Abstract
The tobacco-specific nitrosamine 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone (NNK) is a lung carcinogen in rats and may be a cause of lung cancer in smokers. NNK is metabolized by cytochromes P450 to intermediates that react with DNA forming methyl, pyridyloxobutyl (POB), and pyridylhydroxybutyl (PHB) adducts, which are critical in carcinogenesis. The methyl adduct O6-methylguanine (O6-methyl-G) has miscoding properties, but there are no reports on levels of this adduct in rats treated chronically with NNK in the drinking water, nor has its levels been compared with those of POB- and PHB-DNA adducts. We used liquid chromatography-electrospray ionization-tandem mass spectrometry-selected reaction monitoring to quantify O6-methyl-G in lung and liver DNA of rats treated with a carcinogenic dose of 10 ppm of NNK in the drinking water and sacrificed after 1, 2, 5, 10, 16, and 20 weeks. The maximal level of O6-methyl-G in lung DNA, 2550 ± 263 fmol/mg DNA, was reached at 5 weeks and was significantly greater (P < 0.05) at that point than all other adducts (measured previously) except O2-[4-(3-pyridyl)-4-oxobut-1-yl]thymidine. Overall levels of O6-methyl-G in lung were intermediate between those of total POB- and PHB-DNA adducts. In liver, the wave of O6-methyl-G peaked at 2 weeks while that of total POB-DNA adducts peaked at 10 weeks, and levels of total PHB-DNA adducts were low throughout. The results of this study demonstrate that substantial amounts of O6-methyl-G are formed at various time points in lung and liver DNA of rats treated chronically with NNK, supporting its role in carcinogenesis.
Lung cancer is the leading cause of cancer death in the world, killing approximately 3000 people every day (International Agency for Research on Cancer, 2004). Cigarette smoking causes approximately 90% of lung cancer (International Agency for Research on Cancer, 2004). Although there are multiple pulmonary carcinogens in cigarette smoke, one of the most potent in animal models is 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone (NNK) (Hecht, 1998, 2008). NNK induces adenoma and adenocarcinoma of the lung in rats, mice, hamsters, and ferrets, independent of the route of administration (Hecht, 1998). It is particularly effective in the rat, in which chronic treatment with 1 ppm in the drinking water for 2 years caused a significant incidence of lung tumors (Rivenson et al., 1988).
The major mechanism by which NNK initiates the carcinogenic process is cytochrome P450 (P450)-mediated α-hydroxylation to give intermediates 1 and 2 (Fig. 1) (Hecht, 1998). Multiple P450s including rat P450 2A3, human P450 2A13, mouse P450 2A5, and others are involved in this process, which results in the formation of pyridyloxobutyl (POB)-DNA adducts from intermediate 3 and methyl-DNA adducts from intermediate 4 (Jalas et al., 2005; Hecht, 2008). Similar pathways act upon the NNK metabolite NNAL to produce pyridylhydroxybutyl (PHB)-DNA adducts as well as methyl adducts (Upadhyaya et al., 2008). The POB-DNA adducts are O6-[4-(3-pyridyl)-4-oxobut-1-yl]-2′-deoxyguanosine (O6-POB-dG), 7-[4-(3-pyridyl)-4-oxobut-1-yl]-2′-deoxyguanosine (7-POB-dG), O2-[4-(3-pyridyl)-4-oxobut-1-yl]thymidine (O2-POB-T), and O2-[4-(3-pyridyl)-4-oxobut-1-yl]-2′-deoxycytidine (O2-POB-dC). [7-POB-dG and O2-POB-dC have been quantified as the corresponding bases 7-[4-(3-pyridyl)-4-oxobut-1-yl]guanine (7-POB-G) and O2-[4-(3-pyridyl)-4-oxobut-1-yl]cytosine (O2-POB-C).] The PHB-DNA adducts are the corresponding carbonyl-reduced forms, and the methyl-DNA adducts are O6-methylguanine (O6-methyl-G), 7-methyl-G, and O4-methylthymidine (O4-methyl-T). Among these, O6-methyl-G, O4-methyl-T, O6-POB-dG, and O2-POB-T are known to have miscoding properties (Loechler et al., 1984; Altshuler et al., 1996; Delaney and Essigmann, 2001; Pauly et al., 2002; Sharma et al., 2008). Although the DNA pyridyloxobutylation and methylation pathways of NNK metabolic activation have been well characterized for years, only recently has it become possible to quantify individual POB-DNA and PHB-DNA adducts of NNK. In previous studies, we have demonstrated the formation and persistence of individual POB-DNA and PHB-DNA adducts in the lung and liver of rats treated for 20 weeks with 10 ppm NNK in the drinking water (Lao et al., 2007; Upadhyaya et al., 2008). In the study reported here, we quantified O6-methyl-G in the lung and liver of these rats and compared the levels of this mutagenic DNA adduct with those of the POB- and PHB-DNA adducts previously reported. Although O6-methyl-G has been measured previously in tissues of rats treated with NNK by injection (Hecht, 1998), there are no reports in the literature of its levels in tissues of animals treated chronically with NNK in the drinking water.
Fig. 1.
Overview of NNK metabolic activation to POB- and methyl-DNA adducts. NNAL undergoes analogous activation to produce PHB- and methyl-DNA adducts.
Materials and Methods
Chemicals. (Caution: NNK is carcinogenic. It should be handled in a well ventilated hood with extreme care and with personal protective equipment.) O6-Methyl-G was purchased from Midwest Research Institute (Kansas City, MO; purity >98%), and [CD3]O6-methyl-G was obtained from Toronto Research Chemicals (Toronto, Ontario, Canada). Isotopic purity was greater than 99%. A stock solution of [CD3]O6-methyl-G (29.6 fmol/μl) was prepared as an internal standard.
Animal Experiment. Liver and lung tissues analyzed in this study were those produced in a previous study in which rats were treated with NNK (Lao et al., 2007). In brief, the rats were randomly divided into two groups of 54 rats and treated with either NNK (10 ppm in the drinking water) or nothing. Aqueous solutions of the carcinogen were prepared weekly and stored at 4°C, conditions under which NNK is known to be stable. The NNK solution was placed in the plastic water bottles of the rat cages twice weekly. Three rats per group were sacrificed by CO2 overdose at 1, 2, 5, 10, 16, and 20 weeks. Tissues were harvested and stored at –80°C until DNA isolation.
Quantitation of O6-Methyl-G by High-Performance LC-ESI-MS/MS-SRM. Based on recent reports by Sandercock et al. (2008) and Mijal et al. (2004), we quantified O6-methyl-G instead of O6-methyl-dG.
DNA was isolated from the liver and lung of three rats per group as described previously (Lao et al., 2007). In brief, 0.05 to 1 mg of each DNA sample plus [CD3]O6-methyl-G internal standard (20 μl, 592 fmol) was dissolved in 1 ml of HCl (0.1 N) and heated at 80°C for 30 min, cooled, and neutralized with 1 N NaOH to pH 7.0. A portion of the hydrolysate (50 μl) was reserved for the determination of guanine concentration. The remainder was applied to a solid-phase extraction cartridge (Strata-X; Phenomenex, Torrance, CA). The cartridge was sequentially eluted with 1 ml of H2O, 1 ml of methanol (10%), and 2 ml of methanol (100%). The adduct eluted in the 100% methanol fraction, which was collected and concentrated to dryness under reduced pressure using a SpeedVac (Thermo Fisher Scientific, Waltham, MA) with no heating. The resulting sample was dissolved in 20 μl of NH4OAc (2%) for analysis by capillary LC/ESI-MS/MS-SRM on a TSQ Quantum Discovery Max instrument (Thermo Fisher Scientific) in the positive ion mode with N2 as the nebulizing and drying gas. Mass spectrometry (MS) parameters were set as follows: spray voltage, 4 kV; sheath gas pressure, 20; capillary temperature, 250°C; collision energy, 20 V; scan width, 0.7 amu; Q2 gas pressure, 0.7 mTorr; source CID, 5 V; and tube lens offset, 90 V. Tandem mass spectrometry data were acquired and processed by Xcalibur software version 1.4 (Thermo Electron). Eight microliters of the sample was injected on a 250 × 0.5 mm, 4 μm Synergi C18 column (Phenomenex). The column was isocratically eluted with 88% NH4OAc (25 mM) and a 12% mixture of methanol and acetonitrile (75:25) at a flow rate of 12 μl/min. The column was operated at 40°C. The first 5 min of eluant was directed to waste, and the 5 to 22 min fraction was diverted to the electrospray ionization source. The mass transitions (parent to daughter) monitored were 166.09→149.09 for O6-methyl-G and 169.11→152.12 for [CD3]O6-methyl-G. Quantitation was accomplished by comparing the MS peak area ratio of the adduct with that of the deuterated standard with a calibration curve. Calibration standards were prepared by mixing various quantities of the adduct with a constant amount of the internal standard in 2% NH4OAc. These standards were analyzed by LC-ESI-MS/MS-SRM without undergoing the sample preparation described above. Calibration curves were made by plotting the concentration ratio (adduct: internal standard) versus the MS peak area ratio (adduct: internal standard). The amount of G in each sample was determined by high-performance liquid chromatography, and adduct levels (mean ± S.D. of 3 liver or lung DNA samples per group, each analyzed once) were expressed as femtomole per milligram DNA.
Statistical Analyses. Adduct data were obtained from 3 rats at each of 6 time points (1, 2, 5, 10, 16, and 20 weeks). Comparisons between O6-methyl-G and individual adducts were carried out at each time point with the two-sample t test assuming unequal variances. The comparison among data from three studies, O6-methyl-G, total POB-DNA, and total PHB-DNA adducts, involved the two-way analysis of variance with study and time as the two factors. If the interaction term between study and time was significant, then a one-way analysis of variance was carried out at each time point. The Tukey procedure was used to adjust for multiple comparisons between studies. Due to a highly skewed distribution, the adduct levels from each study were analyzed on the natural log scale.
Results
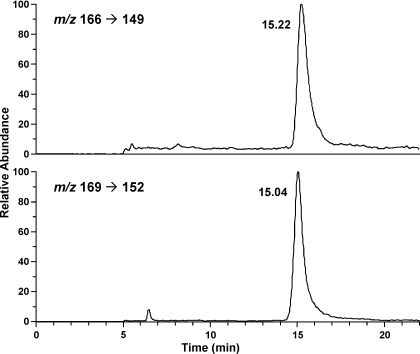
Accuracy was tested by adding various amounts of O6-methyl-G to 1 mg of calf thymus DNA and carrying out the analysis. The results demonstrated excellent agreement between the measured (147, 244, 504, and 1181 fmol) and added (150, 250, 500, and 1250 fmol) amounts. Precision was determined by analyzing 8 aliquots of a sample containing 500 fmol O6-methyl-G/mg calf thymus DNA. The coefficient of variation was 3.1%. The limit of detection was 3.0 fmol/mg DNA. Recoveries averaged 21%. A chromatogram obtained upon analysis of a DNA sample from a rat treated with NNK is illustrated in Fig. 2. A clean peak for O6-methyl-G eluted 0.18 min later than that of the internal standard. O6-Methyl-G was not detected in lung or liver DNA from control rats.
Fig. 2.
Chromatogram obtained upon LC-ESI-MS/MS-SRM analysis of O6-methyl-G in an acid hydrolysate of lung DNA isolated from a rat treated with NNK in the drinking water for 5 weeks. Top, analyte; bottom, internal standard.
Levels of O6-methyl-G in lung and liver DNA of the rats treated with NNK are summarized in Table 1 and compared with levels of the POB-DNA and PHB-DNA adducts of NNK reported previously in these tissues (Lao et al., 2007; Upadhyaya et al., 2008). The maximal level of O6-methyl-G in lung DNA, 2550 ± 263 fmol/mg DNA, was reached at 5 weeks and was significantly greater (P < 0.05) at that point than all other adducts except O2-POB-T. Levels of O6-methyl-G were not significantly different from those of O2-POB-T in weeks 1 to 5, but they were significantly less thereafter (P < 0.05). Levels of O6-methyl-G were not significantly different from those of 7-POB-G and O2-POB-C at most time points other than week 5, whereas they were significantly greater than those of O6-POB-dG and PHB-DNA adducts at most time points (P < 0.05).
TABLE 1.
Comparative DNA adduct levels in lung and liver of F344 rats treated with 10 ppm NNK in the drinking water and sacrificed at various intervalsa,b
| Adduct Levels | ||||||
|---|---|---|---|---|---|---|
| fmol/mg DNA (mean ± S.D.) | ||||||
| Week | 1 | 2 | 5 | 10 | 16 | 20 |
| Lung | ||||||
| O6-Methyl-G | 976 ± 342 | 1020 ± 423 | 2550 ± 263 | 1020 ± 314 | 729 ± 57.5 | 1910 ± 615 |
| O6-POB-dG | 45 ± 7b | 50 ± 5c | 46 ± 13b | 44 ± 14b | 34 ± 17b | 20 ± 5b |
| 7-POB-G | 750 ± 95 | 1180 ± 131 | 1360 ± 214b | 2220 ± 864 | 1700 ± 175b | 1060 ± 169 |
| O2-POB-T | 1080 ± 99 | 2020 ± 150 | 3890 ± 648 | 8260 ± 2730b | 6720 ± 606b | 5070 ± 1060b |
| O2-POB-C | 240 ± 23 | 250 ± 18 | 400 ± 87b | 730 ± 211 | 810 ± 152 | 940 ± 175 |
| O6-PHB-dG | 22 ± 6.4b | 35 ± 10.3d | 28 ± 9.7b | 40 ± 18b | 25 ± 7.5b | 15 ± 1.9b |
| 7-PHB-G | 142 ± 57b | 255 ± 96 | 261 ± 98b | 354 ± 181b | 312 ± 65b | 147 ± 33b |
| O2-PHB-T | 71 ± 13b | 163 ± 69 | 277 ± 60b | 516 ± 255 | 789 ± 303 | 372 ± 95b |
| Week | 1 | 2 | 5 | 10 | 16 | 20 |
| Liver | ||||||
| O6-Methyl-G | 3830 ± 865 | 7120 ± 2080 | 2310 ± 946 | 564 ± 250 | 637 ± 59 | 891 ± 379 |
| 7-POB-G | 490 ± 104b | 880 ± 182b | 1050 ± 90 | 1460 ± 625 | 1170 ± 86b | 730 ± 225 |
| O2-POB-T | 650 ± 121b | 1230 ± 272b | 2190 ± 174 | 3740 ± 1170b | 3540 ± 643b | 2680 ± 643b |
| O2-POB-C | 170 ± 43b | 140 ± 25b | 240 ± 17 | 580 ± 214 | 350 ± 152 | 490 ± 146 |
| 7-PHB-dG | 28 ± 3.6b | 53 ± 23b | 26 ± 12b | 241 ± 337 | ND | ND |
| O2-PHB-T | 33 ± 5b | 92 ± 71b | 38 ± 14b | 407 ± 104 | 431 ± 371 | 454 ± 554 |
ND, not detected.
Data for adducts other than O6-methyl-G are from previous publications (Lao et al., 2007; Upadhyaya et al., 2008).
Significantly different from O6-methyl-G, P < 0.05.
P = 0.058 compared with O6-methyl-G.
P = 0.056 compared with O6-methyl-G.
In liver, the maximal level of 7120 ± 2080 fmol/mg DNA was reached after 2 weeks of treatment with NNK. O6-Methyl-G was the most prevalent adduct during the first 2 weeks of treatment (P < 0.03). Its mean level was similar to that of O2-POB-T at 5 weeks, but thereafter it rapidly declined. O6-POB-dG and O6-PHB-dG were not detected in liver DNA in the previous study.
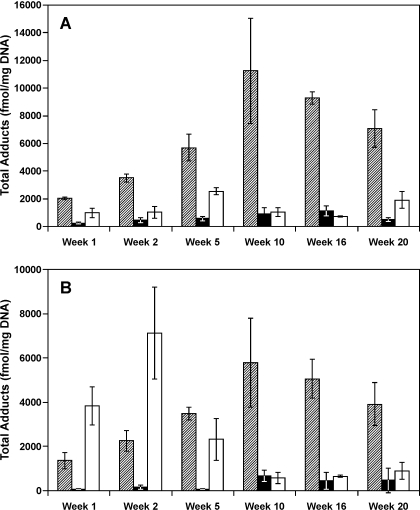
Levels of O6-methyl-G are compared with those of total POB-DNA adducts and PHB-DNA adducts in Fig. 3. The global test for difference in O6-methyl-G, total POB-DNA, and total PHB-DNA adduct levels across all time points was significant (P < 0.001) in both lung and liver. At each time point in lung, levels of O6-methyl-G were significantly lower than those of total POB-DNA adducts (P < 0.05) and greater than or comparable with total PHB-DNA adducts. In liver, the wave of O6-methyl-G peaked at 2 weeks, whereas that of total POB-DNA adducts peaked at 10 weeks, and levels of total PHB-DNA adducts were low throughout.
Fig. 3.
Comparative levels of total POB-DNA adducts
( ), PHB-DNA adducts
(□), and O6-methyl-G (□) in lung (A) and liver (B)
of rats treated with 10 ppm NNK in the drinking water and sacrificed at
various intervals. The data for POB-DNA adducts and PHB-DNA adducts are from
previous publications (Lao et al.,
2007; Upadhyaya et al.,
2008).
), PHB-DNA adducts
(□), and O6-methyl-G (□) in lung (A) and liver (B)
of rats treated with 10 ppm NNK in the drinking water and sacrificed at
various intervals. The data for POB-DNA adducts and PHB-DNA adducts are from
previous publications (Lao et al.,
2007; Upadhyaya et al.,
2008).
Discussion
The results of this study demonstrate that substantial amounts of the promutagenic adduct O6-methyl-G are produced in the lung and liver of rats treated with NNK in the drinking water for 20 weeks. Although overall O6-methyl-G levels were less than those of total POB-DNA adducts in lung, they were comparable with those of O2-POB-T, 7-POB-G, and O2-POB-C at several time points and were greater than those of the other DNA adducts at most time points. In liver, the initial high levels declined rapidly, undoubtedly due to recovery of the O6-alkylguanine-DNA-alkyltransferase (AGT) repair protein. Previous studies have demonstrated that levels of AGT are higher in rat and mouse liver than lung, and that in mice treated with NNK, recovery of AGT occurs more rapidly in liver than in lung (Belinsky et al., 1988; Peterson et al., 2001), which seems to be consistent with our data, because O6-methyl-G levels in liver declined after 2 weeks of NNK treatment, whereas in lung the decline began after 5 weeks. The two waves of DNA adducts in liver (Fig. 3B)—one for O6-methyl-G, peaking at 2 weeks, and the second for total POB-DNA adducts, peaking at 10 weeks—are undoubtedly due to different repair processes, with AGT not being involved in the repair of total POB-DNA adducts other than the relatively minor O6-POB-dG.
Although the critical role of O6-methyl-G in A/J mouse lung tumorigenesis by NNK has been clearly shown in previous studies (Peterson and Hecht, 1991; Peterson et al., 2001), the mechanism in rats may be more complex (Hecht, 1998). Structure-activity studies indicate that both POB-DNA adducts and methyl-DNA adducts are important. Neither N-nitrosodimethylamine, which only methylates DNA, nor N′-nitrosonornicotine, which only pyridyloxobutylates DNA, is as effective as a lung carcinogen as NNK, in direct comparative experiments (Hecht, 1998). A strong correlation of lung tumor incidence and POB-DNA adducts in the target type II cells of the rat lung has been observed over a range of NNK doses, and a similar correlation of O6-methyl-G and tumorigenicity was reported in Clara cells (Belinsky et al., 1990; Staretz et al., 1997). POB-DNA adducts in type II cells and other cell types of the rat lung were diminished by treatment with 2-phenethyl isothiocyanate in tandem with a decrease in lung tumorigenicity (Staretz et al., 1997). Taken together, the presently available data indicate that both DNA pyridyloxobutylation and methylation are important in rat lung tumorigenesis by NNK, and the results of our DNA adduct analysis, carried out at a lung carcinogenic dose of NNK given in the drinking water, support this hypothesis, because substantial amounts of both adduct types are formed. The initially high levels of O6-methyl-G in liver may also have an impact because a significant incidence of liver tumors was observed in rats treated with 5 ppm of NNK for 2 years (Rivenson et al., 1988).
The miscoding properties of O6-methyl-G are well established (Loechler et al., 1984; Delaney and Essigmann, 2001). It stably pairs with thymidine during replication creating an O6-methyl-G-T mismatch leading to G → A transition mutations, which have been observed in the K-ras gene isolated from tumors of mice treated with DNA-methylating agents such as NNK (Belinsky et al., 1989a; Ronai et al., 1993). O4-Methyl-T induces T → C transition mutations, whereas O6-POB-dG causes G → A transition and G → T transversion mutations (Altshuler et al., 1996; Pauly et al., 2002). Multiple mutations due to the presence of O2-POB-T have been reported (Sharma et al., 2008). Mutations have not been reported in K-ras and p53 genes isolated from rat lung tumors induced by NNK, so the relative impact of these miscoding events is unclear (Belinsky et al., 1997).
Levels of O6-methyl-G in lung as measured in this study, peaking at 2550 fmol/mg DNA (Table 1), or approximately 4.2 pmol/μmol G, are quite comparable with those reported previously. Our weekly dose was approximately 3 mg/kg in the drinking water. Belinsky et al. (1990) reported 2.2 pmol/μmol G in lung of rats treated with 3 mg/kg NNK weekly by subcutaneous injection for 4 weeks. Staretz et al. (1997) reported a maximum of approximately 2 pmol/μmol G in lung of rats treated for 8 weeks with 5.28 mg/kg NNK per week by subcutaneous injection. Murphy et al. (1990) reported 1.1 pmol/μmol G in lung of rats treated for 4 days with 0.6 mg/kg/day NNK by i.p. injection.
Levels of O6-methyl-G in lung were considerably higher than those of either O6-POB-dG or O6-PHB-dG. There are limited data in the literature directly comparing in vitro formation of these adducts under comparable conditions and comparative rates of repair, but some studies indicate that alkylation of the O6 position of G is favored in the reaction with methanediazohydroxide (4; Fig. 1) compared with POB-diazohydroxide (3; Fig. 1) (Peterson and Hecht, 1991).
Multiple factors influence levels of O6-methyl-G in the lungs of NNK-treated rats because the different cell types have contrasting activities for NNK metabolic activation, DNA adduct formation, and AGT activity (Belinsky et al., 1988, 1989b, 1990; Staretz et al., 1997). Thus, the results reported here for whole lung are difficult to dissect with respect to these individual components. Nevertheless, the data reported here provide an important comparison of levels of the promutagenic adduct O6-methyl-G with those of the POB- and PHB-DNA adducts formed during chronic treatment with a lung carcinogenic dose of NNK.
Acknowledgments
Mass spectrometric analyses were carried out with the help of Peter W. Villalta in the Analytical Biochem-istry Shared Resource and statistical analyses in the Biostatistics and Informatics Shared Resource of the Masonic Cancer Center, University of Minnesota. We thank Mingyao Wang for helpful discussions.
This work was supported in part by the National Institutes of Health National Cancer Institute [Grants CA81301, CA77598].
Article, publication date, and citation information can be found at http://dmd.aspetjournals.org.
doi:10.1124/dmd.109.027078.
ABBREVIATIONS: NNK, 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone; P450, cytochrome P450; POB, pyridyloxobutyl; NNAL, 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanol; PHB, pyridylhydroxybutyl; O6-POB-dG, O6-[4-(3-pyridyl)-4-oxobut-1-yl]-2′-deoxyguanosine; 7-POB-dG, 7-[4-(3-pyridyl)-4-oxobut-1-yl]-2′-deoxyguanosine; O2-POB-T, O2-[4-(3-pyridyl)-4-oxobut-1-yl]thymidine; O2-POB-dC, O2-[4-(3-pyridyl)-4-oxobut-1-yl]-2′-deoxycytidine; O2-POB-C, O2-[4-(3-pyridyl)-4-oxobut-1-yl]cytosine; 7-POB-G, 7-[4-(3-pyridyl)-4-oxobut-1-yl]guanine; O6-methyl-G, O6-methylguanine; O4-methyl-T, O4-methylthymidine; LC-ESI-MS/MS-SRM, liquid chromatography-electrospray ionization-tandem mass spectrometry-selected reaction monitoring; MS, mass spectrometry; AGT, O6-alkylguanine-DNA-alkyltransferase.
References
- Altshuler KB, Hodes CS, and Essigmann JM (1996) Intrachromosomal probes for mutagenesis by alkylated DNA bases replicated in mammalian cells: a comparison of the mutagenicities of O4-methylthymine and O6-methylguanine in cells with different DNA repair backgrounds. Chem Res Toxicol 9 980–987. [DOI] [PubMed] [Google Scholar]
- Belinsky SA, Devereux TR, Maronpot RR, Stoner GD, and Anderson MW (1989a) The relationship between the formation of promutagenic adducts and the activation of the K-ras proto-oncogene in lung tumors from A/J mice treated with nitrosamines. Cancer Res 49 5305–5311. [PubMed] [Google Scholar]
- Belinsky SA, Dolan ME, White CM, Maronpot RR, Pegg AE, and Anderson MW (1988) Cell specific differences in O6-methylguanine-DNA methyltransferase activity and removal of O6-methylguanine in rat pulmonary cells. Carcinogenesis 9 2053–2058. [DOI] [PubMed] [Google Scholar]
- Belinsky SA, Foley JF, White CM, Anderson MW, and Maronpot RR (1990) Dose-response relationship between O6-methylguanine formation in Clara cells and induction of pulmonary neoplasia in the rat by 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone. Cancer Res 50 3772–3780. [PubMed] [Google Scholar]
- Belinsky SA, Swafford DS, Finch GL, Mitchell CE, Kelly G, Hahn FF, Anderson MW, and Nikula KJ (1997) Alterations in the K-ras and p53 genes in rat lung tumors. Environ Health Perspect 105 (Suppl 4): 901–906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belinsky SA, White CM, Trushin N, and Hecht SS (1989b) Cell specificity for the pulmonary metabolism of tobacco-specific nitrosamines in the Fischer rat. Carcinogenesis 10 2269–2274. [DOI] [PubMed] [Google Scholar]
- Delaney JC and Essigmann JM (2001) Effect of sequence context on O6-methylguanine repair and replication in vivo. Biochemistry 40 14968–14975. [DOI] [PubMed] [Google Scholar]
- Hecht SS (1998) Biochemistry, biology, and carcinogenicity of tobacco-specific N-nitrosamines. Chem Res Toxicol 11 559–603. [DOI] [PubMed] [Google Scholar]
- Hecht SS (2008) Progress and challenges in selected areas of tobacco carcinogenesis. Chem Res Toxicol 21 160–171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- International Agency for Research on Cancer (2004) Tobacco smoke and involuntary smoking, in IARC Monographs on the Evaluation of Carcinogenic Risks to Humans, vol 83, pp 1179–1187, IARC, Lyon. [PMC free article] [PubMed] [Google Scholar]
- Jalas JR, Hecht SS, and Murphy SE (2005) Cytochrome P450 2A enzymes as catalysts of metabolism of 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone (NNK), a tobacco-specific carcinogen. Chem Res Toxicol 18 95–110. [DOI] [PubMed] [Google Scholar]
- Lao Y, Yu N, Kassie F, Villalta PW, and Hecht SS (2007) Formation and accumulation of pyridyloxobutyl DNA adducts in F344 rats chronically treated with 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone and enantiomers of its metabolite, 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanol. Chem Res Toxicol 20 235–245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loechler EL, Green CL, and Essigmann JM (1984) In vivo mutagenesis by O6-methylguanine built into a unique site in a viral genome. Proc Natl Acad Sci U S A 81 6271–6275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mijal RS, Thomson NM, Fleischer NL, Pauly GT, Moschel RC, Kanugula S, Fang Q, Pegg AE, and Peterson LA (2004) The repair of the tobacco specific nitrosamine derived adduct O6-[4-oxo-4-(3-pyridyl)butyl]guanine by O6-alkylguanine-DNA alkyltransferase variants. Chem Res Toxicol 17 424–434. [DOI] [PubMed] [Google Scholar]
- Murphy SE, Palomino A, Hecht SS, and Hoffmann D (1990) Dose-response study of DNA and hemoglobin adduct formation by 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone in F344 rats. Cancer Res 50 5446–5452. [PubMed] [Google Scholar]
- Pauly GT, Peterson LA, and Moschel RC (2002) Mutagenesis by O6-[4-oxo-4-(3-pyridyl)butyl]guanine in Escherichia coli and human cells. Chem Res Toxicol 15 165–169. [DOI] [PubMed] [Google Scholar]
- Peterson LA and Hecht SS (1991) O6-Methylguanine is a critical determinant of 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone tumorigenesis in A/J mouse lung. Cancer Res 51 5557–5564. [PubMed] [Google Scholar]
- Peterson LA, Thomson NM, Crankshaw DL, Donaldson EE, and Kenney PJ (2001) Interactions between methylating and pyridyloxobutylating agents in A/J mouse lungs: implications for 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone-induced lung tumorigenesis. Cancer Res 61 5757–5763. [PubMed] [Google Scholar]
- Rivenson A, Hoffmann D, Prokopczyk B, Amin S, and Hecht SS (1988) Induction of lung and exocrine pancreas tumors in F344 rats by tobacco-specific and Areca-derived N-nitrosamines. Cancer Res 48 6912–6917. [PubMed] [Google Scholar]
- Ronai ZA, Gradia S, Peterson LA, and Hecht SS (1993) G to A transitions and G to T transversions in codon 12 of the Ki-ras oncogene isolated from mouse lung tumors induced by 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone (NNK) and related DNA methylating and pyridyloxobutylating agents. Carcinogenesis 14 2419–2422. [DOI] [PubMed] [Google Scholar]
- Sandercock LE, Hahn JN, Li L, Luchman HA, Giesbrecht JL, Peterson LA, and Jirik FR (2008) Mgmt deficiency alters the in vivo mutational spectrum of tissues exposed to the tobacco carcinogen 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone (NNK). Carcinogenesis 29 866–874. [DOI] [PubMed] [Google Scholar]
- Sharma AK, Gowdahalli K, Krzeminski J, Desai D, Lin J-M, Gowda ASP, Spratt T, and Amin S (2008) Facile syntheses and mutagenicity studies of O2-[4-(3-pryidyl)-4-oxobut-1-yl]thymidine, the major adduct formed by tobacco specific nitrosamine 4-methylnitrosamino-1-(3-pyridyl)-1-butanone (NNK) in vivo, and its site-specifically adducted oligodeoxynucleotide. Proc Am Assoc Cancer Res 49 530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staretz ME, Foiles PG, Miglietta LM, and Hecht SS (1997) Evidence for an important role of DNA pyridyloxobutylation in rat lung carcinogenesis by 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone: effects of dose and phenethyl isothiocyanate. Cancer Res 57 259–266. [PubMed] [Google Scholar]
- Upadhyaya P, Kalscheuer S, Hochalter JB, Villalta PW, and Hecht SS (2008) Quantitation of pyridylhydroxybutyl-DNA adducts in liver and lung of F-344 rats treated with 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone and enantiomers of its metabolite 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanol. Chem Res Toxicol 21 1468–1476. [DOI] [PMC free article] [PubMed] [Google Scholar]