EMBO J 28, 1078–1086 (2009); published online 22 April 2009
Cytosine methylation is widely known for its role in silencing transposable elements and some genes in plants and mammals. However, whereas methylation of promoter sequences can lead to transcriptional repression, the function of gene body methylation remains elusive. This situation is particularly perplexing in the plant Arabidopsis, where many genes are methylated, specifically, over their body. A new study in this issue of The EMBO Journal shows that gene body methylation results from two conflicting activities, one imposing it at CG sites, and one preventing its extension to CHG sites (where H=A,T or C). Importantly, the latter activity is not targeted towards silent transposable elements and is likely coupled to transcription elongation, suggesting that CHG methylation hinders this step.
Transposable elements (TEs), their relics and other repeats are the main targets of cytosine methylation in eukaryotes and multiple lines of evidence indicate that eukaryotic DNA methylation serves primarily to silence these repeat sequences (Suzuki and Bird, 2008). DNA methylation can of course, also silence genes, but the extent to which this modification is used to control gene expression in plants and mammals is unclear. Nonetheless, it is now well established that transcription initiation can be blocked by DNA methylation in these organisms, notably through the masking of promoter sequences by methylcytosine binding proteins or as a consequence of DNA methylation-induced chromatin remodelling. In contrast, the effect of DNA methylation on transcription elongation seems limited, as judged by the fact that most mammalian genes contain methylated TE sequences within their introns.
Unlike their mammalian counterparts, plant introns are almost completely devoid of TEs and it could therefore be expected that gene body methylation should be minimal in plants. However, genome-wide analysis of DNA methylation in Arabidopsis by McrBC digestion, methylcytosine immunoprecipitation (MeDIP) or sequencing of bisulfite-treated DNA, showed that although most genes have unmethylated promoters, 20–30% of genes show a significant amount of body methylation (Zhang et al, 2006; Vaughn et al, 2007; Zilberman et al, 2007; Cokus et al, 2008; Lister et al, 2008). Furthermore, genome-wide bisulfite sequencing indicated that gene body methylation is almost exclusively restricted to CG sites, which is in marked contrast to the methylation of CG, CHG and CHH sites typically seen for repeat sequences in plants.
In this issue, Miura et al (2009) add another piece to this puzzle, by showing that a large fraction of methylated genes in Arabidopsis gain CHG methylation in plants mutated for the gene IBM1 (INCREASE IN BONSAI METHYLATION1). This gene encodes a jumonji-domain protein with putative histone H3 lysine 9 demethylase activity and was identified in a genetic screen for mutants showing ectopic cytosine methylation of the BONSAI (BNS) gene (Saze et al, 2008). BNS was earlier isolated by the Kakutani group as a gene that, paradoxically, tends to become methylated in advanced generations of the ddm1 (decrease in dna methylation1) mutant background, which induces global DNA hypomethylation (Saze and Kakutani, 2007). Whereas BNS methylation in ddm1 likely results because of spreading from a methylated long interspersed nuclear element (LINE) retro-element located immediately downstream and affects Cs in all sequence contexts (Saze and Kakutani, 2007), ibm1-induced methylation of BNS is mostly confined to CHG sites (Saze et al, 2008). In their new work, Miura et al show that ibm1 induces widespread hypermethylation across the genome, which is targeted exclusively to the body of genes and limited to CHG sites. Hypermethylated genes tend to be already methylated at CG sites in wild type plants and are not preferentially associated with the presence of TEs nearby and thus differ from BNS in these two respects. Furthermore, ibm1-induced CHG methylation positively correlates with gene size and intron number, suggesting that transcription elongation is involved. This is in agreement with the initial observation that gene-body methylation is preferentially associated with transcribed genes in wild type plants (Zhang et al, 2006; Zilberman et al, 2007). Miura et al also show that ibm1-induced hypermethylation of genes is not mediated by the de novo DNA methyltransferase DOMAINS REARRANGED METHYLTRANSFERASE2 (DRM2) or other components of the RNAi-dependent de novo methylation machinery. Finally, they provide preliminary evidence that hypermethylation of the body of genes can affect their expression, although not in a consistent manner.
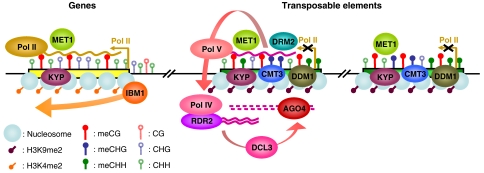
Such results suggest a model in which transcription of genes by the RNA polymerase II (Pol II) attracts, in its wake, the maintenance DNA methyltransferase MET1, which is responsible for most CG methylation across the genome, as well as the histone H3 lysine 9 methyltransferase KRYPTONITE (KYP) or a related enzyme. Gene transcription would also recruit IBM1 that by demethylating lysine 9 of histone H3, would prevent its recognition by the chromodomain-containing, CHG methyltransferase CMT3. Thus, targeting of DNA methylation seems to differ significantly for genes and TEs, despite the fact that many factors are shared by these two processes (Figure 1). This model leaves many questions open, however. For instance, it is not clear as to how highly transcribed genes may escape body-methylation (Zhang et al, 2006; Zilberman et al, 2007), or how CHG body methylation may occur in met1 (Lister et al, 2008) or met1drm1drm2 (Cokus et al, 2008) mutant plants. Nonetheless, the results presented by Miura et al provide the first indication that although gene-body methylation may be an inherent by-product of their transcription, this methylation may need to be limited to CG sites in order that it does not adversely affect transcription.
Figure 1.
A model for the differential DNA methylation of genes and TEs in Arabidopsis. Note that the ATPase chromatin remodeler DDM1 is specifically involved in the methylation of TEs and that only a subset of methylated TEs are also targeted by the RNAi machinery (Teixeira et al, 2009). AGO4: ARGONAUTE4; DCL3: DICER-LIKE3; POLII, IV and V: RNA polymerase II, IV and V; RDR2: RNA-DEPENDENT RNA POLYMERASE2. Other abbreviations: see main text.
References
- Cokus SJ, Feng S, Zhang X, Chen Z, Merriman B, Haudenschild CD, Pradhan S, Nelson SF, Pellegrini M, Jacobsen SE (2008) Shotgun bisulphite sequencing of the Arabidopsis genome reveals DNA methylation patterning. Nature 452: 215–219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lister R, O'Malley RC, Tonti-Filippini J, Gregory BD, Berry CC, Millar AH, Ecker JR (2008) Highly integrated single-base resolution maps of the epigenome in Arabidopsis. Cell 133: 523–536 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miura A, Nakamura M, Inagaki S, Kobayashi A, Saze H, Kakutani T (2009) An Arabidopsis jmjC-domain protein protects transcribed genes from DNA methylation at CHG sites. EMBO J 28: 1078–1086 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saze H, Kakutani T (2007) Heritable epigenetic mutation of a transposon-flanked Arabidopsis gene due to lack of the chromatin-remodeling factor DDM1. EMBO J 26: 3641–3652 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saze H, Shiraishi A, Miura A, Kakutani T (2008) Control of genic DNA methylation by a jmjC domain-containing protein in Arabidopsis thaliana. Science 319: 462–465 [DOI] [PubMed] [Google Scholar]
- Suzuki MM, Bird A (2008) DNA methylation landscapes: provocative insights from epigenomics. Nat Rev Genet 9: 465–476 [DOI] [PubMed] [Google Scholar]
- Teixeira FK, Heredia F, Sarazin A, Roudier F, Boccara M, Ciaudo C, Cruaud C, Poulain J, Berdasco M, Fraga MF, Voinnet O, Wincker P, Esteller M, Colot V (2009) A role for RNAi in the selective correction of DNA methylation defects. Science, published online 29 January 2009 (doi:10.1126/science.1165313) [DOI] [PubMed] [Google Scholar]
- Vaughn MW, Tanurdzic M, Lippman Z, Jiang H, Carrasquillo R, Rabinowicz PD, Dedhia N, McCombie WR, Agier N, Bulski A, Colot V, Doerge RW, Martienssen RA (2007) Epigenetic Natural Variation in Arabidopsis thaliana. PLoS Biol 5: e174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X, Yazaki J, Sundaresan A, Cokus S, Chan SW, Chen H, Henderson IR, Shinn P, Pellegrini M, Jacobsen SE, Ecker JR (2006) Genome-wide high-resolution mapping and functional analysis of DNA methylation in arabidopsis. Cell 126: 1189–1201 [DOI] [PubMed] [Google Scholar]
- Zilberman D, Gehring M, Tran RK, Ballinger T, Henikoff S (2007) Genome-wide analysis of Arabidopsis thaliana DNA methylation uncovers an interdependence between methylation and transcription. Nat Genet 39: 61–69 [DOI] [PubMed] [Google Scholar]