. Taking dendritic cells into medicine. Nature. 2007;449(September(7161)):419–426. doi: 10.1038/nature06175. [DOI] [PubMed] [Google Scholar]
- 12.Apostolopoulos V., McKenzie I.F. Role of the mannose receptor in the immune response. Curr Mol Med. 2001;1(September(4)):469–474. doi: 10.2174/1566524013363645. [DOI] [PubMed] [Google Scholar]
- 13.Cambi A., Figdor C.G. Dual function of C-type lectin-like receptors in the immune system. Curr Opin Cell Biol. 2003;15(October(5)):539–546. doi: 10.1016/j.ceb.2003.08.004. [DOI] [PubMed] [Google Scholar]
- 14.Burgdorf S., Kautz A., Bohnert V., Knolle P.A., Kurts C. Distinct pathways of antigen uptake and intracellular routing in CD4 and CD8 T cell activation. Science. 2007;316(April(5824)):612–616. doi: 10.1126/science.1137971. [DOI] [PubMed] [Google Scholar]
- 15.Burgdorf S., Lukacs-Kornek V., Kurts C. The mannose receptor mediates uptake of soluble but not of cell-associated antigen for cross-presentation. J Immunol. 2006;176(June(11)):6770–6776. doi: 10.4049/jimmunol.176.11.6770. [DOI] [PubMed] [Google Scholar]
- 16.He L.Z., Crocker A., Lee J., Mendoza-Ramirez J., Wang X.T., Vitale L.A. Antigenic targeting of the human mannose receptor induces tumor immunity. J Immunol. 2007;178(May(10)):6259–6267. doi: 10.4049/jimmunol.178.10.6259. [DOI] [PubMed] [Google Scholar]
- 17.Engering A.J., Cella M., Fluitsma D., Brockhaus M., Hoefsmit E.C., Lanzavecchia A. The mannose receptor functions as a high capacity and broad specificity antigen receptor in human dendritic cells. Eur J Immunol. 1997;27(September(9)):2417–2425. doi: 10.1002/eji.1830270941. [DOI] [PubMed] [Google Scholar]
- 18.Sallusto F., Cella M., Danieli C., Lanzavecchia A. Dendritic cells use macropinocytosis and the mannose receptor to concentrate macromolecules in the major histocompatibility complex class II compartment: downregulation by cytokines and bacterial products. J Exp Med. 1995;182(August(2)):389–400. doi: 10.1084/jem.182.2.389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Srinivas O., Larrieu P., Duverger E., Boccaccio C., Bousser M.T., Monsigny M. Synthesis of glycocluster-tumor antigenic peptide conjugates for dendritic cell targeting. Bioconjugate Chem. 2007;18(September–October(5)):1547–1554. doi: 10.1021/bc070026g. [DOI] [PubMed] [Google Scholar]
- 20.Tan M.C., Mommaas A.M., Drijfhout J.W., Jordens R., Onderwater J.J., Verwoerd D. Mannose receptor-mediated uptake of antigens strongly enhances HLA class II-restricted antigen presentation by cultured dendritic cells. Eur J Immunol. 1997;27(September(9)):2426–2435. doi: 10.1002/eji.1830270942. [DOI] [PubMed] [Google Scholar]
- 21.Altmann F., Staudacher E., Wilson I.B., Marz L. Insect cells as hosts for the expression of recombinant glycoproteins. Glycoconjugate J. 1999;16(February(2)):109–123. doi: 10.1023/a:1026488408951. [DOI] [PubMed] [Google Scholar]
- 22.Kost T.A., Condreay J.P. Recombinant baculoviruses as expression vectors for insect and mammalian cells. Curr Opin Biotechnol. 1999;10(October(5)):428–433. doi: 10.1016/s0958-1669(99)00005-1. [DOI] [PubMed] [Google Scholar]
- 23.Harper D.M., Franco E.L., Wheeler C., Ferris D.G., Jenkins D., Schuind A. Efficacy of a bivalent L1 virus-like particle vaccine in prevention of infection with human papillomavirus types 16 and 18 in young women: a randomised controlled trial. Lancet. 2004;364(November(9447)):1757–1765. doi: 10.1016/S0140-6736(04)17398-4. [DOI] [PubMed] [Google Scholar]
- 24.Cox M.M. Progress on baculovirus-derived influenza vaccines. Curr Opin Mol Therap. 2008;10(February(1)):56–61. [PubMed] [Google Scholar]
- 25.Yao Q., Bu Z., Vzorov A., Yang C., Compans R.W. Virus-like particle and DNA-based candidate AIDS vaccines. Vaccine. 2003;21(January(7–8)):638–643. doi: 10.1016/s0264-410x(02)00572-8. [DOI] [PubMed] [Google Scholar]
- 26.Shrestha M.P., Scott R.M., Joshi D.M., Mammen M.P., Jr., Thapa G.B., Thapa N. Safety and efficacy of a recombinant hepatitis E vaccine. N Engl J Med. 2007;356(March(9)):895–903. doi: 10.1056/NEJMoa061847. [DOI] [PubMed] [Google Scholar]
- 27.Zhou Z., Post P., Chubet R., Holtz K., McPherson C., Petric M. A recombinant baculovirus-expressed S glycoprotein vaccine elicits high titers of SARS-associated coronavirus (SARS-CoV) neutralizing antibodies in mice. Vaccine. 2006;24(April(17)):3624–3631. doi: 10.1016/j.vaccine.2006.01.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.van Oers M.M. Vaccines for viral and parasitic diseases produced with baculovirus vectors. Adv Virus Res. 2006;68:193–253. doi: 10.1016/S0065-3527(06)68006-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Neidhart J., Allen K.O., Barlow D.L., Carpenter M., Shaw D.R., Triozzi P.L. Immunization of colorectal cancer patients with recombinant baculovirus-derived KSA (Ep-CAM) formulated with monophosphoryl lipid A in liposomal emulsion, with and without granulocyte-macrophage colony-stimulating factor. Vaccine. 2004;22(January(5–6)):773–780. doi: 10.1016/j.vaccine.2003.08.021. [DOI] [PubMed] [Google Scholar]
- 30.Lodge P.A., Childs R.A., Monahan S.J., McLean J.G., Sehgal A., Boynton A.L. Expression and purification of prostate-specific membrane antigen in the baculovirus expression system and recognition by prostate-specific membrane antigen-specific T cells. J Immunother. 1999;22(July(4)):346–355. doi: 10.1097/00002371-199907000-00008. [DOI] [PubMed] [Google Scholar]
- 31.Soares M., Hanisch F.G., Finn O.J., Ciborowski P. Recombinant human tumor antigen MUC1 expressed in insect cells: structure and immunogenicity. Protein Expr Purif. 2001;22(June(1)):92–100. doi: 10.1006/prep.2001.1414. [DOI] [PubMed] [Google Scholar]
- 32.Tso C.L., Zisman A., Pantuck A., Calilliw R., Hernandez J.M., Paik S. Induction of G250-targeted and T-cell-mediated antitumor activity against renal cell carcinoma using a chimeric fusion protein consisting of G250 and granulocyte/monocyte-colony stimulating factor. Cancer Res. 2001;61(November(21)):7925–7933. [PubMed] [Google Scholar]
- 33.Ullenhag G.J., Frodin J.E., Jeddi-Tehrani M., Strigard K., Eriksson E., Samanci A. Durable carcinoembryonic antigen (CEA)-specific humoral and cellular immune responses in colorectal carcinoma patients vaccinated with recombinant CEA and granulocyte/macrophage colony-stimulating factor. Clin Cancer Res. 2004;10(May(10)):3273–3281. doi: 10.1158/1078-0432.CCR-03-0706. [DOI] [PubMed] [Google Scholar]
- 34.Hurvitz S.A., Timmerman J.M. Recombinant, tumour-derived idiotype vaccination for indolent B cell non-Hodgkin's lymphomas: a focus on FavId. Expert Opin Biol Ther. 2005;5(June(6)):841–852. doi: 10.1517/14712598.5.6.841. [DOI] [PubMed] [Google Scholar]
- 35.Betting D.J., Kafi K., Abdollahi-Fard A., Hurvitz S.A., Timmerman J.M. Sulfhydryl-based tumor antigen-carrier protein conjugates stimulate superior anti-tumor immunity against B cell lymphomas. J Immunol. 2008;181(September(15)):4131–4140. doi: 10.4049/jimmunol.181.6.4131. [DOI] [PubMed] [Google Scholar]
- 36.Campbell M.J., Carroll W., Kon S., Thielemans K., Rothbard J.B., Levy S. Idiotype vaccination against murine B cell lymphoma. Humoral and cellular responses elicited by tumor-derived immunoglobulin M and its molecular subunits. J Immunol. 1987;139(8):2825–2833. [PubMed] [Google Scholar]
- 37.Deslee G., Charbonnier A.S., Hammad H., Angyalosi G., Tillie-Leblond I., Mantovani A. Involvement of the mannose receptor in the uptake of Der p 1, a major mite allergen, by human dendritic cells. J Allergy Clin Immunol. 2002;110(November(5)):763–770. doi: 10.1067/mai.2002.129121. [DOI] [PubMed] [Google Scholar]
- 38.Ma S., Nashabeh W. Carbohydrate analysis of a chimeric recombinant monoclonal antibody by capillary electrophoresis with laser-induced fluorescence detection. Anal Chem. 1999;71(November(22)):5185–5192. doi: 10.1021/ac990376z. [DOI] [PubMed] [Google Scholar]
- 39.Presicce P., Taddeo A., Conti A., Villa M.L., Della Bella S. Keyhole limpet hemocyanin induces the activation and maturation of human dendritic cells through the involvement of mannose receptor. Mol Immunol. 2008;45(February(4)):1136–1145. doi: 10.1016/j.molimm.2007.07.020. [DOI] [PubMed] [Google Scholar]
- 40.Pietrella D., Corbucci C., Perito S., Bistoni G., Vecchiarelli A. Mannoproteins from Cryptococcus neoformans promote dendritic cell maturation and activation. Infect Immun. 2005;73(February(2)):820–827. doi: 10.1128/IAI.73.2.820-827.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Miller R.A., Maloney D.G., Warnke R., Levy R. Treatment of B-cell lymphoma with monoclonal anti-idiotype antibody. N Engl J Med. 1982;306(9):517–522. doi: 10.1056/NEJM198203043060906. [DOI] [PubMed] [Google Scholar]
- 42.Timmerman J.M., Levy R. Linkage of foreign carrier protein to a self-tumor antigen enhances the immunogenicity of a pulsed dendritic cell vaccine. J Immunol. 2000;164(9):4797–4803. doi: 10.4049/jimmunol.164.9.4797. [DOI] [PubMed] [Google Scholar]
- 43.Weng W.K., Czerwinski D., Timmerman J., Hsu F.J., Levy R. Clinical outcome of lymphoma patients after idiotype vaccination is correlated with humoral immune response and immunoglobulin G Fc receptor genotype. J Clin Oncol. 2004;22(December(23)):4717–4724. doi: 10.1200/JCO.2004.06.003. [DOI] [PubMed] [Google Scholar]
- 44.Zhu D., McCarthy H., Ottensmeier C.H., Johnson P., Hamblin T.J., Stevenson F.K. Acquisition of potential N-glycosylation sites in the immunoglobulin variable region by somatic mutation is a distinctive feature of follicular lymphoma. Blood. 2002;99(April(7)):2562–2568. doi: 10.1182/blood.v99.7.2562. [DOI] [PubMed] [Google Scholar]
- 45.Markl J., Lieb B., Gebauer W., Altenhein B., Meissner U., Harris J.R. Marine tumor vaccine carriers: structure of the molluscan hemocyanins KLH and htH. J Cancer Res Clin Oncol. 2001;127(October(Suppl 2)):R3–R9. doi: 10.1007/BF01470992. [DOI] [PubMed] [Google Scholar]
- 46.Favrille Press Release, May 27, 2008: Favrille announces results from phase 3 registration trial of specified in patients with follicular B-cell non-Hodgkin's lymphoma: results fail to show statistically significant improvement in primary endpoint, time to progression, compared to control [www.favrille.com].
- 47.Levy R, Robertson MJ, Ganjoo K, Leonard JP, Vose J, Denney D. Results of a phase 3 trial evaluating safety and efficacy of specific immunotherapy, recombinant idiotype (Id) conjugated to KLH (Id-KLH) with GM-CSF, compared to non-specific immunotherapy, KLH with GM-CSF, in patients with follicular non-Hodgkin's lymphoma (fNHL). In: Proceedings of the 99th annual meeting of the American Association for Cancer Research. San Diego, CA Philadelphia (PA): AACR; 2008 [abstract LB-204 2008].
- 48.Biovest Press Release, July 21, 2008: Biovest reports results for patients treated with anti-cancer vaccine: BiovaxID(R) demonstrates clinically and statistically significant improvement of disease-free survival in non-Hodgkin's lymphoma in pivotal phase 3 clinical trial [www.vcall.com/IC/GenReleaseasp?ID=132297].


