Abstract
Nucleotide excision repair (NER) requires the coordinated sequential assembly and actions of the involved proteins at sites of DNA damage. Following damage recognition, dual incision 5′ to the lesion by ERCC1-XPF and 3′ to the lesion by XPG leads to the removal of a lesion-containing oligonucleotide of about 30 nucleotides. The resulting single-stranded DNA (ssDNA) gap on the undamaged strand is filled in by DNA repair synthesis. Here, we have asked how dual incision and repair synthesis are coordinated in human cells to avoid the exposure of potentially harmful ssDNA intermediates. Using catalytically inactive mutants of ERCC1-XPF and XPG, we show that the 5′ incision by ERCC1-XPF precedes the 3′ incision by XPG and that the initiation of repair synthesis does not require the catalytic activity of XPG. We propose that a defined order of dual incision and repair synthesis exists in human cells in the form of a ‘cut-patch-cut-patch' mechanism. This mechanism may aid the smooth progression through the NER pathway and contribute to genome integrity.
Keywords: DNA repair synthesis, ERCC1-XPF, nucleotide excision repair, xeroderma pigmentosum, XPG
Introduction
Nucleotide excision repair (NER) is a versatile DNA repair pathway that enables cells to eliminate a plethora of helix-distorting lesions caused by different environmental agents. Versatility and specificity in NER are achieved through the sequential and highly coordinated actions of at least 30 polypeptides that detect the lesion and excise a damage-containing oligonucleotide, carry out repair synthesis and ligation events to restore the DNA sequence to its original state (de Laat et al, 1999; Friedberg et al, 2005; Gillet and Schärer, 2006). A subpathway of NER, transcription-coupled NER, preferentially removes damage from the transcribed strand of active genes and is initiated through stalling of an elongating RNA polymerase at DNA lesions (Hanawalt, 2002; Svejstrup, 2002). In bulk DNA, XPC-RAD23B appears to be the initial sensor of DNA damage and is essential for the assembly of all subsequent NER factors in the process known as global genome NER (GG-NER) (Sugasawa et al, 1998, 2001; Volker et al, 2001; Min and Pavletich, 2007). TFIIH, the next factor to be recruited, is responsible for strand separation around the lesion (Evans et al, 1997b; Wakasugi and Sancar, 1998; Tirode et al, 1999), enabling XPA, RPA and XPG to join the complex. ERCC1-XPF is then engaged (Mu et al, 1997; Tapias et al, 2004; Tsodikov et al, 2007) to perform the incision 5′ to the damage (Bardwell et al, 1994; Sijbers et al, 1996), whereas XPG cleaves 3′ to the lesion (O'Donovan et al, 1994). An oligonucleotide of 24–32 nucleotides in length containing the lesion is then released, and the resulting gap is filled by DNA polymerase δ/ɛ (and/or possibly κ (Ogi and Lehmann, 2006)), replication factor C (RFC), PCNA, RPA and the nick is sealed by DNA ligase I or DNA ligase III/XRCC1 (Shivji et al, 1995; Moser et al, 2007) to restore the original DNA sequence. At a higher level of organization, chromatin assembly factor 1 (CAF-1) has been implicated in the restoration of chromatin after the repair reaction (Green and Almouzni, 2003).
Although many recent studies have been concerned with the mechanisms of damage recognition (Schärer, 2007), less is known about the coordination of the two incision and the repair synthesis steps. For repair synthesis to occur, the 5′ incision by ERCC1-XPF is required to generate a free 3′-OH group, the substrate for the DNA polymerase. By contrast, the 3′ incision by XPG may not necessarily be needed to initiate polymerization. If both incisions occurred without any DNA repair synthesis, simple release of the oligonucleotide containing the damaged residue could result in the formation of a single-stranded DNA (ssDNA) gap, another deleterious DNA lesion with a key role in activating DNA damage signalling pathways (Shechter et al, 2004). Furthermore, the occurrence of aberrant DNA breaks is associated with inadvertent NER activity at nondamaged sites in XP/CS cells, underscoring the importance of avoiding the formation of unwanted NER incision reactions (Berneburg et al, 2000; Theron et al, 2005). Therefore, it appears likely that a mechanism ensuring the smooth transition between the dual incision and repair synthesis steps would have evolved.
The similar kinetics of the damage removal and repair synthesis indeed suggests a coordination of these two events (Riedl et al, 2003). Analysis of the literature, however, reveals that there is no consensus concerning the order of the two incisions. Although there is agreement that the 5′ and 3′ incisions are made in a near-synchronous manner (Moggs et al, 1996), both 5′ uncoupled (Matsunaga et al, 1995; Moggs et al, 1996) and 3′ uncoupled (Mu et al, 1996; Evans et al, 1997a, 1997b) incisions have been observed in different experimental contexts in vitro. Using catalytically inactive forms of XPG, it has been shown that the presence of XPG, but not its catalytic activity, is required for the generation of the 5′ incision by ERCC1-XPF (Wakasugi et al, 1997; Constantinou et al, 1999). Another study showed that the efficient 3′ incision by XPG required the presence and catalytic activity of ERCC1-XPF (Tapias et al, 2004).
Here we report the use of catalytically inactive mutants of XPF and XPG to establish the relative temporal order of the two incision reactions and DNA repair synthesis in human cell-free extracts and cells. The results suggest a novel ‘cut-patch-cut-patch' mechanism whereby the dual incision and repair synthesis events of NER are highly coordinated. In turn, this mechanism can explain how the potentially dangerous effects of ssDNA intermediates are minimized or even prevented.
Results
Active site mutants of XPG and XPF do not support dual incision
To try to determine whether there is a strict temporal order to the 5′ and 3′ incisions in human NER, we made use of mutants of ERCC1-XPF and XPG that are catalytically inactive, but retain full DNA binding ability, with the hope of trapping NER intermediates. Three useful active site mutants of XPG have been reported earlier. These D77A, E791A and D812A proteins do not display 3′ nuclease activity and prevent dual incision in NER in vitro but allow 5′ incision by ERCC1-XPF to occur (Wakasugi et al, 1997; Constantinou et al, 1999). We have recently characterized the active site of human XPF and constructed several mutants with severely impaired endonuclease activity that retain full DNA binding activity (Enzlin and Schärer, 2002). Here, we further characterized these mutants to select a catalytically inactive mutant devoid of NER dual incision activity analogous to the previously characterized XPG mutants. Hence, we tested the ability of these purified recombinant proteins, expressed as heterodimers with ERCC1, to restore NER activity in XP-F deficient cell extracts (Moggs et al, 1996).
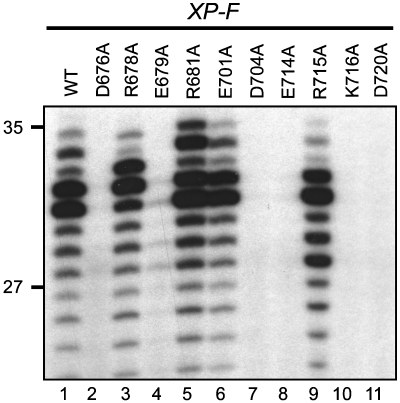
The wild-type, R678A, R681A, E701A and R715A XPF proteins fully restored the excision of a lesion-oligonucleotide from a plasmid containing a site-specific 1,3-intrastrand d(GpTpG) cisplatin DNA crosslink (Figure 1, lanes 1, 3, 5, 6 and 9, respectively), whereas E679A displayed residual activity (Figure 1, lane 4). By contrast, the D676A, D704A, E714A, K716A and D720A XPF mutants (Figure 1, lanes 2, 7, 8, 10 and 11, respectively) failed to restore any NER activity. An earlier study using a fully reconstituted system showed that XPF-D676A was devoid of any NER activity, whereas D720A had some residual activity (Tapias et al, 2004).
Figure 1.
XPF active site mutants deficient in NER in vitro. Cell extracts prepared from XPF-deficient XP2YO cells were incubated with a plasmid containing a single 1,3 cisplatin intrastrand crosslink in the presence of 200 fmol purified recombinant wild-type ERCC1-XPF (lane 1) or ERCC1-XPF proteins with different point mutations in XPF (lanes 2–11). The excision products containing the cisplatin adduct were labelled by the annealing of an oligonucleotide complementary to the excised oligonucleotides with a G4 overhang and filling in with Sequenase 2.0 and [α-32P] dCTP. The products were separated on a 14% denaturing polyacrylamide gel and visualized by autoradiography. The positions of size markers are indicated on the left.
Efficient 3′ incision by XPG is dependent on prior 5′ incision by ERCC1-XPF
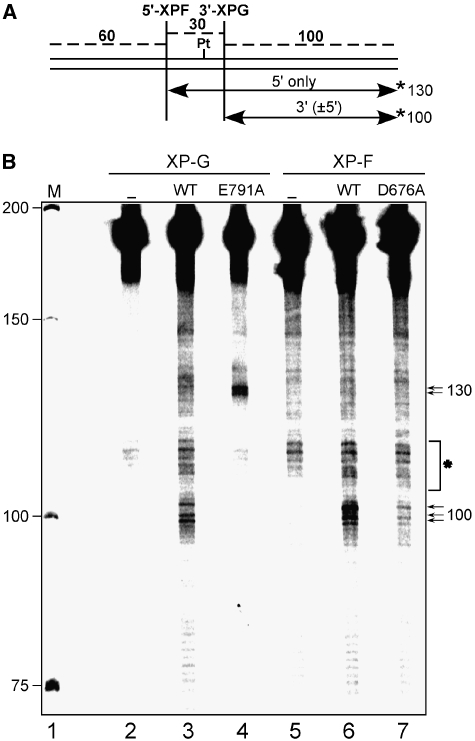
We used ERCC1-XPF-D676A and XPG-E791A for further studies to discern any possible interdependency of the 5′ and 3′ incision steps. Covalently closed circular DNA containing a single 1,3-intrastrand cisplatin DNA crosslink was incubated with an XPF- or XPG-deficient cell-free extract complemented with wild-type or nuclease-deficient ERCC1-XPF and XPG proteins. The reaction products were purified and cleaved with BssHII to excise a 190-bp fragment from the plasmid DNA. Incision products were detected by annealing an oligonucleotide complementary to the BssHII incision site 3′ to the lesion followed by a fill-in reaction to generate a fragment of approximately 130 nt for the 5′ uncoupled incision by XPF and a 100-nt fragment for incision by XPG, in the presence or absence of the 5′ incision (Figure 2A).
Figure 2.
Efficient XPG cleavage is dependent on the catalytic activity of XPF. (A) Schematic representation of a 190-bp BssHII fragment with a single defined cisplatin lesion. Incision sites by ERCC1-XPF and XPG are indicated. Incisions were detected using fill-in reactions with Sequenase 2.0 and [α-32P] dCTP by annealing an oligonucleotide complementary to a BssHII cleavage site containing a G4 overhang allowing for the visualization of the 130 and 100 mer products for 5′ and 3′ incision, respectively. Possible excision products are indicated by arrows and the position of the [α-32P] label is indicated with an asterisk. (B) cccDNA with a single defined cisplatin lesion was incubated with cell extracts lacking XPG- (XPCS1RO, lanes 2–4) or XPF-deficient (XP2YO, lanes 5–7), either alone (lanes 2 and 5) or complemented with wild-type XPG (lane 3), XPG E791A (lane 4), wild-type XPF (lane 6), XPF D676A (lane 7), purified, digested with BssHII, radioactively labelled and analysed on a denaturing PAGE gel. The positions of size markers are indicated on the left, and the position of the reaction products on the right of the gel. Unspecific bands present in all the lanes are marked with an asterisk.
Incubation of the plasmid with XP-G or XP-F cell extract did not yield any incision products (Figure 2B, lanes 2 and 5, respectively). When the extracts were complemented with wild-type XPG or XPF proteins (lanes 3 and 6, respectively), products specific for 3′ incision by XPG (three bands around 100 nt) were visible. Addition of XPF-D676A to the XP-F cell extract only yielded marginal amounts of 3′ incision products (lane 7), suggesting that 3′ uncoupled incision by XPG does not occur efficiently in the presence of catalytically inactive XPF. By contrast, addition of XPG-E791A to the XP-G cell extract resulted in the appearance of two intense specific bands (of around 130 nt in length) corresponding to the product of XPF 5′-uncoupled incision (lane 4). These results indicate that efficient 3′ incision by XPG is dependent on the presence and catalytic activity of ERCC1-XPF, whereas the presence, but not the catalytic activity of XPG is required for the 5′ incision by ERCC1-XPF. Hence, the 5′ incision might precede the 3′ incision.
DNA Repair Synthesis can be initiated in vitro without the 3′ incision by XPG
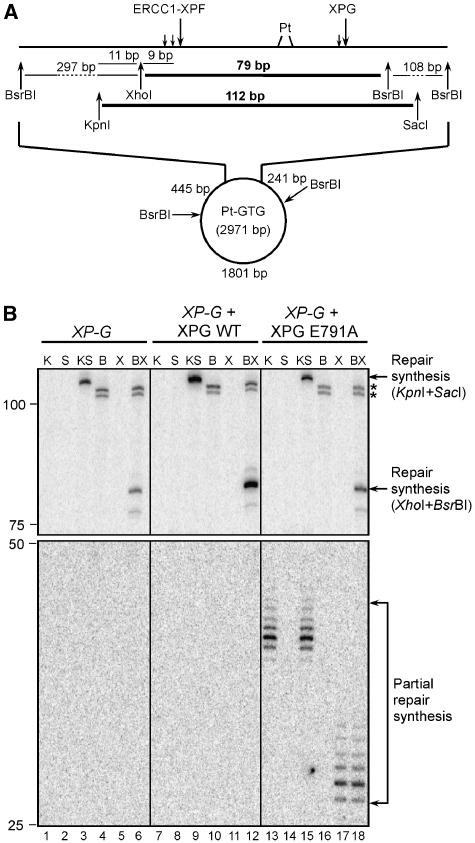
To determine whether both 5′ and 3′ incisions need to occur before DNA repair synthesis can be initiated, we investigated the nature of the repair synthesis products in XP-G cell extracts complemented with wild-type XPG and XPG-E791A, by incubating a plasmid containing a single defined cisplatin lesion in the extracts together with [α-32P]-dCTP and [α-32P]-TTP. After the reaction, DNA was purified and digested with KpnI and/or SacI or XhoI and/or BsrBI (Figure 3A).
Figure 3.
XPG E791A supports partial DNA repair synthesis in vitro. (A) Schematic representation of the lesion-containing plasmid used in the assay. Incision sites by ERCC1-XPF and XPG and the sizes of restriction fragments are indicated. The fragments containing the repair synthesis products are shown in bold (79 and 112 bp). (B) cccDNA with a single defined cisplatin lesion was incubated with a XP-G cell extract, either alone (lanes 1–6) or in the presence of 600 fmol of wild-type XPG (lanes 7–12) or XPG E791A (lanes 13–18) as well as 10 μCi of [α-32P]dCTP and 10 μCi [α-32P]TTP. DNA was further purified, digested with KpnI (lanes 1, 7, 13), SacI (lanes 2, 8, 14), KpnI+SacI (lanes 3, 9, 15), BsrBI (lanes 4, 10, 16), XhoI (lanes 5, 11, 17), BsrBI+XhoI (lanes 6, 12, 18) and analysed on a denaturing PAGE gel. The positions of size markers are indicated on the left, the nature of the observed products on the right of the gel. Two unspecific bands are marked with an asterisk. Abbreviations: K, KpnI; S, SacI; KS, KpnI+SacI; B, BsrBIl X, XhoI; BX, BsrBI+XhoI.
When DNA was incubated with the XP-G cell extract alone, products of nonspecific DNA synthesis were observed with signal intensities roughly proportional to the length of the DNA fragments (Figure 3B, lanes 1–6). These signals are likely due to random nicks produced by topoisomerases and/or nucleases present in the cell-free extracts (Hansson et al, 1989). Addition of wild-type XPG to the mixture led to a significant increase in the intensity of the bands of 112 nt (KpnI and SacI) and 79 nt (BsrBI and XhoI), corresponding to newly synthesized and ligated DNA at the site of the cisplatin lesion (lanes 9 and 12, respectively). Note that the 79 nt signal of the BsrBI/XhoI digestion in lane 12 is much more intense than the nonspecific signal at 108 nt, strongly suggesting that the 79-bp band results from XPG-induced repair synthesis. The specificity of the signal for repair synthesis was further supported by the observation that no increase of the specific band at 79 bp was seen if the reaction was carried out with the parental nondamaged plasmid (data not shown). Addition of nuclease deficient XPG-E791A, which permits incision by ERCC1-XPF, to the XP-G cell extracts, did not result in a change in the intensity of the full-length repair synthesis products of 112 and 79 bp. However, two new products with the most intensive bands of 39 nt (KpnI and SacI) and 28 nt (BsrBI and XhoI) were visible after a longer exposure of the gel (lanes 15 and 18, respectively). These bands were also present after the cleavage with only one restriction enzyme 5′ to the damaged site (lanes 13 and 17), while no products were visible after cutting with restriction enzymes 3′ to the incision sites (lanes 14 and 16). The appearance of these bands is, therefore, consistent with their being partial repair synthesis products, in which the polymerase extended the 3′-OH group generated by ERCC1-XPF about 18–20 nt in the absence of XPG incision. These observations indicate that initiation of repair synthesis is dependent on cleavage by ERCC1-XPF and that it can occur before 3′ cleavage by XPG. No partial repair synthesis products were observed when an extract made from XP-F cells was complemented with wild-type XPF or XPF-D676A (data not shown). These results demonstrate that repair synthesis can be initiated in vitro before the 3′ incision by XPG.
Catalytically inactive XPF persists at sites of UV damage
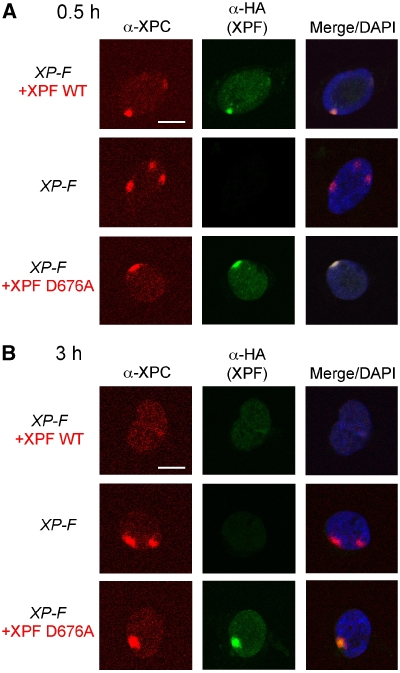
Having observed partial repair synthesis without XPG cleavage in vitro, we wished to test whether repair synthesis could also be initiated in vivo before 3′ incision by XPG. For repair synthesis to take place, the DNA replication machinery has to be recruited to the sites of DNA damage. Therefore, we examined the localization of PCNA, a component of the DNA replication machinery, after local UV irradiation of a XP-G cell line expressing wild-type XPG or XPG-E791A and a XP-F cell line expressing wild-type XPF or XPF-D676A. We have described earlier the generation and characterization of XP-G cell lines expressing wild-type XPG and XPG-E791A using lentiviral transduction. Both XPG proteins were localized to UV-damaged spots in cell nuclei shortly after damage infliction, but only XPG-E791A persisted in these spots, suggesting that completion of NER is required for the dissociation of XPG (Thorel et al, 2004). XP-F cell lines expressing wild-type XPF and XPF-D676A were generated in an analogous fashion by transducing XPF-deficient XP2YO cells with lentiviral recombinants encoding HA-tagged wild-type XPF and XPF-D676A. The resulting cells were subjected to local UV irradiation and the recruitment of XPC, the initial damage recognition factor, and XPF to sites of UV damage was analysed (Volker et al, 2001).
At 0.5 h after irradiation, we observed colocalization of XPC with both HA-tagged wild-type XPF and XPF-D676A (Figure 4A). At 3 h after local UV irradiation, XPC and XPF were no longer present at the damaged sites in the cells expressing wild-type XPF. However, in the mutant XPF transductants, XPC remained colocalized with the catalytically deficient XPF-D676A (Figure 4B). Hence, cleavage by ERCC1-XPF and XPG is needed for both nucleases to dissociate from the damaged site and for the completion of NER.
Figure 4.
Recruitment of XPF to sites of local UV damage in different XP-F cell lines. XP2YO (XP-F) cells, untransduced or transduced with XPF-WT or XPF-D676A, were grown on coverslips and locally irradiated with a UV dose of 150 J/m2 through filters with 5 μm pores and fixed 0.5 h (A) or 3 h (B) after irradiation. The cells were immunolabelled with antibodies against XPC (red) or the HA tag present on the C-terminus of XPF (green). Merged images indicate the overlay of XPC, XPF and DAPI staining. Scale bars, 10 μm.
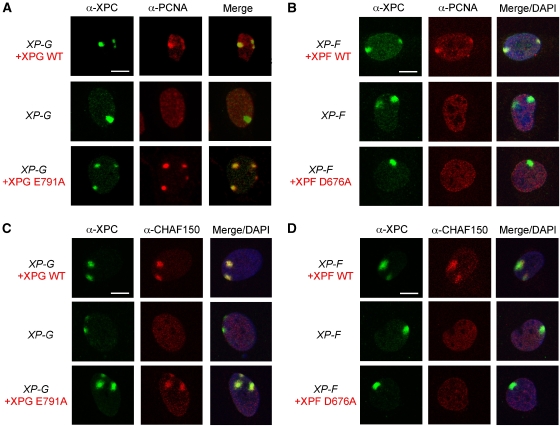
Recruitment of PCNA, and CAF-1 to sites of local UV damage depends on the presence, but not the catalytic activity of XPG
Using the transduced XP-G and XP-F cell lines, we studied how the catalytic activity of the two nucleases correlates with the recruitment of repair synthesis factors to NER sites. It has been shown before that recruitment of PCNA to the sites of local UV damage is severely affected in XPG-deficient cells (Essers et al, 2005). In agreement with this report, we did not observe any colocalization of PCNA with XPC in XP-G cells 0.5 h after UV irradiation (Figure 5A, middle row). However, as expected, XPC and PCNA colocalized at sites of UV damage in XP-G cells expressing wild-type XPG (Figure 5A, top row). PCNA also colocalized with XPC in the XPG-E791A transductants (Figure 5A, bottom row). In principle, the recruitment of PCNA to sites of UV damage in the presence of catalytically inactive XPG could reflect partial DNA repair synthesis or be due to the recruitment of PCNA before incision, possibly by direct interaction with XPG (Gary et al, 1997). To distinguish between these possibilities, we investigated the recruitment of PCNA in various XP-F cells. Although PCNA was found at sites of UV damage in XP-F cells expressing wild-type XPF, PCNA did not colocalize with XPC in untransduced XP-F cells or in the XPF-D676A transductants (Figure 5B). These observations demonstrate that the recruitment of PCNA to sites of UV damage is dependent on the catalytic activity of XPF and, therefore, likely on active DNA repair synthesis. Consistent with this notion, the presence of Polδ at sites of UV damage also required catalytically active XPF (Supplementary Figure 1).
Figure 5.
XPF- and XPG-dependent colocalization of PCNA and CAF-1 with XPC. XPCS1RO (XP-G) cells, untransduced or transduced with XPG-WT or XPG-E791A (A, C) and XP2YO (XP-F) cells, untransduced or transduced with XPF-WT or XPF-D676A (B, D), were grown on coverslips and locally irradiated with a UV dose of 150 J/m2 through filters with 5 μm pores and fixed 0.5 h after irradiation. The cells were immunolabelled with antibodies against XPC (green), PCNA (red) and CHAF150, the largest subunit of CAF-1 (red). Merged images indicate the overlay of XPC, PCNA or CHAF150 and DAPI staining. Scale bars, 10 μm.
Having established that the recruitment of the replication machinery required the catalytic activity of XPF, but not that of XPG, we asked whether factors acting even later in NER could be recruited to sites of UV damage in the absence of XPG incision. We examined the recruitment of CHAF150, a subunit of the CAF-1 that is involved in the restoration of chromatin after an NER reaction (Green and Almouzni, 2003). CHAF150 behaved like PCNA in that its recruitment was dependent on the catalytic activity of XPF, but not on that of XPG (Figure 5D and C). These results are consistent with an earlier observation that CAF-1 is recruited to the sites of DNA damage in a PCNA-dependent manner (Green and Almouzni, 2003). The important novel conclusion is that even factors acting downstream of DNA repair synthesis can be recruited to sites of UV damage before the second incision 3′ to the lesion has occurred.
Partial unscheduled DNA synthesis occurs in the absence of 3′ incision by XPG
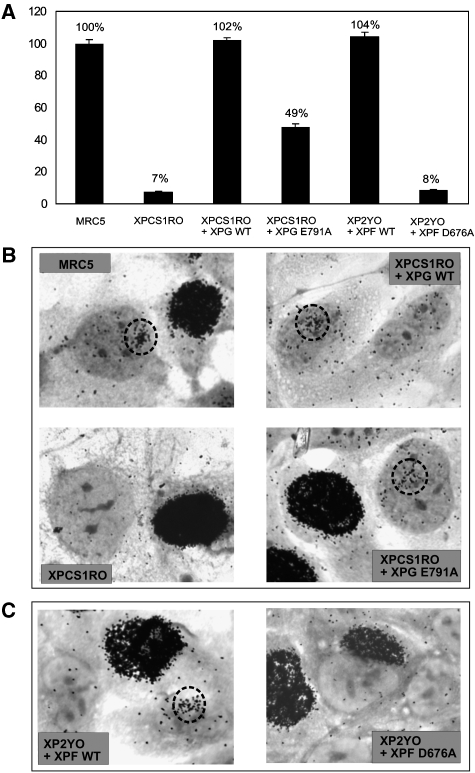
To test whether loading of PCNA to NER sites is able to stimulate DNA repair synthesis in the absence of 3′ incision in vivo, we examined DNA repair by unscheduled DNA synthesis (UDS) after UV irradiation of XP-G transductants expressing wild-type XPG or XPG-E791A. Wild-type XPG complemented the severe UDS defect of untransduced XP-G cells (102 versus 7%, with NER-proficient cells assayed in parallel set at 100%, Figure 6A). In line with the observed partial DNA repair synthesis in vitro and recruitment of PCNA, the very low UDS level of untransduced XP-G cells was significantly increased upon expression of the catalytically inactive XPG (from 7 to 49% UDS, Figure 6A). To try to ensure that this UV-induced DNA synthesis occurs at sites of NER rather than being a nonspecific artifact, we monitored repair synthesis at locally UV-damaged areas (Figure 6B). Although quantification is difficult, significant numbers of autoradiographic grains were found clustered together over non-S phase nuclei in NER-proficient cells and XP-G transductants expressing wild-type XPG (Figure 6B, upper panels). Very few grains were found over comparable nuclear areas of untransduced XP-G cells but the nuclei of the XPG-E791A transductants exhibited a significant amount of grain clustering, roughly mid-way between the wild-type and XP-G levels (Figure 6B, lower panels). In contrast, the levels of UDS in XP-F cells expressing XPF-D676A (8% of wild type, Figure 6A) was at the same background level as untransduced XP-F cells, whereas UDS was restored to normal levels in cells expressing wild-type XPF (104%, Figure 6A). Local UDS experiments confirmed these findings; although UDS sites were clearly observed for XP-F transductants expressing wild-type XPF, they were not evident in XPF-D676A transductants (Figure 6C). Together, these results strongly suggest that the observed UDS is linked to sites of local UV damage, and that partial repair synthesis can occur in living cells in the absence of the catalytic activity of XPG, whereas it does require the catalytic activity of XPF.
Figure 6.
UDS in XP-F and XP-G cells transduced with wild-type and mutant XPF and XPG, respectively. (A). DNA repair synthesis or UV-induced UDS levels of different, as indicated, XP-F and XP-G cells, expressed as percentage of the UDS of an NER-proficient cell line (MRC5) assayed in parallel. (B, C) UDS in cells locally irradiated through a 5-μm microporous filter (60 J/m2), NER-proficient MRC, XPCS1RO, XPCS1RO transduced with wild-type XPG and XPG-E791A (B) and XP2YO transduced with wild-type XPF or XPF-D676A (C). Heavily labelled cells were those in S-phase, incorporating large amounts of tritiated thymidine by replicative DNA synthesis. Dotted circles indicate the position of pores in which UV damage has been induced and UDS has been observed.
Discussion
An unresolved longstanding issue for human NER is whether the 5′ and 3′ incisions, by ERCC1-XPF and XPG, respectively, occur in a strict temporal order and, if so, which one occurs first. A related issue is how these incisions are coordinated with DNA repair synthesis to prevent the exposure of potentially extremely damaging ssDNA gaps. We believe that the following observations described here provide novel and important insight into these issues.
First, the 5′ incision by ERCC1-XPF depends on the presence, but not catalytic activity, of XPG whereas efficient 3′ incision by XPG requires the catalytic activity of ERCC1-XPF (Figure 2), extending previous findings (Wakasugi et al, 1997; Constantinou et al, 1999; Tapias et al, 2004). Second, and most important, partial DNA synthesis is detectable in vitro in the presence of catalytically inactive XPG (Figure 3), thereby demonstrating that the incision 5′ to the lesion by ERCC1-XPF is both necessary and sufficient for the initiation of repair synthesis, whereas 3′ incision by XPG is needed for the completion, but not the initiation of repair synthesis. Third, some late NER factors, including the replication factors PCNA and Polymerase δ as well as the CAF-1, are recruited to sites of local UV damage in cells expressing catalytically inactive XPG, but not in cells expressing catalytically inactive XPF (Figure 5). Fourth, cells expressing catalytically inactive XPG, but not those expressing catalytically inactive XPF, are capable of undergoing intermediate levels of unscheduled DNA repair synthesis (Figure 6).
A defined temporal order for human NER incision and DNA repair synthesis events
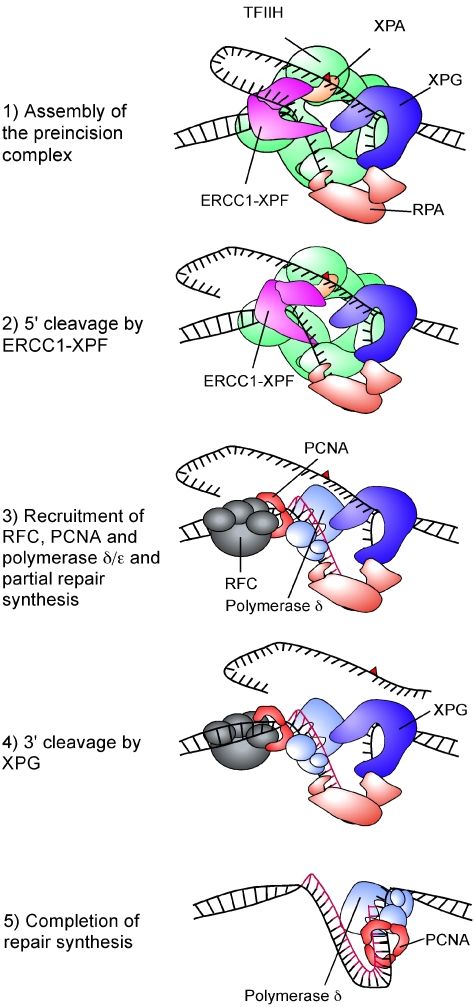
Based on these observations we suggest that the human NER pathway does indeed have a defined temporal order for the 5′ and 3′ incisions and for DNA repair synthesis. Specifically, we propose the following model for the coordination of the dual incision and repair synthesis steps (Figure 7). After assembly of all the factors of the preincision complex, 5′ cleavage by ERCC1-XPF takes place, generating a free 3′-OH group. The repair synthesis machinery consisting minimally of polymerase δ, the clamp loader RFC and the processivity factor PCNA are recruited and repair synthesis is initiated. Which factors and interactions may facilitate this recruitment remains to be established, but it is possible that RPA has an important role in this transition (Riedl et al, 2003). DNA synthesis is then initiated and proceeds about half way through the repair patch. The stalling of the polymerase at this point might trigger the XPG endonuclease activity, allowing the repair synthesis to be completed. We have shown earlier that XPG has distinct requirements for binding and cleaving DNA (Hohl et al, 2003) raising the possibility that a conformational change in the NER complex brought about by the polymerase activity triggers the catalytic activity of XPG.
Figure 7.
Model for the coordination of dual incision and repair synthesis steps in NER. Schematic representation of the proposed sequence of events following the assembly of the preincision complex. The red triangle stands for the DNA lesion. Individual proteins involved in each step are indicated.
Due to the near simultaneous occurrence of the two incision reactions, we have not yet been able to prove that this reaction sequence is also favoured in a situation where wild-type XPF and XPG proteins are present. However, indirect support for our model comes from a recent study that considered the sequence of arrival and release of various NER factors during the dual incision and repair synthesis steps in NER using a fully reconstituted system (Mocquet et al, 2008). This study showed that ERCC1-XPF is released from repair complexes with the arrival of RFC, whereas XPG (and RPA) are only fully displaced once the entire repair synthesis machinery (RFC, PCNA and Polδ) has been recruited.
One prediction of our model is that the addition of DNA polymerase inhibitors might inhibit 3′ incision by XPG. We observed that addition of the Polδ inhibitor aphidicolin to an in vitro NER reaction did not show a significant inhibition of the 5′ or 3′ incision (data not shown), consistent with an earlier study (Moggs et al, 1996). Although further studies will be necessary to fully delineate the biochemical relationship of repair synthesis and the 3′ incision by XPG, it is interesting to note that a recent study found that treatment of cells with HU and AraC inhibited the removal of 6-4PPs (Moser et al, 2007). Although the molecular basis for this observation is currently unknown, it is consistent with our model and the notion that inhibition of repair synthesis blocks at least one of the incisions (presumably by XPG).
The model proposed here does not exclude an involvement of additional protein-protein interactions or protein modifications in the various steps. For example, it is known that XPG has a PIP box and that it can interact with PCNA (Gary et al, 1997). Although it has not yet been shown convincingly that the interaction between PCNA and XPG is required for NER, an interaction between the two proteins may contribute to the activation of the XPG incision. A recent study has implicated polκ in the repair synthesis step of NER (Ogi and Lehmann, 2006). It is possible that two polymerases, polδ and polκ, act at different steps of repair synthesis, for example before and after incision by XPG has taken place. Further studies will be required to determine how the various phases of repair synthesis in NER are regulated by protein-protein interactions and possibly post-translational modifications.
Apparent contradictions
One set of experimental observations appears to be in contrast with our results. Under very similar experimental conditions and using a variety of substrates either 5′ uncoupled (Matsunaga et al, 1995; Moggs et al, 1996) or 3′ uncoupled (Matsunaga et al, 1995; Mu et al, 1996; Evans et al, 1997a, 1997b) incisions have been observed. Our model would predict that 3′ uncoupled incision by XPG should not occur at any appreciable frequency. One possible explanation is that ERCC1-XPF and XPG, can incise NER intermediates under certain conditions in vitro that would be disfavoured in vivo. It is known, for example, that the intrinsic structure-specific endonuclease activity of both ERCC1-XPF and XPG can be readily detected in vitro without the need for additional proteins (O'Donovan et al, 1994; Sijbers et al, 1996). Similarly, high relative concentration of XPG or the absence of ERCC1-XPF in a cell extract or reconstituted system may allow incision by XPG 3′ to the lesion in the absence of a properly assembled NER complex. We have shown earlier that XPG has distinct requirements for binding and cleaving DNA substrates and that the XPG spacer region has a critical role in mediating this substrate preference (Hohl et al, 2003, 2007; Dunand-Sauthier et al, 2005). We suggest that XPG is present in a catalytically inactive conformation before 5′ incision and partial repair synthesis and that its catalytic activity is revealed by a change in the complex brought about by partial repair synthesis. The barrier for XPG to cleave certain substrates does not occur at the level of substrate binding, but likely involves a subsequent rearrangement of an XPG-substrate complex (Hohl et al, 2003). We propose that a lowering of this activation barrier under certain experimental conditions leads to 3′ uncoupled incisions.
A ‘cut-patch-cut-patch' mechanism
Early investigations of excision repair in bacteria focused on the discrimination between two models: ‘patch and cut', involving a first incision close to the damage, followed by repair synthesis and second incision, and ‘cut and patch', invoking excision of the damaged base/s before repair synthesis (Hanawalt, 1966; Hanawalt and Haynes, 1967). Subsequently, in particular with the ability to reconstitute NER in vitro, and the ability to observe dual incision on NER substrates in the absence of repair synthesis, the ‘cut and patch' model became accepted as the way by which NER operates (Aboussekhra et al, 1995; Mu et al, 1995; Moggs et al, 1996; Araujo et al, 2000).
The present work suggests that the human NER machinery operates via a ‘cut-patch-cut-patch' mechanism that includes features of both previous models. Interestingly, long-patch base excision repair (BER) has been shown to proceed in a similar way. In long-patch BER, polymerase δ/ɛ, supported by the replication accessory factors RFC and PCNA or polymerase β, carries out repair synthesis past the abasic site and introduces 2–6 nucleotides. The short oligonucleotide overhang generated in this way is excised by the Flap endonuclease FEN-1, and the nick is sealed by DNA ligase I (Matsumoto et al, 1999; Pascucci et al, 1999). In line with this model, it was demonstrated that PCNA facilitates excision in long-patch BER (Gary et al, 1999). Stimulation of the dual incision by PCNA has also been observed in NER, leading to the proposal that PCNA may promote the turnover of the early NER factors (Nichols and Sancar, 1992) linking the excision and repair synthesis steps. Although many aspects of these important DNA repair processes remain to be discovered, it is interesting and unexpected that both NER and long-patch BER have operational similarities.
Materials and methods
Protein purification
Wild-type XPF, XPF D676A, XPF R678A, XPF E679A, XPF 681A, XPF E701A, XPF D704A, XPF E714A, XPF R715A, XPF K716A, XPF D720A, wild-type XPG and XPG E791A proteins were expressed in Sf9 insect cells and purified, as described earlier (Enzlin and Schärer, 2002; Hohl et al, 2003). The purity of the enzyme preparations was very similar to the ones reported earlier and 0.2–0.5 mg of proteins were obtained at concentrations of 0.2–0.3 mg/ml.
In vitro NER dual incision assay
Covalently closed circular DNA (pBluescript) containing a single 1,3-intrastrand d(GpTpG) cisplatin-DNA crosslink was prepared, as described earlier (Moggs et al, 1996) and additionally purified over two consecutive sucrose gradients. Reactions were carried out in a buffer containing 40 mM HEPES-KOH (pH 7.8), 70 mM KCl, 5 mM MgCl2, 0.5 mM DTT, 2 mM ATP, 0.36 mg/ml BSA, 22 mM phosphocreatine (di-Tris salt) and 50 ng/μl creatine phosphokinase. Each reaction contained 200 ng DNA and 30 μg of cell-free extract prepared from XPG- or XPF-deficient fibroblast cells (XPCS1RO and XP2YO, respectively). Complementation was assayed upon addition of 730 fmol wild-type or mutant protein (XPG or XPF). Reactions were incubated at 30°C for 45 min. 50 nM of an oligonucleotide complementary to the excision product with a G4 5′-overhang (5′-GGGGGAAGAGTGCACAGAAGAAGACCTGGTCGACC) was added, followed by heat inactivation at 95°C for 5 min. For detection of individual incisions, the incision reaction was inactivated by addition of 1 M EDTA, pH 8.0, 3% SDS and 12 μg proteinase K. DNA was extracted with phenol:chloroform and ethanol precipitated, as described earlier (Shivji et al, 1999), then 50 nM of an oligonucleotide complementary to the BssHII restriction site with a G5 5′-overhang was added (5′–GGGGGCAATTAACCCTCACTAAAGGGAACAAAAGCTGG) followed by heat inactivation at 95°C for 5 min. After cooling down the reactions for 15 min at room temperature, 0.5 units of Sequenase and 3.5 μCi of [α-32P]-dCTP (both from Amersham-Pharmacia, diluted in Sequenase dilution buffer) were added. Reactions were incubated for 3 min at 37°C prior the addition of 1.2 μl dNTP mix (100 μM of each dATP, dGTP, TTP and 50 μM dCTP) and incubation for another 12 min at 37°C. This fill-in reaction labelled the product using the G4 overhang provided by the respective complementary oligonucleotides as a template. Reactions were stopped by addition of formamide loading buffer, heated at 95°C for 5 min and analysed on a 12 or 8% denaturing polyacrylamide sequencing gel. The gel was exposed on a phosphor screen and scanned on a PhosphorImager.
In vitro NER repair synthesis assay
The assay was performed using the same substrate, cell extracts and proteins as described for the excision assay. The reaction mixtures additionally contained 10 μM dATP, 10 μM dGTP, 5 μM dCTP, 5 μM TTP, 10 μCi of [α-32P]-dCTP and 10 μCi [α-32P]-TTP. Complementation was assayed upon addition of 600 fmol of wild-type or mutant protein (XPG or XPF). Reactions were incubated at 30°C for 3 h. DNA was purified using MinElute PCR Purification Kit (Qiagen), cleaved with KpnI and SacI or BsrBI and XhoI and analysed on a 10% denaturing polyacrylamide sequencing gel.
Cell culture conditions and preparation of whole cell extracts
For the generation of whole cell extracts, SV40-transformed fibroblast cells XPCS1RO (XPG-deficient (Ellison et al, 1998) and XP2YO (XPF-deficient, GM08437) were cultured in Dulbecco's Modified Eagle's Medium (DMEM, Invitrogen) supplemented with 10% fetal calf serum and 2 mM L-glutamine, 100 U/ml penicillin, and 0.1 mg/ml streptomycin at 37°C in the presence of 5% CO2. Cells were grown to near confluency, and whole cell extract was prepared accordingly to a published procedure (Biggerstaff and Wood, 1999).
Cell transduction with lentiviral recombinants
XPG wild-type cDNA, XPG-E791A cDNA were cloned into the pLOX/EWGFP lentiviral vector and XPF wild type (with a C-terminal HA tag) and XPF-D676A (with a C-terminal HA tag) cDNAs were cloned into the pWPXL lentiviral vector by replacing the GFP cDNA. Lentiviruses containing the different constructs under the control of the EF1α promoter were produced by co-transfecting 293T cells with the following three plasmids: the packaging plasmid pMD2G, the envelop plasmid psPAX2 and the lentiviral vector containing the different XPG and XPF cDNAs. Details of the vectors and protocols are described on the following Web site http://www.lentiweb.com. XP-G/CS (94RD27, patient XPCS1RO) and XP-F (XP2YO) SV40 immortalized fibroblasts at 50% confluency were infected with viral particles containing the different XPG and XPF recombinants. Transduced cells were then cultured in DMEM supplemented with 10% FCS, 2 mM L-glutamine, 100 U/ml penicillin and 0.1 mg/ml streptomycin in a 5% CO2 humidified incubator. The transduction efficiency was further assessed by immunofluorescence.
Local UV irradiation and immunofluorescence
Local DNA damage infliction within cultured cells was performed, as described earlier (Mone et al, 2001). Briefly, cells cultured on coverslips were rinsed with PBS and covered with a micro-porous polycarbonate filter containing 5 μm pores (Millipore). Cells were irradiated through the filter with a Philips TUV lamp (254 nm) with a dose of 150 J/m2. After UV –irradiation, cells were cultured for 0.5 h, washed first with PBS and then with PBS containing 0.05% Triton X-100 for 30 s before fixation with 3% paraformaldehyde for 15 min at room temperature or with ice-cold methanol for 20 min (for PCNA staining). Subsequently, cells were permeabilized by a 2 times 10 min incubation in PBS containing 0.1% Triton X-100, and washed with PBS+ (PBS containing 0.15% glycine and 0.5% bovine serum albumin). For the experiments with Polδ, cells were incubated with Hu-AraC (10 mM HU, 0.1 mM AraC) from 30 min before irradiation until fixation and cells were fixed with MeOH rather than formaldehyde. Cells were incubated at room temperature with the primary antibody (diluted in PBS+) for 2 h in a moist chamber. Subsequently cells were washed 5 times for 10 min. with PBS Triton X-100, washed with PBS+, and incubated at room temperature with the secondary antibody (diluted in PBS+) for 1 h in a moist chamber. Cells were washed 5 times for 10 min in PBS Triton X-100, washed in PBS, and embedded in Vectashield mounting medium (Vector) containing 0.1 mg of DAPI (4′-6′-diamidino-2-phenylindole)/ml.
Primary antibodies were as follows: mouse monoclonal anti-PCNA (Dako, clone PC10), 1:1000, rabbit polyclonal affinity purified anti-XPC (Ng et al, 2003), 1:300, rabbit polyclonal anti-XPB (TFIIH p89, S-19m Santa Cruz), 1:1000, mouse monoclonal anti-Polδ (A-9, Santa Cruz), 1:25, mouse monoclonal anti-CHAF150 (CAF-1) (abcam, ab7655), 1:2000, and mouse monoclonal FITC-conjugated anti-HA (Roche, clone 3F10) Secondary antibodies were as follows: Cy3-conjugated goat anti-mouse (Jackson ImmunoResearch Laboratories), 1:1000, Cy3-conjugated goat anti-rabbit (Jackson ImmunoResearch Laboratories), 1:1000, and Alexa-488 conjugated goat anti-rabbit (Molecular Probes), 1:800.
Confocal microscopy
Confocal images of the cells were obtained using a Zeiss LSM 510 microscope equipped with a 25 mW Ar laser at 488 nm, a He/Ne 543 nm laser, and a 40 × 1.3 NA oil immersion lens. Alexa-488 was detected using a dichroic beam splitter (HFT 488), and an additional 505- to 530-nm bandpass emission filter. Cy3 was detected using a dichroic beam splitter (HFT 488/543) and a 560- to 615-nm bandpass emission filter.
Unscheduled DNA synthesis
To determine GG-NER activity in cultured cells, UV-induced DNA repair synthesis or UDS was measured. Coverslip cultures were rinsed with PBS, UV-irradiated (16 J/m2, Philips 254 nm TUV lamp) and subsequently incubated for 2 h in culture medium supplemented with 20 μCi/ml [3H-1′,2′]-thymidine (120 Ci/mmol, Amersham TRK565). After fixation coverslips were dipped in Ilford K2 photographic emulsion, exposed for three days an after development stained with Giemsa. Autoradiographic grains above the nuclei of 50 cells were counted and compared to the number of grains above nuclei of NER-proficient fibroblasts (MRC5, set at 100% UDS), assayed in parallel. UDS in locally damaged cells (local UDS) with 60 J/m2 was performed in a similar fashion with the exception of an extended exposure time to six days.
Supplementary Material
Supplementary Information
Acknowledgments
We acknowledge Philip C Hanawalt for insightful discussions regarding early models of NER. This work was supported by the Swiss National Science Foundation grants No. 3100A0-00744 and 3130-054873 to ODS and grant No. 3100A0-100487 and the ‘Frontiers in Genetics' NCCR program to SGC, the New York State Office of Science and Technology and Academic Research (NYSTAR) grant No. C040069 and NIH grants No. GM080454 and CA092584 to ODS, the Human Frontier Science Organization grant RGP7/2004 to ODS and WV, ZonMW (Dutch Science Organization, NWO) grants No 912-03-012 and 917-46-364 to WV, NWO grant 805-47-193 to AMG and WV and EU grant MRTN-CT-2003-503618 to WV and AMG. LS was supported in part by EMBO short-term fellowship ASTF191.00-05.
References
- Aboussekhra A, Biggerstaff M, Shivji MK, Vilpo JA, Moncollin V, Podust VN, Protic M, Hübscher U, Egly JM, Wood RD (1995) Mammalian DNA nucleotide excision repair reconstituted with purified protein components. Cell 80: 859–868 [DOI] [PubMed] [Google Scholar]
- Araujo SJ, Tirode F, Coin F, Pospiech H, Syvaoja JE, Stucki M, Hübscher U, Egly JM, Wood RD (2000) Nucleotide excision repair of DNA with recombinant human proteins: definition of the minimal set of factors, active forms of TFIIH, and modulation by CAK. Genes Dev 14: 349–359 [PMC free article] [PubMed] [Google Scholar]
- Bardwell AJ, Bardwell L, Tomkinson AE, Friedberg EC (1994) Specific cleavage of model recombination and repair intermediates by the yeast Rad1-Rad10 DNA endonuclease. Science 265: 2082–2085 [DOI] [PubMed] [Google Scholar]
- Berneburg M, Lowe JE, Nardo T, Araujo S, Fousteri MI, Green MH, Krutmann J, Wood RD, Stefanini M, Lehmann AR (2000) UV damage causes uncontrolled DNA breakage in cells from patients with combined features of XP-D and Cockayne syndrome. EMBO J 19: 1157–1166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biggerstaff M, Wood RD (1999) Assay for nucleotide excision repair protein activity using fractionated cell extracts and UV-damaged plasmid DNA. Methods Mol Biol 113: 357–372 [DOI] [PubMed] [Google Scholar]
- Constantinou A, Gunz D, Evans E, Lalle P, Bates PA, Wood RD, Clarkson SG (1999) Conserved residues of human XPG protein important for nuclease activity and function in nucleotide excision repair. J Biol Chem 274: 5637–5648 [DOI] [PubMed] [Google Scholar]
- de Laat WL, Jaspers NG, Hoeijmakers JHJ (1999) Molecular mechanism of nucleotide excision repair. Genes Dev 13: 768–785 [DOI] [PubMed] [Google Scholar]
- Dunand-Sauthier I, Hohl M, Thorel F, Jaquier-Gubler P, Clarkson SG, Schärer OD (2005) The spacer region of XPG mediates recruitment to nucleotide excision repair complexes and determines substrate specificity. J Biol Chem 280: 7030–7037 [DOI] [PubMed] [Google Scholar]
- Ellison AR, Nouspikel T, Jaspers NG, Clarkson SG, Gruenert DC (1998) Complementation of transformed fibroblasts from patients with combined xeroderma pigmentosum-Cockayne syndrome. Exp Cell Res 243: 22–28 [DOI] [PubMed] [Google Scholar]
- Enzlin JH, Schärer OD (2002) The active site of XPF-ERCC1 forms a highly conserved nuclease motif. EMBO J 21: 2045–2053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Essers J, Theil AF, Baldeyron C, van Cappellen WA, Houtsmuller AB, Kanaar R, Vermeulen W (2005) Nuclear dynamics of PCNA in DNA replication and repair. Mol Cell Biol 25: 9350–9359 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans E, Fellows J, Coffer A, Wood RD (1997a) Open complex formation around a lesion during nucleotide excision repair provides a structure for cleavage by human XPG protein. EMBO J 16: 625–638 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans E, Moggs JG, Hwang JR, Egly JM, Wood RD (1997b) Mechanism of open complex and dual incision formation by human nucleotide excision repair factors. EMBO J 16: 6559–6573 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedberg EC, Walker GC, Siede W, Wood RD, Schultz RA, Ellenberger T (2005) DNA Repair and Mutagenesis, 2nd edn. Washington DC: ASM Press [Google Scholar]
- Gary R, Dale LL, Cornelius HL, MacInnes MA, Park MS (1997) The DNA repair endonuclease XPG binds to Proliferating Cell Nuclear Antigen (PCNA) and shares sequence elements with the PCNA-binding regions of FEN-1 and cyclin-dependent kinase inhibitor p21. J Biol Chem 272: 24522–24529 [DOI] [PubMed] [Google Scholar]
- Gary R, Kim K, Cornelius HL, Park MS, Matsumoto Y (1999) Proliferating cell nuclear antigen facilitates excision in long-patch base excision repair. J Biol Chem 274: 4354–4363 [DOI] [PubMed] [Google Scholar]
- Gillet LC, Schärer OD (2006) Molecular mechanisms of mammalian global genome nucleotide excision repair. Chem Rev 106: 253–276 [DOI] [PubMed] [Google Scholar]
- Green CM, Almouzni G (2003) Local action of the chromatin assembly factor CAF-1 at sites of nucleotide excision repair in vivo. EMBO J 22: 5163–5174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanawalt PC (1966) Repair replication in the bacterial genome. In Genetical Aspects of Radiosensitivity: Mechanisms of Repair Vol. 24, pp 97–104. Vienna: International atomic energy agency [Google Scholar]
- Hanawalt PC (2002) Subpathways of nucleotide excision repair and their regulation. Oncogene 21: 8949–8956 [DOI] [PubMed] [Google Scholar]
- Hanawalt PC, Haynes RH (1967) The repair of DNA. Sci Am 216: 36–43 [DOI] [PubMed] [Google Scholar]
- Hansson J, Munn M, Rupp WD, Kahn R, Wood RD (1989) Localization of DNA repair synthesis by human cell extracts to a short region at the site of a lesion. J Biol Chem 264: 21788–21792 [PubMed] [Google Scholar]
- Hohl M, Dunand-Sauthier I, Staresincic L, Jaquier-Gubler P, Thorel F, Modesti M, Clarkson SG, Schärer OD (2007) Domain swapping between FEN-1 and XPG defines regions in XPG that mediate nucleotide excision repair activity and substrate specificity. Nucleic Acids Res 35: 3053–3063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hohl M, Thorel F, Clarkson SG, Schärer OD (2003) Structural determinants for substrate binding and catalysis by the structure-specific endonuclease XPG. J Biol Chem 278: 19500–19508 [DOI] [PubMed] [Google Scholar]
- Matsumoto Y, Kim K, Hurwitz J, Gary R, Levin DS, Tomkinson AE, Park MS (1999) Reconstitution of proliferating cell nuclear antigen-dependent repair of apurinic/apyrimidinic sites with purified human proteins. J Biol Chem 274: 33703–33708 [DOI] [PubMed] [Google Scholar]
- Matsunaga T, Mu D, Park CH, Reardon JT, Sancar A (1995) Human DNA repair excision nuclease. Analysis of the roles of the subunits involved in dual incisions by using anti-XPG and anti-ERCC1 antibodies. J Biol Chem 270: 20862–20869 [DOI] [PubMed] [Google Scholar]
- Min JH, Pavletich NP (2007) Recognition of DNA damage by the Rad4 nucleotide excision repair protein. Nature 449: 570–575 [DOI] [PubMed] [Google Scholar]
- Mocquet V, Laine JP, Riedl T, Yajin Z, Lee MY, Egly JM (2008) Sequential recruitment of the repair factors during NER: the role of XPG in initiating the resynthesis step. EMBO J 27: 155–167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moggs JG, Yarema KJ, Essigmann JM, Wood RD (1996) Analysis of incision sites produced by human cell extracts and purified proteins during nucleotide excision repair of a 1,3-intrastrand d(GpTpG)-cisplatin adduct. J Biol Chem 271: 7177–7186 [DOI] [PubMed] [Google Scholar]
- Mone MJ, Volker M, Nikaido O, Mullenders LH, van Zeeland AA, Verschure PJ, Manders EM, van Driel R (2001) Local UV-induced DNA damage in cell nuclei results in local transcription inhibition. EMBO Rep 2: 1013–1017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moser J, Kool H, Giakzidis I, Caldecott K, Mullenders LH, Fousteri MI (2007) Sealing of chromosomal DNA nicks during nucleotide excision repair requires XRCC1 and DNA ligase III alpha in a cell-cycle-specific manner. Mol Cell 27: 311–323 [DOI] [PubMed] [Google Scholar]
- Mu D, Hsu DS, Sancar A (1996) Reaction mechanism of human DNA repair excision nuclease. J Biol Chem 271: 8285–8294 [DOI] [PubMed] [Google Scholar]
- Mu D, Park CH, Matsunaga T, Hsu DS, Reardon JT, Sancar A (1995) Reconstitution of human DNA repair excision nuclease in a highly defined system. J Biol Chem 270: 2415–2418 [DOI] [PubMed] [Google Scholar]
- Mu D, Wakasugi M, Hsu DS, Sancar A (1997) Characterization of reaction intermediates of human excision repair nuclease. J Biol Chem 272: 28971–28979 [DOI] [PubMed] [Google Scholar]
- Ng JMY, Vermeulen W, van der Horst GTJ, Bergink S, Sugasawa K, Vrieling H, Hoeijmakers JHJ (2003) A novel regulation mechanism of DNA repair damage-induced and RAD23-dependent stabilization of xeroderma pigmentosum group C protein. Genes Dev 17: 1630–1645 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nichols AF, Sancar A (1992) Purification of PCNA as a nucleotide excision repair protein. Nucleic Acids Res 20: 2441–2446 [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Donovan A, Davies AA, Moggs JG, West SC, Wood RD (1994) XPG endonuclease makes the 3′ incision in human DNA nucleotide excision repair. Nature 371: 432–435 [DOI] [PubMed] [Google Scholar]
- Ogi T, Lehmann AR (2006) The Y-family DNA polymerase kappa (pol kappa) functions in mammalian nucleotide-excision repair. Nat Cell Biol 8: 640–642 [DOI] [PubMed] [Google Scholar]
- Pascucci B, Stucki M, Jonsson ZO, Dogliotti E, Hübscher U (1999) Long patch base excision repair with purified human proteins. DNA ligase I as patch size mediator for DNA polymerases delta and epsilon. J Biol Chem 274: 33696–33702 [DOI] [PubMed] [Google Scholar]
- Riedl T, Hanaoka F, Egly JM (2003) The comings and goings of nucleotide excision repair factors on damaged DNA. EMBO J 22: 5293–5303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schärer OD (2007) Achieving broad substrate specificity in damage recognition by binding accessible nondamaged DNA. Mol Cell 28: 184–186 [DOI] [PubMed] [Google Scholar]
- Shechter D, Costanzo V, Gautier J (2004) Regulation of DNA replication by ATR: signaling in response to DNA intermediates. DNA Repair (Amst) 3: 901–908 [DOI] [PubMed] [Google Scholar]
- Shivji MK, Moggs JG, Kuraoka I, Wood RD (1999) Dual-Incision Assays for Nucleotide Excision Repair Using DNA with a Lesion at a Specific Site. Methods Mol Biol 113: 373–392 [DOI] [PubMed] [Google Scholar]
- Shivji MK, Podust VN, Hübscher U, Wood RD (1995) Nucleotide excision repair DNA synthesis by DNA polymerase epsilon in the presence of PCNA, RFC, and RPA. Biochemistry 34: 5011–5017 [DOI] [PubMed] [Google Scholar]
- Sijbers AM, de Laat WL, Ariza RR, Biggerstaff M, Wei YF, Moggs JG, Carter KC, Shell BK, Evans E, de Jong MC, Rademakers S, de Rooij J, Jaspers NG, Hoeijmakers JH, Wood RD (1996) Xeroderma pigmentosum group F caused by a defect in a structure-specific DNA repair endonuclease. Cell 86: 811–822 [DOI] [PubMed] [Google Scholar]
- Sugasawa K, Ng JMY, Masutani C, Iwai S, van der Spek PJ, Eker APM, Hanaoka F, Bootsma D, Hoeijmakers JHJ (1998) Xeroderma pigmentosum group C protein complex is the initiator of global genome nucleotide excision repair. Mol Cell 2: 223–232 [DOI] [PubMed] [Google Scholar]
- Sugasawa K, Okamoto T, Shimizu Y, Masutani C, Iwai S, Hanaoka F (2001) A multistep damage recognition mechanism for global genomic nucleotide excision repair. Genes Dev 15: 507–521 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Svejstrup JQ (2002) Mechanisms of Transcription-Coupled DNA Repair. Mol Cell Biol 3: 21–29 [DOI] [PubMed] [Google Scholar]
- Tapias A, Auriol J, Forget D, Enzlin JH, Schärer OD, Coin F, Coulombe B, Egly JM (2004) Ordered conformational changes in damaged DNA induced by nucleotide excision repair factors. J Biol Chem 279: 19074–19083 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Theron T, Fousteri MI, Volker M, Harries LW, Botta E, Stefanini M, Fujimoto M, Andressoo JO, Mitchell J, Jaspers NG, McDaniel LD, Mullenders LH, Lehmann AR (2005) Transcription-associated breaks in xeroderma pigmentosum group D cells from patients with combined features of xeroderma pigmentosum and Cockayne syndrome. Mol Cell Biol 25: 8368–8378 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thorel F, Constantinou A, Dunand-Sauthier I, Nouspikel T, Lalle P, Raams A, Jaspers NG, Vermeulen W, Shivji MK, Wood RD, Clarkson SG (2004) Definition of a short region of XPG necessary for TFIIH interaction and stable recruitment to sites of UV damage. Mol Cell Biol 24: 10670–10680 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tirode F, Busso D, Coin F, Egly JM (1999) Reconstitution of the transcription factor TFIIH: assignment of functions for the three enzymatic subunits, XPB, XPD, and cdk7. Mol Cell 3: 87–95 [DOI] [PubMed] [Google Scholar]
- Tsodikov OV, Ivanov D, Orelli B, Staresincic L, Shoshani I, Oberman R, Schärer OD, Wagner G, Ellenberger T (2007) Structural basis for the recruitment of ERCC1-XPF to nucleotide excision repair complexes by XPA. EMBO J 26: 4768–4776 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volker M, Mone MJ, Karmakar P, van Hoffen A, Schul W, Vermeulen W, Hoeijmakers JH, van Driel R, van Zeeland AA, Mullenders LH (2001) Sequential assembly of the nucleotide excision repair factors in vivo. Mol Cell 8: 213–224 [DOI] [PubMed] [Google Scholar]
- Wakasugi M, Reardon JT, Sancar A (1997) The non-catalytic function of XPG protein human nucleotide excision repair. J Biol Chem 272: 16030–16034 [DOI] [PubMed] [Google Scholar]
- Wakasugi M, Sancar A (1998) Assembly, subunit composition, and footprint of human DNA repair excision nuclease. Proc Natl Acad Sci USA 95: 6669–6674 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Information