EMBO J 28, 1016–1028 (2009); published online 22 April 2009
The microtubule cytoskeleton of a mammalian cell originates from the perinuclear region and controls membrane trafficking, organelle positioning, and cell polarisation. Although the centrosome is viewed as the major microtubule organising centre, there is emerging evidence that the Golgi apparatus also has a role in the nucleation of perinuclear microtubules during the interphase. In a recent paper in The EMBO Journal, Rivero and colleagues reported the identification of a microtubule nucleation machinery at the Golgi and provided clues about functional differences between Golgi- and centrosome-nucleated microtubules.
Earlier studies have shown that microtubules can be nucleated by Golgi membranes (Chabin-Brion et al, 2001). Golgi-dependent microtubule nucleation requires γ-tubulin and the γ-TuRC complex, which may be recruited to the Golgi through interactions with the Golgi proteins, GMAP210 and AKAP450 (Chabin-Brion et al, 2001; Takahashi et al, 2002; Rios et al, 2004; Efimov et al, 2007). However, microtubules nucleated at the Golgi differ from centrosomally-nucleated microtubules in several ways. They are asymmetrically organised, with preferential growth towards the leading edge of a migrating cell (Efimov et al, 2007). They become rapidly acetylated, which makes them more stable and resistant to nocodazole-induced depolymerisation (Chabin-Brion et al, 2001) and they are coated with CLASP2, a microtubule (+)-end binding protein that is specifically recruited to the trans-Golgi network (TGN) to stabilise microtubule seeds (Efimov et al, 2007).
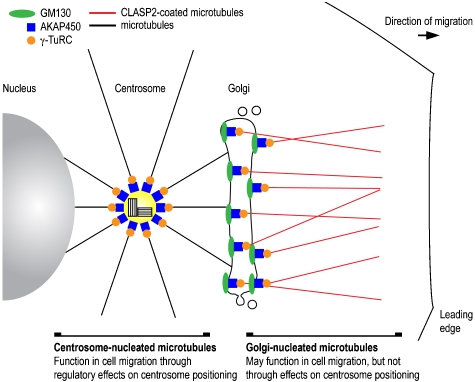
In their study, Rivero and colleagues showed that microtubule nucleation and anchoring at the Golgi is regulated by a machinery consisting of general and Golgi-specific factors (Figure 1). A central component of this machinery is AKAP450, which localises to both the centrosome and the Golgi (Takahashi et al, 1999). Partial knockdown of AKAP450 removed this large scaffold protein from the Golgi, but not from the centrosome, and caused the specific loss of Golgi-nucleated microtubules, indicating that AKAP450 is necessary for the nucleation of this specific pool of microtubules. Additional experiments showed that mislocalisation of AKAP450 to endoplasmic reticulum (ER) exit sites or the cytosol produced microtubule seeds at these specific locations. As AKAP450 also controls microtubule nucleation at the centrosome (Takahashi et al, 2002), these results indicate that AKAP450 may function as a general regulator of cellular microtubule nucleation. Intriguingly, AKAP450-dependent microtubules at the Golgi were specifically stabilized by coating with the TGN-associated protein, CLASP2. The authors also found that microtubule nucleation at the Golgi required GM130, which recruits AKAP450 to the Golgi through physical interactions. The involvement of GM130 is consistent with the role for this peripheral Golgi protein in the organisation of the microtubule cytoskeleton during interphase, identified earlier (Kodani and Sutterlin, 2008).
Figure 1.
Microtubules of the perinuclear region. Model comparing the two populations of microtubules that are formed in the perinuclear region of a mammalian cell.
Rivero and colleagues also reported functional differences between Golgi- and centrosome-nucleated microtubules with regards to cell polarisation and migration (Figure 1). Cells depleted of AKAP450 at the Golgi, but not at the centrosome, were unable to migrate in a wound-healing assay. Unexpectedly, however, they retained the ability to reorient their centrosome and Golgi membranes towards the leading edge of the cell. In contrast, earlier studies have shown that perinuclear microtubules are required for cell migration through regulatory effects on centrosome positioning (Etienne-Manneville and Hall, 2003). Thus, Golgi-nucleated microtubules seem to have specific effects on cell migration that is independent of centrosome and Golgi positioning in motile cells. This model is supported by the finding that CLASP2-coated microtubules are required for directional cell migration (Drabek et al, 2006). However, a direct role for AKAP450 in cell migration and polarisation through a microtubule-independent mechanism has not been excluded.
The data presented in this paper raise numerous questions of which the relationship between Golgi-nucleated microtubules and cell migration is most intriguing. It would be important to confirm the role of Golgi-nucleated microtubules in cell migration and to rule out a microtubule-independent role for AKAP450. One approach would be to measure cell migration when Golgi-nucleated microtubules are removed in an AKAP450-independent manner, such as by depletion of CLASP2 or its TGN-localized receptor, GCC185 (Efimov et al, 2007). Additional studies will be necessary to determine how Golgi-nucleated microtubules control cell migration and whether they regulate other cellular processes.
References
- Chabin-Brion K, Marceiller J, Perez F, Settegrana C, Drechou A, Durand G, Pous C (2001) The Golgi complex is a microtubule-organizing organelle. Mol Biol Cell 12: 2047–2060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drabek K, van Ham M, Stepanova T, Draegestein K, van Horssen R, Sayas CL, Akhmanova A, Ten Hagen T, Smits R, Fodde R, Grosveld F, Galjart N (2006) Role of CLASP2 in microtubule stabilization and the regulation of persistent motility. Curr Biol 16: 2259–2264 [DOI] [PubMed] [Google Scholar]
- Efimov A, Kharitonov A, Efimova N, Loncarek J, Miller PM, Andreyeva N, Gleeson P, Galjart N, Maia AR, McLeod IX, Yates JR III, Maiato H, Khodjakov A, Akhmanova A, Kaverina I (2007) Asymmetric CLASP-dependent nucleation of noncentrosomal microtubules at the trans-Golgi network. Dev Cell 12: 917–930 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Etienne-Manneville S, Hall A (2003) Cdc42 regulates GSK-3beta and adenomatous polyposis coli to control cell polarity. Nature 421: 753–756 [DOI] [PubMed] [Google Scholar]
- Kodani A, Sutterlin C (2008) The Golgi protein GM130 regulates centrosome morphology and function. Mol Biol Cell 19: 745–753 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rios RM, Sanchis A, Tassin AM, Fedriani C, Bornens M (2004) GMAP-210 recruits gamma-tubulin complexes to cis-Golgi membranes and is required for Golgi ribbon formation. Cell 118: 323–335 [DOI] [PubMed] [Google Scholar]
- Takahashi M, Shibata H, Shimakawa M, Miyamoto M, Mukai H, Ono Y (1999) Characterization of a novel giant scaffolding protein, CG-NAP, that anchors multiple signaling enzymes to centrosome and the golgi apparatus. J Biol Chem 274: 17267–17274 [DOI] [PubMed] [Google Scholar]
- Takahashi M, Yamagiwa A, Nishimura T, Mukai H, Ono Y (2002) Centrosomal proteins CG-NAP and kendrin provide microtubule nucleation sites by anchoring gamma-tubulin ring complex. Mol Biol Cell 13: 3235–3245 [DOI] [PMC free article] [PubMed] [Google Scholar]