Abstract
Background
Screening reduces colorectal cancer mortality, but effective screening tests remain underused. Systematic reminders to patients and physicians could increase screening rates.
Methods
We conducted a randomized controlled trial of patient and physician reminders in 11 ambulatory health centers. Participants included 21,860 patients ages 50 to 80 overdue for colorectal cancer screening and 110 primary care physicians. Patients were randomly assigned to receive mailings containing an educational pamphlet, fecal-occult-blood test kit, and instructions for direct scheduling of flexible sigmoidoscopy or colonoscopy. Physicians were randomly assigned to receive electronic reminders during office visits with patients overdue for screening. The primary outcome was receipt of fecal-occult-blood testing, flexible sigmoidoscopy, or colonoscopy over 15 months, and the secondary outcome was detection of colorectal adenomas.
Results
Screening rates were higher for patients who received mailings compared to those who did not (44.0% vs. 38.1%, p<0.001). The effect increased with age: +3.7% for ages 50-59; +7.3% for ages 60-69; and +10.1% for ages 70-80 (p=0.01 for trend). Screening rates were similar among patients of physicians receiving electronic reminders and the control group (41.9% vs. 40.2%, p=0.47). However, electronic reminders tended to increase screening rates among patients with 3 or more primary care visits (59.5% vs. 52.7%, p=0.07). Detection of adenomas tended to increase with patient mailings (5.7% vs. 5.2%, p=0.10) and physician reminders (6.0% versus 4.9%, p=0.09).
Conclusions
Mailed reminders to patients are an effective tool to promote colorectal cancer screening, and electronic reminders to physicians may increase screening among adults who more frequently use primary care.
(ClinicalTrials.gov ID number NCT00355004)
Keywords: Colorectal Cancer Screening, Reminder Systems, Quality Improvement, Randomized Controlled Trial
Colorectal cancer is the second leading cause of cancer mortality in the United States.1 Screening programs involving fecal occult blood testing (FOBT), flexible sigmoidoscopy, and colonoscopy lower the incidence of colorectal cancer by removing precancerous adenomas, detect cancers at more curable early stages, and reduce colorectal cancer mortality.2-6 National guidelines strongly recommend screening for colorectal cancer for average-risk adults 50 years and older.7-9
Unfortunately, only 60% of eligible adults report up-to-date screening.10 Patients cite lack of motivation and awareness of the need for colorectal cancer screening, and many report that their provider did not recommend screening.11 During office visits, physicians may have insufficient time to discuss the growing number of recommended preventive services.12 Physicians report patient requests and reminder systems as two main factors that facilitate screening.13, 14
Patients may benefit from increased awareness of their need for colorectal cancer screening and encouragement to obtain this service, while physicians may benefit from receiving patient-specific, timely information regarding their patients’ screening status. However, except for two studies that compared the effects of colorectal cancer screening reminders focused on patients and physicians nearly 20 years ago,15, 16 most prior interventions to promote colorectal cancer screening have focused on either patients 17-22or physicians.23-28 Therefore, we conducted a randomized controlled trial to compare the individual and joint impact of personalized mailings to patients and electronic reminders to primary care physicians to promote colorectal cancer screening within a multi-site group practice.
Methods
Study Setting
This 15-month trial was conducted from April 2006 to June 2007 at Harvard Vanguard Medical Associates (HVMA), a multi-specialty group practice composed of 14 ambulatory health centers in eastern Massachusetts. Since 1997, clinical practices within HVMA have used a common electronic health record (Epic Systems, www.epicsystems.com) that includes clinical notes, diagnostic codes, procedure codes, and laboratory results. The record also supports computerized ordering of all laboratory tests and referrals. Each primary care physician at HVMA practices at a single health center. Gastroenterologists perform procedures either at an ambulatory endoscopy center operated by HVMA, or within an affiliated hospital-based endoscopy center. Manual chart reviews indicated that electronic documentation of colonoscopies performed at two health centers that contract for this procedure with outside gastroenterologists were incomplete, so these two centers were excluded. After pilot-testing our interventions at one other health center, 11 health centers were included in the randomized trial.
Patient and Physician Eligibility
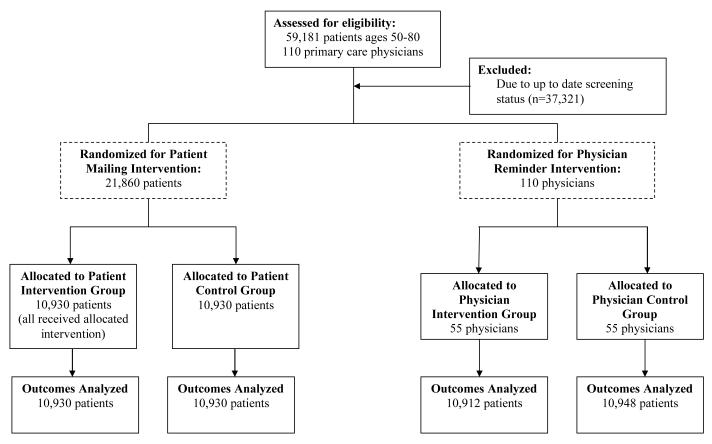
We identified 59,181 patients ages 50 to 80 years of 110 primary care physicians at 11 centers who had a visit with an HVMA primary care physician during the prior 18 months (Figure 1). From this cohort, we excluded 37,321 patients (63%) who had been screened for colorectal cancer in accordance with the HVMA clinical guideline, having received either flexible sigmoidoscopy within 5 years along with FOBT in the prior year or colonoscopy within 10 years. The remaining 21,860 patients (37%) and their 110 primary physicians were eligible for our study.
Figure 1.
CONSORT diagram of patient and physician eligibility and randomization. For the patient mailing, patients were randomized within physician panels; and for the electronic reminders physicians were randomized within each health center.
Screening tests were ascertained via an automated electronic algorithm using laboratory results, diagnostic codes, procedure codes, and outpatient and hospital encounters from the electronic record. Compared to physician medical record review for a random sample of patients, this algorithm was 88% sensitive (95% confidence interval (CI): 79%, 93%) and 96% specific (95% CI: 87%, 100%) in identifying screening tests. Appropriate screening was typically undetected by the automated algorithm when colonoscopy occurred at outside hospitals, particularly before patients received care at HVMA.
The Harvard Medical School and HVMA Human Studies Committees approved the study protocol, including a waiver of informed consent for both patients and physicians because the study was promoting the HVMA standard of care for colorectal cancer screening. The study protocol was registered at ClinicalTrials.gov (ID number NCT00355004).
Patient Intervention
Patients overdue for colorectal cancer screening received a mailing with four components: 1) a cover letter from the HVMA chief medical officer identifying the patient as overdue for screening and indicating the dates of their most recent screening exams (Appendix), 2) an educational pamphlet detailing screening options, 3) an FOBT kit with three Coloscreen stool cards from Helena labs (http://www.helena.com/coloscreen.htm), instructions and a stamped return envelope, and 4) a dedicated phone number to schedule flexible sigmoidoscopy or colonoscopy. The initial mailing occurred during the first month of the intervention, and a repeat mailing was sent to patients still overdue for screening 6 months later. When patients called to schedule a colonoscopy, physician assistants screened them for contraindications and provided instructions. Primary care physicians were notified of patients with potential contraindications.
Physician Intervention
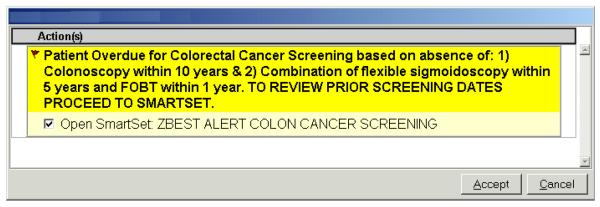
Throughout the 15-month intervention period physicians received electronic reminders during office visits with their patients overdue for colorectal cancer screening. Immediately prior to the intervention, we educated physicians in both the intervention and control groups regarding the use of these reminders via a one-hour presentation and discussion at each center. The alerts were present in both a passive and active form within each patient’s electronic chart. Physicians could view the passive alert at any point during an encounter within the electronic visit summary screen, while the active alert required acknowledgement from physicians attempting to place electronic orders (Figure 2). The alerts provided details regarding the most recent screening tests and facilitated “one-click” electronic ordering of screening exams. Electronic orders for endoscopic procedures were automatically forwarded to the gastroenterology department for scheduling. Physicians were not receiving similar alerts for other preventive services during the intervention period.
Figure 2.
Active electronic reminders were delivered to physicians during office encounters, and facilitated electronic ordering of recommended tests.
Randomization Process
The patient intervention was randomized at the level of individual patients within each physician’s patient panel. Among all 59,181 patients ages 50 to 80, we estimated a multivariable logistic regression model for their propensity to have been screened for colorectal cancer at baseline in accordance with the HVMA clinical guideline (Figure 1). Predictors included patient age, sex, race, insurance coverage, and socioeconomic characteristics based on linking patient 5-digit zip codes to the 2000 US Census, including proportion of high school graduates, median household income, and proportion of households below the federal poverty level. Within each physician panel, we paired patients overdue for screening with similar values of this propensity and randomly assigned one patient in each pair to receive the intervention mailing, thus closely balancing treatment groups on characteristics related to their baseline screening propensity.
The physician intervention was randomized at the physician level. Within each health center, we paired physicians with similar colorectal cancer screening rates and numbers of patients overdue for screening, and then randomly assigned one physician in each pair to receive electronic reminders. We repeated the randomization twenty times and chose the assignment that provided the best overall balance on these two characteristics between the intervention and control groups.
Study Outcomes
All data were collected from the electronic record, and study outcomes were assessed 15 months following the start of the intervention for all randomized patients. The primary study outcome was completion of one of the following 3 options during the 15-month study period: FOBT, flexible sigmoidoscopy, or colonoscopy.7-9 We did not include barium enema because it is rarely used for screening purposes at HVMA. Because the detection and removal of precancerous adenomas is a major objective of colorectal cancer screening,29 the secondary study outcome was detection of adenomas based on diagnostic codes. Visual review of electronic records found these codes to have a positive predictive value of 94% (95% CI: 84%, 99%) and negative predictive value of 96% (95% CI: 86%, 100%) for identifying colorectal adenomas. For a random 10% sample of patients who had colorectal adenomas removed during the study, we conducted chart reviews to identify the following high-risk findings: 1) ≥3 adenomas, 2) adenoma ≥10 mm, or 3) adenoma with villous histology. We also ascertained new diagnoses of colorectal cancer via the presence of a new International Classification of Diseases-Clinical Modification code of 153.0–153.9 or 154.0-154.1. We then conducted chart reviews to verify the diagnosis of colorectal cancer and collect staging data.30
Physician Survey
We surveyed all 43 of the original 55 physicians in the electronic reminder intervention group who were still practicing at HVMA four months after the study ended. The survey instrument assessed perceived effectiveness of colorectal cancer screening modalities and of the electronic reminders using a three-point Likert scale of “very effective”, “somewhat effective”, or “not effective”. Physicians also identified which screening test they most commonly recommended, as well as the perceived proportion of electronic reminders that accurately reflected patients’ screening status. The surveys were administered in a three-stage process that involved an initial paper mailing, followed by a reminder e-mail and a final paper mailing.
Data Analysis
All analyses were conducted on an intention-to-treat basis. Baseline characteristics for patients in the intervention and control groups were compared using the Pearson chi-square test for dichotomous variables and Student’s t test for continuous variables. We analyzed the impact of the interventions by fitting a single linear regression model to predict performance of an appropriate screening exam after adjusting standard errors for clustering of patients by physician. Independent variables included patient intervention status, physician intervention status, and physician baseline screening rate, and we also tested the interaction of patient and physician intervention status.
We fit separate models for pre-specified subgroup analyses according to characteristics known to affect rates of colorectal cancer screening, including age (50-59, 60-69, or 70-80 years), sex, and number of primary care visits (0, 1-2, or ≥3).31-33 All analyses were performed using SAS version 9.1, and we report two-tailed p values or 95% confidence intervals for all comparisons.
Results
Study Subjects
We studied 110 primary care physicians and their 21,860 patients who were overdue for colorectal cancer screening. Patients in the intervention and control group were similar for both the patient-level and physician-level randomizations, except for a borderline significant trend (p=0.08) toward more office visits in the control group for the physician intervention (Table 1). The average age of physicians was 48 (standard deviation 9.7), and 57% were female. Their mean number of eligible patients ages 50 to 80 was 199 (standard deviation 95), with no differences according to intervention status.
Table 1.
Baseline Patient Characteristics
| Patient Mailing Intervention (Patient Level Randomization) |
Physician Reminder Intervention (Physician Level Randomization) |
|||||
|---|---|---|---|---|---|---|
| Patient Characteristics | Intervention (n=10,930) |
Control (n=10,930) |
p value† |
Intervention (n=10,912) |
Control (n=10,948) |
p value† |
| Mean age, years (± SD) | 60.5 (8.3) | 60.4 (8.4) | 0.26 | 60.3 (8.3) | 60.5 (8.4) | 0.55 |
| Female, % | 56.8 | 57.0 | 0.80 | 54.0 | 59.8 | 0.23 |
| Race, % | ||||||
| White | 58.0 | 57.3 | 0.60 | 57.4 | 57.9 | 0.81 |
| Black | 8.3 | 8.4 | 8.7 | 8.0 | ||
| Hispanic | 1.7 | 1.7 | 1.5 | 1.9 | ||
| Asian | 2.3 | 2.7 | 2.2 | 2.8 | ||
| Other | 2.6 | 2.5 | 2.5 | 2.6 | ||
| Unknown | 27.1 | 27.4 | 27.7 | 26.8 | ||
| Insurance, % | ||||||
| Commercial | 68.1 | 68.9 | 0.35 | 69.4 | 67.6 | 0.47 |
| Medicare Fee-For-Service | 9.8 | 9.6 | 9.1 | 10.4 | ||
| Medicare Managed Care | 14.3 | 14.0 | 13.9 | 14.4 | ||
| Medicaid | 3.6 | 3.7 | 3.6 | 3.8 | ||
| Self-pay | 4.2 | 3.7 | 4.0 | 3.9 | ||
| High school graduate, % (± SD)* | 87.1 (8.3) | 87.0 (8.3) | 0.41 | 87.0 (8.4) | 87.1 (8.2) | 0.92 |
| Median household income, $ (± SD)* | 59,605 (21,197) | 59,147 (20,662) | 0.11 | 59,436 (21,068) | 59,316 (20,797) | 0.96 |
| Below poverty level, % (± SD)* | 8.6 (6.8) | 8.8 (6.8) | 0.18 | 8.7 (6.8) | 8.7 (6.8) | 0.94 |
| Number of visits with PCP, % | ||||||
| 0 | 34.9 | 35.0 | 0.96 | 37.2 | 32.7 | 0.08 |
| 1-2 | 41.2 | 41.3 | 40.2 | 42.2 | ||
| 3+ | 23.9 | 23.7 | 22.6 | 25.1 | ||
Based on 2000 US Census data linked to patients’ 5-digit zip codes
Adjusted for clustering of patients within physician panels
Screening Rates
Among this group of patients who were overdue for screening with usual care, patients who received the mailing were significantly more likely to complete colorectal cancer screening than those who did not (44.0% vs. 38.1%, p<0.001). The patient mailing was more effective among older patients, with the absolute increase in screening rates ranging from 3.7% among patients ages 50 to 59 years to 10.1% among patients ages 70 to 80 (p=0.01 for trend, Table 2). The impact of the mailing did not differ between women and men (Table 2). The mailing primarily increased the performance of FOBT among the intervention group compared to the control group (25.4% vs. 20.4%, p<0.001, Table 3). Among patients with a positive FOBT, 73% of this group overall underwent subsequent colonoscopy, with no significant differences by patient or physician intervention group (Table 3).
Table 2.
Receipt of Colorectal Cancer Screening By Intervention Status
| Completed Colorectal Cancer Screening, % | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Patient Mailing Intervention | Physician Reminder Intervention | ||||||||
| No. Patients |
Intervention | Control | Difference (95% CI)* |
p value* |
Intervention | Control | Difference (95% CI)* |
p value* |
|
| All patients | 21,860 | 44.0 | 38.1 | 5.8 (4.5, 7.1) | <0.001 | 41.9 | 40.2 | 1.6 (-2.7, 5.9) | 0.47 |
| Age groups | |||||||||
| 50-59 | 12,240 | 42.1 | 38.4 | 3.7 (2.0, 5.5) | <0.001 | 40.9 | 39.7 | 1.0 (-3.2, 5.1) | 0.64 |
| 60-69 | 5,671 | 45.4 | 38.0 | 7.3 (4.5, 10.1) | <0.001 | 43.2 | 40.4 | 2.7 (-2.4, 7.8) | 0.29 |
| 70-80 | 3,949 | 47.4 | 37.3 | 10.1 (7.0, 13.2) | <0.001 | 43.4 | 41.5 | 2.0 (-3.8, 7.8) | 0.50 |
| Sex | |||||||||
| Female | 12,439 | 44.3 | 38.6 | 5.7 (4.0, 7.4) | <0.001 | 42.8 | 40.2 | 2.2 (-2.6, 7.1) | 0.36 |
| Male | 9,421 | 43.5 | 37.5 | 6.0 (4.1, 7.9) | <0.001 | 40.8 | 40.1 | 0.7 (-4.7, 6.2) | 0.79 |
|
Primary care visits |
|||||||||
| 0 | 7,643 | 19.6 | 15.6 | 3.9 (2.2, 5.6) | <0.001 | 19.1 | 16.0 | 3.0 (-1.1, 7.2) | 0.15 |
| 1-2 | 9,011 | 55.6 | 49.0 | 6.6 (4.7, 8.4) | <0.001 | 53.2 | 51.5 | 1.6 (-3.8, 7.1) | 0.56 |
| 3+ | 5,206 | 59.5 | 52.3 | 7.1 (4.4, 9.8) | <0.001 | 59.4 | 52.7 | 6.0 (-0.5, 12.5) | 0.07 |
Adjusted for clustering by physician and for physician baseline screening rate
Table 3.
Types of Colorectal Cancer Screening Tests and Pathologic Findings by Intervention Status
| Presence of Colorectal Cancer Screening Finding, n (%) | ||||||||
|---|---|---|---|---|---|---|---|---|
| Patient Mailing Intervention | Physician Reminder Intervention | |||||||
| Intervention | Control | Difference (95% CI)* |
p value* |
Intervention | Control | Difference (95% CI)* |
p value* |
|
| Individual tests performed | ||||||||
| Fecal occult blood test (FOBT) | 2,779 (25.4) | 2,225 (20.4) | 5.1 (3.8, 6.3) | <0.001 | 2,505 (23.0) | 2,499 (22.8) | 0.1 (-5.5, 5.7) | 0.96 |
| Positive FOBT† | 47 (1.7) | 12 (0.5) | 1.2 (0.6, 1.7) | <0.001 | 27 (1.1) | 32 (1.3) | -0.2 (-0.8, 0.4) | 0.52 |
| Follow-up colonoscopy‡ | 33 (70.2) | 10 (83.3) | -11.9 (-37.9, 14.1) | 0.36 | 21 (77.8) | 22 (68.8) | 7.8 (-15.4, 31.0) | 0.50 |
| Flexible sigmoidoscopy | 11 (0.1) | 9 (<0.1) | 0.0 (-0.1, 0.1) | 0.66 | 10 (<0.1) | 10 (<0.1) | 0.0 (-0.1, 0.1) | 0.99 |
| Colonoscopy | 2,014 (18.4) | 1,933 (17.7) | 0.7 (-0.3, 1.8) | 0.17 | 2,056 (18.8) | 1,891 (17.3) | 1.6 (-0.7, 3.9) | 0.18 |
| Pathologic findings | ||||||||
| Colonic adenoma | 622 (5.7) | 568 (5.2) | 0.5 (-0.1, 1.1) | 0.10 | 650 (6.0) | 540 (4.9) | 1.0 (-0.1, 2.2) | 0.09 |
| Colorectal cancer | 19 (0.2) | 15 (0.2) | 0.0 (-0.1, 0.1) | 0.43 | 17 (0.2) | 17 (0.2) | 0.0 (-0.1, 0.1) | 0..99 |
Adjusted for clustering by physician
Among those patients who performed a fecal occult blood test
Among those patients with a positive fecal occult blood test
Patients whose physicians received electronic reminders during the study period were not more likely than patients whose physicians did not receive reminders to complete colorectal screening (41.9% vs. 40.2%, p=0.47), but among patients with three or more primary care visits reminders tended to increase screening rates (59.5% vs. 52.7%, p=0.07) (Table 2). Although the overall screening rate and rate of completed colonoscopies did not increase significantly with physician reminders, these electronic reminders did increase the proportion of patients who had an order for colonoscopy placed during the study period (33.1% vs. 29.6%, p=0.004). In contrast, colonoscopy orders did not increase significantly for patients who received mailed reminders (31.8% vs. 30.9%, p=0.12). Among all patients who completed a colonoscopy, the median time from ordering to completion of this test was 49 days (interquartile range: 27 days, 85 days), suggesting adequate capacity for this procedure and acceptable waiting times.
The screening rate among patients who received mailed reminders and whose physicians received electronic reminders was 44.2%, compared with 43.7% for those in the patient intervention but not the physician intervention, 39.6% for those in the physician intervention but not the patient intervention, and 36.7% for those in neither intervention group. The interaction between the patient intervention and the physician intervention was small, negative, and not statistically significant (-0.6%; 95% CI: -1.2%, 0.1%; p=0.08), indicating that the observed effect of the combined patient and physician reminders was 0.6% less than the sum of their effects when applied individually
Detection of Adenomas and Cancers
Detection of colorectal adenomas tended to be greater among patients who received mailings (5.7% vs. 5.2%, p=0.10) and among patients of physicians receiving electronic reminders compared to the respective control groups (6.0% vs. 4.9%, p=0.09 (Table 3). Among patients with adenomas, 15% had 3 or more adenomas removed, 8% had an adenoma ≥10 mm, 1% had villous histology, and 23% had at least one of these high-risk features. Overall, 34 (0.2%) patients were newly diagnosed with colorectal cancer, with no significant differences between intervention groups (Table 3). Among these 34 incident colorectal cancers, 56% were diagnosed at an early stage (Stage 0, 1, or 2), 35% were diagnosed at a later stage (Stages 3 or 4), and 9% lacked definitive stage data.
Physician Survey
Thirty-three of 43 eligible physicians (77%) in the intervention group completed the survey. Nearly all (97%) physicians viewed colonoscopy every 10 years to be “very effective” at reducing colorectal cancer mortality, while only 3% perceived annual FOBT as similarly effective. Accordingly, all respondents (100%) reported colonoscopy as the screening test they most often recommended to patients. Physicians reported that electronic reminders accurately reflected their patients’ screening status for a median of 50% of the reminders (interquartile range: 30%, 80%). Most physicians in the intervention group reported the electronic reminders were “very effective” (9%) or “somewhat effective” (47%) in increasing the colorectal screening rate among their patients.
Comment
In a large cohort of patients who were overdue for screening, we demonstrated that personalized mailings to individual patients produced a modest increase in colorectal cancer screening, particularly by FOBT and among patients in the oldest age group, suggesting patients represent an untapped resource for improving quality of care. Patients frequently report they have not received effective counseling regarding the importance of colorectal cancer screening.11, 34 However, once eligible patients are appropriately informed, most opt to be screened for colorectal cancer.35, 36 Our findings underscore that informed patients can play an active role in completing effective preventive services.37
Electronic reminders to physicians did not significantly increase overall screening rates, in part because over one-third of patients had no visits with their primary physicians during the 15-month study period. However, physician reminders exhibited a trend toward increased overall screening rates among patients with at least 3 primary-care visits over this period. Orders for colonoscopy were modestly increased by reminders to physicians but without a corresponding increase in completed procedures, as nearly half of patients for whom a colonoscopy was ordered did not complete this procedure. This finding underscores the need for more effective communication with patients to encourage them to complete colonoscopy procedures that are scheduled.38, 39
The limited effectiveness of our electronic physician reminders may reflect the challenges primary care physicians face in providing adequate preventive counseling amid competing demands during brief office visits.12 We provided “active” alerts that required physician to respond,40 but some physicians may have disregarded the alerts if they disrupted their workflow or were deemed inaccurate.41 Although we validated the accuracy of our algorithm for detecting whether patients were up-to-date with screening, many physicians viewed the electronic reminders as substantially less accurate, and nearly half of physicians viewed the reminders as ineffective. This suggests that reminders to physicians via electronic health records may require further collaboration with practicing physicians who receive the reminders to achieve a greater impact on screening rates.
Our study highlights an important contrast between the screening strategy pursued by patients and the preferences of their physicians. The patient mailings produced a modest increase in the use of FOBT, but all physicians viewed colonoscopy as the preferred screening test for their patients. This finding is consistent with recent studies indicating a preference for fecal occult blood testing over colonoscopy among patients provided information to make an informed choice,42 whereas physicians report a strong preference to recommend colonoscopy.43 This contrast highlights one potential challenge to engaging patients in quality improvement programs. For services such as colorectal cancer screening for which multiple reasonable options exist, quality improvement programs will need to address the possibly differing preferences of patients and their physicians and develop methods to reconcile such differences.44, 45
Increased screening is essential to reduce the incidence, morbidity, and mortality of colorectal cancer. One recent study estimated that U.S. mortality from this disease could be reduced 23% by 2020 if screening rates rose to 70%.46 The importance of colorectal cancer screening has been recognized through expanded Medicare coverage for this service in 200147 and the endorsement of colorectal cancer screening as a health plan performance measure by the National Committee for Quality Assurance in 2005.48 Published studies of interventions to improve rates of colorectal cancer screening have targeted patients, physicians, or both groups.14, 15, 17-28 Physician-directed interventions such as reminders16, 23, 24, 28 and performance feedback25 have increased screening rates in some settings. Patient-directed interventions including videotaped decision aids,19 educational mailings,20, 21 and nurse counseling18 may also increase screening rates.
Our randomized trial builds on these studies in several important ways. First, these prior studies occurred in a setting where baseline screening rates were much lower than the screening rate of 63% in our population, often produced larger absolute increases in screening rates, and focused on increasing use of FOBT or flexible sigmoidoscopy. These studies may not apply to the current era in which screening rates are higher and colonoscopy has become a preferred screening strategy among physicians43 and is therefore increasingly used.47 In fact, more recent interventions that have included use of colonoscopy in their recommendations have not successfully increased overall screening rates.22, 26, 27
Our study provides important insights into the effect of interventions focused on patients who remain unscreened as screening rates rise through usual care. The modest effect of patient reminders in our study suggests the need to develop more effective strategies to actively engage these remaining patients and encourage them to be screened for colorectal cancer. However, the clear advantage of patient involvement over physician reminders in our study suggests that future strategies should increasingly involve patient-based activity. Promising alternatives include the use of the internet to facilitate patient-provider communication and promote increased patient involvement in their preventive health issues.49 Patient navigators have also been used with success in promoting cancer screening, particularly among low-income and minority groups.50
Second, our intervention simultaneously evaluated the use of personalized mailings to patients and electronic reminders to physicians. We found patient mailings were more effective than physician reminders in raising overall screening rates, and trends of borderline significance in detection of colorectal adenomas were evident with each approach. Involving patients in decisions about colorectal cancer screening fits well with models that promote informed, patients,37 moving them through the “stages of decision” from awareness of screening options through the decision to be screened.19, 51-53 Third, our large sample and rigorous study design allowed reasonably precise estimates of the intervention effects.
Fourth, the use of data from electronic medical records provided relatively complete clinical information on this large patient population, including data on clinical processes and outcomes. Approximately three-quarters of the positive fecal occult blood tests in our study population were followed by a colonoscopy. Although closing this loop is essential to realizing the benefits of a screening program,54 many studies demonstrate a similar gap in care.55-63 Physicians may not recommend appropriate follow-up testing to patients,56, 57, 62 patients may refuse further testing,57 or appropriate systems may not be in place to help clinicians identify abnormal test results and ensure appropriate follow-up.63
The generalizability of our study must also be considered. We implemented our intervention within a single group practice using an advanced electronic health record, so our findings may not apply to less structured settings. In particular, integrated medical groups generally provide higher quality care for screening services.27, 64 However, our patient mailing intervention could be implemented across a wide range of health care settings, and the adoption of electronic health records is being actively promoted to improve ambulatory care.65 Our study demonstrated how electronic data can be used to create clinical registries for outreach to patients, and it assessed the utility of decision support directly integrated with computerized order entry for physicians providing ambulatory care. After our study found that the patient mailings were effective, the integrated group practice instituted a routine protocol to identify patients overdue for colorectal cancer screening (including patients in our control group) and send them mailings regarding their need for screening.
In conclusion, this randomized trial of personalized patient mailings and electronic reminders to physicians in a large integrated group practice found that patient mailings produced modest increases in rates of colorectal cancer screening, whereas electronic physician reminders tended to promote screening only among patients who more frequently use primary care. These complementary approaches have the potential to promote the overarching goal of widespread screening to reduce the incidence, morbidity and mortality of colorectal cancer.
Supplementary Material
Acknowledgements
The authors are grateful to J. Alan Kemp MD for advice on the study design; Jo-Anne Foley, Rebecca Lobb, Amy Marston, and Debby Collins for assisting with project management; Robert Wolf and James Morrissey for data management and analysis; Aimee Shu, MD and Jeffrey Kullgren, MD, MPH for reviewing medical records; and the patients and physicians of Harvard Vanguard Medical Associates for their participation in this study. The study was funded by the National Cancer Institute (R01 CA112367). The funder played no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; or preparation, review, and approval of the manuscript.
Supported by grant R01 CA112367 from the National Cancer Institute. This study was presented at the 2008 Society of General Internal Medicine Annual Meeting in Pittsburgh, PA on April 10, 2008.
References
- 1.Jemal A, Siegel R, Ward E, Murray T, Xu J, Thun MJ. Cancer statistics, 2007. CA Cancer J Clin. 2007;57:43–66. doi: 10.3322/canjclin.57.1.43. [DOI] [PubMed] [Google Scholar]
- 2.Mandel JS, Bond JH, Church TR, et al. Reducing mortality from colorectal cancer by screening for fecal occult blood. Minnesota Colon Cancer Control Study. N Engl J Med. 1993;328:1365–1371. doi: 10.1056/NEJM199305133281901. [DOI] [PubMed] [Google Scholar]
- 3.Hardcastle JD, Chamberlain JO, Robinson MH, et al. Randomised controlled trial of faecal-occult-blood screening for colorectal cancer. Lancet. 1996;348:1472–1477. doi: 10.1016/S0140-6736(96)03386-7. [DOI] [PubMed] [Google Scholar]
- 4.Kronborg O, Fenger C, Olsen J, Jorgensen OD, Sondergaard O. Randomised study of screening for colorectal cancer with faecal-occult-blood test. Lancet. 1996;348:1467–1471. doi: 10.1016/S0140-6736(96)03430-7. [DOI] [PubMed] [Google Scholar]
- 5.Selby JV, Friedman GD, Quesenberry CP, Jr., Weiss NS. A case-control study of screening sigmoidoscopy and mortality from colorectal cancer. N Engl J Med. 1992;326:653–657. doi: 10.1056/NEJM199203053261001. [DOI] [PubMed] [Google Scholar]
- 6.Winawer SJ, Zauber AG, Ho MN, et al. Prevention of colorectal cancer by colonoscopic polypectomy. The National Polyp Study Workgroup. N Engl J Med. 1993;329:1977–1981. doi: 10.1056/NEJM199312303292701. [DOI] [PubMed] [Google Scholar]
- 7.United States Preventive Services Task Force Screening for colorectal cancer: recommendation and rationale. Ann Intern Med. 2002;137:129–131. doi: 10.7326/0003-4819-137-2-200207160-00014. [DOI] [PubMed] [Google Scholar]
- 8.Smith RA, von Eschenbach AC, Wender R, et al. American Cancer Society guidelines for the early detection of cancer: update of early detection guidelines for prostate, colorectal, and endometrial cancers. CA Cancer J Clin. 2001;51:38–75. doi: 10.3322/canjclin.51.1.38. [DOI] [PubMed] [Google Scholar]
- 9.Winawer S, Fletcher R, Rex D, et al. Colorectal cancer screening and surveillance: clinical guidelines and rationale-Update based on new evidence. Gastroenterology. 2003;124:544–560. doi: 10.1053/gast.2003.50044. [DOI] [PubMed] [Google Scholar]
- 10.Use of colorectal cancer tests--United States, 2002, 2004, and 2006. MMWR Morb Mortal Wkly Rep. 2008;57:253–258. [PubMed] [Google Scholar]
- 11.Klabunde CN, Vernon SW, Nadel MR, Breen N, Seeff LC, Brown ML. Barriers to colorectal cancer screening: a comparison of reports from primary care physicians and average-risk adults. Med Care. 2005;43:939–944. doi: 10.1097/01.mlr.0000173599.67470.ba. [DOI] [PubMed] [Google Scholar]
- 12.Yarnall KS, Pollak KI, Ostbye T, Krause KM, Michener JL. Primary care: is there enough time for prevention? Am J Public Health. 2003;93:635–641. doi: 10.2105/ajph.93.4.635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Guerra CE, Schwartz JS, Armstrong K, Brown JS, Halbert CH, Shea JA. Barriers of and facilitators to physician recommendation of colorectal cancer screening. J Gen Intern Med. 2007;22:1681–1688. doi: 10.1007/s11606-007-0396-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Stone EG, Morton SC, Hulscher ME, et al. Interventions that increase use of adult immunization and cancer screening services: a meta-analysis. Ann Intern Med. 2002;136:641–651. doi: 10.7326/0003-4819-136-9-200205070-00006. [DOI] [PubMed] [Google Scholar]
- 15.Turner BJ, Day SC, Borenstein B. A controlled trial to improve delivery of preventive care: physician or patient reminders? J Gen Intern Med. 1989;4:403–409. doi: 10.1007/BF02599691. [DOI] [PubMed] [Google Scholar]
- 16.McPhee SJ, Bird JA, Jenkins CN, Fordham D. Promoting cancer screening. A randomized, controlled trial of three interventions. Arch Intern Med. 1989;149:1866–1872. doi: 10.1001/archinte.149.8.1866. [DOI] [PubMed] [Google Scholar]
- 17.Miller DP, Jr., Kimberly JR, Jr., Case LD, Wofford JL. Using a computer to teach patients about fecal occult blood screening. A randomized trial. J Gen Intern Med. 2005;20:984–988. doi: 10.1111/j.1525-1497.2005.0081.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Stokamer CL, Tenner CT, Chaudhuri J, Vazquez E, Bini EJ. Randomized controlled trial of the impact of intensive patient education on compliance with fecal occult blood testing. J Gen Intern Med. 2005;20:278–282. doi: 10.1111/j.1525-1497.2005.40023.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pignone M, Harris R, Kinsinger L. Videotape-based decision aid for colon cancer screening. A randomized, controlled trial. Ann Intern Med. 2000;133:761–769. doi: 10.7326/0003-4819-133-10-200011210-00008. [DOI] [PubMed] [Google Scholar]
- 20.Myers RE, Sifri R, Hyslop T, et al. A randomized controlled trial of the impact of targeted and tailored interventions on colorectal cancer screening. Cancer. 2007;110:2083–2091. doi: 10.1002/cncr.23022. [DOI] [PubMed] [Google Scholar]
- 21.Church TR, Yeazel MW, Jones RM, et al. A randomized trial of direct mailing of fecal occult blood tests to increase colorectal cancer screening. J Natl Cancer Inst. 2004;96:770–780. doi: 10.1093/jnci/djh134. [DOI] [PubMed] [Google Scholar]
- 22.Zapka JG, Lemon SC, Puleo E, Estabrook B, Luckmann R, Erban S. Patient education for colon cancer screening: a randomized trial of a video mailed before a physical examination. Ann Intern Med. 2004;141:683–692. doi: 10.7326/0003-4819-141-9-200411020-00009. [DOI] [PubMed] [Google Scholar]
- 23.Balas EA, Weingarten S, Garb CT, Blumenthal D, Boren SA, Brown GD. Improving preventive care by prompting physicians. Arch Intern Med. 2000;160:301–308. doi: 10.1001/archinte.160.3.301. [DOI] [PubMed] [Google Scholar]
- 24.Shea S, DuMouchel W, Bahamonde L. A meta-analysis of 16 randomized controlled trials to evaluate computer-based clinical reminder systems for preventive care in the ambulatory setting. J Am Med Inform Assoc. 1996;3:399–409. doi: 10.1136/jamia.1996.97084513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ferreira MR, Dolan NC, Fitzgibbon ML, et al. Health care provider-directed intervention to increase colorectal cancer screening among veterans: results of a randomized controlled trial. J Clin Oncol. 2005;23:1548–1554. doi: 10.1200/JCO.2005.07.049. [DOI] [PubMed] [Google Scholar]
- 26.Walsh JM, Salazar R, Terdiman JP, Gildengorin G, Perez-Stable EJ. Promoting use of colorectal cancer screening tests. Can we change physician behavior? J Gen Intern Med. 2005;20:1097–1101. doi: 10.1111/j.1525-1497.2005.0245.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ganz PA, Farmer MM, Belman MJ, et al. Results of a randomized controlled trial to increase colorectal cancer screening in a managed care health plan. Cancer. 2005;104:2072–2083. doi: 10.1002/cncr.21434. [DOI] [PubMed] [Google Scholar]
- 28.McPhee SJ, Bird JA, Fordham D, Rodnick JE, Osborn EH. Promoting cancer prevention activities by primary care physicians. Results of a randomized, controlled trial. JAMA. 1991;266:538–544. [PubMed] [Google Scholar]
- 29.Levin B, Lieberman DA, McFarland B, et al. Screening and surveillance for the early detection of colorectal cancer and adenomatous polyps, 2008: a joint guideline from the American Cancer Society, the US Multi-Society Task Force on Colorectal Cancer, and the American College of Radiology. Gastroenterology. 2008;134:1570–1595. doi: 10.1053/j.gastro.2008.02.002. [DOI] [PubMed] [Google Scholar]
- 30.Greene FL, Page DL, Fleming ID, Balch CM, Haller DG, Morrow M. AJCC Cancer Staging Manual. 6th ed Lippincott Raven; 2002. [Google Scholar]
- 31.Beydoun HA, Beydoun MA. Predictors of colorectal cancer screening behaviors among average-risk older adults in the United States. Cancer Causes Control. 2007 doi: 10.1007/s10552-007-9100-y. [DOI] [PubMed] [Google Scholar]
- 32.Zapka JG, Puleo E, Vickers-Lahti M, Luckmann R. Healthcare system factors and colorectal cancer screening. Am J Prev Med. 2002;23:28–35. doi: 10.1016/s0749-3797(02)00444-0. [DOI] [PubMed] [Google Scholar]
- 33.Zarychanski R, Chen Y, Bernstein CN, Hebert PC. Frequency of colorectal cancer screening and the impact of family physicians on screening behaviour. CMAJ. 2007;177:593–597. doi: 10.1503/cmaj.070558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wee CC, McCarthy EP, Phillips RS. Factors associated with colon cancer screening: the role of patient factors and physician counseling. Prev Med. 2005;41:23–29. doi: 10.1016/j.ypmed.2004.11.004. [DOI] [PubMed] [Google Scholar]
- 35.Leard LE, Savides TJ, Ganiats TG. Patient preferences for colorectal cancer screening. J Fam Pract. 1997;45:211–218. [PubMed] [Google Scholar]
- 36.Pignone M, Bucholtz D, Harris R. Patient preferences for colon cancer screening. J Gen Intern Med. 1999;14:432–437. doi: 10.1046/j.1525-1497.1999.00018.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bodenheimer T, Wagner EH, Grumbach K. Improving primary care for patients with chronic illness: the chronic care model, Part 2. JAMA. 2002;288:1909–1914. doi: 10.1001/jama.288.15.1909. [DOI] [PubMed] [Google Scholar]
- 38.Denberg TD, Coombes JM, Byers TE, et al. Effect of a mailed brochure on appointment-keeping for screening colonoscopy: a randomized trial. Ann Intern Med. 2006;145:895–900. doi: 10.7326/0003-4819-145-12-200612190-00006. [DOI] [PubMed] [Google Scholar]
- 39.Turner BJ, Weiner M, Berry SD, Lillie K, Fosnocht K, Hollenbeak CS. Overcoming poor attendance to first scheduled colonoscopy: a randomized trial of peer coach or brochure support. J Gen Intern Med. 2008;23:58–63. doi: 10.1007/s11606-007-0445-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Litzelman DK, Dittus RS, Miller ME, Tierney WM. Requiring physicians to respond to computerized reminders improves their compliance with preventive care protocols. J Gen Intern Med. 1993;8:311–317. doi: 10.1007/BF02600144. [DOI] [PubMed] [Google Scholar]
- 41.Ash JS, Berg M, Coiera E. Some unintended consequences of information technology in health care: the nature of patient care information system-related errors. J Am Med Inform Assoc. 2004;11:104–112. doi: 10.1197/jamia.M1471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.DeBourcy AC, Lichtenberger S, Felton S, Butterfield KT, Ahnen DJ, Denberg TD. Community-based preferences for stool cards versus colonoscopy in colorectal cancer screening. J Gen Intern Med. 2008;23:169–174. doi: 10.1007/s11606-007-0480-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Klabunde CN, Frame PS, Meadow A, Jones E, Nadel M, Vernon SW. A national survey of primary care physicians’ colorectal cancer screening recommendations and practices. Prev Med. 2003;36:352–362. doi: 10.1016/s0091-7435(02)00066-x. [DOI] [PubMed] [Google Scholar]
- 44.Klabunde CN, Lanier D, Breslau ES, et al. Improving colorectal cancer screening in primary care practice: innovative strategies and future directions. J Gen Intern Med. 2007;22:1195–1205. doi: 10.1007/s11606-007-0231-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lafata JE, Divine G, Moon C, Williams LK. Patient-physician colorectal cancer screening discussions and screening use. Am J Prev Med. 2006;31:202–209. doi: 10.1016/j.amepre.2006.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Vogelaar I, van Ballegooijen M, Schrag D, et al. How much can current interventions reduce colorectal cancer mortality in the U.S.? Mortality projections for scenarios of risk-factor modification, screening, and treatment. Cancer. 2006;107:1624–1633. doi: 10.1002/cncr.22115. [DOI] [PubMed] [Google Scholar]
- 47.Gross CP, Andersen MS, Krumholz HM, McAvay GJ, Proctor D, Tinetti ME. Relation between Medicare screening reimbursement and stage at diagnosis for older patients with colon cancer. JAMA. 2006;296:2815–2822. doi: 10.1001/jama.296.23.2815. [DOI] [PubMed] [Google Scholar]
- 48.Schneider EC, Nadel MR, Zaslavsky AM, McGlynn EA. Assessment of the Scientific Soundness of Clinical Performance Measures: A Field Test of the National Committee for Quality Assurance’s Colorectal Cancer Screening Measure. Arch Intern Med. 2008;168:876–882. doi: 10.1001/archinte.168.8.876. [DOI] [PubMed] [Google Scholar]
- 49.Poon EG, Wald J, Schnipper JL, et al. Empowering patients to improve the quality of their care: design and implementation of a shared health maintenance module in a US integrated healthcare delivery network. Medinfo. 2007;12:1002–1006. [PubMed] [Google Scholar]
- 50.Christie J, Itzkowitz S, Lihau-Nkanza I, Castillo A, Redd W, Jandorf L. A randomized controlled trial using patient navigation to increase colonoscopy screening among low-income minorities. J Natl Med Assoc. 2008;100:278–284. doi: 10.1016/s0027-9684(15)31240-2. [DOI] [PubMed] [Google Scholar]
- 51.Prochaska JO, DiClemente CC. Stages and processes of self-change of smoking: toward an integrative model of change. J Consult Clin Psychol. 1983;51:390–395. doi: 10.1037//0022-006x.51.3.390. [DOI] [PubMed] [Google Scholar]
- 52.Sarfaty M, Wender R. How to increase colorectal cancer screening rates in practice. CA Cancer J Clin. 2007;57:354–366. doi: 10.3322/CA.57.6.354. [DOI] [PubMed] [Google Scholar]
- 53.Arora NK, Ayanian JZ, Guadagnoli E. Examining the relationship of patients’ attitudes and beliefs with their self-reported level of participation in medical decision-making. Med Care. 2005;43:865–872. doi: 10.1097/01.mlr.0000173560.18607.67. [DOI] [PubMed] [Google Scholar]
- 54.Yabroff KR, Washington KS, Leader A, Neilson E, Mandelblatt J. Is the promise of cancer-screening programs being compromised? Quality of follow-up care after abnormal screening results. Med Care Res Rev. 2003;60:294–331. doi: 10.1177/1077558703254698. [DOI] [PubMed] [Google Scholar]
- 55.Levin B, Hess K, Johnson C. Screening for colorectal cancer. A comparison of 3 fecal occult blood tests. Arch Intern Med. 1997;157:970–976. [PubMed] [Google Scholar]
- 56.Shields HM, Weiner MS, Henry DR, et al. Factors that influence the decision to do an adequate evaluation of a patient with a positive stool for occult blood. Am J Gastroenterol. 2001;96:196–203. doi: 10.1111/j.1572-0241.2001.03475.x. [DOI] [PubMed] [Google Scholar]
- 57.Baig N, Myers RE, Turner BJ, et al. Physician-reported reasons for limited follow-up of patients with a positive fecal occult blood test screening result. Am J Gastroenterol. 2003;98:2078–2081. doi: 10.1111/j.1572-0241.2003.07575.x. [DOI] [PubMed] [Google Scholar]
- 58.Turner B, Myers RE, Hyslop T, et al. Physician and patient factors associated with ordering a colon evaluation after a positive fecal occult blood test. J Gen Intern Med. 2003;18:357–363. doi: 10.1046/j.1525-1497.2003.20525.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Etzioni DA, Yano EM, Rubenstein LV, et al. Measuring the quality of colorectal cancer screening: the importance of follow-up. Dis Colon Rectum. 2006;49:1002–1010. doi: 10.1007/s10350-006-0533-2. [DOI] [PubMed] [Google Scholar]
- 60.Fisher DA, Jeffreys A, Coffman CJ, Fasanella K. Barriers to full colon evaluation for a positive fecal occult blood test. Cancer Epidemiol Biomarkers Prev. 2006;15:1232–1235. doi: 10.1158/1055-9965.EPI-05-0916. [DOI] [PubMed] [Google Scholar]
- 61.Lurie JD, Welch HG. Diagnostic testing following fecal occult blood screening in the elderly. J Natl Cancer Inst. 1999;91:1641–1646. doi: 10.1093/jnci/91.19.1641. [DOI] [PubMed] [Google Scholar]
- 62.Nadel MR, Shapiro JA, Klabunde CN, et al. A national survey of primary care physicians’ methods for screening for fecal occult blood. Ann Intern Med. 2005;142:86–94. doi: 10.7326/0003-4819-142-2-200501180-00007. [DOI] [PubMed] [Google Scholar]
- 63.Klabunde CN, Riley GF, Mandelson MT, Frame PS, Brown ML. Health plan policies and programs for colorectal cancer screening: a national profile. Am J Manag Care. 2004;10:273–279. [PubMed] [Google Scholar]
- 64.Mehrotra A, Epstein AM, Rosenthal MB. Do integrated medical groups provide higher-quality medical care than individual practice associations? Ann Intern Med. 2006;145:826–833. doi: 10.7326/0003-4819-145-11-200612050-00007. [DOI] [PubMed] [Google Scholar]
- 65.Bates DW. Physicians and ambulatory electronic health records. U.S. Physicians are ready to make the transition to EHRs--which is clearly overdue, given the rest of the world’s experience. Health Aff. 2005;24:1180–1189. doi: 10.1377/hlthaff.24.5.1180. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.