Abstract
Objective:
To test the hypothesis that use of antihypertensive medication is associated with lower Alzheimer disease (AD) neuropathology.
Methods:
This was a postmortem study of 291 brains limited to those with normal neuropathology or with uncomplicated AD neuropathology (i.e., without other dementia-associated neuropathology) in persons with or without hypertension (HTN) who were and were not treated with antihypertensive medications. Neuritic plaque (NP) and neurofibrillary tangle (NFT) densities, quantified in selected brain regions according to the Consortium to Establish a Registry for Alzheimer’s Disease (CERAD) neuropathologic criteria, with additional cortical NP counts, yielded 24 neuropathologic regional measures or summaries. Medicated hypertension (HTN-med; n = 77), nonmedicated HTN (HTN-nomed; n = 42), and non-HTN (no-HTN; n = 172) groups were compared by analyses of variance.
Results:
The HTN-med group had significantly less neuropathology than the no-HTN group. The no-HTN group averaged over 50% higher mean NP and NFT ratings, and double the mean NP count, of the HTN-med group. The HTN-nomed group had significantly more neuropathology than the HTN-med group, but not significantly less than the no-HTN group.
Conclusions:
There was substantially less Alzheimer disease (AD) neuropathology in the medicated hypertension group than the nonhypertensive group, which may reflect a salutary effect of antihypertensive medication against AD-associated neuropathology.
GLOSSARY
- AD
= Alzheimer disease;
- ANCOVA
= analysis of covariance;
- ANOVA
= analysis of variance;
- BB
= β-blockers;
- BMI
= body mass index;
- CCB
= calcium channel blockers;
- CDR
= Clinical Dementia Rating scale;
- CERAD
= Consortium to Establish a Registry for Alzheimer’s Disease;
- DBP
= diastolic blood pressure;
- EC
= entorhinal cortex;
- HAAS
= Honolulu Asia Aging Study;
- Hipp
= hippocampus;
- HTN
= hypertension;
- IPL
= inferior parietal lobule;
- JHH
= Jewish Home and Hospital;
- MFG
= midfrontal gyrus;
- MSSM
= Mount Sinai School of Medicine;
- NFT
= neurofibrillary tangle;
- NH
= nursing homes;
- NP
= neuritic plaque;
- OC
= occipital calcarine cortex;
- OFG
= orbital frontal cortex;
- SBP
= systolic blood pressure;
- STG
= superior temporal gyrus.
Hypertension has been associated with cognitive decline, dementia, vascular dementia, and Alzheimer disease (AD) in some studies1–5 but not others.6–9 Elevated systolic blood pressure (SBP) or diastolic blood pressure (DBP) levels, or both, have been reported decades before onset of AD symptoms.4,10–12 Elevated midlife SBP was associated with lower brain weight and increased neuritic plaque (NP) densities in neocortex and hippocampus (Hipp), and elevated DBP was associated with increased NFT densities in Hipp.13 Additionally, elevated midlife SBP and DBP14 were correlated with late-life hippocampal atrophy on MRI in untreated patients clinically diagnosed with AD.
Antihypertensive treatments have been associated with lower incidence of clinically diagnosed AD and better cognitive function15–17; similarly, treatment of hypertensive subjects was associated with lower dementia risk.18–20 However, the specific classes of anti-HTN medication with salutary properties have been inconsistent. The associations between HTN and clinically diagnosed AD4 and hippocampal atrophy14 were less apparent in medicated than nonmedicated subjects. In contrast, several investigators failed to find treatment effects in reducing late-life AD or dementia,21–24 which may reflect methodologic factors including variance in estimates of dementia prevalence,25 and imprecision of clinical diagnoses in distinguishing among the various types of dementia.25,26 Alternatively, overmedication may induce hypotension, which has been correlated with cognitive deficits in persons 80 years and older.27,28 Interestingly, lower dementia risk has been reported in elderly subjects with persistent elevated BP, despite antihypertensive treatments, compared to normotensive subjects29 and to controlled medicated hypertensive subjects.19
This postmortem study was designed to determine the specific association of AD neuropathology with midlife or late-life HTN, and the effect of antihypertensive treatments. The extent of neuropathology was evaluated by Consortium to Establish a Registry for Alzheimer’s Disease (CERAD),30 NP and NFT density ratings, and quantitative neocortical NP counts. Persons with sole or comorbid cerebrovascular disease were excluded to permit direct study of the association of HTN and treatment of HTN with the cardinal neuropathologic lesions of AD.
METHODS
Subjects.
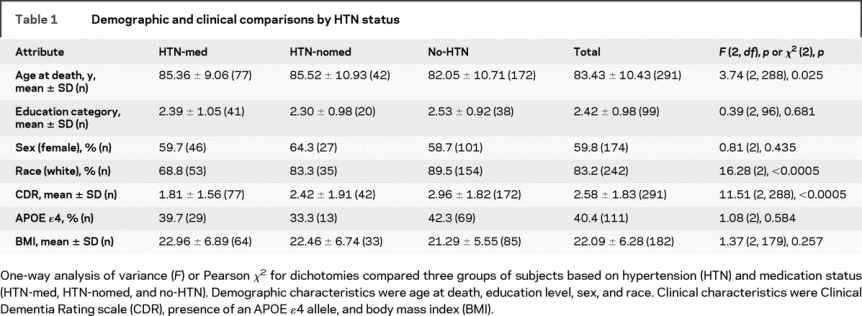
Postmortem brains were received over a period of 20 years by the Mount Sinai School of Medicine (MSSM) Department of Psychiatry Brain Bank. Demographic characteristics from the 291 subjects are shown in table 1. Brains were donated by the next of kin of deceased residents of the Jewish Home and Hospital (JHH) in Manhattan and Bronx, NY, and other New York City area nursing homes (NH) or elder-living facilities participating in studies of aging and early dementia. All assessments were approved by the institutional review boards of the JHH and MSSM; consent for autopsy and neurobiological studies of the brain was obtained from the legal next of kin of all donors. Inclusion criteria were age at death at least 60 years and either normal brain tissue or primary neuropathology with only AD-associated lesions30 as described below.
Table 1 Demographic and clinical comparisons by HTN status
Assessment of HTN.
Extensive medical records, with a medical history and physical examination at admission and medical examinations monthly, were available on all subjects. HTN status was based upon a diagnosis by the admitting or primary care physician (a geriatrician or internist), or from documented treatment with antihypertensive medications. When hypertension was not specifically addressed in the medical record, the American Heart Association criterion for abnormal blood pressure of ≥140/90 mm Hg31 was used, while recognizing that specific values may be less clinically meaningful in the elderly.32 Information on duration and treatment of HTN before NH admission was not consistently available.
Antihypertensive medications.
Hypertensive subjects were further classified as medicated or not medicated according to their recorded medication histories and supervised intake. The sample was grouped into 77 (26.5%) HTN-med, 42 (14.4%) HTN-nomed, and 172 (59.1%) no-HTN. Medications were classified into pharmacologic categories.33 Only four categories that included over 10% of the 77 HTN-med subjects were used for analysis: ACE inhibitors including angiotensin receptor blocking agents, β-blockers (BB), calcium channel blockers (CCB), and thiazide diuretics. For each pair of categories of HTN medications, at least six subjects took both.
Cognitive and functional assessment.
The Clinical Dementia Rating scale (CDR) assesses cognitive and functional impairments associated with dementia and provides specific severity criteria for classifying subjects as no dementia (CDR = 0), questionable dementia (CDR = 0.5), or increasing levels of severity of dementia from CDR = 1 to CDR = 5.34 A previously described multistep approach was applied for the postmortem assignment of CDR for all subjects, based on cognitive and functional status during the last 6 months of life.35,36 Research staff, blind to both the hypotheses being tested and to neuropathology findings, conducted structured CDR assessments of subjects during life, reviewed detailed neuropsychological testing results and medical records, and whenever possible, conducted in-depth structured interviews with staff, family caregivers, or both, to obtain information about antemortem functional and cognitive status. The CDR average score for the 6-month perimortem period was 2.58 ± 1.83, reflecting generally moderate to severe dementia.
Neuropathologic assessment.
The neuropathologic assessment procedures we used have been described in detail.35,36 Standardized representative blocks from superior and midfrontal gyrus (MFG), orbital cortex, basal ganglia with basal forebrain, amygdala, Hipp (rostral and caudal levels with adjacent parahippocampal and inferior temporal cortex), superior temporal gyrus (STG), parietal cortex (angular gyrus), calcarine cortex, hypothalamus with mammillary bodies, thalamus, midbrain, pons, medulla, cerebellar vermis, and lateral cerebellar hemisphere were examined using hematoxylin and eosin, modified Bielschowsky, modified thioflavin S, and anti-β amyloid and anti-tau when necessary. Neuropathologists (e.g., D.P.P.) were blinded to all clinical and psychometric data while evaluating slides for presence and extent of relevant neuropathologic lesions.
Every case was evaluated for the extent of neuropathologic lesions using the CERAD neuropathologic battery30 to quantify NPs and NFTs in seven previously defined regions (Hipp [CA1], entorhinal cortex [EC], amygdala, MFG, STG, the inferior parietal lobule [IPL], and the occipital calcarine cortex [OC]) using the four-point CERAD scale (0 = none, 1 = sparse, 3 = moderate, 5 = severe).35,36 Additionally, quantitative data regarding NP densities were collected in five cortical regions (MFG, orbital frontal cortex, STG, IPL, and OC) using previously published methods36 and mean plaque density per mm2 was calculated for each region.
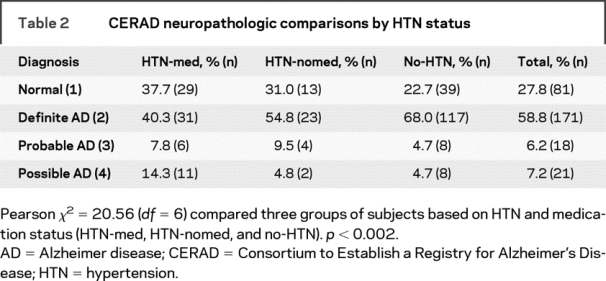
The qualitative CERAD neuropathologic diagnoses were also evaluated (table 2). The neuropathology-based inclusion/exclusion criteria for the selected cases were similar to those already described.37 They consisted of persons whose neuropathologic examination revealed either no significant neuropathology (CERAD30 neuropathology diagnosis of 1) or primary neuropathology of AD (CERAD neuropathology diagnosis of 2 [definite AD], 3 [probable AD], or 4 [possible AD]), with no significant secondary diagnoses (e.g., significant cerebrovascular lesions [as defined by CERAD or Tomlinson et al.38] or Lewy bodies with or without NPs and NFTs).
Table 2 CERAD neuropathologic comparisons by HTN status
Statistical analyses.
One-way analysis of variance (ANOVA), or Pearson χ2 for dichotomies, compared three groups of subjects based on HTN and medication status (HTN-med, HTN-nomed, and no-HTN) on demographic (age at death, educational level, sex, and race) and clinical (CDR, presence of an APOE ɛ4 allele, and body mass index [BMI]) characteristics.
ANOVA compared HTN-med, HTN-nomed, and no-HTN groups on 24 direct neuropathologic measures: regional CERAD NP and NFT ratings from all seven brain regions, sums of NP and NFT ratings from neocortex (MF, SMT, IP, and OV) and all seven regions, and the five NP counts and their mean. To describe the pattern of group differences, similar ANOVAs were calculated for each pair of groups. The Holm39 procedure was used to control for 24 multiple tests of significance of NP and NFT densities, at the 0.05 experiment-wise significance level. For these analyses, comparing the different diagnostic and medication categories (HTN-med, HTN-nomed, and no-HTN), only Holm-corrected significances of p < 0.05 are reported unless otherwise indicated. Additionally, to provide an overall measure of AD-associated neuropathology that was not penalized for multiple comparisons, a quantitative neuropathologic average for AD-associated lesions was calculated from the standardized scores of the 19 distinct regional NP and NFT density measurements.
Analyses of HTN medication categories and pairs of categories were limited to HTN-med subjects, excluding HTN-nomed subjects. Analysis of covariance (ANCOVA) compared subjects using or not using the HTN medication category, controlling for use of the other HTN medication categories. For each pair of HTN medication categories, a two-way ANCOVA assessed the effects on neuropathology of the two categories, with the other HTN medication categories as covariates. ANCOVA was also used to evaluate the effect of controlling for age at death, educational level, sex, race, presence of the APOE ɛ4 allele, or BMI when comparing the three groups on neuropathology measures and the neuropathologic average. CDR was not used as a covariate because the requirement of homogeneous regression coefficients necessary for ANCOVA was often not satisfied.
RESULTS
Comparisons of the three groups on the demographic and clinical characteristics used as covariates in secondary analyses are presented in table 1. The average age at death was 83.4 ± 10.4 years; 174 (59.8%) were female. No-HTN subjects were slightly younger at death than those in the other two groups (p = 0.025; F = 3.74; df = 2,288). The three groups differed on race (p < 0.0005; χ2 = 16.28; df = 2); white subjects included fewer HTN-med and more no-HTN subjects than all others. HTN-med subjects had the lowest level of dementia (lowest CDR) and no-HTN subjects had the highest (p < 0.0005; F = 11.51; df = 2,288). Correspondingly, HTN-med subjects had the highest proportions of CERAD normal, and lowest of CERAD definite AD diagnoses, whereas no-HTN subjects had fewest CERAD normal and most CERAD definite AD (p < 0.002; χ2 = 20.56; df = 6; see table 2). The groups did not differ on educational level, sex, presence of the APOE ɛ4 allele, or BMI.
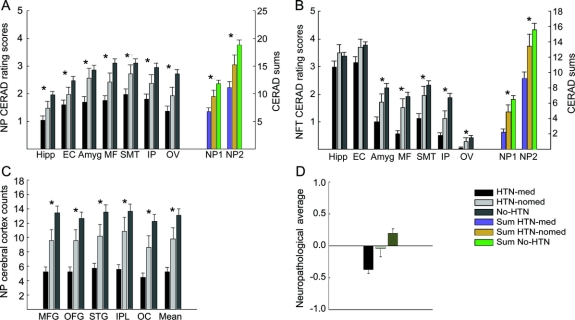
Comparisons of the three groups on the direct neuropathology density measures and the neuropathologic average are presented in the figure. HTN-med subjects had the least and no-HTN subjects had the most NP and NFT neuropathology (p ≤ 0.009 with 20 having p < 0.0005; F = 4.83 to 17.09; df = 2,269 to 2,287), except for NFTs in Hipp and EC, where group differences were not significant. The neuropathologic average for AD-associated lesions also differed (p < 0.0005; F = 13.56; df = 2,259). Controlling for age at death, educational level, sex, race, presence of the APOE ɛ4 allele, or BMI by ANCOVA yielded comparable effect sizes, for analyses of the direct regional neuropathology measures and the neuropathologic average among the three groups.
Figure Means and standard errors of means for three groups based on hypertension (HTN) and medication status
*p < 0.05 for analysis of variance comparing the three groups after Holm correction for multiple tests of significance. (A) Alzheimer disease (AD) neuritic plaque (NP) density scores. Left axis: Consortium to Establish a Registry for Alzheimer’s Disease (CERAD) rating scores reflecting NP density (with and without cores) for specific brain regions: hippocampus (Hipp), amygdala (Amyg), entorhinal cortex (EC), mid frontal cortex (MF), superior middle temporal cortex (SMT), inferior parietal cortex (IP), and occipital primary visual cortex (OC). Right axis: Sum of CERAD rating scores: NP1 sums MF, SMT, IP, and OC; NP2 sums Hipp, Amyg, EC, MF, SMT, IP, and OC. (B) AD neurofibrillary density scores. Left axis: CERAD rating scores reflecting neurofibrillary tangle density in various brain regions (Hipp, Amyg, EC, MF, SMT, IP, and OC). All except NFT in Hipp EC and OV were significant (p < 0.021 to p < 0.0005). Right axis: Sum of CERAD rating scores: NFT1 sums MF, SMT, IP, and OC; NFT2 sums Hipp, Amyg, EC, MF, SMT, IP, and OC. (C) Mean plaque density per mm2: total NP counts (with and without cores) in CERAD-defined neocortical brain regions: middle frontal gyrus (MFG), orbital frontal cortex (OFG), SMT, inferior parietal lobule (IPL), OC, and their mean. (D) Neuropathologic average of standardized NP and NFT density measurements. HTN-med vs no-HTN, p < 0.0005.
The significance of brain regional differences among the three groups reflected differences between pairs of groups. There was significantly less neuropathology in the HTN-med than the no-HTN group except NFT in Hipp (p ≤ 0.011 with 21 comparisons having p < 0.0005; F = 6.56 to 33.89; df = 1,229 to 1,246). The neuropathologic average also differed (p < 0.0005; F = 27.56; df = 1,221). When comparing HTN-med and HTN-nomed groups, lower neuropathology was noted in NFT ratings in MF and NP counts in IPL and the mean (p = 0.002 to 0.001; F = 10.26 to 11.69; df = 1,116 to 1,117). In contrast, there were no significant differences between the HTN-nomed and no-HTN groups. Additionally, 16 of the remaining differences between the HTN-med and HTN-nomed groups had p < 0.05 without correction for multiple testing, but no differences between HTN-nomed and no-HTN groups achieved p < 0.05.
None of the comparisons involving the effects of antihypertensive medication categories or pairs of categories on NP or NFT neuropathology achieved statistical significance after correcting for multiple comparisons. Nonetheless, in all evaluations of medication categories with p < 0.05, medication users of BB or CCB had less neuropathology than nonusers (p = 0.045 to 0.021; F = 4.13 to 5.52; df = 1,87 to 1,88).
DISCUSSION
The results of this study showed consistently and robustly less AD-associated neuropathology in hypertensive persons who had been treated pharmacologically for HTN relative to normotensive subjects. The only exception was no effect of HTN or medication on NFT density in the hippocampus. Since hippocampal involvement has been implicated in the course of AD and in normal aging, the relatively late-life postmortem assessments performed in this study may not have been sensitive to HTN and HTN medication effects in this region.35
The present results identified differences between HTN-med and HTN-nomed groups but not between HTN-nomed and no-HTN groups. Thus differences observed in the severity of AD-associated pathology are more likely to be attributable to HTN medication than to HTN per se. This is consistent with clinical findings of lower dementia risk in subjects with persistent elevated BP, despite antihypertensive treatments.19,29 Furthermore, these results, consistent with the Honolulu Asia Aging Study (HAAS) and Rotterdam findings,13,14 provide a neuropathologic basis to support the clinical18,20 and population studies4,15–17,19 that found significant reductions in dementia and AD risk in persons treated with antihypertensive medications. Antiamyloidogenic and neuroprotective effects of the antihypertensive drug valsartan were reported in the Tg2576 AD mouse model, even at a dose corresponding to half the lowest recommended dose for HTN treatment in humans,33 providing evidence that at least some antihypertensive medications can directly affect biologic processes involved in AD. Together with these results, this study suggests that antihypertensive medications may have a salutary effect against AD-associated neuropathology.
The findings that HTN-nomed subjects had comparable neuropathology to no-HTN subjects differ in part from the HAAS,13 where nonmedicated subjects with normal midlife DBP had less AD neuropathology than nonmedicated subjects who had borderline or high midlife DBP. Many clinical comparisons of hypertensive subjects to nonmedicated normotensive subjects—both medicated and nonmedicated—showed either no effects of HTN or deleterious effects consistent with HAAS. Discrepant results among these studies may be attributed not only to diagnostic criteria differences for HTN and dementia, but also to age-related differences: defining midlife and late life, at study entry, and for hypertension diagnosis. The present findings of uniformly less neuropathology in untreated hypertensive subjects than normotensive subjects, although not significant, are consistent with a clinical report of better cognitive performance in untreated hypertensive subjects compared with normotensive subjects in a very old population.29 Furthermore, the pattern that emerges from a review of the longitudinal and cross-sectional investigations of HTN and cognitive functioning is that, although elevated midlife hypertension is associated with cognitive decline in late life, mild hypertension after 70 years of age may be protective against AD-associated neuropathologic lesions.40 Further studies are necessary to investigate how the possibly nonlinear relationship of BP and cognition decline varies with age.
The differences between the current findings and the HAAS neuropathologic results may be attributable to the operationalization of hypertension as midlife DBP, compared with a late-life or prior diagnosis of hypertension in the present study. Additionally, there were major demographic differences between the two studies: HAAS subjects were community-based, all male, and all of Japanese descent, in contrast to our NH sample, of which the majority had dementia, were women, and were Caucasians. Finally, the no-HTN group was more likely than the HTN-nomed group to include subjects with hypotension, which has been correlated with cognitive deficits in persons 80 years and older.27,28
A potential confound in the present study is that hypertension in the HTN-med group was presumably more severe than in the HTN-nomed group. This possibility suggests the need for caution in attributing the current findings to the beneficial effects of HTN medications alone. Intuitive interpretations and results from the population studies cited above would suggest that the more severely ill subjects should have had more neuropathology. Therefore, this cannot explain the significantly lower levels of AD-associated neuropathology observed in the HTN-med group of this study.
In the present study, the three groups differed significantly in dementia severity (CDR), consistent with the very strong correlations between neuropathology and CDR. A medication effect on neuropathology is consistent with differences in cognitive performance observed in some clinical studies of HTN medication effects.18,20 The groups also differed significantly in age at death, albeit minimally with mean differences less than 3.5 years, but ANCOVA adjusting for the effect of age at death did not change the results. Similarly, ANCOVAs of the three groups, controlling for educational level, sex, race, presence of an APOE ɛ4 allele, or BMI, showed comparable results, suggesting that the conclusion of observed group differences was not appreciably influenced by these characteristics.
The analyses of antihypertensive medication categories and pairs of categories, limited to HTN-med subjects, had reduced power due to the relatively small sample. Thus findings with p < 0.05 that did not reach significance after correction for multiple tests should be interpreted with caution. However, all drug category results with p < 0.05 indicated that treated subjects had less neuropathology than untreated subjects. The exploratory nature of these comparisons warrants caution, but they provide impetus for future prospective investigations of the effects of different classes of potentially beneficial anti-HTN medications as well as interactions among them.
The apparent effects of HTN medication must be interpreted with caution due to the cross-sectional design of this and all postmortem observational studies. This study was further limited by the lack of reliable information on the etiology, extent, and severity of prestudy enrollment hypertension, length and effectiveness of preenrollment therapy, changing criteria for the diagnosis and treatment of HTN over time, medications used before late-life enrollment in our longitudinal study and consequent potential misclassification of subjects. Thus, although the current study suggests that treatment of HTN may beneficially influence the hallmark neuropathologic lesions of AD and AD-associated dementia, firm conclusions must await confirmatory and prospective studies.
In contrast to the limitations of prestudy HTN history outlined above, a strength of this study was that its observations (averaging approximately 3.5 years) regarding HTN status and HTN medications were based on close supervision and well-maintained medical records. Misclassification among the three groups was therefore unlikely, given the comprehensive, supervised medical care and detailed records in the nursing home/hospital setting, as opposed to self-report and often self-medicating practices among community dwellers. Even if some hypertensive subjects were medicated before entering this study but not during it (perhaps due to therapeutic prejudice by physicians reticent to treat a chronic condition in patients with advanced cognitive loss), misclassification of HTN-med subjects as HTN-nomed would only reduce the strength but not change the direction of the observed effects. Similarly, if subjects were diagnosed with hypertension before entering this study and not medicated, but not diagnosed as hypertensive during the study, misclassification of HTN-nomed subjects as no-HTN would tend to reduce the magnitude of any differences between these two groups. Additionally, both types of misclassification would reduce the differences between the HTN-med and no-HTN groups. Therefore, the highly significant findings demonstrating consistently robust differences of lower AD neuropathology in HTN-med than no-HTN subjects can be viewed with relatively high confidence. The relatively large sample sizes used in this study and the use of quantitative counts as well as qualitative CERAD ratings provide additional confidence in the results.
AUTHOR CONTRIBUTIONS
Statistical analysis was performed by James Schmeidler and Lisa B. Hoffman.
Supplementary Material
Address correspondence and reprint requests to Dr. L. Hoffman, Psychiatry, Room 4F-20, Bronx VA Medical Center, 130 West Kingsbridge Road, Bronx, NY 10468 lisa.b.hoffman@mssm.edu
Editorial, page 1716
See also page 1727
e-Pub ahead of print on February 18, 2009, at www.neurology.org.
Supported by NIA grants AG02219 and AG05138 and the Dextra Baldwin McGonagle and Joseph E. and Norma G. Saul Foundations.
Disclosure: The authors report no disclosures.
Received September 4, 2008. Accepted in final form January 5, 2009.
REFERENCES
- 1.Luchsinger JA, Reitz C, Honig LS, Tang MX, Shea S, Mayeux R. Aggregation of vascular risk factors and risk of incident Alzheimer disease. Neurology 2005;65:545–551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Elias MF, Wolf PA, D’Agostino RB, Cobb J, White LR. Untreated blood pressure level is inversely related to cognitive functioning: The Framingham Study. Am J Epidemiol 2008;138:353–364. [DOI] [PubMed] [Google Scholar]
- 3.Peila R, White LR, Petrovich H, et al. Joint effect of the APOE gene and midlife systolic blood pressure on late-life cognitive impairment: the Honolulu-Asia Aging Study. Stroke 2001;32:2882–2887. [DOI] [PubMed] [Google Scholar]
- 4.Launer LJ, Ross GW, Petrovitch H, et al. Midlife blood pressure and dementia: the Honolulu-Asia Aging Study. Neurobiol Aging 2000;21:49–55. [DOI] [PubMed] [Google Scholar]
- 5.Skoog I, Gustafson D. Hypertension and related factors in the etiology of Alzheimer’s disease. Ann NY Acad Sci 2002;977:29–36. [DOI] [PubMed] [Google Scholar]
- 6.Yoshitake T, Kiyohara Y, Kato I, et al. Incidence and risk factors of vascular dementia and Alzheimer’s disease in a defined elderly Japanese population: the Hisayama Study. Neurology 1995;45:1161–1168. [DOI] [PubMed] [Google Scholar]
- 7.Morris MC, Scherr PA, Hebert LE, Glynn RJ, Bennett DA, Evans DA. Association of incident Alzheimer disease and blood pressure measured from 13 years before to 2 years after diagnosis in a large community study. Arch Neurol 2001;58:1640–1646. [DOI] [PubMed] [Google Scholar]
- 8.Yamada M, Kasagi F, Sasaki H, Masunari N, Mimori Y, Suzuki G. Association between dementia and midlife risk factors: the Radiation Effects Research Foundation Adult Health Study. J Am Geriatr Soc 2003;51:410–414. [DOI] [PubMed] [Google Scholar]
- 9.Lindsay J, Laurin D, Verreault R, et al. Risk Factors for Alzheimer’s disease: a prospective analysis from the Canadian Study of Health and Aging. Am J Epidemiol 2002;156:445–453. [DOI] [PubMed] [Google Scholar]
- 10.Skoog I, Lernfelt B, Landahl S, et al. 15-year longitudinal study of blood pressure and dementia. Lancet 1996;347:1141–1145. [DOI] [PubMed] [Google Scholar]
- 11.Kivipelto M, Helkala EL, Laakso MP, et al. Midlife vascular risk factors and Alzheimer’s disease in later life: longitudinal, population based study. BMJ 2001;322:1447–1451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kilander L, Nyman H, Boberg M, Hansson L, Lithell H. Hypertension is related to cognitive impairment: a 20-year follow-up of 999 men. Hypertension 1998;31:780–786. [DOI] [PubMed] [Google Scholar]
- 13.Petrovitch H, White LR, Izmirilian G, et al. Midlife blood pressure and neuritic plaques, neurofibrillary tangles, and brain weight at death: the HAAS: Honolulu-Asia Aging Study. Neurobiol Aging 2000;21:57–62. [DOI] [PubMed] [Google Scholar]
- 14.Korf ESC, White LR, Scheltens P, Launer LJ. Midlife blood pressure and the risk of hippocampal atrophy: the Honolulu Asia Aging Study. Hypertension 2004;44:29–34. [DOI] [PubMed] [Google Scholar]
- 15.Guo ZC, Fratiglioni L, Zhu L, Fastbom J, Winbald B, Viitanen M. Occurrence and progression of dementia in a community population aged 75 years and older: relationship of antihypertensive medication use. Arch Neurol 1999;56:991–996. [DOI] [PubMed] [Google Scholar]
- 16.Khachaturian AS, Zandi PP, Lyketsos CG, et al. Antihypertensive medication use and incident Alzheimer disease: The Cache County Study. Arch Neurol 2006;63:686–692. [DOI] [PubMed] [Google Scholar]
- 17.Hajjar L, Catoe H, Sixta S, et al. Cross-sectional and longitudinal association between antihypertensive medications and cognitive impairment in an elderly population. J Gerontol Ser A Biol Sci Med Sci 2005;60:67–73. [DOI] [PubMed] [Google Scholar]
- 18.Forette F, Seux ML, Staessen JA, et al. The prevention of dementia with antihypertensive treatment: new evidence from the Systolic Hypertension in Europe (Syst-Eur) study. Arch Intern Med 2002;162:2046–2052. [DOI] [PubMed] [Google Scholar]
- 19.Ruitenberg A, Skoog I, Ott A, et al. Blood pressure and risk of dementia: results from the Rotterdam study and the Gothenburg H-70 Study. Dement Geriatr Cogn Disord 2001;12:33–39. [DOI] [PubMed] [Google Scholar]
- 20.Skoog I, Lithell H, Hansson L, et al. Effect of baseline cognitive function and antihypertensive treatment on cognitive and cardiovascular outcomes: Study on Cognition and Prognosis in the Elderly (SCOPE). Am J Hypertens 2005;18:1052–1059. [DOI] [PubMed] [Google Scholar]
- 21.Applegate WB, Pressel S, Wittes J, et al. Impact of the treatment of isolated systolic hypertension on behavioral variables: results from the Systolic Hypertension in the Elderly Program. Arch Intern Med 1994;154:2154–2160. [PubMed] [Google Scholar]
- 22.Prince MJ, Bird AS, Blizard RA, Mann AH. Is the cognitive function of older patients affected by antihypertensive treatment? Results from 54 months of the Medical Research Council’s trial of hypertension in older adults. BMJ 1996;312:801–805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Farmer ME, White LR, Abbott RD, et al. Blood pressure and cognitive performance: The Framingham Study. Am J Epidemiol 1987;126:1103–1114. [DOI] [PubMed] [Google Scholar]
- 24.Peters R, Beckett N, Forette F, et al. Incident dementia and blood pressure lowering in the Hypertension in the Very Elderly Trial cognitive function assessment (HYVET-COG): a double-blind, placebo controlled trial. Lancet Neurol 2008;7:683–689. [DOI] [PubMed] [Google Scholar]
- 25.Pathological correlates of late-onset dementia in a multicentre, community-based population in England and Wales: Neuropathology Group of the Medical Research Council Cognitive Function and Ageing Study (MRC CFAS). Lancet 2001;357:169–175. [DOI] [PubMed] [Google Scholar]
- 26.Galvin JE, Powlishta KK, Wilkins K, et al. Predictors of preclinical Alzheimer disease and dementia: a clinicopathologic study. Arch Neurol 2005;62:758–765. [DOI] [PubMed] [Google Scholar]
- 27.Hestad K, Kveberg B, Engedal K. Low blood pressure is a better predictor of cognitive deficits than the apolipoprotein e4 allele in the oldest old. Acta Neurol Scand 2005;111:323–328. [DOI] [PubMed] [Google Scholar]
- 28.Verghese J, Lipton RB, Hall CB, Kuslansky G, Katz MJ. Low blood pressure and the risk of dementia in very old individuals. Neurology 2003;61:1667–1672. [DOI] [PubMed] [Google Scholar]
- 29.Paran E, Anson O, Reuveni H. Blood pressure and cognitive functioning among independent elderly. Am J Hypertens 2003;16:818–826. [DOI] [PubMed] [Google Scholar]
- 30.Mirra SS, Heyman A, McKeel D, et al. Consortium to Establish a Registry for Alzheimer’s Disease (CERAD): Part II: standardization of the neuropathologic assessment of Alzheimer’s disease. Neurology 1991;41:479–486. [DOI] [PubMed] [Google Scholar]
- 31.Chobanian AV, Bakris GL, Black HR, et al. Seventh report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure. Hypertension 2003;42:1206–1252. [DOI] [PubMed] [Google Scholar]
- 32.Beckett NS, Peters R, Fletcher AE, et al. Treatment of hypertension in patients 80 years of age or older. N Engl J Med 2008;358:1887–1898. [DOI] [PubMed] [Google Scholar]
- 33.Wang J, Ho L, Chen L, et al. Valsartan lowers brain beta-amyloid protein levels and improves spatial learning in a mouse model of Alzheimer disease. J Clin Invest 2007;117:3393–3402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Morris JC, Ernesto C, Schafer K, et al. Clinical dementia rating training and reliability in multicenter studies: the Alzheimer’s Disease Cooperative Study experience. Neurology 1997;48:1508–1510. [DOI] [PubMed] [Google Scholar]
- 35.Haroutunian V, Purohit DP, Perl DP, et al. Neurofibrillary tangles in nondemented elderly subjects and mild Alzheimer disease. Arch Neurol 1999;56:713–718. [DOI] [PubMed] [Google Scholar]
- 36.Haroutunian V, Perl DP, Purohit DP, et al. Regional distribution of neuritic plaques in the nondemented elderly and subjects with very mild Alzheimer disease. Arch Neurol 1998;55:1185–1191. [DOI] [PubMed] [Google Scholar]
- 37.Haroutunian V, Schnaider-Beeri M, Schmeidler J, et al. Alzheimer’s disease neuropathology is not sufficient to explain dementia in the oldest-old. Arch Neurol 2008;65:1211–1217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tomlinson BE, Blessed G, Roth M. Observations on the brains of demented old people. J Neurol Sci 1970;11:205–242. [DOI] [PubMed] [Google Scholar]
- 39.Holm S. A simple sequentially rejective multiple test procedure. Scand J Stat 1979;6:65–70. [Google Scholar]
- 40.Anson O, Paran E. Hypertension and cognitive functioning among the elderly: an overview. Am J Ther 2005;12:359–365. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.